Open Access Article
Open Access ArticlePoly(propene-co-norbornene)s with high molar masses and tunable norbornene contents and properties, obtained in high yields using ketimide-modified half-titanocene catalysts†
Simona
Losio
 a,
Laura
Boggioni
a,
Laura
Boggioni
 a,
Adriano
Vignali
a,
Adriano
Vignali
 a,
Fabio
Bertini
a,
Fabio
Bertini
 a,
Akito
Nishiyama
b,
Kotohiro
Nomura
a,
Akito
Nishiyama
b,
Kotohiro
Nomura
 *b and
Incoronata
Tritto
*b and
Incoronata
Tritto
 *a
*a
aIstituto di Scienze e Tecnologie Chimiche “G. Natta”, Consiglio Nazionale delle Ricerche, Via A. Corti, 12, 20133 Milano, Italy. E-mail: incoronata.tritto@scitec.cnr.it
bDepartment of Chemistry, Tokyo Metropolitan University, 1-1 Minami Osawa, Hachioiji, Tokyo 192-0397, Japan. E-mail: knomura@tmu.ac.jp
First published on 15th July 2025
Abstract
The ketimide-modified half-titanocene catalysts, Cp′TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) [Cp′ = C5H5 (1), C5Me5 (2), Me3SiC5H4 (3)], afford high molecular weight copolymers of propene (P) and norbornene (N) with efficient N incorporation in the presence of MAO. The poly(P-co-N)s obtained were studied in detail by determining the microstructure and comonomer contents using 13C-NMR spectra, molar masses, and thermal and mechanical properties. The ketimide Cp′TiCl2(N
CtBu2) [Cp′ = C5H5 (1), C5Me5 (2), Me3SiC5H4 (3)], afford high molecular weight copolymers of propene (P) and norbornene (N) with efficient N incorporation in the presence of MAO. The poly(P-co-N)s obtained were studied in detail by determining the microstructure and comonomer contents using 13C-NMR spectra, molar masses, and thermal and mechanical properties. The ketimide Cp′TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) catalysts 1 and 3 exhibited higher catalytic activities and yielded the copolymers with higher N contents and molar masses than the ketimide-modified Cp*TiCl2(N
CtBu2) catalysts 1 and 3 exhibited higher catalytic activities and yielded the copolymers with higher N contents and molar masses than the ketimide-modified Cp*TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (2); the resultant copolymer using catalyst 1 contained 61.7 mol% of N and Mw equal to 657 kg mol−1 possessing uniform composition. The copolymers show very different tensile behaviours passing from ductile and tough materials to stiff and fragile ones as the N content increases.
CtBu2) (2); the resultant copolymer using catalyst 1 contained 61.7 mol% of N and Mw equal to 657 kg mol−1 possessing uniform composition. The copolymers show very different tensile behaviours passing from ductile and tough materials to stiff and fragile ones as the N content increases.
Introduction
Polyolefins, accounting for more than 50% of the global commercialized synthetic polymers, are very important industrial materials, and the transition metal catalyzed polymerisation is the core technology for the production. The synthesis of new polyolefin materials with specific functions and properties, such as toughness, barrier properties, miscibility, and rheological properties, thus providing expanded applications, is very difficult even using conventional catalysts, such as metallocenes,1–3 and constrained geometry (linked half-metallocene) catalysts,4,5 but is still of great interest. The progress in nonbridged half-metallocenes6 and so-called non-metallocene type7–11 catalysts offers several new possibilities for the development of new materials. Copolymerisation is unsurpassed for the direct synthesis of polymers with modified physical and mechanical properties. Design of novel catalysts allows us to vary the comonomers incorporated, comonomer ratio and copolymer microstructure and to modulate the desired thermal and mechanical properties.1–19Cyclic olefin copolymers (COCs), such as those synthesized by copolymerisation of ethylene with cyclic olefins, are highly interesting materials with high transparency, thermal resistance, low water absorption, dimensional stability etc. The synthesis of ethylene (E) and norbornene (N) copolymers using ansa-metallocene-based catalysts was first achieved by Kaminsky et al.,20–23 and later, was studied in detail by both academic and industrial groups.24–34 These ethylene/norbornene (E/N) copolymers obtained using metallocene catalysts are commercialized as TOPAS® and are amorphous thermoplastic materials with a broad range of promising properties such as high glass transition temperatures (Tg), excellent humidity barriers, remarkable biocompatibility, thermoformability, stiffness, dead-fold characteristics, and chemical inertness;35–37 these materials are applied in optics, packaging (especially food and medical products), capacitor films, and medical and diagnostic containers.
It is possible to enhance the mechanical and/or other physical properties of COCs for further expanding their applications by the introduction of a comonomer different from ethylene into the polymer backbone. Synthesis and microstructural studies of propene (P) and norbornene (N) copolymers with C2-symmetric metallocenes and MAO as a cocatalyst were performed by Tritto et al.38–43 These studies demonstrated that the copolymers possess rather low Tg values, due to their low molar mass and a significant degree of propene 1,3-misinsertions. Shiono et al. succeeded in the synthesis of P/N copolymers with high norbornene contents up to 71 mol% by using a linked half-titanocene catalyst, (tBuNSiMe2Flu)TiMe2, and Me3Al-free methylaluminoxane (dried MAO) as a cocatalyst.44,45 Recently, investigations on the synthesis of new copolymers of cyclic olefins with propene or higher olefins have received more attention because of expanded properties suitable for new applications and markets.46,47
Ketimide- and phenoxide-modified half-titanocenes, Cp′TiX2(Y) (Cp′ = cyclopentadienyl group, Y = phenoxide, ketimide), are known to efficiently catalyse, especially the syntheses of new COCs.31,32 In particular, these catalysts, Cp′TiCl2(O-2,6-iPr2C6H3) or Cp′TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2), are potentially active for ethylene copolymerisations with sterically hindered olefins or cyclic olefins.48 The ketimide-modified half-titanocenes, Cp'TiCl2(N
CtBu2), are potentially active for ethylene copolymerisations with sterically hindered olefins or cyclic olefins.48 The ketimide-modified half-titanocenes, Cp'TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2), showed high catalytic activity with efficient N incorporation not only in the copolymerisation of N with ethylene32 and α-olefins,49 but also in the terpolymerisation of ethylene and norbornene with 1-octene,48 affording high molecular mass polymers with uniform molecular mass distribution and compositions with various monomer ratios.
CtBu2), showed high catalytic activity with efficient N incorporation not only in the copolymerisation of N with ethylene32 and α-olefins,49 but also in the terpolymerisation of ethylene and norbornene with 1-octene,48 affording high molecular mass polymers with uniform molecular mass distribution and compositions with various monomer ratios.
Recently, we demonstrated that the ketimide-modified half-titanocene catalysts, Cp′TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (Cp′ = Cp, Me3SiC5H4), are highly active for propene polymerisation in the presence of MAO (4 bar, 40 °C). The resultant atactic and highly regioregular polypropenes (aPP) possess high molecular mass, Mw up to 1400 kg mol−1, and the high molar mass amorphous aPP displays properties like high-performance thermoplastic elastomers with exceptionally high ductility and tensile strain at break.50
CtBu2) (Cp′ = Cp, Me3SiC5H4), are highly active for propene polymerisation in the presence of MAO (4 bar, 40 °C). The resultant atactic and highly regioregular polypropenes (aPP) possess high molecular mass, Mw up to 1400 kg mol−1, and the high molar mass amorphous aPP displays properties like high-performance thermoplastic elastomers with exceptionally high ductility and tensile strain at break.50
Thus, we thought to explore the P/N copolymerisation with these half-titanocenes in order to achieve transparent poly(P-co-N)s with variable glass transition temperatures (Tg), high molecular weight (Mw) and possible elastomeric properties. Copolymerisation reactions were performed by using half-titanocene based precursors shown in Scheme 1 activated with a methylaluminoxane (MAO) cocatalyst. The influence of comonomer (norbornene) concentration, at relatively high polymerisation pressure and temperature, on polymerisation behaviour was investigated. The norbornene/propene molar ratio in the feed was maintained at a rather low level in order to observe possible differences in copolymer microstructures. Polymer analysis included determination of the microstructure, comonomer content, molar mass, and thermal and mechanical properties. We demonstrated that these half-titanocene catalysts, unlike ansa-metallocenes, enable the synthesis of poly(P-co-N)s with both high molar masses and yields, and to adjust P and N contents in copolymers and consequently Tg values from 10 to 218 °C. Poly(P-co-N)s with low N content up to 15 mol% are transparent and show a typical elastomeric behaviour with a low initial modulus and a gradual increase in the slope of stress–strain curves and a large amount of immediate strain recovery.
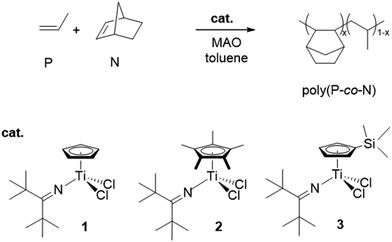 | ||
| Scheme 1 Copolymerisation of propene (P) and norbornene (N) with half-titanocene-based catalyst precursors studied. | ||
Results and discussion
Synthesis and characterization of poly(propene-co-norbornene)s
Selected results of the copolymerisation reactions of propene (P) and norbornene (N) by means of MAO-activated half-titanocene catalysts, [CpTiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (1),12 Cp*TiCl2N
CtBu2) (1),12 Cp*TiCl2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2 (2),12 and (Me3SiC5H4)TiCl2(N
CtBu2 (2),12 and (Me3SiC5H4)TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (3)50,51], are summarized in Table 1. The catalyst precursors for this study have been chosen as representative half-titanocenes which allow one to prepare ethylene–norbornene-1-octene terpolymers with high 1-octene contents, molar masses, and variable Tg values, in high yields48 and atactic high molar mass polypropenes having remarkably high ductility and tensile strain.50
CtBu2) (3)50,51], are summarized in Table 1. The catalyst precursors for this study have been chosen as representative half-titanocenes which allow one to prepare ethylene–norbornene-1-octene terpolymers with high 1-octene contents, molar masses, and variable Tg values, in high yields48 and atactic high molar mass polypropenes having remarkably high ductility and tensile strain.50
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (1), Cp*TiCl2N
CtBu2) (1), Cp*TiCl2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2 (2), and (Me3SiC5H4)TiCl2(N
CtBu2 (2), and (Me3SiC5H4)TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (3)—MAO catalytic systemsa
CtBu2) (3)—MAO catalytic systemsa
| Entry | Catalyst | P/N | Time (h) | Yield (g) | Activity (kg molTi−1 h−1) | N (mol%) | N conv. |
T
g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (°C) c (°C) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (kg mol−1) d (kg mol−1) |
M
w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (kg mol−1) d (kg mol−1) |
Đ |
P
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Polymerisation conditions: catalyst = 10 μmol, [MAO]/[Ti] = 2500, propene pressure = 4.0 bar, solvent = toluene, total volume of solution = 100 mL, temperature = 40 °C. b Determined by 13C-NMR spectra in C2D2Cl4 at 103 °C with HMDS as a reference. c Determined by DSC analysis. d Determined by SEC analysis. e Number-average degree of polymerisation calculated according to ref. 52. f Catalyst = 5 μmol, [MAO]/[Ti] = 5000. g n.d. = not determined. | ||||||||||||
| 1 | 1 | — | 0.25 | 6.08 | 2433 | — | — | 1 | 814 | 1404 | 1.7 | — |
| 2f | 1/0.25 | 0.17 | 2.01 | 1613 | 27.8 | 16.3 | 87 | 331 | 716 | 2.2 | 5865 | |
| 3 | 1/0.5 | 0.5 | 8.03 | 1606 | 32.1 | 37.6 | 117 | 181 | 650 | 3.0 | 3081 | |
| 4f | 1/0.5 | 0.17 | 5.13 | 6153 | 37.3 | 28.1 | 124 | 353 | 717 | 2.0 | 5747 | |
| 5 | 1/1 | 0.5 | 14.22 | 2843 | 44.0 | 46.0 | 162 | 290 | 537 | 1.8 | 4466 | |
| 6f | 1/2 | 0.5 | 11.38 | 4554 | 61.7 | 26.3 | 218 | 357 | 657 | 1.8 | 4820 | |
| 7 | 2 | — | 0.25 | 11.20 | 4480 | — | — | 2 | 315 | 831 | 2.6 | |
| 8 | 1/0.25 | 0.17 | 3.28 | 1966 | 6.0 | 5.6 | 10 | 236 | 584 | 2.5 | 5235 | |
| 9 | 1/0.5 | 1 | 4.49 | 449 | 13.7 | 8.8 | 21 | 189 | 354 | 1.9 | 3835 | |
| 10 | 1/1 | 0.5 | 2.09 | 417.6 | 17.7 | 2.7 | 40 | 124 | 222 | 1.8 | 2424 | |
| 11 | 1/2 | 1 | 0.010 | 0.99 | n.d.g | n.d.g | n.d.g | 63 | 360 | 5.7 | n.d.g | |
| 12 | 3 | — | 0.25 | 9.16 | 3663 | — | — | 1 | 832 | 1240 | 1.5 | — |
| 13 | 1/0.25 | 0.25 | 4.84 | 1938 | 14.1 | 19.7 | 24 | 178 | 539 | 3.0 | 3603 | |
| 14 | 1/0.5 | 0.5 | 7.75 | 1549 | 24.5 | 27.6 | 73 | 309 | 812 | 2.6 | 5632 | |
| 15 | 1/1 | 0.5 | 3.90 | 778 | 36.6 | 10.4 | 117 | 352 | 566 | 1.6 | 5760 | |
| 16f | 1/2 | 0.5 | 13.91 | 5563 | 45.3 | 22.2 | 177 | 380 | 719 | 1.9 | 5794 | |
The [N]/[P] molar ratio was varied to prepare the copolymers with different N contents that thus possess variable glass transition temperatures and possibly high molar mass (Mw). The copolymerisations were conducted in toluene with various feed compositions at 40 °C under a constant propene pressure of 4.0 bar, that is, under the conditions that gave the best results in propene homopolymerisation.50 Their microstructures and monomer contents were evaluated by means of 13C-NMR spectroscopy. The molar masses and Tg values were measured by SEC and DSC, respectively. In this table, entries that gave too high norbornene conversion, due to high activity, are included, since they are instructive about catalyst behaviour in these copolymerisations. The results of P homopolymerisation under identical conditions are also placed for comparison. Table 1 also lists Pn that is the number-average degree of polymerisation and can be described according to ref. 52.
The catalytic activities of the P/N copolymerisation by the ketimide modified half-titanocene catalysts, Cp′TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (1, 2, and 3), were clearly affected by the substituent on the cyclopentadienyl ligand used, as observed in homopolymerisation.
CtBu2) (1, 2, and 3), were clearly affected by the substituent on the cyclopentadienyl ligand used, as observed in homopolymerisation.
Unsubstituted CpTiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (1) is the most active catalyst in the P/N copolymerisation among the catalysts employed (1–3), whereas the permethylated catalyst 2, which is the most active one in the propene homopolymerisation, is the least active in the P/N copolymerisation. Indeed, this catalyst exhibited the lowest activities and Pns (the number-average degrees of polymerisation) with the lowest N incorporation.
CtBu2) (1) is the most active catalyst in the P/N copolymerisation among the catalysts employed (1–3), whereas the permethylated catalyst 2, which is the most active one in the propene homopolymerisation, is the least active in the P/N copolymerisation. Indeed, this catalyst exhibited the lowest activities and Pns (the number-average degrees of polymerisation) with the lowest N incorporation.
A comparison of the entries shows that Pns by catalyst 1 tend to slightly decrease, those by catalyst 3 increase upon increasing the N concentration in the feed, while those by the permethylated catalyst 2 decrease significantly.
A comparison of Pn values provides a clear overview of the catalyst performance in the P/N copolymerisation by using these catalysts, and the values by catalysts 1 and 3 (e.g., entries 5 and 15) were higher than those by catalyst 2 (entry 10, N 17.7 mol%) prepared with the same P/N molar ratio.
It thus seems likely that the activities were related to the capability to incorporate N in the copolymer chain, since catalysts 1 and 3 showed the highest activities with the most efficient N incorporation. Therefore, the N content in the resultant copolymers prepared with Cp′TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) under the same conditions follows the order 1 > 3 ≫ 2, suggesting that the steric hindrance of Cp′ plays a role.
CtBu2) under the same conditions follows the order 1 > 3 ≫ 2, suggesting that the steric hindrance of Cp′ plays a role.
It was revealed that the molar mass distributions (Đ values) of copolymers are narrow, and the molar masses of the resultant copolymers are lower than those of homopolymers for all the catalysts; in the copolymers prepared using catalyst 2 the Mn value clearly decreased with increasing N concentration in the feed.
Interestingly, the molar masses of poly(P-co-N)s with high N contents obtained using catalysts 1 and 3 are very high, and the Mn values in copolymers prepared using 3 are higher than in those prepared using 1. This along with the difficulty to detect the signals of chain end groups in the 1H-NMR spectra (see ESI†) confirm that catalysts 1–3 limit β-H elimination, when an α-olefin is the last inserted monomer.
Microstructure analysis of poly(propene-co-norbornene)s
Poly(P-co-N)s were analyzed by 13C-NMR spectroscopy to evaluate the monomer content and their microstructure. Some of us dedicated much effort in assigning the signals of 13C-NMR spectra of poly(P-co-N), synthesized using metallocene based catalysts.38–43 Poly(P-co-N)s were studied less than poly(E-co-N)s not only because of the high complexity of the spectra, but also because the metallocene-based catalysts show poor ability for copolymerisation of norbornene with propene.38–45,53,54However, it was possible to give a first assignment of signals of an isotactic alternating poly(P-co-N), with N content around 40–45 mol%, synthesized with a rac-Et(Ind)2ZrCl2–MAO catalytic system: methylenes C6 and C5 signals at 30.10 and 27.34 ppm, in sequence, methylene C7 at 31.91 ppm, methines C1 and C4 at 37.17 and 41.32 ppm, in sequence, whereas C3 and C2 signals are at 45.40 and 53.32 ppm, respectively.43 In addition, the methyl Pβ carbon atom was assigned to the resonance at 21.24 ppm. Subsequently, Boggioni et al., by an analytical procedure which uses the observed areas of the assigned signals of 13C-NMR spectra, determined the molar fractions of the sequences defining the microstructure of a poly(P-co-N) at the triad level; all the potential propene insertions (P12, P21, and P13) were considered, although different types of tacticities have been disregarded. This allowed us to achieve new assignments of 13C-NMR spectra.42,43
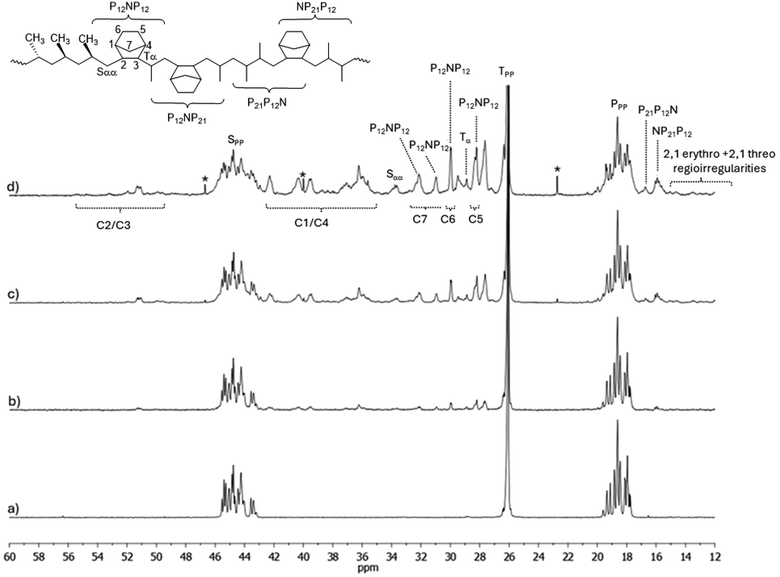
Starting from these assignments, we will attempt to understand the spectra of poly(P-co-N)s synthesized with the ketimide Cp*TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) catalyst 2, the one that allows the lowest N incorporation. Fig. 1 shows the 13C-NMR spectra of polypropene and poly(P-co-N)s prepared at different P/N molar ratios with catalyst 2. A general structure of copolymers, along with the carbon atom labelling based on the common nomenclature,38 and some assignments are also included in the figure. In detail, P, S, and T denote primary, secondary, and tertiary propene carbons, respectively. Greek letters denote the distance of a given carbon from the closest tertiary norbornene carbon.
CtBu2) catalyst 2, the one that allows the lowest N incorporation. Fig. 1 shows the 13C-NMR spectra of polypropene and poly(P-co-N)s prepared at different P/N molar ratios with catalyst 2. A general structure of copolymers, along with the carbon atom labelling based on the common nomenclature,38 and some assignments are also included in the figure. In detail, P, S, and T denote primary, secondary, and tertiary propene carbons, respectively. Greek letters denote the distance of a given carbon from the closest tertiary norbornene carbon.
In the spectra of the copolymers, by increasing the N content in the copolymer, new signals in the region between 14 and 17 ppm appear, which are related to regioirregularities of propene insertion. In particular, two broad signals centred at 15.73 and 16.72 ppm have been assigned to the methyl carbon atom of the central monomer in NP21P12 and P21P12N, respectively.42 This observation seems to indicate that with this catalyst after a norbornene insertion a 2,1 propene insertion occurs. This may explain the strong decrease in polymerisation activity by increasing N concentration in the feed with this catalyst. This catalyst behaviour resembles that of the C2-symmetric rac-Me2Si(2-MeInd)2ZrCl2.42
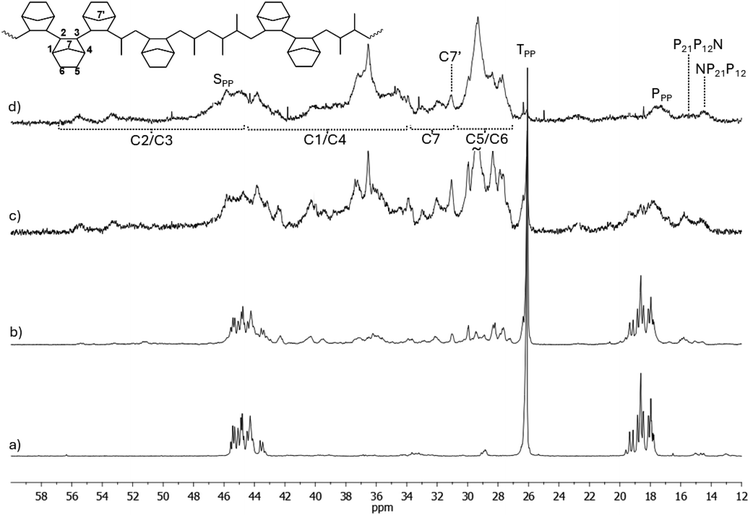
The 13C-NMR spectra of poly(P-co-N) with different P/N molar ratios prepared using catalysts CpTiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (1) and (Me3SiC5H4)TiCl2(N
CtBu2) (1) and (Me3SiC5H4)TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (3) are displayed for comparison in Fig. 2 and 3.
CtBu2) (3) are displayed for comparison in Fig. 2 and 3.
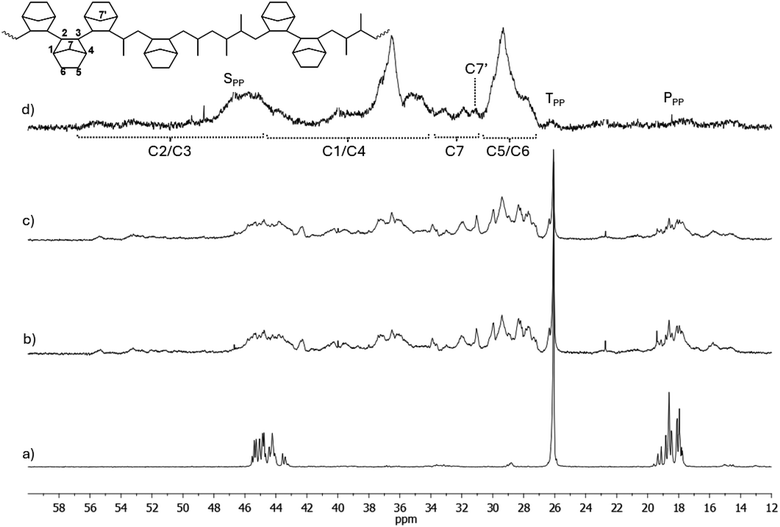 | ||
Fig. 2
13C-NMR spectra of (a) polypropene (entry 1) and poly(P-co-N)s of (b) entry 2 (N = 27.8 mol%), (c) entry 3 (N = 32.1 mol%) and (d) entry 6 (N = 61.7 mol%) obtained using CpTiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (1). CtBu2) (1). | ||
In each figure, the 13C-NMR spectra of the polypropenes prepared with the same catalyst are shown as well. All polypropenes, as already reported, are atactic with a prevalence of rrrr pentads.
Moreover, the homopolymers prepared using catalysts 1 and 3 contain also some regioirregular erythro and threo 2,1 insertions, signals in the region between 15.34 and 14.03 ppm (erythro) and a broad signal centered at 13.01 ppm (threo).
The spectra of copolymers synthesized with catalysts 1 and 3 in Fig. 2 and 3, respectively, have very similar patterns. In the spectra of these copolymers, new methyl signals at 14.73 and 15.80 ppm, 16.79 ppm, and 20.70 and 22.80 ppm appear, and these signals and the methyl signals of the rrrr centered pentad increase with increasing N content. The signals at 16.79 and 15.80 ppm could be due to regioerrors P21P12N and NP21P12 close to N, and differences in chemical shifts may be due to different tacticities. Shiono assigned signals appearing between 12.0 and 17.1 ppm (in our scale) to a Pβ in a NNNPN block, where syndiotacticity prevails both in propene and norbornene blocks.45 Thus, it seems that these catalysts allow the incorporation of regioerrors, as seen from the presence of regioerrors in the spectra of the homopolymers, as well as the incorporation of norbornene after a regioerror, as testified by the high activity of these catalysts (P-co-N) in polymerisation. The methyl signals at 20.70 and 22.80 ppm may be due to Pγγ signals, which result from different tacticities of vicinal P or N units or block □NP12□, where □ could be a P or N unit. The patterns of spectra in Fig. 2d and 3d differ clearly from the others because of N contents of 61.7 and 45.3 mol%, respectively. Indeed, signals of N diads or triads become dominant: indeed, in accordance with literature,45 it is possible to highlight the resonances of the C5 and C6 carbons in the range of 27.1–31.0 ppm, and the signals at about 35.8–41.4 ppm and 43–53 ppm, which were tentatively assigned to C1/C4 and C2/C3 carbons, respectively. The resonances of the methylene C7 carbon appear in the region between 30.8 and 35.4 ppm, in particular the signal observed at 31.0 ppm can be attributed to the C7′ carbon of the norbornene unit close to a propene unit, in accordance with the results from Shiono's work.45
Thermal properties
As already reported, all polypropenes prepared using these half titanocene catalysts are soft solids, elastomeric materials, soluble in hydrocarbons and chlorinated solvents.50 DSC analysis did not show melting events; the measured glass transitions are in the temperature range from 1 to 2 °C.Poly(P-co-N)s with N contents up to 14 mol% are rubbery materials, and those with higher N contents appear as a white powder. DSC thermal characterization of copolymers synthesized with catalysts 1, 2, and 3 revealed single well-defined glass transition temperatures (Fig. 4S†), thus indicating a homogeneous copolymer microstructure with a fully amorphous morphology. Glass transition temperatures varied from 10 °C for the copolymer with the lowest N content prepared with catalyst 2 (entry 8) to 218 °C for the copolymer with the highest N content synthesized with catalyst 1 (entry 6). In Fig. 4, the plots between Tg values of the synthesized copolymers and the N contents calculated from 13C-NMR spectra are shown and compared to those obtained by Shiono with (tBuNSiMe2Flu)TiMe2.45
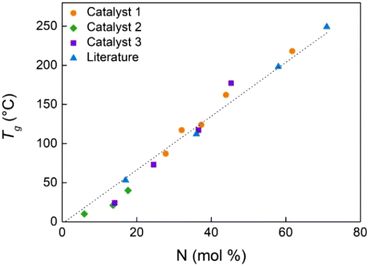 | ||
| Fig. 4 Plot of glass transition temperature (Tg) vs. norbornene content in poly(P-co-N)s from Table 1 and the literature.45 The dotted fitting line interpolates all the points on the graph. | ||
As expected for random copolymers with similar microstructures, Tg values of (P-co-N)s increase linearly with norbornene content. In general, the Tg values of polymers from 2 are the lowest, because of the low N contents ranging from 6 to 18 mol%, while the Tg of the copolymers obtained using the other two ketimide catalysts 1 and 3 can reach very high values due to the high content of the N unit incorporated in the polymer chain.
Mechanical properties and recyclability
We have recently demonstrated that the amorphous polypropenes, prepared using catalysts 1–3 due to their high molar mass, are high-performance thermoplastic elastomers with extraordinarily high ductility, good toughness and a tensile strain at break higher than 2000%, in line with those reported in the literature for amorphous high molecular weight polypropylene samples.55,56 Thus, we have evaluated the influence of norbornene content and molar masses on the mechanical properties of the poly(P-co-N)s synthesized, performing uniaxial tensile and cyclic stress–strain tests. Table 2 summarizes data on the mechanical properties of polypropenes and some selected copolymer samples in Table 1. It was revealed that the introduction in the polymer chain of rigid N units, with the resulting enhancement in Tg, implies noticeably higher Young's moduli (E) and maximum tensile strength (σmax) in poly(P-co-N). The opposite result is observed for the elongation at break (ε): this parameter exhibits a huge decrease and copolymers with N contents higher than 24 mol% break at very low elongation values (about 3%); consequently, a scarce fracture toughness (UT) was detected.| Entry | Catalyst | N (mol%) | M n (kg mol−1) | E (MPa) |
σ
max![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (MPa) a (MPa) |
ε (%) |
U
T![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (MJ m−3) a (MJ m−3) |
|---|---|---|---|---|---|---|---|
| a E: Young's modulus, σmax: tensile strength, ε: tensile strain, and UT: toughness determined by tensile measurements performed at 30 mm min−1. b Recycled sample. | |||||||
| 1 | 1 | — | 814 | 2.5 ± 0.3 | 1.05 ± 0.10 | >2000 | >11 |
| 3 | 32.1 | 181 | 1380 ± 73 | 33.38 ± 3.40 | 3.3 ± 0.3 | 0.63 ± 0.08 | |
| 7 | 2 | — | 315 | 3.1 ± 0.3 | 0.78 ± 0.03 | >2000 | >7 |
| 8 | 6.0 | 236 | 8.9 ± 1.8 | 1.13 ± 0.03 | >2000 | >19 | |
| 9 | 13.7 | 189 | 16.2 ± 2.5 | 5.25 ± 0.21 | 1136 ± 46 | 22.58 ± 2.18 | |
| 10 | 17.7 | 124 | 768 ± 20 | 14.74 ± 1.38 | 65 ± 19 | 5.20 ± 1.03 | |
| 12 | 3 | — | 832 | 4.1 ± 0.1 | 1.24 ± 0.08 | >2000 | >17 |
| 13 | 14.1 | 178 | 142 ± 4 | 8.85 ± 0.47 | 892 ± 27 | 36.69 ± 1.11 | |
| 14 | 24.5 | 309 | 1345 ± 63 | 31.71 ± 2.15 | 2.7 ± 0.4 | 0.58 ± 0.10 | |
| 12rb | — | 832 | 3.4 ± 0.3 | 1.13 ± 0.05 | >2000 | >12 | |
| 13rb | 14.1 | 178 | 187 ± 5 | 9.59 ± 1.37 | 877 ± 8 | 38.41 ± 2.84 | |
| 14rb | 24.5 | 309 | 1330 ± 76 | 28.21 ± 1.87 | 2.4 ± 0.1 | 0.36 ± 0.03 | |
The stress–strain curves in the uniaxial tensile test of some representative copolymers and polypropenes from catalyst 2 are depicted in Fig. 5. Entry 8 with the lowest N content (6 mol%) exhibits a tensile behaviour comparable to that of polypropenes with a low modulus and a very high elongation, typical properties of a tough and highly ductile material. The stress–strain curve of entry 9 with an N content of about 13.7 mol% and a Tg value of 21 °C first shows a linear elastic behaviour and, then, a gradual stress increase with increasing strain, with an elongation at break at 1136% and a high fracture toughness (23 MJ m−3). Very interestingly, a further small increase in N content in the polymer chain strongly modifies the tensile behaviour of the copolymer.
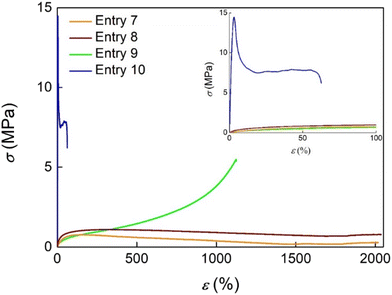 | ||
| Fig. 5 Stress–strain curve in the uniaxial tensile test of polypropene and poly(P-co-N)s using the half-titanocene catalyst 2. | ||
In fact, entry 10 with an N content of about 18 mol% and a Tg value of 40 °C displays a sharp stress maximum at low strain, ascribed to the yield point; after this point a decrease in the stress with a limited enhancement of elongation up to 65% was observed. The transition from uniform stretching peculiar of elastomers to the localized yielding characteristic of thermoplastics took place at an N content close to 15%.
Such abrupt mechanical property variations could be potentially related to mechanisms such as chain entanglement or phase separation. Since entries 8–10, obtained with catalyst 2, exhibit low conversions of norbornene and copolymers have homogeneous composition, phase separation is not probable.
It is likely that by increasing the norbornene content in the chain due to the rigidity of the norbornene moiety, at parity of copolymer microstructure and molecular weight, entanglements are more difficult to be formed. Therefore, the norbornene content remains the dominant factor influencing the variations observed in the mechanical properties.
Similar tensile behaviour was observed for materials prepared with catalysts 1 and 3, so that the presence of the cyclic unit in the polymer chain induces enhancement of strength and rigidity and a decrease of ductility (Fig. 5S†). High values of the Young's modulus (1350–1400 MPa) and maximum tensile strength (31–33 MPa) are achieved for the copolymer samples of entry 14 and entry 3, according to their high content of N units (25–32 mol%). Regarding entry 13 with an intermediate content of N units (14.1 mol%), a strain hardening behaviour accompanied by a very high fracture toughness (37 MJ m−3) was observed.
The elasticity of the materials, namely, the capability to return to the initial shape after the load is removed, was measured from step cycle tensile tests by extending the samples to 300% strain over 10 cycles (Fig. 6). The polypropene and the (P-co-N)s obtained using catalyst 2 exhibit the main amount of unrecovered strain after the 1st cycle with only a small enhancement in the unrecovered strain on each following cycle (Fig. 6a). Polypropene and the copolymer sample of entry 8 show an outstanding elastic recovery over the whole cyclic test, that is, 93 and 92% at the end of the first cycle, and 86 and 84% after being cyclically stretched at 300% strain 10 times, respectively (Fig. 6b). The copolymer with an N content equal to 13.7 mol% (entry 9) exhibits a higher extent of irreversible deformation after the first cycle with SR equal to 85% and a good elastic recovery of 78% after the last cycle (Fig. 6b). Overall, the tensile measurements showed that these random copolymers behave as thermoplastic elastomers with properties close to those reported for ABA block copolymers constituted of polynorbornene as a hard segment and atactic polypropene as a soft segment.57
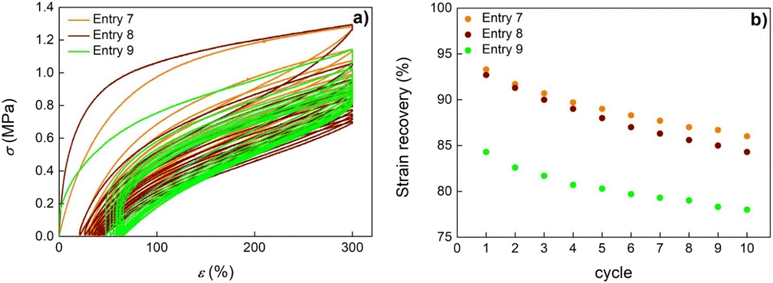 | ||
| Fig. 6 Stress–strain curve in the hysteresis test (a) and (b) strain recovery as a function of the cycle number of polypropene and selected (P-co-N)s by the half-titanocene catalyst. | ||
The progressive enhancement in norbornene content determines very different strain recovery behaviour for the (P-co-N)s. In fact, the copolymer sample of entry 13 with an N content equal to 14.1 mol% recovers not much under standard conditions (39 and 24% after the first and tenth cycles, respectively) but at a low unload rate (10 mm min−1), hence giving it time to recover; the strain recovery is 55 and 40% after the first and last cycle, respectively. The presence of rigid units in the polymer chain delays the relaxation dynamics and the material thus exhibits a long-term elastic recovery.
The reprocessability of the materials prepared with catalyst 3 was evaluated by repeated compression moulding of the broken specimens at the processing parameters of the first preparation (see the Experimental section in the ESI†), to produce recycled films with a thickness of about 200 μm. To assess the mechanical recyclability, the reshaped specimens underwent uniaxial stretching until failure. The stress–strain curves and tensile properties of the pristine and reprocessed materials are reported in Fig. 6S† and Table 2, respectively. The stress–strain curves of the recycled samples almost overlap with the pristine ones; thus, mechanical recycling is an option as the tensile properties are unaltered.
Optical properties
Optically transparent polymers with high refractive indices are well-known for their requirements in various optical applications, including lenses, touch screens, reflection films, and other components used in mobile devices, displays, and optical assemblies. Cyclic olefin copolymers stand out in this context as key optical materials57,58 and thus the optical transmittance of selected copolymers listed in Table 1 was analyzed using UV–vis spectrometry across the visible light wavelength range of 400–800 nm (Fig. 7). Since these materials are amorphous, thin films of poly(P-co-N)s with comparable thicknesses of 20–30 μm and norbornene content up to 32 mol% display high optical transmittance, reaching approximately 85–93%. This outcome highlights the excellent transparency of these copolymers, making them highly valuable for optical applications.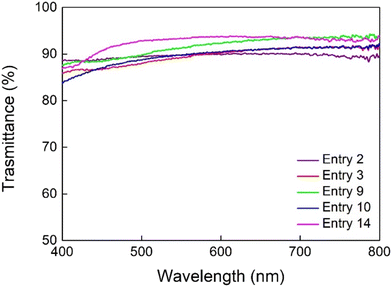 | ||
| Fig. 7 Optical transmittance of selected poly(P-co-N) from Table 1. | ||
Conclusions
Investigation of propene (P) copolymerisation with norbornene (N) using ketimide-modified half-titanocene precatalysts, Cp′TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) (1–3), provided insight into the factors that regulate the copolymerisation with these precatalysts. Activities of copolymerisation by the ketimide modified Cp′TiCl2(N
CtBu2) (1–3), provided insight into the factors that regulate the copolymerisation with these precatalysts. Activities of copolymerisation by the ketimide modified Cp′TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) are much higher than those by ansa-metallocene catalytic systems. The steric hindrance of catalyst ligands influences copolymerisation activities; Cp*TiCl2(N
CtBu2) are much higher than those by ansa-metallocene catalytic systems. The steric hindrance of catalyst ligands influences copolymerisation activities; Cp*TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) 2 is the least active among the ketimide modified catalysts studied. Analysis of the microstructures of propene homopolymers and poly(P-co-N)s reveals that these polymers are mainly atactic, while those by Cp′TiCl2(N
CtBu2) 2 is the least active among the ketimide modified catalysts studied. Analysis of the microstructures of propene homopolymers and poly(P-co-N)s reveals that these polymers are mainly atactic, while those by Cp′TiCl2(N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CtBu2) are slightly more syndiotactic. The high molar masses and low visibility of poly(P-co-N) signals of chain end groups in the 1H-NMR spectra confirmed the ability of catalysts 1–3 to prevent β-H elimination, when an α-olefin is the last inserted monomer.
CtBu2) are slightly more syndiotactic. The high molar masses and low visibility of poly(P-co-N) signals of chain end groups in the 1H-NMR spectra confirmed the ability of catalysts 1–3 to prevent β-H elimination, when an α-olefin is the last inserted monomer.
It is noteworthy that catalysts 1 and 3 display promising N incorporation, affording poly(P-co-N)s with much higher N incorporation and molar mass than those obtained under similar conditions with ansa-metallocenes; catalyst 1 gave the high molar mass poly(P-co-N) with 61.7 mol% of N and Mw equal to 657 kg mol−1 (Table 1, entry 6). DSC thermal analysis showed Tg values ranging between 10 °C and 218 °C and a linear relationship between the Tg values and N content of copolymers synthesized using each catalyst was demonstrated. For copolymers with low N contents up to 15 mol%, a typical elastomeric behaviour is found with a low Young's modulus, a progressive enhancement in the slope of stress–strain curves and a high extent of immediate strain recovery.
Finally, this behaviour, demonstrated by these catalysts (especially by 1 and 3), such as highly efficient N incorporation to afford high molar mass poly(P-co-N)s, is unique and highly promising, and is apparently different from that by well-developed ansa-metallocenes, important catalysts for α-olefin and norbornene copolymerisation. It seems that it is the more open structure of these half titanocenes that allows such unique behaviour. This fact is important for further materials design and design of efficient molecular catalysts.
Author contributions
Simona Losio: investigation, data curation, and writing—original draft preparation; Laura Boggioni: investigation and data curation; Adriano Vignali: investigation and data curation; Fabio Bertini: data curation and writing—original draft preparation; Akito Nishiyama: investigation and data curation; Kotohiro Nomura: conceptualization and validation, supervision, and funding acquisition; Incoronata Tritto: conceptualization and validation, writing—original draft preparation, supervision, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the ESI.† Data for this article are available.Acknowledgements
The project by KN was partly supported by Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS, Grant No. 21H01942). The authors thank Ms Fulvia Greco and Mr Daniele Piovani for their valuable cooperation in NMR and SEC analyses.References
- W. Kaminsky, R. Engehausen and J. A. Kopf, Tailor-Made Metallocene for the Copolymerization of Ethene with Bulky Cycloalkenes, Angew. Chem., Int. Ed. Engl., 1995, 34, 2273–2275, DOI:10.1002/anie.199522731.
- W. Kaminsky, New polymers by metallocene catalysis, Macromol. Chem. Phys., 1996, 197, 3907–3945, DOI:10.1002/macp.1996.021971201.
- W. Kaminsky and M. Arndt, Metallocenes for polymer catalysis, in Polymer Synthesis/Polymer Catalysis, Advances in Polymer Science, Springer Berlin, Heidelberg, 1997, vol. 127, pp. 143–187. DOI:10.1007/BFb0103631.
- A. L. McKnight and R. M. Waymouth, Group 4 ansa-Cyclopentadienyl-Amido Catalysts for Olefin Polymerization, Chem. Rev., 1998, 98, 2587–2598, DOI:10.1021/cr940442r.
- H. Braunschweig and F. M. Breitling, Constrained geometry complexes—Synthesis and applications, Coord. Chem. Rev., 2006, 250, 2691–2720, DOI:10.1016/j.ccr.2005.10.022.
- K. Nomura, Half-titanocenes containing anionic ancillary donor ligands as promising new catalysts for precise olefin polymerization, Dalton Trans., 2009, 41, 8811–8823, 10.1039/B910407K.
- G. J. P. Britovsek, V. C. Gibson and D. F. Wass, The Search for New-Generation Olefin Polymerization Catalysts: Life beyond Metallocenes, Angew. Chem., Int. Ed., 1999, 38, 428–447, DOI:10.1002/(SICI)1521-3773(19990215)38:4<428::AID-ANIE428>3.0.CO;2-3.
- P. D. Bolton and P. Mountford, Transition metal imido compounds as Ziegler-Natta olefin polymerisation catalysts, Adv. Synth. Catal., 2005, 347, 355–366, DOI:10.1002/adsc.200404267.
- H. Makio, H. Terao, A. Iwashita and T. Fujita, FI Catalysts for olefin polymerization—A comprehensive treatment, Chem. Rev., 2011, 111, 2363–2449, DOI:10.1021/cr100294r.
- J. A. Gladysz, Frontiers in Metal-Catalyzed Polymerization: Designer Metallocenes, Designs on New Monomers, Demystifying MAO, Metathesis Déshabillé, Chem. Rev., 2000, 100, 1167–1168, DOI:10.1021/cr000450+.
- H. G. Alt, Metallocene complexes as catalysts for olefin polymerization, Coord. Chem. Rev., 2006, 250, 1–272, DOI:10.1016/j.ccr.2005.05.022.
- K. Nomura, K. Fujita and M. Fujiki, Olefin polymerization by (cyclopentadienyl)(ketimide)-titanium(IV) complexes of the type, J. Mol. Catal. A: Chem., 2004, 220, 133–144, DOI:10.1016/j.molcata.2004.06.010.
- C. Claver and B. Milani, Metal-catalysed Polymerisation, Dalton Trans., 2009, 41, 8769–9076, 10.1039/B918816A.
- B. Liu, M. Terano, V. Busico, W.-Y. Wong and Y. Tang, Metal-catalyzed polymerization of olefins, J. Organomet. Chem., 2015, 798, 291–436 CrossRef CAS.
- G. J. Domski, J. M. Rose, G. W. Coates, A. D. Bolig and M. Brookhart, Living alkene polymerization: New methods for the precision synthesis of polyolefins, Prog. Polym. Sci., 2007, 32, 30–92, DOI:10.1016/j.progpolymsci.2006.11.001.
- M. Delferro and T. J. Marks, Multinuclear olefin polymerization catalysts, Chem. Rev., 2011, 111, 2450–2485, DOI:10.1021/cr1003634.
- J. P. McInnis, M. Delferro and T. J. Marks, Multinuclear group 4 catalysis: Olefin polymerization pathways modified by strong metal–metal cooperative effects, Acc. Chem. Res., 2014, 47, 2545–2557, DOI:10.1021/ar5001633.
- A. Valente, A. Mortreux, M. Visseaux and P. Zinck, Coordinative Chain Transfer Polymerization, Chem. Rev., 2013, 113, 3836–3857, DOI:10.1021/cr300289z.
- H. Harakawa, S. Patamma, A. C. Boccia, L. Boggioni, D. R. Ferro, S. Losio, K. Nomura and I. Tritto, Ethylene Copolymerization with 4-Methyl-cyclohexene or 1-Methylcyclopentene by Half-Titanocene Catalysts: Effect of Ligands and Microstructural Analysis of the Copolymers, Macromolecules, 2018, 51, 853–863, DOI:10.1021/acs.macromol.7b02484.
- W. Kaminsky and A. Noll, Copolymerization of norbornene and ethene with homogeneous zirconocenes/methylaluminoxane catalysts, Polym. Bull., 1993, 31, 175–182, DOI:10.1007/BF00329963.
- W. Kaminsky, A. Bark and M. Arndt, New polymers by homogeneous zirconocene aluminoxane catalysts, Makromol. Chem., Macromol. Symp., 1991, 47, 83–93, DOI:10.1002/masy.19910470108.
- M. Arndt and W. Kaminsky, Microstructure of poly(cycloolefins) produced by metallocene methylaluminoxane (mao) catalysts, Macromol. Symp., 1995, 97, 225–246, DOI:10.1002/masy.19950970123.
- W. Kaminsky and M. Arndt-Rosenau, Homo- and Copolymerization of Cycloolefins by Metallocene Catalysts, in Metallocene-based Polyolefins: Preparation, Properties, and Technology, ed. J. Scheirs and W. Kaminsky, Wiley, 2000, vol. 2, pp. 91–113, and references therein Search PubMed.
- D. Ruchatz and G. Fink, Ethene-Norbornene Copolymerization Using Homogenous Metallocene and Half-Sandwich Catalysts: Kinetics and Relationships between Catalyst Structure and Polymer Structure. 2. Comparative Study of Different Metallocene- and Half-Sandwich Methylaluminoxane Catalysts and Analysis of the Copolymers by 13C Nuclear Magnetic Resonance Spectroscopy, Macromolecules, 1998, 31, 4674–4680, DOI:10.1021/ma971042j.
- M. Arndt-Rosenau and I. Beulich, Microstructure of ethene/norbornene copolymers, Macromolecules, 1999, 32, 7335–7343, DOI:10.1021/ma981813z.
- M. Arndt and I. Beulich, C1-symmetric metallocenes for olefin polymerisation, 1. Catalytic performance of [Me2C(3-tertBuCp)(Flu)]ZrCl2 in ethene/norbornene copolymerisation, Macromol. Chem. Phys., 1998, 199, 1221–1232, DOI:10.1002/(SICI)1521-3935(19980601)199:6<1221::AID-MACP1221>3.0.CO;2-2.
- I. Tritto, L. Boggioni and D. R. Ferro, Metallocene catalyzed ethene- and propene co-norbornene polymerization: Mechanisms from a detailed microstructural analysis, Coord. Chem. Rev., 2006, 250, 212–241, DOI:10.1016/j.ccr.2005.06.019.
- L. Boggioni and I. Tritto, State of the art of cyclic olefin polymers, MRS Bull., 2013, 38, 245–251, DOI:10.1557/mrs.2013.53.
- A. Provasoli, D. R. Ferro, I. Tritto and L. Boggioni, The Conformational Characteristics of Ethylene-Norbornene Copolymers and Their Influence on the 13C Spectra, Macromolecules, 1999, 32, 6697–6706, DOI:10.1021/ma990739x.
- I. Tritto, C. Marestin, L. Boggioni, L. Zetta, A. Provasoli and D. R. Ferro, Ethylene-Norbornene Copolymer Microstructure. Assessment and Advances Based on Assignments of 13C NMR Spectra, Macromolecules, 2000, 33, 8931–8944, DOI:10.1021/ma000795u.
- K. Nomura, M. Tsubota and M. Fujiki, Efficient Ethylene/Norbornene Copolymerization by (Aryloxo)(indenyl)titanium(IV) Complexes – MAO Catalyst System, Macromolecules, 2003, 36, 3797–3799, DOI:10.1021/ma034215f.
- K. Nomura, W. Wang, M. Fujiki and J. Liu, Notable norbornene (NBE) incorporation in ethylene–NBE copolymerization catalysed by nonbridged half-titanocenes: better correlation between NBE incorporation and coordination energy, Chem. Commun., 2006, 25, 2659–2661, 10.1039/B605005K.
- X. Li and Z. Hou, Organometallic catalysts for copolymerization of cyclic olefins, Coord. Chem. Rev., 2008, 252, 1842–1869, 10.1039/B605005K.
- H. Terao, A. Iwashita, S. Ishii, H. Tanaka, Y. Yoshida, M. Mitani and T. Fujita, Ethylene/norbornene copolymerization behavior of bis(phenoxy-imine)Ti complexes combined with MAO, Macromolecules, 2009, 42, 4359–4361, DOI:10.1021/ma900890b.
- TOPAS Advanced COC Polymers | TOPAS, Home page, https://www.topas.com.
- M.-J. Brekner, F. Osan, J. Rohrmann and M. Antberg, Process for the preparation of chemically homogeneous cycloolefin copolymers, U.S. Patent5324801, 1994 Search PubMed.
- Mitsui Chemicals, Home page. https://jp.mitsuichemicals.com/.
- L. Boggioni, F. Bertini, G. Zannoni, I. Tritto, P. Carbone, M. Ragazzi and D. R. Ferro, Propene-Norbornene Copolymers: Synthesis and Analysis of Polymer Structure by 13C NMR Spectroscopy and ab Initio Chemical Shift Computations, Macromolecules, 2003, 36, 882–890, DOI:10.1021/ma0212487.
- L. Boggioni, I. Tritto, M. Ragazzi, P. Carbone and D. R. Ferro, Propene-Norbornene Copolymers: Synthesis and Microstructure, Macromol. Symp., 2004, 218, 39–50, DOI:10.1002/masy.200451405.
- P. Carbone, M. Ragazzi, I. Tritto, L. Boggioni and D. R. Ferro, Ab Initio Molecular Modeling of 13C NMR Chemical Shifts of Polymers. 2. Propene-Norbornene Copolymers, Macromolecules, 2003, 36, 891–899, DOI:10.1021/ma021247e.
- L. Boggioni, C. Zampa, A. Ravasio, D. R. Ferro and I. Tritto, Propene-Norbornene Copolymers by C2-symmetric Metallocene rac-Et(Ind)2ZrCl2: Influence of Reaction Conditions on Reactivity and Copolymer Properties, Macromolecules, 2008, 41, 5107–5115, DOI:10.1021/ma8004235.
- L. Boggioni, A. Ravasio, C. Zampa, D. R. Ferro and I. Tritto, Penultimate Effects and Chain Epimerization in Propene-Norbornene Copolymers by rac-Me2Si(2-Me-Ind)2ZrCl2C2-Symmetric Metallocene, Macromolecules, 2010, 43, 4532–4542, DOI:10.1021/ma1002672.
- L. Boggioni, A. Ravasio, A. C. Boccia, D. R. Ferro and I. Tritto, Propene-Norbornene Copolymers. Toward a Description of Microstructure at Triad Level Based on Assignments of 13C NMR Spectra, Macromolecules, 2010, 43, 4543–4556, DOI:10.1021/ma100268h.
- T. Hasan, K. Nishii, T. Shiono and T. Ikeda, Living Polymerization of Norbornene via Vinyl Addition with ansa-Fluorenylamidodimethyltitanium Complex, Macromolecules, 2002, 35, 8933–8935, DOI:10.1021/ma025586j.
- T. Hasan, T. Ikeda and T. Shiono, Random Copolymerization of Propene and Norbornene with ansa-Fluorenylamidodimethyltitanium-Based Catalysts, Macromolecules, 2005, 38, 1071–1074, DOI:10.1021/ma048117l.
- W. Wang, S. Z. Qu, X. W. Li, J. Chen, Z. F. Guo and W. H. Sun, Transition metal complex catalysts promoting copolymers of cycloolefin with propylene/higher olefins, Coord. Chem. Rev., 2023, 494, 215351, DOI:10.1016/j.ccr.2023.215351.
- B. Qu, Y. Zhao, P. Song, Y. Gao, Y.-Y. Zhou, X.-L. Sun, Q. Fu, X. Ning, J. Li and Y. Tang, Norbornene Monomer Effects and Mechanistic Insights in Binuclear Nickel-Catalyzed Olefin Chain Walking Copolymerizations, ACS Catal., 2023, 13, 12849–12858, DOI:10.1021/acscatal.3c02702.
- L. Boggioni, H. Harakawa, S. Losio, K. Nomura and I. Tritto, Synthesis of ethylene-norbornene-1-octene terpolymers with high 1-octene contents, molar masses, and tunable Tg values, in high yields using half-titanocene catalysts, Polym. Chem., 2021, 12, 4372–4383, 10.1039/D1PY00647A.
- W. Zhao and K. Nomura, Copolymerizations of Norbornene and Tetracyclododecene with α-Olefins by Half-Titanocene Catalysts: Efficient Synthesis of Highly Transparent, Thermal Resistance Polymers, Macromolecules, 2016, 49, 59–70, DOI:10.1021/acs.macromol.5b02176.
- S. Losio, F. Bertini, A. Vignali, T. Fujioka, K. Nomura and I. Tritto, Amorphous Elastomeric Ultra-High Molar Mass Polypropylene in High Yield by Half-Titanocene Catalysts, Polymers, 2024, 16, 512, DOI:10.3390/polym16040512.
- M. Kawatsu, T. Fujioka, S. Losio, I. Tritto and K. Nomura, Trialkylsilyl-cyclopentadienyl)titanium(IV) Dichloride Complexes Containing Ketimide Ligands, Cp'TiCl2(N=Ct,Bu2) (Cp’ = Me3SiC5H4, Et3SiC5H4), as Efficient Catalysts for Ethylene Copolymerisations with Norbornene and Tetracyclododecene, Catal. Sci. Technol., 2025, 15, 2757–2765, 10.1039/d5cy00160a.
- J. C. Jansen, R. Mendichi, M. C. Sacchi and I. Tritto, Kinetic Studies of the Copolymerization of Ethylene with Norbornene by ansa-Zirconocene/Methylaluminoxane Catalysts: Evidence of a Long-Lasting “Quasi-Living” Initial Period, Macromol. Chem. Phys., 2003, 204, 522–530, DOI:10.1002/macp.200390017.
- W. Kaminsky, S. Derlin and M. Hoff, Copolymerization of propylene and norbornene with different metallocene catalysts, Polymer, 2007, 48, 7271–7278, DOI:10.1016/j.polymer.2007.10.013.
- V. N. Dougnac, B. C. Peoples, F. M. Rabagliati, G. B. Galland and R. Quijada, Study on the copolymerization of propylene with norbornene using metallocene catalysts, Polym. Bull., 2012, 69, 925–935, DOI:10.1007/s00289-012-0771-5.
- L. A. Rishina, Y. V. Kissin, S. S. Lalayan, V. G. Krasheninnikov, E. O. Perepelitsina and T. I. Medintseva, A novel efficient Ti(O-iso,-C3H7)4-based olefin polymerization catalyst system, Polym. Sci., Ser. B, 2016, 58, 152–162, DOI:10.1134/S1560090416020056.
- C. G. Collins Rice, J.-C. Buffet, Z. R. Turner and D. O'Hare, Efficient synthesis of thermoplastic elastomeric amorphous ultra-high molecular weight atactic polypropylene (UHMWaPP), Polym. Chem., 2022, 13, 5597–5603, 10.1039/D2PY00708H.
- X. Song, L. Cao, R. Tanaka, T. Shiono and Z. Cai, Optically Transparent Functional Polyolefin Elastomer with Excellent Mechanical and Thermal Properties, ACS Macro Lett., 2019, 8, 299–303, DOI:10.1021/acsmacrolett.9b00005.
- Y. Zhao, L. Cui, Y. Zhang and Z. Jian, All-Hydrocarbon High-Refractive-Index Cyclic Olefin Copolymer Optical Materials, Macromolecules, 2024, 57, 8869–8876, DOI:10.1021/acs.macromol.4c01426.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the Experimental section, chain end analysis and additional 1H NMR spectra, DSC thermograms, and stress–strain curves. See DOI: https://doi.org/10.1039/d5py00549c |
| This journal is © The Royal Society of Chemistry 2025 |