Open Access Article
Open Access ArticleIncreasing the waterproof performance of fatliquored leather by silane modification: a simple and feasible green treatment method
Haojie Qin,
Xiaojun Shen *,
Yuye Chai and
Xiaohong Xu
*,
Yuye Chai and
Xiaohong Xu
Zhejiang Province Key Lab of Leather Engineering, College of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, Zhejiang, China. E-mail: shenxj@wzu.edu.cn
First published on 17th January 2025
Abstract
Waterproof fatliquoring agents can transform leather from a hydrophilic state to a hydrophobic state in the wet process of leather production. However, traditional waterproof fatliquoring agents may cause environmental pollution. Fluorocarbons in fluorinated fatliquoring agents are difficult to degrade, and polyacrylic acid fatliquoring agents require chromium powder fixation. In this work, we proposed a simple and green leather waterproofing strategy through the wet process. The strategy used silane coupling agents, water, and ethanol to waterproof the fatliquored leather. The results indicated that leather exhibited excellent waterproof performance when treated with 20% hexadecyltrimethoxysilane (TMS16), 20% water and 300% ethanol at 30 °C for 6 hours. The water contact angle (WCA) of leather was greater than 120° and maintained lasting hydrophobicity, which can still sustain a high contact angle after 30 minutes. In addition, the physical properties of the leather were significantly improved. After the TMS16 treatment, the tensile strength of the leather increased from 4.82 MPa to 12.45 MPa, the tear strength increased from 77.07 N mm−1 to 125.01 N mm−1, and the elongation at break increased from 47.03% to 66.71%. This leather waterproofing strategy is environmentally friendly, and the process is simple.
1. Introduction
People's demands for leather products are becoming increasingly high and are not only limited to basic leather functions but also focused on properties such as waterproofing, flame retardancy, antibacterial effects and other features.1,2 Waterproof leather has excellent properties, which can effectively prevent water from penetrating the interior of the leather and stop the accumulation of dust and stains.3 Leather can be made waterproof through methods such as fatliquoring and coating. In the wet finishing process, leather becomes hydrophobic after being treated with waterproof fatliquoring agents.4 However, this method causes environmental pollution. Fluorocarbons in the fluorinated fatliquoring agents are difficult to degrade in nature and tend to accumulate in living organisms.5 Polyacrylic acid fatliquoring agents require chromium powder fixation, and hexavalent chromium poses serious environmental and biological hazards.6 Therefore, it is important to develop a simple and green leather waterproofing strategy in the wet process.Leather is formed by interweaving natural fibers, and there are micropores between the fibers. Water can enter the leather through the spaces between the fibers. At the same time, the active polar groups, such as amino and hydroxyl groups, in the side chains of the collagen fibers can be connected with water molecules by hydrogen bonding, thus wetting the fibers.7,8 Therefore, the principle of leather waterproofing treatment must be focused on reducing the surface tension of collagen fibers, closing the gaps between the collagen fibers and increasing the surface roughness of the leather.9
Previously, many treatment schemes for producing hydrophobic leather have been proposed. For instance, Marta Fadda et al. developed a water-protecting coating using only two food byproducts: epoxidized soybean oil and the fatty acid trimer Pripol. These two building blocks were deposited on leather and then crosslinked to create a strong network, making the leather hydrophobic, with a water contact angle >120°.10 Ma et al. prepared a super hydrophobic leather coating by spraying polyacrylate emulsion followed by hydrophobic silica nanoparticles in an ethanol dispersion system.11 However, these treatments only make the surface of the leather hydrophobic, and the inside of the leather is not hydrophobic. Once the hydrophobic coating is damaged by mechanical wear, the leather will be hydrophilic again.12 Luo et al. polymerized oil with maleic anhydride and then grafted dodecafluoroheptanol to prepare a fluorinated polymer fatliquoring agent. Fatliquored leather has excellent waterproof properties.13 Jin et al. synthesized two polymer nanoemulsions containing carboxyl groups and used them as waterproof fatliquoring agents for chrome-tanned leather. However, chromium is required to be fixed during the fatliquoring process.4 Both hexavalent chromium and fluorocarbon will cause environmental pollution.
