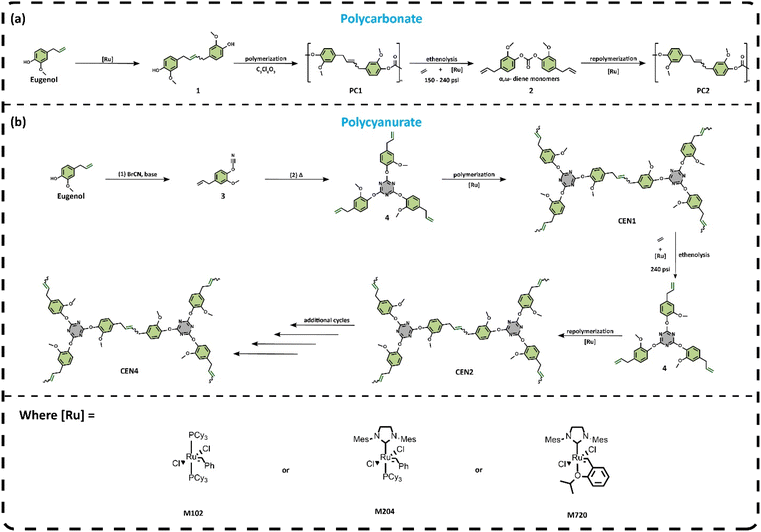
[Ru]-Catalyzed olefin metathesis and ethenolysis for the synthesis and recycling of bio-based polycarbonates and polycyanurates†
Dana M.
Pinson
 ,
Francesca D.
Eckstrom
,
Gregory S.
Ostrom
,
K.
Randall McClain
,
Francesca D.
Eckstrom
,
Gregory S.
Ostrom
,
K.
Randall McClain
 ,
Lawrence C.
Baldwin
,
Lawrence C.
Baldwin
 and
Benjamin G.
Harvey
and
Benjamin G.
Harvey
 *
*
US NAVY, NAWCWD, Research Department, Chemistry Division, China Lake, California 93555, USA. E-mail: benjamin.g.harvey.civ@us.navy.mil
First published on 4th November 2024
Abstract
Eugenol, an abundant, naturally occurring phenolic compound, was converted into a thermoplastic polycarbonate by olefin metathesis followed by interfacial polymerization with triphosghene. This resulted in polymers with Mn ranging from 5300–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1 and an average glass transition temperature (Tg) of 82 °C. The polycarbonates were depolymerized via ethenolysis reactions under modest ethylene pressures (150–240 psi) in the presence of [Ru]-metathesis catalysts to yield a discrete monomer [bis(4-allyl-2-methoxyphenol) carbonate, compound 2]. 2 was then polymerized with a second generation Grubbs catalyst (M204) to produce a recycled polymer with Mn = 7500 g mol−1 and a Tg of 114 °C. The 32 °C increase in Tg was due to the isomerization of the allyl group to internal positions, which then allowed for the formation of stilbene and 3-carbon unsaturated linkages between aromatic groups. To expand the ethenolysis recycling approach to hyperbranched polymers, eugenol was converted into a cyanate ester (3), which was then thermally cyclotrimerized to generate 2,4,6-tris(4-allyl-2-methoxyphenoxy)-1,3,5-triazine (4), a monomer with a triazine core and three pendent aromatic rings with methoxy and allyl substituents. 4 was cross-linked via olefin metathesis (M204 catalyst) to generate a polymer with Mn = 8600 g mol−1 and a Tg of 180 °C. Similar to the polycarbonate, the polycyanurate was efficiently depolymerized in the presence of ethylene to regenerate 4. Compound 4 was then polymerized and depolymerized three additional times, demonstrating full circularity for the triazine monomer/polymer. The recycled polymers exhibited similar Tgs (167–184 °C) and thermal stability compared to the virgin polymer. Overall, this work demonstrates that both linear and hyperbranched polymers can be readily prepared from eugenol and catalytically recycled under standard ethenolysis conditions. Unlike many conventional methods, the recycled polymers described in this work exhibited no significant degradation in thermomechanical properties. This type of approach supports a circular bioeconomy and may help to reduce plastic waste and the accumulation of micro/nanoplastic particles in the environment.
700 g mol−1 and an average glass transition temperature (Tg) of 82 °C. The polycarbonates were depolymerized via ethenolysis reactions under modest ethylene pressures (150–240 psi) in the presence of [Ru]-metathesis catalysts to yield a discrete monomer [bis(4-allyl-2-methoxyphenol) carbonate, compound 2]. 2 was then polymerized with a second generation Grubbs catalyst (M204) to produce a recycled polymer with Mn = 7500 g mol−1 and a Tg of 114 °C. The 32 °C increase in Tg was due to the isomerization of the allyl group to internal positions, which then allowed for the formation of stilbene and 3-carbon unsaturated linkages between aromatic groups. To expand the ethenolysis recycling approach to hyperbranched polymers, eugenol was converted into a cyanate ester (3), which was then thermally cyclotrimerized to generate 2,4,6-tris(4-allyl-2-methoxyphenoxy)-1,3,5-triazine (4), a monomer with a triazine core and three pendent aromatic rings with methoxy and allyl substituents. 4 was cross-linked via olefin metathesis (M204 catalyst) to generate a polymer with Mn = 8600 g mol−1 and a Tg of 180 °C. Similar to the polycarbonate, the polycyanurate was efficiently depolymerized in the presence of ethylene to regenerate 4. Compound 4 was then polymerized and depolymerized three additional times, demonstrating full circularity for the triazine monomer/polymer. The recycled polymers exhibited similar Tgs (167–184 °C) and thermal stability compared to the virgin polymer. Overall, this work demonstrates that both linear and hyperbranched polymers can be readily prepared from eugenol and catalytically recycled under standard ethenolysis conditions. Unlike many conventional methods, the recycled polymers described in this work exhibited no significant degradation in thermomechanical properties. This type of approach supports a circular bioeconomy and may help to reduce plastic waste and the accumulation of micro/nanoplastic particles in the environment.
Introduction
Replacing petroleum-based polymers with bio-based, recyclable, and upcycled polymer materials has been proposed as an important step in the development of a sustainable circular economy.1–3 In addition to sustainability, the use of bio-based phenols may help address toxicity issues related to bisphenol A (BPA).4,5 Polymers containing bisphenols or phenolic moieties are ubiquitous in modern society and include polycarbonates, polysulfones, polycyanurates and epoxy resins.6 Polycarbonates account for a large portion of the polymer industry due to their outstanding thermal, mechanical, and optical properties that enable widespread use in packaging, medical devices, and agriculture.7 Petroleum-based BPA is the most commonly used building block in these polymers, with 4.5 million tons manufactured in 2021.8,9 Unfortunately, BPA is a pervasive contaminant in both the environment and human body. In the latter, it acts as an endocrine disruptor, coordinating to estrogen receptors in place of the hormone estradiol and poses health risks to the reproductive, developmental, and cardiovascular systems.10 Adding to the problem, BPA does not follow standard toxicologic paradigms, namely, lower levels of BPA exposure may cause more severe effects than high exposure levels.11 Despite longstanding knowledge of the complex toxicology and adverse health consequences of BPA, its use has persisted around the globe in consumer and industrial products due to the lack of economical alternatives.12Microplastic accumulation is another key driver for the development of BPA alternatives.13 Microplastics are potential contributors to oxidative stress, metabolic disorders, immune response, and neurotoxicity as well as reproductive and developmental toxicity. These negative health impacts have created a critical need for the study of polymers based on lower toxicity bisphenols. Bio-based alternatives could offer a solution to both BPA toxicity and dependence on unsustainable petrochemicals. Eugenol is an important biorenewable phenolic compound that can be obtained by extraction from plants, such as clove buds, from the valorization of lignin, or through fermentation of sugar substrates.14,15 Currently, approximately 2000 tons of eugenol are produced from clove oil extraction annually.16 In addition to being widely available, eugenol is approved as a food additive, possesses anti-bacterial and antioxidant activities, and has a chemical structure that is ideal for use as a bio-based commodity monomer.17 Eugenol is a phenol with a methoxy group ortho to the hydroxyl group and a three-carbon pendent allyl chain in the position para to the hydroxyl group. This unique functionality provides a synthetic handle for either polymerization or the installation of additional chemical groups. Furthermore, the methoxy group as well as the allylic tail impart unique thermomechanical properties to derivative polymers. Most of the research to date on eugenol-based polymers has focused on developing materials with comparable performance to those prepared from BPA. For example, researchers have prepared a cyanate ester monomer from a eugenol-based bisphenol synthesized by the ruthenium-catalyzed homometathesis of eugenol followed by hydrogenation of the olefin.18 In related work, polycarbonates were synthesized from the same eugenol-based bisphenol and triphosgene with molecular weights up to 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1 and polydispersity indexes (Đ) below 2. Building on these success stories, it would be of significant interest to move beyond the mere utilization of bio-based eugenol polymers to developing methods that leverage the alkene functionality in eugenol for efficient and low-energy recycling protocols.
600 g mol−1 and polydispersity indexes (Đ) below 2. Building on these success stories, it would be of significant interest to move beyond the mere utilization of bio-based eugenol polymers to developing methods that leverage the alkene functionality in eugenol for efficient and low-energy recycling protocols.
