Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Albumin-targeted oxaliplatin(IV) prodrugs bearing STING agonists†
Martijn
Dijkstra‡
ab,
Michael
Gutmann‡
 c,
Mathias
Gradl
c,
Anja
Federa
ab,
Carola
Jaunecker
c,
John Vasco
Breitenstein
c,
Mathias
Gradl
c,
Anja
Federa
ab,
Carola
Jaunecker
c,
John Vasco
Breitenstein
 c,
Petra
Vician
c,
Christine
Pirker
c,
Petra
Vician
c,
Christine
Pirker
 c,
Daniel
Valcanover
c,
Petra
Heffeter
cd,
Bernhard K.
Keppler
ad,
Walter
Berger
c,
Daniel
Valcanover
c,
Petra
Heffeter
cd,
Bernhard K.
Keppler
ad,
Walter
Berger
 *cd and
Christian R.
Kowol
*cd and
Christian R.
Kowol
 *ad
*ad
aUniversity of Vienna, Faculty of Chemistry, Institute of Inorganic Chemistry, Waehringer Str. 42, 1090 Vienna, Austria. E-mail: christian.kowol@univie.ac.at
bUniversity of Vienna, Vienna Doctoral School in Chemistry (DoSChem), Waehringer Str. 42, 1090 Vienna, Austria
cCenter for Cancer Research and Comprehensive Cancer Center, Medical University of Vienna, Borschkegasse 8a, 1090 Vienna, Austria. E-mail: walter.berger@meduniwien.ac.at
dResearch Cluster “Translational Cancer Therapy Research”, 1090 Vienna, Austria
First published on 4th April 2025
Abstract
The anticancer platinum complex oxaliplatin exerts its activity through DNA damage and immune-stimulatory mechanisms, but is associated with adverse treatment side effects. Platinum(IV) complexes represent a promising prodrug strategy to improve tolerability and to enhance antitumor efficacy via attachment of additional bioactive ligands or tumor-targeting moieties. In the present study, oxaliplatin(IV) complexes containing immune-stimulatory STING agonists SR-717 or MSA-2 were synthesized and their biological properties were studied. Whereas the Pt-SR-717 compound was fast reduced, Pt-MSA-2 complexes displayed significantly higher reductive stability reflected by low in vitro cytotoxicity. Although the platinum(IV) complexes activated interferon regulatory factor (IRF) and NF-κB signaling pathways less effectively compared to the free STING agonists, reducing conditions elevated cytotoxicity and STING downstream signaling, particularly for MSA-2-containing prodrugs. Rapid albumin binding of a maleimide-containing Pt-MSA-2 derivative resulted in elevated plasma levels, prolonged blood circulation, and enhanced tumor accumulation of platinum in CT-26 tumor-bearing mice. The Pt-MSA-2 complexes triggered immune activation and cytokine secretion without hematotoxicity usually associated with free oxaliplatin. The albumin-targeted Pt-MSA-2 drug significantly inhibited tumor growth after intravenous application, while the non-maleimide complex was effective only when applied peritumorally. However, the effects were not enhanced compared to mono-treatment with oxaliplatin or MSA-2, indicating a lack of synergism between the two simultaneously released agents. Our results demonstrate that oxaliplatin(IV) complexes represent a valuable strategy for enhanced tumor-targeting and adverse effect reduction, but question the simultaneous release of STING agonists and free oxaliplatin as a potent strategy towards synergistic antineoplastic activity.
Introduction
Since the breakthrough discovery of the anticancer activity of cisplatin, platinum(II) complexes have prevailed as first-line treatment against a variety of cancer types, with approximately half of the present chemotherapies involving a platinum(II) drug.1 Yet, patients suffer from serious side effects, such as systemic toxicity due to the lack of tumor specificity and the development of platinum resistance.2,3 In the past decades, platinum(IV) prodrugs were developed as a promising strategy to overcome the clinical limitations related to the use of platinum(II) drugs.4 It is generally accepted that platinum(IV) complexes are much more inert, which limits premature deactivation by biomolecules. Additionally, platinum(IV) prodrugs release their cytotoxic platinum(II) core via tumor-specific activation by reduction, either in the tumor microenvironment or inside the tumor cells after uptake via endocytosis,5 which reduces systemic toxicity.6 Although several platinum(IV) complexes, e.g. iproplatin and satraplatin, advanced into clinical trials, none have been approved so far.7 Over the past decades, research has increasingly focused on developing multi-action platinum(IV) complexes incorporating additional functional moieties, such as bioactive ligands or targeting groups. These modifications aim to enhance the antitumor efficacy of platinum(IV) drugs by synergistic effects or improving pharmacokinetics.8 In particular, human serum albumin (HSA) attracted significant attention as a versatile carrier for anticancer therapeutics, resulting in prolonged plasma half-lives of HSA–drug conjugates and increased intratumoral accumulation due to the enhanced permeability and retention (EPR) effect.9 Notable examples include the methotrexate–albumin conjugate MTX-HSA, which advanced into phase II clinical trials in combination therapy with cisplatin against advanced bladder cancer.10 Furthermore, aldoxorubicin, a maleimide-functionalized derivative of doxorubicin received orphan drug designation for the treatment of soft tissue sarcomas.11 Our group recently reported that maleimide functionalization of oxaliplatin(IV) complexes distinctly improved anticancer activity as compared to oxaliplatin via passive albumin targeting.5,12–14In the last few decades, cancer immunotherapy reconstituted the field of oncology, manifesting plentiful FDA approvals of diverse novel immunotherapeutics, which is comprehensively reviewed elsewhere.15 There is compelling evidence that oxaliplatin also possesses immunological effects. Specifically, it is capable of inducing immunogenic cell death (ICD), a form of regulated cell death via activation of innate and adaptive immune responses, potentially resulting in potent and long-term antitumor effects.16,17 Accordingly, improving the anticancer activity of oxaliplatin by combinatorial treatment with immunomodulators attracted significant research interest lately. For example, a phase III trial evaluated oxaliplatin in combination with the PD-1 checkpoint inhibitor nivolumab as first-line treatment in patients with advanced gastric cancer, showing superior response rates as compared to cisplatin.18 The cyclic GMP-AMP synthase stimulator of interferon genes (cGAS-STING) pathway plays a crucial role in the activation of innate immunity and is therefore a popular target in modern drug discovery.19 Stimulation of the STING signaling pathway by the endogenous activator 2′,3′-cyclic guanosine monophosphate–adenosine monophosphate (cGAMP) induces the expression of type I interferons (IFNs) and proinflammatory cytokines, ultimately resulting in the activation of cytotoxic CD8+ T-cells. Activation of STING can be exploited therapeutically by small molecule agonists (e.g. cGAMP mimetics) to induce robust anticancer immune responses. Although diverse STING agonists advanced into clinical trials, as extensively reviewed by others,20 their clinical success is significantly challenged by several limiting factors, such as inadequate pharmacology, poor serum stability or high toxicity profiles due to ubiquitous STING expression, necessitating intratumoral administration for the majority of STING-targeting drugs.21 As a consequence, recent efforts focused on the development of delivery systems to improve the pharmacokinetic properties of STING agonists.22 For example, Mersana Therapeutics reported orphan drug designation of a novel HER2-targeting STING agonist antibody–drug conjugate for the treatment of patients with gastric cancer.23 In addition, several metal complexes have been studied for their ability to induce cGAS-STING pathway activation via DNA breakdown products, either from the nucleus or mitochondria.24–27 Recently, reports of two non-nucleotide cGAMP mimetics and STING agonists with promising antitumor activity, SR-71728 and MSA-229, were published. SR-717 treatment led to superior tumor growth inhibition and enhanced overall survival compared to anti-PD-L1 therapy alone in a B16F10 melanoma mouse model. Moreover, MSA-2 induced regression of MC-38 syngeneic colorectal mouse tumors after oral administration and induced long-lasting anticancer immunity. Furthermore, in the case of MSA-2, numerous attempts have been reported to increase its clinical potential, e.g. by diverse structural modifications30,31 or by incorporation into platinum(II) complexes,32 cisplatin(IV)-based prodrugs,33 or nanoparticles.34,35
Encouraged by our recent efforts in the development of tumor-targeted, triple-action platinum(IV) prodrugs bearing immunomodulators, such as the indoleamine 2,3-dioxygenase inhibitor 1-methyl-D-tryptophan,12 and envisioned by combining STING agonists and platinum(IV)-based chemotherapy for achievement of a synergistic antitumor effect, we investigated the potential of oxaliplatin(IV) prodrugs bearing the STING agonists SR-717 and MSA-2. The new complexes were synthesized and investigated in vitro to assess the cytotoxic activity, intracellular accumulation, and STING-dependent activation of the interferon regulatory factor (IRF) and nuclear factor kappa B (NF-κB) pathways. Finally, an albumin-binding maleimide-bearing platinum(IV)-MSA-2 derivative was synthesized and the pharmacokinetics, i.e. platinum tissue distribution as well as the immune activation and anticancer activity in vivo was studied in comparison with the single agents oxaliplatin and MSA-2, using syngeneic mouse models of colorectal cancer.
Results and discussion
Synthesis, stability and reduction of platinum(IV) complexes
To commence our study, we focused on the design of suitable platinum(IV) prodrugs based on the STING agonist SR-717. SR-717, Ox-OH-OAc and Ox-(OH)2 were prepared as described in the literature.28,36 SR-717 was activated with 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethylaminium tetrafluoroborate (TBTU) and coupled to Ox-OH-OAc via an ester linkage to form Ox-SR-OAc (Scheme 1).Unfortunately, the aqueous solubility of Ox-SR-OAc was insufficient in various physiologically relevant media for further in vivo studies. We then shifted our strategy to Ox-SR-717 complexes bearing polyethylene glycol (PEG) linkers to increase solubility. 2,5,8,11-Tetraoxatetradecan-14-oic acid (PEG-COOH) was reacted with isobutyl chloroformate yielding PEG-isoBu anhydride (for synthesis details, see the Experimental section), which was then added dropwise to Ox-(OH)2 overnight to obtain Ox-OH-PEG-Es. Ox-SR-PEG-Es was synthesized via coupling of SR-717 and Ox-OH-PEG-Es using TBTU comparable to the synthesis of Ox-SR-OAc. This new derivative was adequately soluble for subsequent animal studies. Next, we investigated the hydrolytic stability of Ox-SR-PEG-Es in phosphate buffer (PB) at pH 7.4 and 20 °C over 5 h (Fig. 1). No significant hydrolysis was observed, which is well in line with the stability kinetics observed for other multi-action oxaliplatin(IV)-based prodrugs.12,37 The reduction properties were studied by co-incubation with 10 equivalents (eq.) of L-ascorbic acid (AA) as a reducing agent (Fig. 1).
 | ||
| Fig. 1 Stability and reduction kinetics of 1 mM Ox-SR-PEG-Es in 150 mM PB (pH = 7.4) at 20 °C without or with 10 eq. of AA over 5 h, measured with UHPLC. | ||
Unexpectedly, the reduction of Ox-SR-PEG-Es was notably fast, with only traces of the original prodrug remaining after ∼3 h of incubation. This reductive instability of Ox-SR-PEG-Es contradicted the slow reduction of dual-action oxaliplatin(IV) prodrugs bearing e.g. aspirin, which contains a similar acetyl benzoic acid motif.37
We were interested in elucidating the unexpected reduction behavior of Ox-SR-PEG-Es in more detail and investigated the reduction kinetics of a panel of four reference platinum(IV)-acetato complexes containing simplified benzoic acid (Bz) derivatives with alternating substitution patterns in accordance with SR-717: Ox-Bz (Bz = benzoic acid), Ox-Bz-F2 (Bz-F2 = 3,4-difluorobenzoic acid), Ox-Bz-F2-NH2 (Bz-F2-NH2 = 2-amino-4,5-difluorobenzoic acid) and Ox-Bz-F2-NHAc (Bz-F2-NHAc = 2-acetamido-4,5-difluorobenzoic acid, Fig. S1†). Ox-Bz and Ox-Bz-F2 were essentially stable in the presence of AA (approximately 95% left after 5 h), which ruled out that the fluorine substituents are responsible for the fast reduction of Ox-SR-PEG-Es. Interestingly, while ortho-amination in Ox-Bz-F2-NH2 marginally increased the rate of reduction compared to Ox-Bz-F2 (approximately 80% left), acetylation of the ortho-amine (as in Ox-Bz-F2-NHAc) severely impacted the reductive stability, with only 40% of the original compound remaining after 5 h. Although aromatic amines are electron-donating substituents, acetamide-based substituents are electron-withdrawing, which can explain the faster reduction of Ox-Bz-F2-NHAc over Ox-Bz-F2-NH2, although the extent of the effect is unexpected. This trend is in line with the differences in the calculated pKa values between Bz-F2-NHAc (3.1) and Bz-F2-NH2 (4.5).38 Also the Hammet substituent constants σm and σp highlight the stronger electron-withdrawing properties of –NHAc groups (σm: 0.00; σp: 0.21) over –NH2 groups (σm: −0.66; σp: −0.16), although these parameters cannot be directly used to explain the influence of the ortho substituent, because of e.g. steric effects.39 These structure–activity relationships correlate well with the findings of Choi and coworkers that the reduction of platinum(IV) complexes by ascorbate is strongly influenced by the electron-withdrawing power of the axial ligand.40 Thus, the data of the reference complexes suggest that the 3-(1H-imidazol-1-yl)pyridazine subunit of SR-717 is responsible for the fast reduction of Ox-SR-PEG-Es.
Consequently, we focused on the synthesis of platinum(IV) complexes containing the STING agonist MSA-2. MSA-2 was prepared according to the literature,29 and, based on the inadequate aqueous solubility of Ox-SR-OAc, we decided to directly synthesize Ox-MSA-PEG-Esvia a similar synthetic pathway as described for Ox-SR-PEG-Es (Scheme 1). Ox-MSA-PEG-Es was stable in PB at pH 7.4 and 20 °C. However, in contrast to the fast-reducing Ox-SR-PEG-Es, ∼80% of Ox-MSA-PEG-Es remained intact after 24 h of incubation in the presence of 10 eq. of AA (Fig. 2).
 | ||
| Fig. 2 Stability and reduction kinetics of 1 mM Ox-MSA-PEG-Es and Ox-MSA-PEG-Ca in 150 mM PB (pH = 7.4) at 20 °C with or without 10 eq. of AA over 24 h, measured with UHPLC. | ||
In comparison, we also synthesized the respective carbamate analogue Ox-MSA-PEG-Ca. Therefore, Ox-(OH)2 was activated asymmetrically via dropwise addition of the peptide-coupling reagent N,N-disuccinimidyl carbonate (DSC) overnight, and subsequently 3,6,9,12-tetraoxatridecanamine (PEG-NH2) was added to obtain Ox-OH-PEG-Ca. Finally, MSA-2 was coupled with TBTU to yield Ox-MSA-PEG-Ca. The stability and reduction kinetics of Ox-MSA-PEG-Ca were very well comparable to Ox-MSA-PEG-Es (Fig. 2), which is in agreement with the structure–activity relationships described earlier.41
In vitro anticancer activity of platinum(IV) complexes
A key characteristic of platinum(IV) prodrugs is their reduced cytotoxicity in vitro caused by the necessity for activation by reduction. Hence, the reducibility under biological conditions is an important parameter for cellular accumulation and efficacy in vitro. Our group previously published oxaliplatin(IV) complexes with greatly reduced cytotoxicity compared to oxaliplatin in vitro, but superior antitumor efficacy in vivo.5,12,13 To elucidate the impact of the observed differences in the reduction properties (compare Fig. 1 and 2) on the anticancer activity, the respective platinum(IV) complexes Ox-SR-PEG-Es, Ox-MSA-PEG-Es and Ox-MSA-PEG-Ca were studied in detail on cancer cells in vitro. The monocytic THP1-Dual and colorectal cancer cell models of human (HCT116 and HCT116/OxR) and murine (CT-26) origin were used to compare the impact of the platinum(IV) compounds on cell viability in comparison with oxaliplatin, SR-717 and MSA-2 after continuous exposure for 72 h. Oxaliplatin exhibited cytotoxic activity in the low μM range in THP1-Dual, HCT116 and CT-26 cells, indicating sensitivity towards platinum(II)-based chemotherapy. Interestingly, distinct differences in the cytotoxic activities were observed for the investigated platinum(IV) prodrugs (Fig. 3, for all IC50 values, see Table S1†). | ||
| Fig. 3 Cytotoxic activity of platinum(IV) prodrugs and oxaliplatin in various cancer cell models and the impact of AA. (A–C) Impact of 72 h of drug exposure on the viability of cancer cell lines, determined by an MTT-based assay. (A) Dose–response curves of the indicated platinum(IV) prodrugs and oxaliplatin for the human THP1-Dual monocytic cell model. Dose–response curves were modelled using a four parameter logistic (4PL) non-linear regression model. One representative experiment out of at least three performed is shown. (B) IC50 ratios, derived from the IC50 values listed in Table S1,† of the platinum(IV) prodrugs and oxaliplatin in the indicated cell models. Data points in (A) and (B) are shown as mean ± SD. (C) Impact of co-incubation with AA (50 μM) on the IC50 values (μM) of the indicated drugs. For underlying values compare Table S1.† Data in (B) and (C) are derived from at least three independent experiments in triplicate. The statistical significance of differences in IC50 ratios between prodrugs and oxaliplatin (B), and between –AA and + AA (C) was tested using one-way ANOVA (with the Tukey (B) or Sidak (C) multiple comparison test). IC50 = half-maximal inhibitory concentration; AA = L-ascorbic acid; ns = non-significant; and nd = not determinable. *p < 0.05; **p < 0.01; and ***p < 0.001. | ||
Ox-SR-PEG-Es exhibited the lowest reduction of cytotoxic activity at the IC50 level as compared to free oxaliplatin, ranging from a 2.0-fold (THP1-Dual) to a maximum 11.1-fold (CT-26) difference (Fig. 3B). In contrast, Ox-MSA-PEG-Es and Ox-MSA-PEG-Ca were distinctly less active compared to Ox-SR-PEG-Es, indicated by elevated IC50 ratios up to ∼40-fold (Fig. 3B). This observation became evident not only in hematogenic THP1-Dual cells but also in all human and murine colorectal cancer models investigated. Comparable cytotoxic activity between oxaliplatin and Ox-SR-PEG-Es and the massively reduced cytotoxicity of Ox-MSA-PEG-Es and Ox-MSA-PEG-Ca are well in agreement with their stability and reduction kinetics (compare Fig. 1 and 2). Interestingly, the loss of cytotoxic activity of the prodrugs was markedly lower in HCT116/OxR cells as compared to the other models, presumably due to reduced drug accumulation as a consequence of acquired oxaliplatin resistance.42 The oxaliplatin-resistant HCT116/OxR cell line exhibited a 34.3-fold decrease in vulnerability to free oxaliplatin as compared to the parental HCT116 cells (IC50: 27.5 μM vs. 0.8 μM), which is in agreement with previous reports.13 Distinctly reduced resistance factors were found for both Ox-MSA-PEG-Es (>3.1-fold) and Ox-MSA-PEG-Ca (2.7-fold), while a markedly higher factor of 20.5 was observed for Ox-SR-PEG-Es (Fig. S2†). This is again in line with the insufficient complex stability of Ox-SR-PEG-Es under cell culture conditions. Furthermore, reduced resistance levels for the platinum-MSA-2 complexes indicate that stable prodrugs are less affected by the respective resistance mechanisms of HCT116/OxR cells. In addition, all tested cell lines were co-incubated with AA to test the impact of reduction of the platinum(IV)-prodrugs on cell viability. Indeed, this resulted in increased cytotoxic activity for the three platinum(IV)-complexes in all tested cell lines compared to the “non-reducing” conditions (Fig. 3C).
Finally, combination experiments were performed to investigate whether the two released pharmacologically active compounds, STING agonists and oxaliplatin, interact regarding cytotoxicity (Fig. S3†). Of note, none of the investigated cell lines exhibited vulnerability to the free single agent SR-717 or MSA-2 (IC50 > 75 μM, Fig. S3A and B†), although STING and the downstream effectors TANK-binding kinase 1 (TBK1) and interferon regulatory factor 3 (IRF-3) are expressed in all cases (Fig. S4†). In the combination setting, we could identify a weak synergism between STING activation and oxaliplatin cytotoxicity solely in the monocytic THP1-Dual cell model, while no interactions were found in the case of the colorectal cancer cell lines tested (Fig. S3C†).
Intracellular accumulation of platinum(IV) complexes
In the next step, the cellular accumulation of the platinum(IV) complexes was investigated in comparison with oxaliplatin. THP1-Dual cells were exposed to varying drug concentrations for 3 h and the intracellular platinum content was measured with ICP-MS (Fig. 4). Oxaliplatin accumulated dose-dependently, with intracellular platinum levels consistent with literature reports (Fig. 4A).13 While treatment with Ox-SR-PEG-Es resulted in significantly higher platinum levels compared to oxaliplatin, both Ox-MSA-PEG-Es and Ox-MSA-PEG-Ca did not show a comparable amount of platinum hyper-accumulation. These data strongly suggest that Ox-SR-PEG-Es is taken up as the intact platinum(IV) complex within the incubation time of 3 h, despite its comparably high instability, and not as released oxaliplatin. A higher degree of lipophilicity of Ox-SR-PEG-Es compared to both Pt-MSA complexes is indicated by their HPLC retention times of 4.52 min for Ox-SR-PEG-Es, 3.42 min for Ox-MSA-PEG-Es and 3.36 min for Ox-MSA-PEG-Ca, respectively, and provides a plausible explanation for the observed enhanced accumulation.In addition to the clear-cut hyper-accumulation of Ox-SR-PEG-Es, low doses of Ox-MSA-PEG-Es (10 μM) resulted in a significantly higher platinum content in comparison with oxaliplatin as well (Fig. 4B). Interestingly, Ox-MSA-PEG-Es treatment resulted in lower intracellular platinum levels at the highest tested dose (50 μM) compared to oxaliplatin, albeit without statistical significance. In contrast, Ox-MSA-PEG-Ca was accumulating comparable to oxaliplatin at all dose levels. These data prove that minor changes in the design of platinum(IV) complexes, e.g. changing the axial ester linkage to a carbamate, can impact the accumulation dynamics. However, this 3 h short-term cellular accumulation of the platinum compounds does not reflect their cytotoxic activity in 72 h viability assays (compare Fig. 3).
STING-dependent IRF and NF-κB activation by platinum(IV) complexes
Next, we investigated the impact of the reductive release of STING agonists from platinum(IV) prodrugs on IRF and NF-κB pathway activation in the THP1-Dual reporter cell model in comparison to free oxaliplatin, SR-717 and MSA-2 after 24 h of exposure. This reporter cell model enables simultaneous monitoring of activation of both pathways via the detection of secreted reporters. In general, SR-717 and MSA-2 both robustly activated the IRF as well as the NF-κB signaling pathway (Fig. 5A–D). In the case of SR-717, a half-maximal effective concentration (EC50) of 2.9 μM (±0.8) was found to trigger the IRF pathway, which is well in agreement with previous reports (Fig. 5E, EC50: 2.1 μM).28 Furthermore, application of MSA-2 (EC50: 9.1 μM) revealed a ∼3.1-fold lower IRF-inducing activity when compared to SR-717-treated cells (Fig. 5E), which can partially be explained by the required dimerization of MSA-2 for STING activation.29 Oxaliplatin monotherapy did not induce observable activation of IRF or NF-κB signaling pathways (Fig. S5†). Interestingly, this finding contrasts earlier reports suggesting that platinum-induced DNA damage is capable of triggering the STING and NF-κB pathways.43 | ||
| Fig. 5 IRF and NF-κB pathway induction by platinum(IV) prodrugs as compared to reference compounds and the impact of reducing conditions. IRF-inducible luciferase (A and B), as well as NF-κB-inducible secreted embryonic alkaline phosphatase (SEAP) reporter levels (C and D) were measured in the THP1-Dual cell culture supernatant following 24 h of exposure to the indicated drugs or the combinations. Oxaliplatin was always applied at 5 μM. (A–D) Dose–response curves, modelled using a four parameter logistic (4PL) non-linear regression model, were normalized to cell viability (Fig. S6†). Impact of reducing conditions were tested by co-incubation with AA (50 μM). One representative experiment out of at least three performed is shown. (E) EC50 values and EC50 ratios (prodrug/free STING agonist), as well as the maximal induction of the prodrugs in the percent of the maximal response of the single agent SR-717 or MSA-2 (%EMAX), derived from dose–response curves in (A–D) are depicted for IRF and NF-κB as indicated. (F) Impact of reducing conditions is calculated as the area under curve (AUC) ratio of +AA and −AA. Data points in (A–D) and (F) are shown as mean ± SD. Data are derived from at least three independent experiments in triplicate. The statistical significance of differences in the EC50 and Emax values between the indicated combination groups and the respective free STING agonist (SR-717 or MSA-2) was tested using one-way ANOVA (with the Dunnett multiple comparison test) (p values are given). The statistical significance of differences in the AUC between +AA and −AA in (F) was tested using Student's t-test. IRF = Interferon regulatory factor; NF-κB = Nuclear factor kappa-light-chain-enhancer of activated B cells; RLU = relative light units; Abs = absorbance; ctrl = control; EC50 = half-maximal effective concentration; AA = L-ascorbic acid; AUC = area under the curve; ns = non-significant; and nd = not determinable. p < 0.05; **p < 0.01; and ***p < 0.001. | ||
All three platinum(IV) prodrugs were able to significantly stimulate IRF and NF-κB signalling (Fig. 5A–D). Ox-SR-PEG-Es revealed a ∼4.3-fold attenuated activity (EC50: 12.4 μM) for the IRF induction in comparison with free SR-717, with a maximal response (EMAX) of 66% relative to the maximal induction achieved by SR-717 (Fig. 5E). In the case of the NF-κB pathway, EC50 values were more than doubled as compared to the IRF pathway. A distinctly decreased IRF activation was also observed for Ox-MSA-PEG-Es (8.6-fold) and Ox-MSA-PEG-Ca (6.3-fold) as compared to free MSA-2. Notably, while Ox-MSA-PEG-Ca achieved a response of 68% relative to the maximum IRF induction by MSA-2 at the highest tested dose (100 μM), only 24% was achieved by treatment with Ox-MSA-PEG-Es. This trend towards a diminished relative potency was also found in the case of NF-κB activation (%EMAXOx-MSA-PEG-Cavs.Ox-MSA-PEG-Es: 24% vs. 15%). These observations were surprising, given that the two compounds showed comparable AA-induced reduction rates (Fig. 2) which should result in similar amounts of released STING agonist. Nevertheless, despite these differences in downstream signal pathway activation, both MSA-2-containing complexes exerted comparable cytotoxicity after 72 h (Fig. 3).
To establish the STING-dependent specificity of the observed IRF/NF-κB activation, similar studies were conducted with co-administration of the STING inhibitor H-151 (Fig. S6†). Notably, the IRF and NF-κB reporter signals were completely abolished in the presence of the inhibitor, clearly demonstrating that STING-dependent pathway activation occurs with both free STING agonists and STING agonist-releasing platinum(IV) prodrugs.
AA supplementation significantly enhanced the IRF-induced reporter signals for Ox-MSA-2 complexes, especially Ox-MSA-PEG-Es, compared to the non-reductive setting. In contrast, no notable modulation was observed for Ox-SR-PEG-Es, SR-717, MSA-2, or combinations of SR-717 and oxaliplatin. The combination of oxaliplatin and MSA-2 in the presence of AA moderately but significantly raised reporter levels, which was not found with the administration of the individual drugs (Fig. 5F). Of note, the particularly higher STING activation tendency of Ox-MSA-PEG-Es in the presence of AA can be explained by the inherently low downstream pathway-inducing activity (%EMAX of MSA-2: 24%) compared to Ox-MSA-PEG-Ca (%EMAX of MSA-2: 68%). Reducing conditions significantly raised NF-κB-induced reporter levels for Ox-SR-PEG-Es and, to a lesser extent, for the combination of MSA-2 and oxaliplatin (Fig. 5F).
Prodrug activation results in the concomitant liberation of SR-717/MSA-2 and oxaliplatin, which could potentially trigger or enhance pathway activation itself.43 Hence, reporter activations were investigated following administration of free oxaliplatin in combination with SR-717 or MSA-2. While the IRF reporter activity of oxaliplatin combinations reached a maximal response rate (%EMAX) of 60% and 73% relative to the free STING agonists SR-717 and MSA-2, respectively, induction of the NF-κB pathway revealed %EMAX-values of 103% and 98% (Fig. 5). Reporter induction was in all cases normalized to cell viability as determined by MTT assays, considering that platinum-induced cytotoxicity can attenuate reporter levels by reducing viable cell numbers (Fig. S6E–H†). Given that normalized EMAX values remained distinctly below 100% in the case of IRF induction, an inhibitory effect of oxaliplatin especially for STING-mediated IRF pathway activation can be postulated.
Taken together, MSA-2-containing prodrugs presented favorable reduction and activation kinetics and hence were chosen for further in-depth analyses.
Induction of DNA damage and cytokine/chemokine secretion by platinum(IV) complexes
In parallel to IRF- and NF-κB-induced reporter activation, regulation of STING-downstream pathways in the THP1-Dual reporter cell line was studied at the protein level with immunoblots and the results were compared to DNA damage and cell death markers (p-H2A.X, cleaved caspase-3, and cleaved PARP) typically induced by oxaliplatin (Fig. S7†). STING is phosphorylated by TBK1 at Ser366, which leads to the activation of NF-κB (p65) and, subsequently, upregulation of type I IFN and IFN-regulated immune response genes such as ISG15.44 Phosphorylation of STING indicated on-target pathway activation.45 Elevated phosphorylation of NF-κB was only detected in cells treated with MSA-2 and not with the platinum-MSA-2 prodrugs. Induction of ISG15 was only triggered in cells treated with MSA-2 and Ox-MSA-PEG-Ca, with the latter especially upon AA supplementation. Taken together, this confirmed the activation of the STING signaling axis by the investigated compounds. Free MSA-2, Ox-MSA-PEG-Es and Ox-MSA-PEG-Ca induced DNA damage, indicated by upregulation of the DNA damage marker p-H2A.X, as well as cleavage of caspase-3 and PARP. Additionally, DNA damage-inducing activity was distinctly increased for the prodrugs in AA-treated cells, demonstrating enhanced release of the bioactive ligands under reductive conditions (Fig. S7†).We additionally monitored target gene activation downstream of STING at the mRNA level. MSA-2 distinctly induced the immune response genes interferon-beta (IFN-β), ISG15, IFIT1, CXCL10 and CCL5, confirming downstream STING target gene activation (Fig. S8†). Interestingly, Ox-MSA-PEG-Es exerted a low propensity towards the activation of the investigated genes, while Ox-MSA-PEG-Ca distinctly raised transcript levels to a comparable extent as free MSA-2 (Fig. S8A†). The overall higher activation capabilities under reductive conditions (±AA) of Ox-MSA-PEG-Es in comparison with Ox-MSA-PEG-Ca (Fig. S8B†) corroborate our findings concerning IRF pathway induction (compare Fig. 5).
Furthermore, secretion of type I interferons was monitored in cell culture supernatants. Treatment of THP1-Dual cells with MSA-2 and Ox-MSA-PEG-Ca induced distinct secretion of cytokines and chemokines, which is consistent with STING pathway activation at the RNA and protein levels (Fig. S9†). Most pronounced upregulations were found in the case of the chemokines CCL5 and CXCL10, which were both secreted at similar levels upon treatment with MSA-2 or Ox-MSA-PEG-Ca. In addition, MSA-2 as well as MSA-2-bearing prodrugs induced elevation of IFN-α and to some extent IL-1a, while TNF-α and especially IFN-γ were activated predominantly by oxaliplatin.
Synthesis and albumin-binding kinetics of platinum(IV)–maleimide complexes
Albumin binding can strongly improve the anticancer activity of dual-action platinum(IV) prodrugs.13,37 The thorough in vitro characterization study revealed MSA-2-bearing platinum(IV) complexes as promising candidates for maleimide-functionalization and subsequent in vivo testing in mouse models. Given that the carbamate compound Ox-MSA-PEG-Ca presented overall favorable properties in terms of stability and downstream signal activation as compared to the ester analogue Ox-MSA-PEG-Es (compare Fig. 2, 5 and S7, S8†), the albumin-binding complex Ox-MSA-Mal was synthesized utilizing a carbamate linkage between the maleimide ligand and the platinum(IV) core (Scheme 2). In our previous projects, the PEG-maleimide was introduced as a second axial ligand via an isocyanate intermediate prepared from the commercially available carboxylic acid.37 Unfortunately, the yield was low and also attempts to synthesize Ox-OH-Mal were not promising. We recently published a new synthesis strategy towards carbamate-linked, PEG-maleimide-containing oxaliplatin(IV) complexes using dimethyl furane-protected maleimides and employing the coupling agent DSC in a similar fashion to that described for Ox-OH-PEG-Ca (Scheme 1).46 With this strategy the precursor complex Ox-OH-PMal could be successfully synthesized. Consecutive maleimide deprotection via a retro-Diels–Alder reaction at 90 °C and attachment of MSA-2 using TBTU afforded the final complex Ox-MSA-Mal.Subsequently, we focused on investigating the albumin binding properties of Ox-MSA-Mal compared to Ox-MSA-PEG-Ca by incubation in phosphate-buffered fetal calf serum (FCS) at 37 °C over 24 h. 195Pt traces were measured via size-exclusion chromatography-inductively coupled plasma mass spectrometry (SEC-ICP-MS), for which the results are presented in Fig. 6. Serum proteins have a retention span of ∼2–4 min as part of the high-molecular weight fraction with albumin at ∼4 min (Fig. S10†). After incubation with Ox-MSA-Mal, approximately 99% of the platinum was already bound to albumin at the 0 h time point and the drug conjugate was stable over 24 h without any release of platinum from the protein. In contrast, the reference complex Ox-MSA-PEG-Ca lacked the ability to conjugate to albumin (Fig. S11†) and was majorly found in the low-molecular weight fraction, even after an extended incubation time up to 24 h, with only traces of the compound detected in the protein fraction.
 | ||
| Fig. 6 195Pt-traces of incubation of 50 μM Ox-MSA-Mal in FCS (containing 150 mM PB, pH = 7.4) at 37 °C over 24 h, measured by SEC-ICP-MS. | ||
Serum pharmacokinetics and organ distribution of platinum(IV) complexes in vivo
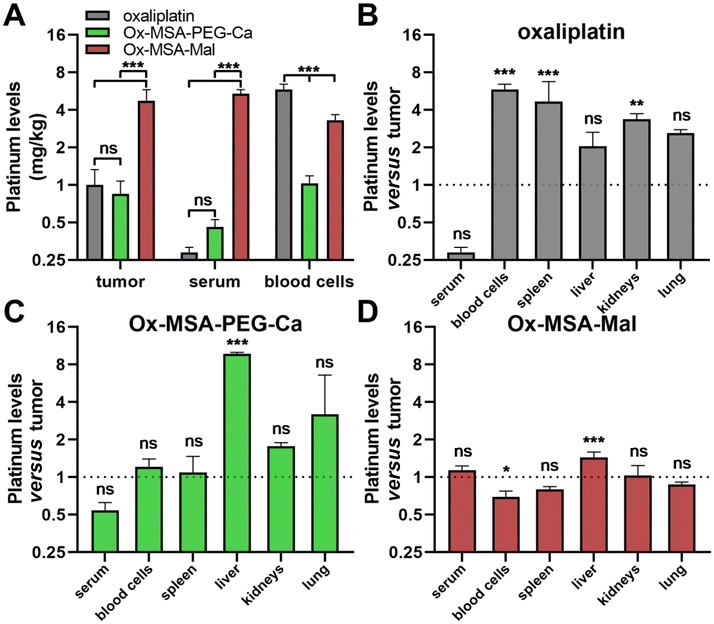
Of note, cell culture assessment of albumin-targeted prodrugs is not feasible due to interactions of the maleimide moiety with multiple cell culture medium components. Consequently, in the next step, we directly investigated how the endogenous albumin binding of Ox-MSA-Mal impacts its pharmacological behavior and organ distribution in CT-26 tumor-bearing mice in comparison with Ox-MSA-PEG-Ca and oxaliplatin. Mice were treated twice a week by intravenous (i.v.) application and platinum levels were determined in serum, organs and tumor tissues with ICP-MS 24 h after the second dose (Fig. 7). Equimolar doses corresponding to the maximum tolerated dose (MTD) of oxaliplatin (= 9 mg kg−1) were administered. Treatment with Ox-MSA-Mal resulted in distinctly elevated platinum levels in the serum and tumor and clearly lowered accumulation in peripheral blood cells as compared to Ox-MSA-PEG-Ca or oxaliplatin-treated animals (Fig. 7A). These findings corroborate previous reports on maleimide-functionalized oxaliplatin(IV) complexes.5,12–14 Low serum platinum levels of oxaliplatin- or Ox-MSA-PEG-Ca-treated animals suggest fast systemic excretion and peripheral distribution. Accordingly, platinum levels in organs of oxaliplatin-treated mice were distinctly higher in comparison to tumor platinum contents, reaching significance in the case of the spleen and kidneys (Fig. 7B). Ox-MSA-PEG-Ca was primarily localized to the liver (9.7-fold higher than in the tumor), while strong accumulation in blood cells, spleen, and kidneys was absent (Fig. 7C). In sharp contrast, Ox-MSA-Mal demonstrated only minor liver accumulation (1.4-fold higher than in the tumor) and even lower platinum levels in blood cells (Fig. 7D). This confirms an improved pharmacological profile and enhanced tumor-targeting properties of the albumin-binding prodrug.Immune activation induced by platinum(IV) complexes in vivo
Next, we analyzed how treatment with Ox-MSA-PEG-Ca and Ox-MSA-Mal impacts the frequencies of selected immune cell populations and their activation status in spleen and tumor tissue as compared to oxaliplatin 24 h after the second dose (Fig. 8 and S12†). In general, none of the investigated drugs significantly affected the frequency of tumor-infiltrating CD45+ immune cells. Oxaliplatin and to a lesser extent Ox-MSA-Mal significantly upregulated CD45+ cells among viable splenic cells (Fig. S12C and D†). Neither treatment with oxaliplatin nor MSA-2-containing prodrugs induced changes in the amount of tumor-infiltrating CD8+ T cells as compared to solvent-treated animals. In contrast, treatment with all investigated drugs significantly elevated splenic CD8+ T cells in the CD45+ cell population, with the strongest effect observed for oxaliplatin. Several immune cell activation markers were enhanced predominantly by the platinum(IV) complexes, including granzyme B (GzmB) on CD8+ T cells in the tumor and on NK cells both in the spleen and tumor, as well as CD69 on splenic and tumor-infiltrating B cells. Additionally, while treatment with Ox-MSA-PEG-Ca and Ox-MSA-Mal selectively elevated the FoxP3+ CD4+ T cell compartment in the spleen, indicative of regulatory T cells (Tregs), reduced levels were observed in the tumor tissue by all investigated compounds (Fig. 8 and S12A, B†). Altogether, these observations suggest that Ox-MSA-PEG-Ca and Ox-MSA-Mal distinctly activate an adaptive immune response. Accordingly, the upregulation of several cytokines and chemokines from the innate immune system was detected in the plasma of treated animals (Fig. S13†). The highest activations were found for IFN-β, IL-6, and M-CSF with Ox-MSA-PEG-Ca, while chemokines CXCL9 and CXCL11 were most strongly activated by Ox-MSA-Mal. These data indicate activation of an anticancer immune cycle by the investigated platinum(IV) compounds with a clear-cut impact of albumin-targeting in the case of Ox-MSA-Mal. | ||
| Fig. 8 Immune cell activation, tumor infiltration and red blood cell parameters following treatment with Ox-MSA-PEG-Ca and Ox-MSA-Mal as compared to free oxaliplatin. (A–C) CT-26-bearing BALB/c mice (n = 4 per group) were dosed twice (day 1 and 4) equimolar to 9 mg kg−1 oxaliplatin. Animals were euthanized 24 h after the second dose. Immediately before euthanization, blood was drawn from the submandibular vein and blood reports were established using a Vet ABC hematology analyzer (C). Immune cell populations were quantified in tumor and spleen tissues by flow cytometry (A and B). Frequency of the indicated immune cell populations (as percentage of CD45+ cells) and their activation state (as a percentage of the respective immune cell compartment) in (A) tumor and (B) spleen are depicted as mean ± SEM. Representative gates of GzmB+ or FoxP3+ cells in (A and B) are depicted in Fig. S12A and B.† The gating strategy used to identify the indicated immune cell populations is depicted in Fig. S14.† The statistical significance between the groups was tested by Student's t-test. (C) Selected red blood cell parameters from full blood reports are depicted as violin plots (the median and quartiles are shown using bold and dotted lines, respectively). The statistical significance between the groups was tested by one-way-ANOVA (with the Tukey multiple comparison test). *p < 0.05; **p < 0.01; and ***p < 0.001. | ||
Hematological analysis of platinum(IV) complexes in vivo
Concerning adverse effects, hematological analysis, performed in parallel to the immune phenotyping experiment in CT-26-bearing animals, revealed anemia in the case of oxaliplatin-treated animals but not in Ox-MSA-PEG-Ca- or Ox-MSA-Mal-treated animals (Fig. 8C). Only treatment with oxaliplatin clearly lowered red blood cell counts (RBCs), levels of hematocrit (HCT) and the red cell distribution width (RDW). In contrast, no significant changes were observed concerning white blood cell parameters by any of the investigated compounds (data not shown). This might be based on the relatively short exposure (24 h after the second dosing) to drug treatment in the immune phenotyping experiment. Consequently, the hematotoxic effects of oxaliplatin and Ox-MSA-Mal were investigated in C57BL/6 mice after long-term treatment (72 h after the fourth dosing; Fig. S15†). In this setting, absolute counts of white blood cells (WBCs), lymphocytes (LYM), monocytes (MO) and granulocytes (GRA) were massively lowered by oxaliplatin therapy. In contrast, treatment with Ox-MSA-Mal induced an increase of white blood cells (WBC) and granulocytes (GRA). Thrombocytopenia, indicated by distinctly reduced platelet counts (PLT) following oxaliplatin therapy, was not evident in animals receiving Ox-MSA-Mal. Distinct RBC toxicity of oxaliplatin was confirmed but was strongly reduced in the case of Ox-MSA-Mal (Fig. S15†).Anticancer efficacy of platinum(IV) complexes in vivo
Based on the promising findings in the pharmacological and immune activation experiments above, we next assessed the anticancer efficacy in vivo of Ox-MSA-PEG-Ca and Ox-MSA-Mal compared to oxaliplatin and MSA-2. For this purpose, the CT-26 tumor-bearing animals were dosed i.v. twice a week for two weeks at equimolar doses to oxaliplatin (9 mg kg−1, Fig. 9A). Generally, the investigated compounds were well tolerated and no significant impact on the weight of the animals was found (Fig. S16A†). Oxaliplatin monotherapy clearly reduced tumor growth (Fig. 9A). MSA-2 also significantly inhibited malignant growth when applied at a low dose of 6.67 mg kg−1 (equimolar to 9 mg kg−1 of oxaliplatin) via the i.v. route, which is well in agreement with previous reports, however using a distinctly higher dose (50 mg kg−1) and different application routes (e.g. subcutaneous and intratumoral).29 Both platinum(IV)-prodrugs significantly reduced tumor growth, with the maleimide-targeted Ox-MSA-Mal exhibiting distinctly stronger anticancer efficacy. However, tumor growth inhibition by Ox-MSA-Mal did not outperform the activity of either of the free components. Considering the higher platinum accumulation in tumors following Ox-MSA-Mal treatment (compare Fig. 7), disadvantageous tumor delivery is unlikely to explain the lack of synergistic effects of the two prodrug components. The rather antagonistic effect of oxaliplatin on MSA-2-mediated STING downstream pathway activation might be considered as one limiting factor (compare Fig. 5). Alternatively, systemic STING agonist application might not reach sufficient levels in the malignant tissue as suggested by the limited immune cell invasion and activation in the tumor in comparison with the spleen (Fig. 8). Consequently, the anticancer activity of Ox-MSA-PEG-Ca was tested in a final experiment with peritumoral (p.t.) application of identical doses used in the i.v. experiment (Fig. 9B). Ox-MSA-Mal could not be employed in this experiment, given the requirement of i.v. administration of maleimide-functionalized prodrugs to enable endogenous albumin-binding. Interestingly, peritumoral application of Ox-MSA-PEG-Ca induced a robust and significant decrease in the tumor volume (Fig. 9B) without negative effects on body weights (Fig. S16B†). These data suggest that i.v. application might indeed lead to insufficient peak levels of free STING agonists inside the complex tumor microenvironment to activate a full anticancer immune cycle.Conclusion
Oxaliplatin is a highly effective chemotherapeutic agent acting mainly via DNA-damage and stimulation of the immune system but at the same time causing severe side effects that greatly diminish patients’ quality of life. Platinum(IV) prodrugs emerged as promising alternatives due to their enhanced kinetic inertness and higher degree of functionalization to improve tumor efficacy, e.g. via the attachment of bioactive ligands or tumor-targeting moieties. In the past decades, albumin emerged as an efficient drug carrier for the enhanced tumor targeting of metal-based drugs either via covalent attachment by using maleimide functional groups47,48 or by non-covalent interactions.49 Our group recently reported several maleimide-functionalized oxaliplatin(IV) prodrugs with distinctly improved anticancer activity over oxaliplatin.12,14 The tumor-specific release of platinum(II) and potentially synergistic, bioactive axial ligands may also mitigate systemic adverse effects of both released compounds and their combination. Since oxaliplatin is considered the strongest inducer of ICD among the approved platinum anticancer drugs16,17 and the cGAS-STING pathway plays a major role in anticancer immune recognition,50 we decided to synthesize and extensively evaluate oxaliplatin(IV) complexes with activators of the STING pathway. Two STING agonists, SR-717 and MSA-2, with promising antitumor activity were recently published.28,29 Hence, in this study, we explored for the first time the synthesis and evaluated the biological properties of a panel of oxaliplatin(IV) prodrugs containing the STING agonists SR-717 or MSA-2. We hypothesized that these complexes should allow synergistic anticancer immune mechanisms and reduce adverse effects characteristic of clinical combination schemes of immune-activating agents and platinum-containing chemotherapy. Indeed, confirming the prodrug concept, platinum(IV) complexes exhibited reduced cytotoxicity, DNA damage, and immune-stimulation in vitro as compared to the free parental compounds, with all these parameters re-activated by the presence of the reducing agent AA. This underscored the necessity of complex activation by reduction especially in the case of the more stable Ox-MSA-PEG-Es and Ox-MSA-PEG-Ca complexes. A maleimide-functionalized Pt-MSA-2 derivative formed stable albumin adducts, translating into promising in vivo pharmacokinetic profiles with strongly enhanced plasma levels and tumor platinum accumulation in CT-26 tumor-bearing mice in comparison with oxaliplatin and the reference non-maleimide complex. In addition, both Pt-MSA-2 complexes distinctly induced immune activation, evident by significant upregulation of cytotoxic CD8+ T cells, NK cell activation and the release of inflammatory cytokines and chemokines, while lacking oxaliplatin-related hematotoxic effects. Interestingly, the albumin-targeted complex Ox-MSA-Mal significantly inhibited colorectal tumor growth via the i.v. route, while the non-targeted Ox-MSA-PEG-Ca failed to do so. The latter, however, exerted distinct anticancer activity after peritumoral application, postulating a clear influence of the application route on the efficacy of Pt-MSA complexes. Unfortunately, the promising results in the pharmacological studies of the Ox-MSA-Mal complex did not translate into improved anticancer activity compared to free oxaliplatin or MSA-2. The underlying reasons seem to be complex. Released intratumoral concentrations of free MSA-2 below the activity threshold, due to the slow reduction of Pt-MSA prodrugs, combined with the necessity for ligand dimerization could explain the lack of synergistic activity via the i.v. route. This hypothesis is in agreement with a previous study showing that the anticancer activity of i.v. applied liposomal oxaliplatin formulations in colorectal cancer-bearing mouse models was significantly enhanced by intratumoral administration of the STING agonist ADU-S100.51 This suggests that achieving sufficiently high intratumoral concentrations of the STING agonist is critical for optimal synergistic anticancer effects. Additionally, the inhibitory effect of oxaliplatin on the MSA-2-mediated STING downstream pathway activity in vitro indicates that the simultaneous release of the STING agonist and oxaliplatin from platinum(IV) complexes might also exert antagonistic effects in vivo. Moreover, a recent study demonstrated that FOLFOX-activated cGAS-STING signaling mediates the upregulation of the immune checkpoint inhibitor PD-L1, thereby dampening the corresponding anticancer immune response.43 Consequently, the combination of Ox-MSA-Mal with PD-1/PD-L1 inhibitory antibodies is currently under investigation. Whether the complex interaction between oxaliplatin and the cGAS-STING signaling pathway is specific to oxaliplatin or extends to other platinum compounds remains to be tested. The group of Guo very recently reported MSA-2-containing cisplatin(IV) prodrugs with distinct anticancer activity in vivo, however, at the same time exerting also massively enhanced in vitro cytotoxicity as compared to free cisplatin.33 This suggests a novel cytotoxic mechanism inherent to this cisplatin(IV) complex.In conclusion, the strategy of combining a STING activator and oxaliplatin within an albumin-targeted platinum(IV) complex showed notable tumor-inhibitory activity with distinctly reduced adverse effects. However, the obtained antitumor activity was not superior to that of free oxaliplatin or the respective STING agonist. Our current efforts focus on developing strategies to investigate and mitigate the inhibitory effect of oxaliplatin on STING agonist-mediated immune responses to optimize the achieved anticancer effects.
Experimental section
Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 and Ox-OH-OAc
23 and Ox-OH-OAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 were synthesized as described in the literature. Electrospray ionization (ESI) mass spectra were recorded using a Bruker Amazon SL ion trap mass spectrometer in the positive and/or negative mode by direct infusion at the Mass Spectrometry Centre of the University of Vienna. One- and two-dimensional 1H and DEPT 13C NMR spectra were recorded using a Bruker AV Neo 500 or AV III 600 spectrometer at 298K. For 1H and 13C NMR spectra, the solvent residual peak was taken as an internal reference. The 1H, DEPT 13C NMR spectra and UHPLC chromatograms of the final compounds are depicted in Fig. S17–20.† Purification by preparative reverse phase (RP) HPLC was performed on an Agilent 1200 series system using a Waters XBridge C18 column (19 × 250 mm). Elemental analysis measurements were carried out using a PerkinElmer 2400 CHN elemental analyzer at the Microanalytical Laboratory of the University of Vienna and are within ±0.4%, confirming >95% purity. The contents of TFA and water can vary between different batches of the same compound.
23 were synthesized as described in the literature. Electrospray ionization (ESI) mass spectra were recorded using a Bruker Amazon SL ion trap mass spectrometer in the positive and/or negative mode by direct infusion at the Mass Spectrometry Centre of the University of Vienna. One- and two-dimensional 1H and DEPT 13C NMR spectra were recorded using a Bruker AV Neo 500 or AV III 600 spectrometer at 298K. For 1H and 13C NMR spectra, the solvent residual peak was taken as an internal reference. The 1H, DEPT 13C NMR spectra and UHPLC chromatograms of the final compounds are depicted in Fig. S17–20.† Purification by preparative reverse phase (RP) HPLC was performed on an Agilent 1200 series system using a Waters XBridge C18 column (19 × 250 mm). Elemental analysis measurements were carried out using a PerkinElmer 2400 CHN elemental analyzer at the Microanalytical Laboratory of the University of Vienna and are within ±0.4%, confirming >95% purity. The contents of TFA and water can vary between different batches of the same compound.
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.65–1.41 (m, 4H, CHcHex), 1.24–1.06 (m, 2H, CHcHex) ppm. MS (m/z): calcd C25H25F2N7O9NaPt (M + H)+, 801.14; found, 801.24.
3), 1.65–1.41 (m, 4H, CHcHex), 1.24–1.06 (m, 2H, CHcHex) ppm. MS (m/z): calcd C25H25F2N7O9NaPt (M + H)+, 801.14; found, 801.24.
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CHi-Pr), 3.67–3.63 (t, J = 6.0 Hz, 2H, C
2CHi-Pr), 3.67–3.63 (t, J = 6.0 Hz, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2OCH3), 3.52–3.47 (m, 10H, CHPEG), 3.44–3.40 (m, 2H, CHPEG), 3.23 (s, 3H, OCH3), 2.81–2.78 (t, J = 5.9 Hz, 2H, CH2C
2OCH3), 3.52–3.47 (m, 10H, CHPEG), 3.44–3.40 (m, 2H, CHPEG), 3.23 (s, 3H, OCH3), 2.81–2.78 (t, J = 5.9 Hz, 2H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2COO), 1.98–1.92 (sp, J = 6.6 Hz, 1H, CHi-Pr), 0.92–0.89 (d, J = 6.7 Hz, 6H, CH3,i-Pr) ppm. MS (m/z): calcd C15H29O8 (M + H)+, 337.39; found, 337.42.
2COO), 1.98–1.92 (sp, J = 6.6 Hz, 1H, CHi-Pr), 0.92–0.89 (d, J = 6.7 Hz, 6H, CH3,i-Pr) ppm. MS (m/z): calcd C15H29O8 (M + H)+, 337.39; found, 337.42.
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2COOPt), 2.12–2.00 (m, 2H, CHcHex), 1.55–1.40 (m, 3H, CHcHex), 1.37–1.26 (m, 1H, CHcHex), 1.18–1.01 (m, 2H, CHcHex) ppm. MS (m/z): calcd C18H34N2O11NaPt (M + Na)+, 672.17; found, 672.22.
2COOPt), 2.12–2.00 (m, 2H, CHcHex), 1.55–1.40 (m, 3H, CHcHex), 1.37–1.26 (m, 1H, CHcHex), 1.18–1.01 (m, 2H, CHcHex) ppm. MS (m/z): calcd C18H34N2O11NaPt (M + Na)+, 672.17; found, 672.22.
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2), 3.52–3.44 (m, 10H, CHPEG), 3.44–3.41 (m, 2H, C
2), 3.52–3.44 (m, 10H, CHPEG), 3.44–3.41 (m, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2OCH3), 3.24 (s, 3H, OCH3), 2.84–2.74 (m, 1H, CHcHex), 2.67–2.59 (m, 1H, CHcHex), 2.57–2.53 (t, 2H, PtOCOCH2C
2OCH3), 3.24 (s, 3H, OCH3), 2.84–2.74 (m, 1H, CHcHex), 2.67–2.59 (m, 1H, CHcHex), 2.57–2.53 (t, 2H, PtOCOCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2), 2.21–2.10 (m, 2H, CHcHex), 1.65–1.55 (m, 1H, CHcHex), 1.54–2.39 (m, 3H, CHcHex), 1.20–1.08 (m, 2H, CHcHex) ppm. 13C NMR (126 MHz, DMSO-d6) δ 178.4 (PtOCO), 171.2 (PtOCO), 163.2 (COOx), 163.2 (COOx), 160.7 (CONHCa), 153.2 (Cq,Ar), 151.8 (Cq,Ar), 150.6 (Cq,Ar), 145.4 (Cq,Ar), 143.8 (Cq,Ar), 136.1 (Cq,Ar), 135.9 (CHAr), 129.7 (2xCHAr), 120.8 (CHAr), 120.1 (CHAr), 117.5 (CHAr), 108.9 (CHAr), 71.2 (CHPEG), 69.8 (2xCHPEG), 69.7 (CHPEG), 69.6 (2xCHPEG), 69.5 (CHPEG), 66.8 (CHPEG), 61.5 (CHcHex), 60.7 (CHcHex), 58.0 (OCH3), 36.4 (PtOCOCH2
2), 2.21–2.10 (m, 2H, CHcHex), 1.65–1.55 (m, 1H, CHcHex), 1.54–2.39 (m, 3H, CHcHex), 1.20–1.08 (m, 2H, CHcHex) ppm. 13C NMR (126 MHz, DMSO-d6) δ 178.4 (PtOCO), 171.2 (PtOCO), 163.2 (COOx), 163.2 (COOx), 160.7 (CONHCa), 153.2 (Cq,Ar), 151.8 (Cq,Ar), 150.6 (Cq,Ar), 145.4 (Cq,Ar), 143.8 (Cq,Ar), 136.1 (Cq,Ar), 135.9 (CHAr), 129.7 (2xCHAr), 120.8 (CHAr), 120.1 (CHAr), 117.5 (CHAr), 108.9 (CHAr), 71.2 (CHPEG), 69.8 (2xCHPEG), 69.7 (CHPEG), 69.6 (2xCHPEG), 69.5 (CHPEG), 66.8 (CHPEG), 61.5 (CHcHex), 60.7 (CHcHex), 58.0 (OCH3), 36.4 (PtOCOCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2), 31.0 (CHcHex), 30.6 (CHcHex), 23.5 (CHcHex), 23.5 (CHcHex) ppm. MS (m/z): calcd C33H41F2N7O13NaPt (M + Na)+, 999.23; found, 999.29. EA calcd C33H41F2N7O13Pt ·1.5H2O: C, 39.49; H, 4.42; N, 9.95. Found: C, 39.24; H, 4.69; N, 10.
H2), 31.0 (CHcHex), 30.6 (CHcHex), 23.5 (CHcHex), 23.5 (CHcHex) ppm. MS (m/z): calcd C33H41F2N7O13NaPt (M + Na)+, 999.23; found, 999.29. EA calcd C33H41F2N7O13Pt ·1.5H2O: C, 39.49; H, 4.42; N, 9.95. Found: C, 39.24; H, 4.69; N, 10.
![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OCq,Ar), 178.0 (PtOCO), 164.2 (PtOCO), 163.4 (COOx), 163.3 (COOx), 150.8 (Cq,Ar), 148.5 (Cq,Ar), 140.6 (Cq,Ar), 135.7 (Cq,Ar), 132.6 (Cq,Ar), 130.3 (CHAr), 106.6 (CHAr), 104.3 (CHAr), 71.2 (CH2,PEG), 69.8 (2xCH2,PEG), 69.7 (CH2,PEG), 69.6 (CH2,PEG), 69.5 (CH2,PEG), 66.8 (CH2,MSA), 60.9 (2xCHcHex), 58.0 (OCH3,PEG), 55.9 (OCH3,MSA), 55.6 (OCH3,MSA), 36.5 (O
OCq,Ar), 178.0 (PtOCO), 164.2 (PtOCO), 163.4 (COOx), 163.3 (COOx), 150.8 (Cq,Ar), 148.5 (Cq,Ar), 140.6 (Cq,Ar), 135.7 (Cq,Ar), 132.6 (Cq,Ar), 130.3 (CHAr), 106.6 (CHAr), 104.3 (CHAr), 71.2 (CH2,PEG), 69.8 (2xCH2,PEG), 69.7 (CH2,PEG), 69.6 (CH2,PEG), 69.5 (CH2,PEG), 66.8 (CH2,MSA), 60.9 (2xCHcHex), 58.0 (OCH3,PEG), 55.9 (OCH3,MSA), 55.6 (OCH3,MSA), 36.5 (O![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH2COOPt), 34.0 (CH2,PEG), 31.0 (2xCH2,cHex), 29.7 (
H2CH2COOPt), 34.0 (CH2,PEG), 31.0 (2xCH2,cHex), 29.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2COOPt), 23.6 (CH2,cHex), 23.4 (CH2,cHex) ppm. MS (m/z): calcd C32H46N2O15NaPt (M + Na)+, 948.22; found, 948.29. EA calcd C32H46N2O15PtS·0.5TFA: C, 40.33; H, 4.77; N, 2.85; S, 3.26. Found: C, 40.32; H, 4.81; N, 2.78; S, 3.55.
H2COOPt), 23.6 (CH2,cHex), 23.4 (CH2,cHex) ppm. MS (m/z): calcd C32H46N2O15NaPt (M + Na)+, 948.22; found, 948.29. EA calcd C32H46N2O15PtS·0.5TFA: C, 40.33; H, 4.77; N, 2.85; S, 3.26. Found: C, 40.32; H, 4.81; N, 2.78; S, 3.55.
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2NHCa), 2.59–2.47 (m, 2H, CHcHex), 2.17–2.00 (m, 2H, CHcHex), 1.61–1.40 (m, 3H, CHcHex), 1.40–1.26 (m, 1H, CHcHex), 1.19–1.05 (m, 2H, CHcHex) ppm. MS (m/z): calcd C18H35N3O11NaPt (M + Na)+, 687.18; found, 687.22.
2NHCa), 2.59–2.47 (m, 2H, CHcHex), 2.17–2.00 (m, 2H, CHcHex), 1.61–1.40 (m, 3H, CHcHex), 1.40–1.26 (m, 1H, CHcHex), 1.19–1.05 (m, 2H, CHcHex) ppm. MS (m/z): calcd C18H35N3O11NaPt (M + Na)+, 687.18; found, 687.22.
![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OCq,Ar), 179.9 (PtOCO), 164.2 (CONHCa), 163.4 (COOx), 163.3 (COOx), 150.8 (Cq,Ar), 148.6 (Cq,Ar), 140.6 (Cq,Ar), 135.7 (Cq,Ar), 132.5 (Cq,Ar), 130.3 (CHAr), 106.5 (CHAr), 104.3 (CHAr), 71.3 (2xCH2,PEG), 69.8 (2xCH2,PEG), 69.6 (2xCH2,PEG), 69.2 (2xCH2,PEG), 60.9 (CHcHex), 60.7 (CHcHex), 58.0 (OCH3,PEG), 55.8 (OCH3,MSA), 55.6 (OCH3,MSA), 40.7 (
OCq,Ar), 179.9 (PtOCO), 164.2 (CONHCa), 163.4 (COOx), 163.3 (COOx), 150.8 (Cq,Ar), 148.6 (Cq,Ar), 140.6 (Cq,Ar), 135.7 (Cq,Ar), 132.5 (Cq,Ar), 130.3 (CHAr), 106.5 (CHAr), 104.3 (CHAr), 71.3 (2xCH2,PEG), 69.8 (2xCH2,PEG), 69.6 (2xCH2,PEG), 69.2 (2xCH2,PEG), 60.9 (CHcHex), 60.7 (CHcHex), 58.0 (OCH3,PEG), 55.8 (OCH3,MSA), 55.6 (OCH3,MSA), 40.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2NHCa), 34.0 (CH2,MSA), 31.0 (CH2,cHex), 30.9 (CH2,cHex), 29.6 (CH2,MSA), 23.7 (CH2,cHex), 23.4 (CH2,cHex) ppm. MS (m/z): calcd C32H47N3O15NaPtS (M + Na)+, 963.23; found, 963.29. EA calcd C32H47N3O15PtS: C, 40.85; H, 5.03; N, 4.47; S, 3.41. Found: C, 40.82; H, 4.87; N, 4.16; S, 3.71.
H2NHCa), 34.0 (CH2,MSA), 31.0 (CH2,cHex), 30.9 (CH2,cHex), 29.6 (CH2,MSA), 23.7 (CH2,cHex), 23.4 (CH2,cHex) ppm. MS (m/z): calcd C32H47N3O15NaPtS (M + Na)+, 963.23; found, 963.29. EA calcd C32H47N3O15PtS: C, 40.85; H, 5.03; N, 4.47; S, 3.41. Found: C, 40.82; H, 4.87; N, 4.16; S, 3.71.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHFurane,exo), 6.34–6.26 + 6.07–5.96 (m, 1H, NHCa), 6.22 (s, 2H,
CHFurane,exo), 6.34–6.26 + 6.07–5.96 (m, 1H, NHCa), 6.22 (s, 2H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHFurane,endo) 3.53–3.40 (m, 18H, CHPEG), 3.27 (s, 2H, CHFurane,endo), 3.10–3.00 (m, 2H, CH2NHCa), 2.89 (s, 2H, CHFurane,exo), 2.58–2.47 (m, 2H, CHcHex), 2.14–2.00 (m, 2H, CHcHex), 1.62 (s, 6H, CH3,Furane,endo), 1.56–1.40 (m, 9H, CHcHex + CH3,Furane,exo), 1.39–1.26 (m, 1H, CHcHex), 1.15–1.05 (m, 2H, CHcHex) ppm. MS (m/z): calcd C29H46N4O14NaPt (M + Na)+, 892.26; found, 892.32.
CHFurane,endo) 3.53–3.40 (m, 18H, CHPEG), 3.27 (s, 2H, CHFurane,endo), 3.10–3.00 (m, 2H, CH2NHCa), 2.89 (s, 2H, CHFurane,exo), 2.58–2.47 (m, 2H, CHcHex), 2.14–2.00 (m, 2H, CHcHex), 1.62 (s, 6H, CH3,Furane,endo), 1.56–1.40 (m, 9H, CHcHex + CH3,Furane,exo), 1.39–1.26 (m, 1H, CHcHex), 1.15–1.05 (m, 2H, CHcHex) ppm. MS (m/z): calcd C29H46N4O14NaPt (M + Na)+, 892.26; found, 892.32.
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2N(CO)2), 3.53–3.49 (t, 2H, CHPEG), 3.48–3.43 (m, 12H, CHPEG), 3.37–3.26 (m, 3H, CH2,MSA + CHPEG), 3.23–3.15 (m, 1H, CHPEG), 3.10–3.00 (m, 2H, CH2NHCa), 2.75–2.53 (m, 2H, CH2,MSA + CHcHex), 2.17–2.07 (m, 2H, CHcHex), 1.55–1.45 (m, 2H, CHcHex), 1.44–1.28 (m, 2H, CHcHex), 1.22–1.08 (m, 2H, CHcHex) ppm. 13C NMR (126 MHz, DMSO-d6) δ 192.8 (
2N(CO)2), 3.53–3.49 (t, 2H, CHPEG), 3.48–3.43 (m, 12H, CHPEG), 3.37–3.26 (m, 3H, CH2,MSA + CHPEG), 3.23–3.15 (m, 1H, CHPEG), 3.10–3.00 (m, 2H, CH2NHCa), 2.75–2.53 (m, 2H, CH2,MSA + CHcHex), 2.17–2.07 (m, 2H, CHcHex), 1.55–1.45 (m, 2H, CHcHex), 1.44–1.28 (m, 2H, CHcHex), 1.22–1.08 (m, 2H, CHcHex) ppm. 13C NMR (126 MHz, DMSO-d6) δ 192.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OCq,Ar), 179.9 (PtOCO), 170.9 (2xCOMal), 164.2 (CONHCa), 163.4 (COOx), 163.3 (COOx), 150.8 (Cq,Ar), 148.5 (Cq,Ar), 140.6 (Cq,Ar), 135.7 (Cq,Ar), 134.5 (2xCHMal), 132.5 (Cq,Ar), 130.3 (CHAr), 106.5 (CHAr), 104.3 (CHAr), 69.7 (2xCH2,PEG), 69.6 (2xCH2,PEG), 69.5 (CH2,PEG), 69.4 (2xCH2,PEG), 69.2 (CH2,PEG), 66.9 (CH2,PEG), 60.9 (CHcHex), 60.7 (CHcHex), 55.8 (OCH3,MSA), 55.6 (OCH3,MSA), 40.7 (
OCq,Ar), 179.9 (PtOCO), 170.9 (2xCOMal), 164.2 (CONHCa), 163.4 (COOx), 163.3 (COOx), 150.8 (Cq,Ar), 148.5 (Cq,Ar), 140.6 (Cq,Ar), 135.7 (Cq,Ar), 134.5 (2xCHMal), 132.5 (Cq,Ar), 130.3 (CHAr), 106.5 (CHAr), 104.3 (CHAr), 69.7 (2xCH2,PEG), 69.6 (2xCH2,PEG), 69.5 (CH2,PEG), 69.4 (2xCH2,PEG), 69.2 (CH2,PEG), 66.9 (CH2,PEG), 60.9 (CHcHex), 60.7 (CHcHex), 55.8 (OCH3,MSA), 55.6 (OCH3,MSA), 40.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2NHCa), 36.7 (
H2NHCa), 36.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2N(CO)2), 34.0 (CH2,MSA), 31.0 (CH2,cHex), 30.9 (CH2,cHex), 29.6 (CH2,MSA), 23.7 (CH2,cHex), 23.3 (CH2,cHex) ppm. MS (m/z): calcd C37H50N4O17NaPtS (M + Na)+, 1072.24; found, 1072.35. EA calcd C37H50N4O17PtS·1TFA: C, 40.24; H, 4.42; N, 4.81; S, 2.75. Found: C, 40.04; H, 4.41; N, 4.98; S, 2.88.
H2N(CO)2), 34.0 (CH2,MSA), 31.0 (CH2,cHex), 30.9 (CH2,cHex), 29.6 (CH2,MSA), 23.7 (CH2,cHex), 23.3 (CH2,cHex) ppm. MS (m/z): calcd C37H50N4O17NaPtS (M + Na)+, 1072.24; found, 1072.35. EA calcd C37H50N4O17PtS·1TFA: C, 40.24; H, 4.42; N, 4.81; S, 2.75. Found: C, 40.04; H, 4.41; N, 4.98; S, 2.88.
Stability and reduction experiments
PB (150 mM, pH 7.4) containing 1 mM platinum compound was incubated at 20 °C, with and without the addition of 10 eq. of AA. The reaction was monitored using a Thermo Scientific Dionex UltiMate 3000 UHPLC system with a Waters Acquity UPLC BEH C18 1.7 μm 3.0 × 50 mm column. Milli-Q water containing 0.1% formic acid and acetonitrile containing 0.1% formic acid were used as eluents. A gradient of 5–95% over 5 min was used with a flow of 0.6 mL min−1. To evaluate the current state of the reaction, the peak area of the parental complex was used. This was done due to the fact that in most cases the reduction products did not have a sufficient retention time to be distinguished from the injection peak.SEC-ICP-MS measurements
FCS was purchased from Sigma-Aldrich and buffered with 150 mM PB pH 7.4 to guarantee a stable pH. The platinum(IV) complexes were dissolved in 150 mM PB (pH 7.4) at a concentration of 5 mM and diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in the buffered serum to obtain a final concentration of 50 μM. The samples were then incubated in an autosampler at 37 °C and analyzed for 24 h. Between each sample, a pure water blank was measured. For SEC-ICP-MS measurements an Agilent 1260 Infinity system coupled to an Agilent 7800 ICP-MS equipped with a dynamic reaction cell was used. Oxygen (purity 5.5, Messer Austria GmbH, Gumpoldskirchen, Austria) was used as a reaction gas. The parameters are given in Table S2.†
100 in the buffered serum to obtain a final concentration of 50 μM. The samples were then incubated in an autosampler at 37 °C and analyzed for 24 h. Between each sample, a pure water blank was measured. For SEC-ICP-MS measurements an Agilent 1260 Infinity system coupled to an Agilent 7800 ICP-MS equipped with a dynamic reaction cell was used. Oxygen (purity 5.5, Messer Austria GmbH, Gumpoldskirchen, Austria) was used as a reaction gas. The parameters are given in Table S2.†
Cell culture and preparation of drug stocks
The human monocytic THP1-Dual™ reporter cell model was purchased from InvivoGen (#thpd-nfis). The human colorectal cancer cell line HCT116 and the murine colorectal CT-26 cell model were purchased from ATCC (Manassas, VA, USA). The oxaliplatin-resistant subline HCT116/OxR was generated as published previously.52 All reagents were obtained from Sigma-Aldrich (Sigma-Aldrich, St Louis, MO, USA) unless stated otherwise. THP1-Dual cells were cultured in RPMI-1640 medium (R6504), supplemented with L-glutamine (2 mM), Normocin™ (InvivoGen), and Pen-Strep (100 U mL−1–100 μg mL−1). Cells were selected once-weekly by additionally supplementing Zeocin® (InvivoGen) and Blasticidin (InvivoGen). HCT116 and HCT116/OxR cells were cultured in McCoy's 5A modified medium (M8403) and CT-26 cells in Dulbecco's modified Eagle's medium/Ham's Nutrient Mixture F12 (DMEM/F12) (SAFC Biosciences Ltd, UK). All cell culture media were supplemented with 10% FCS (BioWest, Riverside, MO, USA). The cells were incubated under standard conditions (37 °C, 5% CO2), regularly checked for Mycoplasma contamination, and authenticated via short tandem repeat analysis (Microsynth, Vienna, Austria). Oxaliplatin (LCLabs; # O-7111) was prepared as 10 mM stock in water. SR-717 was prepared as 10 mM stock in dimethyl sulfoxide (DMSO; Sigma-Aldrich). MSA-2 was prepared as 10 mM stock dimethylformamide (DMF). The STING inhibitor H-151 was obtained from InvivoGen and was prepared as 7.8 mM DMSO stock. Ox-SR-PEG-Es was prepared as a 10 mM stock in DMSO, Ox-MSA-PEG-Es and Ox-MSA-PEG-Ca were prepared as a 100 mM DMF stock. AA was prepared freshly in a cell culture medium. All drug stocks were stored at −20 °C. Dilutions of stocks were prepared in a cell culture medium immediately before the experiment. Corresponding dilutions of DMF or DMSO were used as untreated vehicle controls.MTT-based cell viability assay
Cell models were seeded (2–2.5 × 103 cells per well) in 96-well plates. After 24 h of recovery, the compounds were applied at the concentrations indicated in triplicate. Cell viability was determined after 72 h of continuous drug incubation using an MTT-based cell viability assay (EZ4U, Biomedica, Vienna, Austria) according to the manufacturer's recommendations. Absorbance was measured at 450 nm (vs. 620 nm as reference) using a multimode microplate reader (Tecan Spark, Zurich, Switzerland). Half-maximal inhibitory concentrations (IC50 values) were derived via interpolation from dose–response curves, modeled with the four-parameter logistic (4PL) nonlinear regression model in GraphPad Prism (La Jolla, CA, USA) from at least three independent experiments. Drug interaction dynamics between oxaliplatin and MSA-2 were established using the ZIP synergy scoring model of SynergyFinder (v3.14).53Western blot analysis
The THP1-Dual cell model was seeded at 1 × 106 cells per well in six-well plates. After 24 h of recovery, the compounds were applied at the concentrations indicated. After 24 h of drug exposure, whole-cell protein isolation was performed as described previously.14 Sodium dodecyl sulfate polyacrylamide gel electrophoresis was performed. Proteins were transferred onto polyvinylidene difluoride membranes (Thermo Scientific, Waltham, MA, USA). Antibodies targeting STING (##13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 647; 1
647; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), phospho-STING (#19
1000), phospho-STING (#19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 781; 1
781; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), TBK1 (#3504; 1
500), TBK1 (#3504; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), IRF-3 (#4302; 1
500), IRF-3 (#4302; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), phospho-p65 (#3033; 1
500), phospho-p65 (#3033; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), ISG15 (#2743; 1
500), ISG15 (#2743; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), caspase-3 (#9665; 1
1000), caspase-3 (#9665; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), cleaved caspase-3 (#9661; 1
1000), cleaved caspase-3 (#9661; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), PARP (#9532; 1
1000), PARP (#9532; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), and cleaved PARP (#9541; 1
1000), and cleaved PARP (#9541; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) were purchased from Cell Signaling Technology (Danvers, MA, USA). The p65 antibody (#sc-372; 1
1000) were purchased from Cell Signaling Technology (Danvers, MA, USA). The p65 antibody (#sc-372; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) was purchased from Santa Cruz and the phospho-H2A.X antibody (#05-636-I; 1
1000) was purchased from Santa Cruz and the phospho-H2A.X antibody (#05-636-I; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) was obtained from Merck (Darmstadt, Germany). The anti-β-actin (#5441; 1
500) was obtained from Merck (Darmstadt, Germany). The anti-β-actin (#5441; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000) antibody was obtained from Sigma-Aldrich. The anti-mouse IgG (#A0168; 1
2000) antibody was obtained from Sigma-Aldrich. The anti-mouse IgG (#A0168; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) horseradish peroxidase (HRP)-coupled secondary antibody was purchased from Sigma-Aldrich, and the anti-rabbit IgG (#7074; 1
000) horseradish peroxidase (HRP)-coupled secondary antibody was purchased from Sigma-Aldrich, and the anti-rabbit IgG (#7074; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000) HRP-linked secondary antibody was obtained from Cell Signaling Technology.
5000) HRP-linked secondary antibody was obtained from Cell Signaling Technology.
Intracellular platinum accumulation in vitro by ICP-MS
The THP1-Dual cell model was seeded at 1 × 106 cells per well in six-well plates. After 24 h of recovery, the compounds were applied at the concentrations indicated in triplicate. After 3 h of drug exposure, the cells were prepared as published previously.13 In brief, the cells were detached, washed twice in Dulbecco's Phosphate-Buffered Saline (DPBS) (Cytiva), dried at RT overnight and lysed in 400 μL of nitric acid (68% Rotipuran Supra, Carl Roth, Karlsruhe, Germany) for 2 h at RT. The lysate was filled up to 8 mL with water. The platinum concentration was determined by ICP-MS analysis. Platinum and rhenium standards were derived from CPI International (Amsterdam, the Netherlands). The total platinum content was determined with an Agilent 7800 quadrupole-based ICP-MS instrument (Agilent Technologies, Tokyo, Japan) equipped with an Agilent SPS 4 autosampler (Agilent Technologies, Tokyo, Japan) and a MicroMist nebulizer at a sample uptake rate of approximately 0.2 mL min−1. A radio frequency power of 1550 W was used as well as nickel cones. Argon was used as a plasma gas (15 L min−1) and as a carrier gas (∼1.1 L min−1). The dwell time was set to 0.1 s and the measurements were performed in 12 replicates with 100 sweeps. Rhenium served as an internal standard for platinum. The Agilent MassHunter software package (Workstation Software, version C.01.04, 2018) was used for data processing.IRF and NF-κB-inducible THP1-dual reporter cell model
The THP1-Dual™ reporter cell model for simultaneously monitoring the activity of the IRF and NF-κB pathway contains an IRF-inducible luciferase reporter under the control of an ISG54 minimal promoter in conjunction with five interferon (IFN)-stimulated response elements, and an induced SEAP driven by an IFN-β minimal promoter fused to five copies of the NF-κB consensus transcriptional response element and three copies of the c-Rel binding site. The luciferase and SEAP reporter are measurable in the cell culture supernatant using QUANTI-Luc™ 4 Lucia/Gaussia (InvivoGen) and QUANTI-Blue™ (InvivoGen) solution, respectively. Cells were seeded at 1 × 105 cells per well in 96-well plates in RPMI-1640 medium, supplemented with 10% FCS, L-glutamine and Pen-Strep. After 2 h of recovery, the compounds were applied at the concentrations indicated in triplicate. The cell culture supernatant (SN) was collected after 24 h of drug incubation and secreted luciferase and SEAP reporter levels were measured according to the manufacturer's recommendations. In brief, for the detection of luciferase, 20 μL of SN were transferred into 96-well white opaque plates, and 30 μL of QUANTI-Luc™ reagent were added per well using an injector module in a multimode microplate reader (Tecan Spark, Zurich, Switzerland). Luminescence was recorded at a reading time of 0.1 second. For the detection of SEAP, 10 μL of SN were transferred to 96-well plates and 90 μL of QUANTI-Blue™ were added per well. Absorbance was measured at 620 nm. Half-maximal effective concentrations (EC50 values) were derived via interpolation from dose–response curves, modeled with the four-parameter logistic (4PL) nonlinear regression model in GraphPad Prism. For comparison, the maximal response rate of the prodrug was depicted in the percent of the maximal response rate achieved by SR-717 or MSA-2 (%EMAX) at the respective concentrations tested. Activatability, tested in co-incubation experiments with AA (50 μM), was expressed as the area under the curve (AUC) ratio of +AA vs. −AA-treated cells. The STING-dependency of the reporter induction was tested by co-administration of the STING inhibitor H-151 (10 μM), added to the cell culture medium 2 h before applying the investigated compounds. In addition to monitoring secreted reporter levels, the cell viability of the reporter cells was determined after 24 h of drug treatment by an MTT-based cell viability assay.Quantitative RT-PCR
The THP1-Dual cell model was seeded at 1 × 106 cells per well in six-well plates. After 24 h of recovery, the compounds were applied at the concentrations indicated. After 24 h of drug exposure, total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific, MA, USA), as described previously.12 RNA was reverse transcribed into cDNA using RevertAid Reverse Transcriptase (Thermo Fisher Scientific). Real-time PCR was performed using the GoTaq® RT-qPCR system (Promega) with a CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories). Relative mRNA levels were quantified using the 2−ddCT method.54 RPL41 was used as a housekeeping gene. Human-specific primers were purchased from Eurofins Genomics (Ebersberg, Germany) with the following sequences: RPL41: fwd (5′-CAAGTGGAGGAAGAAGCGA-3′), rev (5′-TTACTTGGACCTCTGCCTC-3′); IFNB1: fwd (5′-CTT GGA TTC CTA CAA AGA AGC AGC-3′), rev (5′-TCCTCCTTCTGGAACTGCTGCA-3′); ISG15: fwd (5′-GAGAGGCAGCGAACTCATCT-3′), rev (5′-CTTCAGCTCTGACACCGACA-3′); IFIT1: fwd (5′-GCCTTGCTGAAGTGTGGAGGAA-3′), rev (5′-ATCCAGGCGATAGGCAGAGATC-3′); CXCL10: fwd (5′-GGTGAGAAGAGATGTCTGAATCC-3′), rev (5′-GTCCATCCTTGGAAGCACTGCA-3′); and CCL5: fwd (5′-ATCCTCATTGCTACTGCCCTC-3′), rev (5′-GCCACTGGTGTAGAAATACTCC-3′).Cytokine and chemokine detection using a Luminex xMAP detection platform
Quantification of secreted protein targets in either cell culture SN or in plasma of treated animals was performed using a ProcartaPlex (96-well) multiplex assay panel (Thermo Fisher Scientific) in the xMAP detection platform (Luminex). For the in vitro detection of secreted proteins, THP1-Dual cells were seeded at 1 × 105 cells per well in 96-well plates in RPMI-1640 medium, supplemented with 10% FCS, L-glutamine and Pen-Strep. After 24 h of recovery, the compounds were applied at the concentrations indicated in triplicate.After 24 h of drug incubation, the cell culture SN of technical triplicate was collected, pooled, centrifuged (400g, 10 min, 4 °C), transferred into a fresh tube, and stored at −20 °C.
For protein detection in vivo, mouse blood was collected from the submandibular vein in K3E K3EDTA-tubes (Greiner Bio-One; Kremsmünster, AUT; #450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530). Following analysis of hematological parameters (see also “In vivo syngraft experiments” in the Experimental section), EDTA-blood was centrifuged (900g, 10 min, 4 °C) twice and the supernatant (EDTA-plasma) was stored at −20 °C. Next, the ProcartaPlex (96-well) multiplex assay panel was performed according to the manufacturer's recommendations. In brief, the capture bead mix was incubated with test samples (undiluted SN or mouse plasma) or standard mix overnight. The SN of three independent experiments or the plasma of four animals per treatment group was loaded onto the multiplex assay plate. Next, biotinylated detection antibody mix and Streptavidin-PE were added to the plate. Following the recommended washing steps, the samples were incubated in a reading buffer and were measured using a Luminex xMAP platform (Bio-Plex MAGPIX Multiplex Reader; Bio-Rad Laboratories) and analyzed using xPONENT software.
530). Following analysis of hematological parameters (see also “In vivo syngraft experiments” in the Experimental section), EDTA-blood was centrifuged (900g, 10 min, 4 °C) twice and the supernatant (EDTA-plasma) was stored at −20 °C. Next, the ProcartaPlex (96-well) multiplex assay panel was performed according to the manufacturer's recommendations. In brief, the capture bead mix was incubated with test samples (undiluted SN or mouse plasma) or standard mix overnight. The SN of three independent experiments or the plasma of four animals per treatment group was loaded onto the multiplex assay plate. Next, biotinylated detection antibody mix and Streptavidin-PE were added to the plate. Following the recommended washing steps, the samples were incubated in a reading buffer and were measured using a Luminex xMAP platform (Bio-Plex MAGPIX Multiplex Reader; Bio-Rad Laboratories) and analyzed using xPONENT software.
In vivo syngraft experiments
Mice were kept in groups of four per cage with sterilized paper-based environmental enrichment and food and water ad libitum, under pathogen-free conditions and a controlled environment with a 12 h light–dark cycle. Syngeneic models of colorectal carcinoma were generated via subcutaneous injection of CT-26 cells (0.5 × 106 cells in 50 μL of serum-free RPMI-1640 medium) into the right flank of 8–12 weeks old BALB/c mice. Oxaliplatin was dissolved in 5% glucose and Ox-MSA-PEG-Ca, Ox-MSA-Mal, and MSA-2 in 15% PEG400 in DPBS (Cytiva). Ox-MSA-PEG-Ca (21.52 mg kg−1), Ox-MSA-Mal (26.37 mg kg−1), and MSA-2 (6.67 mg kg−1) were administered equimolar to the MTD of oxaliplatin (=9 mg kg−1). For the drug distribution study by ICP-MS and immune phenotyping by flow cytometry, animals were treated 6 d after subcutaneous implantation, when tumors reached a volume of ∼140 mm3, intravenously (i.v.) with the respective compounds or solvent twice a week for one week. All animals were euthanized 24 h after the second dose. For examining hematotoxic effects after long-term treatment, C57BL/6 mice were treated i.v. with the relevant compounds or solvent, twice a week for two weeks and full blood was analyzed 72 h after the fourth dose. To determine the anticancer efficacy of the investigated compounds, treatment started 4 d after tumor implantation, when all tumors were measurable (>25 mm3) using a twice-weekly treatment scheme for two weeks. Tumor size was determined daily via manual caliper measurement. The tumor volume was calculated as tumor volume = (length × diameter × diameter)/2. The overall condition, shape, and activity of the animals were assessed daily. Peripheral blood was collected from the submandibular vein in K3E K3EDTA tubes (Greiner Bio-One; Kremsmünster, AUT) on ice and was analyzed for hematological parameters (red blood cells (RBCs), hemoglobin (HGB), hematocrit (HCT), red cell distribution width (RDW), mean corpuscular volume (MCV), white blood cells (WBCs), lymphocytes (LYM), monocytes (MO), granulocytes (GRA), and platelets (PLT) using a Vet ABC hematology analyzer (scil Animal Care GmbH, Viernheim, Germany). EDTA-blood was centrifuged (900g, 10 min) twice and the supernatant (EDTA-plasma) was stored at −20 °C for subsequent cytokine/chemokine analysis using a Luminex detection platform. All animal experiments were performed with humane endpoints based on Federation of European Laboratory Animal Science Associations guidelines and according to the regulations of the Ethics Committee for the Care and Use of Laboratory Animals at the Medical University Vienna (proposal number BMBWF 2023–0.122.324). Animals were euthanized upon reaching a tumor volume of >1500 mm3, a loss in body weight (>20%), tumor ulceration or other indications of deteriorated health. Animals were euthanized via cervical dislocation.Platinum distribution study in vivo by ICP-MS
The generation and treatment of tumor-bearing mice are outlined in the section “In vivo syngraft experiments” described above. Mouse tissues were processed as described previously.13 Briefly, serum was isolated from peripheral blood by centrifugation (900g, 10 min, 4 °C, twice). Tissues were digested in 20% nitric acid and hydrogen peroxide using an open-vessel graphite digestion system. The platinum concentration was determined by ICP-MS analysis; for details see “Intracellular platinum accumulation in vitro by ICP-MS”.Immune activation and infiltration by flow cytometry
The generation and treatment of tumor-bearing mice are outlined in the section “In vivo syngraft experiments” above. Samples were kept on ice during the whole procedure. Tissues were prepared as described previously.55 In brief, tumor and spleen tissues were collected and mechanically dissociated in DPBS buffer containing Ca2+/Mg2+ and FCS (5%). Tumor samples were digested using collagenase VIII (1 mg mL−1) in a DPBS buffer (without Ca2+/Mg2+) supplemented with DNAse I (1 mg mL−1) for 15 min at 37 °C under constant shaking. Samples were filtered using 70 μm cell strainers in a DPBS buffer (without Ca2+/Mg2+) containing FCS (5%), EDTA (5 mM) and DNase I (20 μg mL−1). Subsequent washing steps were performed in DPBS buffer (without Ca2+/Mg2+) containing FCS (5%), EDTA (5 mM) and DNase I (20 μg mL−1), unless indicated otherwise. Red blood cells were removed by short incubation with ACK buffer. For blocking, samples were incubated with the Ultra-LEAF purified anti-mouse CD16/CD32 antibody (clone: 93; #101330; RRID:AB_2783037; dilution: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) (Biolegend, San Diego, CA, USA) in the BD Horizon Brilliant Stain Buffer (#563794; BD Biosciences) for 10 min. Next, the samples were incubated with an antibody mixture in the BD Horizon Brilliant Stain Buffer for 30 min. Fluorescent dye-labeled antibodies targeting murine CD3 (17A2; #100203; RRID:AB_312661; 1
1000) (Biolegend, San Diego, CA, USA) in the BD Horizon Brilliant Stain Buffer (#563794; BD Biosciences) for 10 min. Next, the samples were incubated with an antibody mixture in the BD Horizon Brilliant Stain Buffer for 30 min. Fluorescent dye-labeled antibodies targeting murine CD3 (17A2; #100203; RRID:AB_312661; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), CD8a (53-6.7; #100738; RRID:AB_11204079; 1
50), CD8a (53-6.7; #100738; RRID:AB_11204079; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160), CD19 (6D5; #115528; RRID:AB_493735; 1
160), CD19 (6D5; #115528; RRID:AB_493735; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400), CD49b (DX5; #108924; RRID:AB_2565271; 1
400), CD49b (DX5; #108924; RRID:AB_2565271; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 229), CD11b (M1/70; #101228; RRID:AB_893232; 1
229), CD11b (M1/70; #101228; RRID:AB_893232; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80), and CD69 (H1.2F3; # 104512; RRID:AB_493564; 1
80), and CD69 (H1.2F3; # 104512; RRID:AB_493564; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) were purchased from BioLegend. The antibody targeting murine CD4 (RM4-5; # 85-0042-U100; 1
40) were purchased from BioLegend. The antibody targeting murine CD4 (RM4-5; # 85-0042-U100; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) and the viability dye Ghost Dye Red 780 (#13-0865-T100; 1
80) and the viability dye Ghost Dye Red 780 (#13-0865-T100; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) were purchased from Tonbobio (San Diego, CA, USA). The antibody targeting CD45 (30-F11; #363-0451-82; RRID:AB_2925264; 1
1000) were purchased from Tonbobio (San Diego, CA, USA). The antibody targeting CD45 (30-F11; #363-0451-82; RRID:AB_2925264; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80) was purchased from Life Tech (Invitrogen). For intracellular staining of granzyme B (GzmB) (QA16A02; #372208; RRID:AB_2687032; 1
80) was purchased from Life Tech (Invitrogen). For intracellular staining of granzyme B (GzmB) (QA16A02; #372208; RRID:AB_2687032; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and FoxP3 (MF-14; #320014; RRID:AB_439750; 1
20) and FoxP3 (MF-14; #320014; RRID:AB_439750; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) the True-Nuclear Transcription Factor Buffer Set (Biolegend; #424401) was used according to the manufacturer's recommendations. In brief, samples were incubated with Perm Fix Solution for 60 min at RT. Next, the samples were stained with antibodies targeting GzmB and FoxP3 (diluted in Perm buffer) for 30 min at RT. Then, the samples were resuspended in DPBS buffer containing FCS (5%), EDTA (5 mM) and DNase I (20 μg mL−1) and incubated overnight at 4 °C. Next day, the samples were analyzed using a Cytek Aurora 5L (Cytek Biosciences, CA, USA) spectral flow cytometer. Data were analyzed using SpectroFlo (v3.2.1) (Cytek Biosciences) and FlowJo (v10.8.1) (FlowJo LLC, Ashland, OR, USA) software.
100) the True-Nuclear Transcription Factor Buffer Set (Biolegend; #424401) was used according to the manufacturer's recommendations. In brief, samples were incubated with Perm Fix Solution for 60 min at RT. Next, the samples were stained with antibodies targeting GzmB and FoxP3 (diluted in Perm buffer) for 30 min at RT. Then, the samples were resuspended in DPBS buffer containing FCS (5%), EDTA (5 mM) and DNase I (20 μg mL−1) and incubated overnight at 4 °C. Next day, the samples were analyzed using a Cytek Aurora 5L (Cytek Biosciences, CA, USA) spectral flow cytometer. Data were analyzed using SpectroFlo (v3.2.1) (Cytek Biosciences) and FlowJo (v10.8.1) (FlowJo LLC, Ashland, OR, USA) software.
Data evaluation and statistical analysis
The statistical tests used to determine significance and the corresponding p values are described in the respective figure legends. Significance was set at p < 0.05. All Student's t-tests are unpaired and two-sided unless indicated otherwise. One-way analysis of variance (ANOVA) was performed with the Tukey multiple comparison test unless indicated otherwise. Tumor sizes were compared using two-way ANOVA with Tukey's multiple comparison test. Statistical tests were conducted using GraphPad Prism version 9.3.0 for Windows (GraphPad Software Inc., San Diego, CA, USA). Error bars represent the standard deviation unless indicated otherwise.Author contributions
Conceptualization: CRK and WB; data curation: MD, MG, and AF; formal analysis: MD, MG, and AF; funding acquisition: CRK and WB; investigation: MD, MG, AF, MaG, CJ, and JVB; methodology: MD, MG, AF, MaG, and CJ; project administration: WB and CRK; resources: CRK and WB; supervision: CRK and WB; validation: MD, MG, AF, CRK, WB, MaG, and JVB; visualization: MD, MG, AF CRK, WB, CP, and DV; roles/writing – original draft: MD, MG, CRK, WB, MaG, CP, and DV; and writing – review & editing: MD, MG, MaG, AF, CJ, JVB, PV, CP, DV, PH, CRK, and WB.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We gratefully acknowledge the Austrian Science Fund (FWF) for financial support (grant FG3 to W. B. and C. K. and P37111 to C. K.). We thank Christoph Baumgartinger for tissue distribution ICP-MS measurements as well as Gerhard Zeitler (financed by Obermann-Mahlke Stiftung) for devoted animal care and support with sample digestion for ICP-MS analysis.References
- L. Kelland, The resurgence of platinum-based cancer chemotherapy, Nat. Rev. Cancer, 2007, 7, 573–584 CrossRef CAS PubMed.
- J. Zhou, Y. Kang, L. Chen, H. Wang, J. Liu, S. Zeng and L. Yu, The drug-resistance mechanisms of five platinum-based antitumor agents, Front. Pharmacol., 2020, 11, 343 CrossRef CAS PubMed.
- R. Oun, Y. E. Moussa and N. J. Wheate, The side effects of platinum-based chemotherapy drugs: a review for chemists, Dalton Trans., 2018, 47, 6645–6653 RSC.
- T. C. Johnstone, K. Suntharalingam and S. J. Lippard, The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs, Chem. Rev., 2016, 116, 3436–3486 CrossRef CAS PubMed.
- H. Schueffl, S. Theiner, G. Hermann, J. Mayr, P. Fronik, D. Groza, S. van Schonhooven, L. Galvez, N. S. Sommerfeld, A. Schintlmeister, S. Reipert, M. Wagner, R. M. Mader, G. Koellensperger, B. K. Keppler, W. Berger, C. R. Kowol, A. Legin and P. Heffeter, Albumin-targeting of an oxaliplatin-releasing platinum(IV) prodrug results in pronounced anticancer activity due to endocytotic drug uptake in vivo, Chem. Sci., 2021, 12, 12587–12599 RSC.
- M. D. Hall, H. R. Mellor, R. Callaghan and T. W. Hambley, Basis for design and development of platinum(IV) anticancer complexes, J. Med. Chem., 2007, 50, 3403–3411 CrossRef CAS PubMed.
- S. Dilruba and G. V. Kalayda, Platinum-based drugs: past, present and future, Cancer Chemother. Pharmacol., 2016, 77, 1103–1124 CrossRef CAS PubMed.
- R. G. Kenny and C. J. Marmion, Toward Multi-Targeted Platinum and Ruthenium Drugs-A New Paradigm in Cancer Drug Treatment Regimens?, Chem. Rev., 2019, 119, 1058–1137 CrossRef CAS PubMed.
- F. Kratz, Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles, J. Controlled Release, 2008, 132, 171–183 CrossRef CAS PubMed.
- A. Vis, A. van der Gaast, B. van Rhijn, T. Catsburg, C. Schmidt and G. Mickisch, A phase II trial of methotrexate-human serum albumin (MTX-HSA) in patients with metastatic renal cell carcinoma who progressed under immunotherapy, Cancer Chemother. Pharmacol., 2002, 49, 342–345 CrossRef CAS PubMed.
- J. Gong, J. Yan, C. Forscher and A. Hendifar, Aldoxorubicin: a tumor-targeted doxorubicin conjugate for relapsed or refractory soft tissue sarcomas, Drug Des., Dev. Ther., 2018, 12, 777–786 CrossRef CAS PubMed.
- P. Fronik, I. Poetsch, A. Kastner, T. Mendrina, S. Hager, K. Hohenwallner, H. Schueffl, D. Herndler-Brandstetter, G. Koellensperger, E. Rampler, J. Kopecka, C. Riganti, W. Berger, B. K. Keppler, P. Heffeter and C. R. Kowol, Structure–activity relationships of triple-action platinum(IV) Prodrugs with albumin-binding properties and immunomodulating ligands, J. Med. Chem., 2021, 64, 12132–12151 CrossRef CAS PubMed.
- P. Fronik, M. Gutmann, P. Vician, M. Stojanovic, A. Kastner, P. Heffeter, C. Pirker, B. K. Keppler, W. Berger and C. R. Kowol, A platinum(IV) prodrug strategy to overcome glutathione-based oxaliplatin resistance, Commun. Chem., 2022, 5, 46–59 CrossRef CAS PubMed.
- J. Mayr, P. Heffeter, D. Groza, L. Galvez, G. Koellensperger, A. Roller, B. Alte, M. Haider, W. Berger and C. R. Kowol, An albumin-based tumor-targeted oxaliplatin prodrug with distinctly improved anticancer activity in vivo, Chem. Sci., 2017, 8, 2241–2250 RSC.
- L. Lu, M. Zhan, X.-Y. Li, H. Zhang, D. J. Dauphars, J. Jiang, H. Yin, S.-Y. Li, S. Luo, Y. Li and Y.-W. He, Clinically approved combination immunotherapy: Current status, limitations, and future perspective, Curr. Res. Immunol., 2022, 3, 118–127 CrossRef CAS PubMed.
- B. Englinger, C. Pirker, P. Heffeter, A. Terenzi, C. R. Kowol, B. K. Keppler and W. Berger, Metal Drugs and the Anticancer Immune Response, Chem. Rev., 2019, 119, 1519–1624 CrossRef CAS PubMed.
- A. Terenzi, C. Pirker, B. K. Keppler and W. Berger, Anticancer metal drugs and immunogenic cell death, J. Inorg. Biochem., 2016, 165, 71–79 CrossRef CAS PubMed.
- Y. Y. Janjigian, K. Shitara, M. Moehler, M. Garrido, P. Salman, L. Shen, L. Wyrwicz, K. Yamaguchi, T. Skoczylas, A. C. Bragagnoli, T. Liu, M. Schenker, P. Yanez, M. Tehfe, R. Kowalyszyn, M. V. Karamouzis, R. Bruges, T. Zander, R. Pazo-Cid, E. Hitre, K. Feeney, J. M. Cleary, V. Poulart, D. Cullen, M. Lei, H. Xaio, K. Kondo, M. Li and J. A. Ajani, First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial, Lancet, 2021, 398, 27–40 CrossRef CAS PubMed.
- N. Samson and A. Ablasser, The cGAS–STING pathway and cancer, Nat. Cancer, 2022, 3, 1452–1463 CrossRef CAS PubMed.
- K. Zhao, J. Huang, Y. Zhao, S. Wang, J. Xu and K. Yin, Targeting STING in cancer: Challenges and emerging opportunities, Biochim. Biophys. Acta, Rev. Cancer, 2023, 6, 188983–188999 CrossRef PubMed.
- L. Motedayen Aval, J. E. Pease, R. Sharma and D. J. Pinato, Challenges and opportunities in the clinical development of STING agonists for cancer immunotherapy, J. Clin. Med., 2020, 9, 3323–3352 CrossRef PubMed.
- C. Huang, N. Shao, Y. Huang, J. Chen, D. Wang, G. Hu, H. Zhang, L. Luo and Z. Xiao, Overcoming challenges in the delivery of STING agonists for cancer immunotherapy: a comprehensive review of strategies and future perspectives, Mater. Today Bio, 2023, 23, 100839–100857 CrossRef CAS PubMed.
- Z. Najminejad, F. Dehghani, Y. Mirzaei, A. H. Mer, S. A. Saghi, M. H. Abdolvahab, N. Bagheri, A. Meyfour, A. Jafari, S. Jahandideh, T. Gharibi, Z. Amirkhani, H. Delam, N. Mahatan, H. Shahsavarani and M. Abdollahpour-Alitappeh, Clinical perspective: antibody-drug conjugates (ADCs) for the treatment of HER2-positive breast cancer, Mol. Ther., 2023, 31, 1874–1904 CrossRef CAS PubMed.
- L. Cai, Y. Wang, Y. Chen, H. Chen, T. Yang, S. Zhang, Z. Guo and X. Wang, Manganese(II) complexes stimulate antitumor immunity via aggravating DNA damage and activating the cGAS-STING pathway, Chem. Sci., 2023, 14, 4375–4389 RSC.
- Y. Zheng, X.-X. Chen, D.-Y. Zhang, W.-J. Wang, K. Peng, Z.-Y. Li, Z.-W. Mao and C.-P. Tan, Activation of the cGAS-STING pathway by a mitochondrial DNA-targeted emissive rhodium(III) metallointercalator, Chem. Sci., 2023, 14, 6890–6903 RSC.
- F. Li, Z. Wen, C. Wu, Z. Yang, Z. Wang, W. Diao, D. Chen, Z. Xu, Y. Lu and W. Liu, Simultaneous activation of immunogenic cell death and cGAS-STING pathway by liver-and mitochondria-targeted gold(I) complexes for chemoimmunotherapy of hepatocellular carcinoma, J. Med. Chem., 2024, 67, 1982–2003 CrossRef CAS PubMed.
- B.-F. Liang, S. Jiang, Y.-S. Zhi, Z.-Y. Pan, X.-Q. Su, Q. Gong, Z.-D. He, D.-H. Yao, L. He and C.-Y. Li, Photoactivation of the cGAS-STING pathway and pyroptosis by an endoplasmic reticulum-targeting ruthenium(II) complex for cancer immunotherapy, Inorg. Chem. Front., 2025, 6, 2294–2303 Search PubMed.
- E. N. Chin, C. Yu, V. F. Vartabedian, Y. Jia, M. Kumar, A. M. Gamo, W. Vernier, S. H. Ali, M. Kissai, D. C. Lazar, N. Nguyen, L. E. Perreira, B. Benish, A. K. Woods, S. B. Joseph, A. Chu, K. A. Johnson, P. N. Sander, F. Martinez-Pena, E. N. Hampton, T. S. Young, D. W. Wolan, A. K. Chatterjee, P. G. Schultz, H. M. Petrassi, J. R. Teijaro and L. L. Lairson, Antitumor activity of a systemic STING-activating non-nucleotide cGAMP mimetic, Science, 2020, 369, 993–999 CAS.
- B.-S. Pan, S. A. Perera, J. A. Piesvaux, J. P. Presland, G. K. Schroeder, J. N. Cumming, B. W. Trotter, M. D. Altman, A. V. Buevich, B. Cash, S. Cemerski, W. Chang, Y. Chen, P. J. Dandliker, G. Feng, A. Haidle, T. Henderson, J. Jewell, I. Kariv, I. Knemeyer, J. Kopinja, B. M. Lacey, J. Laskey, C. A. Lesburg, R. Liang, B. J. Long, M. Lu, Y. Ma, E. C. Minnihan, G. O'Donnel, R. Otte, L. Price, L. Rakhilina, B. Sauvagnat, S. Sharma, S. Tyagarajan, H. Woo, D. F. Wyss, S. Xu, D. J. Bennett and G. H. Addona, An orally available non-nucleotide STING agonist with antitumor activity, Science, 2020, 369, 6505–6513 Search PubMed.
- X. Feng, L. Pan, Z. Qian, D. Liu, X. Guan, L. Feng, B. Song, X. Xu, N. Tan, Y. Ma, Z. Li, Z. Wang and J. Bian, Discovery of selenium-containing STING agonists as orally available antitumor agents, J. Med. Chem., 2022, 65, 15048–15065 CrossRef CAS PubMed.
- A. Shen, X. Li, Y. Zhang, J. Ma, R. Xiao, X. Wang, Z. Song, Z. Liu, M. Geng, A. Zhang, Z. Xie and C. Ding, Structure− Activity relationship study of benzothiophene oxobutanoic acid analogues leading to novel stimulator of interferon gene (STING) agonists, Eur. J. Med. Chem., 2022, 241, 114627–114643 CAS.
- M. Wang, Y. Cai, T. He, Y. Zhang, L. Yi, W. Li and P. Zhou, Antitumor Effect of Platinum-Modified STING Agonist MSA-2, ACS Omega, 2024, 9, 2650–2656 CAS.
- S. Zhang, D. Song, W. Yu, J. Li, X. Wang, Y. Li, Z. Zhao, Q. Xue, J. Zhao, J. P. Li and Z. Guo, Combining cisplatin and a STING agonist into one molecule for metalloimmunotherapy of cancer, Natl. Sci. Rev., 2024, 11, nwae020 CAS.
- J. Jiang, M. Zhang, T. Lyu, L. Chen, M. Wu, R. Li, H. Li, X. Wang, X. Jiang and X. Zhen, Sono–Driven STING Activation using Semiconducting Polymeric Nanoagonists for Precision Sono–Immunotherapy of Head and Neck Squamous Cell Carcinoma, Adv. Mater., 2023, 35, 2300854–2300866 CAS.
- J. Yu, S. He, C. Zhang, C. Xu, J. Huang, M. Xu and K. Pu, Polymeric STING Pro–agonists for Tumor–Specific Sonodynamic Immunotherapy, Angew. Chem., 2023, 135, 7272–7280 Search PubMed.
- M. R. Reithofer, S. M. Valiahdi, M. A. Jakupec, V. B. Arion, A. Egger, M. S. Galanski and B. K. Keppler, Novel di-and tetracarboxylatoplatinum(IV) complexes. Synthesis, characterization, cytotoxic activity, and DNA platination, J. Med. Chem., 2007, 50, 6692–6699 CAS.
- A. Kastner, T. Mendrina, F. Bachmann, W. Berger, B. K. Keppler, P. Heffeter and C. R. Kowol, Tumor-targeted dual-action NSAID-platinum(IV) anticancer prodrugs, Inorg. Chem. Front., 2023, 10, 4126–4138 RSC.
- MarvinSketch, Version 20.9 chemical editor of ChemAxon, 2020, https://chemaxon.com/(accessed 18 march 2025) Search PubMed.
- G. Luchini and R. S. Paton, Bottom-Up Atomistic Descriptions of Top-Down Macroscopic Measurements: Computational Benchmarks for Hammett Electronic Parameters, ACS Phys. Chem. Au, 2024, 4, 259–267 CrossRef CAS PubMed.
- S. Choi, C. Filotto, M. Bisanzo, S. Delaney, D. Lagasee, J. L. Whitworth, A. Jusko, C. Li, N. A. Wood, J. Willingham, A. Schwenker and K. Spaulding, Reduction and anticancer activity of platinum(IV) complexes, Inorg. Chem., 1998, 37, 2500–2504 CrossRef CAS.
- S. Chen, H. Yao, Q. Zhou, M.-K. Tse, Y. F. Gunawan and G. Zhu, Stability, reduction, and cytotoxicity of platinum(IV) anticancer prodrugs bearing carbamate axial ligands: comparison with their carboxylate analogues, Inorg. Chem., 2020, 59, 11676–11687 CrossRef CAS PubMed.
- L. Galvez, M. Rusz, M. Schwaiger-Haber, Y. El Abiead, G. Hermann, U. Jungwirth, W. Berger, B. K. Keppler, M. A. Jakupec and G. Koellensperger, Preclinical studies on metal based anticancer drugs as enabled by integrated metallomics and metabolomics, Metallomics, 2019, 11, 1716–1728 CrossRef CAS PubMed.
- B. Liang, X. Xing, H. Storts, Z. Ye, H. Claybon, R. Austin, R. Ding, B. Liu, H. Wen, W. O. Miles, R. Fishel and J. J. Wang, Antagonistic roles of cGAS/STING signaling in colorectal cancer chemotherapy, Front. Oncol., 2024, 14, 1441935–1441946 CrossRef CAS PubMed.
- B. Beernaert and E. E. Parkes, cGAS–STING signalling in cancer: striking a balance with chromosomal instability, Biochem. Soc. Trans., 2023, 51, 539–555 CAS.
- Q. Chen, L. Sun and Z. J. Chen, Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing, Nat. Immun., 2016, 17, 1142–1149 CAS.
- M. Dijkstra, H. Schueffl, A. Federa, C. Kast, A. Unterlercher, B. K. Keppler, P. Heffeter and C. R. Kowol, Novel Maleimide Linkers Based on a Piperazine Motif for Strongly Increased Aqueous Solubility, ACS Omega, 2025, 10, 5047–5064 CAS.
- P. Haquette, B. Talbi, S. Canaguier, S. Dagorne, C. Fosse, A. Martel, G. Jaouen and M. Salmain, Functionalized cationic (η6-arene) ruthenium(II) complexes for site-specific and covalent anchoring to papain from papaya latex. Synthesis, X-ray structures and reactivity studies, Tetrahedron Lett., 2008, 49, 4670–4673 CAS.
- M. Hanif, A. A. Nazarov, A. Legin, M. Groessl, V. B. Arion, M. A. Jakupec, Y. O. Tsybin, P. J. Dyson, B. K. Keppler and C. G. Hartinger, Maleimide-functionalised organoruthenium anticancer agents and their binding to thiol-containing biomolecules, Chem. Commun., 2012, 48, 1475–1477 CAS.
- Y.-R. Zheng, K. Suntharalingam, T. C. Johnstone, H. Yoo, W. Lin, J. G. Brooks and S. J. Lippard, Pt(IV) prodrugs designed to bind non-covalently to human serum albumin for drug delivery, J. Am. Chem. Soc., 2014, 136, 8790–8798 CAS.
- Y. Zhu, X. An, X. Zhang, Y. Qiao, T. Zheng and X. Li, STING: a master regulator in the cancer-immunity cycle, Mol. Cancer, 2019, 18, 1–15 Search PubMed.
- Z. Gu, Y. Hao, T. Schomann, F. Ossendorp, P. Ten Dijke and L. J. Cruz, Enhancing anti-tumor immunity through liposomal oxaliplatin and localized immunotherapy via STING activation, J. Controlled Release, 2023, 357, 531–544 CrossRef CAS PubMed.
- U. Jungwirth, D. N. Xanthos, J. Gojo, A. K. Bytzek, W. Körner, P. Heffeter, S. A. Abramkin, M. A. Jakupec, C. G. Hartinger and U. Windberger, Anticancer activity of methyl-substituted oxaliplatin analogs, Mol. Pharmacol., 2012, 81, 719–728 CAS.
- S. Zheng, W. Wang, J. Aldahdooh, A. Malyutina, T. Shadbahr, Z. Tanoli, A. Pessia and J. Tang, SynergyFinder plus: toward better interpretation and annotation of drug combination screening datasets, Genomics, Proteomics Bioinf., 2022, 20, 587–596 CrossRef PubMed.
- K. J. Livak and T. D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method, Methods, 2001, 25, 402–408 CrossRef CAS PubMed.
- D. Groza, S. Gehrig, P. Kudela, M. Holcmann, C. Pirker, C. Dinhof, H. H. Schueffl, M. Sramko, J. Hoebart, F. Alioglu, M. Grusch, M. Ogris, W. Lubitz, B. K. Keppler, I. Pahkunova-Martic, C. R. Kowol, M. Sibilia, W. Berger and P. Heffeter, Bacterial ghosts as adjuvant to oxaliplatin chemotherapy in colorectal carcinomatosis, OncoImmunology, 2018, 7, e1424676 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5qi00433k |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2025 |