Silane coupling agents can chemically bond with organic and inorganic materials, connecting the two materials with vastly different properties to improve the performance of the composite materials.14 The general formula is Y–Si–(OR)3. When the –OR groups hydrolyze into hydroxyl groups, silanol (Si–OH) groups are generated, which can form a covalent bond with the inorganic/polymer interface with hydroxyl groups.15 Among them, long-chain alkyl siloxanes have flexible long chains, which can significantly reduce the surface energy of substances and make them hydrophobic.16 Therefore, it is an ideal hydrophobic material. Nano SiO2 is hydrophilic due to the presence of hydroxyl groups on its surface, which limits its application in some fields. Xu et al. used hexadecyltrimethoxysilane (HDTMS) to modify the hydrophobicity of nano SiO2 and the WCA reached 170.9°, which was super hydrophobic.17 Adeleke A. Oyekanmi et al. enhanced the hydrophobicity of macroalgae polymer films with silane. Compared with the unmodified membrane, the modified membrane with silane coupling agent not only improves the hydrophobicity of the polymer membrane but also further improves the strength and thermal stability of the polymer membrane.18 Xu et al. tanned the leather with a silane coupling agent, and the treated leather had excellent and long-lasting hydrophobicity and a good tanning effect. However, the crust leather is different from the traditional leather and the post-tanning for this kind of crust leather should be further investigated.19
In this work, we proposed a simple and green leather waterproofing strategy, using silane coupling agents for the waterproofing treatment of fatliquored leather. Under the right reaction conditions, the hydroxyl group hydrolyzed by the silane coupling agent was chemically combined with the hydroxyl group on collagen fiber (Fig. 1). The silicon carbon chain acts as a hydrophobic backbone. The reaction conditions and treatment technology were explored. The hydrophobicity of the treated leather was evaluated. In addition, the physical properties and thermal stability of leather were also investigated.
 | ||
| Fig. 1 Schematic illustration of the waterproofing treatment method and the reaction mechanism of silane coupling agents. | ||
2. Materials and methods
2.1 Materials
The wet-blue sheepskins were obtained from Xinji Runmei. Leather Co., Ltd (Hebei, China). Octyltrimethoxysilane (TMS8, 97%), dodecyltrimethoxysilane (TMS12, 93%), hexadecyltrimethoxysilane (TMS16, 85%) were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd (Shanghai, China). Anhydrous ethanol was purchased from Anhui Ante Food Co., Ltd (Anhui, China). All used chemicals were analytical reagents.2.2 Preparation of hydrophobic leather
The wet-blue sheepskin was firstly fatliquored by sulfonated and succinated lanolin acid fatliquoring agent.20 Then, the fatliquored leather was dried at 50 °C for 1 hour.In a typical waterproofing treatment, 30.0 g of the dried sheepskin, 6.0 g of TMS (TMS8 or TMS12 or TMS16), 6.0 g of water and 90.0 g of ethanol were mixed in a drum. After rotating the mixture in the drum at 30 °C for 6 hours, it was kept standing overnight. Finally, the leather was dried and tumbled. The wet finishing process is shown in Table 1.
