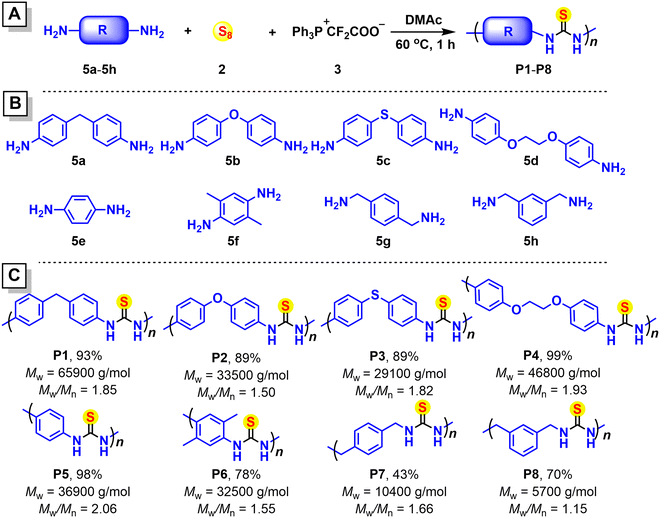
The base-free multicomponent polymerization of elemental sulfur, difluoromethylene phosphobetaine and amines toward electron-deficient aromatic polythioureas†
Yongjiang
Yu
a,
Wang
Chen
a,
Rongrong
Hu
 *a and
Ben Zhong
Tang
*a and
Ben Zhong
Tang
 bc
bc
aState Key Laboratory of Luminescent Materials and Devices, Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates, South China University of Technology, Guangzhou 510640, China. E-mail: msrrhu@scut.edu.cn
bSchool of Science and Engineering, Shenzhen Institute of Aggregate Science and Technology, The Chinese University of Hong Kong, Shenzhen, Guangdong 518172, China
cAIE Institute, Guangzhou 510530, China
First published on 12th February 2025
Abstract
Polythioureas are emerging materials with fascinating properties, such as self-healing and adhesion, high refractive indices, high dielectric constants, and heavy metal ion adsorption.With the increasing requirement for polymer structures and properties suiting a wide range of potential applications, versatile synthetic approaches are demanded to access a great diversity of polythiourea structures efficiently and economically. In this work, a commercially available difluorocarbene precursor, difluoromethylene phosphobetaine (PDFA), was selected to react with sulfur and electron-deficient aromatic amines to enable the efficient syntheses of electron-deficient polythioureas from amine monomers with low reactivity based on the high reactivity of a difluorothiocarbonyl intermediate. A one-pot catalyst-free multicomponent reaction of sulfur, PDFA and an amine was designed, which could take place efficiently in DMAc at 60 °C under nitrogen and was applicable for aromatic amines with both electron-donating and electron-withdrawing groups, producing thioureas in high yields (up to 93%). Most importantly, catalyst-free multicomponent polymerizations of sulfur, PDFA, and diamines were also developed in DMAc at 60 °C with commercially available monomers, showing high efficiency (Mws up to 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1 and yields up to 99%) and wide monomer applicability, providing an efficient synthetic approach for syntheses of polythioureas. Moreover, these polythiourea thin films showed high refractive indices (n633 nm up to 1.8133), suggesting their potential application in optical devices.
900 g mol−1 and yields up to 99%) and wide monomer applicability, providing an efficient synthetic approach for syntheses of polythioureas. Moreover, these polythiourea thin films showed high refractive indices (n633 nm up to 1.8133), suggesting their potential application in optical devices.
Introduction
Polythioureas, with their unique hydrogen bonds, large dipole moment, large molar refractivity, metal-coordination ability, and others, have been developed into a group of promising polymer materials, and have attracted increasing attention owing to their potential applications as self-healing materials,1–3 light refraction materials,4 dielectric materials,5–8 dynamic polymer networks,9 optoelectronic materials,10 and precious metal recovery materials.11 Owing to the different requirements for various applications, polythioureas with different chemical structures and different thermal properties and mechanical properties are required. Versatile synthetic approaches are hence demanded to access a great diversity of polythiourea structures.The commonly adopted synthetic approaches for polythioureas have mainly been divided into two kinds. One is polycondensations between diamine monomers and thiophosgene,7 diimidazolethiocarbonyl,1 or thiourea.12 These sulfur-containing reagents are generally toxic and smelly, difficult to handle, release harmful small molecules, and some even required special synthetic conditions, such as microwave assistance. The other method is polyadditions between diisothiocyanates and diamine monomers.13 While the reaction is efficient, the diisothiocyanate monomers were difficult to synthesize, and there was only limited commercially available diisocyanate compound. Recently, carbon disulfide was utilized for the synthesis of polythioureas;14,15 however, an equivalent amount of sulfur-containing small molecules such as H2S would be released as a byproduct, which was environmentally unfriendly. The limitations of the sulfur-containing monomers have vastly hindered the exploration of the chemical structures of polythioureas.
