Open Access Article
Open Access ArticleEfficient and simplified strategy to access novel polysulfamate materials: from laboratory research to industrial production†
Xingyu
Ma‡
a,
Pengqiang
Liang
b,
Zhongqiang
Zhao
c,
Jinwei
Chen
b,
Xueqing
Wang
c,
Yunbin
Zhou
c,
Xianxing
Jiang
 *a and
Weiwei
Zhu‡
*a and
Weiwei
Zhu‡
 *a
*a
aState Key Laboratory of Anti-Infective Drug Discovery and Development, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006, China. E-mail: zhuww8@mail.sysu.edu.cn
bCollege of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, China
cInner Mongolia Tuwei New Material Technology Co., Ltd, Mengxi Industrial Park, Etoke Banner, Erdos 016062, China
First published on 26th February 2025
Abstract
The development of materials from laboratory research to industrial production is a complex, challenging, but significant process. Polysulfamates have not been industrially available to date because of the absence of efficient and economical synthetic methods. Herein, a comprehensive process for the development of novel polysulfamate (PSA) materials from laboratory research to industrial manufacture is reported. PSAs were prepared with high molecular weight and narrow polydispersity through nucleophilic polycondensation between aryl bisphenols and disulfamoyl difluorides in the presence of an inorganic base. The polymerization process was stable in moisture and air. The industrial production of PSAs was achieved on 100 kg scale with the assistance of a cooperative factory for the first time. The PSAs displayed excellent solvent tolerance, acid/base resistance, thermal stability, machinability and mechanical properties, which were promising for their application in the area of engineering plastics, as well as high-performance resins.
Introduction
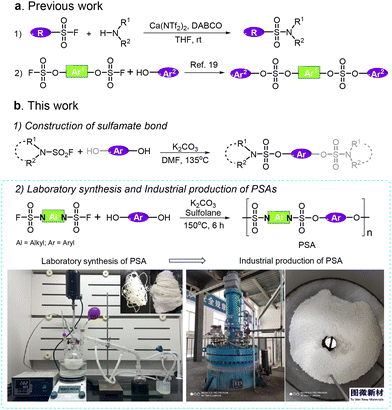
The sulfur(VI) fluoride exchange (SuFEx) reaction is an efficient and rapid method to synthesize functional molecules containing (poly)sulfates, (poly)sulfonates or sulfamates,1,2 which has been widely applied in the fields of organic synthesis, materials science, pharmaceutical chemistry and bio-chemistry.3–5 In materials chemistry, especially, polysulfates and polysulfonates with excellent properties have been successfully synthesized through the SuFEx reaction.6 Additionally, this new click reaction has also been instrumental in the formation of S(VI)–N bonds,7 resulting in the construction of sulfonamides, sulfamates, and sulfamides.8,9 Sulfamides are valuable motifs, which can be found in therapeutic applications10,11 and the catalytic synthesis of pyrimidine systems,12,13 as well as in polymer synthesis.14,15 However, despite their good performance in bioactive compounds, sulfamates are comparatively underexplored because of the challenges posed by their synthesis. In particular, polysulfamate (PSA) materials have not been available to date because of the absence of an efficient and economical synthetic method. S. Mahapatra et al. reported an advanced process to synthesize nitrogenous sulfur(VI) compounds.16 Compared to traditional synthetic protocols, S(VI) fluorides were activated by calcium triflimide and DABCO for SuFEx with amines (Fig. 1a1). However, the stoichiometric utilization of Ca(NTf2)2 and DABCO sets up a barrier against the large-scale synthesis of PSAs.With the progress of science and technology, the modification of existing materials or the development of new materials are of great significance.17,18 We previously reported a nucleophilic process to construct sulfate bonds through the reaction of aryl phenols and aryl fluorosulfates (Fig. 1a2).19 Polysulfates are promising engineering polymers due to their excellent mechanical and chemical properties.19–21 Thus, we hypothesized that the reaction between aryl phenols and R2N–SO2F in the presence of an inorganic base (IOB) could be applicable to the synthesis of sulfamates. We proposed that polymers bearing a sulfamate bond instead of a sulfate bond may lead to a PSA with the properties of tenacity, machinability and mechanical performance.