| Process | Materials | Dosage/% | Temperature/°C | Time/min | pH |
|---|---|---|---|---|---|
| Chrome retanning | |||||
| Neutralizing | Water | 150 | 35 | ||
| Sodium formate | 2 | 30 | |||
| Sodium bicarbonate | 1 | 60 | 5.5–5.8 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Washing | |||||
| Fatliquoring | Water | 200 | 50 | 60 | |
| Fatliquoring | 8 | ||||
| Agent | |||||
| Formic acid | 1.5 | 10 + 30 | 3.3 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Washing, drying | |||||
| Waterproofing | TMS | 20 | 30 | ||
| Water | 20 | ||||
| Ethanol | 300 | 360 | |||
| Drying, softening | |||||
2.3 Characterization
The chemical composition of the samples was monitored using an FTIR spectrometer (Nicolet 6700, PerkinElmer, USA). The spectral region scanned was 4000–500 cm−1 with a resolution of 4 cm−1. The spectra were normalized to their maximum. The mass loss of samples in heating was characterized using a thermal analyzer (TGA, PerkinElmer, USA) from 50 °C to 700 °C with a heating rate of 10 °C min−1 under a nitrogen atmosphere. In order to analyze the chemical composition in detail, the binding energies of the elements were investigated by X-ray photoelectron spectroscopy (PHI 5000 VP III, ULVAC-PHI, Japan). Fiber dispersity of the leather samples was observed using a scanning electron microscope (Nova 200 NanoSEM, FEI, USA). The EDS mapping was performed using the same scanning electron microscope.2.4 Water contact angle
Dynamic water contact angles were measured for leather using a contact angle goniometer (DSA30, Krüss, Germany) at room temperature. Deionized water droplets of 10 μL were deposited on the surface and the contact angle was calculated at specific time points (0, 5, 10, 15, 20, 25, and 30 minutes). The contact angle was calculated from three measurements on each sample.2.5 Physical properties
Softness was measured using a leather softness tester (GT- 300, Gotech, China), and the results are the average of three tests. Tear force, tensile strength, and elongation at the break of all the samples were determined using a leather multifunctional tester (AI- 7000SN, Gotech, China), and the results are the average of the three tests.3. Results and discussion
3.1 Optimization of reaction conditions
Silane coupling agent hydrolyzes to produce silanols. Silanol penetrates into leather.19 Then, silanol crosslinks with itself or collagen fibers. Controlling the reaction conditions is to control the hydrolysis and condensation rates of the silane coupling agents.21,22 The condensation rate was reduced to ensure complete penetration and uniform distribution of the silane coupling agent inside the leather. If the condensation rate is too fast, the silane coupling agent will condense on the surface of the leather, hindering its penetration and distribution inside the leather.With the increase of the TMS16 dosage, the WCAs of leather increases gradually, and the change of WCAs decreases with the extension of time (Fig. 2a). Among them, the leather treated with 20% TMS16 has the best hydrophobic effect. After 30 minutes, WCAs dropped from 126.2° to 111.9°, a decrease of only 14.3°. The 24 h static water absorption rate of 20% TMS16 leather was the lowest, which was 61.2% (Fig. 3a). But excessive use of TMS16 can also have negative effects. Too much condensation of TMS16 on the leather surface, produces white flocculent affecting its penetration in the leather interior, resulting in a decrease in leather hydrophobicity.
Water plays an important role in the hydrolysis process of silane coupling agents. As shown in Fig. 2b, leather treated with 20% water content has the best hydrophobic effect, and the WCA did not significantly decrease over time. After 30 minutes, WCA was 101.9°. However, too much or too little water can affect the hydrophobicity of leather. The more water content in the system, the poorer the hydrophobic durability. After 30 minutes, the WCA of leather treated with 80% water content was only 50.8°. This is because the water content is too high. Silane coupling agent rapidly hydrolyzes on the surface of the leather, undergoing self-condensation, and reacting with collagen fibers. This hinders its penetration and distribution inside the leather. Too little water in the system can also affect the hydrophobicity of leather. Due to little water, the silane coupling agent cannot fully hydrolyze and cannot react with collagen fibers or itself, only physically adsorbing with collagen fibers. Fig. 3b shows the same pattern. The static water absorption of leather treated with 20% water was the lowest.
The carbon chain length of the silane coupling agents also has a significant impact on the hydrophobicity of leather. The influence of the carbon chain length was explored (Fig. 2c), with the amounts of TMS8, TMS12 and TMS16 controlled to 20%. Leather treated with TMS16 had the best hydrophobic effect. After 30 minutes, WCA only decreased by 9°. The hydrophobic effect of the leather treated with TMS8 was worse than that of untreated leather. After 5 minutes, WCA quickly dropped to 0°. The results show that the longer carbon chain in the silane coupling agent is beneficial to the hydrophobic effect.
