 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploring hot melt extrusion in the formation of exemestane/amino acid co-amorphous systems
Ioannis
Partheniadis
 ,
Maria
Tsouka
and
Ioannis
Nikolakakis
,
Maria
Tsouka
and
Ioannis
Nikolakakis
 *
*
Department of Pharmaceutical Technology, School of Pharmacy, Faculty of Health Sciences, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece. E-mail: yannikos@pharm.auth.gr; Tel: +302310997635
First published on 25th August 2025
Abstract
Co-amorphous systems (CAMS) of exemestane (EXE) were prepared with three amino acid (AA) co-formers of increasing hydrophobicity: (i) L-lysine (LYS), (ii) L-valine (VAL) and (iii) L-methionine (MET) using feed solvent pretreatment hot-melt extrusion (mHME). Thermal analysis (DSC and TGA) guided processing parameters confirmed the class III glass-forming ability of EXE (Tg = 91.2 °C). Hansen solubility parameters (Δδt < 4 MPa1/2) predicted favorable drug/co-former miscibility. PXRD and DSC demonstrated successful co-amorphization for molar ratios of EXE/LYS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), EXE/MET (1
2), EXE/MET (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and EXE/VAL (2
1) and EXE/VAL (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 drug/AA). ATR-FTIR indicated co-amorphization predominantly by simple molecular mixing with only weak interactions. The physical stability of CAMS was evaluated by isothermal microcalorimetry, dynamic mechanical analysis (DMA) and crystallographic profiles (pXRD) obtained at different times during accelerated stability tests (40 °C, 75% RH). EXE/LYS systems exhibited the longest relaxation times (
1 drug/AA). ATR-FTIR indicated co-amorphization predominantly by simple molecular mixing with only weak interactions. The physical stability of CAMS was evaluated by isothermal microcalorimetry, dynamic mechanical analysis (DMA) and crystallographic profiles (pXRD) obtained at different times during accelerated stability tests (40 °C, 75% RH). EXE/LYS systems exhibited the longest relaxation times ( ), translating as excellent physical stability, which corroborated the results of accelerated tests. EXE/MET showed moderate stabilization, while EXE/VAL was the least stable. Under non-sink conditions of the dissolution test, EXE/LYS (1
), translating as excellent physical stability, which corroborated the results of accelerated tests. EXE/MET showed moderate stabilization, while EXE/VAL was the least stable. Under non-sink conditions of the dissolution test, EXE/LYS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) presented a pronounced spring–parachute profile with sustained supersaturation, outperforming other EXE/AA CAMS.
1) presented a pronounced spring–parachute profile with sustained supersaturation, outperforming other EXE/AA CAMS.
Introduction
Co-amorphous systems (CAMS) have emerged as a technology to address the low bioavailability of modern drug candidates caused by their poor water solubility, limiting absorption.1–4 By stabilizing the drug in its amorphous form, these systems enhance apparent solubility and drastically improve dissolution performance.Unlike traditional approaches, CAMS utilize small organic molecules (co-formers) instead of polymers, enabling higher drug concentrations while avoiding moisture sorption problems associated with polymers’ hygroscopicity.5 Amino acids (AAs) are frequently employed as co-formers in CAMS.2 To address the low thermal stability of AAs,6 ball milling – which induces crystal defects to achieve amorphization – has been used for preparing such systems. However, ball milling faces challenges such as solvent dependency, scalability constraints, and industrial applicability due to limitations in the size of the equipment.2,7–9
Hot-melt extrusion (HME), a solvent-free, scalable process widely used for amorphous solid dispersions,3,10–12 offers an alternative to prepare CAMS by thermomechanically disrupting the crystalline structure. To address the thermal instability of amino acids (AAs) and enable the processing of drug/AA CAMS, we previously developed a feed solvent pretreatment hot melt extrusion (mHME) method.13,14 In this approach, diluted acetic acid was used to convert drug/AA powder mixtures into extrudable granular feed, enabling extrusion at lower temperatures, thus mitigating thermal degradation risks. Importantly, the CAMS produced by mHME had significantly improved drug solubility and release compared to the crystalline drug.
In a recent study, we prepared CAMS of griseofulvin (GRI) with three AAs differing in hydrophobicity using the mHME method.14 While previous attempts to prepare GRI/AA CAMS using purely mechanical methods were only moderately successful,15 the mHME method produced CAMS at different drug/AA molar ratios. Therefore, the mHME method presents an excellent alternative for the preparation of drug/AA CAMS where mechanical or purely thermal methods fail.
