Direct Suzuki–Miyaura cross-coupling of C(sp2)–B(dan) bonds: designed in pursuit of usability†
Hiroki
Andoh
,
Ryo
Nakagawa
,
Tatsuya
Akutagawa
,
Eiko
Katata
and
Teruhisa
Tsuchimoto
 *
*
Department of Applied Chemistry, School of Science and Technology, Meiji University, 1-1-1 Higashimita, Tama-ku, Kawasaki 214-8571, Japan. E-mail: tsuchimo@meiji.ac.jp
First published on 10th April 2025
Abstract
We developed practical reaction conditions and a procedure for the direct Suzuki–Miyaura cross-coupling (SMCC) of C(sp2)–B(dan) bonds. Below are important notes to successfully execute the direct SMCC: (1) dehydrated conditions that exclude as much H2O as possible are required, (2) LiOH is the base of choice, (3) dppf is the ligand of choice when using electron-deficient (hetero)aryl halides [(Het)ArX], (4) P(t-Bu)3 is the ligand of choice when using electron-rich (Het)ArX, and (5) COD is the ligand of choice when using (Het)ArX with a protic functional group such as NH2 and OH. Taking heed of these notes enables the direct SMCC of the C(sp2)–B(dan) bond by using a wide range of substrates with diverse functional groups, affording the following series of coupling products: Ar–Ar, Ar–HetAr, HetAr–HetAr, alkenyl–Ar, and alkenyl–alkenyl. Sequentially executing distinct types of palladium-catalyzed CCs, such as Buchwald–Hartwig CC + SMCC, Mizoroki–Heck reaction + SMCC, and Sonogashira–Hagihara CC + SMCC, allows access to complex π-conjugated molecules. The B(dan) moiety also exhibits outstanding compatibility with Wittig olefination and Sc(OTf)3-catalyzed acetal-forming reactions, enabling molecular transformations that are otherwise impracticable when using ArB(OH)2. Mechanistic studies suggest the involvement of both path A, wherein a boronate species reacts with an arylpalladium halide, and path B, wherein a boron compound reacts with an arylpalladium hydroxide, at the stage of the transmetalation.
Introduction
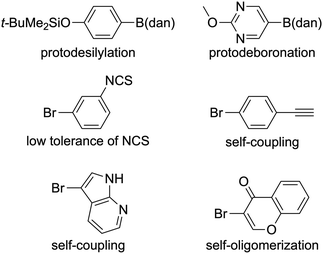
Constructing a C–C bond is a crucial step for the synthesis of complex organic molecules such as drugs, agrochemicals, and optoelectronic materials. Among a lot of synthetic transformations, the transition-metal-catalyzed cross-coupling (CC) reaction of organoboron compounds, that is, the SMCC, has been broadly utilized as one of the most powerful C–C bond-forming protocols.1 This will be due to the low toxicity, commercial availability, high bench stability and good reactivity of organoboron compounds. In particular, the stability and reactivity are exquisitely balanced, unlike other CC reagents based on Mg, Li, Cu, Zn, Sn, and Si.2 Due to these advantages, the exploitation of new organoboron reagents has been actively pursued in the last three decades.3 The organoboron compounds shown in Fig. 1a have been utilized as reactive substrates for the SMCC. In contrast, a unique concept of using organoboron compounds was independently introduced by Suginome4 and Burke5 in 2007. The boryl groups depicted in Fig. 1b are designed to be inactive towards the SMCC, due to the Lewis acidity weakened by the electron donation from the adjacent nitrogen atoms. Accordingly, B(dan),4 B(mida),5 and B(aam)6 behave as though they are masked under the SMCC conditions. A significant achievement with the masked boryl group is iterative SMCCs under the coexistence of a reactive boryl group, allowing access to complex organic molecules, like oligoarenes and natural products (Scheme 1).4–7Among the three masked boryl groups, B(dan) is reportedly the most resistant to hydrolysis8 and used for the SMCC after the conversion to B(OH)2 or B(pin) under strongly acidic conditions.4 This B(dan) technology is now recognized as a boron-masking–unmasking strategy. Despite its robustness, we envisioned that the direct use of B(dan) in the SMCC would contribute to the further progress of CC chemistry in terms of the step economy and substrate scope. The former reduces time, cost, and waste by avoiding the unmasking step. The latter leads to, for instance, solving the 2-pyridyl problem; 2-pyridylB(OH)2 is infamously unstable due to rapid protodeboronation,9 in contrast to 2-pyridylB(dan) (Fig. 2).10 Due to the same reason, the SMCC of 2-heteroarylB(OH)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and perfluoroarylB(OH)2
9 and perfluoroarylB(OH)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9,11 is also challenging. These issues partly apply to their B(pin) variants.12 Accordingly, the SMCC, a Nobel Prize-winning reaction, will be much more practical and reliable by directly utilizing B(dan).
9,11 is also challenging. These issues partly apply to their B(pin) variants.12 Accordingly, the SMCC, a Nobel Prize-winning reaction, will be much more practical and reliable by directly utilizing B(dan).
 | ||
Fig. 2 Stability of 2-pyridylB(OH)2 and 2-pyridylB(dan). a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) At pH 7 and 70 °C in 50% aqueous 1,4-dioxane. At pH 7 and 70 °C in 50% aqueous 1,4-dioxane. | ||
Since 2007, it has been believed that B(dan) is inert under SMCC conditions. Despite this preconception, we disclosed for the first time that the C–B(dan) bond of alkynylB(dan) can be directly activated in the SMCC (Scheme 2a).13 More recently, the Yoshida–Tsuchimoto10 and Mutoh–Saito14 teams independently achieved the direct SMCC of aryl/alkenylB(dan) with more robust C(sp2)–B(dan) bonds (Scheme 2b).15 Both teams employed KOt-Bu as a base, which is crucial for providing the transmetalation-active boronate salt, [(dan)B(Ot-Bu)–Ar]− (conditions A in Scheme 2b).16 However, base-sensitive functional groups are difficult to apply thereto. Ba(OH)2 is an effective alternative in such cases but unsatisfactory in terms of product yields: 33–64% (conditions B in Scheme 2b).10 We have since endeavoured to ensure more practical and reliable direct SMCC of the C(sp2)–B(dan) bond. In this context, we have preliminarily confirmed and reported that one of the reaction conditions presented in this study is effective for the synthesis of optoelectronic molecules.13d After this, the Tsui group reported unique, base-free direct SMCC of fluorinated alkenylB(dan).17 The Yoshida group recently reported a palladium/copper co-catalyzed system for base-sensitive (Het)ArB(dan).18 Herein, we report the details of the direct SMCC catalyzed solely by palladium, using three sets of reaction conditions applicable to couplings of a wide variety of substrate combinations.
 | ||
| Scheme 2 Previous work on direct SMCC of C–B(dan) bonds. Ar covers 5- and 6-membered heteroaryls. DMF = HCONMe2. Cy = cyclohexyl. | ||
Results and discussion
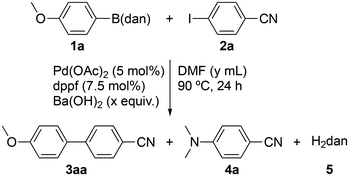
This study started by examining the reaction conditions for the direct SMCC of p-MeOC6H4B(dan) (1a) with p-IC6H4CN (2a) (Table 1). The results that we have previously reported are given in entry 1: 1a (0.20 mmol)/2a/Ba(OH)2 (1/1.5/2 equiv.), Pd(OAc)2 (5 mol%), and dppf (7.5 mol%) in DMF (0.40 mL) at 90 °C for 24 h.10 This reaction afforded the desired product 3aa in 68% yield. Due to the excellent reaction efficiency [yield/conversion (conv.) = 68%/68%], enhancing the conversion of 1a was expected to deliver 3aa in a higher yield. A higher amount of Ba(OH)2 (x = 3) was thus used, but, unfortunately, both the conversion and yield decreased (entry 2). This was thought to be attributed to the difficulty of stirring caused by the heterogeneity of the reaction mixture, including a large amount of solid Ba(OH)2 in 0.4 mL of DMF. To improve the stirring efficiency, using a higher volume of DMF (0.8 mL) increased both the conversion and yield (entry 3). However, side reactions to yield significant amounts of p-Me2NC6H4CN (4a) and H2dan (5) were observed. Higher loadings of Pd(OAc)2/dppf (10/15 mol%) further assisted the formation of 4a (entry 4), which was confirmed to occur via an SNAr reaction as well as under palladium catalysis from 2a and Me2NH that is in situ produced by the hydrolysis of DMF. Another byproduct 5 results from the hydrolysis of 1a. The purpose of this study is the direct SMCC of 1a. Hence, the hydrolysis of 1a, causing the formation of p-MeOC6H4B(OH)2 that can also react with 2a to afford 3aa, must be prevented. We next evaluated the experimental procedure. Procedures A and B are summarized in Fig. 3. The reactions in entries 1–4 of Table 1 have thus far been conducted through procedure A, where 15 min of brief drying of base Ba(OH)2in vacuo is performed before starting the reaction, along with using commercially available dried DMF. We then tested the efficacy of procedure B, where all the chemicals, which are 1, 2, a pre-dried base, a palladium precatalyst, and a ligand, are dried in vacuo for 2 h, followed by the addition of the same grade of DMF. As shown in entry 5 of Table 1, procedure B was superior to procedure A, reducing the generation of 5 (entry 3 vs. 5).| Entry | x | y | Proc. | Conv. (%) | Yield (%) of | ||
|---|---|---|---|---|---|---|---|
| of 1a | 3aa | 4a | 5 | ||||
| a Reagents: 1a (0.20 mmol), 2a (0.30 mmol), Pd(OAc)2 (10 μmol), dppf (15 μmol), Ba(OH)2 (0.40 or 0.60 mmol), and DMF (0.40 or 0.80 mL). See Fig. 3 for procedures A and B. Conversions and yields were determined by 1H NMR. Proc. = procedure. Conv. = conversion. b Isolated yield: 57%. c Performed for 6 h. d Pd(OAc)2 (20 μmol, 10 mol%) and dppf (30 μmol, 15 mol%) were used. | |||||||
| 1 | 2 | 0.4 | A | 68 | 68b | 3 | <1 |
| 2c | 3 | 0.4 | A | 41 | 35 | 7 | <1 |
| 3 | 3 | 0.8 | A | 76 | 69 | 10 | 15 |
| 4d | 3 | 0.8 | A | 77 | 70 | 32 | 17 |
| 5 | 3 | 0.8 | B | 76 | 72 | 10 | 8 |
With procedure B, we continuously examined the effect of other bases (Table 2). Bases that have shown relatively good performance in the preceding study were selected here (see Table S4 in the ESI of ref. 10). The results of entry 5 of Table 1 are presented again in entry 7 of Table 2 for comparison. As a result, LiOH emerged as the most promising option, increasing the yield of 3aa to 85% while remarkably restricting the two side reactions (entries 1–7). Since LiOH (pKb = 0.18) has comparable basicity to Ba(OH)2 (pKb = 0.15),19 the reduction in the side reactions is unlikely to be due to the lower basicity of LiOH, but it could be due, in part, to the lower nucleophilicity of OH− bound to Li+ (73 pm) with a much smaller ionic radius than Ba2+ (149 pm).20,21 However, using LiOH caused poor reproducibility in the yield of 3aa (entries 1 and 8). This could be improved by increasing the loading of Pd(OAc)2 and dppf: using 10/15 mol% of Pd(OAc)2/dppf delivered 3aa in the highest yield of 94% without producing 4a and 5 (entries 9 and 10). Reverting procedure B back to A again caused the side reactions, showing the validity of procedure B irrespective of the base used (entry 11). Entry 12 revealed that dppf is necessary as a ligand. The final tuning of the reaction time indicated that the reaction reaches completion in 8 h (entry 13).
| Entry | Base | Conv. (%) | Yield. (%) of | ||
|---|---|---|---|---|---|
| of 1a | 3aa | 4a | 5 | ||
| a Reagents: 1a (0.20 mmol), 2a (0.30 mmol), Pd(OAc)2/dppf (10/15 μmol) for entries 1–8 or (20/30 μmol) for entries 9–13, base (0.60 mmol), and DMF (0.80 mL). Conversions and yields were determined by 1H NMR. Reactions were performed through procedure B. b Performed through procedure A. c Without dppf. d Performed for 8 h. | |||||
| 1 | LiOH | 93 | 85 | 2 | <1 |
| 2 | KF | 4 | <1 | <1 | <1 |
| 3 | KOAc | 13 | <1 | <1 | <1 |
| 4 | KHCO3 | 30 | 17 | <1 | <1 |
| 5 | K3PO4 | 33 | 11 | <1 | <1 |
| 6 | Cs2CO3 | 35 | 10 | <1 | <1 |
| 7 | Ba(OH)2 | 76 | 72 | 10 | 8 |
| 8 | LiOH | 97 | 71 | 1 | <1 |
| 9 | LiOH | >99 | 94 | <1 | <1 |
| 10 | LiOH | >99 | 93 | <1 | <1 |
| 11b | LiOH | 90 | 89 | 1 | 3 |
| 12c | LiOH | 62 | 25 | <1 | <1 |
| 13d | LiOH | >99 | 93 | <1 | <1 |
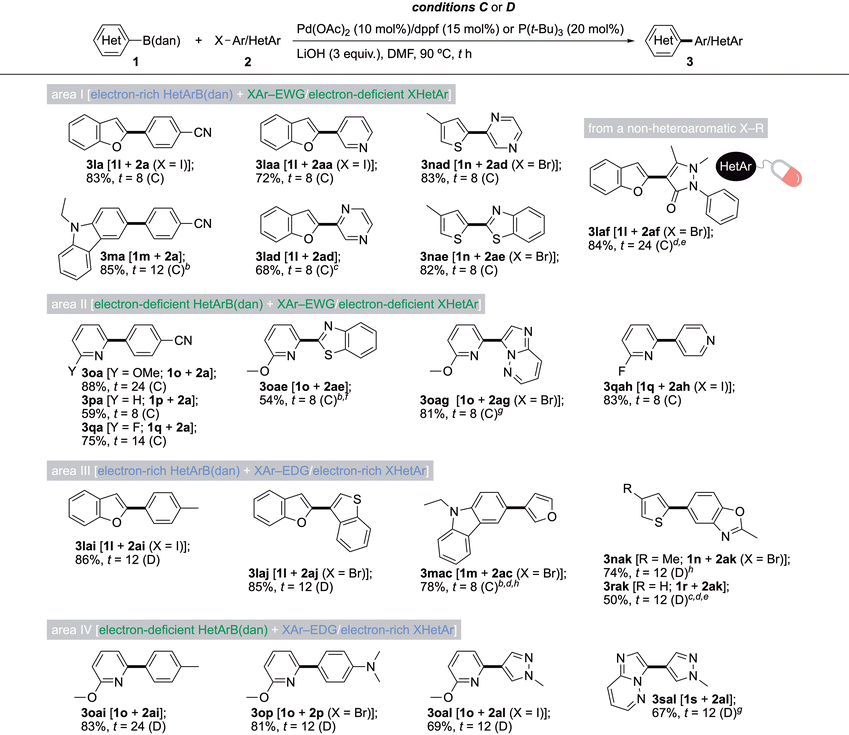
Our strategic framework to clarify the usability of this reaction is depicted in Fig. 4, which is divided into four areas I–IV based on the electronic properties of the two substrates: ArB(dan) 1 and XAr 2 with an EDG or EWG. We expected that filling the four areas with enough coupling products should be a good demonstration of the broad substrate scope of this reaction.
 | ||
| Fig. 4 A strategic framework for the substrate scope. EDG = electron-donating group. EWG = electron-withdrawing group. | ||
We started with the conquest of the area I: EDG–ArB(dan) × XAr–EWG (Table 3). Under conditions C (see the reaction scheme in Table 3) established in the exploration of Tables 1 and 2 and Fig. 3, 1a reacted with 2 bearing CN, Cl, CF3, NO2, COR (R = H, Bu, Ph, indanonyl), and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh groups,22 affording 3aa–3ai in good to high yields. Several important notes are as follows: (1) the careful pre-drying using procedure B may suppress base-mediated hydrolysis of the CN group,23 (2) the Cl–C(sp2) bond of 2′′a participated in the SMCC, but that of 2b remained unreacted by the chemoselective SMCC of the I–C(sp2) bond, (3) the CHO group of 2e was tolerated without undergoing the Cannizzaro side reaction,24 and (4) no aldol side reaction of the COBu group in 2f was observed.25 In area I, ArB(dan) with the sterically congested o-MeO (1b) as well as with p-(i-Pr)S (1c), p-(i-Pr) (1d) and p-(N-carbazolyl) (1e) groups can also be used to obtain 3ba–3ea.
CPh groups,22 affording 3aa–3ai in good to high yields. Several important notes are as follows: (1) the careful pre-drying using procedure B may suppress base-mediated hydrolysis of the CN group,23 (2) the Cl–C(sp2) bond of 2′′a participated in the SMCC, but that of 2b remained unreacted by the chemoselective SMCC of the I–C(sp2) bond, (3) the CHO group of 2e was tolerated without undergoing the Cannizzaro side reaction,24 and (4) no aldol side reaction of the COBu group in 2f was observed.25 In area I, ArB(dan) with the sterically congested o-MeO (1b) as well as with p-(i-Pr)S (1c), p-(i-Pr) (1d) and p-(N-carbazolyl) (1e) groups can also be used to obtain 3ba–3ea.
a Reagents for conditions C and D (unless otherwise noted): 1 (0.20 mmol), 2 (0.30 mmol), Pd(OAc)2 (20 μmol), dppf (30 μmol) or P(t-Bu)3 (40 μmol), LiOH (0.60 mmol), and DMF (0.80 mL). Reagents for conditions E (unless otherwise noted): 1 (0.30 mmol), 2 (0.20 mmol), Pd(OAc)2 (20 μmol), COD (30 μmol), LiOH (0.60 mmol), and DMF (0.80 mL). Conditions used are shown in parentheses. Yields of isolated products are shown here.
b ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2d (0.32 mmol), 2e (0.50 mmol), 2l (0.40 mmol) or 2o (0.36 mmol) was used, respectively.
c LiOH (0.40 mmol) was used.
d At 80 °C.
e
1a (0.30 mmol) and 2i (0.20 mmol) were used.
f At 60 °C.
g Performed with 1a (0.20 mmol) and 2r (0.40 mmol) for 24 h. 2d (0.32 mmol), 2e (0.50 mmol), 2l (0.40 mmol) or 2o (0.36 mmol) was used, respectively.
c LiOH (0.40 mmol) was used.
d At 80 °C.
e
1a (0.30 mmol) and 2i (0.20 mmol) were used.
f At 60 °C.
g Performed with 1a (0.20 mmol) and 2r (0.40 mmol) for 24 h.
|
|---|

|
Inspired by the above results, we worked on capturing area II: EWG–ArB(dan) × XAr–EWG. We conducted the SMCC of p-F3CC6H4B(dan) (1f) with a series of XAr–EWG 2a–2n to obtain 3fa–3fn. The functional groups, F (2j), SO2Me (2k), COMe (2l), and CO2Et (2n), newly proved to be compatible with conditions C. Of note is the tolerance of COMe with higher acidic α-protons than COBu of 2f (see area I). No serious observation of the base-mediated saponification of the CO2Et in 2n is also noteworthy. In addition, the SMCC of 2i selectively occurred to yield 3fi without causing palladium-catalyzed cyclotrimerization of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C part.26 Like other ArB(dan) compounds, 1h was chemoselectively cross-coupled with the I–C(sp2) bond of 2a without losing the Br functionality. Due to the instability of 2,4,5-F3C6H2B(OH)2 [t1/2 = 1 h at 70 °C in H2O/1,4-dioxane (1/1)],11 the compatibility of 1g is an advantage of the dan-protected boronic acid. The uracil framework that is crucial as a pharmacophore is also introducible from non-aromatic 5-iodo-1,3-dimethyluracil (2o).27
C part.26 Like other ArB(dan) compounds, 1h was chemoselectively cross-coupled with the I–C(sp2) bond of 2a without losing the Br functionality. Due to the instability of 2,4,5-F3C6H2B(OH)2 [t1/2 = 1 h at 70 °C in H2O/1,4-dioxane (1/1)],11 the compatibility of 1g is an advantage of the dan-protected boronic acid. The uracil framework that is crucial as a pharmacophore is also introducible from non-aromatic 5-iodo-1,3-dimethyluracil (2o).27
The next concern is area III for the synthesis of biaryls with an EDG on each aryl unit. We carried out the SMCC of 1a with p-BrC6H4NMe2 (2p) under conditions C, but the conditions C were ineffective, providing the desired product 3ap in a low yield of 19% (Scheme 3). GC-MS and 1H NMR analysis of the crude reaction mixture exhibited the formation of some by-products, mainly including p-MeOC6H4–Ph (1a + dppf–Ph) in 24% yield, along with a small amount of Ph–C6H4–p-NMe2 (dppf–Ph + 2p).28 The Ph group of the by-products was considered to come from dppf.29 We therefore explored an appropriate ligand for this case (Scheme 4). Replacing dppf with DPEPhos and XantPhos, both of which still contain the Ph group, did not result in any improvements. With JohnPhos and SPhos, coupling products incorporating the bulky biaryl units were not detected, but the yields of 3ap were still low to moderate. In sharp contrast, P(t-Bu)3 with no aryl group exhibited outstanding performance, affording 3ap in 91% yield; Table 3 shows the isolated yield thereof. Interestingly, COD also worked as a ligand to afford 3ap in a good yield of 72%. These results showed that P(t-Bu)3 is the ligand of choice for the SMCC of 2p. Hereinafter, the reaction conditions using P(t-Bu)3 as a ligand are referred to as conditions D,30 and their generality in area III was examined. However, disappointingly, the SMCC of 1a with 2q having the NH2 group under conditions D resulted in a low yield of 3aq (Scheme 5). Besides 3aq, anisole and a trace amount of its dimer were produced from 1a. As a major by-product, insoluble black materials were obtained quantitatively from 2q. FT-IR analysis implied the generation of polymers probably via the self-Buchwald–Hartwig coupling of 2q.31 Here, the observation in Scheme 4 reminded us that COD may be a good option for 2q, which is also an XAr–EDG. To our delight, switching the ligand to COD prevented the polymerization of 2q, delivering 3aq in a dramatically improved yield of 86% (Table 3).32 Thus, the ligand COD was found to be effective in preventing the 2q-based self-Buchwald–Hartwig coupling that directly lowers the yield of 3aq. Hereinafter, the reaction conditions using COD as a ligand are referred to as conditions E, which also worked well for the SMCC of XAr with NHMe (2r) and OH (2s) groups. These results reveal that COD is a suitable ligand for XAr–YH (Y = NR", O), in which the YH potentially cross-couples with X–Ar. On the other hand, P(t-Bu)3 was again effective in the SMCC of 2t and 2u, indicating that conditions D should be selected for XAr–EDG without the YH.
 | ||
| Scheme 3 Direct SMCC of p-MeOC6H4B(dan) with p-BrC6H4NMe2 under conditions C. Yields determined by 1H NMR are shown here. | ||
 | ||
Scheme 4 Screening of ligands for direct SMCC of p-MeOC6H4B(dan) with p-BrC6H4NMe2. Yields determined by 1H NMR are shown here. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction time = 12 h. Reaction time = 12 h. | ||
At the last corner of area IV, the purpose is the achievement of the SMCC of EWG–ArB(dan) with XAr–EDG. Here as well, conditions D and E established for XAr–EDG and XAr–YH, respectively, in area III remain useful. Thus, 1f was cross-coupled with 2p, 2u, 2v, and 2w under conditions D and with 2x, 2r, and 2y under conditions E to afford the corresponding products in good to high yields. These results show that the functional groups, NHCOMe, N-morpholino, and CH2OH, can also participate in this protocol. The compatibility of NHCOMe without undergoing hydrolysis is noted. Besides 1f, ArB(dan) with m-NO2 (1j) or 2,4,5-F3 (1g) was converted to the respective coupling product 3jp or 3gu in a high yield.
As depicted in the center of Fig. 4, the simplest coupling product, biphenyl (3kz), can also be prepared in an excellent yield under conditions C (Scheme 6).
As Table 4 shows, ArB(dan) 1 can also react with heteroaryl halides (XHetAr) 2, which are classified into two groups of electron-deficient and electron-rich, based on the electron density of the reaction site compared to that of the carbon atom of benzene.33p-MeOC6H4B(dan) (1a) and p-CF3C6H4B(dan) (1f) were used in the SMCC with 3-I–pyridine (2aa), 2-Br–thiophene (2ab), and 3-Br–furan (2ac) under conditions C, affording the corresponding Ar–HetAr 3 in good to high yields. Note that a higher loading is required for the coupling of 2ac due to its rapid consumption (see also the coupling of 2ac in area III of Table 5). It should also be noted that distinct from the couplings of electron-rich XAr–EDG in areas III and IV of Table 3, conditions C with the ligand dppf could be applied to those of electron-rich XHetAr 2ab and 2ac because no incorporation of the dppf-based Ph group into the product was observed (refer to Scheme 3).
| a Reagents (unless otherwise noted): 1 (0.20 mmol), 2 (0.30 mmol), Pd(OAc)2 (20 μmol), dppf (30 μmol) for conditions C or P(t-Bu)3 (40 μmol) for conditions D, LiOH (0.60 mmol), and DMF (0.80 mL). Conditions used are shown in parentheses. b 2a (0.34 mmol), 2ae (0.36 mmol), or 2ac (0.60 mmol) was used, respectively. c1 (0.40 mmol) and 2 (0.20 mmol) were used. dLiOH (0.40 mmol) was used. eAt 70 °C. fPd(OAc)2 (30 μmol) and dppf (45 μmol) were used. g1 (0.30 mmol) and 2 (0.20 mmol) were used. hAt 60 °C. |
|---|
|
Through the investigation conducted so far, we realized 47 reactions, and areas I–IV of Tables 3 and 4 were filled with coupling products to a significant extent. To further expand the scope of this method, we continuously studied the direct SMCC for HetArB(dan) (Table 5) and alkenylB(dan) (Table 6).
| a Reagents (unless otherwise noted): 1 (0.20 mmol), 2 (0.30 mmol), Pd(OAc)2 (20 μmol), dppf (30 μmol) for conditions C or P(t-Bu)3 (40 μmol) for conditions D, LiOH (0.60 mmol), and DMF (0.80 mL). Conditions used are shown in parentheses. b Performed with 1v (0.30 mmol) and 2c (0.20 mmol) at 80 °C. cDetermined by 1H NMR. dDetermined by GC and GC-MS. |
|---|

|
Table 5 summarizing the SMCC of HetArB(dan) with X(Het)Ar also consists of areas I–IV,33 and conditions C and D work well here as well. In area I, we first realized the SMCC of electron-rich benzofuranylB(dan) 1l and carbazolylB(dan) 1m with p-IC6H4CN (2a), affording 3la and 3ma in high yields, respectively. Besides the HetAr–Ar structure, the HetAr–HetAr structure, including benzofuranyl (from 1l), pyridyl (from 2aa), pyrazyl (from 2ad), thienyl (from 1n) or benzothiazolyl (from 2ae) ring, could be constructed. A pharmacologically active antipyrinyl nucleus can also be selected, affording 3laf in a high yield.34
The results from area II in Table 5 reveal that the current protocol is at a satisfactory level to address the 2-pyridyl problem.9 Thus, 2-pyridylB(dan) compounds containing a 6-MeO (1o) or 6-F (1q) group as well as 2-pyridylB(dan) itself (1p) were cross-coupled with electron-deficient X–(Het)Ar. These reactions produced pyridines connected with C6H4–p-CN (3oa, 3pa, and 3qa), 2-benzothiazolyl (3oae), imidazo[1,2-b]pyridazinyl (3oag), and 4-pyridyl (3qah) units in moderate to high yields. Due to the low yield (33%) of a 1q-derived product in a previous study,10 which was attributed to the propensity of 1q for more readily undergoing protodeboronation,9 the results from area II signify that the present method is greatly refined.
A range of HetAr–(Het)Ar molecules were also constructed in areas III and IV, where 1r and 1s as HetArB(dan) and 2ai, 2aj, 2ak, and 2al as X(Het)Ar could be employed as new substrates.
The use of alkenylB(dan) enables access to 1,2-diarylalkenes (3ta and 3tu) and 1,2-alkylarylalkenes (3ua and 3um) as well as 1,1-alkylarylalkenes (3vc and 3vh) in good to high yields (Table 6). With an 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 E/Z mixture of commercially supplied β-bromostyrene (2am), product 3tam also consisted of a mixture of stereoisomers, albeit with higher E selectivity (E/Z = 97
13 E/Z mixture of commercially supplied β-bromostyrene (2am), product 3tam also consisted of a mixture of stereoisomers, albeit with higher E selectivity (E/Z = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). We therefore expected that starting with a single isomer of (E)-2am would afford only (E,E)-3tam and that the classical method of treating (E/Z)-2am with NaOH would be useful to selectively destruct the Z-isomer (Scheme 7a).35 Due to the similar characteristics of NaOH and LiOH, we further expected that, if LiOH can serve as a base for the destruction reaction as well as for the SMCC, the two reactions may be conveniently performed in one pot. This idea worked out nicely (Scheme 7b), and (E,E)-3tam was stereoselectively constructed in a good yield of 76% by the treatment of (E/Z)-2am with LiOH in DMF at 100 °C for 1 h followed by the SMCC with 1t under conditions D.36 This stereoselective one-pot procedure can also be applied to 1-cyclohexenylB(dan) (1w) and p-CF3C6H4B(dan) (1f).
3). We therefore expected that starting with a single isomer of (E)-2am would afford only (E,E)-3tam and that the classical method of treating (E/Z)-2am with NaOH would be useful to selectively destruct the Z-isomer (Scheme 7a).35 Due to the similar characteristics of NaOH and LiOH, we further expected that, if LiOH can serve as a base for the destruction reaction as well as for the SMCC, the two reactions may be conveniently performed in one pot. This idea worked out nicely (Scheme 7b), and (E,E)-3tam was stereoselectively constructed in a good yield of 76% by the treatment of (E/Z)-2am with LiOH in DMF at 100 °C for 1 h followed by the SMCC with 1t under conditions D.36 This stereoselective one-pot procedure can also be applied to 1-cyclohexenylB(dan) (1w) and p-CF3C6H4B(dan) (1f).
 | ||
Scheme 7 [a] Selective destruction of (Z)-β-bromostyrene [(Z)-2am].35 [b] One-pot SMCC starting with an 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 mixture of (E/Z)-2am. 13 mixture of (E/Z)-2am. | ||
We further expected that iterative palladium-catalyzed CCs, including the current direct SMCC, would enable rapid access to complex π-conjugated molecules (Scheme 8). Showing the compatibility of the B(dan) group with other palladium-catalyzed CCs would be one of the advantages of B(dan) chemistry. m-/p-BrC6H4B(dan) 1x and 1h were selected as substrates. Upon the treatment of 1x with benzamide under palladium catalysis, the Buchwald–Hartwig amidation first occurred selectively at the Br group to afford 6 in 80% yield.37 No self-SMCC of 1x was observed under the reaction conditions, showing that the B(dan) group was in the switch-off mode. On the other hand, treating 6 with 2an under conditions C switched the B(dan) group to the "on" mode, providing 7 in 97% yield without an extra step for unmasking B(dan). Moreover, 9 was obtained from 1xvia the sequence of Mizoroki–Heck and SMCC. The iterative CCs starting with the Sonogashira–Hagihara CC38 of 1h followed by the SMCC of 10 yielded 11.
In contrast to ArB(OH)2, ArB(dan) indicates unique adaptability to other organic transformations (Scheme 9). For example, the Wittig olefination of 1′y having the B(OH)2 group under the reaction conditions using t-BuOK yielded no desired product 12′, despite the complete consumption of 1′y.39 Instead, base-promoted protodeboronation occurred as a major side reaction.11a In marked contrast to this, using 1y with the B(dan) group delivered an excellent yield of 12, which was then used for the direct SMCC with 2p to yield 13. Due to the significance of chiral diols as chiral inducers in organic chemistry,40 we attempted to synthesize an arylboron compound having a chiral acetal moiety. The acetalization of boronic acid 1′z with (R,R)-2,3-butanediol in the presence of the acid catalyst Sc(OTf)3 (Tf = SO2CF3) resulted in a complex mixture of products.41 Unlike the B(OH)2 case, the chiral acetal formation of 1z occurred to yield 14, which was then directly reacted with 2h to furnish chiral acetal 15.
Distinct from the successful direct SMCC demonstrated thus far, (Het)ArB(dan) and (Het)ArBr collected in Fig. 5 did not work as substrates: a possible or plausible reason is stated below each substrate.
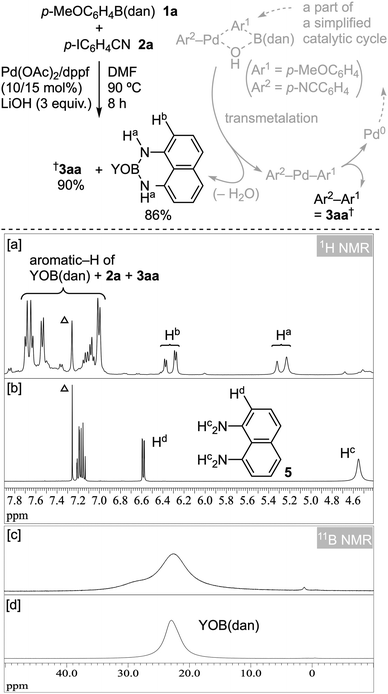
Some experimental observations are available for mechanistic studies. The absence of H2dan (5), already shown in Table 2, can actually be confirmed by the 1H NMR spectrum of the crude reaction mixture after the aqueous work-up of the SMCC of 1a with 2a (Fig. 6a); the 1H NMR spectrum of 5 as an authentic sample is shown in Fig. 6b. Alternatively, a mixture of HOB(dan) and O[B(dan)]2 that retains the B(dan) unit was obtained in 86% yield, close to 90% yield of product 3aa (Fig. 6).42 The formation of this mixture, YOB(dan) [Y = H, B(dan)], in the crude reaction mixture (Fig. 6c) was also confirmed by 11B NMR analysis; the 11B NMR spectrum of isolated YOB(dan) is given in Fig. 6d for comparison. YOB(dan) is considered to form via transmetalation43 followed by partial dehydration,44 as depicted in Fig. 6, supporting the direct SMCC of ArB(dan) 1.
Currently, two distinct courses are recognized to exist prior to the transmetalation event: the boronate pathway A and the oxo-palladium pathway B, as simply illustrated in Scheme 10. There have been studies in support of either path A45 or path B.46 On the other hand, the Denmark group has demonstrated that both path A and path B, including transmetalation-active pre-intermediates, are operative, with path B being faster.47 These earlier studies reveal that whether path A or B is involved depends on the substrates and reaction conditions.
 | ||
| Scheme 10 Simplified generic mechanisms for SMCC. L = ligand; R = H, alkyl; Y = OH, Oalkyl, NHaryl, etc. | ||
To gain insights into the transmetalation, we first examined the possibility of path A via the formation of boronate salts 17. Prior to investigating the above, whether the strongly coordinating solvent DMF interacts with ArB(dan) 1 for enhancing the nucleophilicity of the Ar moiety was examined. The 11B signal of PhB(dan) (1k) was observed at 29 ppm in the non-coordinating solvent CDCl3 (Fig. 7a); a 11B signal of 1 appears at approximately 29 ppm, irrespective of a substituent in the Ar part and a solvent. Adding DMF (5 equiv.) to this solution did not cause any spectral change, showing no formation of a DMF–1k complex (Fig. 7b). Next, a 1/1 mixture of 1k and LiOH was stirred in DMF at 30 °C for 2 h and then monitored by 11B NMR spectroscopy, but no boronate salt 17k–OH was observed (Fig. 7c). The extremely low solubility of LiOH in DMF was assumed to be the reason for the absence of 17k–OH. To improve this, switching from LiOH to LiOEt caused a 5% upfield shift in the 1k signal into the typical range of ArB(dan)-based boronate species (17k–OEt, Fig. 7d).10,14b Replacing 1k with 1f having the CF3 group, which should enhance the Lewis acidity of the boron center, resulted in a higher rate of boronate salt 17f–OEt (Fig. 7e). It was then confirmed that yet untested LiOEt as a base can also promote the direct SMCC of 1k with 2z effectively (Scheme 11). Encouraged by these results, we devoted much effort to purifying 17–OEt for a stoichiometric control reaction with the oxidative adduct BrPd(dppf)Ar2 (16a: Ar2 = C6H4–p-F) but could not purify 17–OEt because 17–OEt readily reverted to its original species, 17 and −OEt. Meanwhile, of note is that, unlike in DMF, 17f–OEt was not formed in toluene (Fig. 7f). This result was consistent with the fact that the direct SMCC of 1f with 2j did not occur in toluene, regardless of whether LiOH or LiOEt was used (Scheme 12), suggesting that the boronate formation is one of the important factors for the progress of the present direct SMCC. Moreover, the Mutoh–Saito group has demonstrated that a stoichiometric reaction between the boronate salt K[PhB(dan)(Ot-Bu)] and the oxidative adduct IPd[(PPh3)2]C6H4–p-OMe successfully yields the coupling product Ph–C6H4–p-OMe.14b Consequently, all the findings collected in this section suggest that path A involving borate salt 17 is a viable option in the current direct SMCC.
 | ||
| Scheme 11 Direct SMCC of PhB(dan) with IPh using LiOEt as a base. The yield of isolated 3kz is shown here. | ||
 | ||
| Scheme 12 Direct SMCC of p-F3CC6H4B(dan) with p-BrC6H4F in toluene. Yields of 3fj determined by 1H NMR are shown here. | ||
The Yoshida group has reported that the H–N moieties of PhB(dan) undergo deprotonation by a Grignard reagent working as a base, followed by methylation with MeOTs (Ts = SO2–p-tolyl).15a Hence, it was assumed that, like 17 in path A, lithium amides 19 possibly produced by LiOH-mediated deprotonation of 1 could be another activated form transmetalating with XPd(Ln)Ar216 (path A′ in Scheme 13a). To verify the possibility of path A′, methylation of 1k under the reaction conditions shown in Scheme 13b was attempted, but 1k was not methylated. In addition, 1kMe2 prepared by a different method was subjected to the direct SMCC with 2a under the standard reaction conditions, delivering 3ka successfully (Scheme 13c). These results clearly indicate that path A′ involving 19 is improbable and thus support path A, in which the reaction partner of 16 is 17.
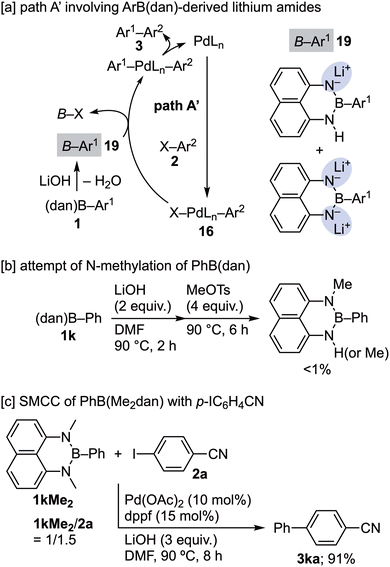 | ||
| Scheme 13 Validation of ArB(dan)-based lithium amides as possible activated species in SMCC. Yields of PhB(Medan) and/or PhB(Me2dan) determined by 1H NMR and of isolated 3ka are shown here. | ||
Subsequently, we looked into the feasibility of path B in which the 6-electron, 3-coordinate boron compound Y2BAr1 reacts with ROPd(Ln)Ar218. Upon the treatment of a stoichiometric mixture of p-F3CC6H4B(dan) (1f) and cis-Pd(OH)(C6H4–p-F)(dppf)(thf)2 [18j(thf)2]47 in DMF at 90 °C for 8 h, the transmetalation occurred to furnish 3fj in 72% yield (Scheme 14).48 This result supports the likelihood of path B.
 | ||
| Scheme 14 Stoichiometric control experiment by reacting p-F3CC6H4B(dan) with cis-Pd(OH)(C6H4–p-F)(dppf)(thf)2. The yield of 3fj determined by 1H NMR is shown here. | ||
There is an additional note in relation to the results of Scheme 14. As shown in Table 3 or Scheme 15, the SMCC of 1f with 2j using LiOH or LiOEt as a base, respectively, was completed in 8 h at 90 °C. These two reactions were thus completed within the same reaction time and at the same reaction temperature as the reaction in Scheme 14, where 1f reacted with intermediate 18j(thf)2 for the subsequent transmetalation. Accordingly, the rate-determining step of the present direct SMCC may be the transmetalation, as proposed in most SMCCs.49
 | ||
| Scheme 15 Direct SMCC of p-F3CC6H4B(dan) with p-BrC6H4F. The yield of 3fj determined by 1H NMR is shown here. | ||
Considering all the experimental results pertaining to the mechanistic studies, we currently regard both path A and path B as possible courses of involvement in the direct SMCC of ArB(dan).
Conclusions
We developed a practical method for the direct SMCC of a C(sp2)–B(dan) bond of (Het)ArB(dan) with (Het)ArX. AlkenylB(dan) and alkenylX can also be used as substrates. The consistent reaction conditions for all reactions are the use of Pd(OAc)2 as a palladium pre-catalyst, LiOH as a base, and DMF as a solvent. The ligand used in combination with the reaction conditions is greatly influenced by (Het)ArX. dppf is appropriate when (Het)ArX is electron-deficient (conditions C). P(t-Bu)3 is suitable when (Het)ArX is electron-rich (conditions D). COD is a good choice when (Het)ArX contains a protic functionality like NH2 and OH (conditions E). Another crucial aspect for the successful direct SMCC is to ensure anhydrous reaction conditions wherever possible (procedure B in Fig. 3).With conditions C, D, and E and procedure B, we carried out 84 SMCCs using a wide variety of substrates with various functional groups. Not only is it merely feasible to conduct the direct SMCC, but also to construct complex aromatic architectures by combining the current direct SMCC with other organic transformations. For example, Buchwald–Hartwig CC, Mizoroki–Heck reaction, Sonogashira–Hagihara CC, Wittig olefination, and Lewis-acid-catalyzed chiral acetal formation can first be used to transform a functional group on the Ar moiety of ArB(dan), followed by the direct SMCC of the remaining B(dan) unit.
Mechanistic studies showed that C(sp2)–B(dan) is directly activated under the reaction conditions established here. At present, both path A and path B illustrated in Scheme 10 are likely the catalytic cycles.
Author contributions
T. T. conceptualized and supervised the research project and reviewed the manuscript and the ESI.† H. A. drafted the manuscript and the ESI.† H. A., R. N., T. A., and E. K. carried out experiments, analyzed the results, and characterized the products. R. N. and E. K. also assisted in preparing the ESI.†Data availability
The data supporting this article have been included in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Partial financial support from Grants-in-Aid for Scientific Research (no. 19K05484 and 23K04739) from the Ministry of Education, Culture, Sports, Science and Technology is acknowledged. We are grateful to Mr Taiki Sakata for his experimental assistance.References
- For selected recent reviews and a book, see: (a) J. C. H. Lee and D. G. Hall, in Metal-Catalyzed Cross-Coupling Reactions and More, ed. A. de Meijere, S. Bräse and M. Oestreich, Wiley-VCH, Weinheim, 2014, vol. 1, pp. 65–132 Search PubMed; (b) J. P. G. Rygus and C. M. Crudden, Enantiospecific and Iterative Suzuki–Miyaura Cross-Couplings, J. Am. Chem. Soc., 2017, 139, 18124–18137 CrossRef CAS PubMed; (c) S. E. Hooshmand, B. Heidari, R. Sedghi and R. S. Varma, Recent advances in the Suzuki–Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media, Green Chem., 2019, 21, 381–405 RSC; (d) N. Tsuchiya, T. D. Sheppard and T. Nishikata, Tertiary Alkylative Suzuki–Miyaura Couplings, Synthesis, 2022, 2340–2349 CAS.
- For a selected review and a book, see: (a) S.-I. Murahashi, Palladium-catalyzed cross-coupling reaction of organic halides with Grignard reagents, organolithium compounds and heteroatom nucleophiles, J. Organomet. Chem., 2002, 653, 27–33 CrossRef CAS; (b) J. Tsuji, Palladium Reagents and Catalysts: New Perspectives for the 21st Century, John Wiley & Sons, Chichester, 2006, pp. 201–231 and 313–338 Search PubMed.
- For selected reviews, see: (a) A. J. J. Lennox and G. C. Lloyd-Jones, Selection of boron reagents for Suzuki–Miyaura coupling, Chem. Soc. Rev., 2014, 43, 412–443 RSC; (b) K. N. Babu, F. Massarwe, R. R. Reddy, N. Eghbarieh, M. Jakob and A. Masarwa, Unsymmetrical 1,1-Bisboryl Species: Valuable Building Blocks in Synthesis, Molecules, 2020, 25, 959 CrossRef CAS PubMed.
- For examples, see: (a) H. Noguchi, K. Hojo and M. Suginome, Boron-Masking Strategy for the Selective Synthesis of Oligoarenes via Iterative Suzuki–Miyaura Coupling, J. Am. Chem. Soc., 2007, 129, 758–759 CrossRef CAS PubMed; (b) N. Iwadate and M. Suginome, Synthesis of B-Protected β-Styrylboronic Acids via Iridium-Catalyzed Hydroboration of Alkynes with 1,8-Naphthalenediaminatoborane Leading to Iterative Synthesis of Oligo(phenylenevinylene)s, Org. Lett., 2009, 11, 1899–1902 CrossRef CAS PubMed.
- For examples, see: (a) E. P. Gillis and M. D. Burke, A Simple and Modular Strategy for Small Molecule Synthesis: Iterative Suzuki–Miyaura Coupling of B-Protected Haloboronic Acid Building Blocks, J. Am. Chem. Soc., 2007, 129, 6716–6717 CrossRef CAS PubMed; (b) J. Li, S. G. Ballmer, E. P. Gillis, S. Fujii, M. J. Schmidt, A. M. E. Palazzolo, J. W. Lehmann, G. F. Morehouse and M. D. Burke, Synthesis of many different types of organic small molecules using one automated process, Science, 2015, 347, 1221–1226 CrossRef CAS PubMed.
- For examples, see: (a) H. Ihara, M. Koyanagi and M. Suginome, Anthranilamide: A Simple, Removable ortho-Directing Modifier for Arylboronic Acids Serving also as a Protecting Group in Cross-Coupling Reactions, Org. Lett., 2011, 13, 2662–2665 CrossRef CAS PubMed; (b) M. Koyanagi, N. Eichenauer, H. Ihara, T. Yamamoto and M. Suginome, Anthranilamide-masked o-Iodoarylboronic Acids as Coupling Modules for Iterative Synthesis of ortho-Linked Oligoarenes, Chem. Lett., 2013, 42, 541–543 CrossRef CAS.
- For selected reviews, see: (a) J. Li, A. S. Grillo and M. D. Burke, From Synthesis to Function via Iterative Assembly of N-Methyliminodiacetic Acid Boronate Building Blocks, Acc. Chem. Res., 2015, 48, 2297–2307 CrossRef CAS PubMed; (b) L. Xu, S. Zhang and P. Li, Boron-selective reactions as powerful tools for modular synthesis of diverse complex molecules, Chem. Soc. Rev., 2015, 44, 8848–8858 RSC; (c) D. Aich, P. Kumar, D. Ghorai, K. K. Das and S. Panda, Recent advances in the synthesis and reactivity of MIDA boronates, Chem. Commun., 2022, 58, 13298–13316 RSC; (d) M. Hirano and S. Kiyota, Ru(0)-catalysed synthesis of borylated polyene building blocks by cross-dimerisation toward cross-coupling, Chem. Commun., 2024, 60, 7672–7686 RSC.
- Ref. 6a shows that the order of hydrolytic stability is B(dan) ≫ B(mida) > B(aam).
- For an important report and review on the stability of 2-pyridylB(OH)2 and 2-heteroarylB(OH)2, see: (a) P. A. Cox, A. G. Leach, A. D. Campbell and G. C. Lloyd-Jones, Protodeboronation of Heteroaromatic, Vinyl, and Cyclopropyl Boronic Acids: pH–Rate Profiles, Autocatalysis, and Disproportionation, J. Am. Chem. Soc., 2016, 138, 9145–9157 CrossRef CAS PubMed; (b) X. A. F. Cook, A. de Gombert, J. McKnight, L. R. E. Pantaine and M. C. Willis, The 2-Pyridyl Problem: Challenging Nucleophiles in Cross-Coupling Arylations, Angew. Chem., Int. Ed., 2021, 60, 11068–11091 CrossRef CAS PubMed (review).
- H. Yoshida, M. Seki, S. Kamio, H. Tanaka, Y. Izumi, J. Li, I. Osaka, M. Abe, H. Andoh, T. Yajima, T. Tani and T. Tsuchimoto, Direct Suzuki–Miyaura Coupling with Naphthalene-1,8-diaminato (dan)-Substituted Organoborons, ACS Catal., 2020, 10, 346–351 CrossRef CAS.
- For an important report and review on the stability of perfluoroarylB(OH)2, see: (a) P. A. Cox, M. Reid, A. G. Leach, A. D. Campbell, E. J. King and G. C. Lloyd-Jones, Base-Catalyzed Aryl-B(OH)2 Protodeboronation Revisited: From Concerted Proton Transfer to Liberation of a Transient Aryl Anion, J. Am. Chem. Soc., 2017, 139, 13156–13165 CrossRef CAS PubMed; (b) Y. P. Budiman, S. A. Westcott, U. Radius and T. B. Marder, Fluorinated Aryl Boronates as Building Blocks in Organic Synthesis, Adv. Synth. Catal., 2021, 363, 2224–2255 CrossRef CAS (review).
- H. L. D. Hayes, R. Wei, M. Assante, K. J. Geogheghan, N. Jin, S. Tomasi, G. Noonan, A. G. Leach and G. C. Lloyd-Jones, Protodeboronation of (Hetero)Arylboronic Esters: Direct versus Prehydrolytic Pathways and Self-/Auto-Catalysis, J. Am. Chem. Soc., 2021, 143, 14814–14826 CrossRef CAS PubMed.
- (a) T. Tsuchimoto, H. Utsugi, T. Sugiura and S. Horio, Alkynylboranes: A Practical Approach by Zinc-Catalyzed Dehydrogenative Coupling of Terminal Alkynes with 1,8-Naphthalenediaminatoborane, Adv. Synth. Catal., 2015, 357, 77–82 CrossRef CAS; (b) T. Tani, Y. Sawatsugawa, Y. Sano, Y. Hirataka, N. Takahashi, S. Hashimoto, T. Sugiura and T. Tsuchimoto, Alkynyl–B(dan)s in Various Palladium-Catalyzed Carbon–Carbon Bond-Forming Reactions Leading to Internal Alkynes, 1,4-Enynes, Ynones, and Multiply Substituted Alkenes, Adv. Synth. Catal., 2019, 361, 1815–1834 CrossRef CAS. See also the following articles: (c) T. Tsuchimoto, T. Johshita, K. Sambai, N. Saegusa, T. Hayashi, T. Tani and M. Osano, In(ONf)3-catalyzed 7-membered carbon-ring-forming annulation of heteroarylindoles with α,β-unsaturated carbonyl compounds, Org. Chem. Front., 2021, 8, 2882–2892 RSC; (d) R. Tokunaga, T. Okusa and T. Tsuchimoto, Synthesis of o-Bis(3-indolyl)arenes under Brønsted Acid Catalysis: Carbonyl Compounds as Sources of Aryl Groups, Adv. Synth. Catal., 2023, 365, 1432–1441 CrossRef CAS.
- (a) K. Yamamoto, Y. Mohara, Y. Mutoh and S. Saito, Ruthenium-Catalyzed (Z)-Selective Hydroboration of Terminal Alkynes with Naphthalene-1,8-diaminatoborane, J. Am. Chem. Soc., 2019, 141, 17042–17047 CrossRef CAS PubMed; (b) Y. Mutoh, K. Yamamoto and S. Saito, Suzuki–Miyaura Cross-Coupling of 1,8-Diaminonaphthalene (dan)-Protected Arylboronic Acids, ACS Catal., 2020, 10, 352–357 CrossRef CAS.
- For reviews, an account, and other related reports, see: (a) J. Li, M. Seki, S. Kamio and H. Yoshida, Transition metal-free B(dan)-installing reaction (dan: naphthalene-1,8-diaminato): H–B(dan) as a B(dan) electrophile, Chem. Commun., 2020, 56, 6388–6391 RSC; (b) S. Kamio and H. Yoshida, Synthetic Chemistry with Lewis Acidity-Diminished B(aam) and B(dan) Groups: Borylation Reactions and Direct Cross-Couplings, Adv. Synth. Catal., 2021, 363, 2310–2324 CrossRef CAS (review); (c) Y. Mutoh, K. Yamamoto, Y. Mohara and S. Saito, (Z)-Selective Hydrosilylation and Hydroboration of Terminal Alkynes Enabled by Ruthenium Complexes with an N-Heterocyclic Carbene Ligand, Chem. Rec., 2021, 21, 3429–3441 CrossRef CAS PubMed (account); (d) K. Tomota, Y. Izumi, K. Nakanishi, M. Nakamoto and H. Yoshida, Efficient one-pot synthesis of dan-substituted organo- and silyl-boron compounds, Org. Biomol. Chem., 2023, 21, 5347–5350 RSC; (e) J. Li, H. Tanaka, T. Imagawa, T. Tsushima, M. Nakamoto, J. Tan and H. Yoshida, Ethynyl-B(dan) in [3 + 2] Cycloaddition and Larock Indole Synthesis: Synthesis of Stable Boron-Containing Heteroaromatic Compounds, Chem. – Eur. J., 2024, 30, e202303403 CrossRef CAS PubMed; (f) H. Yoshida, Modulating Lewis acidity with boronamides and boronates: effect on behavior in borylation and cross-coupling, Chem. Lett., 2024, 53, upae209 CrossRef (review).
- Yoshida and co-workers have also reported the direct SMCC of cyclopropylB(dan) under conditions A, see: M. Koishi, K. Tomota, M. Nakamoto and H. Yoshida, Direct Suzuki–Miyaura Coupling of Naphthalene-1,8-diaminato (dan)-Substituted Cyclopropylboron Compounds, Adv. Synth. Catal., 2023, 365, 682–686 CrossRef CAS.
- Z. Luo, Y. Zong and G. C. Tsui, Palladium-Catalyzed Stereoselective Defluoroborylation of gem-Difluoroalkenes with Unsymmetrical Diboron: Access to Tetrasubstituted Monofluorinated Vinyl–B(dan) Derivatives, Org. Lett., 2023, 25, 4406–4410 CrossRef CAS PubMed.
- K. Tomota, J. Li, H. Tanaka, M. Nakamoto, T. Tsushima and H. Yoshida, Weak Base-Promoted Direct Cross-Coupling of Naphthalene-1,8-diaminato-substituted Arylboron Compounds, JACS Au, 2024, 4, 3931–3941 CrossRef CAS PubMed.
- For the pKb value of LiOH, see: (a) P. Ren, T. Zhang, N. Jain, H. Y. V. Ching, A. Jaworski, G. Barcaro, S. Monti, J. Silvestre-Albero, V. Celorrio, L. Chouhan, A. Rokicińska, E. Debroye, P. Kuśtrowski, S. V. Doorslaer, S. V. Aert, S. Bals and S. Das, An Atomically Dispersed Mn-Photocatalyst for Generating Hydrogen Peroxide from Seawater via the Water Oxidation Reaction (WOR), J. Am. Chem. Soc., 2023, 145, 16584–16596 CrossRef CAS PubMed. For the pKb value of Ba(OH)2, see: (b) N. K. Gupta, P. Palenicek, L. Nortmeyer, G. M. Meyer, T. Schäfer, T. Hellmann, J. P. Hofmann and M. Rose, Modulating Catalytic Selectivity by Base Addition in Aqueous Reductive Amination of 1,6-Hexanediol Using Ru/C, ACS Sustainable Chem. Eng., 2022, 10, 14560–14567 CrossRef CAS.
- A metal salt of a smaller ionic radius has a higher heterolytic bond dissociation energy. For example, see: G. Chen and R. G. Cooks, Estimation of Heterolytic Bond Dissociation Energies by the Kinetic Method, J. Mass Spectrom., 1997, 32, 1258–1261 CrossRef CAS.
- For values of ionic radii, see: R. D. Shannon, Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides, Acta Crystallogr., Sect. A, 1976, 32, 751–767 CrossRef.
- The Hammett and modified Swain–Lupton constant (σp) of the C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh group is reported to be 0.16, and σ values of other various functional groups are also provided in the following review, see: C. Hansch, A. Leo and R. W. Taft, A Survey of Hammett Substituent Constants and Resonance and Field Parameters, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
CPh group is reported to be 0.16, and σ values of other various functional groups are also provided in the following review, see: C. Hansch, A. Leo and R. W. Taft, A Survey of Hammett Substituent Constants and Resonance and Field Parameters, Chem. Rev., 1991, 91, 165–195 CrossRef CAS. - The base-mediated nitrile hydrolysis has been well known as a textbook reaction, see: J. McMurry, Organic Chemistry, Brooks/Cole, Belmont, 7th edn, 2008, pp. 768–769 Search PubMed.
- J. McMurry, Organic Chemistry, Brooks/Cole, Belmont, 7th edn, 2008, pp. 724–725 Search PubMed.
- J. McMurry, Organic Chemistry, Brooks/Cole, Belmont, 7th edn, 2008, pp. 877–879 Search PubMed.
- For the palladium-catalyzed cyclotrimerization of diarylalkynes such as diphenylacetylene, see: A. K. Jhingan and W. F. Maier, Homogeneous Catalysis with a Heterogeneous Pd Catalyst. An Effective Method for the Cyclotrimerization of Alkynes, J. Org. Chem., 1987, 52, 1161–1165 CrossRef CAS.
- For an example of a review, see: D. Ramesh, B. G. Vijayakumar and T. Kannan, Therapeutic potential of uracil and its derivatives in countering pathogenic and physiological disorders, Eur. J. Med. Chem., 2020, 207, 112801 CrossRef CAS PubMed.
- A homo-coupling product of 2p was also formed in 6% NMR yield.
- S.-Q. Wu, S.-Q. Zhang and X. Hong, Understanding the mechanism and reactivity of Pd-catalyzed C–P bond metathesis of aryl phosphines: a computational study, Org. Biomol. Chem., 2020, 18, 5414–5419 RSC.
- Conditions D have been implemented earlier, as demonstrated in our study cited in ref. 13d.
- For selected recent reviews, see: (a) P. Ruiz-Castillo and S. L. Buchwald, Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions, Chem. Rev., 2016, 116, 12564–12649 CrossRef CAS PubMed; (b) R. Dorel, C. P. Grugel and A. M. Haydl, The Buchwald–Hartwig Amination After 25 Years, Angew. Chem., Int. Ed., 2019, 58, 17118–17129 CrossRef CAS PubMed.
- Under identical reaction conditions to those of conditions E except for using a different substrate ratio of 1a/2q = 1/1.5, biaryl 3aq can also be obtained in a good yield of 73%. When the preparation of ArB(dan) is necessary, the choice of 1/2 = 1/1.5 under conditions E would be admissible.
- The grouping was comprehensively made based on the following references, see: (a) M. F. Gotta and H. Mayr, Kinetics of the Friedel–Crafts Alkylations of Heterocyclic Arenes: Comparison of the Nucleophilic Reactivities of Aromatic and Nonaromatic π-Systems, J. Org. Chem., 1998, 63, 9769–9775 CrossRef CAS; (b) S. Pratihar and S. Roy, Nucleophilicity and Site Selectivity of Commonly Used Arenes and Heteroarenes, J. Org. Chem., 2010, 75, 4957–4963 CrossRef CAS PubMed; (c) J. A. Joule and K. Mills, Heterocyclic Chemistry, John Wiley & Sons, Chichester, 5th edn, 2000 Search PubMed.
- J. Sahoo, C. R. Sahoo, P. K. N. Sarangi, S. K. Prusty, R. N. Padhy and S. K. Paidesetty, Molecules with versatile biological activities bearing antipyrinyl nucleus as pharmacophore, Eur. J. Med. Chem., 2020, 186, 111911 CrossRef CAS PubMed.
- (a) L. J. Dolby, C. Wilkins and T. G. Frey, The Mechanism of the Prins Reaction. V. The Prins Reaction of Styrenes, J. Org. Chem., 1966, 31, 1110–1116 CrossRef CAS; (b) J. Liu, Q. Ren, X. Zhang and H. Gong, Preparation of Vinyl Arenes by Nickel-Catalyzed Reductive Coupling of Aryl Halides with Vinyl Bromides, Angew. Chem., Int. Ed., 2016, 55, 15544–15548 CrossRef CAS PubMed.
- Since the homo-coupling product of 2am, (E,E)-1,4-diphenyl-1,3-butadiene, is difficult to separate from (E,E)-3tam, the use of a larger quantity of 2am should be avoided in this case.
- For the reaction conditions, see: J. Yin and S. L. Buchwald, Pd-Catalyzed Intermolecular Amidation of Aryl Halides: The Discovery that Xantphos Can Be Trans-Chelating in a Palladium Complex, J. Am. Chem. Soc., 2002, 124, 6043–6048 CrossRef CAS PubMed.
- For the reaction conditions, see: T. Hundertmark, A. F. Littke, S. L. Buchwald and G. C. Fu, Pd(PhCN)2Cl2/P(t-Bu)3: A Versatile Catalyst for Sonogashira Reactions of Aryl Bromides at Room Temperature, Org. Lett., 2000, 2, 1729–1731 CrossRef CAS PubMed.
- For the reaction conditions, see: T. Dalton, S. Greßies, M. Das, M. Niehues, M. L. Schrader, C. Gutheil, B. J. Ravoo and F. Glorius, Silver-Catalysed Hydroarylation of Highly Substituted Styrenes, Angew. Chem., Int. Ed., 2021, 60, 8537–8541 CrossRef CAS PubMed.
- For example, see: G. Zappia, in Comprehensive Chirality, ed. E. M. Carreira and H. Yamamoto, Elsevier Science, Amsterdam, 2012, vol. 3, pp. 408–485 Search PubMed.
- For the reaction conditions, see: S. Fukuzawa, T. Tsuchimoto, T. Hotaka and T. Hiyama, Direct Synthesis of Chiral Acetals from Carbonyl Compounds and Chiral Diols: Sequential One-Pot Asymmetric Silylcyanation Reaction Catalyzed by Scandium(III) Triflate, Synlett, 1995, 1077–1078 CrossRef CAS.
- The formation of HOB(dan) and (dan)BOB(dan) was also confirmed by HRMS analysis. See the ESI† for details.
- For a part of the catalytic cycle depicted in Fig. 6, see: A. J. J. Lennox and G. C. Lloyd-Jones, Transmetalation in the Suzuki–Miyaura Coupling: The Fork in the Trail, Angew. Chem., Int. Ed., 2013, 52, 7362–7370 CrossRef CAS PubMed.
- It could not be ascertained whether the dehydration of HOB(dan) to yield (dan)BOB(dan) occurred before or after the aqueous work-up.
- For selected reports, see: (a) C. F. R. A. C. Lima, A. S. M. C. Rodrigues, V. L. M. Silva, A. M. S. Silva and L. M. N. B. F. Santos, Role of the Base and Control of Selectivity in the Suzuki–Miyaura Cross-Coupling Reaction, ChemCatChem, 2014, 6, 1291–1302 CrossRef CAS; (b) M. A. Ortuño, A. Lledós, F. Maseras and G. Ujaque, The Transmetalation Process in Suzuki–Miyaura Reactions: Calculations Indicate Lower Barrier via Boronate Intermediate, ChemCatChem, 2014, 6, 3132–3138 CrossRef; (c) C. P. Delaney, D. P. Marron, A. S. Shved, R. N. Zare, R. M. Waymouth and S. E. Denmark, Potassium Trimethylsilanolate-Promoted, Anhydrous Suzuki–Miyaura Cross-Coupling Reaction Proceeds via the “Boronate Mechanism”: Evidence for the Alternative Fork in the Trail, J. Am. Chem. Soc., 2022, 144, 4345–4364 CrossRef CAS PubMed.
- For selected examples, see: (a) B. P. Carrow and J. F. Hartwig, Distinguishing Between Pathways for Transmetalation in Suzuki–Miyaura Reactions, J. Am. Chem. Soc., 2011, 133, 2116–2119 CrossRef CAS PubMed; (b) C. Amatore, A. Jutand and G. L. Duc, Kinetic Data for the Transmetalation/Reductive Elimination in Palladium-Catalyzed Suzuki–Miyaura Reactions: Unexpected Triple Role of Hydroxide Ions Used as Base, Chem. – Eur. J., 2011, 17, 2492–2503 CrossRef CAS PubMed.
- A. A. Thomas, H. Wang, A. F. Zahrt and S. E. Denmark, Structural, Kinetic, and Computational Characterization of the Elusive Arylpalladium(II)boronate Complexes in the Suzuki–Miyaura Reaction, J. Am. Chem. Soc., 2017, 139, 3805–3821 CrossRef CAS PubMed.
- In accordance with the suggestion by one referee, we examined whether cis-PdBr(C6H4–p-F)(dppf) (16j), which is the precursor of 18j in path B, reacts with LiOH in DMF to yield 18j. We hence performed some reactions shown below but failed to observe 18j. At present, we consider that this outcome may be due, in part, to the extremely low solubility of LiOH in DMF as well as the instability of 18j in DMF. NOTE: 18j decomposes in DMF, with an estimated half-life (t1/2) of approximately 12 h at rt. The formation of 18 from 16 thus remains unobserved and awaits future further investigation.
. - M. Iwasaki and Y. Nishihara, in Applied Cross-Coupling Reactions, ed. Y. Nishihara, Springer, Berlin, 2013, pp. 17–39 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, analytical data, characterization data and NMR spectra of compounds. See DOI: https://doi.org/10.1039/d5qo00230c |
| This journal is © the Partner Organisations 2025 |









![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 (1.25 equiv.), Pd(OAc)2 (3 mol%), P(
CH2 (1.25 equiv.), Pd(OAc)2 (3 mol%), P(