Fig. 2d and 3d show the influence of the reaction temperature on the hydrophobicity of leather. Both high and low temperatures can reduce the hydrophobicity of leather. When at high temperatures, the silane coupling agent is rapidly hydrolyzed and condensed on the surface of the leather. This affected the distribution and reaction of silane coupling agents inside the leather. Low temperature led to a slow hydrolysis rate of the silane coupling agent and less reaction between the collagen fibers and themselves.
The reaction time can also affect the hydrophobicity of leather (Fig. 2e). The hydrophobicity of leather was not good when the reaction time was too short. WCA rapidly decreases over time. After 30 minutes, the WCA was less than 100°. Approximatively, the static water absorption rate of leather with a reaction time of 6 hours and overnight is the lowest, at 66.9% (Fig. 3e). This is because when the reaction time is too short, the silane coupling agent is not fully hydrolyzed, and the binding with collagen fibers is lower. Meanwhile, due to the short reaction time, the penetration and distribution of silane coupling agents in leather are uneven.
Fig. 2f shows the effect of proportionally increasing or decreasing the overall dosage on the hydrophobicity of leather. By proportionally reducing the dosage, WCA rapidly decreased over time. After 5 minutes, it had dropped below 90°. Meanwhile, the smaller the dosage, the more significant the decrease in WCA. Fig. 3f shows the same pattern. This is caused by the reduction of silane coupling agents. By proportionally increasing the dosage, the WCAs of the leather slightly increased. It is probably because the amount of the silane coupling agent has reached the critical point, and increasing the amount has little effect on the hydrophobicity of leather. At the same time, due to the proportional increase in dosage, serious condensation occurred on the leather's surface, resulting in a large number of white spots. In addition, leather cannot absorb all ethanol. Excess ethanol dissolved the fatliquoring agent in the leather, affecting its performance.
From Fig. 4a–f, it can be observed that the reaction conditions are not properly controlled, and white particles will deposit on the leather surface. This was caused by the accumulation of silane coupling agents on the surface of the leather. A large amount of silane coupling agent was deposited on the leather surface, which hinders its penetration and distribution inside the leather. It further affected the hydrophobicity and appearance of leather. In too little water, the silane coupling agent cannot be fully hydrolyzed, and only physical adsorption occurs (Fig. 4a). Too much water or too high temperature will cause the hydrolysis rate of the silane coupling agent to be too fast, and white particles will appear on the leather surface (Fig. 4b and c). Excessive silane coupling agent condensation on the leather surface can also cause this phenomenon (Fig. 4d and e). When the reaction conditions are properly controlled, white particles will not appear on the leather surface (Fig. 4f). At the same time, leather had excellent hydrophobicity.
3.2 Water contact angle
Fig. 5 shows the changes of water droplets in the leather grain layer, flesh layer and middle layer over time. It can be found that over time, water droplets on the surface of untreated leather are slowly absorbed and WCA decrease. After 30 minutes, the water droplets are completely absorbed. On the contrary, with the change of time, the water droplets on the TMS16-treated leather surface did not change significantly. Whether it is in the grain layer, flesh layer, or middle layer, the leather has the same hydrophobicity. The phenomenon shows that leather has an overall hydrophobicity. At the same time, it can be found that the WCA of the middle layer and flesh layer are larger than that of the grain layer. This is because the lint increases the roughness, which increases the WCA.23 | ||
| Fig. 5 WCAs of the grain layer, flesh layer and middle layer of untreated leather and TMS16 treated leather. | ||
3.3 Characterization: FTIR, TGA, EDX and XPS