Elemental sulfur, which abundantly exists in nature, is one of the major byproducts of the petroleum and natural gas industries,16–19 and is a non-toxic, safe, odorless solid powder. It could serve as an ideal and economical monomer for the synthesis of sulfur-containing polymers. Great efforts have been made to utilize elemental sulfur to prepare polymeric materials, such as inverse vulcanization to synthesize chalcogenide hybrid inorganic/organic polymers.20–24 Polymerizations of sulfur were also reported for the synthesis of poly(benzothiazole)s,16 polydisulfides,25 poly(O-thiocarbamate)s,26 polythioamides27,28 and others. Among various sulfur-utilization approaches, multicomponent polymerization (MCP), with three or more monomers reacting together in a one-pot procedure to afford the targeted polymeric product,29–33 offers an efficient approach to convert elemental sulfur to various sulfur-containing polymers, such as polythioamides,34,35 polythiocarbamates,26 and polythioureas.11 Additionally, it has shown fascinating advantages, including high efficiency, simple operation, mild conditions, robustness, and, most importantly, great polymer product structural diversity. We have reported the MCPs of elemental sulfur, aliphatic diamines, and diisocyanates to afford polythioureas with various aliphatic or semi-aromatic structures.11,22,27 The MCPs of elemental sulfur, CH2Cl2 and aromatic diamines were also recently developed to afford a series of aromatic polythioureas with high efficiency.36 MCPs of sulfur, chloroform and aromatic diamines were also reported to produce polythioureas with several repeating unit structures through the use of dichlorocarbene active species in the presence of a large amount of the strong base t-BuOK.37 However, for aromatic amines with electron-withdrawing groups, which exhibit lower reactivity and weaker nucleophilicity compared to those with electron-donating groups, it was challenging to realize efficient transformations to thioureas. It is hence still difficult to access electron-deficient aromatic polythioureas, which are required and highly desired for potential applications in organic catalysis38 and for other optical and electronic applications.39
To react efficiently with the less-reactive aromatic diamines, difluorocarbene as an active reaction intermediate could be a promising candidate; it was commonly used for fluoromethylations,40–43 insertion reactions of X–H bonds (X = O, N, S), and [2 + 1] cycloaddition reactions involving multiple bonds.44 Xiao et al. developed a difluoromethylene phosphobetaine (PDFA) reagent to produce difluorocarbene in situ to further participate in N-trifluoromethylation,45C-trifluoromethylthiolation,46,47etc. In these reactions, PDFA was reported to react with sulfur to generate thiocarbonyl fluoride in situ, which then reacted with various amines to afford trifluoromethylated products, isothiocyanates, and HCF2S-substituted heterocycles.45 For example, PDFA, sulfur and an aromatic amine could react in 1,2-dimethoxyethane at 80 °C to afford the isothiocyanate in 85% yield, which could further react with dimethylamine to produce thiourea-containing chloromethiuron; additionally, a vicinal diamine with two neighboring –NH2 groups on a benzene ring was reported to react with PDFA and sulfur to afford cyclic thiourea in 75% yield at 80 °C in 1,2-dimethoxylethane (DME). The highly reactive difluorothiocarbonyl intermediate could hence undergo nucleophilic attack reactions with amines, including less-reactive electron-withdrawing-group-containing aromatic amines, to afford the corresponding polythioureas under suitable reaction conditions (Fig. 1).
 | ||
| Fig. 1 A strategy for polythioureas synthesized from diamines containing electron-withdrawing groups. | ||
In this work, to develop a polymerization approach for the synthesis of polythioureas, the one-pot multicomponent reaction of PDFA, sulfur, and two amine molecules was first investigated to directly produce a thiourea product. As solvents such as DME were not favored for the solubility of polythioureas with abundant intermolecular hydrogen bonds and strong polarity, polar solvents such as dimethylacetamide (DMAc) were adopted. Moreover, utilizing the highly reactive thiocarbonyl fluoride generated from PDFA and sulfur, electron-deficient aromatic amines might react efficiently to produce thiourea from sulfur. Herein, one-pot catalyst-free multicomponent reactions of sulfur, PDFA and electron-withdrawing-group-containing aromatic amines were reported to afford thiourea products in high yields. Efficient and rapid polymerizations of sulfur, PDFA and various aromatic and benzyl diamines were developed without any catalyst under mild conditions, producing a series of polythioureas with high yields and high Mws of up to 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1. In particular, aromatic polythioureas with electron-withdrawing carbonyl groups were successfully prepared in 85% yield and with a Mw of 18
900 g mol−1. In particular, aromatic polythioureas with electron-withdrawing carbonyl groups were successfully prepared in 85% yield and with a Mw of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1, and they proved to possess the highest refractive index of 1.8133 at 633 nm among the tested aromatic polythioureas.
600 g mol−1, and they proved to possess the highest refractive index of 1.8133 at 633 nm among the tested aromatic polythioureas.
Results and discussion
Catalyst-free multicomponent reactions (MCRs) of sulfur, PDFA, and amines
To investigate the one-pot MCR of sulfur, PDFA, and an aromatic amine, an MCR was conducted in DME at 50 °C for 0.5 h, and 3-acetylaniline 1a was studied as an example of an electron-withdrawing-group-containing aromatic amine (Table 1). Luckily, the reaction went smoothly without any catalyst to afford the thiourea product 4a in 64% yield, calculated based on the 1H NMR spectrum, suggesting that the conversion of sulfur, PDFA, and an amine to an isocyanate, and its further reaction with amine to generate a thiourea could be combined in a one-pot MCR. The MCR was then investigated in DME, THF, DMSO, DMF, and DMAc to screen a suitable solvent, and DMAc afforded the optimal yield of 80%. MCRs of sulfur, PDFA, and 1a in DMAc were then conducted at different temperatures ranging from 50 to 80 °C, suggesting that there was no obvious change in yield within this temperature range. The monomer loading ratio was then studied in DMAc at 60 °C, where the ratio of [1a] to [3] was changed from 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 to 2.0
1.2 to 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 while keeping the ratio of sulfur to PDFA fixed; the highest yield of 94% (91% isolated yield) was obtained with [1a]
1.4 while keeping the ratio of sulfur to PDFA fixed; the highest yield of 94% (91% isolated yield) was obtained with [1a]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [3] = 2.0
[3] = 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4.
1.4.
| Entry | [1a]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/8[S8] 1/8[S8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [3] [3] |
Solvent | T (°C) | Yielda (%) |
|---|---|---|---|---|
| The reaction was carried out under nitrogen conditions at the corresponding temperature with 1 mL of solvent; the concentration of 1a was 1.0 M, and the reaction time was 0.5 h.a Calculated based on 1H NMR spectra, using 1,3,5-trimethylbenzene as the internal standard.b Isolated yield. | ||||
| 1 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
DME | 50 | 64 |
| 2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
THF | 50 | 67 |
| 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
DMSO | 50 | 54 |
| 4 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
DMF | 50 | 72 |
| 5 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
DMAc | 50 | 80 |
| 6 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
DMAc | 60 | 83 |
| 7 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
DMAc | 70 | 82 |
| 8 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
DMAc | 80 | 82 |
| 9 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
DMAc | 60 | 87 |
| 10 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
DMAc | 60 | 94 (91)b |
| 11 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
DMAc | 60 | 88 |
| 12 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.0 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
DMAc | 60 | 90 |
With the high efficiency of the catalyst-free MCR, the monomer scope of amines was then studied, and a series of commercially available electron-withdrawing-group-containing aromatic amines 1a–1g was selected to react with sulfur and PDFA in DMAc at 60 °C for 0.5 h (Fig. 2). Under the optimized conditions, these electron-deficient aromatic amines generally reacted efficiently, affording the corresponding thiourea products 4a–4g in high yields. Aromatic amines with benzoyl, ester, –NO2, and sulfonyl groups generally worked well in the MCR, producing rarely reported aromatic thiourea structures in 84–90% isolated yields, demonstrating the good structural tolerance of the MCR. 3-Cyanoaniline 1e showed a lower yield, probably because of a side reaction involving the –CN group. Besides these electron-deficient aromatic amines, p-toluidine 1h with an electron-donating methyl group could also react efficiently under the same conditions, affording the thiourea 4h in 93% yield, showing a similarly high yield to the electron-deficient aromatic amines. Moreover, in addition to aromatic amines, the benzylamine 1i was also investigated, and the MCR of sulfur, PDFA and 1i also proceeded smoothly to afford the thiourea 4i in 62% yield. The catalyst-free MCR hence exhibited a broad amine substrate scope, accommodating electron-deficient and electron-rich aromatic amines as well as aliphatic amine, suggesting its robustness and wide applicability.
 | ||
| Fig. 2 MCRs of sulfur, PDFA and aromatic amines to form thioureas; the single-crystal-based ORTEP diagrams of compounds 4d–4i are given. | ||
During the catalyst-free MCR of p-toluidine 1h, sulfur and PDFA in DMAc, after reacting at 60 °C for 20 min, the reaction solution was cooled in an ice bath and the mixture was filtered for high-resolution mass spectrometry (HRMS) analysis. The characteristic peaks of thiocarbonyl fluoride (found 82.9770, calcd 82.9767) and isothiocyanate (found 150.0374, calcd 150.0377) were observed, indicating the key intermediates involved in the reaction mechanism (Fig. S1†). In this MCR, the difluorocarbene active species A was generated from PDFA upon heating, with the release of Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and CO2; A then reacted with sulfur to produce thiocarbonyl fluoride B according to the literature.45 The nucleophilic substitution of the amine then took place to afford the intermediate C, and after F− was eliminated, the isothiocyanate intermediate D was generated, which then underwent nucleophilic addition with another amine molecule to afford the thiourea product E (Fig. S2†).
S and CO2; A then reacted with sulfur to produce thiocarbonyl fluoride B according to the literature.45 The nucleophilic substitution of the amine then took place to afford the intermediate C, and after F− was eliminated, the isothiocyanate intermediate D was generated, which then underwent nucleophilic addition with another amine molecule to afford the thiourea product E (Fig. S2†).
Moreover, several single crystals of these thiourea compounds, including unreported thiourea compounds, were obtained via solvent evaporation or diffusion. The thioureas containing –CF3, –CN, –NO2, and sulfonyl groups, 4d–4g, generally adopted “W”-shaped molecular configurations in their single crystal structures (Fig. 2). For example, in the single crystal structure of the NO2-containing thiourea 4f, two THF solvent molecules were captured through N–H⋯O hydrogen bonds. The –NO2 groups also contributed to the formation of rich hydrogen bond networks in the crystal structure (Fig. S3†). Different from that, the single crystal of the CH3-containing thiourea 4h was monoclinic with a space group of Pbcn, adopting a “V”-shaped molecular conformation, which was the same as reported crystals prepared via other synthetic approaches.48 A dense hydrogen bond network was formed through –N–H⋯S bonds with a length of 2.496 Å (Fig. S3†). Different from the two above-mentioned types of thioureas, 4i prepared from a benzylamine possessed an asymmetric conformation. Different types of thiourea structures hence adopted different molecular conformations because the hybridization of C–N bonds imparts cis–trans isomeric properties on the C–N bonds of the thiourea moiety, influencing the spatial arrangement of associated N–H bonds. The presence of multiple and diverse hydrogen bonds endows the polythioureas with various hydrogen bond networks.
Catalyst-free multicomponent polymerizations of elemental sulfur, PDFA, and diamines
To explore the multicomponent polymerizations of sulfur, PDFA and diamines, commercially available diphenylmethanediamine 5a was selected as an example to polymerize with sulfur and PDFA. The MCP was conducted in DMF at 40 °C for 3 h under nitrogen with [5a]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/8[S8]
1/8[S8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PDFA] = 1.0
[PDFA] = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6
2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3, and a polymer product with a Mw of 10
1.3, and a polymer product with a Mw of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1 was obtained in 60% yield. It should be noted that besides the thiourea moieties in the polymer product, sulfur atoms were also transformed to Ph3P
200 g mol−1 was obtained in 60% yield. It should be noted that besides the thiourea moieties in the polymer product, sulfur atoms were also transformed to Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S as a byproduct. In solvents with higher polarity, such as DMAc and DMSO, the polymerization yields improved, and the best result was obtained in DMAc, with the polymeric product with a Mw of 16
S as a byproduct. In solvents with higher polarity, such as DMAc and DMSO, the polymerization yields improved, and the best result was obtained in DMAc, with the polymeric product with a Mw of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 g mol−1 obtained in 84% yield (Table S1†). The temperature was found to be crucial for polymerization, and the MCP was conducted at a range of temperatures from 40 to 80 °C (Table 2). The polymerization results obtained upon increasing the temperature from 40 to 60 °C suggested that the Mw of the polymer gradually increased, achieving 53
800 g mol−1 obtained in 84% yield (Table S1†). The temperature was found to be crucial for polymerization, and the MCP was conducted at a range of temperatures from 40 to 80 °C (Table 2). The polymerization results obtained upon increasing the temperature from 40 to 60 °C suggested that the Mw of the polymer gradually increased, achieving 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1 at 60 °C, whereas further heating resulted in a decrease of the Mw. Considering the temperature required for the efficient decomposition of PDFA to form a carbene intermediate and that a high temperature might induce side reactions, such as generating byproduct with N-SCF3 to terminate the polymerization reaction,45 60 °C was found to be the optimal temperature. The monomer feed ratio for polymerization was then optimized at 60 °C with a fixed ratio of PDFA to sulfur (Table 2). Upon increasing the amount of PDFA, both the yield and Mw first increased and then decreased, and when [5a]
900 g mol−1 at 60 °C, whereas further heating resulted in a decrease of the Mw. Considering the temperature required for the efficient decomposition of PDFA to form a carbene intermediate and that a high temperature might induce side reactions, such as generating byproduct with N-SCF3 to terminate the polymerization reaction,45 60 °C was found to be the optimal temperature. The monomer feed ratio for polymerization was then optimized at 60 °C with a fixed ratio of PDFA to sulfur (Table 2). Upon increasing the amount of PDFA, both the yield and Mw first increased and then decreased, and when [5a]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/8[S8]
1/8[S8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [PDFA] = 1
[PDFA] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6
2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3, the highest Mw was obtained, suggesting a balancing of the effects from excess PDFA and sulfur.
1.3, the highest Mw was obtained, suggesting a balancing of the effects from excess PDFA and sulfur.
| Entry | T (°C) | [5a]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/8[S8] 1/8[S8]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [3] [3] |
M
w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (g mol−1) b (g mol−1) |
M w/Mn | Yield (%) |
|---|---|---|---|---|---|
| a The substrate 5a (1.0 mmol) was combined with Ph3P+CF2CO2− and S8 in 1 mL of DMAc and the mixture was stirred for 3 h at the corresponding temperature under N2. b The data were obtained from GPC testing in DMF, with monodisperse PMMA as the standard sample. | |||||
| 1 | 40 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.34 | 84 |
| 2 | 50 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.26 | 82 |
| 3 | 60 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.82 | 94 |
| 4 | 70 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.51 | 98 |
| 5 | 80 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.6 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.50 | 93 |
| 6 | 60 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0 2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.24 | 65 |
| 7 | 60 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.35 | 79 |
| 8 | 60 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.73 | 97 |
| 9 | 60 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.0 3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.47 | 88 |
| 10 | 60 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.73 | 90 |
| 11 | 60 | 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.8 2.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.77 | 94 |
Surprisingly, unlike other sulfur- and aromatic-amine-based polymerizations, the addition of base was not necessary or even beneficial to the MCP, and the incorporation of K2CO3, Na2CO3, NaOH, KF, or triethylamine did not improve the polymerization results (Table S2†), suggesting that simple base-free conditions were optimal, probably due to the reduction of base-promoted side reactions such as the generation of trifluoromethylthio-containing byproducts.42 The progression of the MCP over time was also investigated; it could afford the polymer product with a Mw of 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 in 87% yield within 10 min, reaching 65
000 g mol−1 in 87% yield within 10 min, reaching 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1 in 93% yield after 1 h, suggesting fast and efficient polymerization (Table S3†). Last but not least, the influence of the concentration of the monomer 5a was then investigated while fixing the monomer feed ratio. A low concentration of 0.25 M resulted in relatively low Mws, while the monomer could not be completely dissolved at a high concentration of 1.0 M; 0.5 M was found to be the optimal concentration (Table S4†).
900 g mol−1 in 93% yield after 1 h, suggesting fast and efficient polymerization (Table S3†). Last but not least, the influence of the concentration of the monomer 5a was then investigated while fixing the monomer feed ratio. A low concentration of 0.25 M resulted in relatively low Mws, while the monomer could not be completely dissolved at a high concentration of 1.0 M; 0.5 M was found to be the optimal concentration (Table S4†).
A kinetic study of the MCP
The real-time polymerization process during the MCP of sulfur, PDFA and 5a was then monitored in DMSO at 60 °C using in situ IR measurements (Fig. 3). Due to the interference caused by the coincidence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak of DMAc with the C
O stretching vibration peak of DMAc with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond, DMSO was chosen as the reaction solvent. In the FT-IR spectra of the model compounds 4h and P1 (Fig. S4†), the double peaks at ∼3400 cm−1 associated with the stretching vibration of the –NH2 group of p-toluidine disappeared, while the stretching vibration peak of –NH– appeared at ∼3200 cm−1. Meanwhile, the stretching vibration peaks of the C
S bond, DMSO was chosen as the reaction solvent. In the FT-IR spectra of the model compounds 4h and P1 (Fig. S4†), the double peaks at ∼3400 cm−1 associated with the stretching vibration of the –NH2 group of p-toluidine disappeared, while the stretching vibration peak of –NH– appeared at ∼3200 cm−1. Meanwhile, the stretching vibration peaks of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonds of 4h and P1 were located at 1555 cm−1 and 1537 cm−1, respectively. Through a comparison of the in situ IR spectra of 5a, PDFA and P1, the peak at 1537 cm−1 was attributed to the stretching vibration of C
S bonds of 4h and P1 were located at 1555 cm−1 and 1537 cm−1, respectively. Through a comparison of the in situ IR spectra of 5a, PDFA and P1, the peak at 1537 cm−1 was attributed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bonds, and the peak at 1702 cm−1 was attributed to the stretching vibration of C
S bonds, and the peak at 1702 cm−1 was attributed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in PDFA (Fig. S5†). The stacked time-dependent IR spectra of the polymerization solution of sulfur, PDFA and 5a in DMSO showed that a new increasing peak appeared at 1537 cm−1, and the peak intensity at 1702 cm−1 continuously decreased (Fig. 3A). The time-dependent peak intensities at 1537 cm−1 suggested that the reaction could be completed in 15 min (Fig. 3B and C), proving the rapid and efficient polymerization.
O bonds in PDFA (Fig. S5†). The stacked time-dependent IR spectra of the polymerization solution of sulfur, PDFA and 5a in DMSO showed that a new increasing peak appeared at 1537 cm−1, and the peak intensity at 1702 cm−1 continuously decreased (Fig. 3A). The time-dependent peak intensities at 1537 cm−1 suggested that the reaction could be completed in 15 min (Fig. 3B and C), proving the rapid and efficient polymerization.
To explore the monomer scope of the catalyst-free MCP, a series of commercially available diamines 5a–5h was selected to be used as the monomers; these were polymerized with sulfur and PDFA under the optimal conditions in DMAc at 60 °C under nitrogen (Fig. 4 and Fig. S6†). Polythiourea P1 with a high Mw of 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 g mol−1 was obtained in 93% yield, and good polymerization results were also obtained for the ether- and thioether-containing polythioureas P2 and P3. While these aromatic polythioureas could generally be dissolved in DMF, DMSO, and DMAc, P4 and P5 showed limited solubility; during their synthesis, large amounts of insoluble precipitate were formed, and only the soluble part was used for characterization. The diamine 5f with large steric hindrance might twist the polymer chain and avoid strong interchain stacking, which might produce a polythiourea with improved solubility, while sacrificing yield. Furthermore, the MCPs of aliphatic diamines were not as efficient as those with aromatic diamines, and polythioureas P7 and P8 possessed decreased yields and Mws. The polydispersity of the molecular weights of these polymers was generally low for a step-growth polymerization approach, which was probably associated with the special solubility of these polythioureas.
900 g mol−1 was obtained in 93% yield, and good polymerization results were also obtained for the ether- and thioether-containing polythioureas P2 and P3. While these aromatic polythioureas could generally be dissolved in DMF, DMSO, and DMAc, P4 and P5 showed limited solubility; during their synthesis, large amounts of insoluble precipitate were formed, and only the soluble part was used for characterization. The diamine 5f with large steric hindrance might twist the polymer chain and avoid strong interchain stacking, which might produce a polythiourea with improved solubility, while sacrificing yield. Furthermore, the MCPs of aliphatic diamines were not as efficient as those with aromatic diamines, and polythioureas P7 and P8 possessed decreased yields and Mws. The polydispersity of the molecular weights of these polymers was generally low for a step-growth polymerization approach, which was probably associated with the special solubility of these polythioureas.
Most importantly, considering challenging electron-withdrawing-group-containing aromatic amine monomers, the sulfonyl- and carbonyl-group-containing electron-deficient aromatic diamines 5i and 5j were studied in the catalyst-free MCP (Fig. 5), and they were polymerized with sulfur and PDFA in DMAc at 60 °C for 1 h. The electron-deficient aromatic polythioureas P9 and P10 were obtained in 76% and 85% yields, respectively, with Mws of 7900 g mol−1 (P9) and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1 (P10). These rarely reported results suggested the successful polymerization of electron-deficient aromatic diamine monomers.
600 g mol−1 (P10). These rarely reported results suggested the successful polymerization of electron-deficient aromatic diamine monomers.
 | ||
| Fig. 5 MCPs of the electron-withdrawing-group-containing diamines 5i–5j. Mws are determined using GPC in DMF based on a PMMA standard sample. | ||
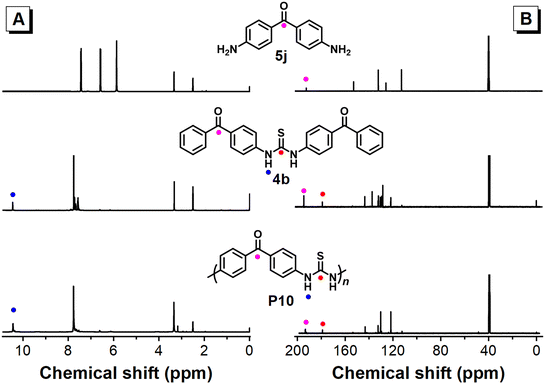
Structural characterization
The chemical structures of these polythioureas were confirmed based on their 1H and 13C NMR spectra (Fig. 6 and S7†). The resonance of –NH2 protons seen in the spectrum of 5j at δ 5.87 disappeared in the 1H NMR spectra of 4b and P10; meanwhile, the –NH– protons of the thiourea groups of 4b and P10 emerged at δ 10.45 and δ 10.44, respectively (Fig. 6A). Similarly, in the 13C NMR spectra of 5j, 4b and P10 (Fig. 6B), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak appeared at δ 192.49, δ 199.83 and δ 193.31, respectively. Most importantly, the C
O peak appeared at δ 192.49, δ 199.83 and δ 193.31, respectively. Most importantly, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S peak emerged at δ 184.33 and δ 178.98 in the spectra of 4b and P10, respectively. The 1H and 13C NMR spectra of P1–P9 are also provided in Fig. S8–S11.† The –NH– proton resonances of the thiourea groups of 4h and P1 are located at δ 9.58 and δ 9.67, respectively, in the 1H NMR spectra, while the C
S peak emerged at δ 184.33 and δ 178.98 in the spectra of 4b and P10, respectively. The 1H and 13C NMR spectra of P1–P9 are also provided in Fig. S8–S11.† The –NH– proton resonances of the thiourea groups of 4h and P1 are located at δ 9.58 and δ 9.67, respectively, in the 1H NMR spectra, while the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S resonances of 4h and P1 are at δ 179.63 and δ 179.49, respectively, in the 13C NMR spectra. Compared with those of P1, the –NH– proton resonance peaks of P9 and P10 appeared at a lower field value of ∼δ 10.5, and the resonance peaks for the aliphatic polythioureas P7 and P8 appeared at a higher field value of δ 8.0. The characteristic C
S resonances of 4h and P1 are at δ 179.63 and δ 179.49, respectively, in the 13C NMR spectra. Compared with those of P1, the –NH– proton resonance peaks of P9 and P10 appeared at a lower field value of ∼δ 10.5, and the resonance peaks for the aliphatic polythioureas P7 and P8 appeared at a higher field value of δ 8.0. The characteristic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S resonance peaks generally emerged at ∼δ 185. Moreover, in the XPS spectra, the binding energies for the S 2p orbitals of 4h and P1 are closely matched at 163.18/161.89 eV and 163.40/162.10 eV, respectively, proving the similar chemical state of sulfur, and also indicating that there was no elemental sulfur residue in the polymer product (Fig. S12†). In the FT-IR spectra of P2–P10, the C
S resonance peaks generally emerged at ∼δ 185. Moreover, in the XPS spectra, the binding energies for the S 2p orbitals of 4h and P1 are closely matched at 163.18/161.89 eV and 163.40/162.10 eV, respectively, proving the similar chemical state of sulfur, and also indicating that there was no elemental sulfur residue in the polymer product (Fig. S12†). In the FT-IR spectra of P2–P10, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond peaks generally emerged at 1530–1594 cm−1 (Fig. S13 and S14†).
S bond peaks generally emerged at 1530–1594 cm−1 (Fig. S13 and S14†).
The thermal stability and refractivity of these polythioureas
Thermogravimetric analysis (TGA) indicated that these polythioureas enjoyed good thermal resistance, with their decomposition temperatures (Td) at 5 wt% weight loss ranging from 191 °C to 246 °C; the intermolecular hydrogen bonds among the abundant thiourea moieties and the large number of aromatic rings might contribute to the good thermal stability (Fig. 7A). Differential scanning calorimetry (DSC) analysis of these polythioureas was also conducted. The glass transition temperature was observed for P10 to be 127 °C (Fig. S15†), and no obvious thermal transition temperatures were observed for the other polymers.Most of the polymers possess satisfying solubility, enabling facile thin film preparation via a spin-coating method. The wavelength-dependent refractive indices of thin films of P1–P3, P6–P7, and P9–P10 were measured within the wavelength range of 400–1700 nm (Fig. 7B). The aromatic polythioureas generally showed higher refractive indices compared with the polythiourea P7 prepared from 1,4-bis(aminomethyl)benzene; and P10 with electron-withdrawing carbonyl groups possessed the highest n633 nm value of 1.8133, which was exceptionally high for organic polymer materials (Table S5†). Moreover, several polymers possessed high n values above 1.7 at 1700 nm, which have rarely been reported. The transmittance spectra of these polymers were also investigated, and their spin-coated thin films on quartz plates were measured. As shown in Fig. S16,† among the tested polythiourea samples of uniform thin films on quartz plates, P1, P2, and P10 showed excellent light transmittance of above 96% beyond 400 nm. Photos of solid samples and thin films on quartz plates of P10 also suggested its high transmittance. These aromatic polythioureas, especially P10 with electron-withdrawing groups, possessed excellent optical properties, and they could be promising optical materials for optical lenses and CMOS systems.
Conclusions
In this work, catalyst-free multicomponent reactions of sulfur, PDFA and amines were developed in DMAc, which proved to be applicable and efficient for both electron-rich and electron-deficient amines, as well as benzylamine. PDFA was then used as a monomer for polymerization with sulfur and diamine without any catalyst; this could react efficiently in DMAc at 60 °C under mild conditions, affording aromatic polythioureas. Notably, aromatic amines containing electron-withdrawing groups could also proceed efficiently, which greatly expanded the amine monomer scope and range of obtained polythiourea structures. Tests of the optical properties of these polythiourea films showed that the electron-deficient polythioureas possessed high refractive indices, reaching 1.8133 at 633 nm, and they could find promising applications in optical sensors, semiconductors, and catalysis.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for compounds 4d–4i have been deposited at the Cambridge Crystallographic Data Centre under the CCDC 2407294–2407296, 2407300, 2245406 and 2407301† and can be obtained from https://www.ccdc.cam.ac.uk.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (22325102 and 52173005), the Ministry of Science and Technology of China (2021YFA1501600), the Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates (2019B030301003), Guangdong Basic and Applied Basic Research Foundation (2023B1515040003), and the Fundamental Research Funds for the Central Universities (2022ZYGXZR107).References
- Y. Yanagisawa, Y. Nan, K. Okuro and T. Aida, Science, 2018, 359, 72 CrossRef CAS PubMed.
- Y. Fujisawa, A. Asano, Y. Itoh and T. Aida, J. Am. Chem. Soc., 2021, 143, 15279–15285 CrossRef CAS PubMed.
- J. Zhu, S. Zhao, J. Luo, W. Niu, J. T. Damron, Z. Zhang, M. A. Rahman, M. A. Arnould, T. Saito, R. Advincula, A. P. Sokolov, B. G. Sumpter and P.-F. Cao, CCS Chem., 2023, 5, 1841–1853 CrossRef CAS.
- S. Watanabe, L. M. Cavinato, V. Calvi, R. van Rijn, R. D. Costa and K. Oyaizu, Adv. Funct. Mater., 2024, 34, 2404433 CrossRef CAS.
- H. Li, Y. Zhou, Y. Liu, L. Li, Y. Liu and Q. Wang, Chem. Soc. Rev., 2021, 50, 6369–6400 RSC.
- C. Wu, Z. Z. Li, G. M. Treich, M. Tefferi, R. Casalini, R. Ramprasad, G. A. Sotzing and Y. Cao, Appl. Phys. Lett., 2019, 115, 163901 CrossRef.
- S. Yoo, H. Park, Y. S. Kim, J. C. Won, D.-G. Kim and Y. H. Kim, J. Mater. Chem. C, 2021, 9, 77–81 RSC.
- S. Wu, W. Li, M. Lin, Q. Burlingame, Q. Chen, A. Payzant, K. Xiao and Q. M. Zhang, Adv. Mater., 2013, 25, 1734–1738 CrossRef CAS PubMed.
- H. Feng, N. Zheng, W. Peng, C. Ni, H. Song, Q. Zhao and T. Xie, Nat. Commun., 2022, 13, 397 CrossRef CAS.
- C. Li, L. Shi, W. Yang, Y. Zhou, X. Li, C. Zhang and Y. Yang, Nanoscale Res. Lett., 2020, 15, 36 CrossRef CAS.
- T. Tian, R. Hu and B. Z. Tang, J. Am. Chem. Soc., 2018, 140, 6156–6163 CrossRef CAS.
- A. Banihashemi, H. Hazarkhani and A. Abdolmaleki, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 2106–2111 CrossRef CAS.
- Y. M. Li, Z. P. Zhang, M. Z. Rong and M. Q. Zhang, Nat. Commun., 2022, 13, 2633 CrossRef CAS.
- S. Wu, M. Luo, D. J. Darensbourg and X. Zuo, Macromolecules, 2019, 52, 8596–8603 CrossRef CAS.
- J. Zhang, F. Ye, J. Huo, J. Peng, R. Hu and B. Z. Tang, Chin. J. Polym. Sci., 2023, 41, 1563–1576 CrossRef CAS.
- S. H. Je, O. Buyukcakir, D. Kim and A. Coskun, Chem, 2016, 1, 482–493 CAS.
- L. He, H. Zhao and P. Theato, Angew. Chem., Int. Ed., 2018, 57, 13012–13014 CrossRef CAS PubMed.
- J. Lim, J. Pyun and K. Char, Angew. Chem., Int. Ed., 2015, 54, 3249–3258 CrossRef CAS.
- D. A. Boyd, Angew. Chem., Int. Ed., 2016, 55, 15486–15502 CrossRef CAS.
- T. Lee, P. T. Dirlam, J. T. Njardarson, R. S. Glass and J. Pyun, J. Am. Chem. Soc., 2022, 144, 5–22 CrossRef CAS PubMed.
- J. Jia, J. Liu, Z.-Q. Wang, T. Liu, P. Yan, X.-Q. Gong, C. Zhao, L. Chen, C. Miao, W. Zhao, S. D. Cai, X.-C. Wang, A. I. Cooper, X. Wu, T. Hasell and Z.-J. Quan, Nat. Chem., 2022, 14, 1249–1257 CrossRef CAS.
- J. J. Griebel, S. Namnabat, E. T. Kim, R. Himmelhuber, D. H. Moronta, W. J. Chung, A. G. Simmonds, K. J. Kim, J. van der Laan, N. A. Nguyen, E. L. Dereniak, M. E. Mackay, K. Char, R. S. Glass, R. A. Norwood and J. Pyun, Adv. Mater., 2014, 26, 3014–3018 CrossRef CAS PubMed.
- M. J. H. Worthington, R. L. Kucera and J. M. Chalker, Green Chem., 2017, 19, 2748–2761 RSC.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS.
- J.-Y. Chao, T.-J. Yue, B.-H. Ren, G.-G. Gu, X.-B. Lu and W.-M. Ren, Angew. Chem., Int. Ed., 2022, 61, e202115950 CrossRef CAS PubMed.
- J. Zhang, Q. Zang, F. Yang, H. Zhang, J. Z. Sun and B. Z. Tang, J. Am. Chem. Soc., 2021, 143, 3944–3950 CrossRef CAS PubMed.
- Y. Hu, L. Zhang, Z. Wang, R. Hu and B. Z. Tang, Polym. Chem., 2023, 14, 2617–2623 RSC.
- A. Yasin, Y. Chen, Y. Liu, L. Zhang, X. Zan and Y. Zhang, Polym. Chem., 2020, 11, 810–819 RSC.
- S. Wang, C. Fu, Y. Wei and L. Tao, Prog. Chem., 2014, 26, 1099–1106 CAS.
- R. Kakuchi, Angew. Chem., Int. Ed., 2014, 53, 46–48 CrossRef CAS.
- P. Stiernet and A. Debuigne, Prog. Polym. Sci., 2022, 128, 101528 CrossRef CAS.
- H. Liu, H.-H. Lu, J. Zhuang and S. Thayumanavan, J. Am. Chem. Soc., 2021, 143, 20735–20746 CrossRef CAS.
- X. Wu, H. Lin, F. Dai, R. Hu and B. Z. Tang, CCS Chem., 2020, 2, 191–202 CrossRef CAS.
- W. Li, X. Wu, Z. Zhao, A. Qin, R. Hu and B. Z. Tang, Macromolecules, 2015, 48, 7747–7754 CrossRef CAS.
- W. X. Cao, F. Dai, R. Hu and B. Z. Tang, J. Am. Chem. Soc., 2020, 142, 978–986 CrossRef CAS PubMed.
- Y. Huang, Y. Yu, R. Hu and B. Z. Tang, J. Am. Chem. Soc., 2024, 146, 14685–14696 CrossRef CAS PubMed.
- N. Zheng, H. Gao, Z. Jiang and W. Song, Sci. China: Chem., 2023, 66, 870–877 CAS.
- F. Schaufelberger, K. Seigel and O. Ramström, Chem. – Eur. J., 2020, 26, 15581–15588 CrossRef CAS PubMed.
- Y. Zhou, Z. Zhu, K. Zhang and B. Yang, Macromol. Rapid Commun., 2023, 44, 2300411 CrossRef CAS PubMed.
- X. Wang, S. Pan, Q. Luo, Q. Wang, C. Ni and J. Hu, J. Am. Chem. Soc., 2022, 144, 12202–12211 CrossRef CAS.
- J. Yu, J.-H. Lin, D. H. Yu, R. B. Du and J.-C. Xiao, Nat. Commun., 2019, 10, 5362 CrossRef.
- J. Zheng, R. Cheng, J.-H. Lin, D.-H. Yu, L. Ma, L. Jia, L. Zhang, L. Wang, J.-C. Xiao and S. H. Liang, Angew. Chem., Int. Ed., 2017, 56, 3196–3200 CrossRef CAS.
- T. Scattolin, K. Deckers and F. Schoenebeck, Angew. Chem., Int. Ed., 2017, 56, 221–224 CrossRef CAS PubMed.
- D. L. S. Brahms and W. P. Dailey, Chem. Rev., 1996, 96, 1585–1632 CrossRef CAS.
- J. Yu, J.-H. Lin and J.-C. Xiao, Angew. Chem., Int. Ed., 2017, 56, 16669–16673 CrossRef CAS.
- J. Zheng, L. Wang, J.-H. Lin, J.-C. Xiao and S. H. Liang, Angew. Chem., Int. Ed., 2015, 54, 13236–13240 CrossRef CAS.
- J.-J. Luo, M. Zhang, J.-H. Lin and J.-C. Xiao, J. Org. Chem., 2017, 82, 11206–11211 CrossRef CAS.
- M. Soriano-Garcia, G. T. Chavez, F. D. Cedillo, A. E. D. Perez and G. A. Hernandez, Anal. Sci., 2003, 19, 1087–1088 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2407294–2407296, 2407300, 2407301, 2245406. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4py01387e |
| This journal is © The Royal Society of Chemistry 2025 |