Olefin metathesis is a powerful and adaptable synthetic tool that generates new carbon–carbon bonds in the presence of transition metal centered carbene complexes. Ethenolysis is a catalytic chemical transformation belonging to the class of olefin metathesis reactions involving ethylene as one of the reactants.19 This versatile technique can be leveraged to cleave internal olefins in polymers in the presence of ethylene to generate compounds with terminal olefins. Acyclic diene metathesis (ADMET) is a step growth polymerization technique where an unconjugated diene polymerizes in the presence of Schrock or Grubbs-type catalysts, typically at temperatures between 40–80 °C.19 With the development of metathesis catalysts exhibiting increased activity and functional group tolerance, combined with the mild nature of the polymerization conditions, ADMET has become an important synthetic tool for the creation of engineering plastics as well as specialty polymers.20,21 In a particularly compelling approach, polymers with internal olefins can be depolymerized via ethenolysis and then repolymerized via ADMET22 resulting in an infinite recycling loop. Several studies have explored the depolymerization of unsaturated polymers such as polybutadiene and polyisoprene via ethenolysis.23–25 However, the recycling of bio-based engineering thermoplastics (e.g. polycarbonates) via ethenolysis has not been explored in detail. Furthermore, additional work is needed to explore the viability of ethenolysis as a depolymerization strategy for cross-linked thermoset networks, which are typically insoluble in common organic solvents, leading to mass transfer issues and reduced reactivity.
In this work, by leveraging the internal alkene in a eugenol-based repeat unit, we demonstrate the ability to depolymerize a high-performance aromatic polycarbonate in the presence of ethylene to generate a monomeric species. This monomer is then repolymerized and upcycled via ADMET chemistry. This methodology is extended to a multi-functional triazine monomer that forms a hyperbranched network upon polymerization. The robustness and versatility of the ethenolysis/metathesis cycle suggests that it has broad applications for polymer recycling in support of a circular plastic economy.26
Experimental
General
Unless otherwise noted, all reagents were purchased and used as received from the manufacturer without further purification. Eugenol, triphosgene, triethylamine (TEA), dichloromethane (DCM), hexanes, tetrahydrofuran (THF), ethyl acetate, Grubbs M102 and M204 catalysts, benzoquinone, and deuterated solvents (CDCl3, DMSO-d6) were purchased from Sigma-Aldrich. Methanol, diethyl ether, cyanogen bromide and ethyl vinyl ether were purchased from Fisher Chemicals. Sodium hydroxide (NaOH) was purchased from PolarChem and M720 catalyst was purchased from Ambeed. Ethylene gas (purity 99.95%) was purchased from Linde. All NMR chemical shifts are reported in ppm downfield from tetramethylsilane and are referenced relative to the NMR solvent according to the literature values: 1H NMR (CDCl3 = 7.26 ppm, DMSO-d6 = 2.50 ppm); 13C NMR (CDCl3 = 77.16 ppm, DMSO-d6 = 39.52 ppm). Fourier transform infrared (FTIR) spectroscopy was performed using a Thermo Scientific Nicolet 6700 FTIR spectrometer equipped with a liquid nitrogen cooled MCTA detector. Experiments were performed using an attenuated total reflectance (ATR) smart accessory with a germanium window. Each FTIR spectrum reported is the average of 32 scans with 4 cm−1 resolution. Background subtraction of the clean germanium crystal and baseline corrections were used to decrease noise from the system. Gel permeation chromatography (GPC) to determine the molecular weight of the samples was performed on a Viscotec TriSEC Model 302 GPC with a refractive index (RI) detector. Differential scanning calorimetry (DSC) analysis was conducted on a DSC Q200 TA instrument under N2 at 50 mL min−1. Approximately 5 mg of sample were tested under a heating scan from 40 to 200 °C with a heating rate of 10 °C min−1. Thermogravimetric analysis (TGA) was performed on a TGA Q5000 TA instrument under N2 flow from 24 to 800 °C with a heating rate of 10 °C min−1. The decomposition temperature is reported as the temperature at which 5% mass loss occurs (Td 5%).Synthesis of (E)-4,4′-(but-2-ene-1,4-diyl)bis(2-methoxyphenol) (compound 1)
The eugenol bisphenol was synthesized as described in previous work.17 Eugenol (25.88 g, 158 mmol) was combined with Grubbs M102 catalyst (0.532 g, 0.4 mol%) under a nitrogen atmosphere to form a thick purple solution. The evolution of bubbles attributed to ethylene gas was immediately observed. After 8 h under nitrogen, the flask was placed under reduced pressure and allowed to react for an additional 24 h with stirring to yield a thick resinous mass. Anhydrous DCM was then added and the resulting solution was allowed to react for an additional 48 h. The solvent was evaporated under reduced pressure and the resulting solid dissolved in an aqueous NaOH solution (1 L, 1.25 M), which was then filtered through a Celite pad to remove a dark black precipitate. After filtration, the solution was acidified with concentrated HCl, which resulted in the precipitation of a pale gray solid. The solid was collected by filtration, redissolved in DCM, washed with brine (6 × 200 mL), and the organic layer dried over MgSO4. The solvent was then removed in vacuo to yield 20.09 g of product (78% yield). Single crystals suitable for X-ray crystallography were grown by slow evaporation of a methanol solution. 1H NMR (DMSO-d6, 400 MHz) δ: 8.70 (s, 1H, OH), 6.72 (s, 1H, Ar–H), 6.66 (s, 1H, Ar–H), 6.56 (s, 1H, Ar–H), 5.59 (bs, 2H, C(sp2)–H), 3.71 (s, 3H, OCH3) 3.21 (d, J = 7.8 Hz, 2H, CH2). 13C NMR (CDCl3, 400 MHz) δ: 146.46, 143.87, 132.73, 130.60, 121.06, 114.27, 111.15, 55.87, 38.60.Polycarbonate synthesis
Compound 1 was converted into a polycarbonate based on a literature method.26,27 In a general procedure, recrystallized bisphenol (0.901 g, 3.0 mmol) was dissolved in a 1.25 M NaOH solution (25 mL) and allowed to stir for a minimum of one h. TEA (0.05 mL, 0.3 mmol) was added to the solution and then triphosgene (0.37 g, 1.25 mmol) dissolved in dichloromethane (10 mL) was added dropwise to yield a biphasic reaction mixture. The mixture was stirred for 10 min at ambient temperature and then diluted with 100 mL of DCM. The heterogeneous mixture was transferred to a separatory funnel, and the organic layer was washed with distilled H2O (6 × 100 mL). The organic phase was then concentrated and added dropwise to stirring MeOH (800 mL) to precipitate the polycarbonate. The precipitate was isolated by filtration, redissolved in minimal DCM, reprecipitated in MeOH, and recollected by filtration. The resulting solid was dried in a vacuum oven (60 °C, ∼50 torr) for 18 h to yield 0.4316 g of the polycarbonate as a white powder (48% yield). Two different batches of polycarbonate were prepared, one with a low molecular weight (PC1low, Mn = 5300), and one with a higher molecular weight (PC1high, Mn = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700). 1H NMR (DMSO-d6, 400 MHz) δ: 7.2 (d, J = 8.3 Hz, Ar–H), 7.0 (bs, Ar–H), 6.8 (d, J = 8.6 Hz, Ar–H), 5.7 (bs, C(sp2)–H), 3.8 (bs, OCH3), 3.3 (bs, CH2).
700). 1H NMR (DMSO-d6, 400 MHz) δ: 7.2 (d, J = 8.3 Hz, Ar–H), 7.0 (bs, Ar–H), 6.8 (d, J = 8.6 Hz, Ar–H), 5.7 (bs, C(sp2)–H), 3.8 (bs, OCH3), 3.3 (bs, CH2).
