Laser irradiation-induced two-photon photolysis of sulfates for photoluminescent sulfur quantum dots†
Shuxian
Wei‡
ab,
Hao
Huang‡
cd,
Ningning
He
ab,
Taiping
Hu
ab,
Jijun
Huang
e,
Yunyu
Cai
 b,
Yixing
Ye
b,
Pengfei
Li
b,
Yixing
Ye
b,
Pengfei
Li
 *b,
Xueling
Lei
*b,
Xueling
Lei
 *e and
Changhao
Liang
*ab
*e and
Changhao
Liang
*ab
aDepartment of Materials Science and Engineering, University of Science and Technology of China, Hefei 230026, China
bKey Laboratory of Materials Physics and Anhui Key Laboratory of Nanomaterials and Nanotechnology, Institute of Solid State Physics, Hefei Institutes of Physical Science, Chinese Academy of Sciences, Hefei 230031, China. E-mail: pfli@issp.ac.cn; chliang@issp.ac.cn
cFair Friend Institute of Intelligent Manufacturing, Hangzhou Vocational & Technical College, Hangzhou 310018, China
dDepartment of Materials Science and Engineering, University of Virginia, Charlottesville 22904-4745, USA
eDepartment of Physics, Jiangxi Normal University, Nanchang, Jiangxi 330022, China. E-mail: xueling@mail.ustc.edu.cn
First published on 15th October 2024
Abstract
Recently, sulfur quantum dots (SQDs) have gained great research interest because of their excellent optical properties and low toxicity, thus inspiring researchers to make efforts to explore a simpler and faster approach for the synthesis of SQDs. Herein, a facile and green bottom-up strategy is first proposed to prepare SQDs via 532 nm laser irradiation of a sulfate-containing solution without any extra additives. The reduction of sulfates to elemental sulfur under visible light is demonstrated for the first time. Furthermore, fluorescence characterization combined with density functional theory calculations revealed that the two-photon dissociation of sulfates plays a critical role in the formation of SQDs under laser irradiation. The nucleation mechanisms of self-assembling of sulfur element were revealed by molecular dynamics.
Introduction
Quantum dots (QDs) are nanocrystals of a semiconductor material whose physical dimensions are smaller than the exciton Bohr radius. The natural quantum confinements endow QDs with many particular optical, electronic, and chemical properties such as size-dependent bandgap, tunable surface chemistry, narrow linewidth, and bright emission that are not found in their bulk counterparts.1–6 Sulfur quantum dots (SQDs) have emerged recently due to their environmental harmlessness, superior biocompatibility, strong photostability, low toxicity, and unique antibacterial characteristics, and thus, they are viewed as promising materials for diverse applications.1,7–10 Li et al. first synthesized SQDs through an acid etching oxidation method involving a three-step physical contact process, in situ dissolution precipitation and a phase interface reaction.11 This work became the milestone for the synthesis of SQDs. Later, Shen et al. proposed a more facile top-down approach to prepare SQDs by hydrogen peroxide etching of sulfur powder,7 which can achieve a higher photoluminescence quantum yield (PLQY). Since then, more efforts have been devoted to improve Shen's method, but the optimized synthetic process is still complicated.8,12–15 Therefore, it is desirable to explore new synthetic methods for SQDs.Laser synthesis in liquids has been demonstrated to be a highly efficient strategy in nanomaterial preparation.16,17 The Stark–Einstein law states that in a photochemical process, one photon can be absorbed by each molecule that causes the main photochemical process. Thus, the photons generated from a nanosecond pulsed laser can also interact with small molecules or groups, thereby inducing photodissociation.18,19 Previous studies have shown that laser radiation-induced decomposition of gaseous chalcogenide-containing compounds can yield chalcogenide elements.20 For instance, laser-induced photolytic and thermolytic decomposition of gas-phase organic selenide or telluride facilitates the extrusion of selenium and tellurium that deposit into a nanostructured powder or thin film.21,22 Photolysis of sulfur-containing compounds such as H2S and SO2 by pulsed laser also enable the generation of sulfur elements.23–26 In principle, this transition is too tough to achieve, which requires very high energy such as two-photon photolysis in the UV band. Motivated by the aforementioned studies, we first propose a strategy regarding the photolysis of compounds containing sulfates under laser irradiation in liquid (LIL) and explore whether the sulfate will undergo photolysis and further assemble into small-sized SQDs with widespread applications.
In this study, the source of SO42− is sodium dodecyl sulfate (SDS) whose molecular formula is C12H25SO4Na, where the S and O atoms form steric tetrahedral structures through sp3 hybridization. The SDS is chosen for the following reasons: (i) it is an organic substance that is feasible for Matrix-free laser desorption/ionization time-of-flight mass spectrometry (LDI-TOF MS); (ii) as a surfactant, it can protect the SQDs by physically adhering to the surface, thus guaranteeing the stability of SQDs in the characterization. The evidenced characterization validates that the photodecomposition of the sulfate in SDS arises under the pulsed laser with a wavelength of 532 nm, thus inducing the generation of well-stable SQDs with a size of 1.5–3 nm. This LIL-based method enables the simple synthesis of SQDs within 30 min. In addition, we explore the photolysis mechanism and show that the sulfate undergoes a two-photon transition, resulting in its dissociation into sulfur elements under the pulsed laser with a wavelength of 532 nm. Then, a molecular dynamics simulation reveals the SQD nucleation processes and mechanisms at the atomistic scale. This work presents a novel approach for the synthesis of SQDs and clarifies the mechanism of the interaction between the pulsed laser and the sulfate in aqueous solutions, which demonstrate the reduction of sulfates to elemental sulfur under visible light for the first time.
Experimental setup and modelling
Synthesis of SQDs
First, 20 ml reaction solution (0.1-M H2SO4, 0.1-M Na2SO4 or 0.1-M C12H25SO4Na) taken in a quartz bottle was irradiated for 30 min with a 532 nm pulsed Nd:Yag laser (INNOLAS, 20 Hz, 7 ns pulse width). The laser beam was parallel with a spot diameter of 7 mm, and the laser energy was approximately 100 mJ per pulse. The fluence was approximately 0.26 J cm−2 and the peak intensity was about 3.71 × 107 W cm−2, which indicates high energy density. The irradiation solution was centrifuged at 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min using an ultracentrifuge (Beckman Coulter, Optima XPN-100-IVD), while the SQDs remained in the sediment. The SQD were then stored in deionized water or freeze-dried.
000 rpm for 30 min using an ultracentrifuge (Beckman Coulter, Optima XPN-100-IVD), while the SQDs remained in the sediment. The SQD were then stored in deionized water or freeze-dried.
Characterizations
The morphology and structure of product were characterized by high-resolution transmission electron microscopy (HRTEM) using an FEI Tecnai TF20. X-Ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250) was performed to obtain the electronic state and chemical state of the samples. Matrix-free laser desorption/ionization time-of-flight mass spectrometry (LDI-TOF MS) was performed using an Autoflex Speed TOF/TOF (Bruker Daltonik, Bremen, Germany). Two-photon fluorescence spectra were recorded via confocal microscopy (ARsiMP-LSM-Kit-Legend Elite-USX, Nikon, Japan). Fluorescence spectroscopy (FS) was performed using a Hitachi F-4600 Spectrophotometer and UV-visible (UV-vis) spectroscopy using a Shimadzu UV-2550 Spectrophotometer. X-Ray diffraction (XRD) analysis was performed using a Philips X'Pert with Cu-Kα radiation. Fourier-transform infrared (FTIR) spectra were recorded using a Thermo Nicolet Corporation NEXUS spectrometer. Raman spectra were recorded using an Analytik Jena RXN1-785 Raman spectrometer. Thermogravimetric analysis (TGA) was performed using a Pyris 1, PerkinElmer.Density functional theory (DFT) calculation
We used the Gussian09 software to perform DFT calculations, in which the B3LYP functional with the 6-31G(d) basis set was employed.27–30 Vibrational frequency calculations at the same theoretical level were performed to ensure that all stable structures had no imaginary frequency. All energies reported in the present work are with zero-point vibrational energy (ZPE) correction.Molecular dynamics model
The accurate determination of the quality of molecular dynamics (MD) simulations hinges on assessing potential energy, which governs atomic interactions. In the MD simulations of S cluster nucleation within a liquid environment, S and water are the primary components. Various models have been proposed to describe the complex behaviors of water, such as TIP3P, TIP4P, SPC/E, and coarse-grained water.31–33 Since our focus is on the interaction between S and water, we chose TIP3P as the potential function to describe water. Regarding sulfur (S), we opt for the Stillinger–Weber potential function proposed by Zhou, which effectively replicates the physical properties of sulfur, particularly cohesive energy, a crucial determinant in the nucleation process.34 The interaction between S and water is described by Leonard Jones (LJ) potential, and the parameters are determined by the Rappe.35 The LJ potential can well reproduce the contact angle of H2O droplet on a S flat plate, as observed in the experiment.36We prepared the solution system by introducing S atoms into the water based on the experimental concentration. To replicate the post-laser irradiation environment, we heated the system to 1000 K for 100 picoseconds under the NVT ensemble. This elevated temperature is used solely to ensure uniform mixing of the S atoms and water, mimicking the rapid diffusion that occurs after laser ablation. Importantly, this process occurs before cooling the system to room temperature, which is more representative of the conditions during nucleation in the experimental setup. After cooling, the system is stabilized at room temperature to simulate the nucleation phase, ensuring consistency with experimental observations. To investigate how heterogeneous nucleation affects the nanoparticle growth process, we inserted a nanoparticle with a 1 nm diameter into the center of the system. To account for fluid flow, we introduced two plates with small surface protrusions that move in opposite directions. Additionally, a paddle was added to the left side of the system and rotated around its center to agitate the solution. The entire simulation had dimensions of 11 nm × 11 nm × 20 nm and consisted of 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 413 water molecules and 1633 S atoms. The simulation was conducted for 800 picoseconds until nanoparticle growth ceased for most nanoparticles.
413 water molecules and 1633 S atoms. The simulation was conducted for 800 picoseconds until nanoparticle growth ceased for most nanoparticles.
Results and discussion
Characterization of the synthesized SQDs
As shown in the TEM images of Fig. 1(a), the SQDs prepared from SDS have restricted the size distribution and high dispersity, with the particle size ranging between 1.5 and 3 nm. The Gaussian distribution curve constructed from the particle size distribution data reveals that the average particle size is centered at 2.15 nm and the full width at half maxima is 0.73 nm, representing a narrow size distribution. Energy-dispersive spectroscopy (EDS) in Fig. 1(a) indicates the existence of the S, C, and Cu elements. The signal of Cu may originate from copper grids, while the signal of C comes from graphene on copper grids. In Fig. 1(b), the lattice spacing was measured to be 0.205 nm, which is the same as the SQDs prepared using other techniques.37,38 XRD analysis was conducted to determine the crystal structure of SQDs, as shown in Fig. 1(c). The diffraction peaks are sharp and numerous, indicating a high degree of crystallinity. The diffraction data matches well with the Joint Committee on Powder Diffraction Standards (JCPDS) cards 00-013-0144, 01-076-0183, and 00-053-1109, confirming the presence of sulfur. A significant peak at 2θ = 44.6° precisely corresponds to the interplanar spacing of 0.205 nm shown in the HRTEM image (Fig. 1(b)), matching the (−442) crystallographic plane. Additional peaks marked by blue dots (JCPDS 01-076-0183) and orange triangles (JCPDS 00-053-1109) further corroborate the monoclinic and rhombohedral crystal phases of the SQDs.In order to investigate the subsurface information of SQDs that are covered by the numerous surface species and surfactants, X-ray photoelectron spectroscopy (XPS) spectrum with ion beam etching was adopted. As shown in Fig. 1(d) for the S2p spectrum of SQDs, the doublet peaks belonging to the sulfur element are barely recognizable before etching. As the etching time increases, doublet peaks of sulfur element are becoming significant and located at 163.6 eV and 164.0 eV, respectively, while the peaks of SO32−/SO42− or S2O32− in sulfate groups (on the surface of sulfur dots, see Fig. S1, ESI†) located at 167.0 eV and 168.4 eV first strengthen and then attenuate. In contrast to that of the sulfur element, the peak intensities of SO42− in SDS located at 169.2 and 170.1 eV are becoming weaker when the etching process continued. The peak locations of these species agree well with those of the previous studies for SQDs.7,8,11,38,39 This indicate that the contents of all the surface species will decrease as the etching process continue. The present XPS spectrum not only confirms the existence of sulfur elements but also demonstrates the component of SQDs that are composed of sulfur dots, subsurface sulfate groups, and SDS coatings.
Fig. 2(a) depicts the ultraviolet (UV) absorption spectrum of the SQDs, which clearly recognize four absorption peaks at 215, 225, 253 and 278 nm, respectively. It is noted that the non-bonded electrons in heteroatoms (S, O) can undergo n → σ* transition, which usually correspond to the range of 150–250 nm.7,40,41 Thus, the absorption peak at 215 nm and 225 nm are mainly contributed by the n → σ* transition of numerous heteroatoms (S, O) on the surface. Most importantly, the absorption peaks at 253 and 278 nm confirm the formation of sulfur particles, which are well consistent with the experimental results of the previous works.40,42–46 The relative weak absorbance may be owing to the surfactants wrapped on the surface of SQDs. As a result, the UV absorption spectrum validate the existence of sulfur particles and S and O heteroatoms on the surface of SQDs. Fig. 2(b) shows the photoluminescence (PL) spectrum of SQDs at an excitation wavelength of 320–340 nm. The fluorescence emission peak is located at 426 nm, which is consistent with those most reported SQDs.7,12,38,47 In addition, the PLQY of the SQDs was 0.519% with quinine sulfate as a reference (Table S2, ESI†). This PLQY is comparable with the PLQY of SQDs prepared from CdSQDs by the bottom-up method.11 The FTIR spectrum of SQDs is presented in Fig. 2(c), covering the wavenumber range of 400–1500 cm−1. A prominent peak at 474 cm−1 is attributed to the S–S stretching vibration, indicating the presence of elemental sulfur in the SQDs.48 The peak labeled around 1400–1500 cm−1 corresponds to the bending vibrations of CH2 groups. Additionally, the peaks around 1000–1300 cm−1 are indicative of the C–C stretching vibrations, which are typical of organic compounds. The absorption band between 600 and 700 cm−1 can be associated with various bending vibrations of C–H bonds, further indicating the presence of organic compounds on the surface of the SQDs. These features confirm the presence of carbon-containing groups, which possibly result from the SDS coating. The Raman spectrum of SQDs (Fig. 2(d)) show a distinct peak at 162 cm−1 that corresponds to the S–S shear vibration, indicating the presence of sulfur in the SQDs.49 However, this peak is absent in the Raman spectrum of SDS. In conclusion, these results demonstrate the formation, composition, absorption, and PL properties of the SQDs.
 | ||
| Fig. 2 (a) UV absorption spectrum of SDS molecules and SQDs, (b) PL spectrum of SQDs excited at 320–340 nm, (c) IR spectrum of SQDs, and (d) Raman spectrum of SDS molecules and SQDs. | ||
Reaction mechanism of SDS under laser irradiation in liquid (LIL)
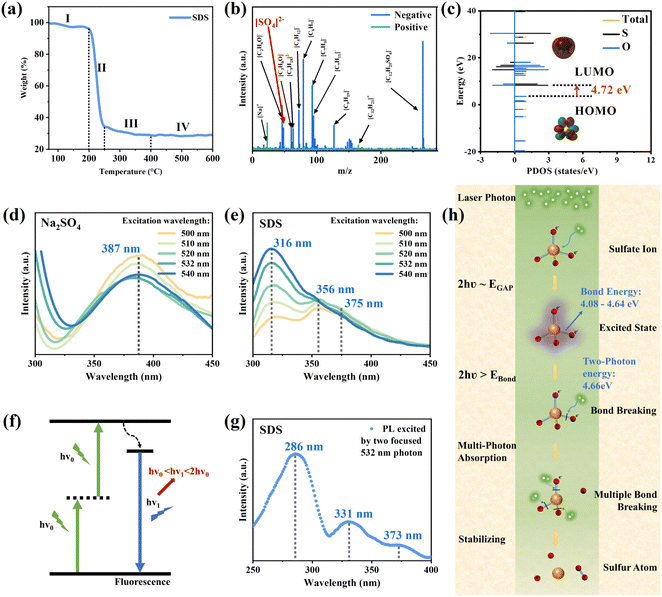
The decomposition behavior of SDS at 80–600 °C can be investigated through the thermogravimetric analysis (TGA) of SDS powder, as shown in Fig. 3(a). As the temperature increases, the weight loss of SDS can be segmented into four stages. Below 200 °C, the weight of the SDS powder decreases slightly, primarily due to the loss of adsorbed water. In the second stage, between 200 °C and 250 °C, SDS undergoes significant decomposition with a sharp decrease in weight. This is due to the breakdown of the molecular structure of SDS, releasing gases and volatile compounds. During this process, carbon chains break and thus result in the release of organic matter and noticeable weight loss. In the third stage, from 250 °C to 400 °C, the weight loss is still observed with a very sluggish rate, indicating that more stable by-products formed during the initial decomposition are degrading. In the final stage, above 400 °C, the weight tends to be stable with the final residue constituting about 29% of the initial sample weight. These residues probably consist of inorganic salts and other stable degradation products, primarily sodium sulfate (Na2SO4) and other inorganic compounds.50 Nanosecond laser exerts both optical and thermal effects, leading to a high-temperature environment surrounding the irradiated material. Therefore, under the influence of a nanosecond laser in liquid, SDS is likely to thermally decompose into sulfates and fragmented carbon chains. To gain insights into the reaction mechanism of SDS under laser irradiation, the LDI-TOF MS was first employed to explore the laser dissociation process of SDS. The ion fragments were directly obtained by irradiating the SDS at a matrix-free baseplate with laser and then detected by the TOF MS. As shown in Fig. 3(b), SDS is mainly dissociated into different ion fragment. The peak of sulfate can be clearly recognized in the specific charge spectrum, which means that the sulfate can be dissociated from the SDS under laser irradiation. Additionally, the sulfate group in SDS is bonded to the alkyl chain via a C–O bond. According to the handbook of chemical bond energies, the bond energy of this C–O bond is approximately 210 kJ mol−1 (2.17 eV).51 A single 532 nm photon (2.33 eV) is sufficient to break the C–O bond between the alkyl chain and the sulfate group. Hence, it can be concluded that the sulfate may be the key species in the transformation from SDS to SQDs under laser irradiation. Here, the density functional theory (DFT) studies based on all-electron basis set was adopted to resolve the electronic structure of sulfate. As plotted in Fig. 3(c), the partial density of state (PDOS) reveals that the energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of sulfate is 4.72 eV, which is much larger than the single photon energy of a 532 nm laser beam (2.33 eV). This means that the sulfate cannot undergo photolysis by adsorbing a single photon of 532 nm laser beam. However, under the condition of high-intensity laser beam, as the laser intensity increases, the probability that a molecule absorbs more than one photon will increase rapidly. When the initial excitation reaches a real intermediate state, subsequent excitation becomes more efficient and may directly result in ionization, providing that the photon energy is sufficient.52 Therefore, it is possible that the sulfate absorbs two photons to reach the excited state and undergoes photolysis under the laser irradiation.In order to verify whether the sulfate absorbs two photons under the irradiation of a 532 nm laser, two different methods were employed to characterize the fluorescence of the sulfate-containing solution. One is the fluorescence spectrophotometer (FS), and another one is two-photon confocal microscopy that enable two-photon excitation. The fluorescence spectra of 0.1 M Na2SO4 and 0.1 M SDS solution were characterized using FS with excitation wavelengths ranging from 500 to 540 nm, as shown in Fig. 3(d) and (e), respectively. In Fig. 3(d), the PL phenomenon for Na2SO4 is observed in the range of 300–450 nm. The fluorescence intensity of the PL peak at 387 nm decreases as the excitation wavelength increases, which indicates a reduction in photon absorption efficiency at longer excitation wavelengths for sodium sulfate. Fig. 3(e) reveals the PL emission in the range of 300–450 nm for SDS, in which three distinct fluorescence peaks at ∼316 nm, 356 nm, and 375 nm are clearly found. Notably, the intensity of the first fluorescence peak at 316 nm increases with the excitation wavelength, suggesting a correlation between the excitation wavelength and the photon absorption efficiency for SDS. Interestingly, the emission wavelengths (300–450 nm) are shorter than the excitation wavelengths (500–540 nm), which indicate that the molecules absorb lower-energy photons (2.3–2.48 eV) and subsequently emit higher-energy photons (2.76–4.13 eV). Thus, it can be inferred that Na2SO4 and SDS reach an electronically excited state through two-photon absorption and subsequently undergo radiative decay by emitting fluorescence, as shown in Fig. 3(f). Meanwhile, the PL spectrum from two-photon confocal microscopy with an excitation wavelength of 532 nm, shown in Fig. 3(g), demonstrates three fluorescence peaks at 286 nm, 331 nm, and 373 nm, respectively. These peaks are consistent with the SDS fluorescence peaks under the excitation wavelength of 230 nm measured by Zhu et al., validating the experimental characterization of photoluminescence properties of SDS after two-photon absorption.53 This indicates that similar excitation effects by a single photon of 230 nm can be achieved through the confocal excitation of two photons of 532 nm. It should be noted that the saturated alkanes contain only σ-bonded electrons and thus only undergo σ → σ* transition whose excitation wavelength is less than 200 nm.54 Therefore, the sulfate is the luminescent group of Na2SO4 and SDS in fluorescence characterization. Based on the aforementioned results, it can be concluded that the sulfate can absorb two photons and reach the excited state under the irradiation of high-intensity 532 nm laser. This excitation is critical because it imparts additional energy within the molecule, which can destabilize bonds and potentially lead to bond breakage.
In an aqueous environment, the sulfur atom in the sulfate ion is coordinated by four oxygen atoms, forming four equivalent sulfur–oxygen bonds.55 These bonds are partially double bond due to resonance, whose bond energies lie between those of S–O bonds (384.9 ± 8.4 kJ mol−1 or 3.90–4.08 eV) and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (460.2 ± 12.6 kJ mol−1 or 4.64–4.90 eV).51,56,57 During photolysis, photon energies that equal or exceed bond energy can cause bond breakage.58 For instance, the energy of two 532 nm photons (4.66 eV) is sufficient to break the equivalent S
O bonds (460.2 ± 12.6 kJ mol−1 or 4.64–4.90 eV).51,56,57 During photolysis, photon energies that equal or exceed bond energy can cause bond breakage.58 For instance, the energy of two 532 nm photons (4.66 eV) is sufficient to break the equivalent S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in the sulfate ion. In high-intensity laser beams, sulfate ions can absorb multiple photons simultaneously and achieve the simultaneous breakage of multiple S
O bond in the sulfate ion. In high-intensity laser beams, sulfate ions can absorb multiple photons simultaneously and achieve the simultaneous breakage of multiple S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. Additionally, the rapid quenching effect of water prevents bond reformation or undesirable side reactions, ensuring the stability of sulfur atoms. The entire photolysis process is illustrated in Fig. 3(h). Under high-energy 532 nm laser irradiation, sulfate ions absorb two photons to reach an excited state followed by the breakage of the equivalent S
O bonds. Additionally, the rapid quenching effect of water prevents bond reformation or undesirable side reactions, ensuring the stability of sulfur atoms. The entire photolysis process is illustrated in Fig. 3(h). Under high-energy 532 nm laser irradiation, sulfate ions absorb two photons to reach an excited state followed by the breakage of the equivalent S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. The high photon density (100 mJ laser) means the great possibility of multi-photon absorption, suggesting the breakage of multiple S
O bond. The high photon density (100 mJ laser) means the great possibility of multi-photon absorption, suggesting the breakage of multiple S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. Ultimately, when all of the four sulfur–oxygen bonds are broken, the produced sulfur atoms are rapidly quenched in water and then self-assembled to be SQDs.
O bonds. Ultimately, when all of the four sulfur–oxygen bonds are broken, the produced sulfur atoms are rapidly quenched in water and then self-assembled to be SQDs.
Atomistic nucleation mechanism of SQDs
In the previous section, we successfully elucidated the reaction mechanism of SDS. However, the nucleation process of single S atoms forming SQDs remains undisclosed. To address this, we conducted a molecular dynamics simulation to investigate the nucleation mechanisms of SQDs, as depicted in Fig. 4(a)–(d). As an illustrative example, we focused on a nanoparticle with a 2 nm diameter to demonstrate how individual S atoms, which are colored in red, move within the liquid environment. As observed in Fig. 4(a), the atoms initially exhibit a homogeneous distribution. Subsequently, they undergo homogeneous nucleation, as evident in Fig. 4(b). These small clusters gradually merge, driven by the motion of the liquid induced by stirring, ultimately resulting in the growth of the nanoparticle. The nanoparticle absorbs smaller clusters and continues to grow until reaching a size sufficient for solidification.While Fig. 4(a)–(d) provide a panoramic view of the nucleation process at the atomic scale, it offers only a glimpse of a few moments. To provide a more comprehensive perspective on how atoms move within the solution and how they undergo growth via various nucleation mechanisms, we turn to Fig. 4(e). In Fig. 4(e), by examining the trajectories of sampled atoms, we observe the nucleation events occurring during the rotation process. These events involve the coalescence of smaller clusters, leading to the formation of larger nanoparticles. Due to the small size of these clusters, their melting temperature is so low that they remain in a liquid state even at room temperature before 400 picoseconds, as demonstrated in Fig. 4(f). However, since the temperature is not high, and the nanoparticles are influenced by water molecules, the resulting nanoparticles do not exhibit a perfect spherical shape. This observation is consistent with experimental results, as shown in Fig. 1(b). It is intriguing to observe that the nanoparticle ceases to grow after merging. This phenomenon is attributed to the sensitivity of the nanoparticle's melting temperature to its size. As the nanoparticle undergoes growth, it also solidifies in the process. Consequently, nanoclusters can merge and continue to grow until they reach a size at which solidification occurs. Once the solidification process commences, further growth halts, even if the nanoparticle comes into contact with other atoms or nanoclusters. This is why the nanoparticle exhibits sustained growth during the simulations, as indicated by the blue line in Fig. 4(g), while larger nanoparticles stop growing when they reach a size sufficient for solidification.
The above-mentioned experimental evidence shows that sulfate plays an essential role in the photolysis under laser irradiation. Not only did the solution matter, but the laser wavelength was also key factor in the entire process since it is closely associated with the energy level transition in the reaction. If the 355 or 1064 nm wavelength is chosen to replace 532 nm, no SQDs and PL peak can be found. Furthermore, irradiation experiments with other inorganic substances containing the sulfate as a precursor were executed to validate that sulfate can be photolyzed to generate SQDs under the laser irradiation. As expected, two SQDs without any surfactant coating were obtained by irradiating aqueous solutions of H2SO4 and Na2SO4 using a 532 nm laser beam, respectively. The TEM image and fluorescence spectrum plotted in Fig. S6 and S7 (ESI†) reveal that these surfactant-free SQDs have wider size distribution, blue-shifted PL excitation wavelengths and red-shifted emission wavelengths (excited at 250 nm and 260 nm, and emitted at around 450 and 460 nm) compared with the SQDs from SDS. Therefore, a reaction mechanism of SDS under the laser irradiation can be proposed, as shown in Fig. 5. Initially, SDS is decomposed into sulfates in the aqueous solution. Then, the sulfate ion adsorbs two photons at a wavelength of 532 nm and thus undergoes two-photon transitions under high-intensity irradiation. The excitation of sulfate ions leads to the breakage of the four equivalent S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and thus the generation of dissociated sulfur elements. Lastly, the dissociated sulfur elements are self-assembled to be SQDs, which are further coated with SDS molecules.
O bonds and thus the generation of dissociated sulfur elements. Lastly, the dissociated sulfur elements are self-assembled to be SQDs, which are further coated with SDS molecules.
 | ||
| Fig. 5 Schematic illustration of SQDs generated through photolysis of SDS molecules under pulsed laser irradiation. | ||
Conclusions
In this work, we proposed a one-step strategy to prepare SQDs via laser irradiation of an SDS solution within 30 min, which is a facile synthesis method with less time consumption. The synthesized small-sized SQDs exhibit excellent physical properties such as narrow size distribution and superb aqueous dispersibility. Further fluorescence characterization combined with DFT calculations has revealed the reaction mechanism of SDS under laser irradiation and validated that two-photon dissociation of sulfates play a critical role in the formation of SQDs from SDS. In addition, we found that stirring is crucial for the nucleation of SQDs based on molecular dynamics. This study presents a universally applicable strategy for synthesizing SQDs using sulfate-containing solutions. Additionally, it pioneers the discovery that sulfates can be reduced to elemental sulfur under visible light, marking a significant first in the field.Data availability
All relevant data are within the manuscript and its additional files.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC, 51971211, 52071313), Chinese Academy of Sciences (No. YZJJ202102), the HFIPS Director's Fund (No. GGZX-GTCX-2023-07), the Youth Innovation Promotion Association of CAS (2020442), the Plan for Anhui Major Provincial Science & Technology Project (Grants 202103a05020015 and 2021d05050006), Guangdong HUST Industrial Technology Research Institute, Guangdong Provincial Key Laboratory of Manufacturing Equipment Digitization (2020B1212060014) and the Natural Science Foundation of Anhui Province (No. 2208085MH256). The computation was supported by Hefei Advanced Computing Center. The authors would like to thank Shiyanjia Lab (https://www.shiyanjia.com) for the XPS measurement.References
- F. P. García de Arquer, D. V. Talapin, V. I. Klimov, Y. Arakawa, M. Bayer and E. H. Sargent, Semiconductor Quantum Dots: Technological Progress and Future Challenges, Science, 2021, 373, eaaz8541 CrossRef.
- B. Gidwani, V. Sahu, S. S. Shukla, R. Pandey, V. Joshi, V. K. Jain and A. Vyas, Quantum Dots: Prospectives, Toxicity, Advances and Applications, J. Drug Delivery Sci. Technol., 2021, 61, 102308 CrossRef CAS.
- D. Sumanth Kumar, B. Jai Kumar and H. M. Mahesh, Quantum Nanostructures (QDs): An Overview, in Synthesis of Inorganic Nanomaterials, ed. S. Mohan Bhagyaraj, O. S. Oluwafemi, N. Kalarikkal and S. Thomas, Woodhead Publishing, 2018, ch. 3, pp. 59–88 Search PubMed.
- W. A. A. Mohamed, H. A. El-Gawad, S. Mekkey, H. Galal, H. Handal, H. Mousa and A. Labib, Quantum dots synthetization and future prospect applications, Nanotechnol. Rev., 2021, 10, 1926–1940 CrossRef CAS.
- S. Savaedi, E. Soheyli, G. Zheng, Q. Lou, R. Sahraei and C. Shan, Excitation-independent deep-blue emitting carbon dots with 62% emission quantum efficiency and monoexponential decay profile for high-resolution fingerprint identification, Nanotechnology, 2022, 33, 445601 CrossRef CAS.
- X. Li, M. Zheng, H. Wang, Y. Meng, D. Wang, L. Liu, Q. Zeng, X. Xu, D. Zhou and H. Sun, Synthesis of carbon dots with strong luminescence in both dispersed and aggregated states by tailoring sulfur doping, J. Colloid Interface Sci., 2022, 609, 54–64 CrossRef CAS.
- L. Shen, H. Wang, S. Liu, Z. Bai, S. Zhang, X. Zhang and C. Zhang, Assembling of Sulfur Quantum Dots in Fission of Sublimed Sulfur, J. Am. Chem. Soc., 2018, 140, 7878–7884 CrossRef CAS PubMed.
- Y. Song, J. Tan, G. Wang, P. Gao, J. Lei and L. Zhou, Oxygen accelerated scalable synthesis of highly fluorescent sulfur quantum dots, Chem. Sci., 2020, 11, 772–777 RSC.
- A. Pal, F. Arshad and M. P. Sk, Emergence of sulfur quantum dots: Unfolding their synthesis, properties, and applications, Adv. Colloid Interface Sci., 2020, 285, 102274 CrossRef CAS PubMed.
- A. Huang, X. Yang, T. Xia, D. He, R. Zhang, Z. Li, S. Yang, Y. Liu and X. Wen, A fluorescence probe of sulfur quantum dots for sensitive detection of copper ions in Paris polyphylla var. yunnanensis, Microchem. J., 2022, 179, 107639 CrossRef CAS.
- S. Li, D. Chen, F. Zheng, H. Zhou, S. Jiang and Y. Wu, Water-Soluble and Lowly Toxic Sulphur Quantum Dots, Adv. Funct. Mater., 2014, 7133–7138 CrossRef CAS.
- H. Wang, Z. Wang, Y. Xiong, S. V. Kershaw, T. Li, Y. Wang, Y. Zhai and A. L. Rogach, Hydrogen Peroxide Assisted Synthesis of Highly Luminescent Sulfur Quantum Dots, Angew. Chem., Int. Ed., 2019, 58, 7040–7044 CrossRef CAS.
- L. Fu, A. Wang, K. Xie, J. Zhu, F. Chen, H. Wang, H. Zhang, W. Su, Z. Wang, C. Zhou and S. Ruan, Electrochemical detection of silver ions by using sulfur quantum dots modified gold electrode, Sens. Actuators, B, 2020, 304, 127390 CrossRef CAS.
- Z. Bai, L. Shen, J. Wei, Y. Li, A. Abbas, Y. Li, M. Qu, D. Zhang and C. Zhang, Layered Sulfur Nanosheets Prepared by Assembly of Sulfur Quantum Dots: Implications for Wide Optical Absorption and Multiwavelength Photoluminescence, ACS Appl. Nano Mater., 2020, 3, 10749–10756 CrossRef CAS.
- F. Arshad and M. P. Sk, Luminescent Sulfur Quantum Dots for Colorimetric Discrimination of Multiple Metal Ions, ACS Appl. Nano Mater., 2020, 3, 3044–3049 CrossRef CAS.
- D. Zhang, B. Gökce and S. Barcikowski, Synthesis and Processing of Colloids: Fundamentals and Applications, Chem. Rev., 2017, 117, 3990–4103 CrossRef CAS PubMed.
- Z. Zhu, S. Wang, Y. Chang, D. Yu and Y. Jiang, Direct photodissociation of toluene molecules to photoluminescent carbon dots under pulsed laser irradiation, Carbon, 2016, 105, 416–423 CrossRef CAS.
- B. Pan, J. Xiao, J. Li, P. Liu, C. Wang and G. Yang, Carbyne with finite length: The one-dimensional sp carbon, Sci. Adv., 2015, 1, e1500857 CrossRef PubMed.
- W. Arpavate, K. Roongraung and S. Chuangchote, Photochemical solid-state reactions, in Green Sustainable Process for Chemical and Environmental Engineering and Science, ed. Inamuddin, R. Boddula, A. M. Asiri and M. M. Rahman, Elsevier, 2021, ch. 10, pp. 189–203 Search PubMed.
- Y. Fujita, S. Fujii and T. Iuchi, Ultraviolet spectra of II–VI organometallic compounds and their application to in situ measurements of the photolysis in a metalorganic chemical vapor deposition reactor, J. Vac. Sci. Technol., A, 1989, 7, 276–280 CrossRef CAS.
- F. S. Guziec and L. J. Sanfilippo, Synthetically useful extrusion reactions of organic sulfur, selenium and tellurium compounds, Tetrahedron, 1988, 44, 6241–6285 CrossRef CAS.
- J. Pola and A. Ouchi, Laser Photolysis and Thermolysis of Organic Selenides and Tellurides for Chemical Gas-phase Deposition of Nanostructured Materials, Molecules, 2009, 14, 1111–1125 CrossRef CAS PubMed.
- J. Steadman, S. K. Cole and T. Baer, Visible and ultraviolet resonance enhance multiphoton ionization photoelectron spectroscopy of H2S in the one-photon wavelength region 143–158 nm, J. Chem. Phys., 1988, 89, 5498–5506 CrossRef CAS.
- J. Steadman and T. Baer, The production and spectroscopy of excited sulfur atoms from the two-photon dissociation of H2S, J. Chem. Phys., 1988, 89, 5507–5513 CrossRef CAS.
- J. R. Appling, M. R. Harbol, R. A. Edgington and A. C. Goren, Photoelectron spectroscopy of sulfur atoms produced via two-photon dissociation of sulfur dioxide, J. Chem. Phys., 1992, 97, 4041–4049 CrossRef CAS.
- L. Zhang, Z. Wang, J. Li, F. Wang, S. Liu, S. Yu and X. Ma, Studies on the photodissociation and symmetry of SO2+ (
![[D with combining tilde]](https://www.rsc.org/images/entities/char_0044_0303.gif) ), J. Chem. Phys., 2003, 118, 9185–9191 CrossRef CAS.
), J. Chem. Phys., 2003, 118, 9185–9191 CrossRef CAS. - M. J. Frisch, G. W. Trucks, et al., Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford, CT, 2010 Search PubMed.
- A. D. Becke, Density-functional thermochemistry. III. The role of exact exchange, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- P. C. Hariharan and J. A. Pople, The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS.
- P. Mark and L. Nilsson, Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K, J. Phys. Chem. A, 2001, 105, 9954–9960 CrossRef CAS.
- D. Paschek, R. Day and A. E. García, Influence of water–protein hydrogen bonding on the stability of Trp-cage miniprotein. A comparison between the TIP3P and TIP4P-Ew water models, Phys. Chem. Chem. Phys., 2011, 13, 19840–19847 RSC.
- H. Huang and L. V. Zhigilei, Computational study of laser fragmentation in liquid: Phase explosion, inverse Leidenfrost effect at the nanoscale, and evaporation in a nanobubble, Sci. China: Phys., Mech. Astron., 2022, 65, 274206 CAS.
- X. W. Zhou, D. K. Ward, J. E. Martin, F. B. Van Swol, J. L. Cruz-Campa and D. Zubia, Stillinger–Weber potential for the II-VI elements Zn-Cd-Hg-S-Se-Te, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 085309 CrossRef.
- A. K. Rappe, C. J. Casewit, K. S. Colwell, W. A. I. Goddard and W. M. Skiff, UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations, J. Am. Chem. Soc., 1992, 114, 10024–10035 CrossRef CAS.
- A. Skłodowska, M. Woźniak and R. Matlakowska, The method of contact angle measurements and estimation of work of adhesion in bioleaching of metals, Biol. Proced. Online, 1999, 1, 114–121 CrossRef.
- S. K. Tammina, R. Priyadarshi and J.-W. Rhim, Dual functions of metal ion detection and antibacterial activity of sulfur quantum dots, New J. Chem., 2023, 47, 7733–7745 RSC.
- F. Arshad, M. P. Sk, S. K. Maurya and H. R. Siddique, Mechanochemical Synthesis of Sulfur Quantum Dots for Cellular Imaging, ACS Appl. Nano Mater., 2021, 4, 3339–3344 CrossRef CAS.
- Z. Wang, C. Zhang, H. Wang, Y. Xiong, X. Yang, Y. Shi and A. L. Rogach, Two-Step Oxidation Synthesis of Sulfur with a Red Aggregation-Induced Emission, Angew. Chem., Int. Ed., 2020, 59, 9997–10002 CrossRef CAS PubMed.
- X. Xie, L. Li, P. Zheng, W. Zheng, Y. Bai, T. Cheng and J. Liu, Facile synthesis, spectral properties and formation mechanism of sulfur nanorods in PEG-200, Mater. Res. Bull., 2012, 47, 3665–3669 CrossRef CAS.
- G. Qiao, L. Liu, X. Hao, J. Zheng, W. Liu, J. Gao, C. C. Zhang and Q. Wang, Signal transduction from small particles: Sulfur nanodots featuring mercury sensing, cell entry mechanism and in vitro tracking performance, Chem. Eng. J., 2020, 382, 122907 CrossRef CAS.
- P. Paralikar and M. Rai, Bio-inspired synthesis of sulphur nanoparticles using leaf extract of four medicinal plants with special reference to their antibacterial activity, IET Nanobiotechnol., 2018, 12, 25–31 CrossRef.
- Z. Huang, Y. Gao, Z. Huang, D. Chen, J. Sun and L. Zhou, Sulfur quantum dots: A novel fluorescent probe for sensitive and selective detection of Fe3+ and phytic acid, Microchem. J., 2021, 170, 106656 CrossRef CAS.
- S. Saedi, M. Shokri and J.-W. Rhim, Antimicrobial activity of sulfur nanoparticles: Effect of preparation methods, Arabian J. Chem., 2020, 13, 6580–6588 CrossRef CAS.
- J. Häcker, D. H. Nguyen, T. Rommel, Z. Zhao-Karger, N. Wagner and K. A. Friedrich, Operando UV/vis Spectroscopy Providing Insights into the Sulfur and Polysulfide Dissolution in Magnesium–Sulfur Batteries, ACS Energy Lett., 2022, 7, 1–9 CrossRef.
- S. Shankar, R. Pangeni, J. W. Park and J.-W. Rhim, Preparation of sulfur nanoparticles and their antibacterial activity and cytotoxic effect, Mater. Sci. Eng., C, 2018, 92, 508–517 CrossRef CAS PubMed.
- S. Kadian, N. Chaulagain, N. N. Joshi, K. M. Alam, K. Cui, K. Shankar, G. Manik and R. J. Narayan, Probe sonication-assisted rapid synthesis of highly fluorescent sulfur quantum dots, Nanotechnology, 2023, 34, 30LT01 CrossRef CAS.
- H.-C. Liu, J.-L. Xia, Z.-Y. Nie, A.-A. Peng, C.-Y. Ma, L. Zheng and Y.-D. Zhao, Comparative study of sulfur utilization and speciation transformation of two elemental sulfur species by thermoacidophilic Archaea Acidianus manzaensis YN-25, Process Biochem., 2013, 48, 1855–1860 CrossRef CAS.
- M. Hagen, P. Schiffels, M. Hammer, S. Dörfler, J. Tübke, M. J. Hoffmann, H. Althues and S. Kaskel, In-Situ Raman Investigation of Polysulfide Formation in Li-S Cells, J. Electrochem. Soc., 2013, 160, A1205 CrossRef CAS.
- V. L. Kett and D. M. Price, Thermogravimetry, in Principles of Thermal Analysis and Calorimetry, ed. S. Gaisford, V. Kett and P. Haines, The Royal Society of Chemistry, 2nd edn, 2016, pp. 18–46 Search PubMed.
- Y.-R. Luo, Comprehensive Handbook of Chemical Bond Energies, CRC Press, 1st edn, 2007 Search PubMed.
- H. H. Telle, A. González Ureña and R. J. Donovan, Laser chemistry: spectroscopy, dynamics and applications, John Wiley & Sons, Chichester, West Sussex, England; Hoboken, NJ, 2007 Search PubMed.
- Y. Zhu, K. Zhong, Y. Qiang, Y. Liu and C. Cai, Study on the Fluorescence Spectra of the Solution of the Lauryl Sodium Sulfate, J. China Univ. Min. Technol., 2006, 35, 799–802 CAS.
- J. X. Mao, P. Kroll and K. A. Schug, Vacuum ultraviolet absorbance of alkanes: an experimental and theoretical investigation, Struct. Chem., 2019, 30, 2217–2224 CrossRef CAS.
- Q. Ma, G. S. Ellis, A. Amrani, T. Zhang and Y. Tang, Theoretical study on the reactivity of sulfate species with hydrocarbons, Geochim. Cosmochim. Acta, 2008, 72, 4565–4576 CrossRef CAS.
- M. Fugel, L. A. Malaspina, R. Pal, S. P. Thomas, M. W. Shi, M. A. Spackman, K. Sugimoto and S. Grabowsky, Revisiting a Historical Concept by Using Quantum Crystallography: Are Phosphate, Sulfate and Perchlorate Anions Hypervalent?, Chem. – Eur. J., 2019, 25, 6523–6532 CrossRef CAS.
- D. Stalke, in The Chemical Bond I: 100 Years Old and Getting Stronger, ed. D. M. P. Mingos, Springer International Publishing, Cham, 2016, pp. 57–88 Search PubMed.
- H. Kuhn, H.-D. Försterling and D. H. Waldeck, Principles of physical chemistry, John Wiley, Hoboken, N.J, 2nd edn, 2009 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qm00733f |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2025 |