Materials science plays an indispensable role in the progress of science, transmission of civilization and the development of human society.22–24 The purpose of laboratory research is to provide primary products and technical services for industrial production. Generally, the development of materials from laboratory research to industrial production and commercial applications is a complex, challenging and protracted process.25 For example, polycarbonates (PCs) were first prepared by Einhorn in 1898, but the widely used PC produced from bisphenol A (BPA) was prepared in 1941.26 The polymerization of vinyl chloride in sealed tubes was reported in 1872, but commercial interest in poly(vinyl chloride) was revealed in 1928.27 Polyethylene (PE) was produced from ethylene in 1936 by Fawcett, but all commercial PE was produced by high-pressure processes until the mid-1950s.28 Therefore, substantial efforts should be made to achieve the scaled-up and industrial production of PSA, which is necessary to support their industrial application.
The goal of our research is to develop an efficient and cost-effective protocol to access polysulfamates with high molecular weight and low polydispersity index (PDI) both in laboratory research and industrial production. Herein, we report a simplified method to access sulfamates (Fig. 1b1) and polysulfamates (Fig. 1b2) efficiently through the reaction between aliphatic sulfonyl fluorides and aryl phenols in the presence of IOBs. We also evaluated the physical and chemical properties of the polysulfamates. This study aims to bridge the gaps between laboratory research, industrial production, and practical applications of PSAs.
Results and discussion
Nucleophilic construction of a sulfamate bond
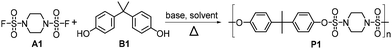
We began our study by treating pyrrolidine-1-sulfonyl fluoride (1a) with 4-(2-phenylpropan-2-yl) phenol (2a) in the presence of K2CO3 in N,N-dimethylformamide (DMF). (For details of the screening of conditions, see ESI section 2.1.†)19 To our delight, the corresponding sulfamates could be obtained in excellent yields when the reaction was conducted at 135 °C. Fig. 2 shows the substrate scope of this nucleophilic sulfamate bond-construction reaction. Various aliphatic sulfonyl fluorides can react with aryl phenols, affording the desired sulfamates in excellent yields. Among them, piperidine-1-sulfonyl fluoride displayed higher reactivity with aryl phenols than pyrrolidine-1-sulfonyl fluoride under the reaction conditions (Fig. 2). These results inspired us to try polycondensation between disulfamoyl difluorides and aryl diphenols.Laboratory research into PSAs
| Entry | Base | Solvent | T/°C | M PSn/kDa | PDI |
|---|---|---|---|---|---|
| a The reaction was carried out with 2.5 mmol A1 (1.00 equiv.) and B1 (1.01 equiv.) in 5 mL of solvent in the presence of 2.2 equiv. of base for 6 h. b 10 mL of DMF was used. c 1.1 equiv. of base was used. d The reaction was carried out for 12 h. T, external temperature. MPSn, number-average molecular weight with polystyrene as standard. PDI, polydispersity index. | |||||
| 1 | Na2CO3 | DMF | 25 | — | — |
| 2 | Na2CO3 | Sulfolane | 80 | 1.26 | 1.80 |
| 3 | K2CO3 | Sulfolane | 80 | 1.48 | 1.93 |
| 4 | Na2CO3 | DMF | 135 | 61.49 | 1.59 |
| 5 | K2CO3 | DMF | 135 | 77.84 | 1.58 |
| 6b | K2CO3 | DMF | 135 | 69.52 | 1.69 |
| 7c | K2CO3 | DMF | 135 | 64.79 | 1.66 |
| 8 | K2CO3 | DMF | 150 | 42.17 | 1.49 |
| 9b | K2CO3 | DMF | 150 | 44.57 | 1.52 |
| 10d | K2CO3 | DMF | 150 | 61.38 | 1.66 |
