Near-infrared luminescence AgPd alloy superatomic clusters†
Xiao-Hong
Ma
a,
Jian-Hua
Qin
 *b,
Fei-Fan
Wang
c,
Peng
Luo
*b,
Fei-Fan
Wang
c,
Peng
Luo
 d and
Xi-Yan
Dong
d and
Xi-Yan
Dong
 *cd
*cd
aCollege of Chemistry and Chemical Engineering, Xinyang Normal University, Xinyang 464000, China
bCollege of Chemistry and Chemical Engineering, Luoyang Normal University, Luoyang 471934, China. E-mail: jh_q128105@126.com
cHenan Key Laboratory of Crystalline Molecular Functional Materials, and College of Chemistry, Zhengzhou University, Zhengzhou 450001, China
dCollege of Chemistry and Chemical Engineering, Henan Polytechnic University, Jiaozuo 454000, China. E-mail: dongxiyan0720@hpu.edu.cn
First published on 24th December 2024
Abstract
Atomically precise superatomic nanoclusters have attracted considerable interest due to their remarkable structures and intriguing photoluminescence properties. Nevertheless, achieving a high photoluminescence quantum yield (PLQY) in the near-infrared (NIR) region of superatomic nanoclusters continues to pose a considerable challenge. Here, we report a novel bimetallic nanocluster prepared utilizing an alloying strategy, formulated as [Ag14Pd(PFBT)6(TPP)8](TPP) (abbreviated as Ag14Pd). Notably, this cluster demonstrates a remarkable NIR emission, achieving a PLQY of 15% in the solid state, which is rare for metal nanoclusters. Both experimental and theoretical analyses indicate that Ag14Pd exhibits a characteristic 8-electron superatomic structure with a 1S21P6 electronic shell closure. Furthermore, due to their bright luminescence and exceptional photostability, Ag14Pd clusters hold promising potential for applications as NIR inks for three-dimensional (3D) printing of various objects and models.
Introduction
Metal nanoclusters, distinguished by their well-defined sizes and meticulously characterized structures, have displayed substantial prospects in multiple application domains, encompassing chemical sensing, bioimaging, and catalysis.1–5 It is ubiquitously recognized that luminescence constitutes one of the most pivotal photophysical attributes of metal clusters,6–12 playing an indispensable role in multifarious applications such as 3D printing and light-emitting diodes.13–17 In recent years, metal nanoclusters that emit within the near-infrared (NIR > 750 nm) region18–23 have garnered increasing scrutiny due to their potential applications in domains such as optoelectronics, bioimaging, sensors, and near-infrared optics. The atomic-level precision of geometries and well-defined compositions inherent to these metal clusters augment our comprehension of their distinctive photophysical mechanisms,24,25 facilitating the design of highly photoluminescent materials.Currently, a substantial number of metal clusters exhibiting high photoluminescence quantum yields (PLQYs) have been reported in the visible light region.26–35 However, achieving comparable performance in the NIR region has been hindered by the significant loss of excitation energy through non-radiative relaxation as dictated by the energy gap law,36 despite ongoing efforts to develop various strategies for enhancing the PLQY of NIR-emitting metal clusters. For instance, strategies such as ligand tailoring and suppression of surface vibrations have been demonstrated to improve the PLQY of metal nanoclusters.37,38 Additionally, aggregation of metal nanoclusters has been observed to contribute positively to PLQY enhancement.39 Importantly, alloying represents an effective strategy for improving photoluminescence properties.40–42 Nevertheless, the PLQY of NIR-emitting metal clusters usually remains below 1%.43–45 Consequently, synthesizing metal nanoclusters exhibiting a high PLQY and efficient NIR emission remains a challenge.
In this study, we report the synthesis, crystal and electronic structures, and optical properties of bimetallic nanoclusters, formulated as [Ag14Pd(PFBT)6(TPP)8](TPP) (referred to as Ag14Pd, PFBT = pentafluorothiophenol and TPP = triphenylphosphine). The crystal structure of Ag14Pd was elucidated through single-crystal X-ray diffraction, electrospray ionization mass spectrometry, thermogravimetric analysis, and X-ray photoelectron spectroscopy. Ag14Pd features a standard icosahedral Ag12Pd metal kernel surrounded by two Ag(PFBT)3 surface motifs at opposite poles, resulting in a superatom structure characterized by an electronic shell closure of 1S21P6. Remarkably, this cluster exhibits a bright NIR emission in both the solution and solid states. In addition, the optical properties and electronic structure of Ag14Pd were comprehensively investigated using density functional theory (DFT). Given their bright luminescence and excellent photostability, Ag14Pd clusters demonstrate significant potential for applications as NIR inks for the 3D printing of different objects and models.
Results and discussion
Ag14Pd was synthesized using a one-pot synthetic method (for details see the ESI†). In a typical synthesis, silver nitrate and palladium-tetrakis(triphenylphosphine) were initially dissolved in a solvent mixture of methanol, acetone, and dichloromethane at room temperature. Following this, PFBT and TPP were introduced into the mixture under vigorous stirring, leading to the formation of Pd–Ag–S–P complexes. After approximately 10 minutes, a freshly prepared aqueous solution of NaBH4 was added to the reaction mixture all at once. The reaction mixture was then aged for 5 hours. Subsequently, the aqueous phase was removed and the organic phase was washed with water several times. After about two days, reddish-black block crystals crystallized from the CH2Cl2/hexane solution at room temperature.The purity and chemical composition of the Ag14Pd clusters were rigorously characterized using an array of analytical techniques, including powder X-ray diffraction (PXRD), thermogravimetric analysis (TGA), electrospray ionization mass spectrometry (ESI-MS) in negative mode, inductively coupled plasma mass spectrometry (ICP-MS), and X-ray photoelectron spectroscopy (XPS). The phase purity of the Ag14Pd crystals was corroborated by the strong agreement between the experimental and simulated PXRD patterns (Fig. S1†). The TGA curve exhibited a weight loss of 67.56 wt%, closely aligning with the theoretical value of 67.07 wt% (Fig. S2†). Further insights into its composition and charge state were obtained through ESI-MS analysis, which revealed a prominent peak at m/z = 2171.3544 (cal. 2171.3802) in negative-ion mode within the range of 1500–4000, corresponding to [Ag14Pd(PFBT)6(TPP)5(CH2Cl2)(CH3OH)(Cl−)2]2−, thereby confirming both the charge state and composition of Ag14Pd (Fig. S3†). Additionally, energy-dispersive spectrometry (EDS) and XPS analyses confirmed the presence of Pd, Ag, S, P, F, and C elements in the Ag14Pd clusters (Fig. S4–5†). High-resolution XPS spectra indicated that the oxidation states of Ag and Pd were close to Ag(0) and Pd(0), respectively (Fig. S5†).46 Finally, the ICP-MS results demonstrated that the average element ratio of Ag to Pd is 13.96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1—remarkably consistent with approximately 14
1—remarkably consistent with approximately 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 determined via single-crystal X-ray analysis (Fig. S6†). Based on superatom theory's electron count rule, its electron count can be computed as 8 (N* = 14–6), suggesting that Ag14Pd is a superatomic cluster with a closed-shell configuration of 1S21P6.47
1 determined via single-crystal X-ray analysis (Fig. S6†). Based on superatom theory's electron count rule, its electron count can be computed as 8 (N* = 14–6), suggesting that Ag14Pd is a superatomic cluster with a closed-shell configuration of 1S21P6.47
Single-crystal structure determination elucidated that Ag14Pd crystallized in the trigonal space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c (Table S1†). As illustrated in Fig. 1, Ag14Pd displays an icosahedral Ag12Pd kernel with two silver atoms capping two triangular Ag3 faces along the three-fold axes of icosahedral Ag12Pd (Fig. 1a and b). These two silver atoms, together with a triangular face of the Ag12Pd icosahedron, give rise to two opposing tetrahedral geometries, wherein each edge of the tetrahedron's waist is bridged by a PFBT ligand (Fig. 1b). Eight TPP ligands are coordinated around the opposite poles of these two silver atoms and six silver atoms arranged in a chair cyclohexane conformation at the waist of the Ag12Pd icosahedron (Fig. 1c, d and S7†). The distances between Ag and Agicosahedron within the Ag12Pd kernel range from 2.864 (5) to 2.939 (7) Å (average 2.900 Å, Table S2†), which correlates well with bulk face-centered cubic silver exhibiting an approximate distance of about 2.889 Å, indicative of pronounced argentophilic interactions (Fig. 1e). The average Ag–Pd distance within Ag12Pd is determined to be 2.757 Å, suggesting a stronger metallophilic interaction (Fig. 1e). Conversely, the average distance between adjacent silver atoms in tetrahedral configurations within the Ag14Pd cluster is approximately 3.228 Å (Ag–Agtetrahedron), and there is also an Ag–Ag interaction smaller than 3.44 Å (the sum of van der Waals radii of the two Ag atoms), indicating weaker interactions compared to those observed between Ag and Agicosahedron. The average bond lengths for both Ag–S and Ag–P are determined to be 2.564 and 2.492 Å, respectively (Fig. 1e). Notably, the structural characteristics of Ag14Pd exhibit similarities to those reported for PdAg14 by Wang et al.46
c (Table S1†). As illustrated in Fig. 1, Ag14Pd displays an icosahedral Ag12Pd kernel with two silver atoms capping two triangular Ag3 faces along the three-fold axes of icosahedral Ag12Pd (Fig. 1a and b). These two silver atoms, together with a triangular face of the Ag12Pd icosahedron, give rise to two opposing tetrahedral geometries, wherein each edge of the tetrahedron's waist is bridged by a PFBT ligand (Fig. 1b). Eight TPP ligands are coordinated around the opposite poles of these two silver atoms and six silver atoms arranged in a chair cyclohexane conformation at the waist of the Ag12Pd icosahedron (Fig. 1c, d and S7†). The distances between Ag and Agicosahedron within the Ag12Pd kernel range from 2.864 (5) to 2.939 (7) Å (average 2.900 Å, Table S2†), which correlates well with bulk face-centered cubic silver exhibiting an approximate distance of about 2.889 Å, indicative of pronounced argentophilic interactions (Fig. 1e). The average Ag–Pd distance within Ag12Pd is determined to be 2.757 Å, suggesting a stronger metallophilic interaction (Fig. 1e). Conversely, the average distance between adjacent silver atoms in tetrahedral configurations within the Ag14Pd cluster is approximately 3.228 Å (Ag–Agtetrahedron), and there is also an Ag–Ag interaction smaller than 3.44 Å (the sum of van der Waals radii of the two Ag atoms), indicating weaker interactions compared to those observed between Ag and Agicosahedron. The average bond lengths for both Ag–S and Ag–P are determined to be 2.564 and 2.492 Å, respectively (Fig. 1e). Notably, the structural characteristics of Ag14Pd exhibit similarities to those reported for PdAg14 by Wang et al.46
Importantly, the photoluminescence of Ag14Pd was systematically investigated in the solid and solution states. Solid state Ag14Pd displayed intense NIR emission at 780 nm with a wide excitation range of 400–650 nm and a photoluminescence quantum yield of 15% (Fig. 2a). The photoluminescence lifetime measured for Ag14Pd was on the microsecond scale, specifically at 5.5 μs (Fig. 2b), indicating that its luminescence behavior is characteristic of phosphorescence.8 However, in CH2Cl2 solution, Ag14Pd also displayed NIR emission centered at 768 nm, with a PLQY of 6% and a lifetime of 4.1 μs. The PLQY in the solid state (15%) significantly surpasses that observed in the CH2Cl2 solution (6%), attributed to the restriction of intermolecular motion inherent to the solid state. Specifically, the packing structure of the Ag14Pd cluster reveals an ABCABC stacking pattern characterized by numerous intermolecular interactions such as C–H⋯F and C–H⋯π between the clusters within each layer and those adjacent to it (Fig. S8†).
 | ||
| Fig. 2 (a and c) NIR emission spectra and (b and d) lifetime decay curves of Ag14Pd in the solid and solution states. | ||
To verify this result, we conducted aggregation-induced emission and dynamic light scattering experiments of the clusters in the solvent systems of dichloromethane and n-hexane, and the results showed that the emission of Ag14Pd in the dilute solution of dichloromethane was weak. The emission intensity gradually enhanced upon further increasing the n-hexane fraction. When the n-hexane fraction increased to 90%, the solution showed strong red emission, indicating the possible presence of a larger aggregate,48–51 which was further demonstrated by dynamic light scattering (Fig. S9†). Meanwhile, the emission intensity at the maximum of solid-state Ag14Pd remains largely unchanged after irradiation for 3 h with a 400 nm xenon lamp (Fig. S10†), demonstrating exceptional photostability and opening new avenues for practical applications including 3D printing, cell imaging, and biological labeling.
The ultraviolet-visible (UV-vis) absorption spectrum of the Ag14Pd cluster was recorded in CH2Cl2, as illustrated in Fig. 3. The UV/vis spectrum of Ag14Pd shows four prominent optical absorption bands at 330 (a), 421 (b), 496 (c), and 679 (d) nm (Fig. 3a). To gain further insights into the electronic structures and absorption characteristics of Ag14Pd, we conducted time-dependent density functional theory (TD-DFT) calculations (Fig. 3b–d and S11†).
The calculated absorption spectrum of Ag14Pd reveals four distinct absorption peaks. The slight shift in the positions of these peaks compared to the experimental data can be attributed to an overestimation of the excitation energy (Fig. 3b). Peak d, corresponding to the highest energy absorption band, primarily arises from transitions between the highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO), predominantly involving metal-to-metal charge transfer (MMCT) transitions associated with Ag and Pd kernel-based orbitals (Fig. 3a and S11†). Similarly, peak c within this higher energy absorption band exhibits MMCT transitions such as HOMO → LUMO+3 and HOMO−1 → LUMO+1 (Fig. 3a and S11†). Peak b in the lower energy absorption band encompasses both HOMO → LUMO+5 and HOMO−1 → LUMO+4 transitions, while peak a is primarily generated from the transition between HOMO−13 and LUMO, which mainly involves ligand to metal charge transfer (LMCT) transitions (Fig. 3a and S11†). Furthermore, the occupied orbitals HOMO, HOMO−1, and HOMO−2 display pronounced superatomic P-like character and are predominantly composed of Ag and Pd atoms (Fig. 3c), whereas the unoccupied orbitals LUMO, LUMO+1, LUMO+2, LUMO+3, and LUMO+4 demonstrate distinct superatomic D-like character largely derived from Ag and Pd atoms (Fig. 3d). Consequently, Ag14Pd qualifies as a typical superatomic cluster.
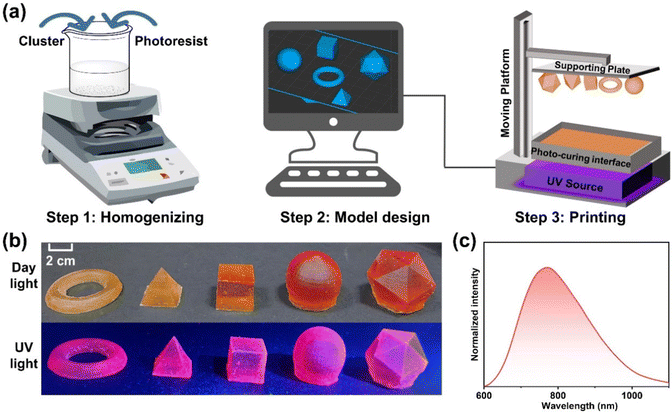
Photo-curing 3D printing involves the layer-by-layer construction of three-dimensional objects through the polymerization or cross-linking of photo-curing resin monomers under UV laser irradiation.52 This method offers significant advantages in terms of resolution, cost-effectiveness, and production efficiency compared to traditional subtractive manufacturing technologies, making it highly attractive. Although a variety of materials can be utilized in photo-curable 3D printing techniques,53 there have been no reports focusing on the application of superatomic clusters with NIR luminescence as inks for 3D printing. Given the excellent solubility, light stability, and bright NIR luminescence exhibited by Ag14Pd clusters, we were motivated to explore their potential for fabricating macroscopic models via 3D printing (Fig. 4a). Initially, the Ag14Pd clusters are thoroughly mixed with photocuring resin and loaded into the liquid storage tank of the printer for subsequent use. Subsequently, a series of model files representing various shapes (such as rings, tetrahedra, cubes, spheres, and icosahedra) are designed using drawing software and converted into the “.CWS” format compatible with 3D printers through slicing software before being uploaded to the printer. The parameters of the printer are adjusted to produce regular and varied macroscopic stereo models (Fig. 4b). Notably, these printed models retain the original NIR luminescence characteristics of the Ag14Pd clusters (Fig. 4c), indicating that superatomic clusters may hold important potential for applications in optoelectronic devices.
Conclusions
In summary, we have synthesized and characterized a novel superatom alloy cluster that demonstrates robust NIR luminescence both in solid and solution states, achieving a maximum PLQY of 15% and exhibiting a microsecond lifetime. For the first time, this near-infrared luminescent superatomic cluster has been employed as an ink for 3D printing of various three-dimensional models. Concurrently, we performed a comprehensive analysis of the electronic structure of the cluster through theoretical calculations. This work expands the application scope of superatomic clusters with near-infrared luminescence and offers a new direction for the application of these clusters.Author contributions
X.-Y. D. conceived and designed the project. X.-H. M. and F.-F. W. conducted the synthesis, characterization, and analysis of experimental results. P. L. performed the calculations. X.-H. M., J.-H. Q., and X.-Y. D. co-wrote the manuscript.Data availability
The X-ray crystallographic coordinates for the structure reported in this work have been deposited at the Cambridge Crystallographic Data Centre (CCDC 2352112†).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. U21A20277 and 22271130), the Excellent Youth Foundation of Henan Scientific Committee (232300421022), and the Program for Innovative Research Team (in Science and Technology) in University of Henan Province (IRTSTHN, 25IRTSTHN001).References
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- I. Chakraborty and T. Pradeep, Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles, Chem. Rev., 2017, 117, 8208–8271 CrossRef CAS PubMed.
- S. Takano and T. Tsukuda, Chemically Modified Gold/Silver Superatoms as Artificial Elements at Nanoscale: Design Principles and Synthesis Challenges, J. Am. Chem. Soc., 2021, 143, 1683–1698 CrossRef CAS PubMed.
- S. Hossain, D. Hirayama, A. Ikeda, M. Ishimi, S. Funaki, A. Samanta, T. Kawawaki and Y. Negishi, Atomically Precise Thiolate-Protected Gold Nanoclusters: Current Status of Designability of the Structure and Physicochemical Properties, Aggregate, 2022, 4, e255 CrossRef.
- S. Li, N.-N. Li, X.-Y. Dong, S.-Q. Zang and T. C. W. Mak, Chemical Flexibility of Atomically Precise Metal Clusters, Chem. Rev., 2024, 124, 7262–7378 CrossRef CAS PubMed.
- G. Li, Z. Lei and Q.-M. Wang, Luminescent Molecular Ag–S Nanocluster [Ag62S13(SBut,)32](BF4)4, J. Am. Chem. Soc., 2010, 132, 17678–17679 CrossRef CAS PubMed.
- L. G. AbdulHalim, M. S. Bootharaju, Q. Tang, S. Del Gobbo, R. G. AbdulHalim, M. Eddaoudi, D. E. Jiang and O. M. Bakr, Ag29(BDT)12(TPP)4: A Tetravalent Nanocluster, J. Am. Chem. Soc., 2015, 137, 11970–11975 CrossRef CAS PubMed.
- R. W. Huang, Y. S. Wei, X. Y. Dong, X. H. Wu, C. X. Du, S. Q. Zang and T. C. W. Mak, Hypersensitive Dual-Function Luminescence Switching of a Silver-Chalcogenolate Cluster-Based Metal-Organic Framework, Nat. Chem., 2017, 9, 689–697 CrossRef CAS PubMed.
- J. W. Liu, Z. Wang, Y. M. Chai, M. Kurmoo, Q. Q. Zhao, X. P. Wang, C. H. Tung and D. Sun, Core Modulation of 70-Nuclei Core-Shell Silver Nanoclusters, Angew. Chem., Int. Ed., 2019, 58, 6276–6279 CrossRef CAS PubMed.
- S. S. Zhang, F. Alkan, H. F. Su, C. M. Aikens, C. H. Tung and D. Sun, [Ag48(C≡CtBu)20(CrO4)7]: An Atomically Precise Silver Nanocluster Co-Protected by Inorganic and Organic Ligands, J. Am. Chem. Soc., 2019, 141, 4460–4467 CrossRef CAS PubMed.
- Y. Xiao, Z. Wu, Q. Yao and J. Xie, Luminescent Metal Nanoclusters: Biosensing Strategies and Bioimaging Applications, Aggregate, 2021, 2, 114–132 CrossRef CAS.
- X.-H. Ma, J. Li, P. Luo, J.-H. Hu, Z. Han, X.-Y. Dong, G. Xie and S.-Q. Zang, Carbene-Stabilized Enantiopure Heterometallic Clusters Featuring EQE of 20.8% in Circularly-Polarized Oled, Nat. Commun., 2023, 14, 4121 CrossRef CAS PubMed.
- J. Ni, C. Zhong, L. Li, M. Su, X. Wang, J. Sun, S. Chen, C. Duan, C. Han and H. Xu, Deep-Blue Electroluminescence from Phosphine-Stabilized Au3 Triangles and Au3Ag Pyramids, Angew. Chem., Int. Ed., 2022, 61, e202213826 CrossRef CAS PubMed.
- H. Zeng, J.-Y. Wang, L.-X. Shi, L.-Y. Zhang and Z.-N. Chen, Platinum(II)-Gold(I) Heterotrinuclear Complexes with N,N′-Diarylamine-Functionalized Acetylide Ligands for Red Electroluminescence, Inorg. Chem. Front., 2022, 9, 3156–3164 RSC.
- X.-H. Ma, Y. Si, J.-H. Hu, X.-Y. Dong, G. Xie, F. Pan, Y.-L. Wei, S.-Q. Zang and Y. Zhao, High-Efficiency Pure Blue Circularly Polarized Phosphorescence from Chiral N-Heterocyclic-Carbene-Stabilized Copper(I) Clusters, J. Am. Chem. Soc., 2023, 145, 25874–25886 CrossRef CAS PubMed.
- J.-J. Wang, L.-Z. Feng, G. Shi, J.-N. Yang, Y.-D. Zhang, H. Xu, K.-H. Song, T. Chen, G. Zhang, X.-S. Zheng, F. Fan, Z. Xiao and H.-B. Yao, High Efficiency Warm-White Light-Emitting Diodes Based on Copper–Iodide Clusters, Nat. Photonics, 2023, 18, 200–206 CrossRef.
- F. Zhang, Y. Gao, P. Lu, Y. Zhong, Y. Liu, X. Bao, Z. Xu, M. Lu, Y. Wu, P. Chen, J. Hu, Y. Zhang, Z. Wu, H. Song and X. Bai, Engineering of Hole Transporting Interface by Incorporating the Atomic-Precision Ag6 Nanoclusters for High-Efficiency Blue Perovskite Light-Emitting Diodes, Nano Lett., 2023, 23, 1582–1590 CrossRef CAS PubMed.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, X. Mu, J. Wang, W. Mi, J. Luo, J. Xie and X. D. Zhang, Atomic-Precision Gold Clusters for NIR-II Imaging, Adv. Mater., 2019, 31, 1901015 CrossRef CAS PubMed.
- L. Jiang, M. Jing, B. Yin, W. Du, X. Wang, Y. Liu, S. Chen and M. Zhu, Bright near-Infrared Circularly Polarized Electrochemiluminescence from Au9Ag4 nanoclusters, Chem. Sci., 2023, 14, 7304–7309 RSC.
- C. Fang, C. Xu, W. Zhang, M. Zhou, D. Tan, L. Qian, D. Hu, S. Jin and M. Zhu, Dual-Quartet Phosphorescent Emission in the Open-Shell M1Ag13 (M = Pt, Pd) Nanoclusters, Nat. Commun., 2024, 15, 5962 CrossRef CAS PubMed.
- W.-Q. Shi, L. Zeng, R.-L. He, X.-S. Han, Z.-J. Guan, M. Zhou and Q.-M. Wang, Near-Unity NIR Phosphorescent Quantum Yield from a Room-Temperature Solvated Metal Nanocluster, Science, 2024, 383, 326–330 CrossRef CAS PubMed.
- W.-D. Si, C. Zhang, M. Zhou, Z. Wang, L. Feng, C.-H. Tung and D. Sun, Arylgold Nanoclusters: Phenyl-Stabilized Au44 with Thermal-Controlled NIR Single/Dual-Channel Phosphorescence, Sci. Adv., 2024, 10, eadm6928 CrossRef CAS PubMed.
- C. Zhang, W.-D. Si, Z. Wang, A. Dinesh, Z.-Y. Gao, C.-H. Tung and D. Sun, Solvent-Mediated Hetero/Homo-Phase Crystallization of Copper Nanoclusters and Superatomic Kernel-Related NIR Phosphorescence, J. Am. Chem. Soc., 2024, 146, 10767–10775 CrossRef CAS PubMed.
- X. Kang, X. Wei, S. Jin, Q. Yuan, X. Luan, Y. Pei, S. Wang, M. Zhu and R. Jin, Rational Construction of a Library of M29 Nanoclusters from Monometallic to Tetrametallic, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 18834–18840 CrossRef CAS PubMed.
- Q. Li, M. Zhou, W. Y. So, J. Huang, M. Li, D. R. Kauffman, M. Cotlet, T. Higaki, L. A. Peteanu, Z. Shao and R. Jin, A Mono-Cuboctahedral Series of Gold Nanoclusters: Photoluminescence Origin, Large Enhancement, Wide Tunability, and Structure–Property Correlation, J. Am. Chem. Soc., 2019, 141, 5314–5325 CrossRef CAS PubMed.
- J. Lu, B. Shao, R.-W. Huang, L. Gutiérrez-Arzaluz, S. Chen, Z. Han, J. Yin, H. Zhu, S. Dayneko, M. N. Hedhili, X. Song, P. Yuan, C. Dong, R. Zhou, M. I. Saidaminov, S.-Q. Zang, O. F. Mohammed and O. M. Bakr, High-Efficiency Circularly Polarized Light-Emitting Diodes Based on Chiral Metal Nanoclusters, J. Am. Chem. Soc., 2024, 146, 4144–4152 CrossRef CAS PubMed.
- Q. Hu, C. Zhang, X. Wu, G. Liang, L. Wang, X. Niu, Z. Wang, W. D. Si, Y. Han, R. Huang, J. Xiao and D. Sun, Highly Effective Hybrid Copper(I) Iodide Cluster Emitter with Negative Thermal Quenched Phosphorescence for X–Ray Imaging, Angew. Chem., Int. Ed., 2023, 62, e202217784 CrossRef CAS PubMed.
- X. Wang, B. Yin, L. Jiang, C. Yang, Y. Liu, G. Zou, S. Chen and M. Zhu, Ligand-Protected Metal Nanoclusters as Low-Loss, Highly Polarized Emitters for Optical Waveguides, Science, 2023, 381, 784–790 CrossRef CAS PubMed.
- S. Horiuchi, S. Moon, A. Ito, J. Tessarolo, E. Sakuda, Y. Arikawa, G. H. Clever and K. Umakoshi, Multinuclear Ag Clusters Sandwiched by Pt Complex Units: Fluxional Behavior and Chiral–at–Cluster Photoluminescence, Angew. Chem., Int. Ed., 2021, 60, 10654–10660 CrossRef CAS PubMed.
- X. H. Ma, J. Y. Wang, J. J. Guo, Z. Y. Wang and S. Q. Zang, Reversible Wide-Range Tuneable Luminescence of a Dual-Stimuli-Responsive Silver Cluster-Assembled Material, Chin. J. Chem., 2019, 37, 1120–1124 CrossRef CAS.
- L. Shi, L. Zhu, J. Guo, L. Zhang, Y. Shi, Y. Zhang, K. Hou, Y. Zheng, Y. Zhu, J. Lv, S. Liu and Z. Tang, Self-Assembly of Chiral Gold Clusters into Crystalline Nanocubes of Exceptional Optical Activity, Angew. Chem., Int. Ed., 2017, 56, 15397–15401 CrossRef CAS PubMed.
- Y. Song, Y. Li, M. Zhou, X. Liu, H. Li, H. Wang, Y. Shen, M. Zhu and R. Jin, Ultrabright Au@Cu14 Nanoclusters: 71.3% Phosphorescence Quantum Yield in Non-Degassed Solution at Room Temperature, Sci. Adv., 2021, 7, eabd2091 CrossRef CAS PubMed.
- Z. Han, X. Y. Dong, P. Luo, S. Li, Z. Y. Wang, S. Q. Zang and T. C. W. Mak, Ultrastable Atomically Precise Chiral Silver Clusters with More Than 95% Quantum Efficiency, Sci. Adv., 2020, 6, eaay0107 CrossRef CAS PubMed.
- Y. Wang, S.-M. Zhai, P. Luo, X.-Y. Dong, J.-Y. Wang, Z. Han and S.-Q. Zang, Vapor- and Temperature-Triggered Reversible Optical Switching for Multi-Response Cu8 Cluster Supercrystals, Chin. Chem. Lett., 2024, 35, 109493 CrossRef CAS.
- J. J. Li, C. Y. Liu, Z. J. Guan, Z. Lei and Q. M. Wang, Anion-Directed Regulation of Structures and Luminescence of Heterometallic Clusters, Angew. Chem., Int. Ed., 2022, 61, e202201549 CrossRef CAS PubMed.
- Y. Wang, C. G. Gianopoulos, Z. Liu, K. Kirschbaum, D. Alfonso, D. R. Kauffman and R. Jin, Au36(SR)22 Nanocluster and a Periodic Pattern from Six to Fourteen Free Electrons in Core Size Evolution, JACS Au, 2024, 4, 1928–1934 CrossRef CAS PubMed.
- X. Kang, S. Jin, L. Xiong, X. Wei, M. Zhou, C. Qin, Y. Pei, S. Wang and M. Zhu, Nanocluster Growth Via “Graft-Onto”: Effects on Geometric Structures and Optical Properties, Chem. Sci., 2020, 11, 1691–1697 RSC.
- Y. Zhou, L. Liao, S. Zhuang, Y. Zhao, Z. Gan, W. Gu, J. Li, H. Deng, N. Xia and Z. Wu, Traceless Removal of Two Kernel Atoms in a Gold Nanocluster and Its Impact on Photoluminescence, Angew. Chem., Int. Ed., 2021, 60, 8668–8672 CrossRef CAS PubMed.
- X. Wei, X. Kang, S. Jin, S. Wang and M. Zhu, Aggregation of Surface Structure Induced Photoluminescence Enhancement in Atomically Precise Nanoclusters, CCS Chem., 2020, 1929–1939 Search PubMed.
- G. Soldan, M. A. Aljuhani, M. S. Bootharaju, L. G. AbdulHalim, M. R. Parida, A. H. Emwas, O. F. Mohammed and O. M. Bakr, Gold Doping of Silver Nanoclusters: A 26-Fold Enhancement in the Luminescence Quantum Yield, Angew. Chem., Int. Ed., 2016, 55, 5749–5753 CrossRef CAS PubMed.
- X. Liu, J. Yuan, C. Yao, J. Chen, L. Li, X. Bao, J. Yang and Z. Wu, Crystal and Solution Photoluminescence of MAg24(SR)18 (M = Ag/Pd/Pt/Au) Nanoclusters and Some Implications for the Photoluminescence Mechanisms, J. Phys. Chem. C, 2017, 121, 13848–13853 CrossRef CAS.
- S. Hossain, Y. Niihori, L. V. Nair, B. Kumar, W. Kurashige and Y. Negishi, Alloy Clusters: Precise Synthesis and Mixing Effects, Acc. Chem. Res., 2018, 51, 3114–3124 CrossRef CAS PubMed.
- M. S. Bootharaju, S. Lee, G. Deng, S. Malola, W. Baek, H. Hakkinen, N. Zheng and T. Hyeon, Ag44(EBT)26(TPP)4 Nanoclusters with Tailored Molecular and Electronic Structure, Angew. Chem., Int. Ed., 2021, 60, 9038–9044 CrossRef CAS PubMed.
- H. Li, C. Zhou, E. Wang, X. Kang, W. W. Xu and M. Zhu, An Insight, at the Atomic Level, into the Intramolecular Metallophilic Interaction in Nanoclusters, Chem. Commun., 2022, 58, 5092–5095 RSC.
- X.-H. Ma, J.-T. Jia, P. Luo, Z.-Y. Wang, S.-Q. Zang and T. C. W. Mak, Layer-by-Layer Alloying of Nir-Ii Emissive M50 (Au/Ag/Cu) Superatomic Nanocluster, Nano Res., 2022, 15, 5569–5574 CrossRef CAS.
- A. Ma, Y. Ren, Y. Zuo, J. Wang, S. Huang, X. Ma and S. Wang, Ligand-Controlled Exposure of Active Sites on the Pd1Ag14 Nanocluster Surface to Boost Electrocatalytic CO2 Reduction, Chem. Commun., 2024, 60, 3162–3165 RSC.
- S. F. Yuan, R. L. He, X. S. Han, J. Q. Wang, Z. J. Guan and Q. M. Wang, Robust Gold Nanocluster Protected with Amidinates for Electrocatalytic CO2 Reduction, Angew. Chem., Int. Ed., 2021, 60, 14345–14349 CrossRef CAS PubMed.
- Z. Wu, Y. Du, J. Liu, Q. Yao, T. Chen, Y. Cao, H. Zhang and J. Xie, Aurophilic Interactions in the Self-Assembly of Gold Nanoclusters into Nanoribbons with Enhanced Luminescence, Angew. Chem., Int. Ed., 2019, 58, 8139–8144 CrossRef CAS PubMed.
- Q. Yao, L. Liu, S. Malola, M. Ge, H. Xu, Z. Wu, T. Chen, Y. Cao, M. F. Matus, An. Pihlajamäki, Y. Han, H. Häkkinen and J. Xie, Supercrystal Engineering of Atomically Precise Gold Nanoparticles Promoted by Surface Dynamics, Nat. Chem., 2023, 15, 230–239 CrossRef CAS PubMed.
- T. Chen, S. Yang, J. Chai, Y. Song, J. Fan, B. Rao, H. Sheng, H. Yu and M. Zhu, Crystallization-Induced Emission Enhancement: A Novel Fluorescent Au-Ag Bimetallic Nanocluster with Precise Atomic Structure, Sci. Adv., 2017, 3, e1700956 CrossRef PubMed.
- Q. Yao, M. Zhu, Z. Yang, X. Song, X. Yuan, Z. Zhang, W. Hu and J. Xie, Molecule-Like Synthesis of Ligand-Protected Metal Nanoclusters, Nat. Rev. Mater., 2024 DOI:10.1038/s41578-024-00741-7.
- H. Liu, W. Ye, Y. Mu, H. Ma, A. Lv, S. Han, H. Shi, J. Li, Z. An, G. Wang and W. Huang, Highly Efficient Blue Phosphorescence from Pillar-Layer MOFs by Ligand Functionalization, Adv. Mater., 2022, 34, e2107612 CrossRef PubMed.
- M. Y. Zheng, Z. B. Jin, Z. Z. Ma, Z. G. Gu and J. Zhang, Photo–Curable 3D Printing of Circularly Polarized Afterglow Metal–Organic Framework Monoliths, Adv. Mater., 2024, 36, 2313749 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: additional material synthesis, experimental methods, data analysis, and supporting tables and figures. CCDC 2352112. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi02655a |
| This journal is © the Partner Organisations 2025 |