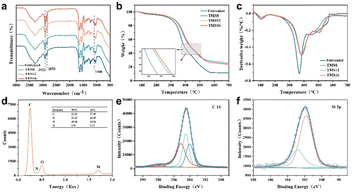
To verify the covalent bond formed between silane coupling agent and collagen fibers, FTIR spectra of the untreated leather and leather treated with TMS with different carbon chains were measured, as displayed in Fig. 6a. Symmetric and asymmetric stretching vibration peaks of – CH3 and – CH2 appeared at 2923 cm−1 and 2853 cm−1, respectively.24 After being treated with a silane coupling agent, the stretching vibration peak intensity of –CH3 and –CH2 increases, and as the carbon chain length of the silane coupling agent increases, its stretching vibration intensity peak gradually becomes stronger, indicating the successful grafting of the silane coupling agent.25 The peak intensity at 1080 cm−1 increased after treatment with a silane coupling agent, indicating the presence of Si–O–Si.26 The TGA and DTG curves of the samples from 50 °C to 800 °C under N2 are shown in Fig. 6b and c. The thermal decomposition curves of the samples were similar. Thermal decomposition was divided into two stages: the evaporation of water and small molecules inside the leather and the collagen fibers undergoing thermal decomposition.27 The residual mass and the thermal decomposition temperature of the leather treated with silane coupling agent are higher than those of untreated leather. The results showed that the silane coupling agent successfully combined with collagen fibers through covalent bonding while improving the thermal stability of leather. Energy dispersive spectroscopy (EDS) analysis was performed to assess the chemical composition of TMS16-treated leather. As shown in Fig. 6d, the peaks of C, N, O and Si were detected in the leather. The element Si and a part of element C were mainly derived from TMS16. The mass percentage of Si was 5.91%. The XPS spectral analysis of the chemical state of the leather surface treated with TMS16 is shown in Fig. 6e and f. Peaks at about 284 eV in the TMS16-treated leather can be associated with Si–C, and the peak at about 284.5 eV can be associated with C–C, the peak at about 285.1 eV can be associated with C–O, and the peak at about 288.1 eV can be associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.28,29 In the low-intensity Si 2p spectrum, the presence of the Si–C group can be observed from the peak around 102.9 eV, and the presence of the Si–O group can be observed the peak at around 102.2 eV.30 The presence of these groups (especially Si–C) indicates that the alkyl chain of the silane coupling agent has successfully integrated into the collagen fibers. Thus, the XPS results supported the FTIR results.
O.28,29 In the low-intensity Si 2p spectrum, the presence of the Si–C group can be observed from the peak around 102.9 eV, and the presence of the Si–O group can be observed the peak at around 102.2 eV.30 The presence of these groups (especially Si–C) indicates that the alkyl chain of the silane coupling agent has successfully integrated into the collagen fibers. Thus, the XPS results supported the FTIR results.
3.4 Morphological analysis
The fiber dispersity of the untreated leather and TMS16-treated leather was observed by SEM (Fig. 7a and b). There was no significant difference in fiber structure between untreated and TMS16-treated leather at low magnification. After magnification, it was found that the fiber bundles treated with TMS16 were better dispersed than the untreated ones. This indicates that the method contributes to fiber dispersion in leather.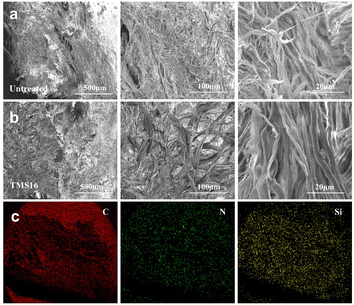 | ||
| Fig. 7 (a) Cross-section SEM images of untreated leather. (b) Cross-section SEM images of TMS16-treated leather. (c) EDS-mapping of the cross-section of TMS16-treated leather. | ||
As shown in the EDS mapping of the cross-section, similar to C and N, Si was almost evenly distributed in the cross-section of the TMS16-treated leather (Fig. 7c), which favored the overall hydrophobicity of the leather. The results indicated that the penetration of the silane coupling agent into leather is comprehensive. The full penetration of the silane coupling agent ensures the overall hydrophobicity of leather.
