 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bioorthogonal activation and mitochondrial targeting of a near-infrared-emitting iridium(III) nitrone complex via cyclooctynylated phosphonium cations for enhanced cellular imaging and photodynamic therapy†
Edward R. H.
Walter‡
 ab,
Lawrence Cho-Cheung
Lee‡
ab,
Lawrence Cho-Cheung
Lee‡
 bc,
Peter Kam-Keung
Leung‡
bc,
Peter Kam-Keung
Leung‡
 cd,
Kenneth Kam-Wing
Lo
cd,
Kenneth Kam-Wing
Lo
 *cd and
Nicholas J.
Long
*cd and
Nicholas J.
Long
 *a
*a
aDepartment of Chemistry, Imperial College London, Molecular Sciences Research Hub, W12 0BZ, UK. E-mail: n.long@imperial.ac.uk
bLaboratory for Synthetic Chemistry and Chemical Biology Limited, Units 1503-1511, 15/F, Building 17 W, Hong Kong Science Park, New Territories, Hong Kong, P. R. China
cDepartment of Chemistry, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong, P. R. China. E-mail: bhkenlo@cityu.edu.hk
dState Key Laboratory of Terahertz and Millimetre Waves, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong, P. R. China
First published on 1st July 2025
Abstract
In this work, we designed and synthesised a new cyclometallated iridium(III) nitrone complex [Ir(bpz)2(bpy-nitrone)](PF6) (1) (Hbpz = benzo[a]phenazine; bpy-nitrone = 4-((methyl(oxido)imino)methyl)-4′-methyl-2,2′-bipyridine) as a bioorthogonally activatable phototheranostic agent. Complex 1 displayed very weak emission and singlet oxygen (1O2) photosensitisation in solutions due to the quenching nitrone moiety. However, upon the strain-promoted alkyne–nitrone cycloaddition (SPANC) reaction with bicyclo[6.1.0]non-4-yne (BCN), which converted the nitrone unit to a non-quenching isoxazoline derivative, the complex exhibited a substantial increase in emission intensity in the near-infrared region and 1O2 generation efficiency. Given that mitochondria are a crucial target in cancer therapy, we prepared a series of BCN-functionalised phosphonium cations (BCN-Phos-n), each bearing different substituents, to serve as mitochondrial-targeting vectors for delivering complex 1 to the mitochondria via the bioorthogonal SPANC reaction. Notably, complex 1 exhibited more significant emission turn-on upon reaction with BCN-Phos-5 and BCN-Phos-6 (I/Io = 24.7 and 14.1, respectively), attributed to their increased hydrophobicity resulting from the methylation or methoxylation of the phenyl rings on the phosphonium cation. Live-cell confocal imaging and flow cytometric analyses revealed that complex 1 showed larger emission enhancement in HeLa cells pretreated with BCN-Phos-5 or BCN-Phos-6 compared to other BCN-Phos-n analogues. Co-staining experiments confirmed that the resultant luminescent isoxazoline cycloadducts predominantly accumulated in the mitochondria. Additionally, both dark and light-induced cytotoxicity of complex 1 increased upon pretreatment of the cells with BCN-Phos-5 or BCN-Phos-6. Our results demonstrate that the theranostic potential of transition metal nitrone complexes can be significantly enhanced via strategic structural manipulation of their bioorthogonal reaction partners.
Introduction
Over the past two decades, the development of bioorthogonal chemistry1,2 has revolutionised the fields of chemical biology3–5 and biomedicine.6–8 Bioorthogonal ligation reactions between two abiotic functionalities have enabled the visualisation of specific biomolecules and associated biological events in live cells using fluorescence microscopy.9 The use of fluorescent probes, however, can lead to unavoidable background fluorescence due to non-specific covalent labelling and the entrapment of unreacted probes within the cellular environment. Thus, there has been significant interest in the development of fluorogenic bioorthogonal probes, whose fluorescence is quenched by the appended bioorthogonal group and can only be restored upon specific bioorthogonal reactions, thereby enhancing the precision of fluorescence imaging.10–12 This design strategy has recently been extended to the development of activatable photosensitisers for targeted photodynamic therapy (PDT).13,14 The controlled activation of the reactive oxygen species (ROS) photosensitisation capabilities of the photosensitisers through bioorthogonal reactions can minimise undesirable off-target photodamage often observed with traditional photosensitisers, which display “always-on” photosensitisation properties and lack target selectivity.Luminescent and photofunctional transition metal complexes have gained significant attention as phototheranostics due to their attractive photophysical and photochemical properties, including high photostability, long-lived and environment-sensitive emission, as well as efficient ROS photosensitisation.15–19 We have a long-standing interest in the development of these complexes as bioorthogonal reagents for various biological and biomedical applications.20 In 2016, we demonstrated for the first time that nitrone, a 1,3-dipole that can selectively react with cyclooctynes via the strain-promoted alkyne–nitrone cycloaddition (SPANC) reaction,21 can serve as an emission quencher for transition metal complexes, providing a new avenue for the development of phosphorogenic bioorthogonal probes.22,23 The nitrone-modified complexes are non-emissive in solutions, but exhibit significant emission enhancement upon reaction with bicyclo[6.1.0]non-4-yne (BCN) derivatives (Scheme 1). This modification also allows for the modulation of the ROS photosensitisation efficiencies of the complexes, enabling controlled activation of their emission and ROS generation behaviour in targeted cells.24,25 Thus, transition metal nitrone complexes represent a promising scaffold for the development of bioorthogonally activatable probes and photosensitisers.
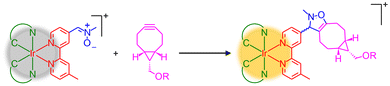 | ||
| Scheme 1 The SPANC reaction of phosphorogenic iridium(III) nitrone complexes with BCN derivatives leading to the formation of luminescent isoxazoline cycloadducts. | ||
Mitochondria are crucial subcellular organelles involved in a wide range of important biological processes including energy production,26 biomolecular synthesis,26 calcium signalling,27 as well as cell proliferation and death.28 Mitochondrial dysfunction can lead to various diseases such as cancer29 and neurodegenerative disorders.30 Given their pivotal role in maintaining cellular functions, mitochondria have become an important target for cancer therapy.31 Mitochondria possess a negative membrane potential (ca. −120 to −180 mV), and it has been reported that the mitochondria in cancer cells are more hyperpolarised than in normal cells due to their higher metabolic activity.32 Thus, lipophilic cations such as triphenylphosphonium cation (TPP+) preferentially accumulate in the mitochondria over other subcellular organelles, resulting in ca. 100–1000-fold higher intramitochondrial concentrations.33 These moieties have been engineered with various bioorthogonal handles to precisely direct fluorescent/fluorogenic bioorthogonal probes to the mitochondria, facilitating the imaging of these organelles in live cells.34–46 This approach enables the monitoring of mitochondrial membrane potential changes47 and mitophagy,48 as well as the activation of mitochondria-enriched prodrugs for applications in cancer therapy49–53 and cardioprotection.54 However, the use of a two-step bioorthogonal approach for delivering photoactive transition metal complexes to the mitochondria, and controlled activation of their emission and ROS photosensitisation properties for bioimaging and PDT applications remains unexplored.
In this work, we designed, synthesised and characterised a new cyclometallated iridium(III) nitrone complex [Ir(bpz)2(bpy-nitrone)](PF6) (1) (Hbpz = benzo[a]phenazine; bpy-nitrone = 4-((methyl(oxido)imino)methyl)-4′-methyl-2,2′-bipyridine) (Scheme 2) as a bioorthogonally activatable phototheranostic agent. The Hbpz ligand was selected because its metal complexes show near-infrared (NIR) emission and high singlet oxygen (1O2) generation efficiencies.55 Additionally, we prepared a series of BCN-modified phosphonium cations (BCN-Phos-n) (Scheme 2) as mitochondrial-targeting vectors to direct the nitrone complex to the mitochondria via the SPANC reaction. Specifically, BCN-Phos-1–BCN-Phos-4 carried varying numbers of cyclohexyl (Cy) and phenyl (Ph) moieties on the phosphonium cation to tune their aromaticity;56–58 while BCN-Phos-5–BCN-Phos-7 contained different substituents on the phenyl rings of the TPP+ unit, including two methyl (BCN-Phos-5) or methoxy groups (BCN-Phos-6) at the meta-positions to enhance their lipophilicity,59–62 or a di(ethylene glycol) pendant at the para-position (BCN-Phos-7) to increase its aqueous solubility and biocompatibility.57,58
Results and discussion
Synthesis and characterisation of complex 1 and BCN-Phos-n
The synthesis of complex 1 involved the reaction of the dichloro-bridged iridium(III) dimer [Ir2(bpz)4Cl2] with the ligand bpy-nitrone in CH2Cl2/MeOH (Scheme 3a), followed by anion exchange with KPF6 and purification by column chromatography and recrystallisation from CH2Cl2/Et2O. The complex was characterised by high resolution (HR)-ESI-MS, 1H and 13C NMR and IR spectroscopy, and gave satisfactory elemental analyses (ESI†).The BCN-modified phosphonium cations BCN-Phos-n were synthesised using the corresponding phosphine precursors (Phos-n) (Scheme 3b), which were either purchased from commercial suppliers (for Phos-1–Phos-4) or prepared according to previously reported protocols (for Phos-5–Phos-7).63,64 The phosphine precursors were reacted with 3-bromopropylamine hydrobromide in n-butanol under reflux for 3 days, in a procedure adapted from Zhou and co-workers.65 The resultant amine-functionalised phosphines (H2N-Phos-n) were obtained as ammonium salts in good yields (44–62%) after purification by recrystallisation from isopropanol/Et2O, except for H2N-Phos-1 which did not precipitate out of Et2O and was, therefore, used in subsequent steps without further purification. Amide coupling of H2N-Phos-n with (1R,8S,9S)-bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate (BCN–NHS) was achieved under mild basic conditions.22–25 All BCN-Phos-n analogues were obtained in average to good yields (45–78%) after purification by silica gel column chromatography. These compounds were characterised by HR-ESI-MS and 1H, 13C and 31P NMR spectroscopy (ESI†).
Photophysical and photochemical properties of complex 1
Complex 1 displayed intense spin-allowed intraligand (1IL) (π → π*) (bpy-nitrone and bpz) absorption in the UV region (ca. 274–431 nm, ε on the order of 104 dm3 mol−1 cm−1) and weaker spin-allowed metal-to-ligand charge-transfer (1MLCT) (dπ(Ir) → π*(bpy-nitrone and bpz)) absorption features in the visible region (ca. 456–550 nm) (Fig. 1a and Table S1, ESI†).25,55 The strong absorption in the region of 500–600 nm is an attractive feature because it allows for efficient photoexcitation using green light. The weaker absorption tailing beyond ca. 615 nm is assigned to spin-forbidden 3MLCT (dπ(Ir) → π*(bpy-nitrone and bpz)) transitions. Upon photoirradiation, the complex exhibited NIR emission in fluid solutions at 298 K (Table 1 and Fig. 1b). Additionally, it showed a vibronically structured emission band with an extraordinarily long emission lifetime (8.79 μs) in an alcohol glass at 77 K (Table 1 and Fig. 1b). These observations suggest that the emission of the complex originates from a predominant 3IL (π → π*) (bpz) excited state with possible mixing of some 3MLCT (dπ(Ir) → π*(bpy-nitrone/bpz)) character.25,55 Similar emission features were observed when the complex was excited at 550 nm, indicating that the emission is largely independent of the excitation wavelength. Importantly, the emission quantum yields of the complex (Φem ≤ 0.0047; Table 1) were significantly lower than those of related bpz complexes,55 indicating efficient emission quenching by the appended nitrone unit.22–25 Additionally, the 1O2 generation quantum yield (ΦΔ) of the complex was determined by monitoring the emission band of 1O2 centred at ca. 1270 nm in aerated CH3CN. The small ΦΔ value (0.05; Table 1) indicates strong suppression of the 1O2 photosensitisation capability of the complex by the quenching nitrone moiety. | ||
| Fig. 1 (a) Electronic absorption and (b) normalised emission spectra of complex 1 in CH2Cl2 (black) and CH3CN (red) at 298 K and in alcohol glass at 77 K (blue). | ||
| Complex | Medium (T/K) | λ em /nm | τ o /μs | Φ em | Φ Δ |
|---|---|---|---|---|---|
a
λ
ex = 350 nm.
b The lifetimes were measured at the emission maxima (λex = 355 nm).
c The Φem values were determined in degassed solvents using [Ru(bpy)3]Cl2 (Φem = 0.040 in aerated H2O, λex = 455 nm)66 as a reference.
d The ΦΔ values were determined in aerated solvents using [Ru(bpy)3]Cl2 (ΦΔ = 0.57 in aerated CH3CN, λex = 450 nm)67 as a reference.
e EtOH/MeOH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1, v/v).
|
|||||
| 1 | CH2Cl2 (298) | 664 | 4.80 | 0.0047 | |
| CH3CN (298) | 668 | 2.97 | 0.0043 | 0.05 | |
| Glasse (77) | 668, 730 sh | 8.79 | |||
| 1-BCN | CH2Cl2 (298) | 666 | 4.28 | 0.072 | |
| CH3CN (298) | 668 | 2.67 | 0.062 | 0.76 | |
| Glasse (77) | 667, 729 sh | 9.63 | |||
Bioorthogonal reactivity and phosphorogenic response of complex 1
We utilised the strained alkyne (1R,8S,9S)-bicyclo[6.1.0]non-4-yn-9-ylmethanol (BCN–OH) as a model substrate to examine the bioorthogonal reactivity of the nitrone complex. The second-order rate constant (k2) of the SPANC reaction of the complex with BCN–OH in MeOH at 298 K was determined to be 0.3309 M−1 s−1 (Fig. S1, ESI†), which is 8.3 times greater than that of the free ligand bpy-nitrone (k2 = 0.040 M−1 s−1).22 The accelerated reaction kinetics can be attributed to the direct coordination of the nitrone ligand to the cationic iridium(III) polypyridine unit, which enhanced its reactivity.22–25 Importantly, upon the SPANC reaction with BCN–OH in aerated phosphate-buffered saline (PBS; pH 7.4)/MeOH (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), the complex showed substantial emission enhancement (I/Io = 5.8; Table 2 and Fig. 2a) and lifetime extension (τ increased from 0.05 to 0.13 μs; Table 2), resulting from the conversion of the quenching nitrone moiety to a non-quenching isoxazoline derivative. The formation of the isoxazoline product 1-BCN was verified by ESI-MS analysis (Fig. S2, ESI†). Conjugate 1-BCN was isolated and purified, and its photophysical and 1O2-photogeneration properties were investigated. Both Φem (0.062–0.072; Table 1) and ΦΔ (0.76; Table 1) values of conjugate 1-BCN are larger than those of complex 1 (Φem ≤ 0.0047, ΦΔ = 0.05; Table 1). These results confirm that both the emission and 1O2-photosensitisation properties of complex 1 can be activated through the bioorthogonal SPNAC reaction with BCN derivatives, which effectively eliminates the nitrone-associated quenching pathway.
1, v/v), the complex showed substantial emission enhancement (I/Io = 5.8; Table 2 and Fig. 2a) and lifetime extension (τ increased from 0.05 to 0.13 μs; Table 2), resulting from the conversion of the quenching nitrone moiety to a non-quenching isoxazoline derivative. The formation of the isoxazoline product 1-BCN was verified by ESI-MS analysis (Fig. S2, ESI†). Conjugate 1-BCN was isolated and purified, and its photophysical and 1O2-photogeneration properties were investigated. Both Φem (0.062–0.072; Table 1) and ΦΔ (0.76; Table 1) values of conjugate 1-BCN are larger than those of complex 1 (Φem ≤ 0.0047, ΦΔ = 0.05; Table 1). These results confirm that both the emission and 1O2-photosensitisation properties of complex 1 can be activated through the bioorthogonal SPNAC reaction with BCN derivatives, which effectively eliminates the nitrone-associated quenching pathway.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at 298 K for 24 h
1, v/v) at 298 K for 24 h
| Entry | λ em | I/Ioa | τ/μs |
|---|---|---|---|
| a I o and I are the emission intensities of the complex (10 μM) in the absence and presence of BCN–OH or BCN-Phos-n (250 μM), respectively. | |||
| 1 | 695 | — | 0.05 |
| 1 + BCN–OH | 684 | 5.8 | 0.13 |
| 1 + BCN-Phos-1 | 687 | 4.2 | 0.15 |
| 1 + BCN-Phos-2 | 690 | 3.8 | 0.14 |
| 1 + BCN-Phos-3 | 703 | 3.1 | 0.12 |
| 1 + BCN-Phos-4 | 686 | 3.3 | 0.13 |
| 1 + BCN-Phos-5 | 674 | 24.7 | 0.33 |
| 1 + BCN-Phos-6 | 677 | 14.1 | 0.25 |
| 1 + BCN-Phos-7 | 706 | 4.5 | 0.19 |
We also investigated the phosphorogenic response of complex 1 towards the BCN-Phos-n derivatives. Incubation of complex 1 with the BCN-Phos-n analogues in aerated aqueous buffers led to substantial emission enhancement (I/Io = 3.1–24.7) and lifetime extension (τ = 0.12–0.33 μs) (Table 2 and Fig. 2b). Notably, the BCN-Phos-5 and BCN-Phos-6 treatment resulted in a larger increase in emission intensity (I/Io = 24.7 and 14.1) and lifetime (τ = 0.33 and 0.25 μs), accompanied by a notable blue shift in the emission maximum from 695 nm to 674 and 677 nm, respectively (Table 2 and Fig. 2b). The more significant photophysical changes compared to other BCN-Phos-n analogues are likely due to the formation of a more hydrophobic pendant after reaction with BCN-Phos-5 and BCN-Phos-6, which feature two lipophilic methyl or methoxy groups on each of the phenyl rings of the TPP+ unit, resulting in a greater reduction in the polarity of the proximal environment of the complex. Such a result aligns with our previous observations that luminescent iridium(III) polypyridine complexes display higher emission intensities and longer lifetimes in less polar solvents or upon bioconjugation to proteins.68–73
Cellular uptake, localisation and (photo)cytotoxicity of complex 1
We then studied the phosphorogenic response of the nitrone complex towards the BCN-Phos-n derivatives in live HeLa cells. The cells were first incubated with BCN-Phos-n (5 μM) for 2 h, washed with PBS (pH 7.4), and then treated with complex 1 (5 μM) for an additional 2 h prior to imaging. Laser-scanning confocal microscopy (LSCM) images reveal negligible emission from HeLa cells incubated with complex 1 (Fig. 3a). However, intense intracellular emission was observed upon pretreatment of the cells with BCN-Phos-5 or BCN-Phos-6 (Fig. 3a). Flow cytometric analysis confirmed that complex 1-treated cells exhibited a 2.15- and 2.94-fold increase in emission intensity when pretreated with BCN-Phos-5 and BCN-Phos-6, respectively (Fig. 3b and Table S2, ESI†). Additionally, ICP-MS analysis indicated that the cellular uptake of complex 1 remained similar without (1.83 fmol per cell) and with pretreatment of BCN-Phos-5 and BCN-Phos-6 (1.77 and 1.74 fmol per cell, respectively) (Table S3, ESI†). These results confirm that the observed intracellular emission enhancement is attributable to the SPANC reaction of complex 1 with BCN-Phos-5 and BCN-Phos-6, rather than an increase in cellular accumulation of the complex. However, similar emission enhancement was not observed for cells pretreated with other BCN-Phos-n derivatives (Fig. 3a and S3 and Table S2, ESI†), consistent with their smaller emission enhancement in solutions (I/Io = 3.1–4.5; Table 2 and Fig. 2b). Co-staining experiments with MitoTracker Green showed that the luminescent isoxazoline cycloadducts formed from the reaction of complex 1 with BCN-Phos-5 and BCN-Phos-6 were enriched in the mitochondrial region of the cells, with Pearson's correlation coefficients (PCC's) of 0.79 and 0.81, respectively (Fig. 4). The mitochondrial accumulation of the isoxazoline cycloadducts is likely due to their cationic and lipophilic character.74–80We also examined the (photo)cytotoxicity of complex 1 towards HeLa cells with or without BCN-Phos-n pretreatment using the Neutral Red uptake (NRU) assay. Complex 1 exhibited moderate dark cytotoxicity (IC50,dark = 18 μM) and substantially enhanced photocytotoxic activity (IC50,light = 0.37 μM) upon irradiation at 525 nm (10 mW cm−2) for 5 min (Fig. 5a and Table S4, ESI†). Notably, the (photo)cytotoxicity of the complex was further increased when the cells were pretreated with BCN-Phos-5 or BCN-Phos-6, with IC50,dark values decreasing to 9.6 and 6.3 μM and IC50,light values decreasing to 0.14 and 0.22 μM, respectively (Fig. 5b and c and Table S4, ESI†). The enhanced dark cytotoxicity of the complex can be attributed to its increased accumulation in the mitochondria after the SPANC reaction with BCN-Phos-5 or BCN-Phos-6, which probably interferes with mitochondrial functions.79 Notably, the photocytotoxicity of the complex was further enhanced following the reaction, which is attributed to the increased 1O2 generation by the resultant isoxazoline cycloadducts. These results highlight that the therapeutic potential of the complex can be enhanced via a judicious selection of its bioorthogonal reaction partners.
Conclusions
In summary, we developed a novel iridium(III) nitrone complex as a bioorthogonally activatable phototheranostic agent, and a series of BCN-modified phosphonium cations serving as mitochondrial-targeting vectors to direct the nitrone complex to the mitochondria via the bioorthogonal SPANC reaction for imaging and PDT applications. Notably, the complex displayed more pronounced emission turn-on upon reaction with BCN-Phos-5 and BCN-Phos-6 compared to other BCN-Phos-n analogues, attributed to the presence of additional hydrophobic methyl or methoxy groups on the phenyl rings of the TPP+ unit that resulted in a more hydrophobic pendant. Similar emission changes were observed in live cells pretreated with BCN-Phos-5 or BCN-Phos-6. Owing to their cationic and lipophilic character, the resultant luminescent isoxazoline cycloadducts were enriched in the mitochondria. Importantly, the (photo)cytotoxicity of the complex further increased when the cells were pretreated with BCN-Phos-5 or BCN-Phos-6. Our findings demonstrate that the theranostic potential of transition metal nitrone complexes can be enhanced through the strategic structural manipulation of their bioorthogonal reaction partners. We believe that our work will contribute to the development of effective mitochondria-targeting agents for diagnostic and therapeutic applications.Author contributions
E. R. H. W., L. C.-C. L., P. K.-K. L., K. K.-W. L. and N. J. L. designed the project. E. R. H. W. carried out the synthesis and characterisation of the phosphonium cations. L. C.-C. L. carried out the synthesis and characterisation of the iridium(III) nitrone complex and the cellular studies. P. K.-K. L. carried out the photophysical measurements and cellular studies. E. R. H. W., L. C.-C. L., P. K.-K. L., K. K.-W. L. and N. J. L. analysed the data and wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
We confirm that all the relevant research data is contained with the manuscript and ESI.† No databases have been used and no references to such databases are contained in the manuscript or ESI.†Acknowledgements
We thank the Laboratory for Synthetic Chemistry and Chemical Biology (LSCCB) under the Health@InnoHK Programme launched by Innovation and Technology Commission, The Government of Hong Kong SAR, P. R. China.References
- E. M. Sletten and C. R. Bertozzi, Bioorthogonal chemistry: fishing for selectivity in a sea of functionality, Angew. Chem., Int. Ed., 2009, 48, 6974 CrossRef CAS.
- S. L. Scinto, D. A. Bilodeau, R. Hincapie, W. Lee, S. S. Nguyen, M. Xu, C. W. am Ende, M. G. Finn, K. Lang, Q. Lin, J. P. Pezacki, J. A. Prescher, M. S. Robillard and J. M. Fox, Bioorthogonal chemistry, Nat. Rev. Methods Primers, 2021, 1, 30 Search PubMed.
- J. C. Jewett and C. R. Bertozzi, Cu-free click cycloaddition reactions in chemical biology, Chem. Soc. Rev., 2010, 39, 1272 RSC.
- B. L. Oliveira, Z. Guo and G. J. L. Bernardes, Inverse electron demand Diels–Alder reactions in chemical biology, Chem. Soc. Rev., 2017, 46, 4895 Search PubMed.
- T. K. Heiss, R. S. Dorn and J. A. Prescher, Bioorthogonal reactions of triarylphosphines and related analogues, Chem. Rev., 2021, 121, 6802 CrossRef CAS.
- X. Ji, Z. Pan, B. Yu, L. K. De La Cruz, Y. Zheng, B. Ke and B. Wang, Click and release: bioorthogonal approaches to “on-demand” activation of prodrugs, Chem. Soc. Rev., 2019, 48, 1077 Search PubMed.
- D. Wu, K. Yang, Z. Zhang, Y. Feng, L. Rao, X. Chen and G. Yu, Metal-free bioorthogonal click chemistry in cancer theranostics, Chem. Soc. Rev., 2022, 51, 1336 RSC.
- Q. Fu, S. Shen, P. Sun, Z. Gu, Y. Bai, X. Wang and Z. Liu, Bioorthogonal chemistry for prodrug activation in vivo, Chem. Soc. Rev., 2023, 52, 7737 Search PubMed.
- V. Rigolot, C. Biot and C. Lion, To view your biomolecule, click inside the cell, Angew. Chem., Int. Ed., 2021, 60, 23084 CrossRef CAS.
- P. Shieh and C. R. Bertozzi, Design strategies for bioorthogonal smart probes, Org. Biomol. Chem., 2014, 12, 9307 RSC.
- Y. Fu, X. Zhang, L. Wu, M. Wu, T. D. James and R. Zhang, Bioorthogonally activated probes for precise fluorescence imaging, Chem. Soc. Rev., 2025, 54, 201 RSC.
- A. Yu, X. He, T. Shen, X. Yu, W. Mao, W. Chi, X. Liu and H. Wu, Chem. Soc. Rev., 2025, 54, 2984, 10.1039/D3CS00520H.
- L. K. B. Tam and D. K. P. Ng, “Click” for precise photodynamic therapy, Mater. Chem. Front., 2023, 7, 3184 RSC.
- E. Kozma, M. Bojtár and P. Kele, Bioorthogonally assisted phototherapy: recent advances and prospects, Angew. Chem., Int. Ed., 2023, 62, e202303198 CrossRef CAS.
- Y. Chen, R. Guan, C. Zhang, J. Huang, L. Ji and H. Chao, Two-photon luminescent metal complexes for bioimaging and cancer phototherapy, Coord. Chem. Rev., 2016, 310, 16 CrossRef CAS.
- J. Li and T. Chen, Transition metal complexes as photosensitizers for integrated cancer theranostic applications, Coord. Chem. Rev., 2020, 418, 213355 CrossRef CAS.
- C.-P. Tan, Y.-M. Zhong, L.-N. Ji and Z.-W. Mao, Phosphorescent metal complexes as theranostic anticancer agents: combining imaging and therapy in a single molecule, Chem. Sci., 2021, 12, 2357 RSC.
- L. C.-C. Lee and K. K.-W. Lo, Luminescent and photofunctional transition metal complexes: from molecular design to diagnostic and therapeutic applications, J. Am. Chem. Soc., 2022, 144, 14420 CrossRef CAS.
- L. C.-C. Lee and K. K.-W. Lo, Leveraging the photofunctions of transition metal complexes for the design of innovative phototherapeutics, Small Methods, 2024, 8, 2400563 CrossRef CAS.
- K. K.-W. Lo, Molecular design of bioorthogonal probes and imaging reagents derived from photofunctional transition metal complexes, Acc. Chem. Res., 2020, 53, 32 CrossRef CAS.
- D. A. Bilodeau, K. D. Margison, M. Serhan and J. P. Pezacki, Bioorthogonal reactions utilizing nitrones as versatile dipoles in cycloaddition reactions, Chem. Rev., 2021, 121, 6699 CrossRef CAS.
- L. C.-C. Lee, J. C.-W. Lau, H.-W. Liu and K. K.-W. Lo, Conferring phosphorogenic properties on iridium(III)-based bioorthogonal probes through modification with a nitrone unit, Angew. Chem., Int. Ed., 2016, 55, 1046 CrossRef CAS.
- T. S.-M. Tang, H.-W. Liu and K. K.-W. Lo, Structural manipulation of ruthenium(II) polypyridine nitrone complexes to generate phosphorogenic bioorthogonal reagents for selective cellular labeling, Chem. – Eur. J., 2016, 22, 9649 CrossRef CAS.
- P. K.-K. Leung and K. K.-W. Lo, Modulation of emission and singlet oxygen photosensitisation in live cells utilising bioorthogonal phosphorogenic probes and protein tag technology, Chem. Commun., 2020, 56, 6074 RSC.
- E. C.-L. Mak, Z. Chen, L. C.-C. Lee, P. K.-K. Leung, A. M.-H. Yip, J. Shum, S.-M. Yiu, V. W.-W. Yam and K. K.-W. Lo, Exploiting the potential of iridium(III) bis-nitrone complexes as phosphorogenic bifunctional reagents for phototheranostics, J. Am. Chem. Soc., 2024, 146, 25589 CrossRef CAS.
- J. B. Spinelli and M. C. Haigis, The multifaceted contributions of mitochondria to cellular metabolism, Nat. Cell Biol., 2018, 20, 745 CrossRef CAS.
- R. Rizzuto, D. De Stefani, A. Raffaello and C. Mammucari, Mitochondria as sensors and regulators of calcium signalling, Nat. Rev. Mol. Cell Biol., 2012, 13, 566 CrossRef CAS.
- D. R. Green and J. C. Reed, Mitochondria and apoptosis, Science, 1998, 281, 1309 CrossRef CAS.
- S. Vyas, E. Zaganjor and M. C. Haigis, Mitochondria and cancer, Cell, 2016, 166, 555 CrossRef CAS.
- M. T. Lin and M. F. Beal, Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases, Nature, 2006, 443, 787 CrossRef CAS.
- S. Fulda, L. Galluzzi and G. Kroemer, Targeting mitochondria for cancer therapy, Nat. Rev. Drug Discovery, 2010, 9, 447 CrossRef CAS.
- L. B. Chen, Mitochondrial membrane potential in living cells, Annu. Rev. Cell Biol., 1988, 4, 155 CrossRef CAS.
- J. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications, Chem. Rev., 2017, 117, 10043 CrossRef CAS.
- S. H. Alamudi, R. Satapathy, J. Kim, D. Su, H. Ren, R. Das, L. Hu, E. Alvarado-Martínez, J. Y. Lee, C. Hoppmann, E. Peña-Cabrera, H.-H. Ha, H.-S. Park, L. Wang and Y.-T. Chang, Development of background-free tame fluorescent probes for intracellular live cell imaging, Nat. Commun., 2016, 7, 11964 CrossRef CAS.
- A. Vázquez, R. Dzijak, M. Dračínský, R. Rampmaier, S. J. Siegl and M. Vrabel, Mechanism-based fluorogenic trans-cyclooctene–tetrazine cycloaddition, Angew. Chem., Int. Ed., 2017, 56, 1334 CrossRef.
- Y. Lee, W. Cho, J. Sung, E. Kim and S. B. Park, Monochromophoric design strategy for tetrazine-based colorful bioorthogonal probes with a single fluorescent core skeleton, J. Am. Chem. Soc., 2018, 140, 974 CrossRef CAS.
- S. H. Alamudi, D. Su, K. J. Lee, J. Y. Lee, J. L. Belmonte-Vázquez, H.-S. Park, E. Peña-Cabrera and Y.-T. Chang, A palette of background-free tame fluorescent probes for intracellular multi-color labelling in live cells, Chem. Sci., 2018, 9, 2376 RSC.
- P. Werther, K. Yserentant, F. Braun, N. Kaltwasser, C. Popp, M. Baalmann, D.-P. Herten and R. Wombacher, Live-cell localization microscopy with a fluorogenic and self-blinking tetrazine probe, Angew. Chem., Int. Ed., 2020, 59, 804 CrossRef CAS.
- M. Bojtár, K. Németh, F. Domahidy, G. Knorr, A. Verkman, M. Kállay and P. Kele, Conditionally activatable visible-light photocages, J. Am. Chem. Soc., 2020, 142, 15164 CrossRef.
- W. Mao, J. Tang, L. Dai, X. He, J. Li, L. Cai, P. Liao, R. Jiang, J. Zhou and H. Wu, A general strategy to design highly fluorogenic far-red and near-infrared tetrazine bioorthogonal probes, Angew. Chem., Int. Ed., 2021, 60, 2393 CrossRef CAS.
- W. Mao, W. Chi, X. He, C. Wang, X. Wang, H. Yang, X. Liu and H. Wu, Overcoming spectral dependence: a general strategy for developing far-red and near-infrared ultra-fluorogenic tetrazine bioorthogonal probes, Angew. Chem., Int. Ed., 2022, 61, e202117386 CrossRef CAS.
- D. Kim, H. Son and S. B. Park, Ultrafluorogenic monochromophore-type BODIPY-tetrazine series for dual-color bioorthogonal imaging with a single probe, Angew. Chem., Int. Ed., 2023, 62, e202310665 CrossRef CAS.
- S. Segawa, J. Wu, R. T. K. Kwok, T. T. W. Wong, X. He and B. Z. Tang, Co-aggregation as a simple strategy for preparing fluorogenic tetrazine probes with on-demand fluorogen selection, Angew. Chem., Int. Ed., 2024, 63, e202313930 CrossRef CAS.
- Y. Deng, T. Shen, X. Yu, J. Li, P. Zou, Q. Gong, Y. Zheng, H. Sun, X. Liu and H. Wu, Tetrazine-isonitrile bioorthogonal fluorogenic reactions enable multiplex labeling and wash-free bioimaging of live cells, Angew. Chem., Int. Ed., 2024, 63, e202319853 CrossRef CAS.
- S. Segawa, X. Ou, T. Shen, T. Ryu, Y. Ishii, H. H. Y. Sung, I. D. Williams, R. T. K. Kwok, K. Onda, K. Miyata, X. He, X. Liu and B. Z. Tang, Matthew effect: general design strategy of ultra-fluorogenic nanoprobes with amplified dark–bright states in aggregates, Aggregate, 2024, 5, e499 CrossRef CAS.
- H. Son, D. Kim, S. Kim, W. G. Byun and S. B. Park, Unveiling the structure-fluorogenic property relationship of Seoul-Fluor-derived bioorthogonal tetrazine probes, Angew. Chem., Int. Ed., 2025, 64, e202421982 CrossRef CAS.
- Z. Xue, R. Zhu, S. Wang, J. Li, J. Han, J. Liu and S. Han, Organelle-directed Staudinger reaction enabling fluorescence-on resolution of mitochondrial electropotentials via a self-immolative charge reversal probe, Anal. Chem., 2018, 90, 2954 CrossRef CAS.
- Y. Shi, X. Zou, S. Wen, L. Gao, J. Li, J. Han and S. Han, An organelle-directed chemical ligation approach enables dual-color detection of mitophagy, Autophagy, 2021, 17, 3475 CrossRef CAS.
- Y. Zheng, X. Ji, B. Yu, K. Ji, D. Gallo, E. Csizmadia, M. Zhu, M. R. Choudhury, L. K. C. De La Cruz, V. Chittavong, Z. Pan, Z. Yuan, L. E. Otterbein and B. Wang, Enrichment-triggered prodrug activation demonstrated through mitochondria-targeted delivery of doxorubicin and carbon monoxide, Nat. Chem., 2018, 10, 787 CrossRef CAS.
- R. Dzijak, J. Galeta, A. Vázquez, J. Kozák, M. Matoušová, H. Fulka, M. Dračínský and M. Vrabel, Structurally redesigned bioorthogonal reagents for mitochondria-specific prodrug activation, JACS Au, 2021, 1, 23 CrossRef CAS.
- M. Liu, Z. Liu, G. Qin, J. Ren and X. Qu, Bioorthogonally activatable autophagy-tethering compounds for aptamer-guided mitochondrial degradation, Nano Lett., 2023, 23, 4965 CrossRef CAS.
- J. Kim, Y. Xu, J. H. Lim, J. Y. Lee, M. Li, J. M. Fox, M. Vendrell and J. S. Kim, Bioorthogonal activation of deep red photoredox catalysis inducing pyroptosis, J. Am. Chem. Soc., 2025, 147, 701 CrossRef.
- B.-L. Li, S. Li, C. Zhang, Y. Zhou, X. Zhao and Z. Yu, Photoclick and release for spatiotemporally localized theranostics of single cells via in situ generation of 1,3-diaryl-1H-benzo[f]indazole-4,9-dione, Angew. Chem., Int. Ed., 2025, 64, e202416111 CrossRef CAS.
- Y. Chen, R. Zhao, C. Tang, C. Zhang, W. Xu, L. Wu, Y. Wang, D. Ye and Y. Liang, Design and development of a bioorthogonal, visualizable and mitochondria-targeted hydrogen sulfide (H2S) delivery system, Angew. Chem., Int. Ed., 2022, 61, e202112734 CrossRef CAS.
- L. Huang, P. K.-K. Leung, L. C.-C. Lee, G.-X. Xu, Y.-W. Lam and K. K.-W. Lo, Photofunctional cyclometallated iridium(III) polypyridine methylsulfone complexes as sulfhydryl-specific reagents for bioconjugation, bioimaging and photocytotoxic applications, Chem. Commun., 2022, 58, 10162 RSC.
- T. I. Rokitskaya, E. A. Kotova, V. B. Luzhkov, R. S. Kirsanov, E. V. Aleksandrova, G. A. Korshunova, V. N. Tashlitsky and Y. N. Antonenko, Lipophilic ion aromaticity is not important for permeability across lipid membranes, Biochim. Biophys. Acta, Biomembr., 2021, 1863, 183483 CrossRef CAS.
- E. R. H. Walter, L. C.-C. Lee, P. K.-K. Leung, K. K.-W. Lo and N. J. Long, Mitochondria-targeting biocompatible fluorescent BODIPY probes, Chem. Sci., 2024, 15, 4846 RSC.
- E. R. H. Walter, P. K.-K. Leung, L. C.-C. Lee, K. K.-W. Lo and N. J. Long, Potent BODIPY-based photosensitisers for selective mitochondrial dysfunction and effective photodynamic therapy, J. Mater. Chem. B, 2024, 12, 10409 RSC.
- Z. Hu, Y. Sim, O. L. Kon, W. H. Ng, A. J. M. Ribeiro, M. J. Ramos, P. A. Fernandes, R. Ganguly, B. Xing, F. García and E. K. L. Yeow, Unique triphenylphosphonium derivatives for enhanced mitochondrial uptake and photodynamic therapy, Bioconjugate Chem., 2017, 28, 590 CrossRef CAS.
- A. J. Smith, P. J. Gawne, M. T. Ma, P. J. Blower, R. Southworth and N. J. Long, Synthesis, gallium-68 radiolabelling and biological evaluation of a series of triarylphosphonium-functionalized DO3A chelators, Dalton Trans., 2018, 47, 15448 RSC.
- A. J. Smith, B. E. Osborne, G. P. Keeling, P. J. Blower, R. Southworth and N. J. Long, DO2A-based ligands for gallium-68 chelation: synthesis, radiochemistry and ex vivo cardiac uptake, Dalton Trans., 2020, 49, 1097 RSC.
- B. E. Osborne, T. T. C. Yue, E. C. T. Waters, F. Baark, R. Southworth and N. J. Long, Synthesis and ex vivo biological evaluation of gallium-68 labelled NODAGA chelates assessing cardiac uptake and retention, Dalton Trans., 2021, 50, 14695 RSC.
- A. Haslop, L. Wells, A. Gee, C. Plisson and N. Long, One-pot multi-tracer synthesis of novel 18F-labeled PET imaging agents, Mol. Pharmaceutics, 2014, 11, 3818 CrossRef CAS.
- M. Jiang, J. Wu, W. Liu, H. Ren, W. Zhang, C.-S. Lee and P. Wang, Self-assembly of amphiphilic porphyrins to construct nanoparticles for highly efficient photodynamic therapy, Chem. – Eur. J., 2021, 27, 11195 CrossRef CAS.
- Q. Zeng, Q. Guo, Y. Yuan, Y. Yang, B. Zhang, L. Ren, X. Zhang, Q. Luo, M. Liu, L.-S. Bouchard and X. Zhou, Mitochondria targeted and intracellular biothiol triggered hyperpolarized 129Xe magnetofluorescent biosensor, Anal. Chem., 2017, 89, 2288 CrossRef CAS.
- K. Suzuki, A. Kobayashi, S. Kaneko, K. Takehira, T. Yoshihara, H. Ishida, Y. Shiina, S. Oishi and S. Tobita, Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector, Phys. Chem. Chem. Phys., 2009, 11, 9850 RSC.
- A. A. Abdel-Shafi, P. D. Beer, R. J. Mortimer and F. Wilkinson, Photosensitized generation of singlet oxygen from vinyl linked benzo-crown-ether–bipyridyl ruthenium(II) complexes, J. Phys. Chem. A, 2000, 104, 192 CrossRef CAS.
- K. K.-W. Lo, J. S.-W. Chan, L.-H. Lui and C.-K. Chung, Novel luminescent cyclometalated iridium(III) diimine complexes that contain a biotin moiety, Organometallics, 2004, 23, 3108 CrossRef CAS.
- K. K.-W. Lo, C.-K. Chung and N. Zhu, Nucleic acid intercalators and avidin probes derived from luminescent cyclometalated iridium(III)–dipyridoquinoxaline and –dipyridophenazine complexes, Chem. – Eur. J., 2006, 12, 1500 CrossRef CAS.
- K. K.-W. Lo, K. Y. Zhang, C.-K. Chung and K. Y. Kwok, Synthesis, photophysical and electrochemical properties, and protein-binding studies of luminescent cyclometalated iridium(III) bipyridine estradiol conjugates, Chem. – Eur. J., 2007, 13, 7110 CrossRef CAS.
- K. K.-W. Lo, K. Y. Zhang, S.-K. Leung and M.-C. Tang, Exploitation of the dual-emissive properties of cyclometalated iridium(III)–polypyridine complexes in the development of luminescent biological probes, Angew. Chem., Int. Ed., 2008, 47, 2213 CrossRef CAS.
- J. S.-Y. Lau, P.-K. Lee, K. H.-K. Tsang, C. H.-C. Ng, Y.-W. Lam, S.-H. Cheng and K. K.-W. Lo, Luminescent cyclometalated iridium(III) polypyridine indole complexes–synthesis, photophysics, electrochemistry, protein-binding properties, cytotoxicity, and cellular uptake, Inorg. Chem., 2009, 48, 708 CrossRef CAS.
- L. C.-C. Lee, H. M.-H. Cheung, H.-W. Liu and K. K.-W. Lo, Exploitation of environment-sensitive luminophores in the design of sydnone-based bioorthogonal imaging reagents, Chem. – Eur. J., 2018, 24, 14064 CrossRef CAS.
- Q. Zhang, R. Cao, H. Fei and M. Zhou, Mitochondria-targeting phosphorescent iridium(III) complexes for living cell imaging, Dalton Trans., 2014, 43, 16872 RSC.
- J. Liu, Y. Chen, G. Li, P. Zhang, C. Jin, L. Zeng, L. Ji and H. Chao, Ruthenium(II) polypyridyl complexes as mitochondria-targeted two-photon photodynamic anticancer agents, Biomaterials, 2015, 56, 140 CrossRef CAS.
- W. Lv, Z. Zhang, K. Y. Zhang, H. Yang, S. Liu, A. Xu, S. Guo, Q. Zhao and W. Huang, A mitochondria-targeted photosensitizer showing improved photodynamic therapy effects under hypoxia, Angew. Chem., Int. Ed., 2016, 55, 9947 CrossRef CAS.
- H. Yuan, Z. Han, Y. Chen, F. Qi, H. Fang, Z. Guo, S. Zhang and W. He, Ferroptosis photoinduced by new cyclometalated iridium(III) complexes and its synergism with apoptosis in tumor cell inhibition, Angew. Chem., Int. Ed., 2021, 60, 8174 CrossRef CAS.
- W. Xu, H. Wang, Y. Guang, Z. Pan, K. Chen, T. Ma and J. Zhang, A mitochondria-targeted iridium(III) phosphorescent probe for selective detection of hypochlorite in living cells, Chem. – Asian J., 2025, 20, e202401351 CrossRef CAS.
- H. Fu, S. Wang, Y. Gong, H. Dong, K. Lai, Z. Yang, C. Fan, Z. Liu and L. Guo, Triphenylphosphine-modified cyclometalated iridiumIII complexes as mitochondria-targeting anticancer agents with enhanced selectivity, Bioorg. Chem., 2025, 155, 108148 CrossRef CAS.
- S. Li, H. Yuan, X.-Z. Yang, X. Xu, W. Yu, Y. Wu, S. Yao, J. Xie, W. He, Z. Guo and Y. Chen, Synergistic antitumor immunotherapy via mitochondria regulation in macrophages and tumor cells by an iridium photosensitizer, ACS Cent. Sci., 2025, 11, 441 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5qi01139f |
| ‡ Equal contribution from these authors. |
| This journal is © the Partner Organisations 2025 |