General polycarbonate depolymerization conditions
The polycarbonate, 5–10 mol% of either M204 or M720, DCM (5 mg polymer per mL) and 10 mol% benzoquinone were charged to a Parr reactor under a nitrogen atmosphere. Ethylene gas (99.95% purity) was then charged to the reactor to a pressure of either 150 or 240 psi at either 25 or 70 °C for either 24 or 72 h. Upon completion of the reaction, pressure was released from the reactor and the contents were transferred to a round bottom flask. An excess of ethyl vinyl ether was added, and the mixture was allowed to stir for 1 h to quench the reaction. An excess of imidazole was then introduced and the mixture was stirred for one hour followed by the addition of activated charcoal and an additional hour of stirring. The reaction mixture was then filtered through silica gel and the solvent removed under reduced pressure. The resulting solid was dried overnight under reduced pressure at 60 °C. In cases where complete depolymerization was obtained, the product of the reaction was bis(4-allyl-2-methoxyphenyl) carbonate (compound 2): 1H NMR (CDCl3, 400 MHz) δ: 7.2 (d, 2H, J = 8.0 Hz, Ar–H), 6.8 (d, 4H, J = 16.0 Hz, Ar–H), 5.97 (septet 2H, J = 6.6 C(sp2)–H), 5.13 (m, 4H, 17.0 Hz), 3.88 (s, 6H, OCH3), 3.39 (d, 4H, J = 6.7 Hz). 13C NMR (DMSO-d6, 400 MHz) δ: 151.00, 150.33, 139.60, 137.77, 137.35, 122.00, 120.34, 116.18, 113.27, 55.92. Single crystals suitable for X-ray crystallography were grown from slow evaporation of a concentrated ethyl acetate solution.Polycarbonate synthesis via ADMET polymerization of bis(4-allyl-2-methoxyphenyl)carbonate
2 (0.1075 g, 0.30 mmol), 1,4 benzoquinone (10 mol%), M204 (10 mol%) and DCM (500 mg mL−1) were charged to a flask under an inert atmosphere and allowed to react for 18 h. Then, 2 mL of ethyl vinyl ether were introduced into the flask and allowed to stir for 1 hour to quench the reaction. The reaction mixture was precipitated in hexanes (50 mL) and the solid precipitate was collected by vacuum filtration. The precipitate was then redissolved in minimal THF, precipitated in cold methanol and collected via vacuum filtration. The solid was dried in a vacuum oven (60 °C, ∼50 torr) for 18 h to yield 0.0916 g of white powder (85.2%). Mn = 7500 g mol−1. 1H NMR (CDCl3, 400 MHz) δ: 6.80–7.15 (m, Ar–H), 5.08–6.45 (m, C(sp2)–H), 3.89 (bs, OCH3), 3.35 (m, CH2).4-Allyl-1-cyanato-2-methoxybenzene (compound 3)
Eugenol (5.00 g, 30.5 mmol) was combined with cyanogen bromide (4.83 g, 1.5 equivalents, 45.6 mmol), placed under a nitrogen atmosphere and cooled to −78 °C. TEA (1.05 equivalents, 32.0 mmol) was diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with diethyl ether and placed in an addition funnel under nitrogen. The TEA solution was added dropwise to the stirring reaction mixture over 30 minutes. The reaction was allowed to proceed for 3 h at −78 °C and then allowed to warm to 0 °C. A large amount of white solids were produced. Once the reaction mixture reached 0 °C, the mixture was filtered through Celite to remove the white solids, diluted with additional ether, washed with brine (6 × 100 mL) and 2M HCl (1 × 100 mL), dried over MgSO4, concentrated under reduced pressure, and dried in a vacuum oven (60 °C, ∼50 torr) for 18 h to yield 4.201 g of a clear, yellow liquid (79.6%). 1H NMR (CDCl3, 400 MHz) δ: 7.21 (d, 1H, J = 7.2 Hz, Ar–H), 6.81 (s, 1H, Ar–H), 6.72 (d, 1H, J = 6.7 Hz, Ar–H), 5.88 (septet, 1H, J = 5.9 Hz, C(sp2)–H), 5.08 (m, 2H J = 5.1 Hz, C(sp2)–H), 3.84 (s, OCH3), 3.34 (d, J = 6.8 Hz, 2H, CH2). 13C NMR (DMSO-d6, 400 MHz) δ: 148.02, 140.79, 138.92, 136.97, 120.64, 117.34, 116.36, 114.14, 109.53, 56.25.
1 with diethyl ether and placed in an addition funnel under nitrogen. The TEA solution was added dropwise to the stirring reaction mixture over 30 minutes. The reaction was allowed to proceed for 3 h at −78 °C and then allowed to warm to 0 °C. A large amount of white solids were produced. Once the reaction mixture reached 0 °C, the mixture was filtered through Celite to remove the white solids, diluted with additional ether, washed with brine (6 × 100 mL) and 2M HCl (1 × 100 mL), dried over MgSO4, concentrated under reduced pressure, and dried in a vacuum oven (60 °C, ∼50 torr) for 18 h to yield 4.201 g of a clear, yellow liquid (79.6%). 1H NMR (CDCl3, 400 MHz) δ: 7.21 (d, 1H, J = 7.2 Hz, Ar–H), 6.81 (s, 1H, Ar–H), 6.72 (d, 1H, J = 6.7 Hz, Ar–H), 5.88 (septet, 1H, J = 5.9 Hz, C(sp2)–H), 5.08 (m, 2H J = 5.1 Hz, C(sp2)–H), 3.84 (s, OCH3), 3.34 (d, J = 6.8 Hz, 2H, CH2). 13C NMR (DMSO-d6, 400 MHz) δ: 148.02, 140.79, 138.92, 136.97, 120.64, 117.34, 116.36, 114.14, 109.53, 56.25.
2,4,6-Tris(4-allyl-2-methoxyphenoxy)-1,3,5-triazine (compound 4)
Compound 3 (4.201 g, 24.25 mmol) was placed in a flask under a nitrogen atmosphere and heated to 150 °C for two h. The temperature was then increased to 210 °C and held at that temperature for 18 h. The resulting brown solid was dissolved in a mixture of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 hexanes
10 hexanes![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate and then filtered. The filtrate was concentrated under reduced pressure and the resulting solid was dried in a vacuum oven (60 °C, ∼50 torr) for 18 h to yield 1.554 g of the product as a white powder (37.0%). Single crystals suitable for X-ray crystallography were grown by slow evaporation of a concentrated solution of 4 in a mixture of 99
ethyl acetate and then filtered. The filtrate was concentrated under reduced pressure and the resulting solid was dried in a vacuum oven (60 °C, ∼50 torr) for 18 h to yield 1.554 g of the product as a white powder (37.0%). Single crystals suitable for X-ray crystallography were grown by slow evaporation of a concentrated solution of 4 in a mixture of 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 heptane
1 heptane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate. 1H NMR (CDCl3, 400 MHz) δ: 6.98 (d, 3H, J = 7.0 Hz, Ar–H), 6.70 (m, 6H, Ar–H), 5.95 (septet, 3H, J = 6.0 Hz, C(sp2)–H), 5.12 (m, 6H, C(sp2)–H), 3.71 (s, 9H, OCH3), 3.35 (d, 6H, J = 3.4 Hz, CH2). 13C NMR (CDCl3, 400 MHz) δ: 173.72, 150.88, 139.07, 138.85, 137.13, 122.13, 120.53, 137.13, 122.13, 120.53, 116.16, 112.89, 55.78, 40.15.
ethyl acetate. 1H NMR (CDCl3, 400 MHz) δ: 6.98 (d, 3H, J = 7.0 Hz, Ar–H), 6.70 (m, 6H, Ar–H), 5.95 (septet, 3H, J = 6.0 Hz, C(sp2)–H), 5.12 (m, 6H, C(sp2)–H), 3.71 (s, 9H, OCH3), 3.35 (d, 6H, J = 3.4 Hz, CH2). 13C NMR (CDCl3, 400 MHz) δ: 173.72, 150.88, 139.07, 138.85, 137.13, 122.13, 120.53, 137.13, 122.13, 120.53, 116.16, 112.89, 55.78, 40.15.
General synthesis of polycyanurates
4 (0.708 g, 1.25 mmol) was combined with M204 (0.053 g, 5.0 mol%) and DCM (50 mL) in a flask under a nitrogen atmosphere. The solution was stirred for 14 h at ambient temperature. Then, 5 mL of ethyl vinyl ether were introduced into the flask and the solution was stirred for 1 h to quench the reaction. The reaction mixture was precipitated in hexanes (500 mL) and the solid precipitate was collected by vacuum filtration. The precipitate was redissolved in minimal THF, precipitated in cold methanol and collected via vacuum filtration. The solid was then dried in a vacuum oven (60 °C, ∼50 torr) for 18 h to yield 0.4675 g of the polymer as a beige powder (66.1%). Reactions conducted for 24 h at ambient temperature resulted in the formation of insoluble cross-linked networks.General ethenolysis conditions for polycyanurates
In an inert atmosphere, the polycyanurate, 10 mol% of either M204 or M720, DCM (5 mg polymer per mL) and 10 mol% benzoquinone were charged to a Parr reactor. Ethylene gas (99.95% purity) was then added to the reactor and the pressure was equilibrated to 240 psi. The reaction was allowed to proceed at 25 °C for 24 h. The pressure was then released from the reactor and the contents were transferred to a round bottom flask. An excess of ethyl vinyl ether was introduced into the flask and the reaction mixture allowed to stir for 1 h to quench the reaction. An excess of imidazole was then added followed by activated charcoal. The reaction mixture was stirred for an additional hour and then filtered through silica gel. The filtrate was concentrated under reduced pressure and the resulting solid was dried in a vacuum oven (60 °C, ∼50 torr) for 18 h. In cases where complete depolymerization was obtained, the product of the reaction was 2,4,6-tris(4-allyl-2-methoxyphenoxy)-1,3,5-triazine (compound 4). The NMR spectra for this product were identical to those obtained for the starting monomer.Results and discussion
Synthesis and characterization of bio-based polymers
The homometathesis of eugenol was performed following previously reported methods to yield 4,4′-(2-butene-1,4-diyl)bis[2-methoxyphenol] (1).11–13 (Scheme 1). A polycarbonate was then synthesized from 1 and triphosgene under biphasic conditions.27,28 Triphosgene was selected as a safer reagent compared to phosgene while interfacial polymerization allowed us to mimic conditions commonly used in the industrial production of polycarbonates. There are literature reports describing polymerization of similar monomers with diphenyl carbonate, but this approach results in low molecular weight oligomers ranging from 3000–4000 g mol−1.29 More importantly, the diphenyl carbonate route is plagued by a side reaction that creates ether linkages. This changes the connectivity of the repeat units in the polymer, which impacts the ability of the polymer to depolymerize to monomer via ethenolysis.20,30,31 The yield of the polycarbonate in our work ranged between 48–52% (Table 1). Despite the solubility of the bisphenol in the NaOH solution, a heterogenous mixture was obtained even after many hours of rigorous stirring. Future research might focus on the use of pyridine or other amines in place of aqueous NaOH to improve yields.30 Extending the reaction time from 10 to 15 min did not impact yield, but increased the molecular weight more than two-fold, from 5300 g mol−1 to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1 (Fig. 1b).