3.5 Physical properties
Collagen fiber is a kind of biological material with good mechanical properties.31 The tensile strength (Fig. 8a), elongation at break (Fig. 8b), and tear strength (Fig. 8c) of the leather treated with the silane coupling agent were significantly improved. At the same time, with the increase of the carbon chain length, the tensile strength and the tear strength also increased. After the TMS16 treatment, the tensile strength of the leather increased from 4.82 MPa to 12.45 MPa, the tear strength increased from 77.07 N mm−1 to 125.01 N mm−1, and the elongation at break increased from 47.03% to 66.71%. This is because the silane coupling agent crosslinks with itself and collagen fibers, enhancing their mechanical properties. The longer the hydrophobic chain, the more effective the dispersion of the collagen fibers, which is beneficial for improving elasticity and ductility. This phenomenon may be due to the hydrophobic modification of the long carbon chains that lubricate and disperse the collagen fibers, providing more space for the fibrils and fibers to slide against each other to cushion the strain.32,33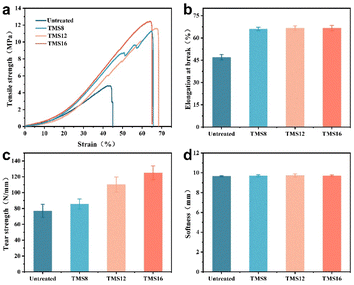 | ||
| Fig. 8 (a) Tensile strength curves, (b) elongation at break, (c) tear strength, and (d) softness of the sheepskins with different treatments. | ||
After treatment with the silane coupling agent, there was no significant change in the softness of the leather (Fig. 8d), and excellent softness was still maintained. This indicates that the introduction of silane coupling agents will not affect the softness of leather. It is worth noting that ethanol needs to be added during the waterproofing treatment, but excessive ethanol can dissolve the fatliquoring agent in the leather, causing the fatliquoring process to lose its effectiveness. Therefore, reasonable control of the amount of ethanol will not affect the fatliquoring process, and leather will still have excellent softness.
In addition, we investigated the water vapor permeability of leather, which impacts the essential functional properties such as the breathability and comfort of leather products.34,35 The water vapor permeability of the untreated leather was 19.6 (mg cm−2 h−1). After the TMS16 hydrophobic treatment, the water vapor permeability was 16.4 (mg cm−2 h−1). After the hydrophobic treatment, the water vapor permeability of the leather decreased slightly. This is because the untreated leather is hydrophilic and has excellent moisture absorption. However, the water vapor permeability decreased a little, which indicates that the hydrophobic treatment has little effect on the water vapor permeability of leather.
3.6 Leather hydrophobicity
In order to verify the hydrophobicity of this waterproofing strategy on leather, the wetting behavior of various liquids on the untreated and TMS16-treated leather surfaces was characterized (Fig. 9a). Water, dye droplets, coffee, milk, tea are easily diffused and absorbed on the leather. However, the various droplets could stand almost in spherical shapes on the leather surface after the TMS16 treatment. These phenomena show that the leather treated by the waterproofing strategy has stable hydrophobicity to different liquids. In addition, TMS16-treated leather floated on water, while the untreated leather sunk to the bottom. The surface of the TMS16-treated leather is covered with fine bubbles, and the silver mirror phenomenon can be observed (Fig. 9b). | ||
| Fig. 9 (a) Various droplets (water, coffee, milk, tea, and dye droplets) on the leather grain layer, (b) the photo of untreated leather and TMS16-treated leather in water. | ||
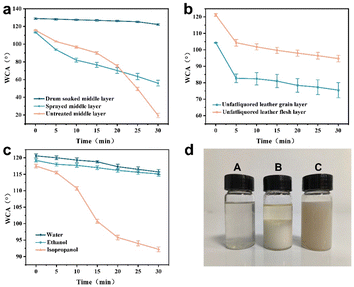
To investigate the effect of different treatment methods on the overall hydrophobicity of leather, we evaluated the hydrophobicity of the middle layer of leather (Fig. 10a). The leather surface after spraying had hydrophobicity. However, the hydrophobicity of the middle layer was poor. After 30 minutes, the WCA dropped to 55.9°. The middle layer of the drum-soaked leather still exhibited excellent hydrophobicity. After 30 minutes, the water contact angle remained at 122.2°. This is because of the spraying method, the silane coupling agent only stays on the surface of the leather and does not penetrate the interior of the leather. The hydrophobicity of drum-soaked leather is all over, and the silane coupling agent completely penetrates and evenly distributes throughout every part of the leather.