| 11b,d | K2CO3 | DMF | 150 | 60.72 | 1.68 |
| 12 | K 2 CO 3 | Sulfolane | 150 | 155.02 | 1.65 |
| 13 | K2CO3 | NMP | 150 | 49.73 | 1.53 |
| 14 | K3PO4 | Sulfolane | 150 | 76.83 | 1.68 |
| 15 | Na3PO4 | Sulfolane | 150 | 65.32 | 1.72 |
| 16 | CaCO3 | Sulfolane | 150 | — | — |
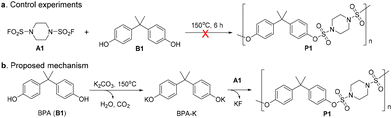
With the optimal conditions in hand, we then examined monomers with various groups to verify the application scope of our process, and the results are shown in Table 2. Both aliphatic chains or rings of disulfamoyl difluorides could react with aryl diphenols bearing different groups to produce PSAs with MPSn ranging from 32 kDa to 167 kDa (for details, see ESI section 4.1†) and narrow PDI (1.34–1.71). Aryl diphenols with large steric hindrance could be applied to this method to afford the target polymers (P7, P8, P12, P13, P19, P20, P26 and P27). We found that the molecular weights of P13 and P27 were relatively low, whereas those of P8 and P20 were relatively high. The results suggested that steric hindrance is not the sole determining factor, and the polymer may also be influenced by the reactivity of the monomers and the polymerization process. In addition, polymerization involving B7 as the bisphenol partner may be unsuitable due to the presence of lactone under the conditions of a base and high temperature (P7, P12 and P26). Copolymerization of A1, B1 and B7 could also provide the polymer with high Mn and narrow PDI (P28).
| PSA | Mon. | M PSn/kDa | PDI | PSA | Mon. | M PSn/kDa | PDI |
|---|---|---|---|---|---|---|---|
a The reaction was carried out on 2.5 mmol scale in sulfolane (0.5 M) at 150 °C for 6 h in the presence of K2CO3 (2.2 equiv.).
b For structures of the polymers, see ESI section 3.2.2.†
c
n
A1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nB1 nB1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nB7 = 1 nB7 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1; MPSn, number-average molecular weight with polystyrene as standard; PDI, polydispersity index; Mon., monomers. 0.1; MPSn, number-average molecular weight with polystyrene as standard; PDI, polydispersity index; Mon., monomers.
|
|||||||
| P1 | A1 + B1 | 155 | 1.65 | P15 | A3 + B2 | 81 | 1.53 |
| P2 | A1 + B2 | 110 | 1.56 | P16 | A3 + B3 | 63 | 1.61 |
| P3 | A1 + B3 | 90 | 1.41 | P17 | A3 + B5 | 103 | 1.61 |
| P4 | A1 + B4 | 45 | 1.43 | P18 | A3 + B6 | 120 | 1.57 |
| P5 | A1 + B5 | 60 | 1.34 | P19 | A3 + B7 | 47 | 1.41 |
| P6 | A1 + B6 | 67 | 1.71 | P20 | A3 + B8 | 72 | 1.45 |
| P7 | A1 + B7 | 45 | 1.27 | P21 | A4 + B1 | 64 | 1.52 |
| P8 | A1 + B8 | 140 | 1.69 | P22 | A4 + B2 | 44 | 1.51 |
| P9 | A2 + B1 | 42 | 1.50 | P23 | A4 + B3 | 37 | 1.71 |
| P10 | A2 + B2 | 85 | 1.64 | P24 | A4 + B5 | 37 | 1.51 |
| P11 | A2 + B3 | 34 | 1.57 | P25 | A4 + B6 | 66 | 1.44 |
| P12 | A2 + B7 | 96 | 1.50 | P26 | A4 + B7 | 40 | 1.38 |
| P13 | A2 + B8 | 35 | 1.50 | P27 | A4 + B8 | 32 | 1.59 |
| P14 | A3 + B1 | 64 | 1.46 | P28 | A1 + B1 + B7 | 167 | 1.60 |
Table 3 shows the mechanical properties of the polymers. P1, P9, and P21 exhibit high mechanical strength, with tensile strengths of 75 MPa, 77 MPa, and 71 MPa, respectively. The tensile and flexural strengths of PSAs with flexible blocks (P14) were lower than those of polymers with rigid blocks (for details of the testing of mechanical properties, see ESI section 4.3†). Compared to polycarbonate,19 polysulfone,19 and polyamide 6, the newly synthesized polysulfamates exhibit high strength and modulus, demonstrating their potential applications as engineering materials.
