 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lanthanide contraction-driven modulation of photoswitchable macrocyclic complexes reveals unprecedented glass-induced re-isomerization and luminescence thermometry†
Dominika
Prętka
 *a,
Dawid
Marcinkowski
*a,
Dawid
Marcinkowski
 a,
Nahir
Vadra
a,
Nahir
Vadra
 a,
Przemysław
Woźny
a,
Przemysław
Woźny
 a,
Marcin
Runowski
a,
Marcin
Runowski
 a,
Maciej
Kubicki
a,
Maciej
Kubicki
 a,
Violetta
Patroniak
a,
Violetta
Patroniak
 a,
Giuseppe
Consiglio
a,
Giuseppe
Consiglio
 b,
Giuseppe
Forte
b,
Giuseppe
Forte
 c and
Adam
Gorczyński
c and
Adam
Gorczyński
 *a
*a
aFaculty of Chemistry, Adam Mickiewicz University in Poznań, Uniwersytetu Poznańskiego 8, 61-614 Poznań, Poland. E-mail: adam.gorczynski@amu.edu.pl
bDepartment of Chemical Science University of Catania, Via S. Sofia 64, 95125, Italy
cDepartment of Drug Science and Health University of Catania, Via S. Sofia 64, 95125, Italy
First published on 10th September 2025
Abstract
Designing light-responsive supramolecular architectures with lanthanide ions offers a promising route towards multifunctional materials with tunable photophysical properties. Here, we report a systematic investigation across the lanthanide series of macrocyclic complexes incorporating azobenzene-functionalized diaza-crown ether ligands. We show that subtle changes in the ionic radius across the Ln3+ series dictate conformational preferences and modulate trans-to-cis photoisomerization efficiency under UV and visible light. Surprisingly, we uncover that the reverse cis-to-trans isomerization, which is here unresponsive to thermal or photonic stimuli, is uniquely triggered upon contact with glass surfaces, revealing a previously overlooked route for controlling molecular photoswitching. Additionally, selected complexes display efficient visible and near-infrared emission leveraged for robust luminescent thermometric behaviour in the solid state, with tunable sensitivity linked to the lanthanide ions. These findings advance the field of light-driven supramolecular materials and demonstrate how careful molecular-level design of lanthanide–azobenzene assemblies enables control over photoswitching, luminescence and thermal sensing properties, highlighting glass-mediated re-isomerization as a novel phenomenon with implications for future photoresponsive materials.
1. Introduction
Light is a fundamental source of energy in photosynthesis, and harnessing light–matter interactions through artificial molecular systems opens pathways towards energy conversion and information processing at the molecular level. The design and synthesis of novel light-responsive supramolecular architectures are therefore of great importance for the development of smart, stimuli-responsive functional materials.1,2 Advancing our understanding of these interactions through the engineering of new materials is a key scientific direction, with potential applications including electronic and optical devices,3,4 data storage,5–7 biological imaging, medical diagnostics, targeted drug delivery,8,9 sensing,10,11 encryption,12 anti-counterfeiting technologies or multicolour QR codes.1,13,14There is growing interest in the development of macrocyclic systems functionalized with photoswitchable units, as light offers a non-invasive and highly tunable stimulus for dynamic control over molecular conformation and properties in supramolecular assemblies.15,16 Among the most widely used photoresponsive motifs is azobenzene, which undergoes reversible photoisomerization between two different geometric isomers – a linear trans-form and a bent cis-form – enabling the modulation of supramolecular photophysical behaviour.13,17,18 Many light-responsive macrocycles incorporating azobenzene have been reported, though predominantly in metal-free systems.19–25 When metal ions are introduced, coordination can either quench the photoswitching activity26 or, conversely, enhance the selectivity and accelerate and improve control over the photoswitching processes.27 This demonstrates a crucial role of matching energy levels and photochromic units to achieve effective and tunable photoswitchable behaviour.
Recent studies have highlighted the impact of transition metal ions in expanding the functionalities of light-responsive systems, enabling properties that are otherwise unattainable in purely organic frameworks.28,29 In particular, integrating luminescent lanthanide ions with light-responsive molecules has emerged as a promising strategy to enhance both emission and photoswitching behaviour.26,30–32 Switchable lanthanide complexes align well with the concept of multi-stimuli-responsive architectures, exhibiting sensitivity to light but also to chemical, thermal, electrical or mechanical inputs. This multifunctionality makes such materials increasingly desirable for applications in chemical sensing, cellular imaging, security tagging, and, most recently, as advanced luminescent optical thermometers.12,33–36 In d-block systems, temperature shifts the balance between ligand- and metal-centered excited states and their nonradiative decay, with ratiometric intensity or lifetime being the thermometric readout (e.g. Re2-L anions bridged with diamine-organic linkers37 or [Bz2NH2]2[Mn(OC6F5)4] ionic assembly38). In f-block complexes, the response often follows redistribution among crystal-field mJ sublevels and thermally assisted antenna-4f energy transfer, enabling self-calibrated intensity ratios, lifetimes or band-shape changes. Examples include a self-calibrated Dy3+ thermometer that also shows single-ion magnet (SIM) behaviour39 or dinuclear single-molecule magnet (SMM) Nd3+ platforms linking ligation to thermometric performance.40 Independent magneto-optical readouts can further improve thermal sensitivity.41 Macrocyclic ligand designs further highlight multifunctionality: a Y3+–diluted Dy3+ complex acts as a bifunctional SIM and luminescent thermometer, with both functions active below its blocking temperature,42 and our Nd3+ macrocyclic SIM operates as a temperature-independent manometer and pressure-independent thermometer.43 These precedents motivate the photoswitchable Nd3+/Yb3+ macrocyclic complexes studied here and frame our analysis of how lanthanide contraction shapes their near-infrared thermometric response.
A number of azobenzene-functionalized lanthanide complexes have been reported to date, and their photoisomerization behaviour and structure–function relationships remain under active investigation.44–53 However, comprehensive studies encompassing a broad range of lanthanide ions with organic ligands are still rare, limiting a deeper understanding of lanthanide contraction effects and their influence on structure and functionality in photoresponsive systems. Although lanthanide–macrocyclic complexes have been widely studied in fields such as biomedical imaging,54,55 antenna-based emission systems,55,56 and molecular nanomagnets (SMMs/SIMs),55,57 the integration of photoswitchable units into such systems remains extremely limited. Lariat-type macrocycles bearing azobenzene moieties have primarily been developed for s-block metal ion coordination or membrane-mimetic applications,24 while analogous systems with lanthanides are exceedingly rare.
In this work, we present a systematic study on a diaza-crown ether macrocycle functionalized with azobenzene units, L-AzoH2, forming a distinct class of lanthanide complexes designed to investigate the structural and photophysical effects of lanthanide contraction. Despite the common quenching of azobenzene switching by metal coordination, our system retains efficient light-induced isomerization while exhibiting high stability of the cis-form—reverting to the trans-isomer unusually fast upon contact with the glass surface. Finally, representative complexes from both the syn- and anti-conformational families were evaluated as luminescent thermometers, establishing a novel correlation among macrocyclic geometry, switching behaviour and near-infrared emissive sensing across the lanthanide series (Fig. 1).
 | ||
| Fig. 1 An overview of key concepts covered in this work, which demonstrate the photoactive Ln-macrocyclic complexes in the context of lanthanide contraction. | ||
2. Results and discussion
2.1. Choice of the ligand, synthesis and characterization
The diaza-crown ether macrocyclic ligand was chosen because (1) macrocycles with N2O4 coordination cavities form stable lanthanide complexes and (2) allow symmetric functionalization with different groups, which should tune the photoresponsive properties. A pendant-arm azobenzene macrocyclic ligand named L-AzoH2 was obtained through the Mannich reaction, based on a previously reported procedure58 with a slight modification of the purification method (section 1.3 in the SI). The purity and homogeneity were confirmed using Fourier transform infrared spectroscopy (FT-IR), electrospray mass spectrometry (ESI-MS), nuclear magnetic resonance spectroscopy (NMR) and thermogravimetric analysis (TGA) (Fig. S1–S7).2.2. Design, synthesis and characterization of complexes
To understand the influence of interactions between the metal centers and the ligand molecule for the development of stimuli responsive azobenzene systems, we focused on the synthesis and investigation of compounds with lanthanide ions, which are interesting due to their emissive properties. To better understand the role of deprotonation of the ligand for photoresponsivity, a neutral CuL-Azo system was also obtained. Simple complexes with a sodium ion, [NaL-AzoH2]CF3SO3, and an uncoordinated ligand L-AzoH2 molecule were also used as references. The [NaL-AzoH2]CF3SO3 complex was synthesized in an acetonitrile solution by the complexation reaction of the NaCF3SO3 salt with the L-AzoH2 ligand. Cu2+ and Ln3+ complexes were synthesized in a similar manner, in an acetonitrile (CuL-Azo) or acetonitrile/methanol (LnL-AzoCF3SO3) mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) by the complexation reaction of the corresponding triflate salts with the L-AzoH2 ligand in the presence of triethylamine (Et3N), used as a deprotonating agent (Fig. 2). A detailed description of the synthesis, isolation, and spectroscopic and solid-state characterization of the complexes can be found in the SI (sections 1.4 and 1.5). The FT-IR spectra (Fig. S8–S12) of the lanthanide complexes from La3+ to Lu3+ are almost identical, which confirmed high isostructurality in the solid state. This is also consistent with the mass spectra of these assemblies, as the peaks observed in the positive mode of the ESI-MS spectra indicate the formation of mononuclear complexes containing one L-Azo ligand molecule with two deprotonated OH groups from the azobenzene pendant-arms of the macrocycle for all complexes (Fig. S13–S24).