In addition, we investigated the effect of the waterproofing treatment sequence on the hydrophobicity of leather (Fig. 10b). Unfatliquored leather treated with TMS16 had poor hydrophobicity. At the same time, after hydrophobic treatment of the unfatliquored leather, there are difficulties in leather wetting and absorption of the fatliquoring agent in the subsequent fatliquoring process (Fig. 10d). Therefore, it is best to arrange the waterproofing treatment in the last process of wet process.
After soaking in water and ethanol for 24 hours, the WCA of the leather treated with TMS16 remained at around 120° (Fig. 10c). Leather had hydrophobic persistence. However, after soaking in isopropanol for 24 hours, the hydrophobicity of the leather decreased. However, after 30 minutes, the water contact angle was still greater than 90°. These results indicate that the combination of the silane coupling agents and collagen fibers endows leather with lasting and stable hydrophobicity, which can resist complex external environments.
4. Conclusions
In this work, we utilized silane coupling agents to crosslink collagen fibers and self-transforming hydrophilic leather into hydrophobic leather. The silicon and long carbon chains in silane coupling agents play a hydrophobic role. By measuring the water contact angle and 24 h static water absorption of leather, the method is proven to be feasible and effective. This waterproof strategy not only gives the leather a durable and stable hydrophobicity but also enhances the mechanical properties of the leather. In addition, we determined the optimal treatment conditions for this waterproofing strategy. The waterproof strategy only uses silane coupling agents, ethanol, and water. It has reference significance for the development of green and environmentally friendly waterproof leather.Data availability
All data generated or analysed during this study are included in this published article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Wenzhou Major Scientific and Technological Innovation Project (ZG2022036) and the Key R&D Program of Zhejiang Province (2017C03055).References
- G. Liu, K. Li, Q. Luo, H. Wang and Z. Zhang, J. Colloid Interface Sci., 2017, 490, 642–651 CrossRef CAS PubMed.
- N. Kamely, Polym. Bull., 2022, 79, 1977–2002 CrossRef CAS.
- Y. Bao, J. Chang, Y. Zhang and L. Chen, Chem. Eng. J., 2022, 446, 136959 CrossRef CAS.
- L. Jin, W. Xu, H. Wen, Y. Wang and F. Zhang, Materials, 2023, 16, 1464 CrossRef CAS PubMed.
- Z. Liu, J. Zhou, Y. Xu, J. Lu, J. Chen and J. Wang, RSC Adv., 2022, 12, 21247–21254 RSC.
- J. Jimenez-Paz, J. J. Lozada-Castro, E. Lester, O. Williams, L. Stevens and J. Barraza-Burgos, J. Environ. Chem. Eng., 2023, 11, 109715 CrossRef CAS.
- X. Huang, X. Kong, Y. Cui, X. Ye, X. Wang and B. Shi, Chem. Eng. J., 2018, 336, 633–639 CrossRef CAS.
- M. Khosravi and S. Azizian, ACS Appl. Mater. Interfaces, 2015, 7, 25326–25333 CrossRef CAS PubMed.
- X. He, Y. Huang, H. Xiao, X. Xu, Y. Wang, X. Huang and B. Shi, Green Chem., 2021, 23, 3581–3587 RSC.
- M. Fadda, A. Zych, R. Carzino, A. Athanassiou and G. Perotto, Green Chem., 2024, 26, 542–555 RSC.
- J. Ma, X. Zhang, Y. Bao and J. Liu, Colloids Surf., A, 2015, 472, 21–25 CrossRef CAS.
- Z. Lin, Z. Sun, C. Xu, A. Zhang, J. Xiang and H. Fan, RSC Adv., 2021, 11, 27620–27626 RSC.