| Polymer | Tensile strength (MPa) | Elastic modulus (MPa) | Flexural strength (MPa) | Flexural modulus (MPa) |
|---|---|---|---|---|
| a PC (polycarbonate), PSU (polysulfone) and PA 6 (polyamide 6) were applied as contrasting polymers. b P1 with MPSn = 155 kDa, P9 with MPSn = 42 kDa, P14 with MPSn = 64 kDa and P21 with MPSn = 64 kDa were applied. | ||||
| P1 | 75 | 3500 | 94 | 3531 |
| P9 | 77 | 3600 | 98 | 3560 |
| P14 | 55 | 2300 | 75 | 2500 |
| P21 | 71 | 3500 | 91 | 3500 |
| PC | 60 | 2500 | 79 | 2500 |
| PSU | 68 | 2600 | 88 | 2600 |
| PA 6 | 60 | 2300 | 65 | 2340 |
Industrial production and applications of PSAs
We evaluated the mechanical properties of industrial P1 products (MPSn = 110 kDa, PDI = 1.61) and found that their mechanical strength was comparable at both the 1000 g and 100 kg production scales. This finding indicates that the mechanical properties of the polymer remain stable during large-scale industrial production (for details, see ESI section 5†).
Conclusions
We have developed an efficient method to construct a sulfamate bond. By applying this method, PSA materials were successfully produced both in the laboratory and in a chemical factory. PSAs possess the properties of lower production costs, higher Tg and mechanical strength than PSEs. This research on PSA from laboratory synthesis to industrial production is a successful example of the combination of study, research and production. Our work would make significant contributions to the development of sulfonic materials, as well as materials science. Research into the application of PSAs is underway in our lab and the cooperative enterprises.Experimental
General procedure for laboratory synthesis of PSA
Aryl phenols (2.50 mmol, 1.0 equiv.) and alkylsulfamoyl fluorides (2.55 mol, 1.02 equiv.) were combined in a 25 mL glass vial equipped with a magnetic stir bar. Sulfolane (5.0 mL) was added, and the vial was placed into a pre-heated 150 °C oil bath with stirring. After 2 min, commercially available anhydrous K2CO3 (2.2 equiv.) was added in one portion. The reaction was run for 6 h, during which the reaction mixture turned highly viscous and moisture appeared. At the end of the reaction, it was allowed to cool to 70 °C and the mixture was slowly poured into 50 mL of cold water under vigorous stirring. Polymers precipitated as white fiber or powder once the sulfolane solution touched the water. The polymers were collected via filtration and then refluxed in water for 1 h to remove the salts and sulfolane. Finally, the polymers were dried at 40 °C for 12 h in vacuo. The molecular weight and polymer distribution were determined on GPC. The thermal properties were determined by DSC and TGA analysis.Industrial production of P1
The 100 kg-scale P1 was produced in Inner Mongolia Tuwei New Material Technology Co., Ltd. Generally, under N2 atmosphere, A1 (70.78 kg) and BPA (64 kg) were dissolved in sulfolane (450 kg) in a 1000 L steel reactor. K2CO3 (85.23 kg) was added (within 30 min) to the reaction mixture when the temperature was raised to 170 °C. The moisture was removed by N2 and collected by condensing. After reaction, the crude product P1 was post-processed, including granulating, washing, removal of salts, solvent recovery and drying. The obtained P1 was used without other modification or processing.Author contributions
