Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Guest-induced gate-opening in a flexible MOF adsorbent that exhibits benzene/cyclohexane selectivity†
Guo-Ao
Li‡
a,
Min
Deng‡
a,
Wei
Guo
a,
Shuang
Yin
a,
Yan-E
Liu
a,
Ai-Xin
Zhu
 *a and
Michael J.
Zaworotko
*a and
Michael J.
Zaworotko
 *b
*b
aKey Laboratory of Modern Separation Analysis and Substance Transformation, Faculty of Chemistry and Chemical Engineering, Yunnan Normal University, Kunming 650500, China. E-mail: zaxchem@126.com
bDepartment of Chemical Sciences, Bernal Institute, University of Limerick, Limerick V94T9PX, Ireland. E-mail: xtal@ul.ie
First published on 10th March 2025
Abstract
We report the synthesis of a 2-fold interpenetrated primitive cubic (pcu) topology network, X-pcu-11-Zn, formulated as [Zn2(DMTDC)2(dpb)] (H2DMTDC = 3,4-dimethylthieno[2,3-b]thiophene-2,5-dicarboxylic acid; dpb = 1,4-di(pyridin-4-yl)benzene). Upon removal of solvent molecules from the as-synthesised form, X-pcu-11-Zn-α, transformation from a large-pore “open” phase to a narrow pore phase, X-pcu-11-Zn-β, occurred. The β-phase subsequently exhibited guest-induced switching as evidenced by a 2-step type F-IV2 adsorption isotherm for N2 at 77 K and a 3-step profile with two gate-opening pressures for CO2 at 195 K. Dynamic vapour sorption studies revealed selective sorption of methanol, ethanol, and CH3CN over H2O at 298 K. Furthermore, X-pcu-11-Zn-β selectively adsorbed benzene over cyclohexane concomitant with a gate-opening effect driven by structural transformations. Importantly, the transformations between the guest-free and guest-loaded structures were found to be reversible over six adsorption/desorption cycles. Single-crystal X-ray diffraction analysis of the benzene-loaded phase indicates that selective benzene binding can be attributed to π–π and C–H⋯π aromatic packing interactions.
Introduction
Metal–organic frameworks (MOFs)1 or porous coordination polymers (PCPs)2 are an evolving class of porous materials that are of interest for key commodity separations, especially for difficult-to-separate hydrocarbons.3 Flexible MOFs (FMOFs), a subset of MOFs, are stimuli-responsive soft porous crystalline solids that undergo structural transformations in response to external stimuli such as pressure, heat, light, cation/anion exchange, or guest (gas/vapour/solvent) uptake/removal.4–7 FMOFs are characterised by atypical stepped or “S-shaped” isotherms that are concomitant with gate-opening phenomena. Hysteresis can be associated with these breathing or swelling processes.8,9 These stimuli-responsive structural transformations can result in useful properties in the context of gas storage and separation,10,11 guest capture and release,12 and molecular sensing,13–15 in some cases setting benchmark performance.16Separation of benzene (Bz) and cyclohexane (Cy) is a challenge for the chemical and petrochemical industries. Cy is primarily obtained through the catalytic hydrogenation of Bz and unreacted Bz must be removed from the reactor's effluent stream to generate high-purity Cy. However, this separation is challenging due to their similar boiling points (Bz, 80.1 °C; Cy, 80.7 °C), molecular geometries (Bz, 3.3 × 6.6 × 7.3 Å3; Cy, 5.0 × 6.6 × 7.2 Å3), and Lennard–Jones collision diameters.17,18 The similar boiling points and the formation of azeotropes render distillation processes poorly effective. Currently, the predominant industrial methods for separating Bz/Cy mixtures are extractive distillation and azeotropic distillation, both of which have high energy footprints and are accompanied by process complexity and high operating costs.19–21 It is therefore desirable to develop separation methods that are easier and more energy-efficient for the separation of Bz and Cy. A similar challenge exists for alcohol/water separations.
To enable selective separation between sorbates with similar physicochemical properties, the development of physisorbents with tight binding sites has proven to be fruitful as exemplified by CO2 and C2H2 selective adsorbents.22,23 For Bz/Cy, π–π, H-π, and H-bonding interactions are likely to drive selective Bz binding and could also induce structural transformations.24–29 FMOFs comprised of aromatic linker ligands would be expected to offer suitable pore chemistry for Bz binding and, because of inherent flexibility, offer the possibility of induced-fit binding for Bz that is reminiscent of enzyme–substrate binding.26–29 This principle was demonstrated by Kitagawa's group in an FMOF featuring a flexible undulating channel that exhibited selective adsorption of Bz over Cy via gate-opening triggered by CH-π interactions.26 Chen et al. reported a flexible MOF with a similar mechanism for selective adsorption of Bz,27 whereas Ghosh et al. reported an FMOF with a π-electron deficient ligand that exhibited Bz/Cy recognition and structural transformation induced by Bz.28
In this study, we report a new 2-fold interpenetrated FMOF, [Zn2(DMTDC)2(dpb)] (X-pcu-11-Zn), comprising two aromatic π-conjugated ligands (Scheme 1): 3,4-dimethylthieno[2,3-b]thiophene-2,5-dicarboxylic acid (H2DMTDC) and 1,4-di(pyridin-4-yl)benzene (dpb). X-pcu-11-Zn was found to undergo reversible structural transformations between its as-synthesized large pore “open” phase, X-pcu-11-Zn-α, and its activated narrow pore phase, X-pcu-11-Zn-β. These transformations were characterised by single-crystal X-ray diffraction (SCXRD) as well as gas and vapor adsorption to provide insight into the Bz binding mechanism.
 | ||
| Scheme 1 X-pcu-11-Zn-α is composed of H2DMTDC and dpb ligands and exhibits a primitive cubic (pcu) topology with 2-fold interpenetration. | ||
Results and discussion
Synthesis and crystal structure
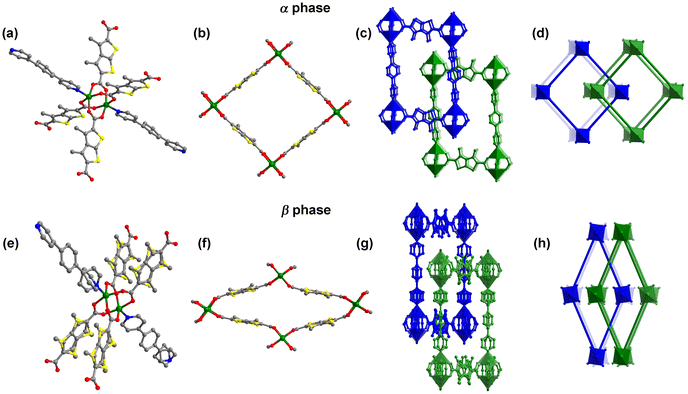
Solvothermal reaction of H2DMTDC and dpb with Zn(NO3)2·6H2O in DMF at 105 °C yielded colourless, block crystals of [Zn2(DMTDC)2(dpb)]·5DMF·0.5H2O, X-pcu-11-Zn-α (full synthetic details are available in the ESI†). SCXRD revealed that X-pcu-11-Zn-α had crystallised in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The resulting coordination network comprises dinuclear Zn(II) tetracarboxylate paddlewheels (Fig. 1a) linked via DMTDC ligands to form a square lattice (sql) network (Fig. 1b). The sql nets are pillared by dpb linkers to generate a pcu topology (Fig. 1c). X-pcu-11-Zn-α is a member of the DMOF-1 family which already has several members with extended (X-) ligands, X-pcu-n-Zn (e.g. n = 5, 6, 7, 8), that exhibit 2-fold offset interpenetration (Fig. 1d).30–32X-pcu-11-Zn-α features ultramicroporous 3D channels with effective pore diameters of ca. 2.3 × 2.5, 3.3 × 5.4, and 4.5 × 6.8 Å2 along the a-, b-, and c-axes, respectively (Fig. S1–S3†). The calculated guest-accessible volume is 49.8%. TGA revealed that as-synthesized X-pcu-11-Zn-α loses guest molecules (observed: 30.02%; calculated: 30.05%) on reaching 150 °C and is thermally stable below 320 °C (Fig. S8†).
. The resulting coordination network comprises dinuclear Zn(II) tetracarboxylate paddlewheels (Fig. 1a) linked via DMTDC ligands to form a square lattice (sql) network (Fig. 1b). The sql nets are pillared by dpb linkers to generate a pcu topology (Fig. 1c). X-pcu-11-Zn-α is a member of the DMOF-1 family which already has several members with extended (X-) ligands, X-pcu-n-Zn (e.g. n = 5, 6, 7, 8), that exhibit 2-fold offset interpenetration (Fig. 1d).30–32X-pcu-11-Zn-α features ultramicroporous 3D channels with effective pore diameters of ca. 2.3 × 2.5, 3.3 × 5.4, and 4.5 × 6.8 Å2 along the a-, b-, and c-axes, respectively (Fig. S1–S3†). The calculated guest-accessible volume is 49.8%. TGA revealed that as-synthesized X-pcu-11-Zn-α loses guest molecules (observed: 30.02%; calculated: 30.05%) on reaching 150 °C and is thermally stable below 320 °C (Fig. S8†).
X-pcu-11-Zn-α underwent single-crystal-to-single-crystal (SCSC) transformation after heating at 120 °C for 12 h to form X-pcu-11-Zn-β (Fig. S11†). SCXRD revealed that the β-form is a contorted version of the α-form with the same connectivity (Fig. 1e and f) but with centred rather than offset interpenetration (Fig. 1g). X-pcu-11-Zn-β crystallized in the orthorhombic space group Ibca with a 32.0% shrinkage in unit-cell volume relative to the α-form (Table S1†). X-pcu-11-Zn-β exhibits 1D channels with an effective pore diameter of ca. 2.1 × 2.6 Å2 along the a-axis (Fig. S4†). TGA and IR studies indicated that guest molecules had indeed been removed (Fig. S8 and S10†) and PLATON calculations revealed that the β-phase has only 4.9% solvent-accessible space. TGA of X-pcu-11-Zn-β showed no weight loss until decomposition at 320 °C (Fig. S8†). The structural transformation associated with guest release is reversible, with PXRD data revealing that the β-form had reverted to the α-form when soaked in DMF at room temperature for one day (Fig. S11†).
Analysis of the crystal structures reveals that the transformation between the large pore and narrow pore phases involves distortion of the sql nets and a sliding motion of the two interpenetrated nets (Fig. 1 and Fig. S7, Table S2†). In the α-phase, the metal–carboxylate junction Zn2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O2 > C approaches linearity with dihedral angles (Table S2 and Fig. S5†) between the Zn2
O2 > C approaches linearity with dihedral angles (Table S2 and Fig. S5†) between the Zn2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O2 plane and the carboxylate group O2 > C ranging from 1.0° to 8.4° for X-pcu-11-Zn-α. Conversely, in the β-phase, the metal–carboxylate junctions bend with dihedral angles ranging from 18.1° to 30.1° for X-pcu-11-Zn-β. There are no π–π interactions (centroid–centroid distance <4.0 Å) or C–H-π interactions in either structure, but C–H⋯O hydrogen bonding exists in both phases (Table S3†).
O2 plane and the carboxylate group O2 > C ranging from 1.0° to 8.4° for X-pcu-11-Zn-α. Conversely, in the β-phase, the metal–carboxylate junctions bend with dihedral angles ranging from 18.1° to 30.1° for X-pcu-11-Zn-β. There are no π–π interactions (centroid–centroid distance <4.0 Å) or C–H-π interactions in either structure, but C–H⋯O hydrogen bonding exists in both phases (Table S3†).
Gas adsorption
That X-pcu-11-Zn-β was prepared from a large pore phase motivated us to determine if switching or breathing would occur when X-pcu-11-Zn-α is exposed to gases. As shown in Fig. 2, the β-form is nonporous to N2 (kinetic diameter: 3.64 Å) at low pressure (P/P0 < 0.1), consistent with its narrow pore dimensions (2.1 × 2.6 Å2) and expected weak framework–N2 interactions. β exhibited a 2-step adsorption profile for N2 at 77 K, with saturation occurring after the second step. The first step, with an onset pressure of approximately P/P0 = 0.10, is indicative of gas-induced gate-opening (switching) behaviour, suggesting a structural transformation from a non-porous phase to an intermediate pore phase. The resulting increase of overall pore volume at a threshold pressure of N2 can result in a negative sorption step as seen in Fig. 2.33 The intermediate phase existed until P/P0 = 0.46. Upon further increasing the pressure to P/P0 = 1.0, the framework transformed to the large pore α-form with an uptake of 337 cm3 g−1. The pore volume calculated by assuming liquid filling of saturated N2 is 0.52 cm3 g−1, consistent with the void volume of X-pcu-11-Zn-α calculated from the crystal structure (0.50 cm3 g−1). These data suggest that X-pcu-11-Zn-β had reverted X-pcu-11-Zn-α under these sorption conditions. To the best of our knowledge, X-pcu-11-Zn-β is the first example of switching between closed and two open phases showing a two-step type F-IV2 isotherm8 (Table S7†) with high capacity (>300 cm3 g−1) although other switching sorbents exhibit one-step type F-IVs isotherms (e.g.DUT-8(Ni),34 rtl-[Cu(HIsa-azdmpz)]35 and ELM-11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36) or even a five-step type F-IV5 isotherm (Co(bpe)) with an uptake >300 cm3 g−1.37
36) or even a five-step type F-IV5 isotherm (Co(bpe)) with an uptake >300 cm3 g−1.37
 | ||
| Fig. 2 Gas sorption isotherms of X-pcu-11-Zn-β. Solid and open symbols represent adsorption and desorption branches, respectively. | ||
Unlike N2 adsorption at 77 K, the adsorption isotherm of CO2 for X-pcu-11-Zn-β at 195 K consists of three adsorption/desorption steps. The initial uptake of CO2 reached a saturated uptake of 33 cm3 g−1. The Langmuir surface area calculated for the first CO2 sorption step is 200 m2 g−1. The next step, which we attribute to gate-opening, occurred at P/P0 = 0.10 with saturated uptake reaching 125 cm3 g−1. The Langmuir surface area calculated from the second-step of CO2 sorption is 754 m2 g−1. At the second gate-opening pressure (P/P0 = 0.70), the CO2 uptake further increased to 267 cm3 g−1 at P/P0 = 1.0. The Langmuir surface area was calculated to be 1677 m2 g−1 (see section 10†).38X-pcu-11-Zn-β represents a rare example of 3-step CO2 adsorption with high capacity (>250 cm3 g−1) (Table S8†). After N2 and CO2 desorption, X-pcu-11-Zn-β retained its original structure (Fig. S12†).
Vapour sorption
Dynamic vapour sorption measurements were conducted at 298 K to further study the dynamic properties of X-pcu-11-Zn-β. Fig. 3 illustrates the adsorption/desorption isotherms of X-pcu-11-Zn-β for H2O, MeOH, EtOH and CH3CN at 298 K. X-pcu-11-Zn-β preferentially adsorbed aliphatic organic molecules over water vapor despite the smaller kinetic diameter of H2O compared to that of the organic molecules. For MeOH and EtOH, gate-opening pressures were found to be P/P0 = 0.7 for MeOH and 0.6 for EtOH, with uptakes of 211 and 140 cm3 g−1, respectively, at P/P0 = 0.96. In contrast, the isotherm for CH3CN shows a 3-step adsorption process, with gate-opening pressures at P/P0 = 0.18 and 0.66, and an uptake of 194 cm3 g−1 at P/P0 = 0.96. These gate-opening phenomena are consistent with guest-induced structural transformations for MeOH, EtOH and CH3CN, whereas H2O did not induce such transformations (Fig. S13†), indicating that X-pcu-11-Zn is hydrophobic. | ||
| Fig. 3 H2O, MeOH, EtOH, and MeCN sorption isotherms measured at 298 K. Solid and open symbols represent adsorption and desorption branches, respectively. | ||
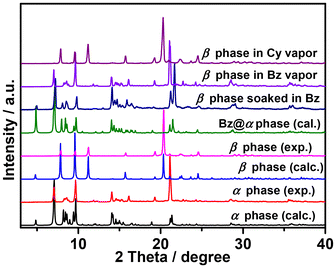
To investigate the adsorption behaviour of larger organic molecules on X-pcu-11-Zn-β, single-component adsorption isotherms for benzene (Bz) and cyclohexane (Cy) were collected at 298 K. As shown in Fig. 4, X-pcu-11-Zn-β adsorbed Bz (92 cm3 g−1) but exhibited minimal adsorption for Cy (5 cm3 g−1) at P/P0 = 0.99. This uptake amount corresponds to 3.56 Bz molecules per formula unit. Although Bz is larger than MeOH, EtOH, and MeCN, its gate-opening adsorption pressure starts at a lower relative pressure (P/P0 = 0.10), which may be attributed to host–guest interactions arising from the π system and the more acidic C(sp2)–H moieties in Bz compared to C(sp3)–H bonds. PXRD patterns of X-pcu-11-Zn-β exposed to Bz for 12 h reveal distinct changes compared to the patterns of unexposed X-pcu-11-Zn-β, whereas the PXRD patterns remained unchanged under Cy vapour for the same duration (Fig. 5). PXRD data suggest that the structure of X-pcu-11-Zn-β after exposure to Bz for 12 h matches well with that of X-pcu-11-Zn-α calculated from SCXRD data, indicating that Bz had induced structural transformation from the β to the α phase, while Cy did not induce such a change (Fig. 5). This makes X-pcu-11-Zn-β a candidate for selective adsorption of Bz over Cy.
To gain insight into this adsorption behaviour, single crystals of Bz@X-pcu-11-Zn were obtained by soaking X-pcu-11-Zn-α in benzene (Bz). PXRD analyses indicate that Bz-loaded X-pcu-11-Zn is indeed isostructural with X-pcu-11-Zn-α as calculated from SCXRD data (Fig. 5). The single crystal structure of Bz-loaded X-pcu-11-Zn reveals the presence of 3.5 Bz molecules per molecular unit, consistent with the uptake from the Bz adsorption isotherm. In the crystal structure of Bz@X-pcu-11-Zn, the framework resembles that of X-pcu-11-Zn-α with similar unit cell parameters (Table S1†). As shown in Fig. 6 and Tables S4, S5,† multiple π–π and C–H⋯π aromatic interactions exist between the benzene molecules and the X-pcu-11-Zn framework, which likely drive the phase transformation of X-pcu-11-Zn-β. The selectivity of X-pcu-11-Zn-β for Bz can be attributed to this host–guest binding. Furthermore, the PXRD pattern of X-pcu-11-Zn-β after Bz capture also matches well with the calculated pattern from the crystal structure of Bz@X-pcu-11-Zn- (Fig. 5), indicating that the crystal structure transforms from X-pcu-11-Zn-β to Bz@X-pcu-11-Zn upon Bz adsorption. The 1H NMR spectra of X-pcu-11-Zn-β exposed to Bz and Cy vapours for 12 h showed that X-pcu-11-Zn-β adsorbs 3.48 Bz and 0.04 Cy molecules per molecular unit (Fig. S19 and S20†), respectively. Additionally, TGA data were collected for Bz and Cy vapour adsorption by X-pcu-11-Zn-β. As shown in Fig. S9,† the TGA curves for X-pcu-11-Zn-β that had been exposed to Bz for 12 h reveal a weight loss of 22.61% below 115 °C, corresponding to 3.26 Bz molecules per molecular unit, again consistent with the SCXRD analysis results. In contrast, there is negligible weight loss (2.61%) for X-pcu-11-Zn-β after exposure to Cy vapor for 12 h before reaching 300 °C. This pronounced adsorption difference suggested to us that X-pcu-11-Zn-β can be utilised for the separation of Bz and Cy.
 | ||
| Fig. 6 The π⋯π and C–H⋯π interactions between benzene molecules and the framework in Bz@X-pcu-11-α viewed along the crystallographic b-axis. | ||
To evaluate the adsorption kinetics of X-pcu-11-Zn-β for benzene (Bz) and cyclohexane (Cy) vapours, 1H NMR spectroscopy was employed (details are available in the ESI†). Samples that had been exposed to Bz and Cy vapours were soaked in CDCl3 to extract adsorbed Bz and Cy, respectively. As depicted in Fig. 7a, the amount of Bz adsorbed by X-pcu-11-Zn-β increased over time, reaching saturation after approximately 1 h at room temperature. At saturation, X-pcu-11-Zn-β adsorbed about 3.48 Bz molecules per unit, while the uptake of Cy was negligible. The adsorption kinetics for Bz were analysed by using pseudo-first-order and pseudo-second-order kinetic models (see section 15 of the ESI†). As shown in Fig. S23, S24 and Table S6,† the adsorption kinetics can be best described by the pseudo-first-order model for X-pcu-11-Zn-β. This suggests that the adsorption process may be governed by physisorption rather than chemisorption. The structural changes induced by Bz adsorption were found to be reversible; adsorbed Bz was removed by heating Bz@X-pcu-11-Zn at 120 °C under vacuum (Fig. S14†). So-formed X-pcu-11-Zn-β retained its ability to adsorb Bz with no loss of performance after six cycles (Fig. 7b) and PXRD data revealed that X-pcu-11-Zn-β maintained phase stability (Fig. S14†).
To explore the potential of X-pcu-11-Zn-β for separating Bz and Cy mixtures, a time-dependent binary-component sorption experiment was conducted with X-pcu-11-Zn-β exposed to an equimolar Bz/Cy vapor mixture (details are available in the ESI†). In contrast to single-component adsorption, binary-component Bz/Cy adsorption resulted in co-adsorption, 2.10 Bz and 0.97 Cy molecules per formula unit, respectively, after 12 h of exposure to equimolar Bz/Cy (Fig. 7c). The separation ratio of the Bz/Cy mixture was calculated to be 2.16. Although the Bz/Cy selectivity is smaller than other flexible MOFs (Table S9†) such as [Zn(TCNQ-TCNQ)bpy]n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 and CID-23,39 this value is comparable or better than several rigid MOFs such as MOF-5,40 HKUST-1,41 and CUB-30.42 Binary-component Bz/Cy adsorption by gas chromatography revealed that X-pcu-11-Zn-β reached saturation for Bz and Cy within 1 h (Fig. S22†), and had adsorbed 76.52% Bz and 23.48% Cy after 1 h (Fig. 7d). The molar ratio of Bz/Cy (3.2
26 and CID-23,39 this value is comparable or better than several rigid MOFs such as MOF-5,40 HKUST-1,41 and CUB-30.42 Binary-component Bz/Cy adsorption by gas chromatography revealed that X-pcu-11-Zn-β reached saturation for Bz and Cy within 1 h (Fig. S22†), and had adsorbed 76.52% Bz and 23.48% Cy after 1 h (Fig. 7d). The molar ratio of Bz/Cy (3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) from gas chromatography is comparable to that obtained from 1H NMR analyses. As expected, the PXRD pattern of X-pcu-11-Zn-β changed upon exposure to the Bz/Cy mixture, matching the calculated pattern from the SCXRD data of Bz@X-pcu-11-Zn (Fig. S15†). These results indicate that X-pcu-11-Zn-β is a soft crystalline material with moderate selectivity for adsorption of Bz from a 1
1) from gas chromatography is comparable to that obtained from 1H NMR analyses. As expected, the PXRD pattern of X-pcu-11-Zn-β changed upon exposure to the Bz/Cy mixture, matching the calculated pattern from the SCXRD data of Bz@X-pcu-11-Zn (Fig. S15†). These results indicate that X-pcu-11-Zn-β is a soft crystalline material with moderate selectivity for adsorption of Bz from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Bz/Cy mixture, the selectivity being ascribed to the structural transformation induced by Bz adsorption.
1 Bz/Cy mixture, the selectivity being ascribed to the structural transformation induced by Bz adsorption.
Conclusions
In summary, we report a flexible, 2-fold interpenetrated coordination network, X-pcu-11-Zn, with π-conjugated thiophene-containing dicarboxylate and benzene-bridged bipyridine ligands. X-pcu-11-Zn exhibited reversible structural transformations between large pore and narrow pore phases. For N2 adsorption, a 2-step N2 F-IV2 isotherm at 77 K was observed with an uptake of 337 cm3 g−1. A 3-step CO2 sorption isotherm was observed at 195 K with the uptake exceeding 260 cm3 g−1. To our knowledge, X-pcu-11-Zn is the first example of switching between closed and two open phases showing a two-step F-IV2 isotherm with high capacity (>300 cm3 g−1). Vapor sorption experiments revealed that X-pcu-11-Zn is selective for methanol, ethanol, and CH3CN over H2O, characterized by stepped vapour sorption isotherms and significant hysteresis. Most notably, it selectively adsorbs Bz over Cy, with gate-opening concomitant with structural transformations. The transformations between the guest-free and guest-containing structures are reversible, with X-pcu-11-Zn maintaining its ability to adsorb benzene without performance loss after six cycles. SCXRD studies indicated that the selective adsorption is driven by host–guest interactions, including multiple π–π and C–H⋯π aromatic interactions, which induce structural transformation and gate-opening upon benzene sorption. This study highlights that two-fold interpenetrated flexible MOFs with π-conjugated ligands, which have many possible variants, offer potential to selectively adsorb/separate Bz over Cy driven by Bz-selective binding sites.Data availability
The data supporting the findings of this study are available in the ESI† or from the authors upon request.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 22161052) and Research Ireland (SFI award 16/IA/4624). We also thank Ji-Kai Chen and Sheng-Ming Xie (Yunnan Normal University) for gas chromatography testing.References
-
L. R. MacGillivray, Metal-organic frameworks: design and application, John Wiley & Sons, 2010 Search PubMed
.
- S. Kitagawa, R. Kitaura and S. Noro, Functional Porous Coordination Polymers, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed
.
- Z. R. Herm, E. D. Bloch and J. R. Long, Hydrocarbon Separations in Metal-Organic Frameworks, Chem. Mater., 2014, 26, 323–338 CrossRef CAS
.
- S. Horike, S. Shimomura and S. Kitagawa, Soft porous crystals, Nat. Chem., 2009, 1, 695–704 CrossRef CAS PubMed
.
- A. Schneemann, V. Bon, I. Schwedler, I. Senkovska, S. Kaskel and R. A. Fischer, Flexible metal-organic frameworks, Chem. Soc. Rev., 2014, 43, 6062–6096 RSC
.
- R. F. Mendes and F. A. A. Paz, Transforming metal-organic frameworks into functional materials, Inorg. Chem. Front., 2015, 2, 495–509 RSC
.
- J. H. Lee, S. Jeoung, Y. G. Chung and H. R. Moon, Elucidation of flexible metal-organic frameworks: Research progresses and recent developments, Coord. Chem. Rev., 2019, 389, 161–188 CrossRef CAS
.
- S. Q. Wang and S. M. J. Zaworotko, Spiers Memorial Lecture: Coordination networks that switch between nonporous and porous structures: an emerging class of soft porous crystals, Faraday Discuss., 2021, 231, 9–50 RSC
.
- X. Yao, K. E. Cordova and Y.-B. Zhang, Flexible Metal-Organic Frameworks as CO2 Adsorbents en Route to Energy-Efficient Carbon Capture, Small Struct., 2022, 3, 2100209 CrossRef CAS
.
- M. K. Taylor, T. Runcevski, J. Oktawiec, J. E. Bachman, R. L. Siegelman, H. Jiang, J. A. Mason, J. D. Tarver and J. R. Long, Near-Perfect CO2/CH4 Selectivity Achieved through Reversible Guest Templating in the Flexible Metal-Organic Framework Co(bdp), J. Am. Chem. Soc., 2018, 140, 10324–10331 CrossRef CAS PubMed
.
- Y. Li, Y. Wang, W. Fan and D. Sun, Flexible metal-organic frameworks for gas storage and separation, Dalton Trans., 2022, 51, 4608–4618 RSC
.
- Z. Chang, D.-H. Yang, J. Xu, T.-L. Hu and X.-H. Bu, Flexible Metal-Organic Frameworks: Recent Advances and Potential Applications, Adv. Mater., 2015, 27, 5432–5441 CrossRef CAS PubMed
.
- N. Yanai, K. Kitayama, Y. Hijikata, H. Sato, R. Matsuda, Y. Kubota, M. Takata, M. Mizuno, T. Uemura and S. Kitagawa, Gas detection by structural variations of fluorescent guest molecules in a flexible porous coordination polymer, Nat. Mater., 2011, 10, 787–793 CrossRef CAS PubMed
.
- J.-H. Wang, M. Li and D. Li, A dynamic, luminescent and entangled MOF as a qualitative sensor for volatile organic solvents and a quantitative monitor for acetonitrile vapour, Chem. Sci., 2013, 4, 1793–1801 RSC
.
- A. Douvali, A. C. Tsipis, S. V. Eliseeva, S. Petoud, G. S. Papaefstathiou, C. D. Malliakas, I. Papadas, G. S. Armatas, I. Margiolaki, M. G. Kanatzidis, T. Lazarides and M. J. Manos, Turn-On Luminescence Sensing and Real-Time Detection of Traces of Water in Organic Solvents by a Flexible Metal–Organic Framework, Angew. Chem., Int. Ed., 2015, 54, 1651–1656 CrossRef CAS PubMed
.
- K. Koupepidou, A. Subanbekova and M. J. Zaworotko, Functional flexible adsorbents and their potential utility, Chem. Commun., 2025, 61, 3109–3126 RSC
.
- S. Mukherjee, D. Sensharma, O. T. Qazvini, S. Dutta, L. K. Macreadie, S. K. Ghosh and R. Babarao, Advances in adsorptive separation of benzene and cyclohexane by metal-organic framework adsorbents, Coord. Chem. Rev., 2021, 437, 213852 CrossRef CAS
.
- Z. Zhang, S. B. Peh, C. Kang, K. Chai and D. Zhao, Metal-organic frameworks for C6-C8 hydrocarbon separations, EnergyChem, 2011, 3, 100057 CrossRef
.
- J. Qin, Q. Ye, X. Xiong and N. Li, Control of Benzene-Cyclohexane Separation System via Extractive Distillation Using Sulfolane as Entrainer, Ind. Eng. Chem. Res., 2013, 52, 10754–10766 CrossRef CAS
.
- P. Navarro, A. Ovejero-Pérez, M. Ayusob, N. Delgado-Mellado, M. Larriba, J. García and F. Rodríguez, Cyclohexane/cyclohexene separation by extractive distillation with cyano-based ionic liquids, J. Mol. Liq., 2019, 289, 111120 CrossRef CAS
.
- M. Ayuso, A. Cañada-Barcala, M. Larriba, P. Navarro, N. Delgado-Mellado, J. Garía and F. Rodríguez, Enhanced separation of benzene and cyclohexane by homogeneous extractive distillation using ionic liquids as entrainers, Sep. Purif. Technol., 2020, 240, 116583 CrossRef CAS
.
- M. Shivanna, K. Otake, B.-Q. Song, L. M. van Wyk, Q.-Y. Yang, N. Kumar, W. K. Feldmann, T. Pham, S. Suepaul, B. Space, L. J. Barbour, S. Kitagawa and M. J. Zaworotko, Benchmark Acetylene Binding Affinity and Separation through Induced Fit in a Flexible Hybrid Ultramicroporous Material, Angew. Chem., Int. Ed., 2021, 60, 20383–20390 CrossRef CAS PubMed
.
- Z.-C. Xu, J. Yu, P.-D. Zhang, Y.-L. Zhao, X.-Q. Wu, M. Zhao, X. Zhang and J.-R. Li, Efficient C2H2 Separation from CO2 and CH4 within a Microporous Metal-Organic Framework of Multiple Functionalities, Ind. Eng. Chem. Res., 2022, 61, 16233–16239 CAS
.
- H. Yao, Y.-M. Wang, M. Quan, M. U. Farooq, L.-P. Yang and W. Jiang, Adsorptive Separation of Benzene, Cyclohexene, and Cyclohexane by Amorphous Nonporous Amide Naphthotube Solids, Angew. Chem., Int. Ed., 2020, 59, 19945–19950 CrossRef CAS PubMed
.
- J. Zhou, G. Yu, Q. Li, M. Wang and F. Huang, Separation of Benzene and Cyclohexane by Nonporous Adaptive Crystals of a Hybrid[3]arene, J. Am. Chem. Soc., 2020, 142, 2228–2232 CrossRef CAS PubMed
.
- S. Shimomura, S. Horike, R. Matsuda and S. Kitagawa, Guest-Specific Function of a Flexible Undulating Channel in a 7,7,8,8-Tetracyano-p-quinodimethane Dimer-Based Porous Coordination Polymer, J. Am. Chem. Soc., 2007, 129, 10990–10991 CrossRef CAS PubMed
.
- J.-P. Zhang and X.-M. Chen, Exceptional Framework Flexibility and Sorption Behavior of a Multifunctional Porous Cuprous Triazolate Framework, J. Am. Chem. Soc., 2008, 130, 6010–6017 CrossRef CAS PubMed
.
- B. Joarder, S. Mukherjee, A. K. Chaudhari, A. V. Desai, B. Manna and S. K. Ghosh, Guest-Responsive Function of a Dynamic Metal-Organic Framework with a π Lewis Acidic Pore Surface, Chem. – Eur. J., 2014, 20, 15303–15308 CrossRef CAS PubMed
.
- A. Karmakar, A. V. Desai, B. Manna, B. Joarder and S. K. Ghosh, An Amide-Functionalized Dynamic Metal-Organic Framework Exhibiting Visual Colorimetric Anion Exchange and Selective Uptake of Benzene over Cyclohexane, Chem. – Eur. J., 2015, 21, 7071–7076 CrossRef CAS PubMed
.
- D. N. Dybtsev, H. Chun and K. Kim, Rigid and Flexible: A Highly Porous Metal-Organic Framework with Unusual Guest-Dependent Dynamic Behavior, Angew. Chem., Int. Ed., 2004, 43, 5033–5036 CrossRef CAS PubMed
.
- A.-X. Zhu, Q.-Y. Yang, A. Kumar, C. Crowley, S. Mukherjee, K.-J. Chen, S.-Q. Wang, D. O'Nolan, M. Shivanna and M. J. Zaworotko, Coordination Network That Reversibly Switches between Two Nonporous Polymorphs and a High Surface Area Porous Phase, J. Am. Chem. Soc., 2018, 140, 15572–15576 CrossRef CAS PubMed
.
- A.-X. Zhu, Q.-Y. Yang, S. Mukherjee, A. Kumar, C.-H. Deng, A. A. Bezrukov, M. Shivanna and M. J. Zaworotko, Tuning
the Gate-Opening Pressure in a Switching pcu Coordination Network, X-pcu-5-Zn, by Pillar-Ligand Substitution, Angew. Chem., Int. Ed., 2019, 58, 18212–18217 CrossRef CAS PubMed
.
- Y.-X. Shi, W.-X. Li, W.-H. Zhang and J.-P. Lang, Guest-Induced Switchable Breathing Behavior in a Flexible Metal–Organic Framework with Pronounced Negative Gas Pressure, Inorg. Chem., 2018, 57, 8627–8633 CrossRef CAS PubMed
.
- N. Klein, H. C. Hoffmann, A. Cadiau, J. Getzschmann, M. R. Lohe, S. Paasch, T. Heydenreich, K. Adil, I. Senkovska, E. Brunner and S. Kaskel, Structural flexibility and intrinsic dynamics in the M2(2,6-ndc)2(dabco) (M = Ni, Cu, Co, Zn) metal-organic frameworks, J. Mater. Chem., 2012, 22, 10303–10312 RSC
.
- S. Millan, B. Gil-Hernández, E. Milles, S. Gökpinar, G. Makhloufi, A. Schmitz, C. Schlüsenera and C. Janiak, rtl-M-MOFs (M = Cu, Zn) with a T-shaped bifunctional pyrazole-isophthalate ligand showing flexibility and S-shaped Type F-IV sorption isotherms with high saturation uptakes for M = Cu, Dalton Trans., 2019, 48, 8057–8067 RSC
.
- H. Noguchi, A. Kondo, Y. Hattori, H. Kajiro, H. Kanoh and K. Kaneko, Evaluation of an Effective Gas Storage Amount of Latent Nanoporous Cu-Based Metal-Organic Framework, J. Phys. Chem. C, 2007, 111, 248–254 CrossRef CAS
.
- H. J. Choi, M. Dinca and J. R. Long, Broadly Hysteretic H2 Adsorption in the Microporous Metal-Organic Framework Co(1,4-benzenedipyrazolate), J. Am. Chem. Soc., 2008, 130, 7848–7850 CrossRef CAS PubMed
.
- L.-H. Xie and M. P. Suh, Flexible Metal-Organic Framework with Hydrophobic Pores, Chem. – Eur. J., 2011, 17, 13653–13656 CrossRef CAS PubMed
.
- Y. Hijikata, S. Horike, M. Sugimoto, H. Sato, R. Matsuda and S. Kitagawa, Relationship between Channel and Sorption Properties in Coordination Polymers with Interdigitated Structures, Chem. – Eur. J., 2011, 17, 5138–5144 CrossRef CAS PubMed
.
- M. Eddaoudi, H. Li and O. M. Yaghi, Highly Porous and Stable Metal-Organic Frameworks: Structure Design and Sorption Properties, J. Am. Chem. Soc., 2000, 122, 1391–1397 CrossRef CAS
.
- F.-J. Ma, S.-X. Liu, D.-D. Liang, G.-J. Ren, F. Wei, Y.-G. Chen and Z.-M. Su, Adsorption of volatile organic compounds in porous metal-organic frameworks functionalized by polyoxometalates, J. Solid State Chem., 2011, 184, 3034–3039 CrossRef CAS
.
- L. K. Macreadie, R. Babarao, C. J. Setter, S. J. Lee, O. T. Qazvini, A. J. Seeber, J. Tsanaktsidis, S. G. Telfer, S. R. Batten and M. R. Hill, Enhancing Multicomponent Metal-Organic Frameworks for Low Pressure Liquid Organic Hydrogen Carrier Separations, Angew. Chem., Int. Ed., 2020, 59, 6090–6098 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, single-crystal XRD data, PXRD patterns, IR spectra, TGA curves, etc. CCDC 2372920–2372922. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5qi00261c |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2025 |