v) by the complexation reaction of the corresponding triflate salts with the L-AzoH2 ligand in the presence of triethylamine (Et3N), used as a deprotonating agent (Fig. 2). A detailed description of the synthesis, isolation, and spectroscopic and solid-state characterization of the complexes can be found in the SI (sections 1.4 and 1.5). The FT-IR spectra (Fig. S8–S12) of the lanthanide complexes from La3+ to Lu3+ are almost identical, which confirmed high isostructurality in the solid state. This is also consistent with the mass spectra of these assemblies, as the peaks observed in the positive mode of the ESI-MS spectra indicate the formation of mononuclear complexes containing one L-Azo ligand molecule with two deprotonated OH groups from the azobenzene pendant-arms of the macrocycle for all complexes (Fig. S13–S24).
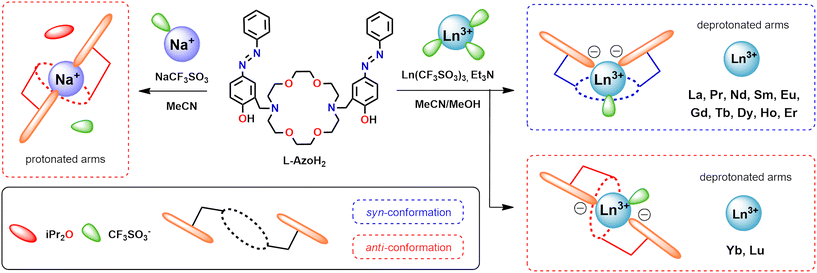 | ||
| Fig. 2 Scheme for the synthesis of macrocyclic complexes with included conformations of the L-AzoH2 ligand scaffold. | ||
Fig. 3 shows the comparison of the crystal structure of the free L-AzoH2 ligand molecule (a), its complex with a sodium ion with a non-deprotonated ligand (b) and a complex with Cu2+ with a deprotonated ligand form (c); the molecular structure of the lanthanide complex in the syn-conformation (d) and the packing diagram (e) of the LaL-AzoCF3SO3 complex. The ligand molecule is Ci-symmetrical in the crystal structure, lying across the center of inversion in the space group P21/c (Fig. 3a). The conformation of the macrocycle changes significantly upon complexation (Fig. 3b–d), and the change directs all N and O atoms towards the center of the complex. For the sodium complexes [NaL-AzoH2]CF3SO3 (A and B), which are observed in both forms of different space group symmetries, CF3SO3− acts as a counterion and is not coordinated to the central metal ion. The coordination number of the sodium cation is in both cases 8 (N2O6), and the crystal lattices contain space which is filled by more (B) or less (A) ordered solvent molecules (Fig. 3b and Fig. S25). Fig. 3c shows the neutral CuL-Azo complex, which is also centrosymmetric (Ci) with the Cu2+ ion located on an inversion centre. The metal ion is six-coordinated and is best described as a Jahn–Teller-elongated octahedron (4 + 2): four donors define a nearly square equatorial plane, while two much longer axial Cu⋯O contacts complete the coordination sphere (see Table S4). Such pronounced axial elongation is typical of d9 Cu2+ and is well precedented with over 4000 six-coordinated Cu2+ entries in the CSD displaying comparably unequal axial Cu–O distances.
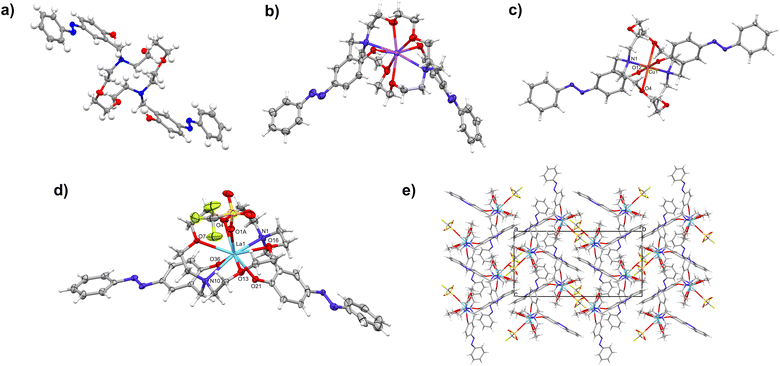 | ||
| Fig. 3 Crystal structures of the (a) L-AzoH2 ligand; (b) [NaL-AzoH2]CF3SO3 (A) complex; (c) CuL-Azo complex; and (d) LaL-AzoCF3SO3 complex; (e) packing diagram of the LaL-AzoCF3SO3 complex. | ||
The determined crystal structures for LaL-AzoCF3SO3, PrL-AzoCF3SO3, NdL-AzoCF3SO3, SmL-AzoCF3SO3, EuL-AzoCF3SO3, GdL-AzoCF3SO3, TbL-AzoCF3SO3 and ErL-AzoCF3SO3 macrocyclic complexes have established that all Ln-assemblies are highly isostructural (Fig. 3d,e and Fig. S26–S32). They crystallize in the same space group (P21/c) and with very similar disposition of molecules in the crystal structure. The isostructurality goes so far that even a similar disorder of one of the dinitrogen bridges is found in all the structures. The lanthanide ion is 9-coordinated (quite typically) by two nitrogen and six oxygen atoms from the ligand molecule, four oxygen atoms from the macrocyclic ring and two oxygen atoms from the phenol moiety of the azobenzene pendant-arms, which are positioned at the same side of the macrocyclic unit resulting in a syn conformation. The coordination sphere is filled additionally by one oxygen atom from the coordinated triflate anion positioned at the opposite side of the two azobenzene pendant-arms of the macrocycle. We have recently demonstrated the crucial effect of a disordered triflate in the Nd3+-macrocyclic assemblies for the implementation of multifunctional characteristics.43 The crystal data, data collection, structure refinement and geometrical characteristics for the ligand and complexes are given in Tables S1–S4. The lanthanide contraction in the synthesized Ln-complexes influences the Ln–N and Ln–O bond distances, resulting in a general trend of gradual shortening of these bonds across the lanthanide series, with some exceptions to the expected trend (Table S4).
The powder X-ray diffraction (PXRD) patterns for LaL-AzoCF3SO3, PrL-AzoCF3SO3, NdL-AzoCF3SO3, SmL-AzoCF3SO3, EuL-AzoCF3SO3, GdL-AzoCF3SO3, TbL-AzoCF3SO3 and ErL-AzoCF3SO3 complexes also confirmed their isostructurality. The experimental powder X-ray diffraction (PXRD) patterns of the lanthanide complexes were in accordance with simulated PXRD patterns based on single crystal diffraction data, indicating phase purity (Fig. S33). The differences in the sharpness and narrowness of the corresponding PXRD signals are due to the poor crystallinity of the powdered samples. In the case of the DyL-AzoCF3SO3, HoL-AzoCF3SO3, YbL-AzoCF3SO3 and LuL-AzoCF3SO3 complexes, the obtained samples are not in the crystalline state but in the amorphous phase, so their PXRD patterns were not possible to determine, but the experimental characterization confirmed their purity and uniformity (see section 1.5).
 | ||
| Fig. 4 Stacked 1H NMR spectra for L-Azo (black), [NaL-AzoH2]CF3SO3 (violet), LaL-AzoCF3SO3 (blue) and LuL-AzoCF3SO3 (green), along with a representation of the coordination around cations. | ||
Besides different conformations of the macrocycle cavity around the Ln centre in La3+ and Lu3+ assemblies, we suppose that the arrangement of coordinated azobenzene pendant-arms is also different. Firstly, the benzylic hydrogen resonances (methylene groups linking the crown moiety and azobenzene pendant-arms) differ between La3+ and Lu3+ complexes. These protons are a broad singlet in La3+, while in Lu3+ they show a typical AX (δ1 = 4.60 ppm; δ2 = 3.35 ppm; J = 12.6 Hz; spectrometer frequency = 600 MHz; Δν/J ∼ 60) spin system and resonate as doublets of doublets with significantly different chemical shifts. It indicates that benzylic protons are diastereotopic thanks to the different chemical environment. This result, together with the difference of the crown-ether signals, indicates that the latter complex is more rigid on the NMR timescale. Moreover, comparing the aromatic region, we can see that chemical shifts in the Lu3+ are more closely related to the ligand, where from the crystal structure data determination we observe that azobenzene pendant-arms are preorganized at the two opposite sides of the macrocyclic ligand skeleton (Fig. 3a). Integration of all these results allows us to predict that the azobenzene pendant arms adopt an anti-conformation in the Lu3+ complex. This is accompanied by a closely coordinated triflate anion near the Lu3+ center, which helps explain the observed conformational differences in solution. A comparison of energetic profiles via density functional theory (DFT) studies also supports the proposed anti-conformation in the LuL-AzoCF3SO3 complex (see section 2.4).
2.3. Photoisomerization studies
These dependencies are typical of the trans-to-cis isomerization of azobenzene molecules.1,66 Therefore, this observation is an obvious confirmation of the UV light-induced isomerization to the cis-conformation of the azobenzene moiety in the Nd3+ macrocyclic complex. The photostationary state for the Nd3+ complex was reached after 3 h of irradiation, as indicated by intensity saturation of the band from the n–π* transition (Fig. 5a). We also revealed that the Nd3+ macrocyclic complex is sensitive to visible light irradiation, as exposure of the chloroform solution of the neodymium complex to 520 nm light irradiation induces the same photochromic and photoisomerization behaviour. This is indicated by identical changes in the absorption spectrum profile (Fig. 5b). The same rate of change from trans to cis-form under irradiation of the Nd3+ complex with visible light (4.00 mW cm−2) compared to UV light (1.68 mW cm−2) was observed after exposure to more intense light irradiation, as under the same conditions, the photoresponsive behaviour was practically invisible (for details, see section 1.1, in the SI). This suggests greater sensitivity to far UV light exposure, as trans-to-cis photoisomerization is faster even at a lower intensity of incident UV light (Fig. 5).
This led us to investigate whether L-AzoH2 photoisomerization properties depend on the nature of the metal ion and protonation state of the ligand. Accordingly, we studied the influence of s-block metal ion–Na+ coordination and d-block metal ion–Cu2+ coordination in comparison with f-electron cation–Nd3+. No evidence of the photoisomerization behaviour was observed for the [NaL-AzoH2]CF3SO3 complex under exposure either to 320 nm or 520 nm light irradiation (Fig. S43a). In situ deprotonation of the ligand in the sodium complex also did not change these results (Fig. 43b). However, we observed that the copper complex (CuL-Azo), with the ligand in its deprotonated form (for details, see sections 2.2.1, 1.4 and 1.5) exhibits reversible, photoswitchable behavior (Fig. S43c), though no visible colour change of the investigated solution was noted. Finally, in situ preparation of [Nd(CF3SO3)3-L-AzoH2] without deprotonation of the phenolic lariat arms also leads to a photoresponsive system, which is switched off after the demetallation of the system with excess trifluoroacetic acid (Fig. S43d). This demonstrates that photoisomerization of the azobenzene moiety is dependent on both the protonation state of the ligand (see also section 2.3.3) and the electronic nature of the metal ion.
Based on these results, we focused our investigation on the stability of the photoinduced cis-isomer and its reversibility to the thermodynamically favoured trans-isomer. We examined the influence of various external stimuli known to trigger cis-to-trans back-isomerization – i.e. light, heat and dark storage – on the stability of the cis-isomer and recovery of the trans form of the Nd3+ macrocyclic complex in solution. The cis-isomer remained highly stable at an elevated temperature (40 °C), showing no significant change in absorption intensity after 2 hours in a chloroform solution (Fig. 6a and Fig. S44b). Further temperature increase led to decomposition (Fig. S44b). Visible light irradiation across 380–720 nm failed to induce re-isomerization (Fig. S45a). Dark storage in a sealed quartz cuvette (wrapped in aluminium foil) also showed minimal recovery of the trans form after 24 hours, indicating high kinetic stability of the cis isomer (Fig. S45b). After 14 days of storage in the dark, the absorption band corresponding to the n–π* transition at ∼505 nm decreased by about 50%, while the π–π* transition band at 403 nm recovered to 82% of its original trans-isomer intensity, indicating a very slow back-isomerization (Fig. 6b and Fig. S45b). These findings demonstrate the remarkable stability of the cis-form in the lanthanide macrocyclic complex, even under conditions typically efficient for the induction of back-isomerization of azobenzene based compounds. Surprisingly, we observed that repeatedly aspirating the cis-rich solution of the Nd3+ complex with a glass Pasteur pipette led to a visible colour change from orange to yellow, suggesting a return to the trans-form. Indeed, after 5 minutes of repeated pipetting, approximately 90% recovery of the trans-isomer was confirmed, regardless of whether the isomerization had originally been induced with 320 nm or 520 nm light (Fig. 6c and Fig. S46a, b).
Intriguingly, although glass has been shown to accelerate certain chemical reactions,67,68 this effect has not been reported for photochemical processes—particularly not for azobenzene-based photoswitches. To probe the origin of the glass-mediated re-isomerization, we examined the effect of individual components of the glass. A large excess of NaCl (500× molar) did not induce re-isomerization over 24 h, excluding Na+ leached from the glass as an operative factor and suggesting that the lack of photoresponsivity of [NaL-AzoH2]CF3SO3 arises from metal identity. Silanized borosilicate slowed the recovery of the trans isomer from ca. 15 s to ca. 5 min (Fig. 6d). Since silanization caps surface OH groups and suppresses formation of basic siloxide sites (SiO−/M+), this attenuation points to basic surface sites – not neutral Si–OH – as the active species. Consistently, adding even an excess of triphenyl silanol, Ph3SiOH, to a cis-enriched solution produced no measurable effect (Fig. S48b). Finally, excess of borax triggered an immediate spectral recovery in a few seconds but led to a slow decomposition of the complex over 24 h (Fig. S48c), consistent with the basic character accelerating re-isomerization.
To assess whether protonation of phenoxide donors affects the photostationary state, we titrated a cis-rich NdL-AzoCF3SO3 complex with acetic acid and trifluoroacetic acid (TFA). Acetic acid produced a modest increase in the cis fraction, and re-isomerization to trans remained inducible by glass contact (Fig. S48d). TFA increased the cis fraction at substoichiometric loadings, but once the acid exceeded ca. 0.5 equiv., it induced demetallation and the spectrum converged to that of the protonated ligand and the trans isomer with a loss of reversibility (Fig. S48e). In a trans-rich solution of NdL-AzoCF3SO3, TFA likewise generated cis, which triethylamine (TEA) cleanly reverted; several cycles were feasible provided the acid remained below the demetallation threshold (Fig. S48f). Similar titration/irradiation cycles can be performed using glass instead of TEA (Fig. S48g). To probe direct protonation, we assembled a [Nd(CF3SO3)3-L-AzoH2] complex in situ and the solution immediately adopted the cis state, remained switchable by glass back to trans, and was again re-enriched to cis upon 320 nm irradiation. Storage in the dark for 2 days increased the cis fraction, again recoverable to trans by glass or TEA (Fig. S48h and i). The spectra of cis generated by protonation and by 320 nm irradiation were indistinguishable within experimental uncertainty (Fig. S48j). To complement the UV-vis studies, we recorded 1H NMR spectra on diamagnetic La3+ analogues (deprotonated LaL-AzoCF3SO3 and the in situ assembled [La(CF3SO3)3-L-AzoH2]). These observations are qualitative only, due to concentration-related aggregation and higher concentrations required for NMR (≥10−3 M vs. ∼10−5 M for UV-vis). Irradiation and acid/base/glass treatments produced diagnostic, reversible changes in the azobenzene-adjacent aromatic region consistent with trans/cis interconversions and protonation/deprotonation (Fig. S48k).
All data converge on the protonation–deprotonation mechanism that influences the photostationary state. Mild protonation (acetic acid; substoichiometric TFA) biases the system toward cis, consistent with the literature showing that protonation can facilitate azobenzene isomerization, in part by enabling the azo–hydrazone tautomerism and altering excited-state pathways.69 In our system, we hypothesize that protonation of the phenolic lariat arms promotes intramolecular proton transfer to the azo group, generating a hydrazone-type tautomer in which the N–N single bond lowers the rotational barrier (Fig. 6e). This proposal is consistent with our observations that mild acid modestly enriches the cis form, strong acid initially increases cis but ≥0.5 equiv. induces demetallation, and that base/glass (SiO−) restores the trans, deprotonated state. The cis state can likewise be generated photochemically (UV/Vis), whereas deprotonation restores the trans state. In this context, glass surfaces act as a benign heterogeneous base: interfacial SiO− sites rapidly and reproducibly reintroduce the trans isomer without increasing the bulk pH or degrading the complex (Fig. 6d and Fig. S48d–k). Controls support this assignment: NaCl is inactive; silanized glass (fewer/basic sites) slows re-isomerization; a molecular silanol (Ph3SiOH) has no effect; and borate/TEA also regenerates trans but can compromise stability with repeated cycles or higher loadings. Thus, glass functions as a solid–liquid “pH probe” and a gentle base, enabling cis/trans recovery that is otherwise difficult to achieve with chemical reagents.
These results demonstrate that NdL-AzoCF3SO3 functions as a dual photoswitch, undergoing efficient trans-to-cis isomerization under both UV and visible light – an uncommon feature in azobenzene-based systems.66,70–74 Strikingly, the reverse cis-to-trans isomerization cannot be triggered by conventional stimuli such as heat (40 °C), visible light (380–720 nm) or O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 highlighting the high kinetic stability of the cis-form. Remarkably, we discovered that contact with glass surfaces induces rapid recovery of the trans-isomer, revealing an unprecedented case of glass surface catalyzed re-isomerization. This behaviour was consistent across all synthesized lanthanide series (see section 2.4), distinguishing our system from typical metal–azobenzene complexes and underscoring the critical role of ligand design and coordination environment in tuning the photoresponsive behaviour. Studies on related photoswitches are underway in our laboratory to ascertain if such an approach is compound specific or can be of general use and was up to now overlooked in the literature.
75 highlighting the high kinetic stability of the cis-form. Remarkably, we discovered that contact with glass surfaces induces rapid recovery of the trans-isomer, revealing an unprecedented case of glass surface catalyzed re-isomerization. This behaviour was consistent across all synthesized lanthanide series (see section 2.4), distinguishing our system from typical metal–azobenzene complexes and underscoring the critical role of ligand design and coordination environment in tuning the photoresponsive behaviour. Studies on related photoswitches are underway in our laboratory to ascertain if such an approach is compound specific or can be of general use and was up to now overlooked in the literature.
2.4. The effect of lanthanide contraction on structural formation and photoisomerization properties: DFT studies
The photoisomerization behaviour of the free L-AzoH2 ligand and the [NaL-AzoH2]CF3SO3 and CuL-Azo complexes, compared with that of the NdL-AzoCF3SO3 system, highlights the critical role of the lanthanide ion in enabling light-responsive functionality. Coordination of Ln3+ ions to the macrocyclic scaffold significantly alters the electronic environment, directly impacting absorption profiles and photoisomerization efficiency.76–79 Furthermore, structural variation across azobenzene-based metal complexes is known to affect their photochemical behaviour28,80,81 so we examined photoisomerization across the lanthanide series accordingly.The LaL-AzoCF3SO3, PrL-AzoCF3SO3, SmL-AzoCF3SO3, EuL-AzoCF3SO3, and GdL-AzoCF3SO3 complexes exhibit photoisomerization behaviour similar to that of the NdL-AzoCF3SO3 macrocyclic complex. All undergo efficient trans-to-cis switching under both 320 nm and 520 nm irradiation, accompanied by pronounced changes in absorption spectra and visible photochromic effects. cis-to-trans back-isomerization occurs rapidly via contact with glass surfaces, consistent across these early lanthanide complexes. Interestingly, they show greater sensitivity to UV light, with low-intensity 320 nm radiation yielding changes comparable to those induced by higher-intensity 520 nm light (Fig. S49–S53). A shift in behaviour appears with the TbL-AzoCF3SO3 complex, which shows reduced switching under UV but improves responsiveness to 520 nm visible light (Fig. S54). For later lanthanides (Dy3+ to Lu3+), 320 nm irradiation induces only minimal spectral changes, indicating suppressed photoisomerization under UV light (Fig. S55a–S59a). The efficiency of photoswitchable behaviour in LnL-AzoCF3SO3 complexes is closely governed by the ionic radius of the lanthanide ion, reflecting the influence of lanthanide contraction. Based on this trend, the complexes can be broadly grouped into two categories. The first group (La3+ to Tb3+) exhibits photoisomerization under both UV (320 nm) and visible (520 nm) light, with generally higher efficiencies under higher energy light, even at lower intensities (Fig. 7a – blue). Within this group, Tb3+ shows a noticeable decline in responsiveness. The second group (Dy3+ to Lu3+) displays little to no response to UV irradiation, though some photoisomerization persists under visible light (Fig. 7a – green). Among the smallest ions (Ho3+, Er3+, Yb3+, Lu3+), the observed spectral changes and photochromism are minimal, underscoring a contraction-driven reduction in switching efficiency. Interestingly, it can be related to even smaller Cu2+ ions, further showing a combined influence of both the size and electronic nature of the chosen cation on the photoresponsiveness. It remains plausible that metal-dependent electronic structures (e.g., heavy-atom/spin–orbit effects, ligand-field coupling, and differences in nonradiative pathways) modulate the azobenzene excited-state landscape. Direct evidence would require time-resolved spectroscopy and electronic-structure calculations, which will be demonstrated in forthcoming studies.
These distinct behaviours prompted further investigation into the kinetics of photoisomerization across the series. The macrocyclic complexes NdL-AzoCF3SO3 and YbL-AzoCF3SO3 were chosen as representatives of the early and late lanthanides, respectively. Notably, the Yb3+ complex reached its photostationary state faster – after 105 minutes of irradiation – compared to 180 minutes for the Nd3+ analogue (Fig. S60 and Fig. 5a). The determined rate constants of the photoisomerization reaction for NdL-AzoCF3SO3 (UV light – 320 nm) and YbL-AzoCF3SO3 (visible light – 520 nm) were calculated as kNd = 7.67 × 10−5 s−1 and kYb = 4.10 × 10−5 s−1 respectively, using a first-order kinetic equation (Fig. S61). These values are lower than those typically reported for photoactive lanthanide complexes.44–46,48,50,51 A comparison of the two reveals that the syn-configured Nd3+ complex switches more rapidly than the anti-configured Yb3+ analogue.
To explore the structural origin of this behaviour and the influence of lanthanide contraction, we optimized the geometry of the LaL-AzoCF3SO3 complex based on its solid-state structure and modeled the lutetium analogue by substituting La3+ with Lu3+—which was not available in crystalline form. In both models, the coordinating triflate anion was included, yielding nine-coordinate structures (Fig. S62). To validate the predicted structures, 1HNMR chemical shifts were calculated for both conformers. The simulated spectra (Fig. S63) matched well with the experimental data. In the Lu3+ complex, methylene protons adjacent to the crown ether nitrogen atoms appeared at 3.2 and 4.6 ppm – consistent with the anti-conformation (compare with Fig. 4). The deshielding of the aromatic protons further supported this assignment, with a para-positioned proton shifting downfield to 7.83 ppm.
This structural differentiation aligns with the DFT-calculated isomerization energy profiles. Since trans/cis photoisomerization of the azobenzene moiety was experimentally observed to be wavelength dependent, the rotational barrier associated with the trans/cis isomerization was calculated. The same procedure was carried out for comparison with the La3+ complex in the syn-conformation, which isomerizes under both 320 nm and 520 nm irradiation and the results are shown in Fig. 7b. As observed and expected, the trans-isomer is more stable in both cases. Notably, the anti-configured Lu3+ complex is thermodynamically less stable than the syn-structure by 8.4 kcal mol−1, suggesting that its formation is kinetically driven by the smaller ionic radius of Lu3+. However, for the La3+ complex, the rotational barrier enabling cis-to-trans conversion is 16.49 kcal mol−1, whereas for the Lu3+ complex, a higher barrier of 23.48 kcal mol−1 was calculated. The computed energy profiles reveal that the anti-LuL-AzoCF3SO3 complex exhibits significantly higher isomerization barriers (∼35 kcal mol−1) than the syn-LaL-AzoCF3SO3 analogue, consistent with the experimentally observed differences in photoisomerization efficiency. These findings collectively indicate that lanthanide contraction imposes rigidity and steric constraints around the azobenzene moiety, reducing conformational flexibility and suppressing photoisomerization.
2.5. Photoluminescence studies and luminescent temperature sensors
Based on the absorption characteristics, the luminescence properties of the sequence of lanthanide-based macrocyclic complexes were investigated in a solid-state form, using a 370 nm excitation source. It is noted that the investigated solid materials have a rigid structure; hence, they do not undergo isomerization and are stable under elevated temperature conditions, at least up to 220–270 °C (see the TGA – Fig. S64–S75), making them suitable candidates for optical sensing applications.All of the lanthanide-based macrocyclic complexes show the characteristic broad emission band of the L-AzoH2 macrocyclic ligand (see the spectra for the L-AzoH2 ligand and complexes in Fig. S76 and S77), with a maximum at around 650 nm, as a result of the π* → π transitions within the macrocyclic ligand (Fig. 8a). Photoluminescence intensity of the macrocyclic complexes decreases when Ln3+ ions are incorporated into the complex, which is evidence of ligand-to-metal energy transfer (LMET) from the L-Azo ligand to Ln3+ ions, as depicted in the energy level diagram in Fig. 8b. First, the deprotonated L-Azo ligand is excited by UV light from the singlet S0 to S1 state, then the intersystem crossing (ISC) process occurs, and the energy is transferred to the triplet state of the ligand. Finally, ligand emission from the excited T1 state can be observed, together with a simultaneous LMET process, which results in the characteristic emission from Ln3+ ions.
Importantly, three of the Ln-based macrocyclic complexes show the characteristic Ln3+ ion emission bands, i.e. SmL-AzoCF3SO3 (λ ≈ 680 nm), NdL-AzoCF3SO3 (λ ≈ 890 nm) and YbL-AzoCF3SO3 (λ ≈ 1000 nm), as a result of the most efficient LMET process. These low-energy NIR-red emissions of the mentioned Ln3+ ions are observable because these Ln3+ ions have their emitting states located below (in energy) the lowest excited triplet state of the ligand. It is noteworthy that due to the same excitation mechanism for all complexes and inefficient direct excitation of the Ln3+ in complexes (forbidden 4f–4f transitions), the excitation spectra for all compounds are almost identical and exhibit the same excitation band, as shown in the exemplary spectrum shown in Fig. S76, which was used to estimate energies of S1 and T1 for the purpose of drawing the mentioned energy level diagram, indicating the possible radiative and nonradiative processes occurring in the studied system.
Following an initial evaluation, two materials with the most intense lanthanide emissions, i.e. NdL-AzoCF3SO3 and YbL-AzoCF3SO3 macrocyclic complexes, were selected, and their emission properties were investigated under varied temperature conditions, from cryogenic to high temperature, i.e. from −180 to 160 °C (Fig. 9). The first NdL-AzoCF3SO3 macrocyclic complex shows a broad emission band in the visible (red) range, centred at ≈650 nm, associated with π* → π transition from the macrocyclic part of the complex, as well as sharp emission bands from Nd3+ at around 890 nm (4F3/2 → 4I9/2) and 1050 nm (4F3/2 → 4I11/2), accompanied by characteristic narrow peaks due to crystal-field split mJ sublevels (Fig. 9a). It is noteworthy that, in this case, the influence of the Nd3+ absorption is also clearly visible in the form of intensity drops (due to Nd3+ reabsorption) at around 580, 670, 750 and 800 nm (4I9/2 → 2G9/2, 4F9/2, 2G7/2 and 4F5/2 Nd3+ transitions, respectively), altering the expected round shape of the broad emission band (π* → π) from the organic component.
The gradual decrease of luminescence intensity of the deprotonated L-Azo ligand and Nd3+ as a function of temperature is a result of thermal quenching of emission in a non-radiative way (inset in Fig. 9a).82,83 A similar tendency was observed for the YbL-AzoCF3SO3 macrocyclic complex in Fig. 9b. However, the change of intensity for both bands (L-Azo and Ln3+) was not the same; hence, we could calculate the luminescence intensity ratio (LIR) parameter for both macrocyclic complexes, which can work as a thermometric parameter utilized in optical thermometry. The LIR between two emission bands of the L-Azo ligand and Ln3+ ions (i.e.L-Azo/Nd3+ and L-Azo/Yb3+) changes monotonously from around −70 to 110 °C for the Nd3+ complex and from 20 to 160 °C for the Yb3+ complex, allowing their use as luminescent thermometers in the indicated operating temperature ranges (Fig. 9c and d). Based on the calculated thermometric parameters, we also calculated the relative thermal sensitivity (Sr) of the developed sensors using eqn (1):
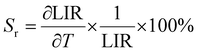 | (1) |
The Sr parameter for the NdL-AzoCF3SO3 complex reaches the maximum value of ≈0.89%/°C at around 55 °C (see the inset in Fig. 9c). However, for the YbL-AzoCF3SO3 complex, the Sr parameter has the maximum value of ≈1.289% per °C at the highest operating temperature, i.e. at 160 °C (see the inset in Fig. 9d).
Another observed temperature-dependent spectroscopic effect is the spectral shift with temperature. In both materials the spectral position of the L-Azo moiety emission band changes non-monotonously with temperature (data not shown), in contrast to the emission band centroids for the Nd3+ and Yb3+, which linearly change with temperature. Both emission bands, centered at λ ≈ 890 nm for Nd3+ and at λ ≈ 1000 nm for Yb3+, show a linear blue-shift, with a rate Δλ = −0.0151 and −0.0381 nm °C−1, respectively, caused by the thermal expansion of the unit cell. Specifically, the observed spectral shift is due to the static contribution caused by the changes in the site geometry, which is occupied by the lanthanide ion in the crystal, as a result of lattice thermal expansion.82,84 The observed spectral shifts, together with the discussed LIRs allow multi-parameter temperature sensing in a relatively broad T-range. It is worth noting that due to severe quenching of Ln3+ luminescence by the solvent molecules, the characteristic emission bands originating from Ln3+ ions could be observed only in the solid-state form of the metal–organic complexes studied (see Fig. S78 for comparison). Nonetheless, broad ligand centered emission overlaps the region typically used to excite the cis n → π* band and could possibly be the reason visible irradiation does not drive efficient cis/trans re-isomerization. While metal coordination, aggregation effects and matrix rigidity may also play a role in this, a definitive mechanism will require time-resolved and solvent-dependent studies that are beyond the scope of this study.
3. Conclusions
We report the development of a new class of photoswitchable lanthanide-based macrocyclic complexes, where trans-to-cis isomerization is induced by light and reversed through an unprecedented glass surface-mediated mechanism. Structural and spectroscopic studies across the LnL-AzoCF3SO3 series revealed two distinct structural regimes. Complexes from La3+ to Tb3+ and Er3+ adopt a consistent nine-coordinate geometry comprising donor atoms from the diazacrown ether, azobenzene moiety and a monodentate triflate anion. Despite this apparent structural consistency, 1H NMR and DFT studies identified a conformational shift from a syn- to anti-arrangement as the lanthanide series progresses – correlated with increased ligand rigidity and higher isomerization barriers.All synthesized Ln-complexes display light-responsive behaviour, with most undergoing reversible trans-to-cis photoisomerization in solution under UV and/or visible light irradiation. Switching efficiency depends on the nature of the lanthanide ion and its electronic structure, with suppression observed in certain cases, particularly for smaller lanthanides, aligning with computational findings and highlighting the impact of lanthanide contraction on the photoswitching dynamics. Notably, the reverse cis/trans isomerization proved unresponsive to conventional triggers such as heat, visible light, or oxygen, but it occurred rapidly upon contact with the glass surface. This unusual recovery combined with the high stability of the cis-isomer introduces an unprecedented case of glass-driven re-isomerization of diazobenzene photoswitches and can be explained on the basis of protonation/deprotonation behaviour.
In addition to the photoresponsive solution behaviour, the solid-state luminescence properties of the complexes were also investigated, with Nd3+ and Yb3+ complexes exhibiting promising thermosensitive emissions. These systems function as dual VIS/NIR luminescent thermometers, based on LIR and band shifts, representing the first example of such dual photoswitchable and luminescent macrocyclic systems. The combined study of isomerization and emission behaviour under light irradiation underscores the potential of these macrocycles as multi-stimuli-responsive lanthanide platforms. This work provides new insights into the interplay between macrocyclic conformation, metal ion size and external stimuli, offering a valuable design strategy for future development of functional lanthanide-based supramolecular materials.
Author contributions
Dominika Prętka: conceptualization, data curation, investigation, visualization, writing – original draft, and writing – review & editing; Dawid Marcinkowski: formal analysis and resources; Nahir Vadra: investigation and resources; Przemysław Woźny: data curation, investigation, and writing – original draft; Marcin Runowski: conceptualization, visualization, writing – review & editing, and resources; Maciej Kubicki: data curation, formal analysis, visualization, and writing – original draft; Violetta Patroniak: supervision, resources, visualization, and writing – review & editing; Giuseppe Consiglio: formal analysis, methodology, and writing – review & editing; Giuseppe Forte: formal analysis, methodology, and writing – review & editing; Adam Gorczyński: conceptualization, methodology, investigation, visualization, writing – original draft, writing – review & editing, project administration, supervision, resources, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the SI and are also available in the public open repository Zenodo at https://doi.org/10.5281/zenodo.15790031.Supplementary information is available. See DOI: https://doi.org/10.1039/d5qi01461a.
CCDC 2406250–2406258, 2435298, 2435299 and 2480151 contain the supplementary crystallographic data for this paper.85a–l
Acknowledgements
We acknowledge B.Sc. Antoni Stefaniak for help in the synthesis of the CuL-Azo complex. This work was supported by the National Science Centre, Poland (grant no. 2020/39/D/ST4/01182 (PI: A. G.), 2022/45/N/ST4/00344 (PI: D. M.), 2023/50/E/ST5/00021 (PI: M. R.), 2024/52/C/ST5/00047 (PI: N. V.)).References
- M. Baroncini, J. Groppi, S. Corra, S. Silvi and A. Credi, Light-Responsive (Supra)Molecular Architectures: Recent Advances, Adv. Opt. Mater., 2019, 7, 1900392 CrossRef.
- Z. Yang, Z. Liu and L. Yuan, Recent Advances of Photoresponsive Supramolecular Switches, Asian J. Org. Chem., 2021, 10, 74–90 CrossRef CAS.
- T. Zheng, M. Runowski, I. R. Martín, K. Soler-Carracedo, L. Peng, M. Skwierczyńska, M. Sójka, J. Barzowska, S. Mahlik, H. Hemmerich, F. Rivera-López, P. Kulpiński, V. Lavín, D. Alonso and D. Peng, Mechanoluminescence and Photoluminescence Heterojunction for Superior Multimode Sensing Platform of Friction, Force, Pressure, and Temperature in Fibers and 3D-Printed Polymers, Adv. Mater., 2023, 35, 2304140 CrossRef CAS PubMed.
- C. Hernández-Álvarez, P. I. Martín-Hernández, I. R. Martín, F. Rivera-López, H. Hemmerich, M. Grzegorczyk, S. Mahlik and M. Runowski, Optical Temperature Sensor Evaluation in a Working Gear Motor: Application of Luminescence Thermometry in Industrial Technology, Adv. Opt. Mater., 2024, 12, 2303328 CrossRef.
- X. Yao, T. Li, J. Wang, X. Ma and H. Tian, Recent Progress in Photoswitchable Supramolecular Self-Assembling Systems, Adv. Opt. Mater., 2016, 4, 1322–1349 CrossRef CAS.
- A. Goulet-Hanssens, F. Eisenreich and S. Hecht, Enlightening Materials with Photoswitches, Adv. Mater., 2020, 32, 1905966 CrossRef CAS PubMed.
- X. Huang and T. Li, Recent progress in the development of molecular-scale electronics based on photoswitchable molecules, J. Mater. Chem. C, 2020, 8, 821–848 RSC.
- I. M. Welleman, M. W. H. Hoorens, B. L. Feringa, H. H. Boersma and W. Szymański, Photoresponsive molecular tools for emerging applications of light in medicine, Chem. Sci., 2020, 11, 11672–11691 RSC.
- S. Jia, W.-K. Fong, B. Graham and B. J. Boyd, Photoswitchable Molecules in Long-Wavelength Light-Responsive Drug Delivery: From Molecular Design to Applications, Chem. Mater., 2018, 30, 2873–2887 CrossRef CAS.
- T. Zheng, J. Luo, D. Peng, L. Peng, P. Woźny, J. Barzowska, M. Kamiński, S. Mahlik, J. Moszczyński, K. Soler-Carracedo, F. Rivera-López, H. Hemmerich and M. Runowski, Persistent Photoluminescence and Mechanoluminescence of a Highly Sensitive Pressure and Temperature Gauge in Combination with a 3D-Printable Optical Coding Platform, Adv. Sci., 2024, 11, 2408686 CrossRef CAS PubMed.
- L. Marciniak, P. Woźny, M. Szymczak and M. Runowski, Optical pressure sensors for luminescence manometry: Classification, development status, and challenges, Coord. Chem. Rev., 2024, 507, 215770 CrossRef CAS.
- M. Runowski, P. Woźny, I. R. Martín, K. Soler-Carracedo, T. Zheng, H. Hemmerich, F. Rivera-López, J. Moszczyński, P. Kulpiński and S. Feldmann, Multimodal Optically Nonlinear Nanoparticles Exhibiting Simultaneous Higher Harmonics Generation and Upconversion Luminescence for Anticounterfeiting and 8-bit Optical Coding, Adv. Funct. Mater., 2024, 34, 2307791 Search PubMed.
- M. Younis, S. Ahmad, A. Atiq, M. Amjad Farooq, M.-H. Huang and M. Abbas, Recent Progress in Azobenzene-Based Supramolecular Materials and Applications, Chem. Rec., 2023, 23, e202300126 CrossRef CAS PubMed.
- Z.-H. Liao and F. Wang, Light-controlled smart materials: Supramolecular regulation and applications, Smart Mol., 2024, e20240036 CrossRef CAS PubMed.
- E. Nieland, J. Voss and B. M. Schmidt, Photoresponsive Supramolecular Cages and Macrocycles, ChemPlusChem, 2023, 88, e202300353 CrossRef CAS PubMed.
- C. Ludwig and J. Xie, The Growing Field of Photoswitchable Macrocycles: A Promising Way to Tune Various Properties with Light, ChemPhotoChem, 2023, 7, e202300126 CrossRef CAS.
- C. Fedele, T.-P. Ruoko, K. Kuntze, M. Virkki and A. Priimagi, New tricks and emerging applications from contemporary azobenzene research, Photochem. Photobiol. Sci., 2022, 21, 1719–1734 CrossRef CAS PubMed.
- F. A. Jerca, V. V. Jerca and R. Hoogenboom, Advances and opportunities in the exciting world of azobenzenes, Nat. Rev. Chem., 2022, 6, 51–69 CrossRef PubMed.
- S. Song, H. Zhang and Y. Liu, Light-Controlled Macrocyclic Supramolecular Assemblies and Luminescent Behaviors, Acc. Mater. Res., 2024, 5, 1109–1120 CrossRef CAS.
- J. Yu, D. Qi and J. Li, Design, synthesis and applications of responsive macrocycles, Commun. Chem., 2020, 3, 189 CrossRef CAS PubMed.
- Z. Li, J. Liang, W. Xue, G. Liu, S. H. Liu and J. Yin, Switchable azo-macrocycles: from molecules to functionalisation, Supramol. Chem., 2014, 26, 54–65 CrossRef CAS.
- W.-C. Geng, H. Sun and D.-S. Guo, Macrocycles containing azo groups: recognition, assembly and application, J. Inclusion Phenom. Macrocyclic Chem., 2018, 92, 1–79 CrossRef CAS.
- H. Roithmeyer, M. Uudsemaa, A. Trummal, M.-L. Brük, S. Krämer, I. Reile, V. Rjabovs, K. Palmi, M. Rammo, R. Aav, E. Kalenius and J. Adamson, Large Azobenzene Macrocycles: Formation and Detection by NMR and MS Methods, Supramol. Chem., 2023, 34, 77–86 CrossRef.
- E. Wagner-Wysiecka, N. Łukasik, J. F. Biernat and E. Luboch, Azo group(s) in selected macrocyclic compounds, J. Inclusion Phenom. Macrocyclic Chem., 2018, 90, 189–257 CrossRef CAS PubMed.
- P. Tecilla and D. Bonifazi, Configurational Selection in Azobenzene-Based Supramolecular Systems Through Dual-Stimuli Processes, ChemistryOpen, 2020, 9, 529–544 Search PubMed.
- O. Galangau, L. Norel and S. Rigaut, Metal complexes bearing photochromic ligands: photocontrol of functions and processes, Dalton Trans., 2021, 50, 17879–17891 Search PubMed.
- R. G. DiNardi, A. O. Douglas, R. Tian, J. R. Price, M. Tajik, W. A. Donald and J. E. Beves, Visible-Light-Responsive Self-Assembled Complexes: Improved Photoswitching Properties by Metal Ion Coordination, Angew. Chem., Int. Ed., 2022, 61, e202205701 Search PubMed.
- G. C. Thaggard, J. Haimerl, K. C. Park, J. Lim, R. A. Fischer, B. K. P. Maldeni Kankanamalage, B. J. Yarbrough, G. R. Wilson and N. B. Shustova, Metal–Photoswitch Friendship: From Photochromic Complexes to Functional Materials, J. Am. Chem. Soc., 2022, 144, 23249–23263 CrossRef CAS PubMed.
- M. A. Soto and M. J. MacLachlan, Responsive macrocyclic and supramolecular structures powered by platinum, Chem. Sci., 2024, 15, 431–441 Search PubMed.
- L. Su, X. Liu, Q. Niu and Z. Li, Photoresponsive lanthanide luminescent materials, J. Mater. Chem. C, 2024, 12, 10759–10774 RSC.
- Y. Fréroux, L. Caussin, N. El Beyrouti, S. Rigaut and L. Norel, in Handb. Phys. Chem. Rare Earths, ed. J.-C. G. Bünzli and S. M. Kauzlarich, Elsevier, 2024, vol. 65, pp. 35–91 Search PubMed.
- Y. Hasegawa, T. Nakagawa and T. Kawai, Recent progress of luminescent metal complexes with photochromic units, Coord. Chem. Rev., 2010, 254, 2643–2651 CrossRef CAS.
- H. Nie, J. L. Self, A. S. Kuenstler, R. C. Hayward and J. Read de Alaniz, Multiaddressable Photochromic Architectures: From Molecules to Materials, Adv. Opt. Mater., 2019, 7, 1900224 CrossRef.
- L. Norel, O. Galangau, H. Al Sabea and S. Rigaut, Remote Control of Near Infrared Emission with Lanthanide Complexes, ChemPhotoChem, 2021, 5, 393–405 CrossRef CAS.
- R. Li, F.-F. Xu, Z.-L. Gong and Y.-W. Zhong, Thermo-responsive light-emitting metal complexes and related materials, Inorg. Chem. Front., 2020, 7, 3258–3281 RSC.
- Y. Hasegawa and Y. Kitagawa, Thermo-sensitive luminescence of lanthanide complexes, clusters, coordination polymers and metal–organic frameworks with organic photosensitizers, J. Mater. Chem. C, 2019, 7, 7494–7511 Search PubMed.
- M. Liberka, M. Zychowicz, L. Vasseur, J. Hooper and S. Chorazy, Governing efficiency and thermoresponsivity of luminescence in dirhenium(V) molecules by a highly tunable emission mechanism, Inorg. Chem. Front., 2024, 11, 8047–8069 RSC.
- L. Labella, G. Bottaro, F. Marchetti, S. Samaritani and L. Armelao, Luminescent Tetrahedral Manganese(II) Pentaphluorophenolate Complex as a Highly Sensitive Molecular Thermometer, Inorg. Chem., 2025, 64, 7960–7969 CrossRef CAS PubMed.
- D. Errulat, R. Marin, D. A. Gálico, K. L. M. Harriman, A. Pialat, B. Gabidullin, F. Iikawa, O. D. D. Couto Jr., J. O. Moilanen, E. Hemmer, F. A. Sigoli and M. Murugesu, A Luminescent Thermometer Exhibiting Slow Relaxation of the Magnetization: Toward Self-Monitored Building Blocks for Next-Generation Optomagnetic Devices, ACS Cent. Sci., 2019, 5, 1187–1198 Search PubMed.
- A. G. Bispo-Jr, D. A. Gálico, R. M. Diaz-Rodriguez, J. S. Ovens, F. A. Sigoli and M. Murugesu, The role of terminal ligands in the slow relaxation of magnetisation and luminescence thermometry of dinuclear NdIII complexes, Inorg. Chem. Front., 2023, 10, 3929–3939 RSC.
- S. Zanella, M. Aragon-Alberti, C. D. S. Brite, F. Salles, L. D. Carlos and J. Long, Luminescent Single-Molecule Magnets as Dual Magneto-Optical Molecular Thermometers, Angew. Chem., Int. Ed., 2023, 62, e202306970 Search PubMed.
- J. Corredoira-Vázquez, C. González-Barreira, M. Fondo, A. M. García-Deibe, J. Sanmartín-Matalobos, S. Gómez-Coca, E. Ruiz, C. D. S. Brites and L. D. Carlos, An air-stable high-performance single-molecule magnet operating as a luminescent thermometer below its blocking temperature, Inorg. Chem. Front., 2025, 12, 5506–5516 RSC.
- D. Marcinkowski, D. Prętka, P. Woźny, J. Kobylarczyk, A. Siwiak, D. Pakulski, V. Patroniak, K. Roszak, A. Katrusiak, R. Herchel, R. Podgajny, S. Sobczak, N. Majewska, S. Mahlik, M. Runowski and A. Gorczyński, Nd(III)-Based Temperature-Independent Manometer and Pressure-Independent Thermometer with Slow Relaxation of Magnetization, Adv. Opt. Mater., 2025, 2500495 Search PubMed.
- L.-R. Lin, X. Wang, G.-N. Wei, H.-H. Tang, H. Zhang and L.-H. Ma, Azobenzene-derived tris-β-diketonate lanthanide complexes: reversible trans-to-cis photoisomerization in solution and solid state, Dalton Trans., 2016, 45, 14954–14964 Search PubMed.
- L.-R. Lin, H.-H. Tang, Y.-G. Wang, X. Wang, X.-M. Fang and L.-H. Ma, Functionalized Lanthanide(III) Complexes Constructed from Azobenzene Derivative and β-Diketone Ligands: Luminescent, Magnetic, and Reversible Trans-to-Cis Photoisomerization Properties, Inorg. Chem., 2017, 56, 3889–3900 CrossRef CAS PubMed.
- Y.-G. Wang, Y.-Q. Li, H.-H. Tang, L.-R. Lin and L.-H. Ma, Near-Infrared Photoluminescence and Reversible Trans-to-Cis Photoisomerization of Mononuclear and Binuclear Ytterbium(III) Complexes Functionalized by Azobenzene Groups, ACS Omega, 2018, 3, 5480–5490 CrossRef CAS PubMed.
- G. Huang, X. Yi, F. Gendron, B. Le Guennic, T. Guizouarn, C. Daiguebonne, G. Calvez, Y. Suffren, O. Guillou and K. Bernot, A supramolecular chain of dimeric Dy single molecule magnets decorated with azobenzene ligands, Dalton Trans., 2019, 48, 16053–16061 RSC.
- C.-Y. Fu, L. Chen, X. Wang and L.-R. Lin, Synthesis of Bis-β-Diketonate Lanthanide Complexes with an Azobenzene Bridge and Studies of Their Reversible Photo/Thermal Isomerization Properties, ACS Omega, 2019, 4, 15530–15538 CrossRef CAS PubMed.
- M. Cieslikiewicz-Bouet, S. V. Eliseeva, V. Aucagne, A. F. Delmas, I. Gillaizeau and S. Petoud, Near-infrared emitting lanthanide(iii) complexes as prototypes of optical imaging agents with peptide targeting ability: a methodological approach, RSC Adv., 2019, 9, 1747–1751 Search PubMed.
- J. Xie, T. Wu, X. Wang, C. Yu, W. Huang and D. Wu, Azo-Label Heterometal–Organic Rhomboids Exhibiting Photoswitchable NIR Luminescence in Crystalline State, Inorg. Chem., 2020, 59, 15460–15466 Search PubMed.
- C. Qian, Y. Ma, Y. Zhang, L. Yuan, D. Zhang, L. Zhao, J. Luo and X. Wang, A multi-input/multi-output molecular system based on lanthanide(iii) complexes, Inorg. Chem. Front., 2022, 9, 2668–2675 RSC.
- A. Guesdon-Vennerie, P. Couvreur, F. Ali, F. Pouzoulet, C. Roulin, I. Martínez-Rovira, G. Bernadat, F.-X. Legrand, C. Bourgaux, C. L. Mazars, S. Marco, S. Trépout, S. Mura, S. Mériaux and G. Bort, Breaking photoswitch activation depth limit using ionising radiation stimuli adapted to clinical application, Nat. Commun., 2022, 13, 4102 Search PubMed.
- C. H. Simms, V. R. M. Nielsen, T. J. Sørensen, S. Faulkner and M. J. Langton, Photoswitchable luminescent lanthanide complexes controlled and interrogated by four orthogonal wavelengths of light, Phys. Chem. Chem. Phys., 2024, 26, 18683–18691 Search PubMed.
- A. J. Amoroso and S. J. A. Pope, Using lanthanide ions in molecular bioimaging, Chem. Soc. Rev., 2015, 44, 4723–4742 RSC.
- K. Atal, U. Phageria, S. Kumari, Y. Dhayal and S. Bugalia, A review on designing and synthesis of lanthanide based macrocyclic complexes and their potential applications, Inorg. Chim. Acta, 2024, 561, 121857 Search PubMed.
- W.-L. Zhou, Y. Chen, W. Lin and Y. Liu, Luminescent lanthanide–macrocycle supramolecular assembly, Chem. Commun., 2021, 57, 11443–11456 Search PubMed.
- Y. Gil, A. Castro-Alvarez, P. Fuentealba, E. Spodine and D. Aravena, Lanthanide SMMs Based on Belt Macrocycles: Recent Advances and General Trends, Chem. – Eur. J., 2022, 28, e202200336 CrossRef CAS PubMed.
- N. Su, J. S. Bradshaw, X. X. Zhang, P. B. Savage, K. E. Krakowiak and R. M. Izatt, Syntheses of diaza-18-crown-6 ligands containing two units each of 4-hydroxyazobenzene, benzimidazole, uracil, anthraquinone, or ferrocene groups, J. Heterocycl. Chem., 1999, 36, 771–775 CrossRef CAS.
- J. A. Peters, K. Djanashvili, C. F. G. C. Geraldes and C. Platas-Iglesias, The chemical consequences of the gradual decrease of the ionic radius along the Ln-series, Coord. Chem. Rev., 2020, 406, 213146 CrossRef CAS.
- S. Li, S. Jansone-Popova and D.-e. Jiang, Insights into coordination and ligand trends of lanthanide complexes from the Cambridge Structural Database, Sci. Rep., 2024, 14, 11301 Search PubMed.
- S. A. Cotton and P. R. Raithby, Systematics and surprises in lanthanide coordination chemistry, Coord. Chem. Rev., 2017, 340, 220–231 CrossRef CAS.
- A. Hu, S. N. MacMillan and J. J. Wilson, Macrocyclic Ligands with an Unprecedented Size-Selectivity Pattern for the Lanthanide Ions, J. Am. Chem. Soc., 2020, 142, 13500–13506 Search PubMed.
- A. Roca-Sabio, M. Mato-Iglesias, D. Esteban-Gómez, É. Tóth, A. d. Blas, C. Platas-Iglesias and T. Rodríguez-Blas, Macrocyclic Receptor Exhibiting Unprecedented Selectivity for Light Lanthanides, J. Am. Chem. Soc., 2009, 131, 3331–3341 Search PubMed.
- N. A. Thiele, V. Brown, J. M. Kelly, A. Amor-Coarasa, U. Jermilova, S. N. MacMillan, A. Nikolopoulou, S. Ponnala, C. F. Ramogida, A. K. H. Robertson, C. Rodríguez-Rodríguez, P. Schaffer, C. Williams Jr., J. W. Babich, V. Radchenko and J. J. Wilson, An Eighteen-Membered Macrocyclic Ligand for Actinium-225 Targeted Alpha Therapy, Angew. Chem., Int. Ed., 2017, 56, 14712–14717 CrossRef CAS PubMed.
- W.-C. Xu, S. Sun and S. Wu, Photoinduced Reversible Solid-to-Liquid Transitions for Photoswitchable Materials, Angew. Chem., Int. Ed., 2019, 58, 9712–9740 Search PubMed.
- P. Weis and S. Wu, Light-Switchable Azobenzene-Containing Macromolecules: From UV to Near Infrared, Macromol. Rapid Commun., 2018, 39, 1700220 CrossRef PubMed.
- Y. Li, K.-H. Huang, N. M. Morato and R. G. Cooks, Glass surface as strong base, ‘green’ heterogeneous catalyst and degradation reagent, Chem. Sci., 2021, 12, 9816–9822 RSC.
- Y. Li, T. F. Mehari, Z. Wei, Y. Liu and R. G. Cooks, Reaction Acceleration at Solid/Solution Interfaces: Katritzky Reaction Catalyzed by Glass Particles, Angew. Chem., Int. Ed., 2021, 60, 2929–2933 CrossRef PubMed.
- H. M. D. Bandara and S. C. Burdette, Photoisomerization in different classes of azobenzene, Chem. Soc. Rev., 2012, 41, 1809–1825 RSC.
- A. A. Beharry, O. Sadovski and G. A. Woolley, Azobenzene Photoswitching without Ultraviolet Light, J. Am. Chem. Soc., 2011, 133, 19684–19687 CrossRef PubMed.
- M. Gao, D. Kwaria, Y. Norikane and Y. Yue, Visible-light-switchable azobenzenes: Molecular design, supramolecular systems, and applications, Nat. Sci., 2023, 3, e220020 CrossRef.
- E. Nieland, J. Voss, A. Mix and B. M. Schmidt, Photoresponsive Dissipative Macrocycles Using Visible-Light-Switchable Azobenzenes, Angew. Chem., Int. Ed., 2022, 61, e202212745 CrossRef PubMed.
- Y. Huang, X. Zeng, X. Ma, Z. Lin, J. Sun, W. Xiao, S. H. Liu, J. Yin and G.-F. Yang, A visible light-activated azo-fluorescent switch for imaging-guided and light-controlled release of antimycotics, Nat. Commun., 2024, 15, 8670 CrossRef PubMed.
- J. Volarić, J. Buter, A. M. Schulte, K.-O. van den Berg, E. Santamaría-Aranda, W. Szymanski and B. L. Feringa, Design and Synthesis of Visible-Light-Responsive Azobenzene Building Blocks for Chemical Biology, J. Org. Chem., 2022, 87, 14319–14333 CrossRef PubMed.
- K. Kuntze, J. Isokuortti, A. Siiskonen, N. Durandin, T. Laaksonen and A. Priimagi, Azobenzene Photoswitching with Near-Infrared Light Mediated by Molecular Oxygen, J. Phys. Chem. B, 2021, 125, 12568–12573 CrossRef PubMed.
- J. Baldwin, A. Brookfield, G. F. S. Whitehead, L. S. Natrajan, E. J. L. McInnes, M. S. Oakley and D. P. Mills, Isolation and Electronic Structures of Lanthanide(II) Bis(trimethylsilyl)phosphide Complexes, Inorg. Chem., 2024, 63, 18120–18136 CrossRef PubMed.
- W. A. Rabanal-León, D. Páez-Hernández and R. Arratia-Pérez, Covalent lanthanide(iii) macrocyclic complexes: the bonding nature and optical properties of a promising single antenna molecule, Phys. Chem. Chem. Phys., 2014, 16, 25978–25988 RSC.
- M. del C. Fernández-Fernández, R. Bastida, A. Macías, P. Pérez-Lourido, C. Platas-Iglesias and L. Valencia, Lanthanide(III) Complexes with a Tetrapyridine Pendant-Armed Macrocyclic Ligand: 1H NMR Structural Determination in Solution, X-ray Diffraction, and Density-Functional Theory Calculations, Inorg. Chem., 2006, 45, 4484–4496 CrossRef PubMed.
- A. Cruz-Navarro, J. M. Rivera, J. Durán-Hernández, S. Castillo-Blum, A. Flores-Parra, M. Sánchez, I. Hernández-Ahuactzi and R. Colorado-Peralta, Luminescence properties and DFT calculations of lanthanide(III) complexes (Ln = La, Nd, Sm, Eu, Gd, Tb, Dy) with 2,6-bis(5-methyl-benzimidazol-2-yl)pyridine, J. Mol. Struct., 2018, 209–216 CrossRef.
- J. Pang, C. Gao, L. Shu, X. Hu and M. Li, DFT calculations: Bridged-azo working with visible light, Comput. Theor. Chem., 2020, 1191, 113041 CrossRef.
- Z. Wang, L. Heinke, J. Jelic, M. Cakici, M. Dommaschk, R. J. Maurer, H. Oberhofer, S. Grosjean, R. Herges, S. Bräse, K. Reuter and C. Wöll, Photoswitching in nanoporous, crystalline solids: an experimental and theoretical study for azobenzene linkers incorporated in MOFs, Phys. Chem. Chem. Phys., 2015, 17, 14582–14587 RSC.
- C. D. S. Brites, R. Marin, M. Suta, A. N. Carneiro Neto, E. Ximendes, D. Jaque and L. D. Carlos, Spotlight on Luminescence Thermometry: Basics, Challenges, and Cutting-Edge Applications, Adv. Mater., 2023, 35, 2302749 Search PubMed.
- X. Qiu, T. Zheng, M. Runowski, P. Woźny, I. R. Martín, K. Soler-Carracedo, C. E. Piñero, S. Lebedkin, O. Fuhr and S. Bräse, Constructing [2.2]Paracyclophane-Based Ultrasensitive Optical Fluorescent-Phosphorescent Thermometer with Cucurbit[8]uril Supramolecular Assembly, Adv. Funct. Mater., 2024, 34, 2313517 CrossRef.
- M. Runowski, A. Shyichuk, A. Tymiński, T. Grzyb, V. Lavín and S. Lis, Multifunctional Optical Sensors for Nanomanometry and Nanothermometry: High-Pressure and High-Temperature Upconversion Luminescence of Lanthanide-Doped Phosphates—LaPO4/YPO4:Yb3+–Tm3+, ACS Appl. Mater. Int, 2018, 10, 17269–17279 CrossRef PubMed.
- (a) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2406250: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lrwz7; (b) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2406251: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lrx09; (c) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2406252: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lrx1b; (d) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2406253: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lrx2c; (e) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2406254: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lrx3d; (f) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2406255: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lrx4f; (g) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2406256: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lrx5g; (h) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2406257: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lrx6h; (i) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2406258: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2lrx7j; (j) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2435298: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2mr40k; (k) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2435299: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2mr41l; (l) D. Prętka, D. Marcinkowski, N. Vadra, P. Woźny, M. Runowski, M. Kubicki, V. Patroniak, G. Consiglio, G. Forte and A. Gorczyński, CCDC 2480151: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p7swm.
Footnote |
| † Dedicated to Professor Bronisław Marciniak on the occasion of his 75th birthday. |
| This journal is © the Partner Organisations 2025 |




![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. Both profiles display distinct minima at
N bond. Both profiles display distinct minima at 
