Open Access Article
Open Access ArticlePost-synthetic engineering of covalent organic frameworks with thiophene and naphthalimide units for enhanced oxygen reduction electrocatalysis
Elena
Gala
 ab,
Emiliano
Martínez-Periñán
ab,
Emiliano
Martínez-Periñán
 *cd,
Marcos
Martínez-Fernández
a,
Marta
Gordo-Lozano
a,
José I.
Martínez
*cd,
Marcos
Martínez-Fernández
a,
Marta
Gordo-Lozano
a,
José I.
Martínez
 e and
José L.
Segura
e and
José L.
Segura
 *a
*a
aDepartamento de Química Orgánica I, Facultad de Ciencias Químicas, Universidad Complutense de Madrid, 28040, Madrid, Spain. E-mail: segura@ucm.es
bDepartamento de Tecnología Química y Ambiental, Escuela Superior de Ciencias Experimentales y Tecnología, Universidad Rey Juan Carlos, 28933, Móstoles (Madrid), Spain
cDepartamento de Química Analítica y Análisis Instrumental, Facultad de Ciencias, Universidad Autónoma de Madrid, 28049, Madrid, Spain. E-mail: emiliano.martinez@uam.es
dInstitute for Advanced Research in Chemical Sciences (IAdChem), Universidad Autónoma de Madrid, 28049, Madrid, Spain
eDepartamento de Nanoestructuras, Superficies, Recubrimientos y Astrofísica Molecular, Instituto de Ciencias de Materiales de Madrid (ICMM-CSIC), 28049, Madrid, Spain
First published on 29th October 2025
Abstract
The development of efficient metal-free electrocatalysts for the oxygen reduction reaction (ORR) is essential for advancing sustainable energy technologies. In this work, we report the post-synthetic functionalization of covalent organic frameworks (COFs) with donor–acceptor (D–A) motifs incorporating thiophene and naphthalimide derivatives, yielding two novel materials. These COFs were synthesized via CuAAC click chemistry and thoroughly characterized. Electrochemical analyses revealed enhanced ORR activity in both materials, with one COF exhibiting near-ideal four-electron selectivity and remarkable stability. Density functional theory (DFT) calculations corroborated the experimental results, demonstrating that the electronic structure of COFs facilitates efficient O–O bond cleavage and electron transfer. These findings underscore the potential of rationally designed D–A COFs as high-performance, metal-free ORR electrocatalysts, contributing to the development of next-generation sustainable energy conversion technologies.
Introduction
Molecular oxygen (O2) is one of the most abundant components of breathable air, and despite its high-bond-energy (498 kJ mol−1), the cleavage of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond is essential for aerobic respiration.1,2 Thus, the oxygen reduction reaction (ORR) is an inspiring counter reaction for sustainable energy conversion technologies (e.g., hydrogen fuel cells and metal–air batteries).3,4 However, this process shows two main drawbacks: (i) its slow kinetics and (ii) the H2O2/H2O selectivity of the process, which is related to the energy conversion efficiency.4–6 Both problems can be solved using suitable catalysts, enhancing the reaction rates and controlling the selectivity. However, the most widely used catalysts in energy conversion are platinum-based catalysts due to the high-performances offered by this noble metal. Nevertheless, the scarcity and high cost of platinum hinder their commercial viability and, consequently, recent research has focused on alternative electrocatalysts.2,7
O bond is essential for aerobic respiration.1,2 Thus, the oxygen reduction reaction (ORR) is an inspiring counter reaction for sustainable energy conversion technologies (e.g., hydrogen fuel cells and metal–air batteries).3,4 However, this process shows two main drawbacks: (i) its slow kinetics and (ii) the H2O2/H2O selectivity of the process, which is related to the energy conversion efficiency.4–6 Both problems can be solved using suitable catalysts, enhancing the reaction rates and controlling the selectivity. However, the most widely used catalysts in energy conversion are platinum-based catalysts due to the high-performances offered by this noble metal. Nevertheless, the scarcity and high cost of platinum hinder their commercial viability and, consequently, recent research has focused on alternative electrocatalysts.2,7
In this field, metal-free electrocatalysts (MFEs) are gaining increasing attention due to their enhanced environmental compatibility.8 These materials typically consist of polarized sp2 carbon—light heteroatom bonds embedded within a conductive carbon matrix, generating active sites for the ORR.9,10 Conventional MFE synthesis strategies include: (i) heteroatom doping of carbon materials, often resulting in heterogeneous distributions,10 and (ii) pyrolysis of organic precursors.11 However, these methods often compromise key catalyst properties such as porosity and atomic order, and the formation of active sites during doping or pyrolysis remains difficult to control.8
To address these limitations, covalent organic frameworks (COFs) have emerged as an ideal platform for the design of MFEs. In this sense, COFs combine intrinsic crystallinity, high surface areas (up to 4000 m2 g−1), and tunable architectures, making them excellent model systems for studying catalytic conversions.1 Among the various active sites motifs investigated for ORR electrocatalysis —including thiophene derivatives or naphthalenediimides12,13 —donor–acceptor (D–A) active sites remain largely underexplored, despite their potential to enhance catalytic performance through electronic charge redistribution (e.g., Tafel slope reduction).14,15
Herein, we report the post-synthetic modification of an “innocent” COF with various D–A motifs incorporating thiophene and naphthalenediimide-based active sites. In particular, we evaluated two new donor units: (i) unsubstituted thiophenes, which represent a benchmark in the design of state-of-the-art MFEs for ORR, and (ii) novel thienopyrrole derivatives, which are emerging as promising building blocks in fields such as solar-cells16 and field effect transistors.17 The resulting frameworks were characterized by solid-state techniques, confirming the successful integration of the new active sites within the porous network. Notably, incorporation of the D–A system led to enhanced electrocatalytic kinetics compared to the previously reported pure acceptor-based COF.13
Results and discussion
Synthesis and characterization of NDI-3T-COF and NDI-2TP-COF
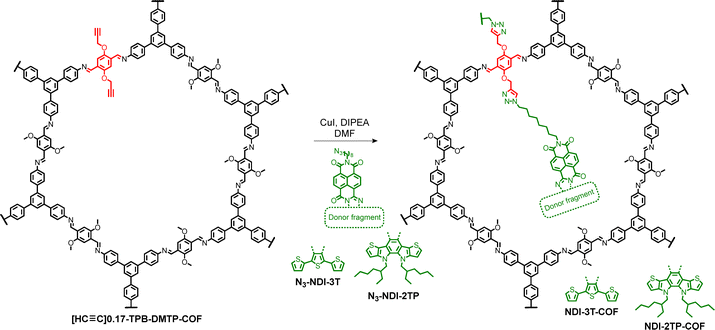
The incorporation of ambipolar naphthalimide fragments into the COF pores required the preliminary synthesis of azide-functionalized building blocks. Accordingly, the preparation of the compound N3-NIA (Scheme 1) represented a key step toward obtaining the desired materials. To the best of our knowledge, no prior examples of naphthalenemonoimide derivatives bearing an azido group on the alkyl chain have been reported. Based on the method described by Dan Pantos and co-workers,18N3-NIA was synthesized via microwave-assisted reaction between naphthalenetetracarboxylic dianhydride (NTDA) and 8-azidooctan-1-amine (1) in DMF (Scheme 1). Under these conditions, the naphthalimide derivative N3-NIA was obtained as the sole detectable product in excellent yield (85%).Following the synthesis of N3-NIA, we proceeded with the preparation of the conjugated donor–acceptor molecules N3-NDI-3T and N3-NDI-2TP (Scheme 2). N3-NDI-3T was obtained via condensation of N3-NIA with the diaminoterthiophene derivative 2 under the same conditions previously reported.17 In contrast, the synthesis of N3-NDI-2TP proved more challenging due to the instability of diamine 2, which was generated in situ by reduction of the benzothiadiazole derivative BTD-2TP using zinc powder in acetic acid (Scheme 2). Upon completion of the reduction, the resulting solution of compound 2 was transferred to a solution of N3-NIA in acetic acid via cannula filtration.17 This approach was adopted to prevent the introduction of residual zinc, which could potentially reduce the azido groups present in both N3-NIA and the final product, N3-NDI-2TP. Characterization of both compounds by NMR, FTIR, and mass spectrometry confirmed that the azido functionality remained intact throughout the condensation processes (see SI).
The incorporation of donor–acceptor molecules N3-NDI-3T and N3-NDI-2TP into the pores of [HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TPB-DMTP-COFvia copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction click chemistry was successfully achieved (Scheme 2). The covalent attachment of these units was initially confirmed by solid-state cross-polarization magic angle spinning carbon-13 nuclear magnetic resonance (13C-CP/MAS-NMR) spectroscopy. As shown in Fig. 1, the most significant changes are observed in the aliphatic region of the spectrum. Notably, the disappearance of signals corresponding to sp-hybridized carbon (70–80 ppm) and the appearance of a strong signal at ∼30 ppm, attributed to the methylene groups of the ambipolar fragments, confirm the successful modification. In the aromatic region, the broadening of the signal around 130 ppm is consistent with the presence of NDI-3T and NDI-2TP moieties (Fig. S6 and S8). Additional spectroscopic characterization was carried out using UV-Vis and FTIR spectroscopy (Fig. S16–S18). In the FTIR spectra, the disappearance of the characteristic azide stretching band at 2100 cm−1, along with the retention of the carbonyl stretching bands from the NDI units, confirmed complete coupling and the absence of residual N3-NDI-3T or N3-NDI-2TP. The disappearance of both azide and alkyne vibrational features in the FTIR spectra, together with the complete loss of the sp-carbon signal in the 13C CP/MAS NMR, confirms that the CuAAC post-synthetic modification proceeded to completion within the sensitivity limits of these analyses.
C]0.17-TPB-DMTP-COFvia copper(I)-catalyzed azide–alkyne cycloaddition (CuAAC) reaction click chemistry was successfully achieved (Scheme 2). The covalent attachment of these units was initially confirmed by solid-state cross-polarization magic angle spinning carbon-13 nuclear magnetic resonance (13C-CP/MAS-NMR) spectroscopy. As shown in Fig. 1, the most significant changes are observed in the aliphatic region of the spectrum. Notably, the disappearance of signals corresponding to sp-hybridized carbon (70–80 ppm) and the appearance of a strong signal at ∼30 ppm, attributed to the methylene groups of the ambipolar fragments, confirm the successful modification. In the aromatic region, the broadening of the signal around 130 ppm is consistent with the presence of NDI-3T and NDI-2TP moieties (Fig. S6 and S8). Additional spectroscopic characterization was carried out using UV-Vis and FTIR spectroscopy (Fig. S16–S18). In the FTIR spectra, the disappearance of the characteristic azide stretching band at 2100 cm−1, along with the retention of the carbonyl stretching bands from the NDI units, confirmed complete coupling and the absence of residual N3-NDI-3T or N3-NDI-2TP. The disappearance of both azide and alkyne vibrational features in the FTIR spectra, together with the complete loss of the sp-carbon signal in the 13C CP/MAS NMR, confirms that the CuAAC post-synthetic modification proceeded to completion within the sensitivity limits of these analyses.
Nitrogen sorption isotherms measured at 77 K were used to determine the Brunauer–Emmett–Teller (BET) surface areas and pore size distributions of the alkyne-containing precursor and the functionalized COFs, NDI-3T-COF and NDI-2TP-COF (Fig. 1 and Fig. S19–S21). The mesoporous nature of all three materials was confirmed by the presence of type IV isotherms. As expected, the incorporation of naphthalimide units within the pores resulted in a reduction of both surface area and pore volume parameters (Fig. 1). Thermogravimetric analysis (TGA) demonstrated that NDI-3T-COF and NDI-2TP-COF exhibit good thermal stability, with decomposition temperatures above 400 °C.
As result of the mild conditions employed in the CuAAC reaction, both NDI-3T-COF and NDI-2TP-COF retained their crystalline structure, as evidenced by the powder X-ray diffraction (PXRD) patterns shown in Fig. 2 and Fig. S23–S25. However, a set of DFT-based calculations performed using Gaussian 16 C.0119 and QUANTUM ESPRESSO20 revealed an unexpected change in the stacking mode of one of the materials. For NDI-3T-COF, the eclipsed AA stacking of the parent [HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TPB-DMTP-COF was preserved, as indicated by the characteristic diffraction peaks at 2.89°, 4.92°, 5.70°, 7.51° and 9.83°, corresponding to the (100), (110), (200), (210) and (220) reflections typical of imine-based COFs (Fig. 2).21 In contrast, the incorporation of the bulky NDI-2TP fragment into the pores of [HC
C]0.17-TPB-DMTP-COF was preserved, as indicated by the characteristic diffraction peaks at 2.89°, 4.92°, 5.70°, 7.51° and 9.83°, corresponding to the (100), (110), (200), (210) and (220) reflections typical of imine-based COFs (Fig. 2).21 In contrast, the incorporation of the bulky NDI-2TP fragment into the pores of [HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TPB-DMTP-COF disrupts the eclipsed stacking, resulting in a lateral displacement of the COF layers. Despite this shift, NDI-2TP-COF maintains an ordered macromolecular structure, as evidenced by the PXRD data (Fig. 2 and Fig. S23). This change in stacking can be attributed to the presence of 2-ethylhexyl chains in the NDI-2TP moiety, which, due to their steric bulk, must be accommodated outside the COF plane—an arrangement only achievable through interlayer displacement. Simulations of NDI-2TP-COF with enforced AA stacking produced significantly larger interlayer distances and a PXRD pattern inconsistent with the experimental data thereby conclusively ruling out the eclipsed stacking model. To quantitatively support the structural assignment, Pawley refinements were performed for both COFs, yielding unit cell parameters and R-factors in good agreement with the experimental data (Fig. S24 and S25). These results confirm the preservation of crystallinity and validate the proposed stacking models for NDI-3T-COF and NDI-2TP-COF.
C]0.17-TPB-DMTP-COF disrupts the eclipsed stacking, resulting in a lateral displacement of the COF layers. Despite this shift, NDI-2TP-COF maintains an ordered macromolecular structure, as evidenced by the PXRD data (Fig. 2 and Fig. S23). This change in stacking can be attributed to the presence of 2-ethylhexyl chains in the NDI-2TP moiety, which, due to their steric bulk, must be accommodated outside the COF plane—an arrangement only achievable through interlayer displacement. Simulations of NDI-2TP-COF with enforced AA stacking produced significantly larger interlayer distances and a PXRD pattern inconsistent with the experimental data thereby conclusively ruling out the eclipsed stacking model. To quantitatively support the structural assignment, Pawley refinements were performed for both COFs, yielding unit cell parameters and R-factors in good agreement with the experimental data (Fig. S24 and S25). These results confirm the preservation of crystallinity and validate the proposed stacking models for NDI-3T-COF and NDI-2TP-COF.
Additional morphological characterization by SEM and TEM (Fig. S26–S29) confirmed that both NDI-3T-COF and NDI-2TP-COF retain their characteristic morphology after post-synthetic modification.
Electrochemical characterization of ORR electrocatalysts
The electrochemical behavior of new synthesized COFs was evaluated both in the absence and in the presence of O2 in alkaline medium (0.1 M KOH), in order to assess their potential as ORR electrocatalysts. Initially, cyclic voltammetry (CV) measurements under static conditions were performed. In the absence of O2 (Fig. 3(A)), both COF materials exhibited two redox couples, which are attributed to reversible redox processes involving the enolization of the diimide carbonyl groups.22–24 For NDI-2TP-COF, the redox couples appear at E1red processes associated with the reversible enolation of the diimide carbonyl groups, the slight differences in redox potentials can be attributed to the distinct donor moieties of the newly synthesized COFs, cyclic voltammetry was performed under O2-saturated conditions (Fig. 3(B)). All COFs materials exhibited higher electrocatalytic activity compared to the Carbon SuperP/GC electrode alone (Eonset = −0.32 V vs. SCE), as evidenced by more positive onset potential and increased current densities during the ORR electrocatalytic process. The most positive onset potential was observed for the NDI-3T-COF/Carbon SuperP/GC electrode (Eonset = −0.21 V vs. SCE) followed by the 2TP-COF/Carbon SuperP/GC electrode (Eonset = −0.23 V vs. SCE). The pristine COF backbone ([HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TPB-DMTP-COF Carbon SuperP/GC) exhibited an onset potential of Eonset = −0.27 V vs. SCE, supporting the notion that the COF framework contributes to ORR enhancement. However, the superior performance of the NDI-functionalized COFs suggests that the redox-active NDI moieties play a key role in improving electrocatalytic activity. The highest current intensity was also achieved with the NDI-3T-COF/Carbon SuperP/GC electrode, further confirming its superior electrocatalytic performance under static electrochemical conditions. To conclude the static electrochemical characterization, electrochemical impedance spectroscopy (EIS) was performed using GC-modified electrodes in O2-saturated 0.1 M KOH, and the corresponding Nyquist plots were obtained (Fig. 3(C)). This technique enables evaluation of electron transfer resistance during the ORR process for each modified electrode. As anticipated from the cyclic voltammetry results, the NDI-3T-COF/Carbon SuperP/GC electrode exhibited the lowest charge transfer resistance (Rp = 3.9 kΩ), indicating the most efficient electron transfer. The NDI-2TP-COF/Carbon SuperP/GC and [HC
C]0.17-TPB-DMTP-COF Carbon SuperP/GC) exhibited an onset potential of Eonset = −0.27 V vs. SCE, supporting the notion that the COF framework contributes to ORR enhancement. However, the superior performance of the NDI-functionalized COFs suggests that the redox-active NDI moieties play a key role in improving electrocatalytic activity. The highest current intensity was also achieved with the NDI-3T-COF/Carbon SuperP/GC electrode, further confirming its superior electrocatalytic performance under static electrochemical conditions. To conclude the static electrochemical characterization, electrochemical impedance spectroscopy (EIS) was performed using GC-modified electrodes in O2-saturated 0.1 M KOH, and the corresponding Nyquist plots were obtained (Fig. 3(C)). This technique enables evaluation of electron transfer resistance during the ORR process for each modified electrode. As anticipated from the cyclic voltammetry results, the NDI-3T-COF/Carbon SuperP/GC electrode exhibited the lowest charge transfer resistance (Rp = 3.9 kΩ), indicating the most efficient electron transfer. The NDI-2TP-COF/Carbon SuperP/GC and [HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TPB-DMTP-COF Carbon SuperP/GC showed comparable values (Rp = 5.2 kΩ and Rp = 4.9 kΩ respectively). All COF-based electrodes displayed significantly lower charge transfer resistance than the Carbon SuperP/GC reference (Rp = 20.1 kΩ), confirming the benefit of COF structures in enhancing electron transfer during ORR and supporting their potential as efficient metal-free electrocatalysts.
C]0.17-TPB-DMTP-COF Carbon SuperP/GC showed comparable values (Rp = 5.2 kΩ and Rp = 4.9 kΩ respectively). All COF-based electrodes displayed significantly lower charge transfer resistance than the Carbon SuperP/GC reference (Rp = 20.1 kΩ), confirming the benefit of COF structures in enhancing electron transfer during ORR and supporting their potential as efficient metal-free electrocatalysts.
In addition to impedance measurements, the turnover frequency (TOF) was estimated for both NDI-functionalized COFs to further evaluate their intrinsic catalytic activity. The calculated TOF values were 0.1148 s−1 for NDI-2TP-COF and 0.1017 s−1 for NDI-3T-COF, confirming the superior performance of the former. These values are among the highest reported for metal-free COF-based ORR catalysts, underscoring the effectiveness of the donor–acceptor design strategy in enabling rapid catalytic turnover at the molecular level.
The electrocatalytic activity toward the oxygen reduction reaction was further investigated through hydrodynamic electrochemical experiments. Linear sweep voltammograms (LSVs) were recorded using modified rotating ring-disk electrodes (RRDEs) (Fig. 4(A)). The best performance was observed for the NDI-3T-COF/Carbon SuperP/GC and NDI-2TP-COF/Carbon SuperP/GC electrodes, both exhibiting an onset potential of −0.26 V vs. SCE and reaching a current limit of −3.04 mA cm−2 at −0.60 V vs. SCE. These results clearly outperform those obtained with the Carbon SuperP/GC electrode alone. In this case kinetics limitation during the ORR generates low current broad peaks at high reduction potentials because of different redox process overlaps, such as ORR and hydrogen peroxide reduction. Moderate performance was observed for the COF backbone without electroactive groups ([HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TPB-DMTP-COF/Carbon SuperP/GC).
C]0.17-TPB-DMTP-COF/Carbon SuperP/GC).
The platinum ring currents obtained at 0.25 V vs. SCE (Fig. 4(A) dashed lines) were used to calculate the number of electrons transferred during the ORR (Fig. 4(B)). A higher electron transfer number was observed for the NDI-2TP-COF/Carbon SuperP/GC electrode, reaching 3.7 electrons—close to the ideal 4-electron pathway desired for fuel cell applications. This result highlights the promising potential of this material as a cathodic electrocatalyst in energy devices.
In contrast, the NDI-3T-COF/Carbon SuperP/GC electrode exhibited an average electron transfer number of 2.9, improving over the Carbon SuperP/GC baseline (2.4 electrons), yet still indicating a mixed 2- and 4- electron ORR mechanism. While this represents performance enhancement, it falls short of selectivity toward a single desirable pathway. Consequently, this material is not optimal either for selective hydrogen peroxide production (via 2-electron ORR) or for maximum energy output in fuel cell cathodes (via 4-electron ORR).
At this point, it is important to highlight the contribution of the electrode used as a support Carbon SuperP/GC during the electrocatalytic ORR process. Carbon SuperP/GC is fundamental during the electrocatalytic process, as it allows the electrocatalysts (NDI-3T-COF and NDI-2TP-COF) to possess sufficient conductivity to carry out the electrochemical reaction. As we have demonstrated, the ORR process using Carbon SuperP/GC presents slow kinetic, and the ORR mechanism is a mixture of the two possible pathways 2 and 4 electrons, resulting in an average of 2.4 electrons. Furthermore, the limiting current barely exceeds 1 mA cm−2 at −0.5 V. Therefore, the contribution of the NDI-3T-COF and NDI-2TP-COF materials is significant, both in terms of the number of electrons, approaching the ORR via the 4-electron mechanism in both cases, and in terms of the limiting current, which is greater than 3 mA cm−2 at −0.5 V.
A similar electron transfer number (3.1) was obtained using the pristine COF backbone, [HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TPB-DMTP-COF/Carbon SuperP/GC, further supporting the presence of a non-selective combination of 2- and 4- electron pathways in the absence of redox-active functionalization.
C]0.17-TPB-DMTP-COF/Carbon SuperP/GC, further supporting the presence of a non-selective combination of 2- and 4- electron pathways in the absence of redox-active functionalization.
Tafel slopes (Fig. 4(C)) were derived from the hydrodynamic linear sweep voltammograms shown in Fig. 4(A). The NDI-2TP-COF/Carbon SuperP/GC and NDI-3T-COF/Carbon SuperP/GC electrodes exhibited Tafel slopes of 69.9 and 69.5 mV dec−1, respectively. These values represent a clear improvement over those obtained with the Carbon SuperP/GC electrode (98.9 mV dec−1) and the pristine COF backbone ([HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TPB-DMTP-COF/Carbon SuperP/GC (76.7 mV dec−1)). These results confirm the enhanced electrocatalytic performance of the NDI-functionalized COFs.
C]0.17-TPB-DMTP-COF/Carbon SuperP/GC (76.7 mV dec−1)). These results confirm the enhanced electrocatalytic performance of the NDI-functionalized COFs.
Notably, the Tafel slopes of NDI-2TP-COF/Carbon SuperP/GC and NDI-3T-COF/Carbon SuperP/GC are very close to that of a 10% Pt-C/SuperP/GC electrode (68.5 mV dec−1), obtained in other reported works by our group.23 This is a remarkable result, highlighting that the developed COF-based materials function as highly active, entirely metal-free electrocatalysts for the oxygen reduction reaction.
The stability of the newly developed electroactive COFs materials as ORR electrocatalysts was evaluated by applying a constant potential of −0.5 V vs. SCE for 25 ks under hydrodynamic conditions in O2-saturated 0.1 M NaOH. For the NDI-3T-COF/Carbon SuperP/GC electrode, an initial current drop of 20% was observed within the first 2500 s, after which the current stabilized at approximately 75% of its initial value for the remainder of the experiment. In contrast, the NDI-2TP-COF/Carbon SuperP/GC electrode showed a smaller initial decrease of 10% over the first 5 ks, maintaining around 87% of the initial current throughout the remaining test period. This represents an excellent stability profile.
These results are particularly noteworthy given the organic nature of the materials and the fact that they are metal-free and non-pyrolyzed, highlighting their robustness as ORR electrocatalysts under continuous operation.
Table 1 contains the main electrochemical results of all the electrodes studied in this work.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TPB-DMTP-COF/Carbon SuperP/GC and Carbon SuperP/GC)
C]0.17-TPB-DMTP-COF/Carbon SuperP/GC and Carbon SuperP/GC)
| Electrode | E onset vs. SCE/mV | j lim/(mA cm−2) | Tafel slope/(mV dec−1) | R p/kΩ | ORR number of electrons | η experimental/mV |
|---|---|---|---|---|---|---|
| 2TP-COF/Carbon SuperP/GC | −264 | −2.97 | −69.9 | 5.2 | 3.7 | 484 |
| NDI-3T-COF/Carbon SuperP/GC | −265 | −2.95 | −68.5 | 3.9 | 2.9 | 485 |
[HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TPB-DMTP-COF/Carbon SuperP/GC C]0.17-TPB-DMTP-COF/Carbon SuperP/GC |
−307 | −2.70 | −76.7 | 4.9 | 3.1 | 527 |
| Carbon SuperP/GC | −330 | −2.08 | −98.9 | 20.1 | 2.4 | 550 |
Computed O2 reduction reaction (ORR) mechanisms
To provide a theoretical basis for the experimentally observed differences in oxygen reduction reaction (ORR) performance between NDI-2TP-COF and NDI-3T-COF, we performed a comprehensive series of density functional theory (DFT) calculations, complemented by Gibbs free energy analyses. These calculations were aimed at evaluating the thermodynamic viability of both the 4e− ORR pathway: | (1) |
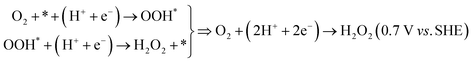 | (2) |
For the NDI-2TP system, theoretical modeling reveals seven distinct 4e− ORR active sites exhibiting overpotentials |η| < 1.2 V (C1–C4, N1, S1 and S2) able to catalyze the complete reduction of O2 to H2O, with overpotentials ranging from 0.53 to 1.07 V (see Fig. 5a and b). Fig. 5c shows the DFT-optimized structure for the 4e− ORR intermediates for the most active site (C1). In all cases except for N1, the rate-limiting step (establishing the η4e-ORR overpotential value) is the proton-coupled electron transfer from adsorbed atomic oxygen to adsorbed OH*, O* + (H+ + e−) → OH*, which is typically associated with strong oxygen adsorption that hinders O desorption. For the N1 site the limiting step shifts to the initial activation of O2, O2 + * + (H+ + e−) → OOH*, indicating a weaker initial O2 binding affinity at that site. Despite the moderately high overpotentials observed in some sites, all values lie within the range considered viable for practical electrocatalysis. In addition, theoretical analysis predicts six sites yielding |η| < 0.5 V (most of them coincident with the 4e− ORR active sites) exhibiting activity toward the 2e− ORR pathway, with significantly lower overpotentials: 0.09–0.39 V for the C1–C5 and S1 sites (see Fig. 5d and e). In all cases the rate-limiting step (establishing the η2e-ORR overpotential value) is the final proton-coupled electron transfer OOH* + (H+ + e−) → H2O2 sub-reaction. Fig. 5f shows the DFT-optimized structure for the 2e− ORR intermediate for the most active site (C5). However, a key mechanistic insight is that most of the active sites show exceptionally strong O* binding, with ΔGO* values all below 2 eV, compared to the ideal value of 2.46 eV for the ideal/balanced 4e− ORR catalyst. This strong binding energetically favors O–O bond cleavage from the OOH* intermediate, steering the reaction toward full O2 reduction to H2O even when the 2e− route is thermodynamically accessible. From a kinetic standpoint, the reaction barriers for OOH* dissociation (leading to O* and eventually to OH*) are expected to be lower due to the stabilization of intermediates by the electronic structure of the COF framework. This accelerates the 4e− pathway relative to the 2e− route, where OOH* desorption would otherwise dominate. Consequently, despite the favourable thermodynamics of the 2e− route at some sites, the kinetics clearly favour almost complete O–O bond scission and full reduction, leading to the experimentally observed average electron transfer number of 3.7, remarkably close to the ideal 4e− ORR process. Such a high degree of selectivity is rare for metal-free COFs, underscoring NDI-2TP-COF as a high-performance ORR catalyst for fuel cell cathodes, where selective and almost complete reduction to H2O is essential to avoid H2O2 accumulation.
In contrast, NDI-3T system displays a more heterogeneous electrocatalytic behaviour. Eight 4e− ORR active sites similarly exhibiting |η| < 1.2 V were theoretically identified (C1–C4, N1, S1–S3) with overpotentials ranging from 0.47 to 1.13 V (see Fig. 5g and h). Fig. 5i shows the DFT-optimized structure for the 4e− ORR intermediates for the most active site (C1). However, the distribution of these sites includes several with relatively high kinetic barriers, which could bottleneck the overall catalytic performance in the 4e− pathway. Again, in all cases except for C1, the rate-limiting step (establishing the η4e-ORR overpotential value) is O* + (H+ + e−) → OH*. For the C1 site the limiting step shifts to the final OH* + (H+ + e−) → H2O sub-reaction.
Moreover, remarkably, a large amount of twelve 2e− ORR active sites (double than for the NDI-2TP case) yielding |η| < 0.5 V exhibit favorable 2e− ORR activity (C1–C8, N1, S1–S3) with overpotentials ranging from −0.15 to 0.47 V (see Fig. 5j and k). Fig. 5l shows the DFT-optimized structure for the 2e− ORR intermediate for the most active site (C5). While low ΔGO* values are also observed in this system, as in NDI-2TP-COF, the stronger thermodynamic driving force for the 2e− pathway at a larger number of sites suggests a broader mechanistic competition between O–O bond cleavage and retention. In all cases except for C8, the rate-limiting step (establishing the η2e-ORR overpotential value) is the final proton-coupled electron transfer OOH* + (H+ + e−) → H2O2 sub-reaction. For the C8 site the limiting step is the initial activation of O2, O2 + * + (H+ + e−) → OOH*. This dual-pathway behavior is confirmed experimentally by an average electron transfer number of 2.9, reflecting a non-selective mixture of the 2e− and 4e− ORR mechanisms. Although this represents a significant improvement over the baseline SuperP/GC system (2.4 electrons), it falls short of ideal behavior for either complete water production or selective H2O2 synthesis. The kinetic scenario here likely involves competition between OOH* dissociation and desorption, with site-to-site variability preventing a dominant pathway from emerging, ultimately reducing overall selectivity.
Further comparison with [HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C]0.17-TPB-DMTP-COF, a structurally distinct material with an intermediate value of 3.1 electrons, reinforces the importance of structural and electronic design in governing ORR selectivity. Specifically, the extended conjugation, electron-deficient NDI core, and strategic placement of heteroatoms in NDI-2TP-COF collectively enable stronger O* stabilization and lower kinetic barriers for O–O bond cleavage, thereby favoring the 4e− mechanism. In contrast, the more delocalized and less directional reactivity of NDI-3T-COF leads to non-selective behavior.
C]0.17-TPB-DMTP-COF, a structurally distinct material with an intermediate value of 3.1 electrons, reinforces the importance of structural and electronic design in governing ORR selectivity. Specifically, the extended conjugation, electron-deficient NDI core, and strategic placement of heteroatoms in NDI-2TP-COF collectively enable stronger O* stabilization and lower kinetic barriers for O–O bond cleavage, thereby favoring the 4e− mechanism. In contrast, the more delocalized and less directional reactivity of NDI-3T-COF leads to non-selective behavior.
In conclusion, DFT and Gibbs free energy analyses provide robust mechanistic evidence supporting the nearly exclusive 4e− ORR selectivity in NDI-2TP-COF. The confluence of strong O* binding, favorable kinetics for O–O cleavage, and moderate overpotentials establishes a highly active and selective catalytic environment for full O2 reduction. In contrast, NDI-3T-COF lacks this mechanistic coherence, resulting in reduced selectivity and efficiency. These findings highlight the power of rational COF design in tuning both thermodynamic and kinetic parameters for targeted electrochemical applications in sustainable energy conversion.
Conclusions
In this study, we successfully developed two novel metal-free electrocatalysts, NDI-3T-COF and NDI-2TP-COF, via post-synthetic functionalization of a covalent organic framework with donor–acceptor (D–A) motifs. Structural and spectroscopic analyses confirmed the effective integration of active sites into the COF backbone, while preserving crystallinity and porosity. Electrochemical measurements revealed that both materials exhibit enhanced oxygen reduction reaction (ORR) activity compared to the pristine COF and carbon-based benchmarks, with NDI-2TP-COF displaying superior onset potential, electron transfer number, and long-term stability. Density functional theory (DFT) calculations provided mechanistic insights, showing that the electronic structure of NDI-2TP-COF promotes a 4-electron ORR pathway with low overpotentials and efficient O–O bond cleavage. These results highlight the potential of rational D–A design in COFs for the development of high-performance, metal-free electrocatalysts for sustainable energy conversion technologies.Author contributions
E. G.: investigation, data curation, visualization, writing – original draft, writing – review and editing. E. M.-P.: investigation, data curation, visualization, writing – original draft, writing – review and editing, funding acquisition, project administration. M. M.-F. data curation, writing – original draft, writing – review and editing. J. I. M.: conceptualization, investigation, data curation, visualization, writing – original draft, writing – review and editing. M. G.-L.: investigation, data curation, writing – review and editing. J. L. S.: conceptualization, investigation, visualization, supervision, writing – review and editing, funding acquisition, project administration.Conflicts of interest
There are no conflicts to declare.Data availability
The experimental and theoretical data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5qm00655d.Acknowledgements
This work was financially supported by MICINN (PID2022-138908NB-C33, TED2021-129886BC43 and PID2022-142262OA-I00) and the UCM (INV.GR.00.1819.10759). J. L. S. acknowledge the MICINN for the REDES project “RED2022-134503-T” and the Comunidad de Madrid (TEC-2024/ECO-332).References
- M. Martínez-Fernández and J. L. Segura, Exploring Advanced Oxygen Reduction Reaction Electrocatalysts: The Potential of Metal-Free and Non-Pyrolyzed Covalent Organic Frameworks, ChemSusChem, 2024, 17, e202400558 CrossRef PubMed.
- R. Ma, G. Lin, Y. Zhou, Q. Liu, T. Zhang, G. Shan, M. Yang and J. Wang, A review of oxygen reduction mechanisms for metal-free carbon-based electrocatalysts, npj Comput. Mater., 2019, 5, 78 CrossRef.
- M. Shao, Q. Chang, J. P. Dodelet and R. Chenitz, Recent Advances in Electrocatalysts for Oxygen Reduction Reaction, Chem. Rev., 2016, 116, 3594–3657 CrossRef PubMed.
- T. Xu, H. Zhou, X. Zhang, T. S. Herng, J. Ding, C. Chi and J. Zhu, Covalent Organic Frameworks with Carbon-Centered Radical Sites for Promoting the 4e− Oxygen Reduction Reaction, Angew. Chem., Int. Ed., 2025, 64, e202424449 CrossRef PubMed.
- D. Grumelli, B. Wurster, S. Stepanow and K. Kern, Bio-inspired nanocatalysts for the oxygen reduction reaction, Nat. Commun., 2013, 4, 2904 CrossRef PubMed.
- M. K. Debe, Electrocatalyst approaches and challenges for automotive fuel cells, Nature, 2012, 486, 43–51 CrossRef PubMed.
- Y. Yang, Y. Lu, H. Y. Zhang, Y. Wang, H. L. Tang, X. J. Sun, G. Zhang and F. M. Zhang, Decoration of Active Sites in Covalent-Organic Framework: An Effective Strategy of Building Efficient Photocatalysis for CO2 Reduction, ACS Sustainable Chem. Eng., 2021, 9, 13376–13384 CrossRef.
- Q. Xu, Y. Tang, X. Zhang, Y. Oshima, Q. Chen and D. Jiang, Template Conversion of Covalent Organic Frameworks into 2D Conducting Nanocarbons for Catalyzing Oxygen Reduction Reaction, Adv. Mater., 2018, 30, 1706330 CrossRef PubMed.
- X. Yan, B. Wang, J. Ren, X. Long and D. Yang, An Unsaturated Bond Strategy to Regulate Active Centers of Metal-Free Covalent Organic Frameworks for Efficient Oxygen Reduction, Angew. Chem., Int. Ed., 2022, 61, e202209583 CrossRef PubMed.
- P. Bai and L. Xu, Toward a unified pH-performance picture of active sites in nitrogen-doped carbon materials, Appl. Catal., B, 2025, 365, 124908 CrossRef.
- J. Barrio, J. Li and M. Shalom, Carbon Nitrides from Supramolecular Crystals: From Single Atoms to Heterojunctions and Advanced Photoelectrodes, Chem. – Eur. J., 2023, 29, e202302377 CrossRef PubMed.
- R. Bao, Z. Xiang, Z. Qiao, Y. Yang, Y. Zhang, D. Cao and S. Wang, Designing Thiophene-Enriched Fully Conjugated 3D Covalent Organic Framework as Metal-Free Oxygen Reduction Catalyst for Hydrogen Fuel Cells, Angew. Chem., Int. Ed., 2023, 62, e202216751 CrossRef PubMed.
- M. Martínez-Fernández, E. Martínez-Periñán, J. I. Martínez, M. Gordo-Lozano, F. Zamora, J. L. Segura and E. Lorenzo, Evaluation of the Oxygen Reduction Reaction Electrocatalytic Activity of Postsynthetically Modified Covalent Organic Frameworks, ACS Sustainable Chem. Eng., 2023, 11, 1763–1773 CrossRef.
- S. Huang, S. Lu, Y. Hu, Y. Cao, Y. Li, F. Duan, H. Zhu, Y. Jin, M. Du and W. Zhang, Covalent Organic Frameworks with Molecular Electronic Modulation as Metal-Free Electrocatalysts for Efficient Hydrogen Peroxide Production, Small Struct., 2023, 4, 2200387 CrossRef.
- G. Kumar, S. K. Das, T. R. K. Rana, S. Samui, L. Billon and R. S. Dey, Donor–acceptor covalent organic frameworks propel the oxygen reduction reaction with push–pull dynamics, J. Mater. Chem. A, 2024, 12, 28085–28094 RSC.
- F. Suárez-Blas, L. Pandolfi, M. J. Alonso-Navarro, S. Riera-Galindo, J. I. Martínez, B. Dörling, A. Funes, A. Harillo-Baños, E. Venuti, M. M. Ramos, M. Campoy-Quiles and J. L. Segura, Tailoring the Electron-Deficient Central Core on Fused-Ring Nonfullerene Acceptors: Deciphering the Relationships Between Structure, Property, and Photovoltaic Performance, Adv. Energy Sustainable Res., 2024, 5, 2400028 CrossRef.
- R. González-Núñez, M. J. Alonso-Navarro, F. Suárez-Blas, E. Gala, M. M. Ramos, J. L. Segura and R. Ponce Ortiz, Tuning the charge stabilization and transport in naphthalimide-based semiconductors via a fused-ring and core-engineering strategy, Mater. Chem. Front., 2024, 8, 1981–1992 RSC.
- K. Tambara, N. Ponnuswamy, G. Hennrich and G. D. Pantoş, Microwave-assisted synthesis of naphthalenemonoimides and N-desymmetrized naphthalenediimides, J. Org. Chem., 2011, 76, 3338–3347 CrossRef PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, 2016 Search PubMed.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. Dal Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef.
- H. Xu, J. Gao and D. Jiang, Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts, Nat. Chem., 2015, 7, 905–912 CrossRef PubMed.
- X. Wu, Y. Qi, J. J. Hong, Z. Li, A. S. Hernandez and X. Ji, Rocking-Chair Ammonium-Ion Battery: A Highly Reversible Aqueous Energy Storage System, Angew. Chem., Int. Ed., 2017, 56, 13026–13030 CrossRef PubMed.
- M. Martínez-Fernández, E. Martínez-Periñán, J. I. Martínez, M. Gordo-Lozano, F. Zamora, J. L. Segura and E. Lorenzo, Evaluation of the Oxygen Reduction Reaction Electrocatalytic Activity of Postsynthetically Modified Covalent Organic Frameworks, ACS Sustainable Chem. Eng., 2023, 11, 1763–1773 CrossRef.
- H. H. Tsai, T. J. Lin, B. Vedhanarayanan, C. C. Tsai, T. Y. Chen, X. Ji and T. W. Lin, A 1.9-V all-organic battery-supercapacitor hybrid device with high rate capability and wide temperature tolerance in a metal-free water-in-salt electrolyte, J. Colloid Interface Sci., 2022, 612, 76–87 CrossRef PubMed.
| This journal is © the Partner Organisations 2025 |