700 g mol−1 (Fig. 1b).
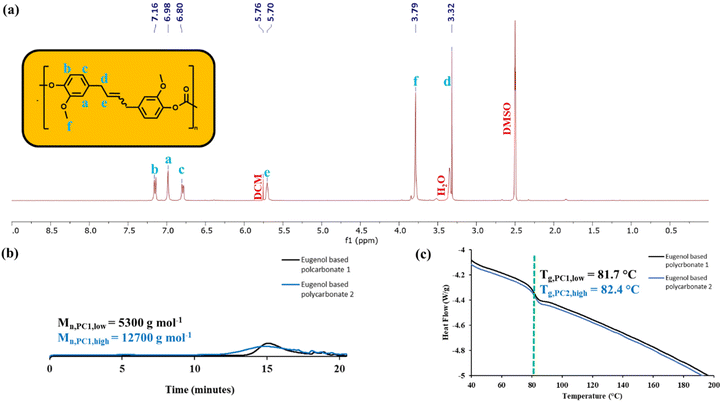 | ||
| Fig. 1 (a) 1H NMR spectrum of PC1high in DMSO-d6. (b) GPC data for PC1low and PC1high. (c) DSC traces for PC1low and PC1high. Heating cycles ran from 40 °C–200 °C at 10 °C min−1. | ||
The polycarbonate was characterized by 1H NMR as well as ATR FTIR spectroscopy (Fig. 1a and S5†).1H NMR spectroscopy was used to verify successful polymerization. The 1H NMR spectrum of the polymer did not contain the peak at 8.70 ppm attributed to the –OH of the bisphenol. In addition, all of the peaks were shifted slightly downfield. For example, the methoxy peak in the monomer was seen at 3.71 ppm while the methoxy peak for the polymer occurred at 3.79 ppm.
The FTIR spectrum of the polycarbonate (Fig. S5†) exhibited an intense peak at 1780 cm−1 attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the carbonyl group while a peak at 1236 cm−1 was consistent with C–O–C stretching. A broad peak observed from 1442–1477 cm−1 was attributed to both a Csp2–H bending vibration and C
O stretching of the carbonyl group while a peak at 1236 cm−1 was consistent with C–O–C stretching. A broad peak observed from 1442–1477 cm−1 was attributed to both a Csp2–H bending vibration and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching. Other C
C stretching. Other C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching peaks attributed to aromatic ring carbons appeared at 1510 and 1006 cm−1.32,33
C stretching peaks attributed to aromatic ring carbons appeared at 1510 and 1006 cm−1.32,33
The molecular weights of the polycarbonates were measured by gel permeation chromatography (GPC) and found to be dependent on reaction time (Table 1). The number-average molecular weight (Mn) for the polycarbonates in the current work are higher than those reported by Trita et al. for a similar polycarbonate synthesized by ester exchange with diphenyl carbonate.29 The molecular weight ranges achieved with triphosghene allow for a better comparison to commercially available BPA-based polycarbonates such as Lexan™ (typical Mn = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1).34
000 g mol−1).34
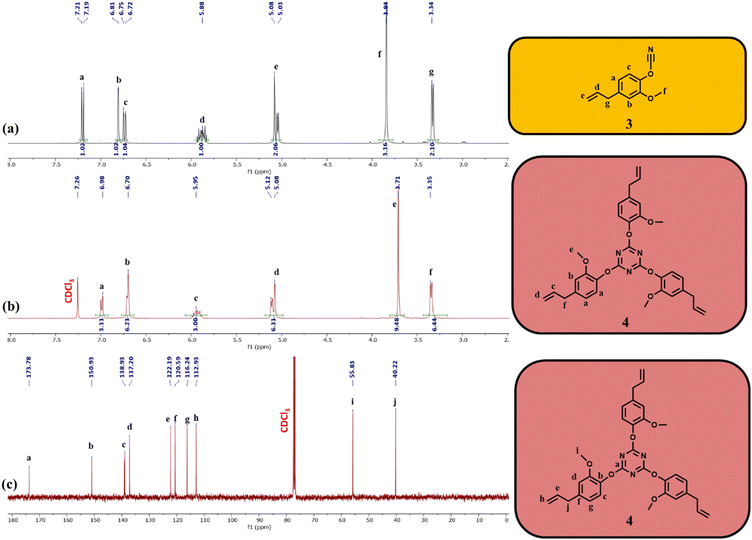
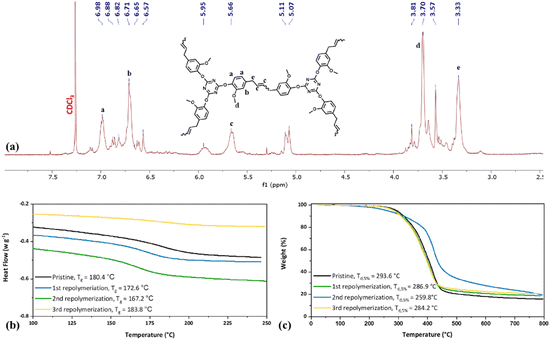
In addition to the thermoplastic polycarbonate, a trifunctional monomer was synthesized from eugenol by cyanation followed by thermal cyclotrimerization (Scheme 1). First, eugenol was reacted with cyanogen bromide in the presence of base to yield 4-allyl-1-cyanato-2-methoxybenzene (3). Successful synthesis of 3 was confirmed by 1H NMR (Fig. 2a) and FTIR spectroscopy (Fig. 3). In the 1H NMR spectrum, peaks ranging from 7.20–6.74 ppm were attributed to the aromatic protons. A septet and mulitplet attributed to the alkene protons were observed at 5.88 ppm and 5.06 ppm, respectively. A singlet attributed to the methoxy protons occurred at 3.84 ppm and a doublet for the methylene protons was present at 3.35 ppm (Fig. 2a). Thermal trimerization of 3 resulted in the formation of 2,4,6-tris(4-allyl-2-methoxyphenoxy-1,3,5-triazine) (compound 4), a monomer with three pendent alkenes. Successful creation of the triazine ring was confirmed by both 1H and 13C NMR spectroscopy (Fig. 2b and c). In the 1H NMR spectrum, the aromatic protons were shifted downfield upon successful triazine formation. The peak attributed to the terminal alkene protons shifted slightly downfield (Fig. 2b) while the peak for the alkene shifted from 3.84 ppm to 3.71 ppm. In the 13C NMR spectrum, a peak attributed to the carbons of the triazine ring can be seen at 173.8 ppm (Fig. 2c). FTIR spectroscopy provided further evidence of the successful synthesis of 3 and complete conversion of the cyanate ester group during the thermal cyclotrimerization reaction (Fig. 3a and b). In the FTIR spectrum for 3, a prominent peak at 2257 cm−1 was observed, confirming successful installation of the cyanate group on eugenol.35 Upon thermal cyclotrimerization, this peak was no longer visible, indicating complete cyclotrimerization to generate 4 (Fig. 3b). In the FTIR spectra for both 3 and 4, peaks for the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups were observed at 1500, 1270 and 1190 cm−1 while a peak for the C–O stretching vibration appeared at 1030 cm−1.36,37 Upon thermal trimerization, peaks for the triazine moiety were seen at 1610 cm−1 (C
C groups were observed at 1500, 1270 and 1190 cm−1 while a peak for the C–O stretching vibration appeared at 1030 cm−1.36,37 Upon thermal trimerization, peaks for the triazine moiety were seen at 1610 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) as well as 1565 and 1506 cm−1 (C
N) as well as 1565 and 1506 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C).32,33 Finally, single crystals of 4 were grown from a mixture of heptane/ethyl acetate, and the structure of 4 was confirmed through X-ray crystallography (Fig. 3c).
C).32,33 Finally, single crystals of 4 were grown from a mixture of heptane/ethyl acetate, and the structure of 4 was confirmed through X-ray crystallography (Fig. 3c).
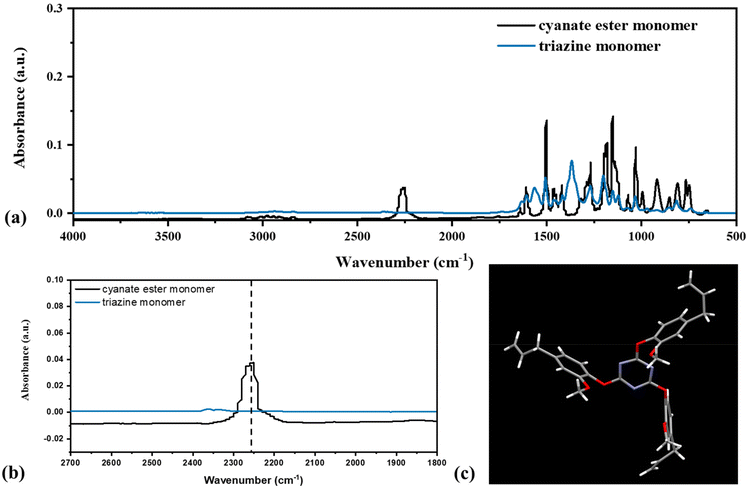 | ||
| Fig. 3 (a) ATR-FTIR spectrum of 3 before and after thermal cyclotrimerization. (b) Expanded view of the –OCN region in the FTIR spectrum. (c) X-ray crystal structure of compound 4. Ortep drawings with thermal ellipsoids at the 50% probability level are included in the ESI.† | ||
Compound 4 was polymerized via olefin metathesis using the M204 catalyst to yield a hyperbranched polymer (CEN1). The polymerization was conducted at ambient temperature and the reaction time was carefully controlled (14 h) to ensure the arrested polycyanurate network was of low enough molecular weight to be fully soluble in organic solvents. This enabled straightforward GPC and NMR characterization, as well as ethenolysis of the network. When the reaction time was extended to 24 h, a solid insoluble material precipitated out of solution, which suggested the formation of a highly cross-linked network. 1H NMR spectroscopy of the soluble hyperbranched polymer showed significant peak broadening when compared to the monomer spectrum, which was indicative of successful polymerization (Fig. 4a). The degree of polymerization was evaluated by GPC and CEN1 was found to have an Mn (based on polystyrene standards) of 8600 g mol−1 (Fig. 4b and Table 1).
The thermoplastic polycarbonates and CEN1 were analyzed by TGA and DSC to characterize thermal stability and measure glass transition temperatures (Tg), melting points (Tm) and crystallization temperatures (Tc). The eugenol-based polycarbonates exhibited similar Tgs (82 °C) irrespective of molecular weight (Fig. 1c and Table 1). This value is higher than previously reported values of 52–71 °C for a saturated eugenol-based polycarbonate27,37 likely due to the presence of the alkene bridge located between the aromatic rings in the current work. However, the Tgs measured for PC1low and PC1high were much lower than commercially available Lexan™, which is based on BPA and has a Tg = 146 °C.38 Despite this shortcoming, the Tg of the eugenol polycarbonate was still well above room temperature, which should make it suitable for a number of commercial applications.33,36 The moderate Tg of PC1low and PC1high was attributed to the 4-carbon bridges between the aromatic rings, which allow for greater flexibility compared to the isopropylidene linkage in Lexan™. Interestingly, no Tm or Tc was observed for PC1low or PC1highvia DSC. Examination of the material in a standard melting temperature apparatus revealed a Tm between 160–173 °C. CEN1 was found to have a Tg of 180 °C (Table 1), which is close to the reported Tg of a saturated polycyanurate network derived from eugenol (186 °C).18 The similarity in these values despite the modest molecular weight in the current case suggests that a fully cross-linked network derived from the unsaturated eugenol-based monomer would have a higher Tg compared to the network obtained from the saturated version. The Tg for CEN1 is much lower, approximately 100 °C, compared to the Tg of a polycyanurate network derived from bisphenol A dicyanate.39,40 As discussed above for the polycarbonates, this difference is likely due to the more flexible 4-carbon linker between aromatic rings. As expected, no Tm or Tc was observed for CEN1 due to the amorphous branched structure.
Catalytic recycling via ethenolysis and olefin metathesis
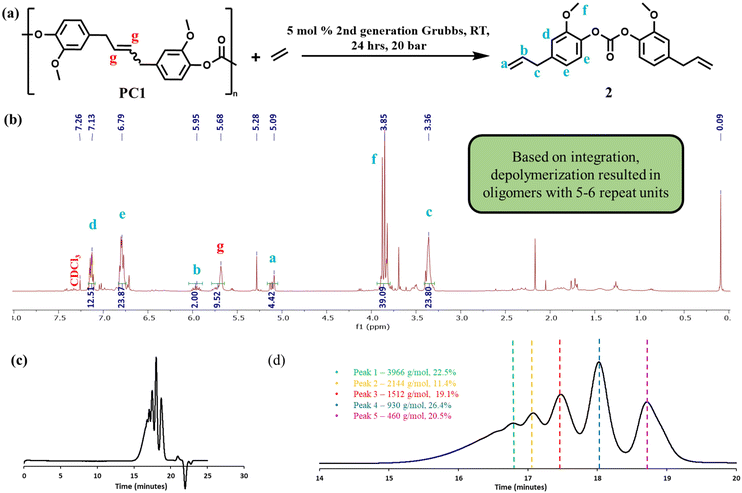
After characterizing the polymers, we first explored the depolymerization of the polycarbonates via ethenolysis (Fig. 5a). Closed loop recycling requires the ability to convert used polymers back to monomers followed by repolymerization. Initial ethenolysis conditions were chosen based on previous work with methyl oleate.41 Catalytic depolymerization to monomer or low molecular weight oligomers is desirable for recycling as it prevents damage to the polymer chains that can occur during mechanical recycling. In addition, this approach creates α,ω-diene monomers that can be used in the synthesis of other polymers.42,43 The impact of reaction conditions as well as the molecular weight of the starting polymer were explored to optimize ethenolysis conditions for obtaining monomeric product. The products of ethenolysis were purified and characterized by 1H NMR and GPC. For the depolymerized products derived from PC1low and PC1high, the terminal alkene peaks of the product were clearly visible at 5.95 and 5.09 ppm (Fig. 5b). Integration was performed and normalized to the terminal alkene resonance at 5.95 ppm. The peak at 5.68 ppm was attributed to the internal alkene along the backbone of the oligomers. Based on the 1H NMR integration, it was determined that the ethenolysis reaction yielded oligomers with an average length of approximately 6 repeat units. Complimentary to the 1H NMR analysis, GPC analysis revealed 5 peaks attributable to different oligomers (Fig. 5c and d). The average Mn for all of the experiments ranged from 1700–2600 g mol−1, which is consistent with the molecular weight determined by 1H NMR spectroscopy. It should be noted that the molecular weights determined by 1H NMR are quantitative whereas the molecular weights determined by GPC are estimates as they are based on elution time related to polystyrene standards. In addition, the GPC method has less fidelity compared to NMR analysis, particularly for low molecular weight species.The ethenolysis of the eugenol-based polycarbonate was repeated several times while varying the starting molecular weight, catalyst type, time, temperature, pressure, and use of a radical inhibitor (Table 2). In every case, the product of ethenolysis was an oligomeric distribution with an Mn of ∼1500 g mol−1 (as determined by 1H NMR spectroscopy). Although it is unclear exactly why the initial depolymerization step is limited to the formation of oligomers, one possible explanation is that the catalyst is deactivated due to irreversible binding of the oligomeric product as the reaction proceeds. Alternatively, there may be some impurity in the reaction mixture that poisons the catalyst prior to complete depolymerization. In an attempt to drive the reaction to completion, the depolymerization products of several ethenolysis trials were combined and subjected to further ethenolysis with fresh catalyst. This resulted in complete depolymerization to bis(4-allyl-2-methoxyphenyl) carbonate (compound 2) as confirmed by 1H NMR spectroscopy (Fig. 6a). The GPC chromatogram of the depolymerization product exhibited one narrow peak providing further evidence for complete conversion to the monomer (Fig. S4†). Finally, large single crystals of 2 were grown from ethyl acetate (Fig. 6b) and an X-ray crystal structure was obtained (Fig. 6c). Although two rounds of ethenolysis were required, this work demonstrated that a high molecule weight bio-derived polycarbonate could be selectively depolymerized to a monomeric species via [Ru]-catalyzed ethenolysis.