Z. W. and J. X. conceived the experiments and led the project. M. X., L. P., C. J., Z. Z., W. X. and Z. Y. performed most experiments. Z. W. and M. X. wrote the manuscript.Data availability
All relevant data are available from the corresponding author upon reasonable request. The authors declare that all data generated or analyzed during this study are included in this article and its ESI.†Conflicts of interest
The authors have filed a patent application (CN111072966B), on materials reported in this manuscript and are working to commercialize advanced PSAs.Acknowledgements
We appreciate the financial support from the National Natural Science Foundation of China (No. 91853106), Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery (No. 2023B1212060022), Guangdong Provincial Key Laboratory of Construction Foundation. Shenzhen Science and Technology Program (No. JSGG20200225153121723), and the Department of Education of Guangdong Province, China (No. 2020KQNCX016), and Fundamental Research Funds for the Central Universities, Sun Yat-Sen University (No. 23ptpy40). We also wish to thank Inner Mongolia Tuwei New Material Technology Co., Ltd for its support.References
- J. Dong, K. B. Sharpless, L. Kwisnek, J. S. Oakdale and V. V. Fokin, Angew. Chem., Int. Ed., 2014, 53, 9466–9470 CrossRef CAS PubMed.
- S. Li, P. Wu, J. E. Moses and K. B. Sharpless, Angew. Chem., Int. Ed., 2017, 56, 2903–2908 CrossRef CAS PubMed.
- Z. Liu, G. Meng, T. Guo, J. Dong and P. Wu, Curr. Protoc. Chem. Biol., 2019, 11, e64 CrossRef PubMed.
- J. Yatvin, K. Brooks and J. Locklin, Chem. – Eur. J., 2016, 22, 16348–16354 CrossRef CAS PubMed.
- D. E. Mortenson, G. J. Brighty, L. Plate, G. Bare, W. Chen, S. Li, H. Wang, B. F. Cravatt, S. Forli, E. T. Powers, K. B. Sharpless, I. A. Wilson and J. W. Kelly, J. Am. Chem. Soc., 2018, 140, 200–210 CrossRef CAS PubMed.
- B. Gao, L. Zhang, Q. Zheng, F. Zhou, L. M. Klivansky, J. Lu, Y. Liu, J. Dong, P. Wu and K. B. Sharpless, Nat. Chem., 2017, 9, 1083–1088 CrossRef CAS PubMed.
- J. Dong, L. Krasnova, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2014, 53, 9430–9448 CrossRef CAS PubMed.
- M. Chrominski, K. Ziemkiewicz, J. Kowalska and J. Jemielity, Org. Lett., 2022, 24, 4977–4981 CrossRef CAS PubMed.
- Z. Zhan and M. C. Willis, Chem, 2022, 8, 1137–1146 Search PubMed.
- D. Moi, P. Foster, L. Rimmer, A. Jaffri, A. Deplano, G. Balboni, V. Onnis and B. Potter, Eur. J. Med. Chem., 2019, 182, 111614 CrossRef CAS PubMed.
- A. Narayanan and L. H. Jones, Chem. Sci., 2015, 6, 2650–2659 RSC.
- S. A. Sundararaman and A. R. Odom John, Cell Host Microbe, 2022, 30, 1074–1076 CrossRef CAS PubMed.
- K. Debbabi, F. Guittard and S. Geribaldi, J. Colloid Interface Sci., 2008, 326, 235–239 CrossRef CAS PubMed.
- C. J. Smedley, M. C. Giel, A. Molino, A. S. Barrow, D. J. D. Wilson and J. E. Moses, Chem. Commun., 2018, 54, 6020–6023 RSC.
