 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-catalyzed Buchner reaction and phenyl cyclopropanation through diyne cyclization†
Tong
Li‡
a,
Jia-Juan
He‡
a,
Kai-Yu
Fan
a,
Long-Wu
Ye
 *abc,
Bo
Zhou
*abc,
Bo
Zhou
 b and
Xin-Qi
Zhu
b and
Xin-Qi
Zhu
 *a
*a
aYunnan Key Laboratory of Modern Separation Analysis and Substance Transformation, College of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming 650500, China. E-mail: longwuye@xmu.edu.cn; xinqizhu@ynnu.edu.cn
bKey Laboratory of Chemical Biology of Fujian Province, State Key Laboratory of Physical Chemistry of Solid Surfaces, and College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
First published on 22nd January 2025
Abstract
The Buchner reaction, as an exclusive category of expansive dearomatization, represents a powerful approach for the construction of valuable functionalized cycloheptatrienes in a direct manner from readily available aromatic precursors. However, the traditional Buchner reaction almost relies on using explosive diazo compounds as carbene precursors. Herein, we disclose a copper-catalyzed Buchner reaction through diyne cyclization, and bicycle-fused cycloheptatrienes bearing all carbon quaternary stereogenic centers are obtained in moderate to excellent yields with a broad substrate scope. In addition, norcaradiene derivatives can also be selectively produced by substrate control through a phenyl cyclopropanation reaction, thus constituting a copper-catalyzed controllable cyclization of diynes.
The Buchner reaction, since its discovery in 1885 by Buchner and Curtius,1 has become a feasible protocol for the straightforward synthesis of valuable functionalized cycloheptatrienes from ubiquitous aromatic precursors.2 In addition, norcaradiene, as an arene cyclopropanation intermediate in the Buchner reaction, has also been extensively employed to synthesize versatile cyclopropane derivatives.3 In the past decades, transition-metal-catalyzed reactions via metal carbenes4 have proven to be the most important method to realize the Buchner reaction. However, most of the transition-metal-catalyzed Buchner reactions have to rely on the use of explosive diazo compounds as carbene precursors (Scheme 1a),5 which strictly restrain their further synthetic applications. By comparison, the Buchner reaction based on alkynes via a non-diazo approach was far less explored (Scheme 1a). In 2014, Panek and co-workers first reported the Buchner reaction based on alkynes via a rhodium-catalyzed nitrene–alkyne cycloaddition.6a Such a similar strategy was nicely exploited by Zhang6b and Huang6c respectively via a gold-catalyzed intermolecular oxidation of alkynes. In 2021, the first example of the asymmetric Buchner reaction based on alkynes7 was demonstrated by Nemoto and Harada via a non-diazo approach.7a Despite these significant advances (Scheme 1a), these protocols are almost limited to noble-metal catalysts, and they are not atom-economical synthesis. Therefore, the exploration of novel approaches for the non-noble-metal-catalyzed Buchner reaction from alkynes, especially in an atom-economical manner, is highly desirable.
The vinyl cation, as a prominent intermediate in organic synthesis, has attracted widespread interest for its unique carbene-like reactivity in recent years.8 On the basis of this, in 2022, Zhang and co-workers first disclosed a hypervalent iodine-promoted Buchner reaction via a vinyl cation (Scheme 1b).9 In the past several years, our group has established a unique copper-catalyzed cyclization of diynes for the generation of vinyl cations. By using this methodology, a variety of valuable asymmetric conversions have been developed through the remote control of enantioselectivity, enabling the divergent synthesis of enantioenriched N-heterocycles.10 Inspired by the above results and by our recent study on developing ynamide chemistry for heterocycle synthesis,11 we envisaged that vinyl cations, generated from copper-catalyzed diyne cyclization, could react with intramolecular arene moieties, delivering the corresponding dearomatized products (Scheme 1c). Very recently, our group also reported an asymmetric Buchner reaction and arene cyclopropanation via a copper-catalyzed controllable cyclization of diynes.12 However, in this protocol, the diyne substrates were limited to phenyl-fused diynes for the Buchner reaction and only naphthyl-substituted diynes were suitable for arene cyclopropanation. Herein, we disclose a Buchner reaction and phenyl cyclopropanation via a copper-catalyzed cyclization of phenyl linked diynes, furnishing a series of valuable bicycle-fused cycloheptatrienes and norcaradienes bearing all carbon quaternary carbon centers in generally moderate to excellent yields with a wide substrate scope.
We started our investigations by using the phenethyl-substituted N-propargyl ynamide 1a as the model substrate using the copper-catalyzed method for the Buchner reaction (Table 1). To our delight, the desired cycloheptatriene 2a bearing an all carbon quaternary carbon center could be readily prepared in 76% yield by employing Cu(MeCN)4PF6 (10 mol%) as a catalyst, NaBArF4 (12 mol%) as an additive and (±)-SEGPHOS (12 mol%) as a ligand in toluene (Table 1, entry 1). Stimulated by this primary attempt, we next screened several typical solvents such as CH2Cl2, DCE and THF (Table 1, entries 2–4), and CH2Cl2 was found to be the optimal solvent with 80% yield (Table 1, entry 2). Then, various typical copper catalysts, such as Cu(MeCN)4BF4, CuOTf, CuCl, CuBr, Cu(OTf)2 and CuCl2, were also evaluated (Table 1, entries 5–10). We were pleased to find that the use of CuCl as a catalyst led to a slightly increased yield, and the anticipated cycloheptatriene 2a was delivered in 84% yield (Table 1, entry 7). Finally, significantly decreased yields of 2a were detected in the absence of a ligand and NaBArF4 (Table 1, entries 11–13).
| Entry | Catalyst | Reaction conditions | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1 (0.05 mmol), catalyst (0.005 mmol), (±)-SEGPHOS (0.006 mmol), NaBArF4 (0.006 mmol), solvent (1 mL), PMP = 4-methoxyphenyl, NaBArF4 = sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate. b Measured by 1H NMR using diethyl phthalate as an internal standard. c Without a ligand. d Without NaBArF4. | |||
| 1 | Cu(MeCN)4PF6 | Toluene, rt, 23 h | 76 |
| 2 | Cu(MeCN)4PF6 | CH2Cl2, rt, 23 h | 80 |
| 3 | Cu(MeCN)4PF6 | DCE, rt, 23 h | 58 |
| 4 | Cu(MeCN)4PF6 | THF, rt, 23 h | <5 |
| 5 | Cu(MeCN)4BF4 | CH2Cl2, rt, 23 h | 10 |
| 6 | CuOTf | CH2Cl2, rt, 23 h | <5 |
| 7 | CuCl | CH 2 Cl 2 , 40 °C, 4 d | 84 |
| 8 | CuBr | CH2Cl2, rt, 23 h | 70 |
| 9 | Cu(OTf)2 | CH2Cl2, rt, 23 h | 20 |
| 10 | CuCl2 | CH2Cl2, rt, 4 d | <5 |
| 11c | CuCl | CH2Cl2, 40 °C, 4 d | 67 |
| 12d | CuCl | CH2Cl2, 40 °C, 4 d | <5 |
| 13c,d | CuCl | CH2Cl2, 40 °C, 4 d | 68 |
Having established the optimized reaction conditions (Table 1, entry 7), we next sought to explore the scope of this Buchner reaction. As shown in Table 2, we initially investigated the scope of different N-protecting groups of N-propargyl ynamides. Apart from the Ts-protected ynamide, the reaction also proceeded smoothly with alkyl sulfonyl groups, such as Ms and SO2Et, providing the corresponding cycloheptatriene-fused pyrroles 2b and 2c in good yields. Then, we changed PMP to other O-containing electron-donating groups (EDGs). For instance, different O-containing arylsubstituted N-propargyl ynamides encompassing functional groups (e.g. ethyl, benzyl, silyl, and phenyl) were suitable substrates for this tandem cyclization, affording the desired cycloheptatrienes 2d–2g in 74–90% yields. In addition, the Me- and Et-substituted diynes 1h and 1i could react smoothly in this protocol, and the cyclic products 2h and 2i were produced in 68% and 75% yields, respectively. Subsequently, other heteroatom-containing EDGs, such as NMe2, NBn2, and SMe, were also applicable for this reaction to deliver the desired cycloheptatriene-fused pyrroles 2j–2l in moderate to good yields. The simple phenyl-substituted cycloheptatriene 2m could be readily prepared from diyne 1m as well. In particular, we were surprised to find that N-propargyl ynamides bearing electron-withdrawing groups (EWGs) on the aryl ring (e.g. F, Cl, Br, and CO2Me) could participate in this transformation as well, giving rise to 2n–2q in 77–83% yields. It is worth noting that EWG-substituted diynes were incompatible in the asymmetric Buchner reaction previously reported by our group.13 Furthermore, various substitutions at the meta-position of the phenyl ring had little effect on this protocol, delivering the related cycloheptatrienes 2r–2t in 74–94% yields. Besides the mono-substituted aryl groups, di-substituted aromatic groups were also readily tolerated, leading to products 2u–2w in excellent yields. Heteroaryl-substituted N-propargyl ynamide 1x was proved to be the applicable substrate in this reaction, providing the anticipated product 2x in 76% yield. Finally, substitutions at the phenyl of the phenethyl moiety were studied. It was found that para-substitutions including both electron-donating and electron-withdrawing groups worked efficiently, and the corresponding cycloheptatriene products 2y–2aa were obtained with receivable yields. We also attempted to realize this asymmetric Buchner reaction by using various chiral ligands. However, only low enantioselectivities were obtained at this stage (for details, see the ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
Intriguingly, during the substrate scope study of the above Buchner reaction, it was found that when the diphenyl-substituted diynes 3 were used as substrates, only the phenyl cyclopropanation products 4 were obtained and no seven-membered Buchner cyclization products were isolated. Notably, the desymmetrization13 and dearomatization14 processes were simultaneous in this phenyl cyclopropanation. After the optimization study (for details, see the ESI†), we confirmed the optimized reaction conditions as follows: CuCl (10 mol%) as a catalyst, NaBArF4 (12 mol%) as an additive and BINAP (12 mol%) as a ligand in CH2Cl2. The substrate scope for the synthesis of norcaradiene-derived pyrroles was then examined. As depicted in Table 3, this phenyl cyclopropanation was remarkably tolerant of various N-protected ynamides, including Ts, MBS, Bs, Ns, and SO2-2-naphthyl, affording the corresponding norcaradienes 4a–4e in 70–99% yields. Moreover, this dearomatization process readily accommodated substitutions at different positions of the phenyl ring with a variety of electron-rich substituents such as Me, OMe, OEt, OBn, OPh, OTBS, and SMe, enabling the assembly of the expected norcaradiene-derived pyrroles 4f–4p in generally good yields. Then, the reaction was also extended to di-substituted diynes 3q–3s, enabling an approach for the synthesis of the anticipated norcaradienes 4q–4s in 72–87% yields. Next, the thienyl-substituted N-propargyl ynamide 3t performed well in this phenyl cyclopropanation, and the corresponding product 4t was isolated in 87% yield. Ultimately, further modifications of the substituents (R substituent) of diphenyl groups with electron-rich and electron-deficient substituents encompassing 4-Me, 4-F, 4-Cl and 4-Br were also tolerated to furnish the desired norcaradiene derived pyrroles 4u–4x in moderate to excellent yields. However, the reaction of 3-fluorophenyl-substituted diyne 3y under the standard conditions only gave a complex mixture. Of note, excellent diastereoselectivities (dr > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were achieved in all cases. The enantioselective phenyl cyclopropanation was also demonstrated, but only low enantioselectivities were detected by employing various chiral ligands (for details, see the ESI†). The relative configuration of product 4a was confirmed by X-ray crystallographic analysis.15
1) were achieved in all cases. The enantioselective phenyl cyclopropanation was also demonstrated, but only low enantioselectivities were detected by employing various chiral ligands (for details, see the ESI†). The relative configuration of product 4a was confirmed by X-ray crystallographic analysis.15
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
a ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (0.2 mmol), CuCl (0.02 mmol), (±)-BINAP (0.024 mmol), NaBArF4 (0.024 mmol), CH2Cl2 (2 mL), 40 °C, 11 h–4 d; yields are those for the isolated products. 3 (0.2 mmol), CuCl (0.02 mmol), (±)-BINAP (0.024 mmol), NaBArF4 (0.024 mmol), CH2Cl2 (2 mL), 40 °C, 11 h–4 d; yields are those for the isolated products.
|
|---|

|
Interestingly, it was found that the treatment of phenyl and methyl substituted diyne 3z under the standard reaction conditions afforded the cyclopropanation product 4z as a single product in 94% yield (eqn (1)).
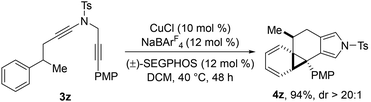 | (1) |
Gram-scale synthesis and further synthetic applications of the as-synthesized cycloheptatriene 2a and norcaradiene 4a were then investigated, as shown in Scheme 2. First, the cycloheptatriene-derived pyrrole 2a could be prepared on a gram scale in 92% yield under the standard conditions. The protecting group of 2a could be efficiently removed by treatment with KOH, followed by protection with the Me group to form the desired 5a in 75% yield in 2 steps. Then, the treatment of 2a with NaBH3CN as the reductant led to the tricyclic dihydropyrrole 5b in a good yield. Interestingly, 2a could undergo the Diels–Alder reaction with maleic anhydride to furnish the polycyclic bridged product 5c with an excellent yield and diastereoselectivity. Of note, 6π-electrocyclization of 2a occurred first to form norcaradiene at a high temperature, followed by the subsequent Diels–Alder reaction of norcaradiene with maleic anhydride to produce 5c. In addition, the Ts group in the norcaradiene-derived pyrrole 4a could be readily removed under basic conditions to produce the unprotected pyrrole 5d in 83% yield. Finally, reduction of 4a with NaBH3CN led to the norcaradiene-derived dihydropyrrole 5e in a good yield.
Based on our previous research studies,10,13 a plausible mechanism for this copper-catalyzed controllable Buchner reaction and phenyl cyclopropanation was proposed and is presented in Scheme 3. First, the coordination of copper complexes with diyne 1a or 3a forms complex A, which induces an intramolecular cyclization to afford the vinyl cation intermediate containing alkenyl copper species B. Subsequently, the vinyl cation is trapped by the phenyl group via an intramolecular nucleophilic attack to provide the phenyl cation intermediate C, which undergoes further intramolecular cyclization to generate the copper carbene D. Then, intermediate D undergoes a formal [1,4]-H shift and demetallation to produce norcaradiene E with the regeneration of the copper catalyst. When the R substitution is phenyl, only the phenyl cyclopropanation product 4a is achieved. Alternatively, cycloheptatriene 2a is delivered selectively via a retro-6π electro-ring opening when the R substitution is hydrogen. For this unique chemoselectivity, we assumed that when the R substitution is aryl or methyl, the steric-hindrance effect may hinder the ring opening of cyclopropanes to give the 7-membered ring products.
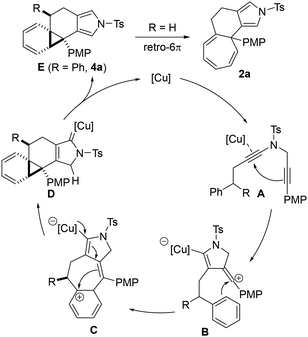 | ||
| Scheme 3 Plausible catalytic cycle for the copper-catalyzed controllable Buchner reaction and arene cyclopropanation. | ||
In conclusion, we have developed an efficient copper-catalyzed controllable Buchner reaction and phenyl cyclopropanation of phenyl or diphenyl tethered diynes, providing a series of valuable bicycle-fused cycloheptatrienes and norcaradienes bearing all carbon quaternary carbon centers in generally moderate to excellent yields with a wide substrate scope under mild reaction conditions. In particular, the diynes can tolerate various EWGs on the aryl ring in the Buchner reaction, and a desymmetrization/dearomatization process is involved in the phenyl cyclopropanation. Further investigation of catalytic asymmetric cyclization of these diynes is undergoing in our laboratory.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (22101238, 22125108, 22331004), the Yunnan Fundamental Research Project (202401CF070024), and Yunnan Normal University.References
- E. Buchner and T. Curtius, Ueber die Einwirkung von Diazoessigäther auf aromatische Kohlenwasserstoffe, Ber. Dtsch. Chem. Ges., 1885, 18, 2377 CrossRef.
- For selected reviews on the Buchner reaction, see: (a) C.-Y. Shi, G.-Y. Zhu, Y. Xu, M.-Y. Teng and L.-W. Ye, Recent Advances in Catalytic Asymmetric Buchner Reaction, Chin. Chem. Lett., 2023, 34, 108441 CrossRef CAS; (b) G. Ma, K.-F. Wei, M. Song, Y.-L. Dang, Y. Yue, B. Han, H. Su and W.-B. Shen, Recent Advances in Transition-metal-catalyzed Buchner Reaction of Alkynes, Org. Biomol. Chem., 2023, 21, 5150 RSC; (c) S. E. Reisman, R. R. Nani and S. Levin, Buchner and Beyond: Arene Cyclopropanation as Applied to Natural Product Total Synthesis, Synlett, 2011, 2437 CrossRef CAS.
- For selected examples of the arene cyclopropanation reaction, see: (a) F. Guan, R. Zhou, X. Ren, Z. Guo, C. Wang and C.-Y. Zhou, Asymmetric Dearomative Cyclopropanation of Naphthalenes to Construct Polycyclic Compounds, Chem. Sci., 2022, 13, 13015 RSC; (b) N. Otog, B. Gantogos, I. Fujisawa and S. Iwasa, Highly Enantioselective Synthesis of Norcaradiene Derivatives from Naphthyl Diazoacetamides Using a Ru(II)-Pheox Complex, Chem. Commun., 2022, 58, 12325 RSC; (c) S. Zhao, X.-X. Chen, N. Gao, M. Qian and X. Chen, Visible-Light-Mediated Cyclopropanation Reactions of 3-Diazooxindoles with Arenes, J. Org. Chem., 2021, 86, 7131 CrossRef; (d) K. L. Smith, C. L. Padgett, W. D. Mackay and J. S. Johnson, J. Am. Chem. Soc., 2020, 142, 6449 CrossRef; (e) R. R. Nani and S. E. Reisman, Catalytic Asymmetric Dearomative Synthesis of Complex Cyclohexanes via a Highly Regio- and Stereoselective Arene Cyclopropanation Using α-Cyanodiazoacetates, J. Am. Chem. Soc., 2013, 135, 7304 CrossRef; (f) M. P. Doyle, D. G. Ene, D. C. Forbes and T. H. Pillow, Chemoselectivity and Enantiocontrol in Catalytic Intramolecular Metal Carbene Reactions of Diazo Acetates Linked to Reactive Functional Groups by Naphthalene-1,8-Dimethanol, Chem. Commun., 1999, 1691 RSC.
- For recent selected reviews on the transition-metal-catalyzed reaction via metal carbenes, see: (a) Y. Chen and S. Zhu, Recent Advances in Metal Carbene-Induced Semipinacol Rearrangements, Chem. Commun., 2024, 60, 11253 RSC; (b) T. R. Roose, D. S. Verdoorn, P. Mampuys, E. Ruijter, B. U. W. Maes, R. V. A. Orru and T. R. Roose, Transition Metal-Catalysed Carbene- And Nitrene Transfer To Carbon Monoxide and Isocyanides, Chem. Soc. Rev., 2022, 51, 5842 RSC; (c) X. Qi and Y. Lan, Recent Advances in Theoretical Studies on Transition-Metal-Catalyzed Carbene Transformations, Acc. Chem. Res., 2021, 54, 2905 CrossRef; (d) T. Wang and A. S. K. Hashmi, 1,2-Migrations onto Gold Carbene Centers, Chem. Rev., 2021, 121, 8948 CrossRef; (e) L.-W. Ye, X.-Q. Zhu, R. L. Sahani, Y. Xu, P.-C. Qian and R.-S. Liu, Nitrene Transfer and Carbene Transfer in Gold Catalysis, Chem. Rev., 2021, 121, 9039 CrossRef PubMed; (f) S. W. Roh, K. Choi and C. Lee, Transition Metal Vinylidene- and Allenylidene-Mediated Catalysis in Organic Synthesis, Chem. Rev., 2019, 119, 4293 CrossRef PubMed.
- For recent selected examples of the Buchner reaction by using diazo compounds as carbene precursors, see: (a) B. Darses, P. Maldivi, C. Philouze, P. Dauban and J.-F. Poisson, Asymmetric Intramolecular Buchner Reaction: From High Stereoselectivity to Coexistence of Norcaradiene, Cycloheptatriene, and an Intermediate form in the Solid State, Org. Lett., 2021, 23, 300 CrossRef PubMed; (b) Q. Zeng, K. Dong, C. Pei, S. Dong, W. Hu, L. Qiu and X. Xu, Divergent Construction of Macrocyclic Alkynes via Catalytic Metal Carbene C(sp2)–H Insertion and the Buchner Reaction, ACS Catal., 2019, 9, 10773 CrossRef; (c) T. Hoshi, E. Ota, Y. Inokuma and J. Yamaguchi, Asymmetric Synthesis of a 5,7-Fused Ring System Enabled by an Intramolecular Buchner Reaction with Chiral Rhodium Catalyst, Org. Lett., 2019, 21, 10081 CrossRef; (d) N. P. T. Thanh, M. Tone, H. Inoue, I. Fujisawa and S. Iwasa, Highly Stereoselective Intramolecular Buchner Reaction of Diazoacetamides Catalyzed by a Ru(II)-Pheox Complex, Chem. Commun., 2019, 55, 13398 RSC; (e) H. Nakayama, S. Harada, M. Kono and T. Nemoto, Chemoselective Asymmetric Intramolecular Dearomatization of Phenols with α-Diazoacetamides Catalyzed by Silver Phosphate, J. Am. Chem. Soc., 2017, 139, 10188 CrossRef; (f) H. Wang, C.-Y. Zhou and C.-M. Che, Cobalt-Porphyrin-Catalyzed Intramolecular Buchner Reaction and Arene Cyclopropanation of in situ Generated Alkyl Diazomethanes, Adv. Synth. Catal., 2017, 359, 2253 CrossRef; (g) G. S. Fleming and A. B. Beeler, Regioselective and Enantioselective Intermolecular Buchner Ring Expansions in Flow, Org. Lett., 2017, 19, 5268 CrossRef PubMed; (h) X. Xu, X. Wang, P. Z. Zavalij and M. P. Doyle, Straightforward Access to the [3.2.2]Nonatriene Structural Framework via Intramolecular Cyclopropenation/Buchner Reaction/Cope Rearrangement Cascade, Org. Lett., 2015, 17, 790 CrossRef PubMed; (i) Z. Liu, H. Tan, L. Wang, T. Fu, Y. Xia, Y. Zhang and J. Wang, Transition-Metal-Free Intramolecular Carbene Aromatic Substitution/Buchner Reaction: Synthesis of Fluorenes and [6,5,7]Benzo-fused Rings, Angew. Chem., Int. Ed., 2015, 54, 3056 CrossRef; (j) X. C. Wang, Q. M. Abrahams, P. J. Zavalij and M. P. Doyle, Highly Regio- and Stereoselective Dirhodium Vinylcarbene Induced Nitrone Cycloaddition with Subsequent Cascade Carbenoid Aromatic Cycloaddition/N–O Cleavage and Rearrangement, Angew. Chem., Int. Ed., 2012, 51, 5907 CrossRef; (k) P. Panne and J. M. Fox, Rh-Catalyzed Intermolecular Reactions of Alkynes with α-Diazoesters that Possess β-Hydrogens: Ligand-Based Control over Divergent Pathways, J. Am. Chem. Soc., 2007, 129, 22 CrossRef PubMed; (l) B. R. Galan, M. Gembicky, P. M. Dominiak, J. B. Keister and S. T. Diver, Carbon Monoxide-Promoted Carbene Insertion into the Aryl Substituent of an N-Heterocyclic Carbene Ligand: Buchner Reaction in a Ruthenium Carbene Complex, J. Am. Chem. Soc., 2005, 127, 15702 CrossRef.
- (a) R. A. Brawn, K. Zhu and J. S. Panek, Rhodium(II)-Catalyzed Alkyne Amination of Homopropargylic Sulfamate Esters: Stereoselective Synthesis of Functionalized Norcaradienes by Arene Cyclopropanation, Org. Lett., 2014, 16, 74 CrossRef; (b) K. Ji and L. Zhang, Cyclopropanation of Benzene Rings by Oxidatively Generated α-Oxo Gold Carbene: One-Pot Access to TetrahydropyranoneFused Cycloheptatrienes from Propargyl Benzyl Ethers, Adv. Synth. Catal., 2018, 360, 647 CrossRef; (c) J. Xia, J. Liu, Y. Yu, J. Zhang and X. Huang, Divergent Access to Polycyclic N-Heterocyclic Compounds through Büchner-Type Dearomatization Enabled Cycloisomerization of Diynamides under Gold Catalysis, Org. Lett., 2022, 24, 4298 CrossRef PubMed.
- (a) T. Ito, S. Harada, H. Homma, H. Takenaka, S. Hirose and T. Nemoto, Asymmetric Intramolecular Dearomatization of Nonactivated Arenes with Ynamides for Rapid Assembly of Fused Ring System under Silver Catalysis, J. Am. Chem. Soc., 2021, 143, 604 CrossRef PubMed; (b) D. Zhu, T. Cao, K. Chen and S. Zhu, Rh2(II)-Catalyzed Enantioselective Intramolecular Buchner Reaction and Aromatic Substitution of Donor-Donor Carbenes, Chem. Sci., 2022, 13, 1992 RSC.
- For selected reviews on vinyl cations, see: (a) X.-J. Liu, Y. Xu, C. Tang, P.-C. Qian and L.-W. Ye, Unactivated C(sp3)–H Functionalization via Vinyl Cations, Sci. China: Chem., 2022, 65, 20 CrossRef; (b) M. Niggemann and S. Gao, Are Vinyl Cations Finally Coming of Age?, Angew. Chem., Int. Ed., 2018, 57, 16942 CrossRef. For selected examples, see: (c) A. Ahrens, J. Schwarz, D. M. Lustosa, R. Pourkaveh, M. Hoffmann, F. Rominger, M. Rudolph, A. Dreuw and A. S. K. Hashmi, Synthesis of Fulvene Vinyl Ethers by Gold Catalysis, Chem. – Eur. J., 2020, 26, 5280 CrossRef PubMed; (d) S. Tavakkolifard, K. Sekine, L. Reichert, M. Ebrahimi, K. Museridz, E. Michel, F. Rominger, R. Babaahmadi, A. Ariafard, B. F. Yates, M. Rudolph and A. S. K. Hashmi, Gold-Catalyzed Regiospecific Annulation of Unsymmetrically Substituted 1,5-Diynes for the Precise Synthesis of Bispentalenes, Chem. – Eur. J., 2019, 25, 12180 CrossRef CAS PubMed; (e) T. Wurm, J. Bucher, S. B. Duckworth, M. Rudolph, F. Rominger and A. S. K. Hashmi, On the Gold-Catalyzed Generation of Vinyl Cations from 1,5-Diynes, Angew. Chem., Int. Ed., 2017, 56, 3364 CrossRef CAS.
- D.-F. Yuan, Z.-C. Wang, R.-S. Geng, G.-Y. Ren, J. S. Wright, S.-F. Ni, M. Li, L.-R. Wen and L.-B. Zhang, Hypervalent Iodine Promoted the Synthesis of Cycloheptatrienes and Cyclopropanes, Chem. Sci., 2022, 13, 478 RSC.
- (a) F.-L. Hong, Z.-S. Wang, D.-D. Wei, T.-Y. Zhai, G.-C. Deng, X. Lu, R.-S. Liu and L.-W. Ye, Generation of Donor/Donor Copper Carbenes through Copper-Catalyzed Diyne Cyclization: Enantioselective and Divergent Synthesis of Chiral Polycyclic Pyrroles, J. Am. Chem. Soc., 2019, 141, 16961 CrossRef CAS; (b) F.-L. Hong, Y.-B. Chen, S.-H. Ye, G.-Y. Zhu, X.-Q. Zhu, X. Lu, R.-S. Liu and L.-W. Ye, Copper-Catalyzed Asymmetric Reaction of Alkenyl Diynes with Styrenes by Formal [3 + 2] Cycloaddition via Cu-Containing All-Carbon 1,3-Dipoles: Access to Chiral Pyrrole-Fused Bridged [2.2.1]Skeletons, J. Am. Chem. Soc., 2020, 142, 7618 CrossRef CAS; (c) X.-Q. Zhu, P. Hong, Y.-X. Zheng, Y.-Y. Zhen, F.-L. Hong, X. Lu and L.-W. Ye, Copper-Catalyzed Asymmetric Cyclization of Alkenyl Diynes: Method Development and New Mechanistic Insights, Chem. Sci., 2021, 12, 9466 RSC; (d) F.-L. Hong, C.-Y. Shi, P. Hong, T.-Y. Zhai, X.-Q. Zhu, X. Lu and L.-W. Ye, Copper-Catalyzed Asymmetric Diyne Cyclization via [1,2]-Stevens-Type Rearrangement for the Synthesis of Chiral Chromeno[3,4-c]pyrroles, Angew. Chem., Int. Ed., 2022, 61, e202115554 CrossRef CAS; (e) L.-J. Qi, C.-T. Li, Z.-Q. Huang, J.-T. Jiang, X.-Q. Zhu, X. Lu and L.-W. Ye, Enantioselective Copper-Catalyzed Formal [2 + 1] and [4 + 1] Annulations of Diynes with Ketones via Carbonyl Ylides, Angew. Chem., Int. Ed., 2022, 61, e202210637 CrossRef CAS; (f) J.-J. Zhou, Y.-N. Meng, L.-G. Liu, Y.-X. Liu, Z. Xu, X. Lu, B. Zhou and L.-W. Ye, Copper-Catalyzed Enantioselective Diyne Cyclization via C(sp2)–O Bond Cleavage, Chem. Sci., 2023, 14, 3493 RSC; (g) Y.-B. Chen, L.-G. Liu, C.-M. Chen, Y.-X. Liu, B. Zhou, X. Lu, Z. Xu and L.-W. Ye, Construction of Axially Chiral Arylpyrroles via Atroposelective Diyne Cyclization, Angew. Chem., Int. Ed., 2023, 62, e202303670 CrossRef CAS; (h) C.-T. Li, L.-J. Qi, L.-G. Liu, C. Ge, X. Lu, L.-W. Ye and B. Zhou, Asymmetric Formal C–C Bond Insertion into Aldehydes via Copper-Catalyzed Diyne Cyclization, Nat. Commun., 2023, 14, 7058 CrossRef CAS PubMed; (i) H.-J. Xu, C.-T. Li, C.-M. Chen, J. Chen, X.-Q. Zhu, B. Zhou and L.-W. Ye, Copper-Catalyzed Formal [4 + 1] Annulation of N-Propargyl Ynamides with Diketones, Org. Chem. Front., 2023, 10, 203 RSC; (j) Y.-B. Chen, L.-G. Liu, Z.-Q. Wang, R. Chang, X. Lu, B. Zhou and L.-W. Ye, Enantioselective Functionalization of Unactivated C(sp3)–H Bonds through Copper-Catalyzed Diyne Cyclization by Kinetic Resolution, Nat. Commun., 2024, 15, 2232 CrossRef CAS PubMed; (k) F.-S. Li, X.-Y. Zou, T.-Q. Hu, Q. Sun, Z. Xu, B. Zhou and L.-W. Ye, Asymmetric One-Carbon Ring Expansion of Diverse N-Heterocycles via Copper-Catalyzed Diyne Cyclization, Sci. Adv., 2024, 10, eadq7767 CrossRef CAS; (l) H.-H. Chen, Y.-B. Chen, J.-Z. Gao, L.-W. Ye and B. Zhou, Copper-Catalyzed Enantioselective Dehydro-Diels–Alder Reaction: Atom-Economical Synthesis of Axially Chiral Carbazoles, Angew. Chem., Int. Ed., 2024, 63, e202411709 CrossRef CAS; (m) B. Prabagar, R. K. Mallick, R. Prasad, V. Gandon and A. K. Sahoo, Umpolung Reactivity of Ynamides: An Unconventional [1,3]-Sulfonyl and [1,5]-Sulfinyl Migration Cascade, Angew. Chem., Int. Ed., 2019, 58, 2365 CrossRef CAS PubMed; (n) S. Dutta, R. K. Mallick, R. Prasad, V. Gandon and A. K. Sahoo, Alkyne Versus Ynamide Reactivity: Regioselective Radical Cyclization of Yne-Ynamides, Angew. Chem., Int. Ed., 2019, 58, 2289 CrossRef CAS.
- For recent selected reviews on ynamide chemistry, see: (a) L. Hu and J. Zhao, Ynamide Coupling Reagents: Origin and Advances, Acc. Chem. Res., 2024, 57, 855 CrossRef CAS PubMed; (b) Y.-C. Hu, Y. Zhao, B. Wan and Q.-A. Chen, Reactivity of Ynamides in Catalytic Intermolecular Annulations, Chem. Soc. Rev., 2021, 50, 2582 RSC; (c) C. C. Lynch, A. Sripada and C. Wolf, Asymmetric Synthesis with Ynamides: Unique Reaction Control, Chemical Diversity and Applications, Chem. Soc. Rev., 2020, 49, 8543 RSC; (d) Y.-B. Chen, P.-C. Qian and L.-W. Ye, Brønsted Acid-Mediated Reactions of Ynamides, Chem. Soc. Rev., 2020, 49, 8897 RSC; (e) F.-L. Hong and L.-W. Ye, Transition Metal-Catalyzed Tandem Reactions of Ynamides for Divergent N-Heterocycle Synthesis, Acc. Chem. Res., 2020, 53, 2003 CrossRef CAS; (f) J. Luo, G.-S. Chen, S.-J. Chen, J.-S. Yu, Z.-D. Li and Y.-L. Liu, Exploiting Remarkable Reactivities of Ynamides: Opportunities in Designing Catalytic Enantioselective Reactions, ACS Catal., 2020, 10, 13978 CrossRef CAS; (g) B. Zhou, T.-D. Tan, X.-Q. Zhu, M. Shang and L.-W. Ye, Reversal of Regioselectivity in Ynamide Chemistry, ACS Catal., 2019, 9, 6393 CrossRef CAS; (h) G. Evano, C. Theunissen and M. Lecomte, Ynamides: Powerful and Versatile Reagents for Chemical Synthesis, Aldrichimica Acta, 2015, 48, 59 CAS; (i) X.-N. Wang, H.-S. Yeom, L.-C. He, S. Fang, Z.-X. Ma, B. L. Kedrowski and R. P. Hsung, Ynamides in Ring Forming Transformations, Acc. Chem. Res., 2014, 47, 560 CrossRef CAS; (j) S. Shandilya, M. P. Gogoi, S. Dutta and A. K. Sahoo, Gold-catalyzed transformation of ynamides, Chem. Rec., 2021, 21, 4123 CrossRef CAS; (k) B. Prabagar, N. Ghosh and A. K. Sahoo, Cyclization and Cycloisomerization of π-Tethered Ynamides: An Expedient Synthetic Method to Construct Carbo- and Heterocycles, Synlett, 2017, 2539 CAS.
- Y.-X. Zheng, L.-G. Liu, T.-Q. Hu, X. Li, Z. Xu, X. Hong, X. Lu, B. Zhou and L.-W. Ye, Asymmetric Buchner Reaction and Arene Cyclopropanation via Copper-Catalyzed Controllable Cyclization of Diynes, Nat. Commun., 2024, 15, 9227 CrossRef CAS PubMed.
- For recent selected reviews on the desymmetrization reaction, see: (a) X.-P. Zeng, Z.-Y. Cao, Y.-H. Wang, F. Zhou and J. Zhou, Catalytic Enantioselective Desymmetrization Reactions to All-Carbon Quaternary Stereocenters, Chem. Rev., 2016, 116, 7330 CrossRef CAS; (b) A. Borissov, T. Q. Davies, S. R. Ellis, T. A. Fleming, M. S. W. Richardson and D. J. Dixon, Organocatalytic Enantioselective Desymmetrisation, Chem. Soc. Rev., 2016, 45, 5474 RSC; (c) M.-Y. Teng, T. Han, E.-H. Huang and L.-W. Ye, Research Progress on Enantioselective Desymmetrization Reactions Involving Metal Carbenes, Chin. J. Org. Chem., 2022, 42, 3295 CrossRef CAS; (d) Y. Xu, T.-Y. Zhai, Z. Xu and L.-W. Ye, Recent Advances towards Organocatalytic Enantioselective Desymmetrizing Reactions, Trends Chem., 2022, 4, 191 CrossRef CAS; (e) Y. Zhang, H. Cai, X. Gan and Z. Jin, N-Heterocyclic Carbene-Catalyzed Enantioselective (dynamic) Kinetic Resolutions and Desymmetrizations, Sci. China: Chem., 2024, 67, 482 CrossRef CAS.
- For selected reviews on the dearomatization reaction of arenes, see: (a) W. C. Wertjes, E. H. Southgate and D. Sarlah, Recent Advances in Chemical Dearomatization of Nonactivated Arenes, Chem. Soc. Rev., 2018, 47, 7996 RSC; (b) A. R. Pape, K. P. Kaliappan and E. P. Kündig, Transition-Metal-Mediated Dearomatization Reactions, Chem. Rev., 2000, 100, 2917 CrossRef CAS.
- CCDC 2410274† (4a) contains the supplementary crystallographic data for this paper.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2410274. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qo02371d |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2025 |