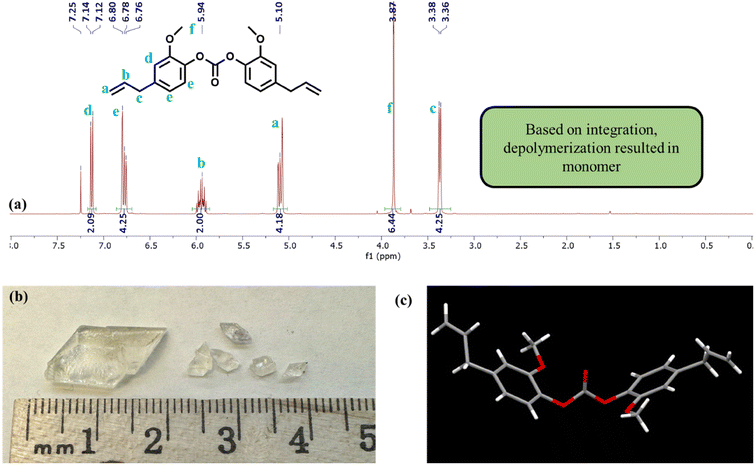 | ||
| Fig. 6 (a) 1H NMR spectrum of the ethenolysis product generated from oligomers of the eugenol-based polycarbonates CDCl3. (b) Photograph showing crystalline monomer obtained via ethenolysis. (c) X-ray crystal structure of the monomer. Ortep drawings with thermal ellipsoids at the 50% probability level are included in the ESI.† | ||
| Polymer | Starting Mn (g mol−1) | M n after ethenolysis (g mol−1) | Yield (%) | Catalyst type and loading | Time (hours) | Temperature (°C) | Benzo-quinone (mol%) |
|---|---|---|---|---|---|---|---|
| a Starting material is a combination of products of the 4 previous reactions. b Pure monomer obtained from the ethenolysis reaction. | |||||||
| PC1low | 5300 | 1700 | 62 | 5 mol% M204 | 24 | 25 | None |
| PC1low | 5300 | 2500 | 67 | 10 mol% M720 | 24 | 70 | 10 |
| PC1low | 5300 | 2600 | 64 | 10 mol% M204 | 72 | 25 | 10 |
| PC1high | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2000 | 59 | 10 mol% M204 | 24 | 25 | 10 |
| 5-mer PC | 1600–2600a | 294.35b | 64 | 10 mol% M204 | 24 | 25 | 10 |
| CEN1a | 8600 | 1000 | 58 | 10 mol% M204 | 24 | 25 | 10 |
| CEN1b | 1000 | 600 | 71 | 10 mol% M204 | 24 | 25 | 10 |
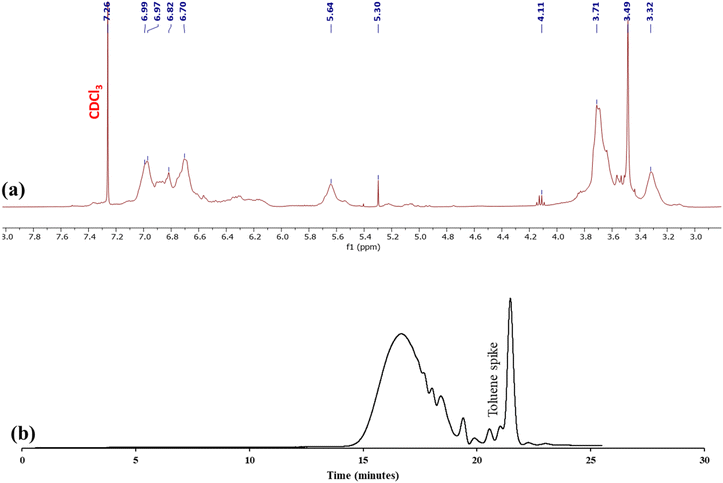
Having found great success in depolymerizing a thermoplastic material to a monomeric building block, the ethenolysis of CEN1 was attempted (Table 2). The 1H NMR spectrum of the depolymerized network and structure of the depolymerized product (4) are shown in Fig. 7a and b, respectively. Unlike the polycarbonate example, it was not possible to estimate the molecular weight of the depolymerized product via NMR spectroscopy. The internal alkene peak appearing at 5.64 ppm was not present in the spectrum, suggesting complete depolymerization. However we did observe some broadening of the peaks which suggested some polymeric impurity. GPC analysis revealed primarily monomer with a shoulder due to the presence of oligomers (Fig. 7c). However, based on the NMR data it did not appear that the shoulder was due to oligomers with unsaturated alkenes in the backbone.
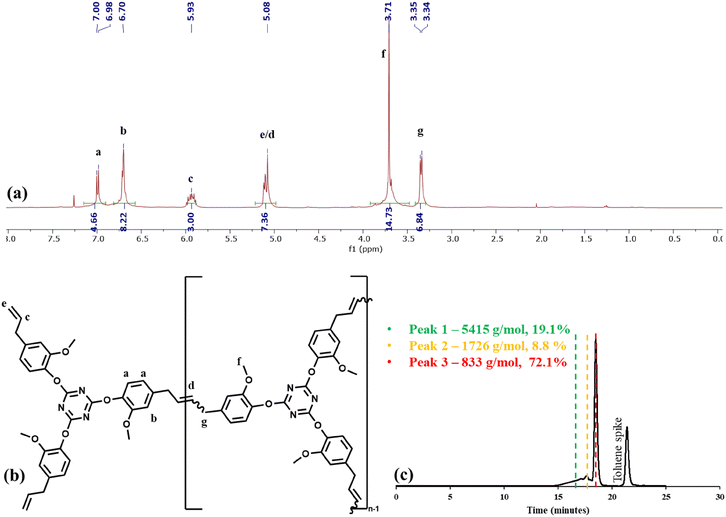 | ||
| Fig. 7 (a) 1H NMR spectrum of the CEN1a ethenolysis product in CDCl3. (b) Chemical structure of the ethenolysis product. (c) GPC chromatogram of the ethenolysis product. | ||
Although the triazine monomer could be easily purified from the product mixture after one ethenolysis cycle, we typically conducted a second round of ethenolysis to ensure product composition across multiple experiments for subsequent polymerization reactions (Fig. 8). The triazine product obtained after two rounds of ethenolysis was analyzed via1H NMR spectroscopy and found to be consistent with an authentic sample of 4 (Fig. 8a). Similarly, GPC analysis of the product revealed one narrow peak attributable to the monomer (Fig. 8b). The successful depolymerization of the polycyanurate is significant as it shows that a cross-linked structure does not prevent depolymerization via ethenolysis. Thus, this work may open the door to depolymerization of thermoset networks.
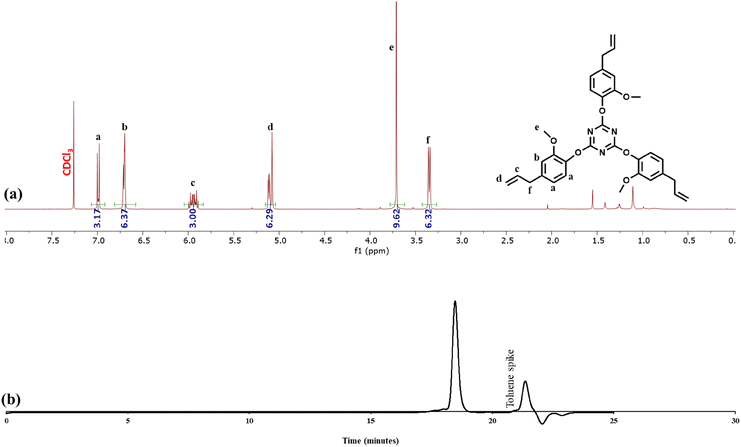 | ||
| Fig. 8 (a) 1H NMR spectrum of fully depolymerized polycyanurate (CEN1b) in CDCl3. (b) GPC chromatogram of CEN1b. | ||
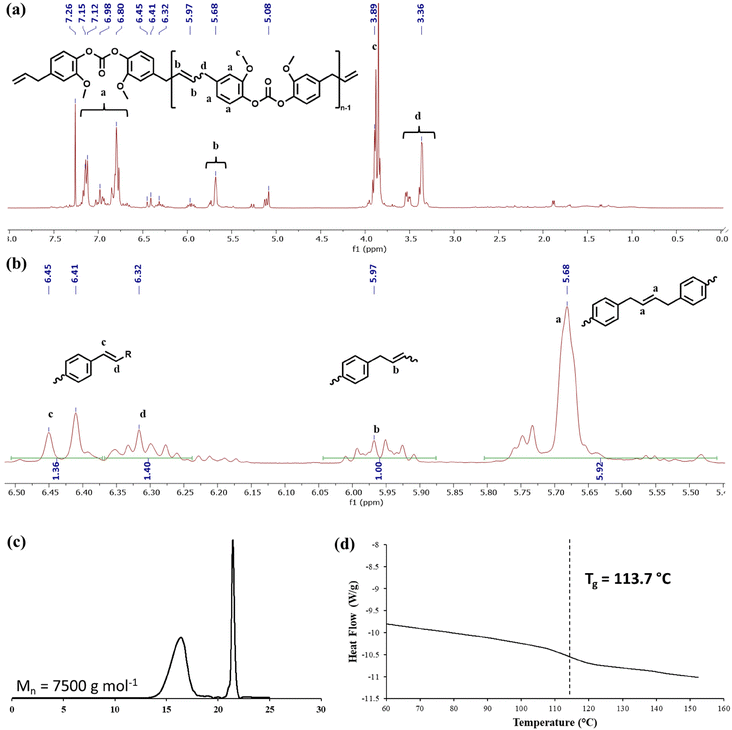
The monomeric ethenolysis products from both the polycarbonate and triazine network were repolymerized via olefin metathesis in dichloromethane at ambient temperature in the presence of 10% M204 to generate PC2 and CEN2 from 2 and 4, respectively (Table 3). During the repolymerization of 2, a partial isomerization of the primary olefin to the internal position was observed by NMR spectroscopy (Fig. 9a). The 1H NMR spectrum showed a new stilbene linkage at 6.45 and 6.32 ppm derived from the metathesis reaction of two internal alkenes. In addition, we observed a second set of alkene hydrogens at 5.97 ppm attributable to the product obtained by a metathesis reaction between an internal and primary alkene (Fig. 9b).44 Finally, we observed the conventional linkage from the parent polymer at 5.68 ppm (Fig. 9b). Careful integration of these regions allowed us to quantify the relative amount of each type of linkage in the polycarbonate. 61% of the linkages were between two allyl groups, 10% were asymmetric (between an internal alkene and a primary alkene), and 29% of the linkages were of the stilbene type. An attempt was made to prevent isomerization of the allyl group to an internal position by the addition of 1,4-benzoquinone. However, we saw no difference in the product distribution, despite a contrary result in the literature.45 GPC analysis was performed on the recycled polymer, and Mn was found to be 7500 g mol−1 (Fig. 9c). This is substantially lower than the initial Mn of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1 for PC1high, however, direct molecular weight comparisons are not warranted because the original polycarbonate was polymerized via step-growth interfacial polymerization, whereas the recycled polymer was polymerized via ADMET.
700 g mol−1 for PC1high, however, direct molecular weight comparisons are not warranted because the original polycarbonate was polymerized via step-growth interfacial polymerization, whereas the recycled polymer was polymerized via ADMET.
| Polymer | Time (min) | Yield (%) | M n GPC (g mol−1) | Đ | T g (°C) |
|---|---|---|---|---|---|
| PC2 | 1080 | 59 | 7500 | 1.49 | 114 |
| CEN2 | 840 | 70 | 8600 | 1.73 | 173 |
Further evidence of alkene isomerization prior to polymerization was provided by DSC analysis of the recycled material. The Tg of the repolymerized polycarbonate was found to be 114 °C, an increase of 32 °C compared to the virgin polycarbonate (Fig. 9d). Mechanically recycled polymers typically exhibit lower Tgs due in part to dangling chain ends created during the recycling process.1,43 Similarly, harsh chemical depolymerization mechanisms can generate “dirty mixtures” which negatively impact the properties of the repolymerized material. In the current case, the substantial increase in Tg was attributed to the partial isomerization of the allyl group, which resulted in the formation of more rigid linking groups, particularly stilbenes. Despite the structural differences, it is remarkable that the recycled polymer product has an improved Tg compared to the initial material. It is likely that subsequent cycles of de- and repolymerization would result in additional isomerization of the alkene, leading to further increases in Tg.44
Circularity of catalytic recycling
The repolymerized polycarbonate was not subjected to further ethenolysis cycles due to the isomerization of the allyl group under the established conditions. The isomerization process would generate a different polycarbonate product over time, resulting in changing material properties. Additionally, the solubility of the material would be expected to diminish as the stilbenic linkages increase. While the polycarbonate can be efficiently recycled, the isomerization process would make it hard to evaluate if the catalytic recycling process detrimentally affected the material properties for this particular system. In contrast, we did not observe any isomerization during repolymerization of 4. After the initial depolymerization and repolymerization cycle, two additional cycles of catalytic recycling were successfully performed. In each cycle, the network was fully depolymerized to 4 (two steps, a and b) and subsequently repolymerized to generate a polycyanurate (Tables 4 and 5). For clarity, polymers are identified by their polymerization round number. For example, CEN1 represents the first round of polymerization while CEN2 is the network repolymerized from the first round of ethenolysis products, CEN3 is the network repolymerized from the second depolymerization step, and so on.| Polymer identity | Starting Mn (g mol−1) | M n after ethenolysis (g mol−1) | Yield (%) | Catalyst typeb | Time (hours) |
|---|---|---|---|---|---|
| a 240 PSI, 25 °C, 10 mol% benzoquinone. b 10 mol% catalyst; pure monomer required 2 consecutive ethenolysis reactions. | |||||
| CEN2a | 8600 | 1800 | 67 | M204 | 24 |
| CEN2b | 1800 | 700 | 68 | M204 | 24 |
| CEN3a | 7500 | 1900 | 72 | M204 | 24 |
| CEN3b | 1900 | 600 | 85 | M204 | 24 |
| CEN4a | 8000 | 1500 | 59 | M204 | 48 |
| CEN4b | 1540 | 600 | 83 | M204 | 24 |
| Polymer | Reaction time (minutes) | % yield | M n, GPC (g mol−1) | Đ | T g, C |
|---|---|---|---|---|---|
| CEN2 | 840 | 68 | 8600 | 1.73 | 173 |
| CEN3 | 840 | 90 | 7500 | 4.21 | 167 |
| CEN4 | 840 | 82 | 8000 | 1.33 | 184 |
After each polymerization, the thermomechanical properties of the reconstituted networks were evaluated. Neither the Tg nor the molecular weight were significantly different in the repolymerized samples (Table 5 and Fig. 10). For CEN3, the 2nd repolymerization of the polycyanurate, Đ was extremely high. Careful examination of the GPC trace revealed a small amount of high molecular weight material combined with a peak attributed to unpolymerized monomer present in this sample. The effects of residual monomer greatly impacted Tg, which was 13 °C lower than the virgin polymer. However, in the next cycle the Tg recovered to 184 °C, an increase in 4 °C over the virgin polymer. Overall, these results show full circularity for the eugenol-based polycyanurate network, and suggest that this novel recycling method may be applied to insoluble thermoset networks, potentially addressing a long-standing challenge within the polymer industry.
Conclusions
A thermoplastic polycarbonate and a hyperbranched polycyanurate with alkenes incorporated in the backbone were synthesized from the biobased phenol eugenol and fully characterized. The resulting materials were then depolymerized via a two-step [Ru]-catalyzed ethenolysis process to generate monomers with pendent allyl groups. These monomers were subsequently repolymerized via olefin metathesis to obtain high molecular weight linear and hyperbranched polymers. The depolymerization products were purified without rigorous effort and found to be suitable for repolymerization. For the depolymerization products of the linear polycarbonate, isomerization of the allyl group during ADMET polymerization was observed, preventing direct comparison of the thermo-mechanical properties of the recycled polycarbonate with the initial polycarbonate. Future work may explore alternative catalysts or lower catalyst loading as a possible route to prevent isomerization of the allyl group. Interestingly, the isomerization during repolymerization resulted in a dramatic increase in Tg (32 °C), which essentially providing a route to upcycle the polycarbonate. In contrast, most polymers recycled via either mechanical routes or treatment with harsh chemicals at elevated temperature result in materials with decreased Tgs and poorer mechanical properties.In parallel with the polycarbonate work we demonstrated efficient depolymerization/repolymerization protocols for a polycyanurate. No isomerization was observed during the repolymerization of this monomer, enabling full circularity. Three cycles of depolymerization and repolymerization were performed and no significant decrease in thermo-mechanical properties was observed. Future work in our laboratory will focus on recycling the ethenolysis catalyst, using more environmentally friendly solvents, and attempting the ethenolysis of insoluble thermoset networks. Overall, this work demonstrates the potential of sustainable and fully recyclable eugenol-based polymers for commercial applications. This approach can be readily extended to other alkene containing thermoplastics and cross-linked networks, offering a versatile recycling strategy to support the creation of a circular economy.
Data availability
The data supporting this article are included in the ESI.† Crystallographic data for compounds 1, 2, and 4 have been deposited in the Cambridge Structural Database (CCDC deposition numbers 2360344, 2360346, and 2360345, respectively†).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the National Research Council for administering the postdoctoral fellowship for DMP and the Office of Naval Research for supporting FDE through the Naval Research Enterprise Internship Program (NREIP).References
- J. Payne and M. D. Jones, The Chemical Recycling of Polyesters for a Circular Plastics Economy: Challenges and Emerging Opportunities, ChemSusChem, 2021, 14(19), 4041–4070, DOI:10.1002/cssc.202100400.
- C. G. Schirmeister and R. Mülhaupt, Closing the Carbon Loop in the Circular Plastics Economy, Macromol. Rapid Commun., 2022, 43(13), 220–247, DOI:10.1002/marc.202200247.
- C. R. Bening, J. T. Pruess and N. U. Blum, Towards a circular plastics economy: Interacting barriers and contested solutions for flexible packaging recycling, J. Cleaner Prod., 2021, 302, 126966, DOI:10.1016/j.jclepro.2021.126966.
- A. Tarafdar, R. Siroh, P. A. Balakumaran, R. Reshmy, A. Madhavan, R. Sindhu, P. Binod, Y. Kumar, D. Kumar and S. J. Sim, The hazardous threat of Bisphenol A: Toxicity, detection and remediation, J. Hazard. Mater., 2022, 423, 127097, DOI:10.1016/j.jhazmat.2021.127097.
- L. Trullemans, S. F. Koelwijn, I. Scodeller, T. Hendrickx, P. V. Puyvelde and B. F. Sels, A guide towards safe, functional and renewable BPA alternatives by rational molecular design: structure–property and structure–toxicity relationships, Polym. Chem., 2021, 12(41), 5870–5901, 10.1039/D1PY00909E.
- X. Wu, J. Li, L. Yao and Z. Xu, Auto-sorting commonly recovered plastics from waste household appliances and electronics using near-infrared spectroscopy, J. Cleaner Prod., 2020, 246(10), 118732, DOI:10.1016/j.jclepro.2019.118732.
- S. Thomas and P. M. Visakh, Handbook of Engineering and Specialty Thermoplastics, Volume 3: Polyethers and Polyesters, ed. S. T. a. V. P. M., John Wiley & Sons, 3rd edn, 2011, ISBN: 978-0-470-63926-9 Search PubMed.
- G. Warner and J. A. Flaws, Bisphenol A and Phthalates: How Environmental Chemicals Are Reshaping Toxicology, Toxicol. Sci., 2018, 166(2), 246–249, DOI:10.1093/toxsci/kfy232.
- M. Kumar, D. K. Sarma, S. Shubham, M. Kumawat, V. Verma, A. Prakash and R. Tiware, Environmental Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases, Front. Public Health, 2020, 8, 553850, DOI:10.3389/fpubh.2020.553850.
- L. N. Vandenberg, T. Colborn, T. B. Hayes, J. J. Heindel, D. R. Jacobs, D. H. Lee, T. Shioda, A. M. Soto, S. F. Saal, W. V. Welshons, R. T. Zoeller and J. P. Myers, Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses, Endocr. Rev., 2012, 33(3), 378–455, DOI:10.1210/er.2011-1050.
- D. Melzer, N. J. Osborne, W. E. Henley, R. Cipelli, A. Young, C. Money, P. McCormack, R. Luben, K. Khaw, N. Wareham and T. S. Galloway, Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women, Circulation, 2012, 125(12), 1482–1490, DOI:10.1161/circulationaha.111.069153.
- K. Pelch, J. A. Wignall, A. E. Goldstone, P. K. Ross, R. B. Blain, A. J. Shapiro, S. D. Holmgren, J. Hsieh, D. Svoboda, S. S. Auerback, F. M. Parham, S. A. Masten, V. Walker, A. Rooney and K. A. Thayer, A scoping review of the health and toxicological activity of bisphenol A (BPA) structural analogues and functional alternatives, Toxicology, 2019, 424, 152235, DOI:10.1016/j.tox.2019.06.006.
- Y. Li, L. Tao, Q. Wang, F. Wang, G. Li and M. Song, Potential Health Impact of Microplastics: A Review of Environmental Distribution, Human Exposure, and Toxic Effects, Environ. Health, 2023, 1(4), 249–257, DOI:10.1021/envhealth.3c00052.
- M. Fache, E. Darroman, V. Besse, R. Auvergne, S. Caillol and B. Boutevin, Vanillin, a promising biobased building-block for monomer synthesis, Green Chem., 2014, 16(4), 1987–1998, 10.1039/C3GC42613K.
- E. K. R. Hanko, K. N. G. Valdehuesa, K. J. A. Verhagen, J. Chromy, R. A. Stoney, J. Chua, C. Yan, J. A. Roubos, J. Schmitz and R. Breitling, Carboxylic acid reductase-dependent biosynthesis of eugenol and related allylphenols, Microb. Cell Fact., 2023, 22(238) DOI:10.1186/s12934-023-02246-4.
- K. Hüsnü, C. Başer and F. Demirci, Chemistry of Essential Oils, Flavours Fragrances, 2007, 43–86, DOI:10.1007/978-3-540-49339-6_4.
- P. Prakash and N. Gupta, Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review, Indian J. Physiol. Pharmacol., 2005, 49(2), 125–131, DOI:10.3390/molecules28031193.
- B. G. Harvey, C. M. Sahagun, A. J. Guenthner, T. J. Groshens, L. R. Cambrea, J. T. Reams and J. M. Mabry, A High-Performance Renewable Thermosetting Resin Derived from Eugenol, ChemSusChem, 2014, 7(7), 1964–1969, DOI:10.1002/cssc.201400019.
- G. Odian, Step Polymerization, Principles of Polymerization, 2004, pp. 39–197. DOI:10.1002/047147875X.
- J. Pribyl, K. B. Wagener and G. Rojas, ADMET polymers: synthesis, structure elucidation, and function, Mater. Chem. Front., 2021, 5(1), 14–43, 10.1039/D0QM00273A.
- A. Kajetanowicz and K. Grela, Nitro and Other Electron Withdrawing Group Activated Ruthenium Catalysts for Olefin Metathesis Reactions, Angew. Chem., Int. Ed., 2021, 60(25), 13738–13756, DOI:10.1002/anie.202008150.
- J. C. Foster, J. Zheng, M. Arifuzzaman, M. Rahman, J. Damron, C. Guan, I. Popovs, N. Galan, Z. Demchuk and T. Saito, Closed-loop recycling of semi-aromatic polyesters upcycled from poly(ethylene terephthalate), Cell Rep. Phys. Sci., 2023, 4(12), 101734, DOI:10.1016/j.xcrp.2023.101734.
- K. B. Wagener and J. C. Marmo, Acyclic diene metathesis (ADMET) depolymerization: The synthesis of 1,4-polybutadiene telechelics, Macromol. Rapid Commun., 1995, 16(8), 557–561, DOI:10.1002/marc.1995.030160804.
- K. L. Sedransk, C. F. Kaminkski, L. R. Hutchings and G. D. Moggridge, The metathetic degradation of polyisoprene and polybutadiene in block copolymers using Grubbs second generation catalyst, Polym. Degrad. Stab., 2011, 96(6), 1074–1080, DOI:10.1016/j.polymdegradstab.2011.03.007.
- K. B. Wagener, J. M. Boncella and J. G. Nel, Acyclic diene metathesis (ADMET) polymerization, Macromolecules, 1991, 24(10), 2649–2657, DOI:10.1016/j.polymdegradstab.2011.03.007.
- S. Utekar, V. K. Suriya, N. More and A. Rao, Comprehensive study of recycling of thermosetting polymer composites – Driving force, challenges and methods, Composites, Part B, 2021, 207, 108596, DOI:10.1016/j.compositesb.2020.108596.
- M. D. Garrison, P. J. Storch, W. S. Eck, V. H. Adams, P. W. Fedick and B. G. Harvey, BPA-free high-performance sustainable polycarbonates derived from non-estrogenic bio-based phenols, Green Chem., 2021, 23(20), 8016–8029, 10.1039/D1GC01500A.
- Q. Chen, W. Huang, P. Chen, C. Peng, H. Xie, Z. K. Zhao, M. Sohail and M. Bao, Synthesis of Lignin-Derived Bisphenols Catalyzed by Lignosulfonic Acid in Water for Polycarbonate Synthesis, ChemCatChem, 2015, 7(7), 1083–1089, DOI:10.1002/cctc.201500010.
- A. S. Trita, L. C. Over, J. Pollini, S. Riegsinger, M. A. R. Meier and L. J. Gooben, Synthesis of potential bisphenol A substitutes by isomerising metathesis of renewable raw materials, Green Chem., 2017, 19(13), 3051–3060, 10.1039/C7GC00553A.
- W. B. Kim, U. A. Joshi and J. S. Lee, Making Polycarbonates without Employing Phosgene: An Overview on Catalytic Chemistry of Intermediate and Precursor Syntheses for Polycarbonate, Ind. Eng. Chem. Res., 2004, 43(9), 1897–1914, 10.1039/C7GC00553A.
- B. G. Harvey, A. J. Guenthner, T. A. Koontz, P. J. Storch, J. T. Reams and T. J. Groshens, Sustainable hydrophobic thermosetting resins and polycarbonates from turpentine, Green Chem., 2016, 18(8), 2416–2423, 10.1039/C5GC02893K.
- B. C. Smith, Infrared Spectral Interpretation, A Systematic Approach. CRC Press, 1999, pp. 119–221. DOI:10.5860/choice.36-5697.
- G. Socrates, Infrared and Raman Characteristic Group Frequencies: Tables and Charts, The University of West London, U.K. J. Wiley and Sons, Chichester, Middlesex, 2001, vol. 3, ISBN: 0-471-85298-8 Search PubMed.
- D. Kyriacos, Chapter 17 – Polycarbonates, Brydson's Plastics Materials, 2017, vol. 8, pp. 457–485. DOI:10.1016/C2009-0-30435-3.
- H. R. Abuzeid, A. F. M. El-Mahdy, M. M. M. Ahmed and S. Kuo, Triazine-functionalized covalent benzoxazine framework for direct synthesis of N-doped microporous carbon, Polym. Chem., 2019, 10(44), 6010–6020, 10.1039/c9py01231a.
- Z. Yang, Y. Chai, X. Yao and H. Ji, Mechanism for efficient separation of eugenol and eugenol acetate with β-cyclodextrin as a selective solvent, Supramol. Chem., 2019, 31(12), 767–775, DOI:10.1080/10610278.2019.1702663.
- B. G. Harvey, A. J. Guenthner, G. R. Yandek, L. R. Cambrea, H. A. Meylemans, L. C. Baldwin and J. T. Reams, Synthesis and characterization of a renewable cyanate ester/polycarbonate network derived from eugenol, Polymer, 2014, 55(20), 5073–5079, DOI:10.1016/j.polymer.2014.08.034.
- J. L. DeRudder, Handbook of Polycarbonate Science and Technology, CRC Press, 2000, vol. 1, pp. 101–144. DOI:10.1201/9781482273694.
- R. Kessler, Cyante Ester Resins, Wiley Encyclopedia of Composites, 2012, vol. 1. DOI:10.1002/9781118097298.weoc062.
- A. Shimp, Chemistry and Technology of Cyanate Ester Resins, Chapman and Hall, 1994, 1. DOI:10.1007/978-94-011-1326-7.
- J. Spekreijse, J. P. Sanders, J. H. Bitter and E. L. Scott, The Future of Ethenolysis in Biobased Chemistry, ChemSusChem, 2017, 10(3), 470–482, DOI:10.1002/cssc.201601256.
- F. P. La Mantia, Polymer Mechanical Recycling: Downcycling or Upcycling?, Prog. Rubber, Plast. Recycl. Technol., 2004, 20(1), 11–24, DOI:10.1177/147776060402000102.
- H. Jin, J. Gonzalez-Guitierrez, P. Oblak, B. Zupancic and I. Emri, The effect of extensive mechanical recycling on the properties of low density polyethylene, Polym. Degrad. Stab., 2012, 97(11), 2262–2272, DOI:10.1016/j.polymdegradstab.2012.07.039.
- S. Günther, P. Lamprecht and G. A. Luinstra, ADMET-Polymerization of Dienes based on Sustainable Chemicals, Macromol. Symp., 2010, 293(1), 15–19, DOI:10.1016/j.polymdegradstab.2012.07.039.
- M. Huang, D. Bai, Q. Chen, C. Zhao, T. Ren, C. Huang, M. North and H. Zie, Facile preparation of polycarbonates from bio-based eugenol and 2-methoxy-4-vinylphenol, Polym. Chem., 2020, 11(32), 5133–5139, 10.1039/D0PY00291G.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2360344–2360346. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4py00940a |
| This journal is © The Royal Society of Chemistry 2025 |