- Z. Luo, H. Fan, Y. Lu, H. Shi and B. Shi, China Leather, 2007, 23, 12–16 Search PubMed.
- P. Yiren, Z. Meng, Z. Jian, Z. Xiaoyao, B. Huiguang and W. Chuansheng, Mater. Des., 2020, 13, 4896 Search PubMed.
- T. Aziz, A. Ullah, H. Fan, M. I. Jamil, F. U. Khan, R. Ullah, M. Iqbal, A. Ali and B. Ullah, J. Polym. Environ., 2021, 29, 3427–3443 CrossRef CAS.
- X. Shang, Y. Zhu and Z. Li, Appl. Surf. Sci., 2017, 394, 169–177 CrossRef CAS.
- B. Xu and Q. Zhang, ACS Omega, 2021, 6, 9764–9770 CrossRef CAS PubMed.
- A. A. Oyekanmi, N. I. Saharudin, C. M. Hazwan, H. P. S. A. Khalil, N. G. Olaiya, C. K. Abdullah, T. Alfatah, D. A. Gopakumar and D. Pasquini, Molecules, 2021, 26, 2254 CrossRef CAS PubMed.
- S. Xu and B. Shi, J. Cleaner Prod., 2023, 383, 135526 CrossRef CAS.
- H. Qin, G. Qian, J. Wu, X. Shen and J. Soc, Leather Technol. Chem., 2024, 108, 51–56 CAS.
- Q. Zhai, W. Zhao, Z. Lu, J. Wang, D. Zhao and G. Zhou, Silicon, 2023, 15, 3945–3957 CrossRef CAS.
- M.-C. B. Salon and M. N. Belgacem, Phosphorus Sulfur Relat. Elem., 2011, 186, 240–254 CrossRef.
- S. Ge-Zhang, T. Cai, H. Yang, Y. Ding and M. Song, Front. Bioeng. Biotechnol., 2022, 10, 1033514 CrossRef PubMed.
- H. J. Perera, R. Latifi and F. D. Blum, J. Phys. Chem. C, 2019, 123, 19005–19012 CrossRef CAS.
- S. Xu, H. Xiao and B. Shi, J. Leather Sci. Eng., 2022, 4, 26 CrossRef CAS.
- S. Li, Y. Wang, W. Xu and B. Shi, ACS Sustainable Chem. Eng., 2020, 8, 5091–5099 CrossRef CAS.
- E. Ayse, Mater. Chem. Phys., 2023, 295, 127194 CrossRef.
- F. Zhao, H. Li, L. Ji, Y. Mo, W. Quan, W. Du, H. Zhou and J. Chen, Surf. Coat. Technol., 2009, 203, 981–985 CrossRef CAS.
- A. Avila, I. Montero, L. Galán, J. M. Ripalda and R. Levy, J. Appl. Phys., 2000, 89, 212 CrossRef.
- M. Zhao, K. Cao, M. Liu, J. Zhang, R. Chen, Q. Zhang and Z. Xia, Angew. Chem., Int. Ed., 2020, 59, 12938–12943 CrossRef CAS PubMed.
- K. H. Sizeland, H. C. Wells, S. J. R. Kelly, R. L. Edmonds, N. M. Kirby, A. Hawley, S. T. Mudie, T. M. Ryan and R. G. Haverkamp, RSC Adv., 2017, 7, 40658–40663 RSC.
- B. Depalle, Z. Qin, S. J. Shefelbine and M. J. Buehler, J. Mech. Behav. Biomed. Mater., 2015, 52, 1–13 CrossRef PubMed.
- W. Yang, M. A. Meyers and R. O. Ritchie, Prog. Mater. Sci., 2019, 103, 425–483 CrossRef.
- Y. Bao, C. Shi, Y. Yang, J. Ma and R. Sha, RSC Adv., 2015, 5, 11485–11493 RSC.
- S. Sängerlaub, M. Schmid and K. Müller, Food Packag. Shelf Life, 2018, 17, 80–84 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |