Construction of a CoP/MnP/Cu3P heterojunction for efficient methanol oxidation-assisted seawater splitting†
Weijia
Liu‡
 a,
Min
Zhou‡
b,
Jingwen
Zhang
c,
Wenxian
Liu
a,
Min
Zhou‡
b,
Jingwen
Zhang
c,
Wenxian
Liu
 *a,
Doudou
Qin
ac,
Qian
Liu
d,
Guangzhi
Hu
*a,
Doudou
Qin
ac,
Qian
Liu
d,
Guangzhi
Hu
 e and
Xijun
Liu
e and
Xijun
Liu
 *c
*c
aCollege of Materials Science and Engineering, Zhejiang University of Technology, Hangzhou 310014, China. E-mail: liuwx@zjut.edu.cn
bSino-German College of Intelligent Manufacturing, Shenzhen Technology University, Shenzhen 518118, China
cMOE Key Laboratory of New Processing Technology for Nonferrous Metals and Materials, Guangxi Key Laboratory of Processing for Non-ferrous Metals and Featured Materials, School of Resources, Environment and Materials, Guangxi University, Nanning, 530004 Guangxi, China. E-mail: xjliu@gxu.edu.cn
dInstitute for Advanced Study, Chengdu University, Chengdu 610106, Sichuan, China
eInstitute for Ecological Research and Pollution Control of Plateau Lakes School of Ecology and Environmental Science, Yunnan University, Kunming 650504, China
First published on 22nd January 2025
Abstract
Methanol oxidation-assisted direct seawater electrolysis has emerged as a potent technology for efficient hydrogen (H2) production alongside high-value chemicals such as formic acid and formaldehyde. However, the large-scale application of this technology heavily relies on developing highly active and robust bifunctional electrocatalysts for methanol oxidation and hydrogen evolution reactions (MOR/HER). Herein, we report a simple hydrothermal-phosphorylation method to synthesize a heterostructured catalyst on copper foam, comprising CoP, MnP, and Cu3P (CoP/MnP/Cu3P@CF). The synergistic interaction among the heterogeneous components endowed CoP/MnP/Cu3P@CF with excellent MOR, oxygen evolution reaction (OER), and HER performance in alkaline seawater electrolytes. Notably, the MOR-assisted CoP/MnP/Cu3P@CF-based seawater electrolyzer catalyst required only 1.410 V to achieve a current density of 10 mA cm−2, significantly lower than the 1.681 V required for an OER–HER seawater electrolyzer. Additionally, the MOR-assisted electrolyzer exhibits high faradaic efficiency and cycling stability, offering the potential for sustainable energy-efficient H2 production.
1. Introduction
The global surge in energy demand, compounded by climate change and reliance on fossil fuels, has spurred the quest for sustainable and clean energy solutions.1–7 Hydrogen (H2) is emerging as a formidable renewable energy carrier with its high energy capacity and clean combustion.8–10 Traditional H2 production methods, however, are both energy-intensive and emit greenhouse gases.11–14 Utilizing renewable sources for water electrolysis offers a greener alternative. Particularly critical is developing technologies for continuous seawater electrolysis to produce H2 on a large scale.15,16 A primary challenge in the electrocatalytic splitting of seawater is the slow kinetics and high potential required for the oxygen evolution reaction (OER) at the anode, which leads to significant energy consumption and potential competitive chlorine evolution reactions.17–20 Researchers have thus explored alternative anodic reactions that are thermodynamically favorable, enhancing the energy efficiency of H2 production at the cathode. During electrolysis, a promising strategy involves oxidizing small organic molecules like alcohols, urea, glucose, 5-hydroxymethylfurfural, and hydrazine.21–27 Methanol oxidation, in particular, offers lower overpotentials and faster kinetics than the OER, potentially enabling more efficient H2 production. Additionally, methanol oxidation can yield value-added chemicals like formaldehyde, formic acid, and dimethyl ether, making it a viable option for low-energy, large-scale H2 production.Recent advances have significantly boosted the performance of non-precious metal electrocatalysts for methanol oxidation-assisted seawater electrolysis.28,29 Innovations include crystal phase engineering, chemical doping, and the creation of heterostructures.30–33 Constructing heterostructures promotes electron redistribution at interfaces, producing synergistic effects and enhanced electrocatalytic performance due to lattice mismatches.34–36 Transition metal phosphides (TMPs), known for their abundant availability, high conductivity, and catalytic activity, have garnered significant attention in this context.37–39 As a result, constructing heterostructures based on TMPs has become a promising approach to enhance the energy efficiency of methanol oxidation-assisted hydrogen production. For example, Zhang et al.40 developed a CoP@NiFe LDH via a straightforward electrodeposition–phosphorization–electrodeposition method. This structure optimized the electronic configuration and improved the catalyst's corrosion resistance in seawater, achieving 100 mA cm−2 at just 260 mV. Kandel et al.41 synthesized a Ni2P-MnP@Co2P@NF heterostructured catalyst that enhanced charge transfer and exposed more active sites, exhibiting 10 mA cm−2 at only 255 mV during OER tests. Chang et al.42 designed an amorphous NixCoyFez–P heterostructured catalyst via mild electro-deposition. Adding 2 M methanol to the electrolysis system reduced the potential by 110 mV at 20 mA cm−2. Nonetheless, crafting phosphide-based heterostructures that are both highly active and stable remains a formidable challenge.
In this study, we prepared a heterostructured catalyst on copper foam composed of CoP, MnP, and Cu3P (CoP/MnP/Cu3P@CF) using a hydrothermal-phosphorization method. The synergistic interaction among each component optimized the electronic structures at the active centers, enhancing their MOR, OER, and HER activities. As a result, a MOR–HER seawater electrolyzer equipped with the CoP/MnP/Cu3P@CF catalyst exhibited a current density of 10 mA cm−2 at a low cell voltage of 1.410 V, which is 0.271 V higher than that required for an OER–HER electrolyzer. Moreover, the methanol oxidation-assisted electrolysis cell demonstrated high Faraday efficiency and stability, indicating its potential for practical applications.
2. Experimental
2.1 Chemicals and reagents
In the experiments, copper foam (CF), ethanol (C2H5OH), deionized water, cobalt nitrate hexahydrate (Co(NO3)2·6H2O), manganese nitrate solution (50 wt%), urea, ammonium fluoride, sodium hypophosphite monohydrate, potassium hydroxide, and methanol were used. Seawater was collected from the beach in Rizhao, Shandong Province.2.2 Material preparation
2.3 Material characterization
X-ray diffraction (XRD) tests were performed using a Bruker D8 instrument from Germany. Scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HRTEM) were conducted using a Carl Zeiss Sigma 300 and a Thermo Fisher Talos F200X, respectively. X-ray photoelectron spectroscopy (XPS) was performed using a Thermo Fisher ESCALAB 250Xi, calibrated with C 1s at 284.8 eV. Fourier-transform infrared spectroscopy (FTIR) was conducted with a DEEP-FTIR-2 by Zhonghuan Technologies Beijing.2.4 Electrochemical analysis
Electrochemical measurements were performed on a CHI760E electrochemical workstation from Shanghai Chenhua using a three-electrode system with Cu3P@CF, MnP/Cu3P@CF, CoP/Cu3P@CF, and CoP/MnP/Cu3P@CF serving as the working electrodes. Seawater + 1 M KOH and seawater + 1 M KOH + 1 M methanol were used as electrolytes. The main ions in the seawater were Cl− (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ppm), K+ (500 ppm), Na+ (11
800 ppm), K+ (500 ppm), Na+ (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm), Ca2+ (300 ppm), Br− (50 ppm), and Mg2+ (1500 ppm). In situ IR measurements were conducted using a specialized electrolyzer equipped with an MCT detector and a DEEP-FTIR-2 Fourier transform infrared spectrometer. The in situ IR data were collected using an electrochemical workstation (CHI 760E) within a potential range of 1.2 to 1.8 V vs. RHE.
000 ppm), Ca2+ (300 ppm), Br− (50 ppm), and Mg2+ (1500 ppm). In situ IR measurements were conducted using a specialized electrolyzer equipped with an MCT detector and a DEEP-FTIR-2 Fourier transform infrared spectrometer. The in situ IR data were collected using an electrochemical workstation (CHI 760E) within a potential range of 1.2 to 1.8 V vs. RHE.
The electrocatalytic activity of the samples was primarily evaluated using linear sweep voltammetry (LSV) at a scan rate of 5 mV s−1 with a 95% iR compensation. All potentials were recalibrated to the reversible H2 electrode potential using the following equation:
| ERHE = EHg/HgO + 0.098 V + 0.0592 pH | (1) |
The polarization curve was converted to a Tafel slope plot using the following equation:
η = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log[j] log[j] | (2) |
The turnover frequency (TOF) was calculated as follows:
 | (3) |
The measurement and calculation of N followed previously reported methods.43,44 Cyclic voltammetry (CV) measurements were conducted at a fixed scan rate of 50 mV s−1 within a potential range relative to the RHE. The integral of the charge over the entire potential range of the CV curve provided the surface charge density (Qs), calculated as half of the charge value.
The formula for calculating N is:
 | (4) |
The electrochemical impedance spectroscopy (EIS) measurements were typically conducted within a frequency range of 10−2 to 106 Hz, with an AC voltage amplitude disturbance of 5–10 mV.
The electrochemical surface area (ECSA) was calculated using CV measurements at different scan rates in the non-faradaic region to obtain the Cdl value, plotted as a slope that was further used to deduce the ECSA. The ECSA can be calculated using:45,46
 | (5) |
3. Results and discussion
3.1 Morphology and structure characterization
As illustrated in Fig. 1a, the CoP/MnP/Cu3P@CF samples were synthesized through a hydrothermal-phosphating method. Specifically, CF is added to an aqueous solution containing Mn2+, and the Mn precursor is obtained after a hydrothermal process. Subsequently, the Mn precursor is immersed in an aqueous solution containing Co2+, to deliver the CoMn precursor. After a phosphating process, CoP/MnP/Cu3P@CF was obtained. | ||
| Fig. 1 (a) Schematic diagram of the preparation route of CoP/MnP/Cu3P@CF. (b) TEM, (c) HRTEM images, and (d) EDS mapping of CoP/MnP/Cu3P@CF. | ||
The morphologies of the Mn precursor, MnCo precursor, and the CoP/MnP/Cu3P@CF were analyzed using scanning electron microscopy (SEM). As shown in Fig. S1a and b (ESI†), MnCO3 (Mn precursor) spheres with a diameter of approximately 3 μm were grown on the copper foam skeleton. In the second step of the hydrothermal process, Co(CO3)0.5(OH)·0.11H2O particles were grown on MnCO3 spheres (Fig. S1c and d, ESI†). After the phosphatizing process, CoP/MnP/Cu3P@CF was obtained, and the spherical morphology is basically preserved (Fig. S1e, ESI†). The transmission electron microscopy (TEM) image in Fig. 1b confirms the irregular morphology of CoP/MnP/Cu3P@CF. A high-resolution TEM (HRTEM) image depicted lattice spacings of 0.282 nm, 0.202 nm, 0.196 nm, and 0.200 nm, corresponding to the (011) plane of CoP, (021) and (220) planes of MnP, and the (300) plane of Cu3P, respectively (Fig. 1c). Energy dispersive spectroscopy (EDS) elemental mapping demonstrated the homogeneous Co, Mn, Cu, and P distribution within the particles (Fig. 1d).
The X-ray diffraction (XRD) patterns in Fig. 2a suggest that CoP/MnP/Cu3P@CF comprises CoP (PDF No. 65-1474), MnP (PDF No. 51-0942), and Cu3P (PDF No. 71-2261). X-ray photoelectron spectroscopy (XPS) was employed to characterize the elemental composition and electronic structure of the CoP/MnP/Cu3P@CF electrocatalyst (Fig. 2b). The high-resolution XPS spectrum for Co 2p (Fig. 2c) displayed satellite peaks at 803.09 and 786.54 eV, while Co 2p1/2 and Co 2p3/2 satellite peaks were observed at 798.24 and 782.25 eV, respectively.47 The Co-P satellite peaks were located at 793.81 and 778.83 eV. In the Mn 2p spectrum (Fig. 2d), satellite peaks appeared at 648.51 eV, with MnP-related peaks at 653.65 (Mn 2p1/2) and 641.85 eV (Mn 2p3/2). Additional peaks at 655.76 and 643.96 eV were attributed to Mn 2p1/2 and Mn 2p3/2 of Mn4+, respectively.48 The Cu 2p spectrum (Fig. 2e) shows peaks at 952.82 (Cu 2p1/2) and 933.11 eV (Cu 2p3/2) for Cu3P, while peaks at 955.27 and 935.92 eV were assigned to Cu 2p1/2 and Cu 2p3/2 of Cu2+, respectively.49Fig. 2f shows the P 2p spectrum of CoP/MnP/Cu3P@CF, in which the P–O peak was observed at 134.38 eV, and the P 2p1/2 and P 2p3/2 peaks were located at 130.82 eV and 129.77 eV, respectively.50 The presence of P–O peaks is ascribed to the partial surface oxidation of the catalyst.51,52
 | ||
| Fig. 2 (a) XRD pattern and (b) survey XPS spectra of CoP/MnP/Cu3P@CF. High-resolution (c) Co 2p, (d) Mn 2p, (e) Cu 2p, (f) P 2p of CoP/MnP/Cu3P@CF. | ||
3.2 OER performance in alkaline seawater
The oxygen evolution reaction (OER) performance of CoP/MnP/Cu3P@CF was investigated in a seawater electrolyte containing 1 M KOH using a three-electrode system.53Fig. 3a shows that the CoP/MnP/Cu3P@CF catalyst exhibits superior OER catalytic activity in the alkaline seawater electrolyte. Fig. 3b shows that the CoP/MnP/Cu3P@CF requires 1.530 V, 1.590 V, and 1.621 V to achieve current densities of 10, 100, and 200 mA cm−2, respectively, lower than 1.636 V, 1.776 V, and 1.809 V for the benchmark Cu3P@CF, 1.586 V, 1.678 V, and 1.736 V for MnP/Cu3P@CF, and 1.533 V, 1.617 V, and 1.658 V for CoP/Cu3P@CF. To evaluate the reaction kinetics of the synthesized catalyst during the OER process, Tafel plots were created (Fig. 3c). The Tafel slope for CoP/MnP/Cu3P@CF in alkaline seawater is 43 mV dec−1, lower than those of Cu3P@CF (135.6 mV dec−1), MnP/Cu3P@CF (70.8 mV dec−1), and CoP/Cu3P@CF (60.9 mV dec−1), indicating enhanced reaction kinetics and suggesting the synergistic effect of each component in the electrocatalyst.54–56 For further assessment of the intrinsic activity of the electrocatalysts in the OER, TOF values were calculated at specific potentials.57 CV curves (Fig. S2a–d, ESI†) measured in the range of 0–0.6 V (vs. RHE) were used to compute the Qs values for Cu3P@CF, MnP/Cu3P@CF, CoP/Cu3P@CF, and CoP/MnP/Cu3P@CF. As shown in Fig. 3d, at working potentials of 1.6 V, 1.625 V, and 1.65 V, the TOF values for CoP/MnP/Cu3P@CF are 0.113, 0.191, and 0.305 s−1, respectively, much higher than 0.013, 0.028, and 0.046 s−1 of Cu3P@CF, 0.018, 0.038, and 0.063 s−1 of MnP/Cu3P@CF, and 0.073, 0.112, and 0.172 s−1 of CoP/Cu3P@CF. These results confirm the superior OER catalytic activity of CoP/MnP/Cu3P@CF. Electrochemical impedance spectroscopy (EIS) results confirm that CoP/MnP/Cu3P@CF exhibits the lowest charge transfer resistance of 1.101 Ω compared to 13.940 Ω for Cu3P@CF, 3.068 Ω for MnP/Cu3P@CF, and 1.289 Ω for CoP/Cu3P@CF (Fig. 3e and Fig. S3, ESI†), indicating faster electron transfer during OER electrolysis with CoP/MnP/Cu3P@CF. Finally, constant current tests performed for 24 h at 20 mA cm−2 during the OER, as shown in Fig. 3f, indicate stable catalytic performance with no significant degradation, demonstrating the remarkable corrosion resistance and catalytic longevity of the CoP/MnP/Cu3P@CF electrode in the alkaline seawater electrolyte during the OER. Moreover, the OER performance of CoP/MnP/Cu3P@CF is superior to those reported in many recent reports (Table S1, ESI†).3.3 Methanol oxidation reaction performance in alkaline seawater
Similarly, we explored the methanol oxidation reaction (MOR) performance of CoP/MnP/Cu3P@CF in a seawater electrolyte containing 1 M KOH and 1 M methanol, employing a three-electrode setup. Fig. 4a reveals the superior MOR catalytic activity of the CoP/MnP/Cu3P@CF catalyst compared to that of the comparison samples. The introduction of methanol significantly lowers the onset potentials for oxidation reactions compared to the OER process. As depicted in Fig. 4b, the specific potentials required to achieve current densities of 10, 100, and 200 mA cm−2 are 1.356 V, 1.443 V, and 1.489 V, respectively. In contrast, the potential values for Cu3P@CF stand at 1.447 V, 1.556 V, and 1.612 V; for MnP/Cu3P@CF at 1.375 V, 1.543 V, and 1.603 V; and for CoP/Cu3P@CF at 1.380 V, 1.474 V, and 1.525 V. These results highlight the pronounced reactivity of CoP/MnP/Cu3P@CF during the MOR process. Fig. 4c presents the Tafel plots, where the CoP/MnP/Cu3P@CF catalyst exhibits a lower slope of 76.2 mV dec−1 in alkaline seawater compared to 165.4 mV dec−1 for Cu3P@CF, 150.3 mV dec−1 for MnP/Cu3P@CF, and 91.5 mV dec−1 for CoP/Cu3P@CF. CV curves were plotted in the range of 0–0.6 V (vs. RHE) (Fig. S4a–d, ESI†) to calculate the Qs values for Cu3P@CF, MnP/Cu3P@CF, CoP/Cu3P@CF, and CoP/MnP/Cu3P@CF, and the calculated values are 0.0407, 0.0574, 0.190, and 0.142, respectively. Fig. 4d shows the calculated TOF for the MOR at working potentials of 1.45, 1.475, and 1.5 V. The TOF values for CoP/MnP/Cu3P@CF were calculated to be 0.132, 0.195, and 0.268 s−1, respectively, higher than 0.044, 0.074, and 0.127 s−1 for Cu3P@CF, 0.071, 0.103, and 0.153 s−1 for MnP/Cu3P@CF, and 0.057, 0.089, and 0.128 s−1 for CoP/Cu3P@CF. As depicted in Fig. 4e, EIS tests were carried out in methanol-containing alkaline seawater at an applied potential of 1.6 V. The CoP/MnP/Cu3P@CF catalyst demonstrated an Rct value of 0.378 Ω, approximately 2.9 times lower than the resistance observed during the OER at the same potential, indicating that the introduction of methanol accelerates charge transfer (Fig. 4e and Fig. S3, ESI†). To assess the corrosion resistance of the CoP/MnP/Cu3P@CF catalyst in a methanol-containing seawater electrolyte, a constant current test was performed at 20 mA cm−2 (Fig. 4f). The electrolyte was replaced after 12 hours due to methanol consumption, and no discernible deterioration in catalyst performance was observed, demonstrating excellent stability throughout the MOR process. It is worth mentioning that after 24 h of cycling, there is no significant change in Rct, further suggesting the satisfactory stability of the catalyst (Fig. S5, ESI†). Moreover, the MOR performance of CoP/MnP/Cu3P@CF is superior to those reported in many recent reports (Table S2, ESI†), revealing excellent catalytic performance.3.4 HER performance in alkaline seawater
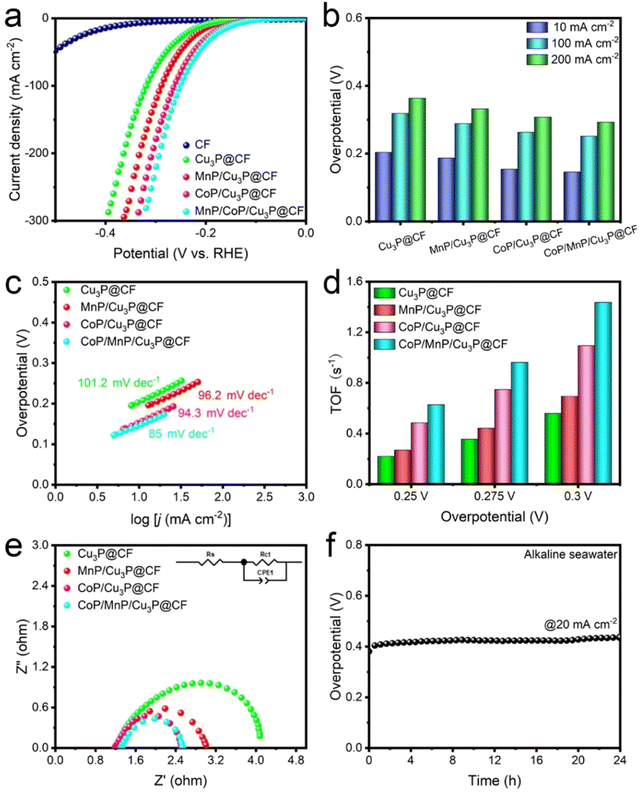
We assessed the H2 evolution reaction (HER) performance of CoP/MnP/Cu3P@CF using a three-electrode system in a seawater electrolyte containing 1 M KOH.58 As shown in Fig. 5a, the polarization curves reveal that CoP/MnP/Cu3P@CF exhibits superior HER performance compared to Cu3P@CF, MnP/Cu3P@CF, and CoP/Cu3P@CF. Notably, Fig. 5b illustrates that at current densities of 10, 100, and 200 mA cm−2, the overpotentials of CoP/MnP/Cu3P@CF are 0.146, 0.252, and 0.293 V, respectively, which are significantly lower than those of Cu3P@CF (0.204, 0.319, 0.364 V), MnP/Cu3P@CF (0.187, 0.289, 0.332 V), and CoP/Cu3P@CF (0.154, 0.263, 0.308 V). Further analysis through Tafel results, presented in Fig. 5c, reveals a notable reduction in the slope for CoP/MnP/Cu3P@CF (85 mV dec−1) compared to Cu3P@CF (101.2 mV dec−1), MnP/Cu3P@CF (96.2 mV dec−1), and CoP/Cu3P@CF (94.3 mV dec−1). Additionally, cyclic voltammetry (CV) curves measured within the range of 0 to 0.6 V vs. RHE (Fig. S6, ESI†) yielded Qs values of 6.49 × 10−2, 8.79 × 10−2, 8.28 × 10−2, and 7.78 × 10−2 for Cu3P@CF, MnP/Cu3P@CF, CoP/Cu3P@CF, and CoP/MnP/Cu3P@CF, respectively, with corresponding N values of 6.72 × 10−7, 9.11 × 10−7, 8.58 × 10−7, and 8.06 × 10−7.As depicted in Fig. 5d, the turnover frequency (TOF) values for the CoP/MnP/Cu3P@CF catalyst at potentials of 0.25, 0.275, and 0.3 V are 0.627, 0.962, and 1.436 s−1, respectively, higher than 0.219, 0.354, and 0.559 s−1 for Cu3P@CF, 0.269, 0.443, and 0.693 s−1 for MnP/Cu3P@CF, and 0.485, 0.746, and 1.093 s−1 for CoP/Cu3P@CF. Furthermore, the electrochemical surface area (ECSA) was calculated via Cdl to determine the number of exposed active sites.59 As shown in Fig. S7 (ESI†), the Cdl value for the CoP/MnP/Cu3P@CF heterostructure catalyst is approximately 1.74, 1.55, and 1.49 times higher than those of Cu3P@CF, MnP/Cu3P@CF, and CoP/Cu3P@CF, respectively, suggesting that more active sites are exposed in CoP/MnP/Cu3P@CF compared to other samples. Moreover, EIS tests were performed for Cu3P@CF, MnP/Cu3P@CF, CoP/Cu3P@CF, and CoP/MnP/Cu3P@CF during the HER in alkaline seawater electrolysis (Fig. 5e). The results indicate that the CoP/MnP/Cu3P@CF catalyst exhibits a lower charge transfer resistance (1.256 Ω) compared to Cu3P@CF (3.058 Ω), MnP/Cu3P@CF (1.818 Ω), and CoP/Cu3P@CF (1.348 Ω), suggesting that the CoP/MnP/Cu3P heterostructures are more favorable for electron transfer (Fig. S3, ESI†).60 Additionally, a stability test was conducted at 20 mA cm−2 for 24 hours during the HER (Fig. 5f). Remarkably, the overpotential exhibits no obvious changes, indicating its potential applicability in seawater electrolysis. Importantly, the HER performance of CoP/MnP/Cu3P@CF is superior to those reported in many recent reports (Table S3, ESI†).
3.5 Methanol oxidation assisted seawater electrolysis performance
Based on the excellent catalytic performance of the CoP/MnP/Cu3P@CF catalyst in the MOR, HER, and OER, we developed a two-electrode seawater electrolyzer using seawater + 1 M KOH and seawater + 1 M KOH + 1 M methanol as electrolytes (Fig. 6a), respectively. Polarization curves in Fig. 6b show that the MOR-assisted seawater electrolyzer requires cell voltages of 1.410 V and 1.697 V to achieve current densities of 10 and 100 mA cm−2, much lower than 1.681 V and 1.873 V for a seawater electrolyzer. Fig. 6c shows a linear correlation between H2 production and operational time. The H2 production rate at 20 mA cm−2 is calculated to be 10 mL h−1. Importantly, the CoP/MnP/Cu3P@CF-based MOR-assisted seawater electrolyzer exhibits high Faraday efficiency during the test period. To gain insight into the MOR mechanism, electrochemical in situ Fourier transform infrared spectroscopy (FTIR) was applied to investigate intermediate products on the CoP/MnP/Cu3P@CF catalyst. As shown in Fig. 6d, in the alkaline seawater electrolyte, a band centered around 1190 cm−1 corresponding to OOH* was observed. Notably, in the electrolyte containing methanol, a characteristic peak of COOH* appears at 1484 cm−1, indicating the formation of the corresponding species.61,62 The constant current test results show that CoP/MnP/Cu3P@CF-based electrolyzers with and without methanol exhibit negligible performance degradation after 24 hours of continuous operation at 20 mA cm−2, suggesting satisfactory stability (Fig. 6e). Moreover, the performance of the MOR-assisted seawater electrolyzer using CoP/MnP/Cu3P@CF both as the anode and cathode is superior to those reported in many recent reports (Table S4, ESI†).4. Conclusion
In summary, a unique heterostructured electrocatalyst comprising CoP, MnP, and Cu3P on copper foam (CoP/MnP/Cu3P@CF) is designed and successfully synthesized via a hydrothermal-phosphating process. The synergistic interaction among heterogeneous components endows CoP/MnP/Cu3P@CF with impressive OER, MOR, and HER performance. Consequently, the CoP/MnP/Cu3P@CF-based MOR-assisted seawater electrolyzer needs only 1.410 V to achieve a current density of 10 mA cm−2, much lower than the 1.681 V of the seawater electrolyzer without methanol. Moreover, the MOR-assisted seawater electrolyzer shows high faradaic efficiency and stability, providing a feasible way to produce H2 by energy-saving seawater electrolysis.Data availability
Data will be made available on request.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by the Guangxi Natural Science Fund for Distinguished Young Scholars (2024GXNSFFA010008), the National Natural Science Foundation of China (22469002 and 22275166), and the Fundamental Research Funds for the Provincial Universities of Zhejiang (RF-B2023002).References
- I. Masood ul Hasan, L. Peng, J. Mao, R. He, Y. Wang, J. Fu, N. Xu and J. Qiao, Carbon-based metal-free catalysts for electrochemical CO2 reduction: Activity, selectivity, and stability, Carbon Energy, 2021, 3(1), 24–49 CrossRef CAS
.
- X. Liu, M. Chen, J. Ma, J. Liang, C. Li, C. Chen and H. He, Advances in the synthesis strategies of carbon-based single-atom catalysts and their electrochemical applications, China Powder Sci. Technol., 2024, 30(05), 35–45 Search PubMed
.
- Y. Hao, S.-F. Hung, L. Wang, L. Deng, W.-J. Zeng, C. Zhang, Z.-Y. Lin, C.-H. Kuo, Y. Wang, Y. Zhang, H.-Y. Chen, F. Hu, L. Li and S. Peng, Designing neighboring-site activation of single atom via tunnel ions for boosting acidic oxygen evolution, Nat. Commun., 2024, 15(1), 8015 CrossRef CAS
.
- W. Ma, T. Jian, J. Ma, X. Li, H. Gao and H. Liu, Research progress on transition metals and their alloy catalysts for Li-CO2 batteries, China Powder Sci. Technol., 2024, 30(6), 1–14 Search PubMed
.
- H. Shang and D. Liu, Atomic design of carbon-based dual-metal site catalysts for energy applications, Nano Res., 2023, 16(5), 6477–6506 CrossRef CAS
.
- S. Li, G. Xing, S. Zhao, J. Peng, L. Zhao, F. Hu, L. Li, J. Wang, S. Ramakrishna and S. Peng, Fe-N co-doped carbon nanofibers with Fe3C decoration for water activation induced oxygen reduction reaction, Natl. Sci. Rev., 2024, 11(10), nwae193 CrossRef PubMed
.
- J.-D. Musah, A. M. Ilyas, S. Venkatesh, S. Mensah, S. Kwofie, V. A. L. Roy and C.-M. L. Wu, Isovalent substitution in metal chalcogenide materials for improving thermoelectric power generation - A critical review, Nano Res. Energy, 2022, 1, e9120034 Search PubMed
.
- C. Jin Hyuk, K. Youngho, Y. Hak Ki and K. Soo Young, Advancements in two-dimensional covalent organic framework nanosheets for electrocatalytic energy conversion: current and future prospects, Energy Mater., 2024, 4(2), 400013 Search PubMed
.
- D. Yu, Y. Hao, S. Han, S. Zhao, Q. Zhou, C.-H. Kuo, F. Hu, L. Li, H.-Y. Chen, J. Ren and S. Peng, Ultrafast Combustion Synthesis of Robust and Efficient Electrocatalysts for High-Current-Density Water Oxidation, ACS Nano, 2023, 17(2), 1701–1712 CrossRef CAS PubMed
.
- R. Yin, Z. Wang, J. Zhang, W. Liu, J. He, G. Hu and X. Liu, Tunable NiSe-Ni3Se2 Heterojunction for Energy-Efficient Hydrogen Production by Coupling Urea Degradation, Small Methods, 2025, 2401976 CrossRef PubMed
.
- S. Zhao, F. Hu, L. Yin, L. Li and S. Peng, Manipulating electron redistribution induced by asymmetric coordination for electrocatalytic water oxidation at a high current density, Sci. Bull., 2023, 68(13), 1389–1398 CrossRef CAS PubMed
.
- K. H. Modi, P. M. Pataniya and C. K. Sumesh, 2D Monolayer Catalysts: Towards Efficient Water Splitting and Green Hydrogen Production, Chem. – Eur. J., 2024, 30(23), e202303978 CrossRef CAS PubMed
.
- S. Mathur, Y. Gönüllü, M. Pyeon and M. Wang, Perspectives of Nanostructured Metal Oxides and Their Heterostructures in Photoelectrochemical Water Splitting for Solar Hydrogen Production, Eng. Ceram., 2016, 457–495 CAS
.
-
S. A. Grigoriev and V. N. Fateev, Hydrogen Production by Water Electrolysis, Hydrogen Production Technologies, 2017, pp. 231–276 Search PubMed
.
- W. Liu, W. Liu, T. Hou, J. Ding, Z. Wang, R. Yin, X. San, L. Feng, J. Luo and X. Liu, Coupling Co-Ni phosphides for energy-saving alkaline seawater splitting, Nano Res., 2024, 17(6), 4797–4806 CrossRef CAS
.
- W. Liu, J. Tang, C. Kong, R. Yin, W. Guo, J. Dai, F. Wu, W. Shi and X. Cao, A p-block dopant enables energy-efficient hydrogen production from biomass, Chem. Commun., 2024, 60(38), 5058–5061 RSC
.
- I. Hasan and F. A. Alharthi, Synthesis of Cobalt Oxide (Co3O4) Nanoparticles for Efficient Photocatalytic Water Splitting and Hydrogen Production, ChemistrySelect, 2023, 8(34), e202302685 CrossRef CAS
.
- Y. Surendranath and D. G. Nocera, Oxygen Evolution Reaction Chemistry of Oxide-Based Electrodes, Prog. Inorg. Chem., 2011, 505–560 Search PubMed
.
- S. N. Hussain, Y. Men, Z. Li, P. Zhao, G. Cheng and W. Luo, Molybdenum-induced tuning 3d-orbital electron filling degree of CoSe2 for alkaline hydrogen and oxygen evolution reactions, Chin. Chem. Lett., 2023, 34(2), 107364 CrossRef CAS
.
- M. Biegun, X. Chen and E. Mijowska, Cobalt/Carbon Nanocomposite as Oxygen Evolution Reaction Electrocatalyst, ChemElectroChem, 2018, 5(18), 2681–2685 CrossRef CAS
.
- G. Ma, X. Zhang, G. Zhou and X. Wang, Hydrogen production from methanol reforming electrolysis at NiO nanosheets supported Pt nanoparticles, Chem. Eng. J., 2021, 411, 128292 CrossRef CAS
.
- H. Wang, X. Niu, W. Liu, R. Yin, J. Dai, W. Guo, C. Kong, L. Ma, X. Ding, F. Wu, W. Shi, T. Deng and X. Cao, S-Block Metal Mg-Mediated Co-N-C as Efficient Oxygen Electrocatalyst for Durable and Temperature-Adapted Zn–Air Batteries, Adv. Sci., 2024, 11(34), 2403865 CrossRef CAS
.
- X. Fu, D. Cheng, A. Zhang, J. Zhou, S. Wang, X. Zhao, J. Chen, P. Sautet, Y. Huang and X. Duan, Ag-Ru interface for highly efficient hydrazine assisted water electrolysis, Energy Environ. Sci., 2024, 17(6), 2279–2286 RSC
.
- T. Wei, P. Zhou, W. Liu, X. Liu and T. Kuang, Catalytic recycling of plastics into value-added products, Nano Res., 2024, 17, 9428–9445 CrossRef CAS
.
- G.-L. Li, Y.-Y. Miao, F. Deng, S. Wang, R.-X. Wang, W.-H. Lu and R.-L. Chen, Highly-dispersed 2D NiFeP/CoP heterojunction trifunctional catalyst for efficient electrolysis of water and urea, J. Colloid Interface Sci., 2024, 667, 543–552 CrossRef CAS PubMed
.
- X. Zhang, Y. Li, J. Wang, G. Zeng and Q. Zhong, H2O2-assisted fabricate nickel cobalt sulfide as the efficient catalyst for the novel strategy of 5-hydroxymethylfurfural stepwise electrolysis, Appl. Surf. Sci., 2023, 639, 158198 CrossRef CAS
.
- N. Thakur, D. Mehta, A. Chaturvedi, D. Mandal and T. C. Nagaiah, Glucose oxidation assisted hydrogen and gluconic/glucaric acid production using NiVP/Pi bifunctional electrocatalyst, J. Mater. Chem. A, 2023, 11(29), 15868–15877 RSC
.
- B. Zhu, J. Xiong, S. Wu, K. You, B. Sun, Y. Liu, M. Chen, P. Jin and L. Feng, Core@Shell Heterostructured NiMoP@Ni5P4 Nanorod Arrays Promoting Direct Electro-Oxidation of Methanol and Hydrogen Evolution under Industry Conditions, Adv. Funct. Mater., 2024, 2407236 CrossRef CAS
.
- X. Du, M. Tan, T. Wei, H. Kobayashi, J. Song, Z. Peng, H. Zhu, Z. Jin, R. Li and W. Liu, Highly efficient and robust nickel-iron bifunctional catalyst coupling selective methanol oxidation and freshwater/seawater hydrogen evolution via CO-free pathway, Chem. Eng. J., 2023, 452, 139404 CrossRef CAS
.
- S. Ding, H. Wang, X. Dai, Y. Lv, X. Niu, R. Yin, F. Wu, W. Shi, W. Liu and X. Cao, Mn-modulated Co-N-C oxygen electrocatalysts for robust and temperature-adaptative zinc-air batteries, Chin. J. Struct. Chem., 2024, 43(7), 100302 CrossRef CAS
.
- P. Zhang, S. Hong, N. Song, Z. Han, F. Ge, G. Dai, H. Dong and C. Li, Alloy as advanced catalysts for electrocatalysis: From materials design to applications, Chin. Chem. Lett., 2024, 35(6), 109073 CrossRef CAS
.
- Y. Li, T. Wang, M. Asim, L. Pan, R. Zhang, Z.-F. Huang, Z. Chen, C. Shi, X. Zhang and J.-J. Zou, Manipulating Spin Polarization of Defected Co3O4 for Highly Efficient Electrocatalysis, Trans. Tianjin Univ., 2022, 28(3), 163–173 CrossRef CAS
.
- H. Zhang, R. Li, M. Humayun, Z. Huang, Y. Fu, Y. Cao, J. Duan, Y. A. Attia and C. Wang, Recent progress in Mott–Schottky junction electrocatalysts for the pH-universal hydrogen evolution reaction, Mater. Chem. Front., 2024, 8(17), 2811 RSC
.
- J. Yang, F. Zhang and J. Chen, Structural design and application of fiber-based electrocatalytic materials, China Powder Sci. Technol., 2024, 30(04), 161–170 Search PubMed
.
- K. Wang, P. Liu, M. Wang, T. Wei, J. Lu, X. Zhao, Z. Jiang, Z. Yuan, X. Liu and J. He, Modulating d-d orbitals coupling in PtPdCu medium-entropy alloy aerogels to boost pH-general methanol electrooxidation performance, Chin. Chem. Lett., 2024 DOI:10.1016/j.cclet.2024.110532
.
- T. Kuang, H. Guo, W. Guo, W. Liu, W. Li, M. R. Saeb, M. Vatankhah-Varnosfaderani and S. S. Sheiko, Boosting the Strength and Toughness of Polymer Blends via Ligand-Modulated MOFs, Adv. Sci., 2024, 2407593 CrossRef CAS PubMed
.
- M. Pahuja, S. G. Dastider, Jyoti, K. Alam, S. Rani, S. Das, R. Urkude, M. Afshan, D. Rani, N. Chaudhary, S. A. Siddiqui, S. K. Riyajuddin, R. Ghosh, K. Mondal and K. Ghosh, Harvesting Green Hydrogen from the Deep Blue: Seawater-Compatible SnSe-P Decorated Graphene-CNTs Based Electrocatalyst Under Universal pH, Small, 2024, 2406113 CrossRef CAS PubMed
.
- L. Zhang, Y. Lei, W. Xu, D. Wang, Y. Zhao, W. Chen, X. Xiang, X. Pang, B. Zhang and H. Shang, Highly active and durable nitrogen-doped CoP/CeO2 nanowire heterostructures for overall water splitting, Chem. Eng. J., 2023, 460, 141119 CrossRef CAS
.
- W. Wu, S. Luo, Y. Huang, H. He, P. K. Shen and J. Zhu, Recent advances in transition metal phosphide-based heterostructure electrocatalysts for the oxygen evolution reaction, Mater. Chem. Front., 2024, 8(4), 1064–1083 RSC
.
- Z. Zhang, K. Ye, H. Du and X. Li, In situ electrodeposition synthesis of CoP@NiFe LDH heterostructure as high-performance electrocatalyst for enhanced seawater electrolysis, Int. J. Hydrogen Energy, 2024, 62, 722–731 CrossRef CAS
.
- M. R. Kandel, U. N. Pan, P. P. Dhakal, R. B. Ghising, T. T. Nguyen, J. Zhao, N. H. Kim and J. H. Lee, Unique heterointerface engineering of Ni2P-MnP nanosheets coupled Co2P nanoflowers as hierarchical dual-functional electrocatalyst for highly proficient overall water-splitting, Appl. Catal., B, 2023, 331, 122680 CrossRef CAS
.
- J. Chang, W. Wang, D. Wu, F. Xu, K. Jiang, Y. Guo and Z. Gao, Self-supported amorphous phosphide catalytic electrodes for electrochemical hydrogen production coupling with methanol upgrading, J. Colloid Interface Sci., 2023, 648, 259–269 CrossRef CAS PubMed
.
- D. Merki, S. Fierro, H. Vrubel and X. Hu, Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water, Chem. Sci., 2011, 2(7), 1262–1267 RSC
.
- X. Liu, J. He, S. Zhao, Y. Liu, Z. Zhao, J. Luo, G. Hu, X. Sun and Y. Ding, Self-powered H2 production with bifunctional hydrazine as sole consumable, Nat. Commun., 2018, 9(1), 4365 CrossRef PubMed
.
- Z. Liu, R. Wang, S. Li, Y. Gu, J. Lan, Q. Zhou and W. Xu, Oxygen vacancy-rich ultrafine CoP/Co3O4 nanoparticles as high-efficiency trifunctional electrocatalyst, Electrochim. Acta, 2022, 412, 140134 CrossRef CAS
.
- T. Xiong, Z. Zhu, Y. He, M. S. Balogun and Y. Huang, Phase Evolution on the Hydrogen Adsorption Kinetics of NiFe-Based Heterogeneous Catalysts for Efficient Water Electrolysis, Small Methods, 2023, 7(4), 2201472 CrossRef CAS PubMed
.
- F. Huang, J. Wang, M. Wang, C. Zhang, Y. Xue, J. Liu, T. Xu, N. Cai, W. Chen and F. Yu, Core-shell Ni2P@CoP nanoarrays supported on NF as a highly efficient electrocatalyst for hydrogen evolution reaction, Colloids Surf., A, 2021, 623, 126526 CrossRef CAS
.
- Y. Jiang, Z. Song, M. Qu, Y. Jiang, W. Luo and R. He, Co-Mn Bimetallic Nanowires by Interfacial Modulation with/without Vacancy Filling as Active and Durable Electrocatalysts for Water Splitting, Small, 2024, 20(33), 2400859 CrossRef CAS PubMed
.
- J. Chen, X. Li, B. Ma, X. Zhao and Y. Chen, Cu3P@Ni core-shell heterostructure with modulated electronic structure for highly efficient hydrogen evolution, Nano Res., 2022, 15(4), 2935–2942 CrossRef CAS
.
- C. Huang, Z. Xia, J. Wang, J. Zhang, C. Zhao, X. Zou, S. Mu, J. Zhang, X. Lu, H. J. Fan, S. Huo and Y. Zhao, Highly efficient and stable electrocatalyst for hydrogen evolution by molybdenum doped Ni-Co phosphide nanoneedles at high current density, Nano Res., 2024, 17(3), 1066–1074 CrossRef CAS
.
- L. Wang, W. Huan, F. Jia, L. Lu, J. Zhu, P. Zhu, X. Zhao, G. Wang and S. Liu, Tailored Crafting of CoP/Ni2P Nanoparticles Coupled with N-Doped Nanoporous Carbon to Enable Efficient Hydrogen and Oxygen Evolution, Energy Fuels, 2022, 36(19), 12245–12252 CrossRef CAS
.
- T. Shu, H. Gao, Q. Li, F. Wei, Y. Ren, Z. Sun, J. Qi and Y. Sui, One-step phosphating synthesis of CoP nanosheet arrays combined with Ni2P as a high-performance electrode for supercapacitors, Nanoscale, 2020, 12(40), 20710–20718 RSC
.
- Z. Lu, H. Yang, Q. Liu, J. Luo, L. Feng, L. Chu and X. Liu, Nb2AlC MAX Nanosheets Supported Ru Nanocrystals as Efficient Catalysts for Boosting pH-Universal Hydrogen Production, Small, 2024, 20(17), 2305434 CrossRef CAS PubMed
.
- G. Afshan, S. Karim, Y. P. Kharwar, T. Aziz, S. Saha, S. Roy and A. Dutta, Green H2 Generation from Seawater Deploying a Bifunctional Hetero-Interfaced CoS2-CoFe-Layered Double Hydroxide in an Electrolyzer, Small, 2024, 2406431 Search PubMed
.
- Y. Hao, S.-F. Hung, C. Tian, L. Wang, Y.-Y. Chen, S. Zhao, K.-S. Peng, C. Zhang, Y. Zhang, C.-H. Kuo, H.-Y. Chen and S. Peng, Polarized Ultrathin BN Induced Dynamic Electron Interactions for Enhancing Acidic Oxygen Evolution, Angew. Chem., Int. Ed., 2024, 63(18), e202402018 CrossRef CAS PubMed
.
- Y. Du, L. Zhan, Y. Liu, R. Chen, Y. Fu, B. Li and L. Wang, Morphology engineering induces the increase of FeP/CoP heterointerface density for efficient alkaline water splitting driven by interfacial dual active sites, Mater. Chem. Front., 2023, 7(19), 4573–4583 RSC
.
- M. Wu, X. Fan, W. Zhang, B. Chen, T. Ye, Q. Zhang, Y. Fang, Y. Wang and Y. Tang, Highly dispersed Ru nanospecies on N-doped carbon/MXene composite for highly efficient alkaline hydrogen evolution, Chin. Chem. Lett., 2024, 35(4), 109258 CrossRef CAS
.
- B. Zhang and Y. Yu, Breaking Ordered Atomic Arrangement into Disordered Amorphous Structure for High Electrocatalytic Performance, Trans. Tianjin Univ., 2024 DOI:10.1007/s12209-024-00422-0
.
- J. Ding, Z. Peng, Z. Wang, C. Zeng, Y. Feng, M. Yang, G. Hu, J. Luo and X. Liu, Phosphorus-tungsten dual-doping boosts acidic overall seawater splitting performance over RuOx nanocrystals, J. Mater. Chem. A, 2024, 12(41), 28023–28031 RSC
.
- D. Malhotra, T. H. Nguyen, D. T. Tran, V. A. Dinh, N. H. Kim and J. H. Lee, Triphasic Ni2P-Ni12P5-Ru with Amorphous Interface Engineering Promoted by Co Nano-Surface for Efficient Water Splitting, Small, 2024, 20(27), 2309122 CrossRef CAS PubMed
.
- H. Wang, K. Xie, Q. Fang, L. Zheng, X. Yang, L. Ling, Y. Wen, L. Lin, X. Yang, W. Gu, C. He and C. Zhu, Ni2P Aerogels with Pt Single-Atom Doping Tune Competitive Adsorption for Boosted Methanol Electrooxidation, ACS Mater. Lett., 2024, 6(6), 2446–2454 CrossRef CAS
.
- Q. Wang, X. Yang, H. Zang, C. Liu, J. Wang, N. Yu, L. Kuai, Q. Qin and B. Geng, InBi Bimetallic Sites for Efficient Electrochemical Reduction of CO2 to HCOOH, Small, 2023, 19(41), 2303172 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm01067a |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2025 |