- B. Gao, S. Li, P. Wu, J. E. Moses and K. B. Sharpless, Angew. Chem., Int. Ed., 2018, 57, 1939–1943 CrossRef CAS PubMed.
- S. Mahapatra, C. P. Woroch, T. W. Butler, S. N. Carneiro, S. C. Kwan, S. R. Khasnavis, J. Gu, J. K. Dutra, B. C. Vetelino, J. Bellenger, C. W. am Ende and N. D. Ball, Org. Lett., 2020, 22, 4389–4394 CrossRef CAS PubMed.
- Y. Gan, Engineering, 2024, 32, 10–13 CrossRef.
- M. A. Elkodous, S. O. Olojede, S. Sahoo and R. Kumar, Chem.-Biol. Interact., 2023, 379, 110517 CrossRef CAS PubMed.
- W. Zhu, F. Li, J. Liu, X. Ma and X. Jiang, React. Chem. Eng., 2019, 4, 2074–2080 RSC.
- T. Guo, G. Meng, X. Zhan, Q. Yang, T. Ma, L. Xu, K. B. Sharpless and J. Dong, Angew. Chem., Int. Ed., 2018, 57, 2605–2610 CrossRef CAS PubMed.
- F. Liu, H. Wang, S. Li, G. A. L. Bare, X. Chen, C. Wang, J. E. Moses, P. Wu and K. B. Sharpless, Angew. Chem., Int. Ed., 2019, 58, 8029–8033 CrossRef CAS PubMed.
- S. H. Ali, Nat. Mater., 2018, 17, 1052–1053 CrossRef CAS PubMed.
- M. Ayman, C. Chen and J. T. Jesper, Adv. Sci., 2024, 11, 2401401 CrossRef PubMed.
- P. J. J. Alvarez, C. K. Chan, M. Elimelech, N. J. Halas and D. Villagrán, Nat. Nanotechnol., 2018, 13, 634–641 CrossRef CAS PubMed.
- N. Wang, S. Song, W. Wu, Z. Deng and C. Tang, Adv. Energy Mater., 2024, 14, e2303451 CrossRef.
- D. Kyriacos, Brydson's Plastics Materials, 2017, vol. 17, pp. 457–485 Search PubMed.
- M. Gilbert and S. Patrick, Brydson's Plastics Materials, 2017, vol. 13, pp. 329–388 Search PubMed.
- S. Ronca, Brydson's Plastics Materials, 2017, vol. 10, pp. 247–278 Search PubMed.
- W. C. Firth Jr., US Pat, 3895045, 1973 Search PubMed.
- X. Ru, H. Zhang, Y. Wang, Z. Zhi, Y. Guo, K. Zhang and L. He, J. Mater. Cycles Waste Manage., 2024, 26, 2304–2316 CrossRef CAS.
- Z. Li, H. Zhang, X. Zhang, J. Wang and Y. Wen, Polym. Chem., 2022, 13, 1260–1266 RSC.
- G. Cheng, M. Sahli, J. C. Gelin and T. Barriere, Int. J. Adv. Des. Manuf. Technol., 2014, 75, 225–235 CrossRef.
- Q. Wei, J. Huang, D. Jia, J. Lei, H. Huang, H. Lin, J. Xu, G. Zhong and Z. Li, Macromolecules, 2024, 57, 3706–3718 CrossRef CAS.
- Y. Zhang, G. Ren, G. Nie, S. Hu, D. Li, W. Sun, Z. Li, Z. Cui, D. Lu, X. Shi, L. Li, H. Yu and J. He, Colloids Surf., A, 2024, 685, 133310 CrossRef CAS.
- S. H. Yu, H. Jeon, H. Ko, J. H. Cha, S. Jeon, M. Jae, G. Nam, K. Kim, Y. Gil, K. Lee and D. S. Chung, Chem. Eng. J., 2023, 470, 144102 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4py01383b |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |