Open Access Article
Open Access ArticleAchieving red-light anticancer photodynamic therapy under hypoxia using Ir(III)–COUPY conjugates†
Enrique
Ortega-Forte
 ac,
Anna
Rovira
b,
Pezhman
Ashoo
ac,
Anna
Rovira
b,
Pezhman
Ashoo
 a,
Eduardo
Izquierdo-García
a,
Eduardo
Izquierdo-García
 b,
Cormac
Hally
c,
Diego
Abad-Montero
b,
Cormac
Hally
c,
Diego
Abad-Montero
 b,
Mireia
Jordà-Redondo
c,
Gloria
Vigueras
b,
Mireia
Jordà-Redondo
c,
Gloria
Vigueras
 a,
Alba
Deyà
d,
José Luis
Hernández
d,
Jorge
Galino
d,
Manel
Bosch
e,
Marta E.
Alberto
a,
Alba
Deyà
d,
José Luis
Hernández
d,
Jorge
Galino
d,
Manel
Bosch
e,
Marta E.
Alberto
 f,
Antonio
Francés-Monerris
f,
Antonio
Francés-Monerris
 g,
Santi
Nonell
g,
Santi
Nonell
 c,
José
Ruiz
c,
José
Ruiz
 *a and
Vicente
Marchán
*a and
Vicente
Marchán
 *b
*b
aDepartamento de Química Inorgánica, Universidad de Murcia, Biomedical Research Institute of Murcia (IMIB-Arrixaca), E-30100 Murcia, Spain. E-mail: jruiz@um.es
bDepartament de Química Inorgànica i Orgànica, Secció de Química Orgànica, Universitat de Barcelona (UB), Institut de Biomedicina de la Universitat de Barcelona (IBUB), Martí i Franquès 1-11, E-08028 Barcelona, Spain. E-mail: vmarchan@ub.edu
cInstitut Químic de Sarrià, Universitat Ramon Llull, Vía Augusta 390, E-08017 Barcelona, Spain
dHealth and Biomedicine Department, Leitat Technological Center, Carrer de la Innovació 2, E-08225 Terrassa, Spain
eUnitat de Microscòpia Òptica Avançada, Centres Científics i Tecnològics, Universitat de Barcelona, Av. Diagonal 643, E-08028 Barcelona, Spain
fDipartimento di Chimica e Tecnologie Chimiche, Università della Calabria, Arcavacata di Rende I-87036, Italy
gInstitut de Ciència Molecular, Universitat de València, P.O. Box 22085, València 46071, Spain
First published on 4th March 2025
Abstract
Despite the potential of photodynamic therapy (PDT), this oxygen-dependent oncological treatment is greatly restricted in the clinic by the well-known hypoxic feature of solid tumors. Here we provide new insights into the development of PDT agents based on conjugates between COUPY fluorophores and cyclometalated iridium(III) complexes with the aim of overcoming this limitation. The structural modifications carried out within the metal core of Ir(III)–COUPY conjugates, based on the incorporation of trifluorobenzyl groups at the cyclometalating ligands, enabled efficient exploitation of type I PDT mechanisms while retaining operativity under long-wavelength visible light, which facilitated deeper tissue penetration compared with short wavelengths. Photobiological evaluation revealed that Ir(III)–COUPY conjugate 3c achieved potent photocytotoxicity towards cisplatin-resistant ovarian (A2780cis) and mammary (EO771) cancer cell lines, efficiently photogenerated type I and type II ROS, and photoinduced apoptotic cell death using red light irradiation (620 nm). Importantly, this Ir(III)–COUPY conjugate retained such potent photoactivity under low-oxygen environment conditions (2% O2), delivering equipotent photocytotoxicity towards normoxic and hypoxic adherent cancer cells. Compound 3c was found to be highly phototoxic against EO771 multicellular tumor spheroids and showed no signs of toxicity or adverse effects in mice, which could facilitate in vivo phototherapeutic applications. Taken together, this study demonstrates that the conjugation between COUPY dyes and rationally designed Ir(III) complexes is a strategy at the frontier of the development of new red light-activated photosensitizers capable of operating under hypoxia, showing the promise of achieving satisfactory anticancer PDT effects.
Introduction
Photodynamic therapy (PDT) is a clinically approved form of cancer treatment in which a light-activated drug, commonly referred to as photosensitizer (PS), and molecular oxygen are involved in the process of tumor cell killing.1–3 Many PSs have been developed in different chemical platforms, including porphyrins, chlorins, organic fluorophores and diverse nanosystems and biomaterials.4–9 Recently, transition metal complexes emerged as promising systems for anticancer PDT, with Ru(II)- and Ir(III)-based PSs leading to early-stage preclinical and clinical applications.10–18 However, despite these achievements, two major challenges posed by the chemical properties of current commercial PS drugs limit the efficacy of PDT: (1) photoactivation at long wavelengths i.e., red or near-infrared (NIR) light, and (2) photoactivity retention under low concentrations of oxygen (hypoxia), conditions inevitably encountered in growing solid tumors. Overcoming the former challenge, which limits the reachable penetration depth of PDT in biological tissues, has been attempted, for example, by using strategies that integrate or conjugate metal complexes with low-molecular weight organic fluorophores to red-shift the absorption of the PS to the phototherapeutic window. Examples of this include cyclometalated Ru(II) and Ir(III) complexes conjugated to boron-dipyrromethene (BODIPY),19,20 porphyrin,21 xanthene,22 and rhodamine derivatives.23 The hypoxia limitation, however, is more difficult to tackle since most PSs operate via the type II PDT mechanism, which relies on the presence of oxygen to produce highly toxic singlet oxygen (1O2).24–26 In view of this limitation, we succeeded in developing type I PDT agents based on the conjugation of cyclometalated Ir(III) complexes to coumarin–pyridine (COUPY) fluorophores, which are less oxygen-sensitive and exhibit potent photoactivity upon blue or green light irradiation.27,28 A structure–activity relationship study further revealed key structural modifications to the coumarin scaffold that improved the hypoxia performance of the Ir(III)–COUPY PSs.29 Recently, we also disclosed a method to red-shift the operability of a cyclometalated Ru(II) polypyridyl system towards the NIR region through the conjugation of a COUPY fluorophore with operativity in the phototherapeutic window.30Photoactivation via low-energy wavelength irradiation is more difficult to achieve for single Ir(III)-based systems bearing polypyridyl ligands since their absorption tail typically lies within the blue region of the electromagnetic spectrum.13,31 Yet the development of cyclometalated cationic Ir(III)-based PDT agents is highly desirable because they generally possess larger Stokes’ shifts and longer luminescence lifetimes, which add bioimaging capacity to the PDT effects.32–36 We therefore envisioned new PSs based on Ir(III)–COUPY conjugates in which modifications of the cyclometalated Ir complex coupled to a far-red/NIR-emitting COUPY fluorophore could both defeat hypoxic environments and enable photoactivation at long wavelengths.
With this idea in mind, herein we explored the photobiological properties of two PSs based on the conjugation of COUPY dyes 1a and 1b to Ir(III) complex 2a (compounds 3a and 3b, respectively; Fig. 1) upon 620 nm light irradiation, and reported a new Ir(III)–COUPY PS (3c) bearing trifluorobenzyl groups at the cyclometalating ligand of the metal complex (2b). The incorporation of trifluorobenzyl groups was made with the aim of exploiting type I PDT mechanisms, since some Ir(III) complexes containing these groups were reported to be superoxide radical generators under hypoxic conditions.32
 | ||
| Fig. 1 Structure of the compounds investigated in this work: COUPY coumarins 1a and 1b, cyclometalated Ir(III) complexes 2a and 2b, and the corresponding Ir(III)–COUPY conjugates 3a–3c. | ||
Results and discussion
Synthesis and characterization
The Ir(III) complexes 2a and 2b and conjugates 3a and 3b were prepared as previously reported.27,29,37 The Ir(III)–COUPY conjugate 3c (Fig. 1) was synthesized by following our previously described methodology, which was based on the formation of an amide bond between the primary amino group of a suitable coumarin derivative and the carboxylic acid group of the required conjugatable Ir(III) complex 4 (Schemes S1 and S2†). The compounds were purified by column chromatography on silica gel and fully characterized by NMR spectroscopy and HRMS.Photophysical and photochemical characterization
The photophysical and photochemical properties [absorption and emission spectra, molar absorption coefficients (ε), fluorescence (ΦF) or phosphorescence (ΦP) quantum yields, fluorescence (τF) or phosphorescent (τP) lifetimes, and singlet oxygen quantum yield (ΦΔ)] were studied for the newly synthesized Ir(III)–COUPY conjugate (3c) and the parent Ir(III) complex (2b) in three solvents of different polarities (PBS, ACN and DCM), and compared with those of the previously reported conjugates (3a and 3b) and the non-conjugated parent compounds (1a, 1b and 2a) (Fig. 2a, Fig. S5 and S6, and Tables S1 and S2†).29 As expected, the Ir(III)–COUPY conjugate 3c displayed similar absorption and emission maxima to those of 3a, since both PSs share the same coumarin (e.g. in ACN, λabs = 556 nm and λem = 606 nm for 3cvs. λabs = 555 nm and λem = 615 nm for 3a). In contrast, conjugate 3b bearing the julolidine-fused system in the coumarin moiety showed the largest red-shift in the absorption and emission maxima (e.g. in ACN, λabs = 580 nm and λem = 647 nm). Notably, the new Ir(III)–COUPY conjugate 3c showed higher values of molar absorption coefficients in all the solvents studied and a higher luminescent quantum yield, especially in organic solvents (e.g. in ACN, ΦP = 0.08 for 3a and 3bvs. ΦP = 0.43 for 3c). In PBS, both the coumarins and, especially, the Ir(III)–COUPY conjugates, showed aggregation, as revealed by the lower absorption coefficients, broadening of the absorption bands, and decrease in the fluorescence quantum yields. | ||
| Fig. 2 (a) Comparison of the absorption spectra of Ir(III)–COUPY conjugates 3a–3c in ACN. For other solvents, please see Fig. S5 and S6.† (b) Fluorescence spectra of ROS probe DHR123 in PBS induced by irradiation with red light (620 ± 15 nm; 130 mW cm−2) in the presence of 3c. | ||
Singlet oxygen quantum yields (ΦΔ) were determined by direct observation of the 1O2 phosphorescence (λexc = 355 or 532 nm). As shown in Table S1,† the Ir(III) complex 2b shows similar 1O2 quantum yields to those of 2a in all solvents. However, conjugation of the Ir(III) complex 2b with the COUPY fluorophore 1a, a very poor 1O2 photosensitizer, resulted in an increase of the 1O2 quantum yield of the Ir(III)–COUPY conjugate 3c in all organic solvents (Table S2†), which reproduced the previously observed trends with conjugates 3a and 3b, leading to comparable quantum yields for all three Ir(III)–COUPY PSs in organic solvents. In PBS, the production of 1O2 was negligible for all compounds.
Given the capability of 3a and 3b to generate the superoxide anion radical (O2˙−) within cells upon exposure to green light,27,29 we focused on investigating if these Ir(III)–COUPY conjugates, along with the newly synthesized 3c, could produce this specific type-I ROS under red light irradiation in PBS. To our delight, the use of the fluorogenic probe dihydrorhodamine 123 (DHR123) showed that all of them were capable of generating O2˙− under red light irradiation (620 nm), contrasting with the behavior of the parent Ir(III) complexes (2a and 2b) and COUPY derivatives (1a and 1b) alone, which did not produce significant O2˙− under the same irradiation conditions (Fig. 2b and Fig. S7†). Notably, the enhancement of the DHR123 fluorescence signal by the different Ir(III)–COUPY conjugates could be prevented using the O2˙−-specific scavenger tiron (Fig. S8 and S9†).
The generation of type I and type II ROS by conjugates 3a and 3c was also evidenced by electron paramagnetic resonance (EPR). The spin traps 4-amino-2,2,6,6-tetramethylpiperidine (4-amino-TEMP) and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) were used to detect 1O2 and O2˙−, respectively. As shown in Fig. 3a, while no EPR signal was observed in the dark, the triplet signal characteristic of the TEMPO spin adduct (peak integral ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) appeared upon irradiation with green light, confirming that compound 3a, and even more so compound 3c, could effectively photogenerate 1O2. Furthermore, the detection of the four distinctive peaks of the DMPO-O2˙− adduct (peak integral ratio 1
1) appeared upon irradiation with green light, confirming that compound 3a, and even more so compound 3c, could effectively photogenerate 1O2. Furthermore, the detection of the four distinctive peaks of the DMPO-O2˙− adduct (peak integral ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) upon irradiation with green light also confirmed the ability of compounds 3a and 3c to produce superoxide (Fig. 3b). The fact that no paramagnetic signal was observed in the absence of light confirmed that, as in the case of 1O2, the production of O2˙− was a light-induced process.
1) upon irradiation with green light also confirmed the ability of compounds 3a and 3c to produce superoxide (Fig. 3b). The fact that no paramagnetic signal was observed in the absence of light confirmed that, as in the case of 1O2, the production of O2˙− was a light-induced process.
 | ||
| Fig. 3 EPR spectra of Ir(III)–COUPY conjugates 3a and 3c trapped by 4-amino-TEMP (a) or DMPO (b) in MeOH, in the dark and upon irradiation with green light (505 nm, 2 min, 100 mW cm−2). | ||
Additional insights into the viability of the type I PDT mechanism and intramolecular photoinduced electron transfer between the coumarin moiety and the Ir(III) complex to generate a geminate radical ion pair was ascertained through density functional theory (DFT) calculations38–42 (Tables 1, S3 and S4 and Fig. S10†). Our data show that the electron transfer processes from excited state S1 of both COUPY coumarins (1a, 1b) to the corresponding Ir(III) complexes (2a, 2b) in Ir(III)–COUPY conjugates 3a–3c are slightly thermodynamically favorable (reactions 1–3, Table 1). Even more significant is the favorable reduction of molecular dioxygen by the obtained radicals 22a0 and 22b0 forming superoxide, as confirmed by the higher electron affinity of oxygen than the Ir(III) complexes (reactions 4 and 5, Table S3†), supporting the occurrence of type I PDT activity for conjugates 3a–3c, as experimentally observed (vide infra).
| # | Conjugate | Reaction | ΔE (eV) |
|---|---|---|---|
| Photoinduced electron transfer | |||
| (1) | 3a | 11a+ (S1) + 12a+ → 21a2+ + 22a0 | −0.100 |
| (2) | 3b | 11b+ (S1) + 12a+ → 21b2+ + 22a0 | −0.298 |
| (3) | 3c | 11a+ (S1) + 12b+ → 21a2+ + 22b0 | −0.102 |
| Superoxide formation | |||
| (4) | 3a,3b | 22a0 + 3O2 → 12a+ + 2(˙O2−) | −0.163 |
| (5) | 3c | 22b0 + 3O2 → 12b+ + 2(˙O2−) | −0.159 |
As expected, electron transfer mechanisms from the ground state of COUPYs toward the Ir(III) complex can definitely be ruled out on the basis of our computed thermodynamic data (see Table S4†), confirming the necessity to reach the excited state localized over the coumarin fragment to promote such a mechanism.
The optimized structure of 3c in water reveals specific non-covalent interactions that reduce the molecular volume of the conjugate (Fig. 4). These include the C–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C H-bonding-like interaction, C–H⋯π (T-shape)43 interactions involving the C–H bonds of the tertiary amine ethyl groups, and C–F⋯H–C electrostatic interactions. Overall, this molecular packing should favour small distances between the COUPY moiety and the Ir(III) metal centre, facilitating photoinduced electron transfer processes between both components of the Ir(III)–COUPY conjugate.
C H-bonding-like interaction, C–H⋯π (T-shape)43 interactions involving the C–H bonds of the tertiary amine ethyl groups, and C–F⋯H–C electrostatic interactions. Overall, this molecular packing should favour small distances between the COUPY moiety and the Ir(III) metal centre, facilitating photoinduced electron transfer processes between both components of the Ir(III)–COUPY conjugate.
Dark and light stability of Ir(III)–COUPY conjugates in biological media
The stability of the Ir(III)–COUPY conjugates 3a–c in DMEM culture medium supplemented with 10% FBS was evaluated by reversed-phase HPLC in the dark and upon red light irradiation. The three compounds were remarkably stable in the dark even for 48 h at 37 °C (Fig. S11–S15†). Furthermore, while compound 3b displayed considerable photodegradation after 1 h under irradiation with red light (620 ± 15 nm; 130 mW cm−2), less than 20% photobleaching was observed for compounds 3a and 3c (Fig. S11 and S16–S18†), which indicated that the incorporation of the julolidine moiety into the coumarin backbone had a negative effect on the overall photostability of the compounds.Cellular uptake and subcellular localization
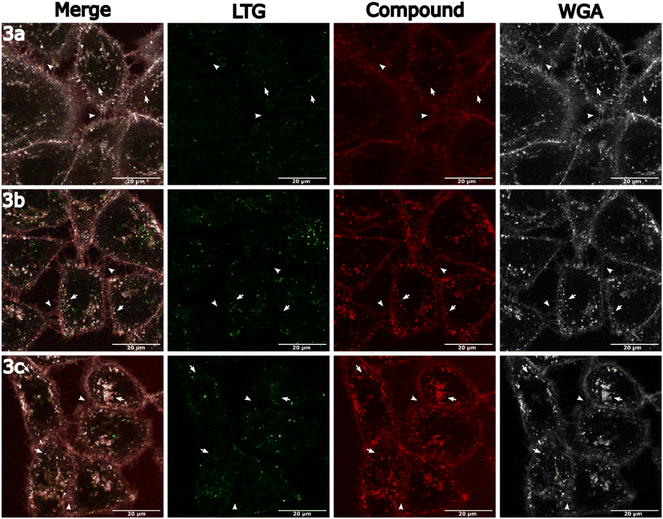
The cellular accumulation of the Ir(III)–COUPY conjugates 3a–3c in cancer cells was first studied by inductively coupled plasma mass spectrometry (ICP-MS). As shown in Fig. S19,† intracellular amounts of metal content determined by ICP-MS following 2 h incubation with 3c was approximately 6-fold higher than those found in cisplatin-treated cancer cells but comparable to other Ir(III)–COUPY conjugates i.e., 3a and 3b, and similar to the Ir(III) parent complexes,29 indicating good membrane permeability and cellular accumulation despite their relatively high molecular weight. Confocal microscopy was then used to gain more insights into cellular uptake and subcellular localization of the compounds in living cancer cells by taking advantage of the luminescence properties of the coumarin moiety, allowing the use of yellow light laser (λex = 561 nm) excitation. As shown in Fig. 5, the Ir(III)–COUPY conjugate 3c efficiently stained HeLa cells after 30 min of incubation, predominantly accumulating in the plasma membrane and, to a lesser extent, in intracellular vesicles. This behavior is consistent with the previously observed staining patterns of other Ir(III)–COUPY conjugates, including 3a and 3b.To better understand the nature of the intracellular vesicles stained by conjugates 3a–c, a series of colocalization experiments were conducted using the lysosome-specific fluorescent marker LysoTracker Green DND (LTG), and the fluorescent probe wheat germ agglutinin Alexa Fluor 633 (WGA), which had affinity for glycoproteins and stained the extracellular membrane and endosomes as they formed by endocytosis. Colocalization was measured using Pearson's correlation coefficient (Table S5†).44 As shown in Fig. 6, the three Ir(III)–COUPY conjugates exhibited a significant correlation with WGA staining (3a: PCC = 0.70; 3b: PCC = 0.51; 3c: PCC = 0.69; Table S5†). We then performed a more detailed colocalization analysis at the vesicle level and observed a lower correlation between the vesicular component of the fluorescent signals of compounds 3a–3c and that of WGA (3a: PCC = 0.42; 3b: PCC = 0.44; 3c: PCC = 0.36; Table S5†), however, this correlation was clearly higher than that with LTG (3a: PCC = 0.07; 3b: PCC = 0.03; 3c: PCC = 0.12; Table S5†). These results suggest that the intracellular vesicles stained by compounds 3a–3c correspond to endosomes, rather than lysosomes.
Photobiological studies
To evaluate the photocytotoxicity of the compounds, cisplatin-resistant ovarian cancer (A2780cis) cells were first chosen. The resistance of A2780cis cells is caused by elevated levels of the antioxidant glutathione tripeptide and altered DNA repair mechanism.45,46 This provides an interesting biological system to model the efficacy of Ir(III)–COUPY PSs in a challenging intracellular reductant environment that scavenges excess reactive oxygen species (ROS) and fails to activate apoptosis in response to oxidative damage caused by ROS. As shown in Table 2 and Fig. S21,† all compounds including the parent Ir(III) complexes (2a, 2b), COUPY coumarin 1a and Ir(III)–COUPY conjugates (3a–3c) were deemed to be inactive (>250 μM) against A2780cis cells under dark conditions, with the exception of COUPY 1b, which bore the julolidine-fused system. Next, we determined the half-maximal inhibitory concentration (IC50) values of the compounds upon 1 h irradiation with red light centered at 620 nm and compared them with our previously reported results using green light (520 nm).29 The phototherapeutic index (PI), calculated as the ratio of dark to light IC50 values, was considered as the measure for light-induced cytotoxicity. Interestingly, the photocytotoxicities of the Ir(III)–COUPY conjugates upon 620 nm light irradiation were very similar to those obtained with 520 nm light (IC50 values between 0.7 and 1.2 μM compared to 0.7–1.9 μM, respectively) using similar light doses (5.4 and 6.7 J cm−2 with 520 nm and 620 nm light, respectively). In contrast, a 3- to 5-fold loss in photocytotoxicity was found for the parent compounds (Ir(III) complexes and COUPY dyes) when changing from 520 nm light to 620 nm light irradiation (Table 2). As a result, similar PI values were obtained for 3a and 3b following red light treatment (>352 and >208, respectively) compared to green light treatment (>357 and >240, respectively, Table 2 and Fig S21†). Strikingly, the photocytotoxicity of 3c under red light provided a higher PI than that under green light (>208 vs. >134). This in vitro anticancer photoactivity retention upon irradiation with longer wavelengths of light indicated the potential of Ir(III)–COUPY conjugates for red-light PDT.| IC50 (μM), dark | IC50 (μM), 520 nm | PIb | IC50 (μM), 620 nm | PIb | |
|---|---|---|---|---|---|
| a Cells were treated for 2 h (1 h incubation and 1 h irradiation with green or red light at 520 nm or 620 nm, respectively) followed by 48 h of incubation in drug-free medium. Dark analogues were kept in the dark. Data expressed as mean ± SD from three independent experiments. b PI = phototherapeutic index defined as IC50 (dark-non-irradiated cells)/IC50 (irradiated cells). c Data reported in ref. 29. | |||||
| 1a | >250 | 2.1 ± 0.2c | >119 | 7.1 ± 0.4 | >35 |
| 1b | 15 ± 2 | 0.15 ± 0.04c | 100.0 | 0.7 ± 0.1 | 21.4 |
| 2a | >250 | 1.5 ± 0.1 | >166.7 | 4.5 ± 0.3 | >56 |
| 2b | >250 | 3.5 ± 0.4c | >71 | 9 ± 2 | >28 |
| 3a | >250 | 0.7 ± 0.06c | >357 | 0.71 ± 0.02 | >352 |
| 3b | >250 | 1.04 ± 0.02c | >240 | 1.2 ± 0.1 | >208 |
| 3c | >250 | 1.9 ± 0.3 | >134 | 1.2 ± 0.2 | >208 |
In view of this, we decided to explore the ability of the Ir(III)–coumarin PSs to overcome the hypoxia limitation. The A2780cis cells were incubated under hypoxic conditions (2% O2) and dosed with compounds 3a–3c under dark and light treatment regimens (Table S6†). As depicted in Fig. 7, all three conjugates exhibited potent photocytotoxicity under hypoxia, with IC50 values in the low micromolar range. Notably, 5-aminolevulinic acid (5-ALA), the clinically approved precursor of the protoporphyrin IX (PpIX) PS, was barely active under normoxic conditions and inactive under hypoxic conditions. To better illustrate the oxygen sensitivity of the studied PSs, we used the hypoxia index (HI), i.e., the ratio of the light IC50 value under normoxia to that under hypoxia.29 Strikingly, the HI for 3a was 2.3, indicating that hypoxic conditions halved its photocytotoxicity, whereas the HI for 3b was ∼4, meaning a 4-fold loss in photoactivity under low oxygen concentration (Fig. 6a). In contrast, 3c displayed a HI close to 1, which indicated that the photocytotoxicity of 3c was not entirely dependent on high oxygen concentrations.
Based on the promising results obtained in A2780cis cells, we focused on evaluating the biological photoactivity of Ir(III)–COUPY conjugates 3a–3c in the mammary cancer cell line EO771 with the aim of further validating their efficacy for red-light under hypoxic conditions. To our delight, the conjugates showed a similar trend in photocytotoxicity (Table 3). Notably, 3c exhibited the largest PI values (>227) under hypoxia and the smallest HI (1.1), which led us to select 3c as the best PS.
| Normoxia (21% O2) | Hypoxia (2% O2) | ||||||
|---|---|---|---|---|---|---|---|
| IC50 (μM), Dark | IC50 (μM), 620 nm | PIb | IC50 (μM), Dark | IC50 (μM), 620 nm | PIb | HIc | |
| a Cells were treated for 2 h (1 h incubation and 1 h irradiation with red light) followed by 48 h of incubation in drug-free medium either under normoxic (21% O2) or hypoxic (2% O2) conditions. Dark analogues were kept in the dark. Data expressed as mean ± SD from three independent experiments. b PI = phototherapeutic index defined as IC50 (dark-non-irradiated cells)/IC50 (irradiated cells). c HI = hypoxia index defined as the ratio of IC50 values obtained under hypoxic (2% O2) and normoxic (21% O2) conditions after light irradiation. | |||||||
| 3a | >250 | 1.7 ± 0.3 | >147 | >250 | 3.1 ± 0.9 | >81 | 1.8 |
| 3b | >250 | 2.3 ± 0.1 | >109 | >250 | 3.8 ± 0.6 | >66 | 1.7 |
| 3c | >250 | 0.98 ± 0.17 | >255 | >250 | 1.1 ± 0.1 | >227 | 1.1 |
To gain insights into the ability of 3c to overcome the barrier of hypoxia, the levels of intracellular ROS were measured after red light irradiation under normoxic and hypoxic conditions in A2780cis and EO771 cells and were then compared to those raised by the two previously reported conjugates (3a, 3b). Interestingly, all Ir(III)–COUPY conjugates rapidly photogenerated a large amount of ROS under both conditions, as revealed by strong intracellular fluorescent signals of the DCFH-DA probe compared to non-treated cells (Fig. 7b and Fig. S22 and S23†). As such, if 1O2 generation was the primary mediator of photocytotoxicity, then hypoxia would diminish ROS levels because the lack of oxygen would dramatically limit type II PDT reactions. However, although ROS photogeneration was considerably lower under hypoxic conditions, significant differences between the control and treated groups were still found (Fig. 7b). Such differences, ranging from 2- to 3-fold increases in overall ROS levels, were somewhat comparable to those found under normoxic conditions, which showed approximately 3-fold changes in both A2780cis and EO771 cell lines (Fig. S22 and S23†). This situation would be compatible with type I and type II PDT mechanisms simultaneously operating under low oxygen environments. To further verify this hypothesis, selective scavengers of reactive species were used, i.e., sodium azide (NaN3) for 1O2, MnTBAP for O2˙− and terephthalic acid for ˙OH, upon PDT treatment with 3a–3c (Fig. S23 and S24†). We found that MnTBAP and terephthalic acid partially inhibited fluorescence enhancement of DCF regardless of the low oxygen concentration, which suggested that type I superoxide and hydroxyl radical species participated in the reaction mechanism. As expected, NaN3 treatment only prevented ROS photogeneration with 3a–3c under normoxic conditions but not under hypoxic conditions, which could imply a minor role of 1O2 under hypoxia and an overall shift toward type I PDT.
Photoredox catalysis in cells could also be the main underlying mechanism of PDT, especially in oxygen-depleted environments.47 Nicotinamide adenine dinucleotide (NADH), a key reducing metabolite that participates in the mitochondrial electron transport chain, is considered to be the main biological electron donor in photosensitization.35,47 As a result, photocatalytic conversion of NADH in cancer cells has been proposed as a promising anticancer strategy.35,48–50 In this context, we evaluated the photocatalytic oxidation of NADH by Ir(III)–COUPY conjugates 3a–3c in PBS by UV-Vis spectroscopy, according to previously described methods.50 As shown in Fig. S26,† the spectrum of NADH (160 μM) remained unchanged after 5 min of incubation with conjugates 3a–3c (10 μM) in the dark at 37 °C. Conversely, upon irradiation with red light (620 ± 15 nm; 130 mW cm−2), compounds 3a–c dramatically reduced the absorption of NADH (Fig. S26†). The turnover frequency (TOF) values of 3a–3c after 5 min of light irradiation were 179, 164 and 152 h−1 (Fig. S27†), respectively, which were in the same range as those for other Ir(III) complexes described in the literature.49,50 Therefore, the high photocytotoxicity of Ir(III)–COUPY conjugates could be attributed not only to the photogeneration of ROS but also to the photocatalytic oxidation of NADH.
The high photocytotoxicity of Ir(III)–COUPY conjugates was already revealed in confocal microscopy studies by monitoring the morphological changes to the cells after laser irradiation. As shown in Fig. S20,† plasma membrane rupture and blebbing were observed in all cases. Additionally, flow cytometry analysis revealed secondary populations of A2780cis with reduced cell size as detected in forward light scattering (FSC) and nuclear and cytoplasmic condensation as shown by the transient increase in side scattering (SCC), indicating an overall disruption of normal cell morphology accompanied by cell shrinkage after red-light treatments (Fig. S28 and S29†). To discern the type of cell death induced, dual annexin V/propidium iodide (AV/PI) staining was employed (Fig. 7c and Fig. S30†), showing large A2780cis populations undergoing phosphatidylserine translocation after PDT treatments (AV+/PI− and AV+/PI+ populations). Altogether, these morphological signatures indicated beyond doubt that apoptosis was the main cell death mode of action induced by red-light PDT with these PSs. This was somewhat intriguing because our previous results using the same irradiation protocol but with 520 nm light (1.5 mW cm−2) showed necrosis photoinduction.29 We note that the cell death mode of PSs depends on the PDT dose applied, i.e., PS concentration and light fluence, but the fact that opposite types of cell death (necrosis vs. apoptosis) can be induced depending on the wavelength and intensity of light applied using structurally similar PSs is interesting for several reasons. On the one hand, the ability to cause effective cancer cell death upon irradiation at different wavelengths of light demonstrates that Ir(III)–COUPYs behave as polychromatic compounds, which might enable optimization of the light parameters and protocols according to treatment depth requirements. On the other hand, activation of apoptosis upon red-light irradiation in A2780cis cells, which intrinsically show resistance to cisplatin-induced apoptosis (Fig. 7c), is promising for resistant cancers where chemotherapy alone might be inefficient.
Phototherapeutic efficacy in 3D tumor spheroids and in vivo toxicity
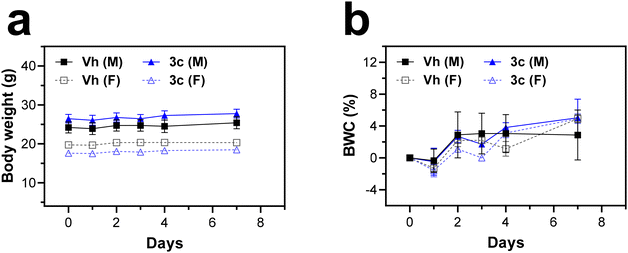
Encouraged by these promising results with the best performing Ir(III)–COUPY conjugate, 3c, we decided to evaluate its phototherapeutic efficacy in a 3D tumor spheroid model, as well as that of 3a and 3b for comparison purposes. Tumor spheroids mimic hypoxia features of growing tumors under pathophysiological conditions owing to oxygen gradients towards the center of the sphere.51 The efficacy of the PSs in EO771 multicellular tumor spheroids (MCTS) was evaluated by monitoring growth in volume as well as spheroid viability after PDT over a span of 8 days. After red-light irradiation, the growth of these MCTS treated with 3a and 3c, and to a lesser extent with 3b was impaired (Fig. 8 and S31†). To confirm cell death induction in spheroids with Ir(III)–COUPY PDT, calcein-AM/propidium iodide dual staining was performed. As illustrated in Fig. S32,† a reduction in calcein-AM fluorescence with a concomitant increase in propidium iodide fluorescence was observed in PDT-treated MCTS, particularly with 3c, revealing a high proportion of dead cells with compromised membrane integrity.Given the phototherapeutic performance of 3c-PDT, we conducted a preliminary in vivo toxicological study to assess its biocompatibility. In this study, conjugate 3c was administered intraperitoneally at a dose of 5 mg kg−1 to both male and female C57BL6/Hsd mice. The mice were monitored for 7 days for mortality, morbidity, body weight changes, and other signs of treatment-related toxicity. To our delight, Ir(III)–COUPY conjugate 3c was well tolerated by both sexes throughout the study. As shown in Fig. 9, there were no significant differences in body weight between the control (Vh) and 3c-treated groups. Post-mortem examination of organs revealed no significant abnormalities. Additionally, biochemistry analysis of the plasma samples showed no significant differences between the control and treated groups (Fig. S33†). Overall, there were no signs of toxicity or adverse effects, indicating that the 5 mg kg−1 dose was well tolerated. These preliminary studies will serve as the basis for the design of an in vivo phototherapeutic efficacy study with Ir(III)–COUPY conjugates using red light.
Experimental section
Synthesis of Ir(III)–COUPY conjugate 3c
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) in a Schlenk flask. The reaction was stirred at 58 °C for 24 h under a nitrogen atmosphere. After cooling the solution to room temperature, an excess of KPF6 (125 μmol) was added and it was stirred for 30 min. The solvent was concentrated under reduced pressure, and the product was washed with water and recrystallized using DCM and diethyl ether to give 58 mg of a red solid (yield: 84%). 1H NMR (400 MHz, DMSO-d6): δ 8.91 (d, J = 8.6 Hz, 1H), 8.68 (d, J = 8.6 Hz, 1H), 8.20 (d, J = 8.0 Hz, 1H), 8.10–8.01 (m, 3H), 7.84 (d, J = 7.6 Hz, 1H), 7.76 (d, J = 7.6 Hz, 1H), 7.72–7.68 (m, 2H), 7.65 (t, J = 7.6 Hz, 1H), 7.56 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 8.4 Hz, 2H), 7.21 (t, J = 8.3 Hz, 2H), 7.13–7.10 (m, 3H), 7.02 (d, J = 8.3 Hz, 3H), 6.98–6.92 (m, 2H), 6.90–6.83 (m, 4H), 6.44 (d, J = 6.7 Hz, 1H), 6.35–6.30 (m, 2H), 6.25–6.13 (m, 4H), 6.05 (d, J = 8.4 Hz, 1H), 5.16–4.95 (m, 2H), 1.75–1.54 (m, 2H), 0.94–0.72 (m, 2H), 0.54 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, DMSO-d6): δ 166.4, 162.8, 162.1, 156.0, 154.2, 149.8, 148.1, 145.0, 141.6, 140.9, 140.7, 139.1, 139.0, 138.4, 138.3, 134.9, 134.8, 133.3, 132.5, 131.4, 130.5, 130.3, 129.3, 129.0, 128.4, 128.1, 126.6, 126.5, 126.0, 125.8, 125.5, 125.4, 125.3, 124.4, 123.9, 123.1, 122.7, 122.6, 122.0, 120.8, 120.1, 113.8, 113.7, 112.9, 111.9, 111.8, 46.7, 46.7, 45.9, 31.7, 18.8, 13.3. HRMS (ESI-TOF) m/z: [M − PF6]+ calcd for C63H47F12IrN7O2P 1240.332; found 1240.3284.
3, v/v) in a Schlenk flask. The reaction was stirred at 58 °C for 24 h under a nitrogen atmosphere. After cooling the solution to room temperature, an excess of KPF6 (125 μmol) was added and it was stirred for 30 min. The solvent was concentrated under reduced pressure, and the product was washed with water and recrystallized using DCM and diethyl ether to give 58 mg of a red solid (yield: 84%). 1H NMR (400 MHz, DMSO-d6): δ 8.91 (d, J = 8.6 Hz, 1H), 8.68 (d, J = 8.6 Hz, 1H), 8.20 (d, J = 8.0 Hz, 1H), 8.10–8.01 (m, 3H), 7.84 (d, J = 7.6 Hz, 1H), 7.76 (d, J = 7.6 Hz, 1H), 7.72–7.68 (m, 2H), 7.65 (t, J = 7.6 Hz, 1H), 7.56 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 8.4 Hz, 2H), 7.21 (t, J = 8.3 Hz, 2H), 7.13–7.10 (m, 3H), 7.02 (d, J = 8.3 Hz, 3H), 6.98–6.92 (m, 2H), 6.90–6.83 (m, 4H), 6.44 (d, J = 6.7 Hz, 1H), 6.35–6.30 (m, 2H), 6.25–6.13 (m, 4H), 6.05 (d, J = 8.4 Hz, 1H), 5.16–4.95 (m, 2H), 1.75–1.54 (m, 2H), 0.94–0.72 (m, 2H), 0.54 (t, J = 7.2 Hz, 3H). 13C NMR (150 MHz, DMSO-d6): δ 166.4, 162.8, 162.1, 156.0, 154.2, 149.8, 148.1, 145.0, 141.6, 140.9, 140.7, 139.1, 139.0, 138.4, 138.3, 134.9, 134.8, 133.3, 132.5, 131.4, 130.5, 130.3, 129.3, 129.0, 128.4, 128.1, 126.6, 126.5, 126.0, 125.8, 125.5, 125.4, 125.3, 124.4, 123.9, 123.1, 122.7, 122.6, 122.0, 120.8, 120.1, 113.8, 113.7, 112.9, 111.9, 111.8, 46.7, 46.7, 45.9, 31.7, 18.8, 13.3. HRMS (ESI-TOF) m/z: [M − PF6]+ calcd for C63H47F12IrN7O2P 1240.332; found 1240.3284.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 (12,4 mg, 23.9 μmol) and DIPEA (7 μL, 40.3 μmol) in anhydrous DMF (3 mL), the reaction mixture was stirred for 2.5 h at room temperature under Ar and protected from light. After evaporation under reduced pressure, the crude product was purified by column chromatography (silica gel, 0–12% MeOH in DCM) to give 10.7 mg of a purple solid (yield: 73%). TLC: Rf (10% MeOH in DCM) 0.54. 1H NMR (400 MHz, DMSO-d6) δ (ppm) 9.03 (1H, br. s), 8.92 (1H, d, J = 8.8 Hz), 8.76 (1H, t, J = 5.4 Hz), 8.69 (1H, d, J = 8.9 Hz), 8.56 (2H, d, J = 7.4 Hz), 8.18 (4H, m), 8.04 (1H, d, J = 9.1 Hz), 7.94 (2H, t, J = 8.9 Hz), 7.81 (1H, d, J = 8.4 Hz), 7.72 (4H, m), 7.62 (1H, t, J = 7.9 Hz), 7.54 (4H, d, J = 8.4 Hz), 7.21 (4H, m), 7.03 (4H, m), 6.88 (8H, m), 6.61 (1H, s), 6.23 (7H, m), 5.90 (1H, d, J = 8.3 Hz), 5.30 (2H, s), 5.09 (2H, m), 3.53 (4H, m), 3.15 (4H, m), 2.55 (3H, s), 1.67 (4H, m), 1.15 (5H, m), 0.85 (2H, m), 0.56 (3H, t, J = 7.2 Hz). 13C NMR (101 MHz, DMSO-d6) δ (ppm) 166.8, 165.6, 165.0, 162.8, 162.2, 155.6, 154.9, 154.3, 152.8, 152.0, 149.8, 149.2, 148.0, 145.5, 144.2, 141.5, 140.8, 139.1, 138.6, 138.1, 138.0, 135.0, 134.8, 133.5, 133.4, 132.9, 132.2, 132.1, 131.2, 130.5, 130.3, 129.2, 129.0, 128.4, 128.1, 128.0, 127.0, 127.0, 126.3, 125.9, 125.7, 125.7, 125.7, 125.6, 125.3, 124.8, 124.3, 123.9, 123.7, 122.7, 122.6, 122.0, 120.7, 120.1, 118.2, 117.5, 114.1, 113.4, 112.8, 111.9, 111.8, 110.4, 110.4, 96.5, 78.1, 59.6, 53.4, 46.2, 44.2, 41.6, 37.1, 37.0, 31.8, 29.0, 28.9, 18.9, 18.7, 18.4, 18.0, 16.7, 13.3, 12.4, 12.3. HRMS (ESI-TOF) m/z: [M]2+ calcd for C89H77F6IrN12O3 834.2885, found 834.2887. Analytical HPLC (10 to 100% B in 6 min, formic acid additive): tR = 2.85 min.
27 (12,4 mg, 23.9 μmol) and DIPEA (7 μL, 40.3 μmol) in anhydrous DMF (3 mL), the reaction mixture was stirred for 2.5 h at room temperature under Ar and protected from light. After evaporation under reduced pressure, the crude product was purified by column chromatography (silica gel, 0–12% MeOH in DCM) to give 10.7 mg of a purple solid (yield: 73%). TLC: Rf (10% MeOH in DCM) 0.54. 1H NMR (400 MHz, DMSO-d6) δ (ppm) 9.03 (1H, br. s), 8.92 (1H, d, J = 8.8 Hz), 8.76 (1H, t, J = 5.4 Hz), 8.69 (1H, d, J = 8.9 Hz), 8.56 (2H, d, J = 7.4 Hz), 8.18 (4H, m), 8.04 (1H, d, J = 9.1 Hz), 7.94 (2H, t, J = 8.9 Hz), 7.81 (1H, d, J = 8.4 Hz), 7.72 (4H, m), 7.62 (1H, t, J = 7.9 Hz), 7.54 (4H, d, J = 8.4 Hz), 7.21 (4H, m), 7.03 (4H, m), 6.88 (8H, m), 6.61 (1H, s), 6.23 (7H, m), 5.90 (1H, d, J = 8.3 Hz), 5.30 (2H, s), 5.09 (2H, m), 3.53 (4H, m), 3.15 (4H, m), 2.55 (3H, s), 1.67 (4H, m), 1.15 (5H, m), 0.85 (2H, m), 0.56 (3H, t, J = 7.2 Hz). 13C NMR (101 MHz, DMSO-d6) δ (ppm) 166.8, 165.6, 165.0, 162.8, 162.2, 155.6, 154.9, 154.3, 152.8, 152.0, 149.8, 149.2, 148.0, 145.5, 144.2, 141.5, 140.8, 139.1, 138.6, 138.1, 138.0, 135.0, 134.8, 133.5, 133.4, 132.9, 132.2, 132.1, 131.2, 130.5, 130.3, 129.2, 129.0, 128.4, 128.1, 128.0, 127.0, 127.0, 126.3, 125.9, 125.7, 125.7, 125.7, 125.6, 125.3, 124.8, 124.3, 123.9, 123.7, 122.7, 122.6, 122.0, 120.7, 120.1, 118.2, 117.5, 114.1, 113.4, 112.8, 111.9, 111.8, 110.4, 110.4, 96.5, 78.1, 59.6, 53.4, 46.2, 44.2, 41.6, 37.1, 37.0, 31.8, 29.0, 28.9, 18.9, 18.7, 18.4, 18.0, 16.7, 13.3, 12.4, 12.3. HRMS (ESI-TOF) m/z: [M]2+ calcd for C89H77F6IrN12O3 834.2885, found 834.2887. Analytical HPLC (10 to 100% B in 6 min, formic acid additive): tR = 2.85 min.
Photophysical and photochemical characterization of the compounds
 | (1) |
Phosphorescence quantum yields (ΦP) of the iridium complex were determined analogously to ΦF, using argon-saturated meso-tetra-5,10,15,20-phenylporphine as the reference (ΦF = 0.11 in toluene).55
 | (2) |
The ΦΔ values of different samples were obtained by comparing S0 values of optically matched samples and using an appropriate reference, by means of eqn (3).
 | (3) |
The same setup was used to monitor the phosphorescence of the complex and the conjugate, except that the red-sensitive Hamamatsu H5783 photosensor module was used for detection.
The turnover number (TON) and the turnover frequency (TOF) values were calculated following eqn (4) and (5), respectively. The concentration of NADH was obtained following Lambert–Beer's law. The extinction coefficient of NADH in water at 339 nm is 6220 M−1 cm−1.56
 | (4) |
 | (5) |
The adiabatic electron affinities (EAs) and ionization potentials (IPs) listed in Table S3† are computed as follows:
| EA = E(An+1) − E(An) |
| IP = E(An−1) − E(An) |
Cellular take up and accumulation studies
For colocalization experiments, HeLa cells were incubated with the corresponding Ir(III)–COUPY conjugate (5 μM) in supplemented DMEM for 30 min at 37 °C. After removing excess conjugate with DPBS, the cells were incubated with WGA (2 μg mL−1) in non-supplemented DMEM for 20 min at 37 °C. LTG (0.2 μM) was added to the medium 5 min before the end of this incubation period. Finally, the cells were washed once more with DPBS and maintained in low glucose DMEM without phenol red supplemented with 10 mM HEPES for fluorescence imaging.
All microscopy observations were performed using a Zeiss LSM 880 confocal microscope equipped with an argon-ion laser, a 561 nm laser, and a 633 nm laser. The microscope was also equipped with a heating insert (P Lab-Tek S, Pecon). Cells were observed using a 63 × 1.4 oil-immersion objective. Ir(III)–COUPY conjugates 3a–c were excited using the 561 nm laser and emission was detected from 570 to 660 nm. In colocalization studies, LTG was excited using the 488 nm laser line of the argon-ion laser and detected from 500 to 550 nm, while WGA was excited using the 633 nm laser and detected from 660 to 760 nm. Image analysis was performed using Fiji.64 All images are colorized using the Fire lookup table from Fiji.64
For colocalization analysis of the signal located in vesicles, images were processed by median filtering (radius = 1), Gaussian filtering (sigma = 1), and background subtraction (rolling ball radius = 10). Then images were segmented using the Otsu thresholding algorithm and the resulting binary images were processed to remove larger and non-circular particles with a size filter between 0 and 1 μm2 and a circularity filter between 0.7 and 1. Processed binary images were converted to 16-bit and multiplied by 257 to obtain images with two grey levels (i.e., 0 and 65535) that finally were used to mask the original 16-bit images. Colocalization was then measured on the masked images using the JaCoP plugin.65
Photobiological evaluation
 | (6) |
Phototherapeutic activity in tumor spheroids
Toxicological in vivo study
On day 0, the mice received a single intraperitoneal injection at a volume of 10 mL kg−1. The “Vh” groups, serving as the non-treated control, were administered the vehicle (DMEM culture medium). In contrast, the “3c” groups, serving as the treated group, received a solution of compound 3c in DMEM (5 mg kg−1). Following dose administration, the animals were carefully observed for 7 days and routinely monitored for mortality, morbidity, changes in body weight, and other signs of toxicity related to the treatment.
All animals were sacrificed 7 days post-treatment. Each animal underwent a detailed gross pathological examination, including careful inspection of both external and internal body surfaces, as well as all vital organs. The animals were euthanized using CO2 asphyxiation, and blood samples (600–800 μL) were collected via cardiac puncture in labeled microcentrifuge tubes containing EDTA as an anticoagulant. The samples were then centrifuged for 10 min at 5000 rpm at 4 ± 2 °C to obtain plasma. The plasma samples were then analyzed using a VetScan® comprehensive diagnostic profile reagent rotor with the VetScan VS2 chemistry analyzer (Zoetis). This analysis determined the levels of total proteins, albumin, globulins, total bilirubin, alanine aminotransferase, alkaline phosphatase, amylase, creatinine, glucose, cholesterol, total bile acids, blood urea nitrogen, sodium, potassium, calcium, and phosphorus.
Conclusions
In summary, in this work we achieved potent in-cell PDT effects via red-light activated Ir(III)–COUPY conjugates under hypoxic conditions. The incorporation of trifluorobenzyl groups at the cyclometalated ligands of the novel Ir(III)–COUPY photosensitizer 3c produces an increase in the molar absorption coefficients values in all solvents studied and raises the luminescent quantum yield compared to conjugates 3a and 3b (which do not contain such groups). This new conjugate, 3c, is photostable and triggers type I and type II ROS production under irradiation with red light (>600 nm), as demonstrated by means of spectroscopic methods and EPR and further confirmed by theoretical calculations. Moreover, 3c exhibits a favorable profile for anticancer PDT since it delivers equipotent photocytotoxicity towards normoxic and hypoxic A2780cis and EO771 cancer cells, with high phototherapeutic indexes (>208) and a hypoxia index close to 1 in both cell lines. Importantly, Ir(III)–COUPY conjugate 3c was found to be highly phototoxic against EO771 multicellular tumor spheroids under red-light irradiation and showed no signs of toxicity in vivo. Taken together, this study demonstrated that the conjugation between COUPY dyes and rationally designed Ir(III) complexes was a strategy at the frontier of the development of new red light-activated photosensitizers capable of operating under hypoxic conditions, showing promise for achieving satisfactory anticancer PDT effects. Similar to our previous results, where we found that small changes to the coumarin scaffold and/or the linker moiety improved the PDT activity of the Ir(III)–COUPY PSs,29 herein we demonstrated that minimal modifications of the cyclometalated Ir(III) system could also have a profound impact on the resulting conjugate, especially on their hypoxia performance and their dark toxicity. As a result, we conclude that both components of the Ir(III)–COUPY dyad play key roles in conditioning the photobiological properties of the PSs as shown for conjugate 3c. This opens up new possibilities for designing other Ir(III)–COUPY PSs that operate in the red and near-infrared region of the electromagnetic spectrum with the intent of overcoming the hypoxia limitation.Author contributions
Conceptualization: E. O.-F., V. M., J. R., S. N. Data curation: E. O.-F., G. V., A. R., P. A., E. I.-G., D. A.-M., M. E. A., A. F.-M. Formal analysis: E. O.-F., P. A., E. I.-G., G. V., M. B., V. M., J. R., S. N., M. E. A., A. F.-M. Funding acquisition: V. M., J. R., S. N., M. E. A., A. F.-M. Investigation: E. O.-F., A. R., P. A., E. I.-G., D. A.-M., G. V., C. H., M. J.-R., A. D., J. G., M. B., M. E. A., A. F.-M. Methodology: E. O.-F., A. R., P. A., E. I.-G., D. A.-M., G. V., C. H., M. J.-R., A. D., J. G., M. B., M. E. A., A. F.-M. Project administration: E. O.-F., G. V., V. M., J. R., S. N., M. E. A., A. F.-M. Resources: V. M., J. R., S. N., J.-L. H., M. E. A., A. F.-M. Software: E. O.-F., G. V., P. A., E. I.-G., M. B., M. E. A., A. F.-M. Supervision: V. M., J. R., S. N. Validation: E. O.-F., A. R., P. A., E. I.-G., D. A.-M., G. V., C. H., M. J.-R., A. D., J. G., M. B., M. E. A., A. F.-M. Writing – original draft: E. O.-F., V. M. Writing – review & editing: E. O.-F., G. V., P. A., E. I.-G., M. B., V. M., J. R., S. N., M. E. A., A. F.-M.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by funds from the Spanish Ministerio de Ciencia, Innovación e Universidades and Agencia Estatal de Investigación (MICIU/AEI/10.13039/501100011033) (PID2020-117508RB-I00 and PID2023-146161OB-I00 to V. M.; PID2021-122850NB-I00 to J. R.; PID2021-127554NA-I00 to A. F.-M.; PID2020-115801RB-C22 to S. N.), by “ERDF A way of making Europe”. (PID2023-146161OB-I00 to V. M.; PID2021-122850NB-I00 to J. R.; PID2021-127554NA-I00 to A. F.-M.), and Fundación Séneca-CARM (project 21989/PI/22 to J. R.). A. F.-M. is grateful for support from Generalitat Valenciana Project No. CIAICO/2022/121. M. E. A. acknowledges financial support under the PRIN D.D. 104/2022 PNRR M4.C2.1.1. – Project Code: 2022AN47CA – CUP: H53D23003820001, funded by the European Union – NextGenerationEU– Project Title “La-G4-DACA” – Grant Assignment DD 862, 16/06/2023. M. E. A. is also grateful for the CINECA award under the ISCRA initiative, for the availability of high-performance computing resources and support (IsCc4_PAC-DACA project). E. O-F. thanks AECC (Project PRDMU19003ORTE). E. O.-F. was supported by JDC2023-050936-I funded by MICIU/AEI/10.13039/501100011033 and FSE+. A. R. was a recipient of a fellowship from the University of Barcelona (APIF). C. H. thanks the SUR del DEC de la Generalitat de Catalunya and the FSE for his predoctoral fellowship (Grants No. 2017 FI_B_00617, 2018 FI_B1 00174 and 2019 FI_B2 00167). E. I.-G. acknowledges support from a Margarita Salas postdoctoral grant at the University of Barcelona, funded by the Spanish Ministerio de Universidades with European Union funds – NextGenerationEU. S. N. thanks the Departament de Recerca i Universitats de la Generalitat de Catalunya for the support given to his research group (2021 SGR 01023) and the ICREA-Catalan Institution for Research and Advanced Studies for grant no. Ac2232308. The valuable collaboration of the technicians from the NMR, HRMS, ICP-MS, confocal microscopy and EPR facilities of the Scientific and Technological Centers (CCiTUB) of the Universitat de Barcelona is also appreciated.References
- X. Wang, J. Peng, C. Meng and F. Feng, Recent advances for enhanced photodynamic therapy: from new mechanisms to innovative strategies, Chem. Sci., 2024, 15, 12234–12257 RSC.
- W. Jiang, M. Liang, Q. Lei, G. Li and S. Wu, The Current Status of Photodynamic Therapy in Cancer Treatment, Cancers, 2023, 15, 585 CrossRef CAS.
- G. Li, Q. Wang, J. Liu, M. Wu, H. Ji, Y. Qin, X. Zhou and L. Wu, Innovative strategies for enhanced tumor photodynamic therapy, J. Mater. Chem. B, 2021, 9, 7347–7370 RSC.
- T. C. Pham, V.-N. Nguyen, Y. Choi, S. Lee and J. Yoon, Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy, Chem. Rev., 2021, 121, 13454–13619 CrossRef CAS.
- X. Zhao, J. Liu, J. Fan, H. Chao and X. Peng, Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application, Chem. Soc. Rev., 2021, 50, 4185–4219 RSC.
- E. Ortega-Forte, A. Rovira, A. Gandioso, J. Bonelli, M. Bosch, J. Ruiz and V. Marchán, COUPY Coumarins as Novel Mitochondria-Targeted Photodynamic Therapy Anticancer Agents, J. Med. Chem., 2021, 64, 17209–17220 CrossRef CAS PubMed.
- J. Bonelli, E. Ortega-Forte, A. Rovira, M. Bosch, O. Torres, C. Cuscó, J. Rocas, J. Ruiz and V. Marchán, Improving Photodynamic Therapy Anticancer Activity of a Mitochondria-Targeted Coumarin Photosensitizer Using a Polyurethane-Polyurea Hybrid Nanocarrier, Biomacromolecules, 2022, 23, 2900–2913 CrossRef CAS.
- Y. Wen, W. Zhang, N. Gong, Y.-F. Wang, H.-B. Guo, W. Guo, P. C. Wang and X.-J. Liang, Carrier-free, self-assembled pure drug nanorods composed of 10-hydroxycamptothecin and chlorin e6 for combinatorial chemo-photodynamic antitumor therapy in vivo, Nanoscale, 2017, 9, 14347–14356 RSC.
- A. Escudero, C. Carrillo-Carrión, M. C. Castillejos, E. Romero-Ben, C. Rosales-Barrios and N. Khiar, Photodynamic therapy: photosensitizers and nanostructures, Mater. Chem. Front., 2021, 5, 3788–3812 RSC.
- L. M. Lifshits, J. A. R. Iii, P. Konda, S. Monro, H. D. Cole, D. von Dohlen, S. Kim, G. Deep, R. P. Thummel, C. G. Cameron, S. Gujar and S. A. McFarland, Near-infrared absorbing Ru(II) complexes act as immunoprotective photodynamic therapy (PDT) agents against aggressive melanoma, Chem. Sci., 2020, 11, 11740–11762 RSC.
- R. Bevernaegie, B. Doix, E. Bastien, A. Diman, A. Decottignies, O. Feron and B. Elias, Exploring the Phototoxicity of Hypoxic Active Iridium(III)-Based Sensitizers in 3D Tumor Spheroids, J. Am. Chem. Soc., 2019, 141, 18486–18491 CrossRef CAS.
- F. J. Ballester, E. Ortega, D. Bautista, M. D. Santana and J. Ruiz, Ru(ii) photosensitizers competent for hypoxic cancers via green light activation, Chem. Commun., 2020, 56, 10301–10304 RSC.
- J. Pracharova, G. Vigueras, V. Novohradsky, N. Cutillas, C. Janiak, H. Kostrhunova, J. Kasparkova, J. Ruiz and V. Brabec, Exploring the Effect of Polypyridyl Ligands on the Anticancer Activity of Phosphorescent Iridium(III) Complexes: From Proteosynthesis Inhibitors to Photodynamic Therapy Agents, Chem. – Eur. J., 2018, 24, 4607–4619 CrossRef CAS.
- L. Lilge, M. Roufaiel, S. Lazic, P. Kaspler, M. A. Munegowda, M. Nitz, J. Bassan and A. Mandel, Evaluation of a Ruthenium coordination complex as photosensitizer for PDT of bladder cancer: Cellular response, tissue selectivity and in vivo response, Transl. Biophotonics, 2020, 2, e201900032 CrossRef.
- L. C.-C. Lee, K.-K. Leung and K. K.-W. Lo, Recent development of luminescent rhenium(I) tricarbonyl polypyridine complexes as cellular imaging reagents, anticancer drugs, and antibacterial agents, Dalton Trans., 2017, 46, 16357–16380 RSC.
- C. Imberti, P. Zhang, H. Huang and P. J. Sadler, New Designs for Phototherapeutic Transition Metal Complexes, Angew. Chem., Int. Ed., 2020, 59, 61–73 CrossRef CAS.
- Y. Zhang, B.-T. Doan and G. Gasser, Metal-Based Photosensitizers as Inducers of Regulated Cell Death Mechanisms, Chem. Rev., 2023, 123, 10135–10155 CrossRef CAS.
- Y. Wu, S. Li, Y. Chen, W. He and Z. Guo, Recent advances in noble metal complex based photodynamic therapy, Chem. Sci., 2022, 13, 5085–5106 RSC.
- L. Qiao, J. Liu, S. Kuang, X. Liao, J. Kou, L. Ji and H. Chao, A mitochondrion-targeted BODIPY-Ir(III) conjugate as a photoinduced ROS generator for the oxidative destruction of triple-negative breast cancer cells, Dalton Trans., 2021, 50, 14332–14341 RSC.
- B. Liu, S. Monro, M. A. Jabed, C. G. Cameron, K. L. Colón, W. Xu, S. Kilina, S. A. McFarland and W. Sun, Neutral iridium(III) complexes bearing BODIPY-substituted N-heterocyclic carbene (NHC) ligands: synthesis, photophysics, in vitro theranostic photodynamic therapy, and antimicrobial activity, Photochem. Photobiol. Sci., 2019, 18, 2381–2396 CrossRef CAS PubMed.
- L. Zhang, Y. Geng, L. Li, X. Tong, S. Liu, X. Liu, Z. Su, Z. Xie, D. Zhu and M. R. Bryce, Rational design of iridium–porphyrin conjugates for novel synergistic photodynamic and photothermal therapy anticancer agents, Chem. Sci., 2021, 12, 5918–5925 RSC.
- Y. Wu, J. Wu and W.-Y. Wong, Biomater. Sci., 2021, 9, 4843–4853 RSC.
- C. Liu, L. Zhou, F. Wei, L. Li, S. Zhao, P. Gong, L. Cai and K. M.-C. Wong, Versatile Strategy To Generate a Rhodamine Triplet State as Mitochondria-Targeting Visible-Light Photosensitizers for Efficient Photodynamic Therapy, ACS Appl. Mater. Interfaces, 2019, 11, 8797–8806 CrossRef CAS PubMed.
- K.-X. Teng, W.-K. Chen, L.-Y. Niu, W.-H. Fang, G. Cui and Q.-Z. Yang, BODIPY-Based Photodynamic Agents for Exclusively Generating Superoxide Radical over Singlet Oxygen, Angew. Chem., Int. Ed., 2021, 60, 19912–19920 CrossRef CAS.
- B. Lu, L. Wang, H. Tang and D. Cao, Recent advances in type I organic photosensitizers for efficient photodynamic therapy for overcoming tumor hypoxia, J. Mater. Chem. B, 2023, 11, 4600–4618 RSC.
- J. An, S. Tang, G. Hong, W. Chen, M. Chen, J. Song, Z. Li, X. Peng, F. Song and W.-H. Zheng, An unexpected strategy to alleviate hypoxia limitation of photodynamic therapy by biotinylation of photosensitizers, Nat. Commun., 2022, 13, 2225 CrossRef CAS.
- V. Novohradsky, A. Rovira, C. Hally, A. Galindo, G. Vigueras, A. Gandioso, M. Svitelova, R. Bresolí-Obach, H. Kostrhunova, L. Markova, J. Kasparkova, S. Nonell, J. Ruiz, V. Brabec and V. Marchán, Towards Novel Photodynamic Anticancer Agents Generating Superoxide Anion Radicals: A Cyclometalated IrIII Complex Conjugated to a Far-Red Emitting Coumarin, Angew. Chem., Int. Ed., 2019, 58, 6311–6315 CrossRef CAS PubMed.
- V. Novohradsky, L. Markova, H. Kostrhunova, J. Kasparkova, J. Ruiz, V. Marchán and V. Brabec, A Cyclometalated IrIII Complex Conjugated to a Coumarin Derivative Is a Potent Photodynamic Agent against Prostate Differentiated and Tumorigenic Cancer Stem Cells, Chem. – Eur. J., 2021, 27, 8547–8556 CrossRef CAS.
- A. Rovira, E. Ortega-Forte, C. Hally, M. Jordà-Redondo, D. Abad-Montero, G. Vigueras, J. I. Martínez, M. Bosch, S. Nonell, J. Ruiz and V. Marchán, Exploring Structure–Activity Relationships in Photodynamic Therapy Anticancer Agents Based on Ir(III)-COUPY Conjugates, J. Med. Chem., 2023, 66, 7849–7867 CrossRef CAS.
- E. Ortega-Forte, A. Rovira, M. López-Corrales, A. Hernández-García, F. J. Ballester, E. Izquierdo-García, M. Jordà-Redondo, M. Bosch, S. Nonell, M. D. Santana, J. Ruiz, V. Marchán and G. Gasser, A near-infrared light-activatable Ru(II)-coumarin photosensitizer active under hypoxic conditions, Chem. Sci., 2023, 14, 7170–7184 RSC.
- G. Vigueras, L. Markova, V. Novohradsky, A. Marco, N. Cutillas, H. Kostrhunova, J. Kasparkova, J. Ruiz and V. Brabec, A photoactivated Ir(III) complex targets cancer stem cells and induces secretion of damage-associated molecular patterns in melanoma cells characteristic of immunogenic cell death, Inorg. Chem. Front., 2021, 8, 4696–4711 RSC.
- V. Novohradsky, G. Vigueras, J. Pracharova, N. Cutillas, C. Janiak, H. Kostrhunova, V. Brabec, J. Ruiz and J. Kasparkova, Molecular superoxide radical photogeneration in cancer cells by dipyridophenazine iridium(III) complexes, Inorg. Chem. Front., 2019, 6, 2500–2513 RSC.
- L. Wang, J. Karges, F. Wei, L. Xie, Z. Chen, G. Gasser, L. Ji and H. Chao, A mitochondria-localized iridium(III) photosensitizer for two-photon photodynamic immunotherapy against melanoma, Chem. Sci., 2023, 14, 1461–1471 RSC.
- B. Liu, S. Monro, Z. Li, M. A. Jabed, D. Ramirez, C. G. Cameron, K. Colón, J. I. Roque, S. Kilina, J. Tian, S. A. McFarland and W. Sun, New Class of Homoleptic and Heteroleptic Bis(terpyridine) Iridium(III) Complexes with Strong Photodynamic Therapy Effects, ACS Appl. Bio Mater., 2019, 2, 2964–2977 CrossRef CAS.
- H. Huang, S. Banerjee, K. Qiu, P. Zhang, O. Blacque, T. Malcomson, M. J. Paterson, G. J. Clarkson, M. Staniforth, V. G. Stavros, G. Gasser, H. Chao and P. J. Sadler, Targeted photoredox catalysis in cancer cells, Nat. Chem., 2019, 11, 1041–1048 CrossRef CAS PubMed.
- C. Huang, C. Liang, T. Sadhukhan, S. Banerjee, Z. Fan, T. Li, Z. Zhu, P. Zhang, K. Raghavachari and H. Huang, In vitro and In vivo Photocatalytic Cancer Therapy with Biocompatible Iridium(III) Photocatalysts, Angew. Chem., 2021, 133, 9560–9565 CrossRef.
- J. Yellol, S. A. Pérez, G. Yellol, J. Zajac, A. Donaire, G. Vigueras, V. Novohradsky, C. Janiak, V. Brabec and J. Ruiz, Highly potent extranuclear-targeted luminescent iridium(III) antitumor agents containing benzimidazole-based ligands with a handle for functionalization, Chem. Commun., 2016, 52, 14165–14168 RSC.
- C. Adamo and V. Barone, Toward reliable density functional methods without adjustable parameters: The PBE0 model, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Gaussian, Inc., Wallingford, CT, 2016 Search PubMed.
- D. Andrae, U. Häußermann, M. Dolg, H. Stoll and H. Preuß, Energy-Adjusted ab initio Pseudopotentials for the Second and Third Row Transition Elements, Theor. Chim. Acta, 1990, 77, 123–141 CrossRef CAS.
- J. Tomasi, B. Mennucci and R. Cammi, Quantum Mechanical Continuum Solvation Models, Chem. Rev., 2005, 105, 2999–3093 CrossRef CAS PubMed.
- R. U. Kadam, D. Garg, J. Schwartz, R. Visini, M. Sattler, A. Stocker, T. Darbre and J.-L. Reymond, CH-π “T-Shape” Interaction with Histidine Explains Binding of Aromatic Galactosides to Pseudomonas aeruginosa Lectin LecA, ACS Chem. Biol., 2013, 8, 1925–1930 CrossRef CAS.
- S. Bolte and F. P. Cordelières, A guided tour into subcellular colocalization analysis in light microscopy, J. Microsc., 2006, 224, 213–232 CrossRef CAS.
- B. C. Behrens, T. C. Hamilton, H. Masuda, K. R. Grotzinger, J. Whang-Peng, K. G. Louie, T. Knutsen, W. M. McKoy, R. C. Young and R. F. Ozols, Characterization of a cis-Diamminedichloroplatinum(II)-resistant Human Ovarian Cancer Cell Line and Its Use in Evaluation of Platinum Analogues, Cancer Res., 1987, 47, 414–418 CAS.
- D. Fink, S. Aebi and S. B. Howell, The role of DNA mismatch repair in drug resistance, Clin. Cancer Res., 1998, 4, 1–6 CAS.
- M. Li, Y. Xu, Z. Pu, T. Xiong, H. Huang, S. Long, S. Son, L. Yu, N. Singh, Y. Tong, J. L. Sessler, X. Peng and J. S. Kim, Photoredox catalysis may be a general mechanism in photodynamic therapy, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2210504119 CrossRef CAS.
- C. Huang, C. Liang, T. Sadhukhan, S. Banerjee, Z. Fan, T. Li, Z. Zhu, P. Zhang, K. Raghavachari and H. Huang, In vitro and In vivo Photocatalytic Cancer Therapy with Biocompatible Iridium(III) Photocatalysts, Angew. Chem., Int. Ed., 2021, 60, 9474–9479 CrossRef CAS.
- T. Fan, J. Song and L.-Z. Gong, Asymmetric Redox Allylic Alkylation to Access 3,3′-Disubstituted Oxindoles Enabled by Ni/NHC Cooperative Catalysis, Angew. Chem., Int. Ed., 2022, 61, e202201678 CrossRef CAS.
- T. Wei, W. Liu, S. Zhang, Q. Liu, J. Luo and X. Liu, A dual-functional Bi-doped Co3O4 nanosheet array towards high efficiency 5-hydroxymethylfurfural oxidation and hydrogen production, Chem. Commun., 2023, 59, 442–445 RSC.
- S. J. Han, S. Kwon and K. S. Kim, Challenges of applying multicellular tumor spheroids in preclinical phase, Cancer Cell Int., 2021, 21, 152 CrossRef PubMed.
- D. Magde, J. H. Brannon, T. L. Cremers and J. Olmsted, Absolute luminescence yield of cresyl violet. A standard for the red, J. Phys. Chem., 1979, 83, 696–699 CrossRef CAS.
- C. Würth, M. Grabolle, J. Pauli, M. Spieles and U. Resch-Genger, Relative and absolute determination of fluorescence quantum yields of transparent samples, Nat. Protoc., 2013, 8, 1535–1550 CrossRef PubMed.
- A. M. Brouwer, Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report), Pure Appl. Chem., 2011, 83, 2213–2228 CrossRef CAS.
- P. G. Seybold and M. Gouterman, Porphyrins: XIII: Fluorescence spectra and quantum yields, J. Mol. Spectrosc., 1969, 31, 1–13 CrossRef CAS.
- Y. Han, X. Liu, Z. Tian, X. Ge, J. Li, M. Gao, Y. Li, Y. Liu and Z. Liu, Half-sandwich Iridium(III) Benzimidazole-Appended Imidazolium-Based N-heterocyclic Carbene Complexes and Antitumor Application, Chem. – Asian J., 2018, 13, 3697–3705 CrossRef CAS PubMed.
- J. A. Roque III, H. Cole, P. Barrett, L. Lifshits, R. Hodges, S. Kim, A. Francés-Monerris, M. E. Alberto, C. Cameron and S. McFarland, Intraligand Excited States Turn a Ruthenium Oligothiophene Complex into a Light-Triggered Ubertoxin with Anticancer Effects in Extreme Hypoxia, J. Am. Chem. Soc., 2022, 144, 8317–8336 CrossRef CAS.
- H. D. Cole, A. Vali, J. A. Roque III, G. Shi, G. Kaur, R. O. Hodges, A. Francés-Monerris, M. E. Alberto, C. G. Cameron and S. A. McFarland, Ru(II) Phenanthroline-Based Oligothienyl Complexes as Phototherapy Agents, Inorg. Chem., 2023, 62, 21181–21200 CrossRef CAS.
- H. D. Cole, A. Vali, J. A. Roque III, G. Shi, A. Talgatov, G. Kaur, A. Francés-Monerris, M. E. Alberto, C. G. Cameron and S. A. McFarland, Ru(II) Oligothienyl Complexes with Fluorinated Ligands: Photophysical, Electrochemical, and Photobiological Properties, Inorg. Chem., 2024, 63, 9735–9752 CrossRef CAS.
- J. A. Roque, P. C. Barrett, H. D. Cole, L. M. Lifshits, G. Shi, S. Monro, D. von Dohlen, S. Kim, N. Russo, G. Deep, C. G. Cameron, M. E. Alberto and S. A. McFarland, Breaking the barrier: an osmium photosensitizer with unprecedented hypoxic phototoxicity for real world photodynamic therapy, Chem. Sci., 2020, 11, 9784–9806 RSC.
- I. S. Ufimtsev and T. J. Martínez, Quantum Chemistry on Graphical Processing Units. 1. Strategies for Two-Electron Integral Evaluation, J. Chem. Theory Comput., 2008, 4, 222–231 CrossRef CAS.
- I. S. Ufimtsev and T. J. Martinez, Quantum Chemistry on Graphical Processing Units. 2. Direct Self-Consistent-Field Implementation, J. Chem. Theory Comput., 2009, 5, 1004–1015 CrossRef CAS.
- I. S. Ufimtsev and T. J. Martinez, Quantum Chemistry on Graphical Processing Units. 3. Analytical Energy Gradients, Geometry Optimization, and First Principles Molecular Dynamics, J. Chem. Theory Comput., 2009, 5, 2619–2628 CrossRef CAS.
- J. Schindelin, I. Arganda-Carreras, E. Frise, V. Kaynig, M. Longair, T. Pietzsch, S. Preibisch, C. Rueden, S. Saalfeld, B. Schmid, J.-Y. Tinevez, D. J. White, V. Hartenstein, K. Eliceiri, P. Tomancak and A. Cardona, Fiji: an open-source platform for biological-image analysis, Nat. Methods, 2012, 9, 676–682 CrossRef CAS PubMed.
- S. Bolte and F. P. Cordelières, A guided tour into subcellular colocalization analysis in light microscopy, J. Microsc., 2006, 224, 213–232 CrossRef CAS.
- N. Otsu, A threshold selection method from gray-level histograms, IEEE Trans. Syst., Man, Cybern., 1979, 9, 62–66 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi03369h |
| This journal is © the Partner Organisations 2025 |