Innovative synthesis of PPC-P-co-PLA multi-block copolymers via one-pot copolymerization and transesterification catalysed by alkyl boron and diverse Lewis bases†
Mei
Meng
ab,
Min
Xiao
b,
Jintao
Wang
a,
Peiyuan
Li
a,
Xianli
Wu
 *a and
Yuezhong
Meng
*a and
Yuezhong
Meng
 *abc
*abc
aCollege of Chemistry, Zhengzhou University, Zhengzhou 450001, China. E-mail: wuxianli@zzu.edu.cn; mengyzh@mail.sysu.edu.cn
bThe Key Laboratory of Low-carbon Chemistry & Energy Conservation of Guangdong Province/State Key Laboratory of Optoelectronic Materials and Technologies, School of Materials Science and Engineering, Sun Yat-sen University, Guangzhou 510275, China
cInstitute of Chemistry, Henan Academy of Sciences, Zhengzhou 450000, China
First published on 20th May 2025
Abstract
Carbon dioxide (CO2) has been utilized for synthesizing biodegradable polymers to promote sustainable energy conservation and mitigate emissions. Nonetheless, the low glass transition temperature of amorphous CO2-based polyester–polycarbonate (PPC-P) significantly impedes its practical application. Herein, semi-crystalline copolymers, PPC-P-co-PLA, were successfully synthesized via copolymerization of propylene oxide (PO), phthalic anhydride (PA), and CO2 in combination with PLA through transesterification catalysed by Lewis acid–base pairs in a one-pot/one-step method. Metal-free catalysts were utilized for the first time to catalyse both ring-opening copolymerization and transesterification reactions for copolymer synthesis. The mechanism study of PLA transesterification catalysed by triethyl borane (TEB)/Lewis bases revealed that effective depolymerization and transesterification of PLA hinge on α-H chemical shift alterations induced by the Lewis bases. Lewis acid–base pairs facilitate the formation of PPC-P-co-PLA multi-block copolymers by terminating one end of PPC-P with PLA segments via continuous transesterification reactions. Compared to PPC-P, the copolymers exhibit an increase in glass transition temperature by 5–7 °C and an elevation in thermal decomposition temperature by 18–45 °C. The optimal mechanical and rheological properties of PPC-P-co-PLA multi-block copolymers are achieved at a PLA concentration of 8 wt%. This study opens new avenues for the synthesis of semi-crystalline polyester–polycarbonate-based copolymers with high glass transition temperatures, thereby enriching the theoretical foundation for the synthesis of block copolymers.
1. Introduction
Employing carbon dioxide (CO2) as a raw material for the synthesis of biodegradable polymers promotes energy savings, reduces emissions, and effectively addresses environmental concerns arising from single-use plastics.1–4 The utilization of CO2-based biodegradable polyesters and polycarbonates as raw materials for the preparation of eco-friendly polymers has proven to be a highly effective and promising strategy, particularly following the seminal work on alternating copolymerization of CO2 and epoxides to produce polycarbonates, published in 1969.5 Considerable research has been conducted to enhance the selectivity and productivity of polycarbonate synthesis. Various catalytic systems have been investigated, including metal complex catalysts, organic base cocatalysts and metal-free catalysts. Many metal complex catalysts are employed in ring-opening copolymerization (ROCOP) of epoxides and CO2 such as zinc-based composites5–9 and Zn–Co double metal cyanide complexes.10,11 Homogeneous catalytic systems mainly consist of metal complexes and often necessitate the inclusion of organic bases, such as ammonium salts and amines, as cocatalysts.12–15 Metal-free catalysts based on alkyl boron are undergoing rapid development, enabling their complete removal in acidified ethanol to produce non-toxic, colorless polycarbonates without the necessity for multistep catalyst/ligand synthesis.16–21 Furthermore, diverse monomers, such as epoxides, anhydrides, and lactones, have been used to synthesize polycarbonates and their copolymers with enhanced properties, ultimately broadening their potential applications.22–25 For instance, the ROCOP of propylene oxide (PO), phthalic anhydride (PA) and CO2 led to the synthesis of polyester–polycarbonate (PPC-P) exhibiting exceptional strength and thermal stability.26,27 This was attributed to the Lewis acid–base pairs formed through the coordination of chloride (Cl−) to boron. PPC-P exhibits excellent performance and biodegradability, with a degradation rate surpassing that of PBAT in a 58 ± 2 °C composting test.26 The glass transition temperature of 41 °C (PE 27%) makes PPC-P susceptible to deformation during transportation under high-temperature conditions, thereby limiting its potential applications. Incorporating semi-crystalline polymers to PPC-P could improve its thermal stability and performance.Polylactide (PLA), also referred to as poly(lactic acid), is a biodegradable, semi-crystalline polyester derived from renewable resources. It exhibits high strength, transparency, and non-toxicity. PLA exhibits biodegradability and disintegration under actual composting conditions, with minimal impact on the composting process.28 PLA has found widespread applications in various fields, including packaging, disposable straws, textiles, biomedicine, and architecture.29 Copolymerization is a key technique for synthesizing high-performance polymers. Several kinds of copolymers incorporating PLA segments have been synthesized through the ROCOP process using lactide, a subtype of lactone, as the feedstock.30–32 Lactide also serves as a common monomer for synthesizing CO2-based copolymers, which results in semi-crystalline degradable polymers and enhances the thermal properties of CO2-based polycarbonates.33–35 Both metal-based and metal-free catalysts have been attempted for the preparation of polycarbonates and PLA copolymers via copolymerization of lactide, CO2 and epoxides, such as PO,33,34 cyclohexene oxide,36 and citronellyl glycidyl ether.35 A semicrystalline copolymer of poly(propylene carbonate) (PPC) and poly(L-lactide) (PLLA) with a high molecular weight (Mn 127.0 kg mol−1) was synthesized via simultaneous feeding of PO and L-lactide utilizing zinc adipate as the catalyst.37 A crystallization melting peak was observed in the PPC-co-PLLA copolymer when the molar content of lactide exceeded 12%, with a melting temperature (Tm) of 164.5 °C. The highest molecular weight PPC-co-PLLA copolymer reported to date (Mn 698.0 kg mol−1) was synthesized using a heterogeneous ternary catalyst system comprising SalenCoIII, zinc glutarate and PPNCl.38 The catalyst system exhibited intermolecular collaboration between cobalt and zinc, resulting in enhanced catalytic activity. Metal-free catalysts, comprised of Lewis acid–base pairs formed by triethylboron (TEB) and Lewis bases, facilitated the ROCOP of PO, CO2 and lactide. A one-pot/one-step strategy was employed using these catalysts to prepare low-molecular-weight PPCLAs (Mn < 15 kg mol−1). In the synthesis of PLA copolymers through ROCOP of lactide, PLA transesterification acts as a side reaction that considerably affects the molecular weight of the resultant copolymer. Meanwhile, a one-pot/two-step strategy was utilized, involving the initial synthesis of PPC followed by the addition of lactide to form PLA chains, yielding high-molecular-weight PLA-co-PPC (Mn 46.5 kg mol−1).34 Triblock copolymers of PLA-b-PPC-b-PLA can be synthesized using TEB and difunctional initiators, exhibiting a transition from brittle to ductile to elastomeric properties. The synthesis process involves the initial preparation of PPC, followed by the addition of lactide to form PLA segments, with diphenylurea serving as a hydrogen-bonding activator.33 The incorporation of high-strength semi-crystalline PLA copolymers offers dual advantages: preserving the benefits of PPC-P while enabling the creation of novel semi-crystalline copolymers.
Block copolymers can be synthesized via a transesterification approach. PLA-PCL-PLA triblock copolymers were synthesized using a sequential transesterification process. Specifically, a macroinitiator HO-PCL-OH, terminated with α,ω-hydroxyl groups, was first obtained from PCL. This macroinitiator then underwent transesterification with PLA in the presence of 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) and diols.39 The transesterification is primarily governed by the interaction between alcohols and carboxyl groups, with diols39 or triols40 as the transesterification reagent. Catalysed by appropriate agents, the ester groups in polyesters and polyols facilitate the cleavage of polyester chains, resulting in the formation of hydroxyl-terminated polyesters. These intermediates then undergo further reactions with additional polyesters, ultimately resulting in the formation of block copolymers through transesterification. Our study demonstrates, for the first time, the successful transesterification of PLA without the need for additional diols, marking a significant advancement in the transesterification process of polyester materials.
Semi-crystalline PPC-P-co-PLA multi-block copolymers were successfully synthesized via transesterification, leveraging commercial high-modulus PLA as the raw material. The inclusion of PLA markedly enhanced the thermal properties of the material with 18–45 °C higher decomposition temperature than that of PPC-P. The one-pot/one-step approach was utilized to carry out ROCOP of PO, PA and CO2 in conjunction with the transesterification of PLA (ROCOP-T), wherein the reactants were simultaneously fed into the reaction system utilizing metal-free catalysts. The successful synthesis of PPC-P-co-PLA multiblock copolymers requires a balance between the copolymerization reaction rate and the rates of PLA depolymerization and transesterification. Multiple factors can affect the initiation of ROCOP-T, including the selection of Lewis acid–base pairs, ion porosity, deprotonation processes, and solvent effects. In this study, we investigated the transesterification reaction mechanism of ester groups catalysed by metal-free organic catalysts, with a focus on the open-loop processes involved in PLA chemical recycling. Specifically, we emphasized the partial depolymerization of PLA and its subsequent incorporation into PPC-P-co-PLA copolymers, rather than its conversion to LA. Utilizing PLA transesterification for copolymer preparation has important implications for the reuse and recycling of PLA, offering novel insights into polyester recycling and utilization.
2. Experimental materials and methods
2.1 Materials
Polylactide (PLA; Macklin, ∼80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da) was dried at 80 °C for 96 hours in a blast air oven. Propylene oxide (PO; Energy, 99%) was stirred and refluxed for 24 h over CaH2 and then distilled at constant pressure prior to use. 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU; TCI, 98%) was stirred and refluxed for 24 h over CaH2 and then distilled by reduced-pressure distillation. High-purity CO2 (Guangqi Gas Co. Ltd, Guangzhou, China, >99.9999%), triethyl borane (TEB; Energy, 1 M in THF), bis(triphenylphosphine)iminium chloride (PPNCl; Alfa, 97%), triethylamine (TEA; Aladdin, ≥99.5%), benzyl alcohol (BnOH; TCI, 98%), 1,4-benzenedimethanol (BDM; Aladdin, 99%), 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD; J&K, 98%), dicyclohexylurea (U1; TCI, >98%), 4-dimethyl aminopyridine (DMAP; Alfa, 99%), benzyl triethyl ammonium chloride (TEBAC; TCI, 98%), tetrapropyl ammonium chloride (TPACl; J&K, >97%), tetrabutyl ammonium chloride (TBACl; Alfa, 97%), tetraamyl ammonium chloride (TPNAC; TCI, >98%), tetrabutylammonium bromide (TEABr; J&K, 99%), tetrabutyl phosphonium chloride (TBPC; Macklin, 96%), benzyl triphenyl phosphonium chloride (BPP; TCI, >98%) and tetraphenylphosphonium chloride (TTPP; J&K, 98.5%) were used as received.
000 Da) was dried at 80 °C for 96 hours in a blast air oven. Propylene oxide (PO; Energy, 99%) was stirred and refluxed for 24 h over CaH2 and then distilled at constant pressure prior to use. 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU; TCI, 98%) was stirred and refluxed for 24 h over CaH2 and then distilled by reduced-pressure distillation. High-purity CO2 (Guangqi Gas Co. Ltd, Guangzhou, China, >99.9999%), triethyl borane (TEB; Energy, 1 M in THF), bis(triphenylphosphine)iminium chloride (PPNCl; Alfa, 97%), triethylamine (TEA; Aladdin, ≥99.5%), benzyl alcohol (BnOH; TCI, 98%), 1,4-benzenedimethanol (BDM; Aladdin, 99%), 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD; J&K, 98%), dicyclohexylurea (U1; TCI, >98%), 4-dimethyl aminopyridine (DMAP; Alfa, 99%), benzyl triethyl ammonium chloride (TEBAC; TCI, 98%), tetrapropyl ammonium chloride (TPACl; J&K, >97%), tetrabutyl ammonium chloride (TBACl; Alfa, 97%), tetraamyl ammonium chloride (TPNAC; TCI, >98%), tetrabutylammonium bromide (TEABr; J&K, 99%), tetrabutyl phosphonium chloride (TBPC; Macklin, 96%), benzyl triphenyl phosphonium chloride (BPP; TCI, >98%) and tetraphenylphosphonium chloride (TTPP; J&K, 98.5%) were used as received.
2.2 Characterization
1H NMR and 13C NMR spectroscopy and diffusion-ordered NMR spectroscopy (DOSY) of PPC-P-co-PLA copolymers in deuterated chloroform (CDCl3) were performed on a Bruker DRX-400 MHz NMR spectrometer with tetramethylsilane (TMS) as an internal reference. Molecular weights were determined by Waters gel permeation chromatography (GPC) using tetrahydrofuran (THF) as the eluent (1 mL min−1 at 40 °C) and polystyrene (PS) as the standard. Fourier transform infrared spectroscopy (FT-IR) was conducted using a PerkinElmer Spectrum 100 system within the wavelength range of 4000 cm−1 to 500 cm−1. To enhance the visualization of variations in functional groups, we focused on the specific wavelength range of 2000 cm−1 to 500 cm−1.Thermogravimetric analysis (TGA) was performed using a PerkinElmer Pyris Diamond TG/DTA analyser under a nitrogen atmosphere (200 mL min−1) at a heating rate of 10 °C min−1 in the temperature range of 30 to 650 °C. Differential scanning calorimetry (DSC) measurements were carried out on a DSC Model 204 (Netzsch) under nitrogen. The samples (around 5–10 mg) were first heated at a heating rate of 10 °C min−1 from room temperature to 200 °C and then cooled at a cooling rate of 10 °C min−1 to −30 °C using liquid nitrogen, followed by a second heating at a heating rate of 10 °C min−1 in the temperature range of −30 to 200 °C. The crystalline melting temperature (Tm) was measured from the first heating cycle. The glass transition temperature (Tg) was measured from the second heating cycle. An X-ray diffractometer (XRD) model D/Max-IIIA, manufactured by Rigaku in Japan, was utilized to assess the crystallinity of the resulting polymer. This assessment employed Cu-Kα radiation, scanning at a rate of 1° min−1 within a 2θ range spanning from 5° to 40°.
Mechanical properties were assessed using a computer-controlled Instron tester (Model 5566) at 25 °C and 50% ± 5% relative humidity, conforming to the ASTM E104 standard. Each sample was tested using five dumbbell-shaped specimens measuring 25 mm × 4 mm × 1 mm, with average results reported. The stretching rate was set to 50 mm min−1. The rheological properties were characterized using a rotational rheometer (Haake MARS III, Thermo Fisher Scientific). Circular specimens, 1 mm thick and 25 mm in diameter, were prepared for rheological testing. The test parameters comprised a frequency range of 0.1–100 rad s−1, an amplitude of 1%, and a temperature of 170 °C.
2.3 The effect of PPNCl on PLA depolymerization
PLA (10 wt% or 5 wt%) and PPNCl were dissolved in PO at ratios ranging from 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 200
1 to 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (PO
1 (PO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PPNCl). The solution was then heated to 70 °C, cooled to room temperature, and subsequently analysed by 1H NMR and GPC.
PPNCl). The solution was then heated to 70 °C, cooled to room temperature, and subsequently analysed by 1H NMR and GPC.
2.4 The effect of initiators on PLA transesterification
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of PO
1 ratio of PO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEB
TEB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LB. The mixture was heated at 70 °C for 2 hours, cooled, and then re-analysed by 1H NMR.
LB. The mixture was heated at 70 °C for 2 hours, cooled, and then re-analysed by 1H NMR.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (PO
1 (PO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEB
TEB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LB). 1H NMR spectra were analysed after heating the solution at 70 °C for 2 hours.
LB). 1H NMR spectra were analysed after heating the solution at 70 °C for 2 hours.
2.5 Initiator activity for both PO/PA/CO2 copolymerization and PLA transesterification
PLA/PO homogeneous solution was prepared by heating 1.1 g of PLA in 12.6 g of PO at 70 °C for 1 hour, followed by cooling to room temperature. Within the glove box, PO, PA, TEB, and LB in a ratio of 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (PO
1 (PO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA
PA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEB
TEB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LB) and the PLA homogeneous solution were rapidly introduced into a dry reactor, which was then sealed tightly. The reactor was removed from the glove box and placed in a fume hood. Throughout the heating reaction at 65 °C, the CO2 pressure was maintained below 1 Megapascal. After continuous copolymerization and PLA transesterification, the reactor was cooled to room temperature, allowing CO2 to be released. A small aliquot of the mixture was then sampled for 1H NMR analysis. The crude product was dissolved in CH2Cl2, and the reaction was quenched using a 1 mol L−1 hydrochloric acid/ethanol solution. The polymer solution was precipitated by adding an 8-fold excess of ethanol. The product was dried in a blast drying oven at 60 °C for 24 hours, followed by vacuum drying at 80 °C for an additional 24 hours until constant weight was achieved. The mass of the weighed product is mproduct.
LB) and the PLA homogeneous solution were rapidly introduced into a dry reactor, which was then sealed tightly. The reactor was removed from the glove box and placed in a fume hood. Throughout the heating reaction at 65 °C, the CO2 pressure was maintained below 1 Megapascal. After continuous copolymerization and PLA transesterification, the reactor was cooled to room temperature, allowing CO2 to be released. A small aliquot of the mixture was then sampled for 1H NMR analysis. The crude product was dissolved in CH2Cl2, and the reaction was quenched using a 1 mol L−1 hydrochloric acid/ethanol solution. The polymer solution was precipitated by adding an 8-fold excess of ethanol. The product was dried in a blast drying oven at 60 °C for 24 hours, followed by vacuum drying at 80 °C for an additional 24 hours until constant weight was achieved. The mass of the weighed product is mproduct.
2.6 One-pot one-step synthesis of PPC-P-co-PLA
The specific reaction ratios utilizing PPNCl as the Lewis base are presented in Table 3. As detailed in entry 19 of Table 3, the crucial steps involved in synthesizing the copolymers are outlined below: A PLA/PO homogeneous solution was prepared by heating 2.2 g of PLA in 12.6 g of PO at 70 °C for 1 hour, and then cooled to room temperature. Inside the glove box, 108 μL of TEB, 31.2 mg of PPNCl, 4 g of PA, and the PLA/PO homogeneous solution were quickly transferred into a 50 ml reactor, which was then tightly sealed. The reactor was then removed from the glove box and placed in a fume hood. During the heating reaction at 65 °C, the CO2 pressure was maintained at 1 MPa. After washing and drying, the 1H NMR spectra, 13C NMR spectra, GPC, and FT-IR spectrum were analysed.2.7 Geometric structure optimization calculation methods
The geometries of organic bases (TPACl, TBACl, TPNAC, TBPC, and TBABr) interacting with PLA chain segments were optimized with the M06-2X density functional and the 6-21G basis set using Gaussian.41 To streamline the calculations, we focused solely on the impact of Lewis bases on a single PLA chain segment. Following the structural optimization of the TBACl–PLA interaction, the structural optimization calculation for the interaction between Lewis bases and PLA, including TBABr, TBPC, TPACl and TPNCl was conducted subsequently. The SMD implicit solvation model was used to evaluate the effect of PO as the solvent (eps = 16, epsinf = 1.9) on the distance between ion pairs, taking into account the solvation effect of PO. The carbon atom carrying the α-H of PLA exhibits stereoisomerism. To investigate the impact of stereoisomerism, we altered the positions of hydrogen and methyl groups on a specific carbon atom to optimize the geometry of PLLA (S,S) in complex with TBACl.3. Results and discussion
3.1 Mechanism of PLA depolymerization and transesterification by metal-free catalysts
In the presence of THF as a solvent, the addition of a Lewis base can also induce the depolymerization of PLA. The degree of PLA depolymerization is enhanced upon the addition of PO (Fig. S8†). The chemical shift (δ5.05–5.12) of hydrogen is observed exclusively with PO as the solvent, indicating that the interaction between PO and PPNCl preceded its action on PLA (Fig. S9b–e†). PLA depolymerization occurs through a nucleophilic substitution reaction under alkaline catalytic conditions. An incremental increase in PPNCl addition positively correlates with the proportion of rightward-shifted α-H, suggesting that the observed chemical shift variations are not attributable to PLA decomposition into lactide (δ5.06–5.14, Fig. S6 and S14g, h†) but rather result from interactions between the initiator and PLA (Fig. 1a and b). These interactions subsequently alter the chemical environment surrounding proximal hydrogen atoms. Over time, the molecular weights of the depolymerized ester exchange products decrease slightly. The peak shape in the GPC curves remained constant (Fig. S10†). The incorporation of a Lewis base and PO initiates the depolymerization of PLA. The length of depolymerized PLA segments is intimately linked to the quantity of the Lewis base. Subsequently, the newly formed anions are less likely to attack PLA segments further. This observation supports the subsequent use of PLA transesterification, a displacement reaction, for the preparation of copolymers.
After adding PPNCl, there were no significant changes in the chemical shift or peak shape of the α-H of PLA, but a large amount of PPO was produced. Our results suggest that TEB selectively binds to PO, and the addition of PPNCl initiates the ring-opening of PO, resulting in the formation of a substantial amount of PPO (Fig. S12b–d†). When TEB and PPNCl are combined, the GPC curves mimic those observed solely with PPNCl during the initial stage (0–1 h) (Fig. 2a). This implies that PLA depolymerization occurs in the initial reaction stage, facilitating copolymer synthesis in subsequent experiments. As the reaction progresses, the PPO content continues to increase (Fig. S13†). Following a 4-hour reaction, the depolymerization of PLA into various chain segment lengths was confirmed by the DOSY spectrum (Fig. 2b). Furthermore, the formation of PPO-co-PLA copolymers is apparent from the linkage between PPO and PLA (Fig. 2b and Fig. S12e, f†). The mere addition of a Lewis base promotes PLA depolymerization, whereas the inclusion of Lewis acid–base pairs enhances the formation of PPO-co-PLA copolymers through a displacement reaction.
Upon the concurrent addition of TEB and Lewis base systems to the homogeneous PLA/PO solution, DBU and TBD, two specific initiators, induced a rightward shift in the chemical shift of α-H in PLA (Fig. 3d). DBU binds to both oxygen anions and carbonyl groups of PLA via the alcohol activation pathway and nucleophilic attack pathway (Fig. S15†).47–50 The incorporation of TEB mainly influences the α-H in PLA positioned near oxygen anions, with minimal effect on the α-H attached to the carbonyl group adjacent to DBU or TBD. The chemical shift in the partial α-H of PLA, induced by DBU or TBD, is observable in Fig. 3b, 2c and d. Therefore, the mechanisms underlying PLA depolymerization and transesterification, initiated by ionic and non-ionic species, differ. During depolymerization, ionic Lewis bases aid in the splitting of PLA into two chains, which then associate with anions and cations, respectively. Non-ionic Lewis bases, exemplified by DBU, exhibit robust protonation and deprotonation capabilities, allowing them to interact with the carbonyl groups and oxygen anions of PLA after depolymerization.
By analyzing the hydrogen spectrum of α-H in the PLA 1H NMR spectra, preliminary predictions can be made regarding the potential occurrence of depolymerization and transesterification. Specific Lewis bases, including TEA, TEA/BnOH, and U1, do not induce a chemical shift towards the higher-frequency side of α-H in PLA, nor do they initiate PLA transesterification when used as initiators. The results support this hypothesis. Using TEA and TEA/BnOH as initiators can solely initiate ROCOP of PO, PA and CO2 to produce PPC-P/PLA blends without inducing PLA transesterification (Fig. 5b, Fig. S16† and Fig. 3e). U1, when used as an initiator, cannot initiate copolymerization or transesterification of PLA (Fig. 3e). Therefore, if a LB does not cause shifts in the chemical resonance of partial α-H in PLA, it indicates that PLA depolymerization has not taken place, implying that transesterification of PLA is improbable.
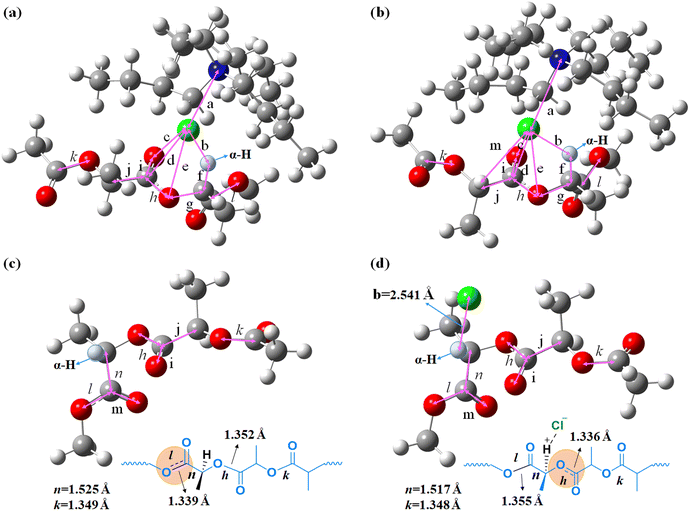
| Distance/Å | PLA | TPACl | TBACl | TPNCl | TBPC | TBABr | TBAClPO | PLLA | TBAClPLLA |
|---|---|---|---|---|---|---|---|---|---|
| PO: the SMD implicit solvation model employed to assess the influence of PO as the solvent (eps = 16, epsinf = 1.9). PLLA: M06-2X/6-21G optimized geometries of TBACl and the chain segment of PLLA (S,S).a The detailed data pertaining to the computational outcomes are presented in Data S1–S9.† | |||||||||
| a (anion⋯cation) | — | 3.9283 | 3.9568 | 3.9660 | 3.8496 | 4.0442 | 4.2229 | — | 3.9522 |
| b (anion⋯H) | — | 2.5748 | 2.5412 | 2.5374 | 2.5384 | 2.6092 | 2.7309 | — | 2.6387 |
| c (anion⋯O) | — | 3.6462 | 3.7701 | 3.8265 | 3.8251 | 3.8044 | 3.6433 | — | 3.5382 |
| d (anion⋯C) | — | 3.3732 | 3.5052 | 3.5466 | 3.5486 | 3.5116 | 3.3978 | — | 3.1613 |
| e (anion⋯O) | — | 3.3313 | 3.3647 | 3.3589 | 3.4039 | 3.3701 | 3.3900 | — | 3.2350 |
| f (C–H) | 1.0934 | 1.0934 | 1.0937 | 1.0934 | 1.0937 | 1.0933 | 1.0948 | 1.0935 | 1.0948 |
| g (C–O) | 1.4208 | 1.4240 | 1.4238 | 1.4246 | 1.4255 | 1.4239 | 1.4311 | 1.4197 | 1.4222 |
| h (O–C) | 1.3518 | 1.3363 | 1.3355 | 1.3344 | 1.3363 | 1.3353 | 1.3364 | 1.3532 | 1.3393 |
i (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
1.1998 | 1.2088 | 1.2083 | 1.2086 | 1.2078 | 1.2084 | 1.2096 | 1.1998 | 1.2085 |
| j (C–C) | 1.5176 | 1.5168 | 1.5148 | 1.5144 | 1.5159 | 1.5151 | 1.5169 | 1.5141 | 1.5122 |
| k (O–C) | 1.3492 | 1.3458 | 1.3482 | 1.3496 | 1.3471 | 1.3483 | 1.3458 | 1.3486 | 1.3479 |
| l (O–C) | 1.3386 | 1.3552 | 1.3544 | 1.3538 | 1.3537 | 1.3543 | 1.3316 | 1.3373 | 1.3549 |
m (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
1.2024 | 1.2009 | 1.2007 | 1.2008 | 1.2010 | 1.2009 | 1.2112 | 1.2036 | 1.2008 |
| n (C–C) | 1.5249 | 1.5181 | 1.5166 | 1.5167 | 1.5168 | 1.5149 | 1.5188 | 1.5254 | 1.5184 |
| m (anion⋯H) | — | — | — | — | — | — | — | — | 2.9110 |
An increase in steric hindrance, such as TPACl, TBACl, and TPNCl, leads to a progressive increase in the distance between chloride ions and the positive charge center and a decrease of the hydrogen bond formed between anions and the α-H of PLA (Table 1a and b). The difference in hydrogen bond distances between TBACl and TPNCl is negligible. Our experimental results align with the findings, suggesting that further elongation of LB's carbon chains is unlikely to yield beneficial outcomes in the synthesis of PPC-P-co-PLA multi-block copolymers (entries 10 and 11, Table 2). Ionic bonds are intrinsically related to the electrostatic attraction between anions and cations. Compared to TBACl, TBABr exhibits a larger distance between anions and cations, as well as weaker hydrogen bonding between anions and the α-H of PLA, due to the larger atomic radius of Br compared to Cl (Table 1a and b). The shorter distance between the anion of TBABr and the carbonyl group indicates a positional shift of the anion (Table 1c and d). The positional shift likely contributes to the weaker attraction of TBABr to the α-H of PLA compared to TBACl, resulting in the absence of the ester exchange reaction. Substituting the nitrogen (N) in TBACl with phosphorus (P) results in a shortened distance between anions and cations, enhanced hydrogen bonding between anions and the α-H of PLA, and increased distances between the anion and the carbonyl group in TBPC compared to TBACl. TBPC exhibits a stronger attraction to the α-H of PLA than TBACl (Table 1 a–d). An enlargement of the cation radius leads to reduced separation between anions and cations. Furthermore, this enlargement prompts anions to shift towards the α-H region of PLA. The close proximity of anions in the initiator to the α-H of PLA enhances transesterification, ultimately facilitating copolymer synthesis.
| Entry | Initiator | α-H chemical shifta | Product | m Product (g) | Yieldb (%) | Conv. PEc (%) | PEc (%) | PPCc (%) | PPOc (%) | PLAc (%) | CO2c (wt%) | Selectivityc (%) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (kg mol−1)
(kg mol−1) |
PDId | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h | +TEB2 h | ||||||||||||||
| a The results are sourced from Fig. 3b and c. b m reactant = 12.55 g(PO) + 1.10 g(PLA) + 4.00 g(PA) + CO2wt% × mProduct; copolymers: yield % = mProduct/mreactant × 100; blends: yield % = (mProduct − mPLA)/(mreactant − mPLA) × 10. c Calculated by 1H NMR; PE% = A1/4/(A1/4 + A4 + (A6,7)/3 + A8) × 100; PPC% = A4/(A1/4 + A4 + (A6,7)/3 + A8) × 100; PPO% = (A6,7)/3/(A1/4 + A4 + (A6,7)/3 + A8) × 100; PLA% = A8/(A1/4 + A4 + (A6,7)/3 + A8 + Ac) × 100; selectivity% = 100 − CC% = 100 − A9/(A2 + A4 + (A6,7)/3 + A8 + Ac). d Determined by GPC in tetrahydrofuran (THF). | |||||||||||||||
| 1 | TEA | NO | NO | PPC-P/PLA blends | 9.53 | 53.3 | 100 | 36.7 | 42.6 | 2.3 | 18.5 | 14.0 | 97.6 | 49.1 | 1.47 |
| 2 | TEA/BnOH | NO | NO | PPC-P/PLA blends | 7.24 | 42.3 | 100 | 52.2 | 27.0 | 1.4 | 19.4 | 7.89 | 97.6 | 28.8 | 1.50 |
| 3 | DBU | YES | YES | PPC-P-co-PLA/PLA blends | 8.17 | 43.4 | 100 | 35.8 | 44.0 | 0 | 20.3 | 14.5 | 100 | 24.6 | 1.38 |
| 4 | TBD | YES | NO | PE-co-PLA | 3.79 | 21.5 | 54.7 | 43.9 | 0 | 0.3 | 55.8 | 0 | 100 | 17.3 | 1.51 |
| 5 | U1 | NO | NO | — | 1.10 | — | 0 | 0 | 0 | 0 | 100 | 0 | 0 | — | — |
| 6 | DMAP | YES | NO | — | 0.93 | — | 0 | 0 | 0 | 0 | 100 | 0 | 0 | — | — |
| 7 | U1/DMAP/BDM | YES | NO | PE/PLA blends | 1.12 | — | 19.2 | 14.9 | 0 | 1.6 | 83.5 | 0 | — | — | — |
| 8 | TEBAC | YES | YES | PPC-P-co-PLA/PLA blends | 9.95 | 52.1 | 100 | 34.4 | 42.9 | 3.0 | 19.7 | 14.5 | 90.9 | 33.8 | 1.46 |
| 9 | TPACL | YES | YES | PPC-P-co-PLA | 8.68 | 45.6 | 100 | 32.7 | 46.5 | 1.4 | 19.3 | 15.8 | 95.2 | 39.1 | 1.59 |
| 10 | TBACl | YES | YES | PPC-P-co-PLA | 11.7 | 59.7 | 100 | 31.7 | 48.6 | 1.2 | 18.6 | 16.6 | 96.2 | 53.7 | 1.42 |
| 11 | TPNAC | YES | YES | PPC-P-co-PLA | 10.3 | 53.1 | 100 | 30.1 | 49.1 | 2.5 | 18.3 | 17.0 | 95.2 | 58.2 | 1.37 |
| 12 | TEABr | YES | NO | PPC-P/PLA blends | 8.38 | 45.2 | 100 | 42.2 | 32.9 | 1.5 | 23.4 | 10.5 | 90.7 | 30.3 | 1.41 |
| 13 | TBPC | YES | YES | PPC-P-co-PLA/PLA blends | 7.82 | 42.5 | 91.4 | 43.3 | 29.8 | 0.9 | 26.0 | 9.41 | 98.2 | 39.8 | 1.54 |
| 14 | BPP | YES | YES | PPC-P-co-PLA | 11.7 | 58.5 | 100 | 25.3 | 55.6 | 3.3 | 15.8 | 20.0 | 95.7 | 46.5 | 1.36 |
| 15 | TTPP | YES | YES | PPC-P-co-PLA | 10.7 | 55.8 | 100 | 32.1 | 41.1 | 1.2 | 25.6 | 14.2 | 100 | 46.0 | 1.59 |
| 16 | PPNCl | YES | YES | PPC-P-co-PLA | 12.3 | 61.4 | 100 | 27.6 | 54.8 | 2.9 | 14.8 | 19.3 | 99.4 | 60.5 | 1.59 |
In summary, analyzing the 1H NMR chemical shift of α-H aids in predicting and identifying concurrent transesterification and copolymerization processes. Transesterification of PLA will not occur if Lewis bases fail to induce changes in the chemical shift of specific α-H sites. Lewis bases capable of modifying the α-H chemical shift in PLA are considered viable candidates. Adding only Lewis bases results in PLA depolymerization, whereas the integration of Lewis acid–base pairs leads to the production of copolymers. The extent of PLA depolymerization and transesterification is intimately related to the ionic distance between Lewis bases and α-H of PLA. Anions in the initiator, positioned in close proximity to the α-H of PLA, facilitate copolymer formation due to deprotonation, leading to modifications in the bond lengths of ester groups. As a polar solvent and an active epoxide, PO has the potential to cause depolymerization and transesterification in PLA.
3.2 Initiator activity for both PO/PA/CO2 copolymerization and PLA transesterification
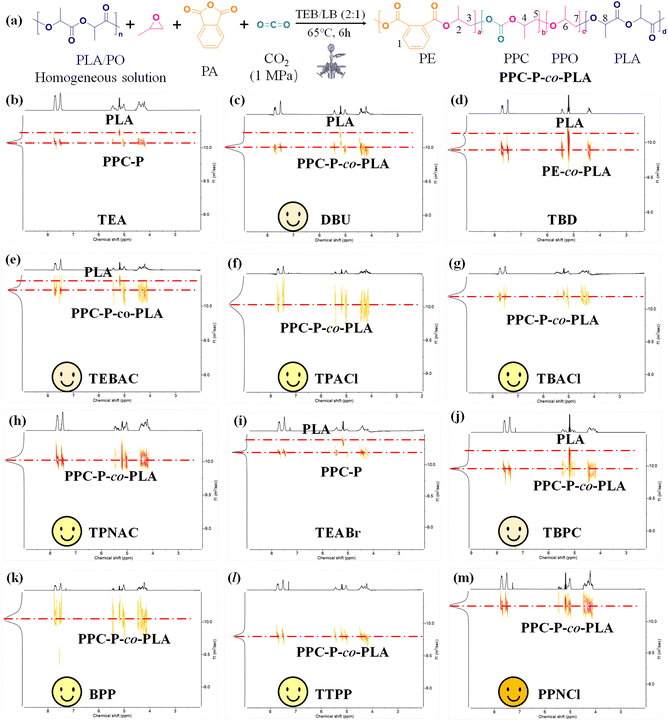
The Lewis bases mentioned earlier were utilized in the study of ROCOP-T. The PPC-P-co-PLA multi-block copolymers were identified using the notation provided in Fig. 5. When combined with TEB, the catalytic system consisting of TPACl, TBACl, TPNAC, BPP, TTPP, and PPNCl enabled the conversion of PLA into PPC-P-co-PLA multiblock copolymers via transesterification within 6 h. Under identical conditions, using DBU, TEBAC, and TBPC as initiators resulted in only partial conversion of PLA, resulting in PPC-P-co-PLA/PLA blends. When TBD was used as the initiator, PE-co-PLA copolymers were obtained without chain propagation of CO2. TEA and TEABr were ineffective in initiating the transesterification of PLA, leading solely to the production of PPC-P. U1 and DMAP exhibited no activity towards ROCOP of PA, PO, and CO2, resulting in no formation of CO2-based polymers.Amidine, a nitrogen-rich organic compound, exhibits stronger alkalinity than amines and has the ability to form stable salts with acids.48,54 With DBU serving as the initiator, PPC-P-co-PLA multiblock copolymers with a highly regular composition and minimal PPO content were synthesized (Fig. 3e). This was achieved due to DBU's ability to effectively minimize side reactions arising from the nucleophilic nature of bases. PPC-P-co-PLA/PLA blends were obtained using DBU as the initiator, given the presence of some unreacted PLA during the same reaction time. In contrast, TBD resulted in the production of PE-co-PLA copolymers. TBD significantly influenced the depolymerization of PLA. During the same time frame, incomplete conversion of PA and absence of CO2 chain propagation were observed (Fig. 5d). When compared to its effects on the depolymerization and transesterification of PLA, TBD demonstrated reduced activity in catalyzing the ROCOP of PO, PA, and CO2. Consequently, with TBD as the initiator, PLA transesterification preceded the chain propagation reactions of PE and PPC. The pKa value of TBD surpasses that of DBU,52 suggesting that higher alkalinity does not inherently correlate with superior performance in copolymerization and PLA transesterification reactions.
Organic ammonium chloride exhibits a regular effect on ROCOP-T. As the carbon chain length on the positive ion of the initiator increased, the molecular weight and the PPC content of the resulting product gradually increased: TPNAC (58.2 kDa, 49.1%) > TBACl (53.7 kDa, 48.6%) > TPACl (39.1 kDa, 46.5%) (entries 9–11, Table 2). This suggests a positive correlation between the increasing carbon chain length (≤5) of the initiator and the PPC content, which enhances the efficient insertion of CO2 into the polymer chain, thereby promoting the chain propagation of PPC. The similarity in molecular weight and composition of the products initiated by TBACl and TPNAC suggests that the impact plateaus once the carbon chain length reaches a certain level for initiating ROCOP-T. Compared to TBACl, TPNAC leads to an elevated PPO content in PPC-P-co-PLA multi-block copolymers, likely due to the increased ion looseness. Upon substitution of bromide ions for chloride ions, TBABr, as the initiator for copolymerization and transesterification, results in minimal ester exchange within PLA, yielding PPC-P/PLA blends as the final product. Using TBPC as the initiator yielded PPC-P-co-PLA/PLA blends with a 91.4% conversion rate of PA. TBPC exhibits lower initiation efficiency than TBACl for ROCOP-T. When the carbon chain lengths are equal, the initiation efficiency for ROCOP-T follows the order: TBACl > TBPC > TBABr.
All four currently utilized organic phosphine salts exhibit initiator activity in ROCOP-T. Analysis of the molecular weights of the obtained products indicates a specific activity order of PPNCl > TTPP ≈ BPP > TBPC within the reaction system (entries 13–16, Table 2). When three linear carbon chains of TBPC were substituted with phenyl groups, BPP exhibited increased efficiency in ROCOP-T. However, when all straight-chain carbon chains of TBPC were substituted with phenyl groups, the PPC content in the copolymer composition decreased, impeding the subsequent incorporation of carbon dioxide. In comparison with TTPP, the anions in PPNCl led to enhanced hydrogen delocalization,41 making it a more efficient Lewis base for ROCOP-T. Among the ionic initiators suitable for ROCOP-T, TBACl, TPNCl and PPNCl were found to be the most efficacious. PPNCl exhibited the highest efficiency in enhancing the insertion of CO2 into the chain, yielding PPC-P-co-PLA multi-block copolymers with the highest PPC content. Consequently, among the tested ionic initiators, PPNCl emerges as the preferred choice for ROCOP-T.
The formation of PPC-P-co-PLA multi-block copolymers necessitates a balance between the copolymerization reaction rate and the rates of PLA depolymerization and transesterification. In the entire initiator research system, the listed initiators exhibit activity for ROCOP-T, with their efficiency ranked as follows: PPNCl > TPNAC > TBACl > TTPP ≈ BPP > TPACl > TEBAC > TBPC > DBU. PPNCl was selected as the initiator for further experiments.
3.3 Reaction process to synthesize PPC-P-co-PLA multi-block copolymers
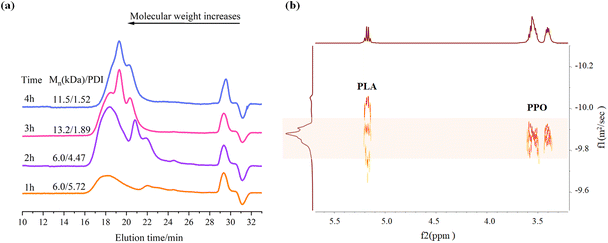
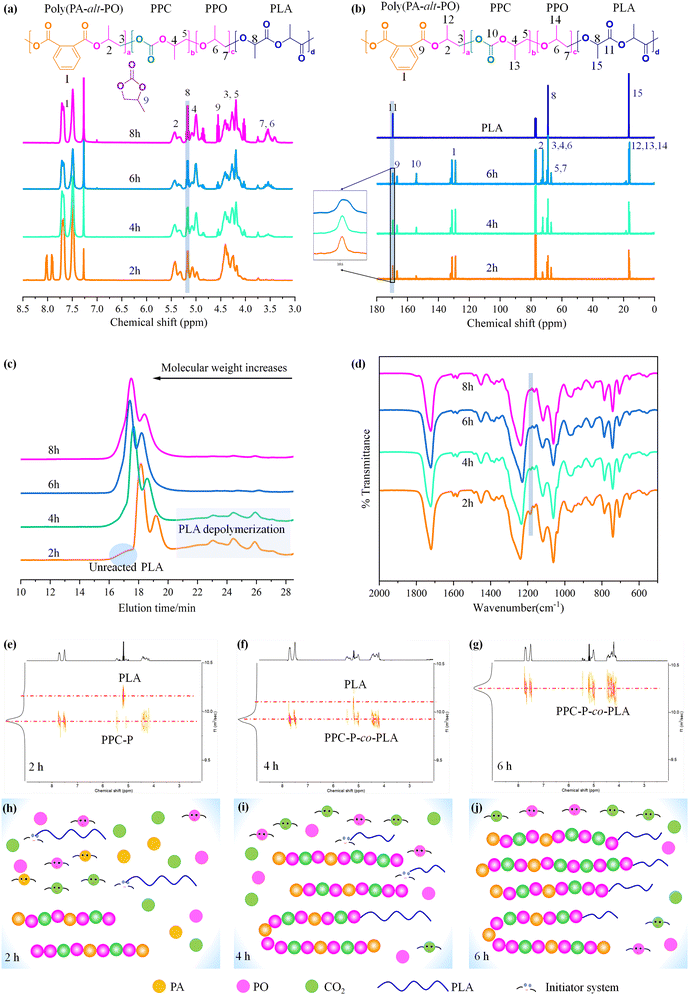
To monitor the reaction process, samples were collected at various time points and analysed using 1H NMR spectra, 13C NMR spectra, GPC, and FT-IR spectrum (Fig. 6a–d). DOSY spectra were used to differentiate between the obtained PPC-P-co-PLA copolymer and PPC-P/PLA blends (Fig. 6e–g). The schematic illustrates the sequential process of monomer reduction, PPC-P chain extension, and subsequent transesterification reactions between PPC-P and PLA during the reaction (Fig. 6h–j).The copolymerization of PO, PA, and CO2 is the key process during the 2 h reaction to produce PPC-P. After 2 h of reaction, residual PA peaks persisted, with a conversion rate to PE of 86.4%, suggesting significant unreacted PA (Fig. 6a). Initially, the concentrations of cyclic carbonate and PPO were low (entry 1, Table S1†). At this stage, the majority of PPC-P polymer chain segments in the system was evident (Fig. 6e). The prominent antisymmetric stretching vibration peak of COC (νas COC) at 1185 cm−1 indicates the presence of unreacted PLA (Fig. 6d). Notably, a substantial proportion of PLA remained unreacted at this stage. The unreacted PLA may experience fragmentation and shortening due to interactions with Lewis bases (Fig. 6e). Consequently, PPC-P/PLA blends emerged as the primary products during this phase. Analysis of the 13C NMR spectra (Fig. 6b) indicates that PLA exhibited isotactic stereoisomerism.55 After 2 h of reaction, the carbonyl peak shape in 13C NMR remained unchanged, suggesting that transesterification does not affect the stereoisomeric regularity of PLA (Fig. 6b).
After 4 hours of reaction, the primary products obtained were PPC-P-co-PLA/PLA blends (Fig. 6f). The main reactions were ROCOP of PO, PA, and CO2 to form PPC-P, accompanied by transesterification between PPC-P and PLA to yield PPC-P-co-PLA multi-block copolymers. After 4 h, PA was fully converted to PE (Fig. 6a), resulting in an increase in molecular weight to 46.0 kg mol−1 (entry 2, Table S1†). Following the complete conversion of PA, the PPC content in the polymer segments increased with consistent CO2 injection, accompanied by an increase in cyclic carbonate (CC) content due to back-biting. Consequently, the PE content decreased, whereas the PPC and CC contents increased. PLA long chains, which have undergone depolymerization with Lewis bases and activation via Lewis acid–base pairs, are continuously linked to PPC-P segments via transesterification, resulting in the formation of block copolymers. The notable reduction of the νas COC peak at 1185 cm−1 suggests alterations in the stretching vibration of PLA, which can be attributed to depolymerization and transesterification (Fig. 6d). At this stage, it is noted that some PLA segments in the system have not undergone transesterification to form block copolymers (Fig. 6f).
After 6 hours of reaction, transesterification successfully converted all PLA into PPC-P-co-PLA multi-block copolymers (Fig. 6g). The molecular weight of the PPC-P-co-PLA multi-block copolymers was determined to be 48.4 kg mol−1 (entry 3, Table S1†). The increase in molecular weight was insignificant when compared to the 4-hour reaction. Consequently, transesterification of PLA remained the dominant reaction throughout, with continuous introduction of CO2. The carbonyl peak corresponding to the 6 h product showed a broadened profile in the 13C NMR spectra. The broadening is presumably attributed to changes in the chemical environment of PLA following transesterification to PPC-P-co-PLA, despite the carbonyl peak retaining its singlet nature. As an active anion reaction, the continuous addition of CO2 occurs when chain termination is absent. The side effects associated with the back-biting reaction increased substantially, resulting in a concurrent rise in CC levels (entry 4, Table S1†). Hence, precise control of reaction time and conditions is crucial for the successful outcome of this reaction. 6 h was identified as the optimal reaction time with PPNCl as the initiator, which was maintained for all subsequent reactions.
3.4 Competitive polymerization rate and reaction mechanism
PPC-P-co-PLA multi-block copolymers were synthesized via ROCOP-T, incorporating PE derived from PA, PPC generated from CO2, a minor quantity of PPO, and PLA through transesterification. As the content of TEB increased, the interaction between TEB and PO intensified, leading to an enhanced PPO concentration and a corresponding decrease in PPC. The enhanced binding of TEB with Lewis bases simultaneously reduced the affinity of the Lewis base for PLA, mitigating PLA depolymerization into oligomers and ultimately increasing PLA content (Fig. 7a). From a holistic perspective, it is notable that despite using more reaction materials compared to ROCOP of PO, PA, and CO2, a TEB molar ratio of 2.0 adequately fulfills the experimental prerequisites for copolymerization and PLA transesterification. Upon maintaining a constant molar ratio of PA, an elevation in PO content resulted in a corresponding increase in PPC content, while PE content decreased significantly and PLA content exhibited a slight decrease (Fig. 7b). At a constant molar ratio of PO, an increase in PA content led to an elevation in PE content, a reduction in PPC content, and minimal changes in PLA content (Fig. 7c). The PE and PPC contents are closely related to the PO/PA ratio. PA has the highest reaction rate, slightly surpassing CO2. After the termination of PA chain propagation, the continuous chain growth with CO2 proceeds to produce the random copolymer PPC-P.26,27 Altering the feeding ratio modifies the structure of PPC-P. When the PO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA ratio is adjusted from 3000
PA ratio is adjusted from 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 to 3000
400 to 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250, there is a notable increase in PO/CO2 chain segments in PPC (Fig. S17†). The presence of PA has little effect on the depolymerization and transesterification of PLA. With a constant PO
250, there is a notable increase in PO/CO2 chain segments in PPC (Fig. S17†). The presence of PA has little effect on the depolymerization and transesterification of PLA. With a constant PO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA ratio, an increase in copolymer raw material content led to an increase in PE content and a corresponding decrease in PPC, PPO, and PLA content (entries 8 and 9, Table 3). An increase in PLA content resulted in a continuous decrease in the PPC mole fraction, suggesting a decrease in CO2 conversion, while PE mole content remained stable, suggesting unaffected PA conversion (Fig. 7d). At a PLA mass fraction of 16%, some PLA molecules were not fully ester-exchanged with PPC-P, resulting in the formation of PPC-P-co-PLA/PLA blends (Fig. S18†). PLA segments are covalently attached to the PPC termini within PPC-P, serving as end-capping agents through anion transfer (Fig. S19†). Consequently, the resultant block copolymer consists of random PPC-P and depolymerized PLA by transesterification. Furthermore, increased PLA content suppresses CO2 chain growth, resulting in shorter PPC-P segments and a subsequent decrease in the PPC-P-co-PLA copolymer molecular weight.
PA ratio, an increase in copolymer raw material content led to an increase in PE content and a corresponding decrease in PPC, PPO, and PLA content (entries 8 and 9, Table 3). An increase in PLA content resulted in a continuous decrease in the PPC mole fraction, suggesting a decrease in CO2 conversion, while PE mole content remained stable, suggesting unaffected PA conversion (Fig. 7d). At a PLA mass fraction of 16%, some PLA molecules were not fully ester-exchanged with PPC-P, resulting in the formation of PPC-P-co-PLA/PLA blends (Fig. S18†). PLA segments are covalently attached to the PPC termini within PPC-P, serving as end-capping agents through anion transfer (Fig. S19†). Consequently, the resultant block copolymer consists of random PPC-P and depolymerized PLA by transesterification. Furthermore, increased PLA content suppresses CO2 chain growth, resulting in shorter PPC-P segments and a subsequent decrease in the PPC-P-co-PLA copolymer molecular weight.
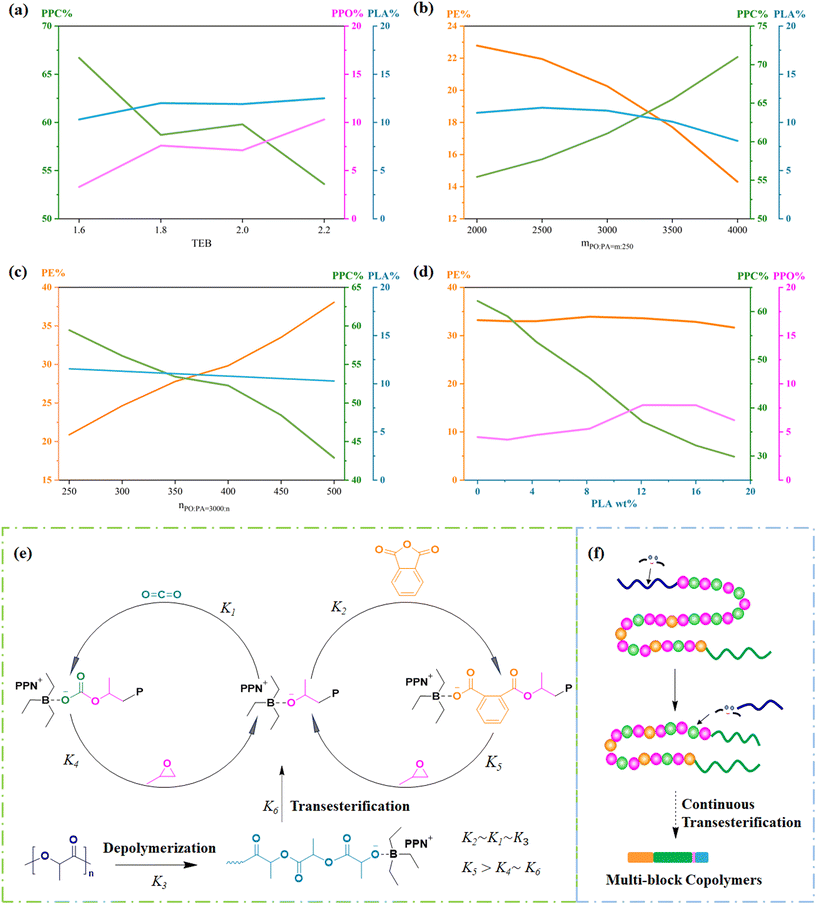 | ||
| Fig. 7 Compositional variation charts of PPC-P-co-PLA multi-block copolymers by varying the molar ratios of TEB (a), PO (b), and PA (c), as well as adjusting the PLA content (d). The data utilized for creating the charts originated from the following entries in Table S4:† (a) entries 1–4; (b) entries 5, 6, 1 and 7–11; (c) entries 3 and 11–15. (e) Proposed reaction mechanism of PO, PA, and CO2 copolymerization and PLA transesterification. (f) Proposed reaction mechanism for generating multi-block copolymers through continuous transesterification. | ||
| Entry | PO/PA/TEB/PPNCl | PO/PA | PEa (%) | PPCa (%) | PPOa (%) | PLAa (%) | CCa (%) | PLAa (wt%) | CO2a (wt%) | M n (kg mol−1) | PDIb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Calculated by 1H NMR; PE% = A1/4/(A1/4 + A4 + (A6,7)/3 + A8) × 100; PPC% = A4/(A1/4 + A4 + (A6,7)/3 + A8) × 100; PPO% = (A6,7)/3/(A1/4 + A4 + (A6,7)/3 + A8) × 100; PLA% = A8/(A1/4 + A4 + (A6,7)/3 + A8 + Ac) × 100; CC% = A9/(A2 + A4 + (A6,7)/3 + A8 + Ac). PLAwt% = 72 × PLA%/(72 × PLA% + 206 × PE% + 102 × PPC% + 58 × PPO%) × 100; CO2wt% = 44 × PPC%/(72 × PLA% + 206 × PE% + 102 × PPC% + 58 × PPO%) × 100.b Determined by GPC in tetrahydrofuran (THF). | |||||||||||
| 1 | 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 19.7 | 66.7 | 3.3 | 10.3 | 8.2 | 6.3 | 24.9 | 33.7 | 1.58 |
| 2 | 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 21.7 | 58.7 | 7.6 | 12.0 | 7.5 | 7.3 | 22.0 | 37.6 | 1.51 |
| 3 | 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 20.9 | 59.8 | 7.4 | 11.9 | 7.8 | 7.3 | 22.5 | 37.6 | 1.53 |
| 4 | 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 23.5 | 53.6 | 10.3 | 12.5 | 9.1 | 7.6 | 20.0 | 32.5 | 1.58 |
| 5 | 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
16 | 14.2 | 70.6 | 7.1 | 8.1 | 4.5 | 5.2 | 27.9 | 53.1 | 1.54 |
| 6 | 3500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14 | 17.7 | 66.7 | 5.9 | 9.8 | 7.9 | 6.1 | 25.5 | 42.8 | 1.43 |
| 7 | 2500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 21.1 | 58.2 | 9.8 | 10.9 | 9.0 | 6.7 | 22.0 | 42.8 | 1.58 |
| 8 | 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 23.1 | 55.4 | 10.3 | 11.2 | 6.4 | 6.8 | 20.6 | 31.9 | 1.46 |
| 9 | 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 33.6 | 52.4 | 4.2 | 9.9 | 5.3 | 5.4 | 17.4 | 48.8 | 1.38 |
| 10 | 3500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7 | 35.4 | 49.9 | 4.7 | 10.1 | 4.5 | 5.4 | 16.4 | 48.0 | 1.37 |
| 11 | 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 38.7 | 42.3 | 7.8 | 11.1 | 5.7 | 5.9 | 13.7 | 36.0 | 1.47 |
| 12 | 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.7 | 31.9 | 49.9 | 8.1 | 10.0 | 6.3 | 5.6 | 17.1 | 42.6 | 1.51 |
| 13 | 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7.5 | 30.9 | 51.4 | 7.1 | 10.6 | 4.7 | 6.0 | 17.7 | 38.0 | 1.46 |
| 14 | 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8.6 | 27.2 | 54.1 | 8.6 | 10.1 | 6.7 | 5.9 | 19.3 | 37.5 | 1.47 |
| 15 | 3000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 24.8 | 55.2 | 10.3 | 11.8 | 4.8 | 7.0 | 19.9 | 42.8 | 1.58 |
| 16 | 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 34.0 | 62.5 | 3.4 | 0.0 | 7.4 | 0.0 | 20.2 | 74.3 | 1.47 |
| 17 | 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 31.1 | 58.3 | 6.6 | 4.0 | 6.3 | 2.2 | 19.7 | 67.3 | 1.46 |
| 18 | 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 34.0 | 53.9 | 3.9 | 8.2 | 6.0 | 4.3 | 19.2 | 61.8 | 1.44 |
| 19 | 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 33.8 | 46.8 | 4.6 | 14.8 | 3.9 | 8.2 | 15.8 | 55.7 | 1.34 |
| 20 | 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 33.2 | 36.7 | 8.8 | 21.2 | 3.1 | 12.1 | 12.8 | 42.2 | 1.36 |
| 21 | 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 33.5 | 30.7 | 8.1 | 27.7 | 3.9 | 16.0 | 10.8 | 35.5 | 1.39 |
| 22 | 4000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 31.4 | 30.7 | 5.9 | 32.0 | 5.5 | 18.8 | 11.0 | 38.3 | 1.46 |
The copolymerization reaction rate between PA and CO2 exhibits insignificant variation (K2 ≈ K1) and resembles that of PLA depolymerization catalysed by PPNCl (K1 ≈ K3). Competitive insertion of PA and CO2 results in the formation of carboxylate and carbonate species. Subsequently, the insertion of PO into these species yields alkoxide intermediates.26 During chain propagation, carboxylate exhibits the fastest chain extension and remains relatively unaffected by PLA (K5 > K4). PLA transesterification and CO2 chain growth compete, with CO2 chain propagation closely approaching the equilibrium constant of PLA transesterification (K4 ≈ K6). Transesterification between PPC-P and PLA terminates one end of PPC-P with PLA. The proposed reaction mechanism is depicted in Fig. 7e. The released initiator system undergoes nucleophilic substitution reactions with PLA in PPC-P-PLA copolymers, leading to the formation of shorter PLA segments. The newly formed shorter PLA segments with active ends will undergo further transesterification with PPC segments in PPC-P. Continuous transesterification reactions ultimately yield multi-block copolymers with similar PLA chain lengths (Fig. 7f). If the initiator remains active, the continuous reaction will result in PLA segments in the copolymers of similar length to those obtained in the corresponding proportion in Fig. 1c.
3.5 Thermal properties and crystallinity of PPC-P-co-PLA multi-block copolymers
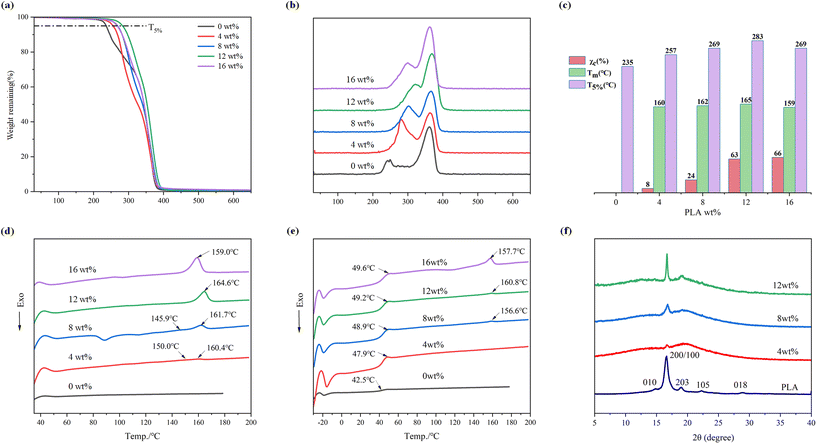
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements were conducted to assess the thermal behavior of the copolymers. When compared to PPC-P, the T5% of the copolymer increased by 18 to 45 °C (Fig. 8a), indicating that the incorporation of PLA enhances the overall structural stability of the copolymer. At a PLA mass fraction of 12 wt%, the copolymer achieved a maximum T5% value of 280.4 °C. The DTG curves, derived from the thermogravimetric curve through differentiation, provide insights into the rate of weight loss. The decomposition of the PPC-P-co-PLA multi-block copolymers occurs in two distinct stages (Fig. 8b). With increasing PLA content, the decomposition temperature of the PPC chain segment in the first stage consistently increases. This implies an enhancement in the thermal decomposition resistance of the chain segment, due to interactions between PLA and one terminus of PPC (Fig. S18†). Consequently, an increase in PLA content results in enhanced thermal stability of the copolymer.The PPC-P-co-PLA multi-block copolymers synthesized via ROCOP-T exhibit crystalline domains, qualifying them as semi-crystalline copolymers (Fig. 8d and e). DSC analysis of the PPC-P-co-PLA multi-block copolymers revealed two crystallization melting peaks at a PLA content of approximately 4 wt%. When the PLA mass fraction was increased to 8 wt%, two crystallization melting peaks emerged during the first heating cycle, while only one peak persisted during the second cycle. At a PLA mass fraction of 12 wt%, a single crystallization melting peak was observed during the first heating cycle, reaching a maximum temperature of 164.6 °C. When the PLA mass fraction reached 16 wt%, both the crystallization melting peak and the thermal decomposition temperature decreased. The decrease could be attributed to PLA acting as a capping agent, which hindered the incorporation of CO2, resulting in reduced copolymer molecular weight and incomplete attachment of PLA to PPC-P during copolymer formation. The DSC second heating curve showed that the incorporation of PLA increased the glass transition temperature (Tg) of the PPC-P-co-PLA multiblock copolymers to above 47.9 °C, exhibiting distinct relaxation peaks (Fig. 8e). At a PLA mass fraction of 12 wt%, the Tg of PPC-P-co-PLA multi-block copolymers increased to 49.2 °C, expanding the potential applications. An increase in the PLA mass fraction leads to the expansion of crystalline domains within the copolymer, consequently reducing its transmittance. The transmittance of PPC-P-co-PLA multi-block copolymers consistently exceeded 80% when the PLA content in the polymer remained below 16 wt% (Table S2†).
The crystallinity of the PLA segments was calculated using DSC curves and the crystallinity of the overall copolymer was determined using XRD patterns (Fig. 8f). Crystallinity from the DSC curves was calculated based on the endothermic enthalpy of a single crystallization melting peak and the exothermic enthalpy related to cold crystallization during the initial heating cycle. A gradual increase in crystallinity was observed with an increasing mass fraction of PLA (Table S2†). In essence, an increase in PLA content corresponds to an increase in crystalline regions within the copolymer. The commercial high-modulus PLA used after drying exhibits an exceptionally high crystallinity of ∼99.5%. At a mass fraction of 12.1 wt%, the crystallinity is 63.0%. This indicates that over 60% of the PLA segments in the copolymer have effectively crystallized. XRD analysis reveals that the PLA employed exhibits a fully isomorphic structure, encompassing five distinct crystal forms. The diffraction peaks are observed at 2θ values of 14.8°, 16.6°, 18.9°, 22.3°, and 28.8° correspond to the (010), (200/100), (203), (105), and (018) lattice planes, respectively.55–57 At a concentration of 4 wt% PLA, a distinct peak appears at 16.6°, indicating the subsequent formation of a crystalline region within the copolymer. Increasing the PLA content in the copolymer to 12 wt% leads to a pronounced and distinct 2θ peak at 16.6° in the XRD pattern. The relative crystallinity is calculated as the ratio of the crystallization peak area to the total area in the XRD pattern. To analyse the proportion of the crystalline region within the copolymer, we examined the XRD pattern of PLA containing 12 wt% and determined a crystallinity of 12.3% using integral area calculations.
3.6 Mechanical and rheological properties of PPC-P-co-PLA multi-block copolymers
Mechanical performance testing revealed that PPC-P, composed of 34% PE and having a molecular weight of 74.3 kg mol−1, exhibited a tensile strength of 44.5 ± 0.4 MPa and an elongation at break of 6.6 ± 1.8%. The incorporation of 4 wt% PLA into PPC-P-co-PLA multiblock copolymers resulted in a slight decrease in tensile strength to 42.3 ± 0.6 MPa. Increasing the PLA content to 8 wt% led to enhancement of the tensile strength to 46.3 ± 0.9 MPa and elongation at break to 8.3 ± 1.6%. The percentage content of each component in this copolymer PE/PPC/PPO/PLA was 34/47/4/15. By calculating the number of each repeating unit within the polymer, the composition was as follows: PE 92, PPC 257, PPO 38, and PLA 116. At a PLA content of 12 wt%, the PE content remained stable, while the PPC content decreased by 10%. The material exhibited brittle fracture and minimal deformation, potentially due to a decrease in molecular weight.Rheological testing reveals that changes in storage modulus G′, loss modulus G′′ and complex viscosity η* as the PLA content increases mirrored those observed in tensile strength. Complex viscosity (η*) encapsulates both elastic and viscous characteristics of the material. Analysis of composite viscosity revealed a decrease followed by an increase when the PLA content was 4 wt%. The initial decrease was attributed to the rheological properties of PPC-P, whereas the subsequent increase was likely due to PLA crystallization. When the PLA content reached 8 wt%, a substantial rise in the composite material's viscosity was observed as the angular frequency increased from 1 to 10 rad s−1. During tensile hardening of plastics, changes in molecular chain orientation and crystallinity enhance the material's elasticity, with the elastic modulus typically increasing as a result of strain or time. As the tensile strain intensifies, molecular chain orientation and entanglement become more pronounced, enhancing the material's resistance to flow and, consequently, increasing complex viscosity. The phenomenon diminished as the PLA content further increased. Based on their mechanical and rheological properties, copolymers with a PLA content of 4–8 wt% showed superior performance compared to those with 12–16 wt% PLA. Among them, copolymers containing 8 wt% PLA exhibited the best performance and had the highest application potential (Fig. 9).
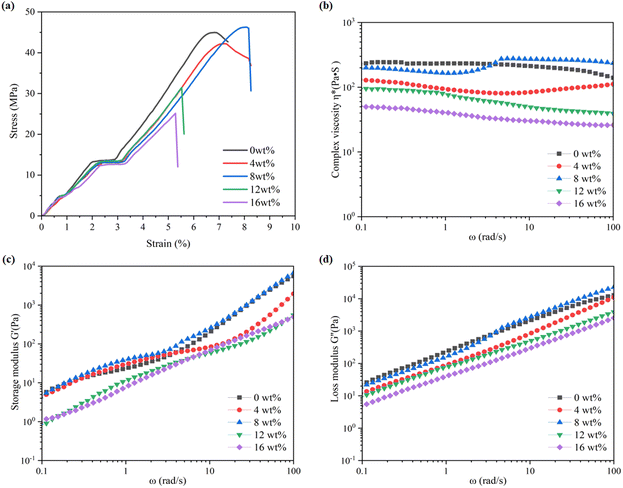 | ||
| Fig. 9 (a) Stress–strain curves, (b) storage modulus G′, (c) loss modulus G′′ and (d) complex viscosity η*of PPC-P-co-PLA multi-block copolymers with different mass fractions of PLA. | ||
4. Conclusions
Semi-crystalline PPC-P-co-PLA multi-block copolymers were successfully synthesized using ROCOP of PO, PA and CO2 in conjunction with the transesterification of PLA via a one pot/one-step method. The sole addition of Lewis bases leads to the depolymerization of PLA, whereas Lewis acid–base pairs as catalysts promote the formation of copolymers. Lewis bases capable of inducing changes in the α-H chemical shift of PLA, which can be further altered by the addition of TEB, are suitable for concurrent transesterification and copolymerization processes. The depolymerization and transesterification of PLA are closely related to the degree of ion pair loosening, deprotonation and solvation effects. For the ROCOP-T process, the sequence of initiator activity is as follows: PPNCl > TPNAC > TBACl > TTPP ≈ BPP > TPACl > TEBAC > TBPC > DBU. TEB/PPNCl shows the highest catalytic activity in the synthesis of the semi-crystalline copolymer. Initially, PPC-P is formed, followed by transesterification with PLA to yield PPC-P-co-PLA multi-block copolymers. The copolymer containing 12 wt% PLA exhibits optimal thermal performance, with a thermal decomposition temperature of 280.4 °C, a maximum crystallization melting temperature of 164.6 °C, and a glass transition temperature of 49.2 °C. The crystallinity of PLA in the PPC-P-co-PLA copolymer with 12 wt% PLA reaches 63%, and the overall crystallinity of the copolymer is 12%. Copolymers containing 8 wt% PLA demonstrated superior performance, with a tensile strength of 46.3 ± 0.9 MPa and an elongation at break of 8.3 ± 1.6%. This research significantly enhanced the chemical utilization efficiency of carbon dioxide and, meanwhile, offers a novel perspective on the sustainable reuse of polyesters and the synthesis of semi-crystalline copolymers.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
Financial support was provided by the Fundamental Research Fund of Henan Academy of Sciences (230603001 & No. 240618065), High-level Talent Research Start-up Project Funding of Henan Academy of Sciences (232018002 & No. 241818084), the Scientific and Technological Research Project of Henan Academy of Sciences (242102230180), Joint Fund of Henan Province Science and Technology R&D Program (235200810027), Research and Development Project of Henan Academy of Science (241603011 & 231818020), Henan Provincial Natural Science Foundation General Project (242300420193), the National Natural Science Foundation of China (No. 22075254), and the International Science and Technology Cooperation Project of Henan Province (242102521042). We also appreciate the financial supports from industries: Shandong Lecron Industrial Development Group, Co., Ltd, China, Zhuhai Zhongguan Petrochemical, Co., Ltd, China, Guangdong Tianxin New Material Technology Co., Ltd, China, Hebei CNC Risun Energy Co., Ltd, China, and Huanghua Xinnuolixing Fine Chemicalstock Co., Ltd, China.References
- Y. Xu, L. Lin, M. Xiao, S. Wang, A. T. Smith, L. Sun and Y. Meng, Prog. Polym. Sci., 2018, 80, 163–182 CrossRef CAS.
- H. Dau, G. R. Jones, E. Tsogtgerel, D. Nguyen, A. Keyes, Y.-S. Liu, H. Rauf, E. Ordonez, V. Puchelle, H. Basbug Alhan, C. Zhao and E. Harth, Chem. Rev., 2022, 122(18), 14471–14553 CrossRef CAS PubMed.
- G. Hayes, M. Laurel, D. MacKinnon, T. Zhao, H. A. Houck and C. R. Becer, Chem. Rev., 2022, 123(5), 2609–2734 CrossRef PubMed.
- Z. Zhang, Y. Ren, J. Liang, M. Xiao, S. Wang, S. Huang, D. Han and Y. Meng, Energy Storage Mater., 2024, 71, 103667 CrossRef.
- S. Inoue, H. Koinuma and T. Tsuruta, J. Polym. Sci., Part B:Polym. Phys., 1969, 7(4), 287–292 CrossRef CAS.
- Y. Wang and D. J. Darensbourg, Coord. Chem. Rev., 2018, 372, 85–100 CrossRef CAS.
- T. Ohkawara, K. Suzuki, K. Nakano, S. Mori and K. Nozaki, J. Am. Chem. Soc., 2014, 136(30), 10728–10735 CrossRef CAS PubMed.
- L. Yang, S. Liu, Z. Zhou, R. Zhang, H. Zhou, C. Zhuo and X. Wang, Macromolecules, 2023, 57(1), 150–161 CrossRef.
- L. Tang, M. Xiao, Y. Xu, S. Wang and Y. Meng, J. Polym. Res., 2013, 20, 190 CrossRef.
- X.-H. Zhang, R.-J. Wei, Y. Y. Zhang, B.-Y. Du and Z.-Q. Fan, Macromolecules, 2015, 48(3), 536–544 CrossRef CAS.
- Q. Wang, X. Li, H. Li, L. Liu, X. Zhang, Y. Li, M. Kang, Q. Li and J. Wang, J. Environ. Chem. Eng., 2025, 13, 115928 CrossRef CAS.
- C. A. L. Lidston, S. M. Severson, B. A. Abel and G. W. Coates, ACS Catal., 2022, 12(18), 11037–11070 CrossRef CAS.
- D. J. Darensbourg, R. M. Mackiewicz, J. L. Rodgers, C. C. Fang, D. R. Billodeaux and J. H. Reibenspies, Inorg. Chem., 2004, 43(19), 6024–6034 CrossRef CAS PubMed.
- J. Liu, W. Ren, Y. Liu and X. Lu, Macromolecules, 2013, 46(4), 1343–1349 CrossRef CAS.
- R. Chiarcos, M. Laus, K. Sparnacci, R. Po, P. Biagini, I. Tritto, L. Boggioni and S. Losio, Eur. Polym. J., 2023, 192, 112058 CrossRef CAS.
- D. Zhang, S. K. Boopathi, N. Hadjichristidis, Y. Gnanou and X. Feng, J. Am. Chem. Soc., 2016, 138(35), 11117–11120 CrossRef CAS PubMed.
- Y.-Y. Zhang, G.-W. Yang, R. Xie, X.-F. Zhu and G.-P. Wu, J. Am. Chem. Soc., 2022, 144(43), 19896–19909 CrossRef CAS PubMed.
- J. Liang, S. Ye, S. Wang, S. Wang, D. Han, S. Huang, Z. Huang, W. Liu, M. Xiao, L. Sun and Y. Meng, Macromolecules, 2022, 55(14), 6120–6130 CrossRef CAS.
- P. Du, Y. Li and X. Lu, Macromolecules, 2023, 56(17), 6783–6789 CrossRef CAS.
- Y. Ren, T. Zhang, S. Wang, D. Han, S. Huang, H. Guo, M. Xiao and Y. Meng, J. CO2 Util., 2024, 85, 102853 CrossRef CAS.
- C. Chen, Y. Gnanou and X. Feng, Polym. Chem., 2022, 13(45), 6312–6321 RSC.
- C. Hu, X. Pang and X. Chen, Macromolecules, 2022, 55(6), 1879–1893 CrossRef CAS.
- A. C. Deacy, G. L. Gregory, G. S. Sulley, T. T. D. Chen and C. K. Williams, J. Am. Chem. Soc., 2021, 143(27), 10021–10040 CrossRef CAS PubMed.
- W. Yu, E. Maynard, V. Chiaradia, M. C. Arno and A. P. Dove, Chem. Rev., 2021, 121(18), 10865–10907 CrossRef CAS PubMed.
- S. J. Poland and D. J. Darensbourg, Green Chem., 2017, 19(21), 4990–5011 RSC.
- J. Liang, S. Ye, W. Wang, C. Fan, S. Wang, D. Han, W. Liu, Y. Cui, L. Hao, M. Xiao and Y. Meng, J. CO2 Util., 2021, 49, 101558 CrossRef CAS.
- V. K. Chidara, S. K. Boopathi, N. Hadjichristidis, Y. Gnanou and X. Feng, Macromolecules, 2021, 54(6), 2711–2719 CrossRef CAS.
- N. Kawashima, T. Yagi and K. Kojima, Sustainability, 2021, 13(1654), 1–18 Search PubMed.
- M. Meng, S. Wang, M. Xiao and Y. Meng, Sustainable Polym. Energy, 2023, 1(1), 1–43 Search PubMed.
- H. Li, H. Luo, J. Zhao and G. Zhang, ACS Macro Lett., 2018, 7(12), 1420–1425 CrossRef CAS PubMed.
- H. Kudo, S. Nishioka, H. Jin, H. Maekawa, S. Nakamura and T. Masuda, Polymer, 2022, 240, 124489 CrossRef CAS.
- C. Hu, X. Chen, M. Niu, Q. Zhang, R. Duan and X. Pang, Macromolecules, 2022, 55(2), 652–657 CrossRef CAS.
- J. Liu, M. Jia, Y. Gnanou and X. Feng, Macromolecules, 2023, 56(4), 1615–1624 CrossRef CAS.
- S. Ye, J. Liang, Y. Ren, S. Wang, D. Han, S. Huang, Z. Huang, M. Xiao and Y. Meng, Sustainable Polym. Energy, 2023, 1(1), 1–13 Search PubMed.
- S. Schüttner, C. Gardiner, F. S. Petrov, N. Fotaras, J. Preis, G. Floudas and H. Frey, Macromolecules, 2023, 56(20), 8247–8259 CrossRef.
- G. Huang, Y. Zou, M. Xiao, S. Wang, W. Luo, D. Han and Y. Meng, Polym. Degrad. Stab., 2015, 117, 16–21 CrossRef CAS.
- L. Tang, W. Luo, M. Xiao, S. Wang and Y. Meng, J. Polym. Sci., Part A: Polym. Chem., 2015, 53(14), 1734–1741 CrossRef CAS.
- X. Li, R.-l. Duan, C.-y. Hu, X. Pang and M.-x. Deng, Polym. Chem., 2021, 12(11), 1700–1706 RSC.
- B. Dong, G. Xu, R. Yang, X. Guo and Q. Wang, Macromolecules, 2023, 56(24), 10143–10152 CrossRef CAS.
- T. M. McGuire, A. Buchard and C. Williams, J. Am. Chem. Soc., 2023, 145(36), 19840–19848 CrossRef CAS PubMed.
- S. H. Dempsey and S. R. Kass, J. Org. Chem., 2022, 87(22), 15466–15482 CrossRef CAS PubMed.
- W. N. Sit, S. M. Ng, K. Y. Kwong and C. P. Lau, J. Org. Chem., 2005, 70, 8583–8586 CrossRef CAS PubMed.
- D. J. Darensbourg, R. M. Mackiewicz, A. L. Phelps and D. R. Billodeaux, Acc. Chem. Res., 2004, 37, 836–844 CrossRef CAS PubMed.
- D. J. Darensbourg and D. R. Billodeaux, Inorg. Chem., 2005, 44, 1433–1442 CrossRef CAS PubMed.
- J. K. Varghese, N. Hadjichristidis, Y. Gnanou and X. Feng, Polym. Chem., 2019, 10(27), 3764–3771 RSC.
- L. Lin, J. Liang, Y. Xu, S. Wang, M. Xiao, L. Sun and Y. Meng, Green Chem., 2019, 21(9), 2469–2477 RSC.
- N. J. Sherck, H. C. Kim and Y.-Y. Won, Macromolecules, 2016, 49(13), 4699–4713 CrossRef CAS PubMed.
- H. A. Brown, A. G. De Crisci, J. L. Hedrick and R. M. Waymouth, ACS Macro Lett., 2012, 1(9), 1113–1115 CrossRef CAS PubMed.
- N. Fanjul-Mosteirín, C. Jehanno, F. Ruipérez, H. Sardon and A. P. Dove, ACS Sustainable Chem. Eng., 2019, 7(12), 10633–10640 CrossRef.
- G. Scoponi, N. Francini, V. Paradiso, R. Donno, A. Gennari, R. d'Arcy, C. Capacchione, A. Athanassiou and N. Tirelli, Macromolecules, 2021, 54(20), 9482–9495 CrossRef CAS PubMed.
- T. S. Stukenbroeker, J. S. Bandar, X. Zhang, T. H. Lambert and R. M. Waymouth, ACS Macro Lett., 2015, 4(8), 853–856 CrossRef CAS PubMed.
- M. Hong, J. Chen and E. Y. X. Chen, Chem. Rev., 2018, 118(20), 10551–10616 CrossRef CAS PubMed.
- E. F. de Lima, J. W. de M. Carneiro, C. Fenollar-Ferrer, S. Miertus, S. Zinoviev, N. C. Om Tapanes and D. A. G. Aranda, Fuel, 2010, 89(3), 685–690 CrossRef.
- M. Castillo-Santillan, D. Maniar, M. C. Gutiérrez, P. Quiñonez-Angulo, J. R. Torres-Lubian, K. Loos and J. D. Mota-Morales, ACS Appl. Polym. Mater., 2023, 5(7), 5110–5121 CrossRef CAS.
- H. Tsuji and Y. Arakawa, Polym. Chem., 2018, 9(18), 2446–2457 RSC.
- Y. Su, Y. Zhao, W. Zheng, H. Yu, Y. Liu and L. Xu, ACS Appl. Mater. Interfaces, 2020, 12(49), 55520–55526 CrossRef CAS PubMed.
- Y. Zhang, M. T. Müller, R. Boldt and M. Stommel, ACS Appl. Polym. Mater., 2023, 6(1), 419–432 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5py00272a |
| This journal is © The Royal Society of Chemistry 2025 |