In the present study, we applied the mHME process for the development of CAMS of the poorly water-soluble drug exemestane (EXE) with three amino acids of different hydrophobicity and molecular side chains as co-formers: L-lysine (polar, positively charged), L-valine (hydrophobic, small aliphatic chain) and L-methionine (more hydrophobic, large aliphatic chain). Since in our previous work,14 we had shown that griseofulvin with 6 hydrogen bond acceptor groups forms stable CAMS with L-lysine, L-methionine and L-valine and with improved drug release, in the present work, we wanted to see to what extent this is possible with a drug of lower H-bonding potential such as exemestane (only 2 hydrogen bond acceptor groups). As in our previous publications,13,14 CAMS were prepared using only drugs and amino acids without polymers. The produced CAMS were examined based on three critical attributes: co-formability, physical stability, and dissolution performance.2
Experimental
Materials
Exemestane (EXE; Mw = 296.40 g mol−1) was chosen as a poorly water-soluble drug and L-lysine (LYS; Mw = 146.19 g mol−1), L-methionine (MET; Mw = 149.21 g mol−1) and L-valine (VAL; Mw = 117.25 g mol−1) as the amino acid (AA) co-formers for CAMS. 30% w/w aqueous acetic acid solution (AcOH; CAS No. 64-19-7, Sigma-Aldrich Inc., Saint Louis, MO, U.S.A) was the feed pretreatment solvent.Pretreatment of EXE/AA powder mixtures for hot melt extrusion processing
Drug/AA powder mixtures at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratios were prepared and 30% aqueous acetic acid (AcOH) solution (9 to 12 mL g−1) was added. The AcOH solution was selected because it enabled the formation of a paste-consistency mass, resulting in granules after drying/sieving. This was subsequently dried in an air circulation oven (Heraeus, Germany) at 100 °C for 3 h, followed by sieving (850 μm sieve) to give a granular feed that was added to the extruder. More details on the feed pretreatment process are given elsewhere.13,14
2 molar ratios were prepared and 30% aqueous acetic acid (AcOH) solution (9 to 12 mL g−1) was added. The AcOH solution was selected because it enabled the formation of a paste-consistency mass, resulting in granules after drying/sieving. This was subsequently dried in an air circulation oven (Heraeus, Germany) at 100 °C for 3 h, followed by sieving (850 μm sieve) to give a granular feed that was added to the extruder. More details on the feed pretreatment process are given elsewhere.13,14
Hot melt extrusion with feed solvent pretreatment (HME)
The extruder was a bench-type vertical single-screw (Model RCP-0250 Microtruder, Randcastle Extrusion Systems, NJ, USA) fitted with a 2 mm orifice die, operated at 20 rpm screw speed. Feeds were processed at extrusion zone temperatures ranging from 145 to 150 °C in the feeding zone, 165 to 175 °C in the mixing/melting/shearing/compression zone, and 170 to 185 °C in the extrusion zone. More details about the preparation method are given elsewhere.13 Extrudate codes, drug/AA molar ratios of the nine extruded feeds (three amino acids at three EXE/AA molar ratios), together with % drug content in the extrudates determined by high liquid pressure chromatography, are listed in Table 1. The zone temperatures applied for the extrusion of the nine EXE/AA feeds together with the decomposition temperatures (Tdec) of the feeds measured by thermogravimetry (TGA) are given in Table 2.| Code | Amino acid (AA) | Molar ratio | Composition (% w/w) of the feed | Drug content (% w/w) | ||
|---|---|---|---|---|---|---|
| Drug | AA | Drug | AA | |||
EXE/LYS 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
L-Lysine | 2 | 1 | 80.25 | 19.75 | 98.8 ± 0.3 |
EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 1 | 67.00 | 33.00 | 97.9 ± 0.4 | |
EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1 | 2 | 50.25 | 49.75 | 98.7 ± 0.3 | |
EXE/MET 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
L-Methionine | 2 | 1 | 80.00 | 20.00 | 99.3 ± 0.6 |
EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 1 | 66.50 | 33.50 | 99.0 ± 0.2 | |
EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1 | 2 | 49.75 | 50.25 | 98.9 ± 0.4 | |
EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
L-Valine | 2 | 1 | 83.50 | 16.50 | 99.1 ± 0.3 |
EXE/VAL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 | 1 | 71.50 | 28.50 | 98.6 ± 0.2 | |
EXE/VAL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1 | 2 | 55.75 | 44.25 | 99.4 ± 0.1 | |
| Drug/AA molar ratio | Temperature zones (°C) | Decomposition temp. (Tdec °C) | Difference Tdec − T3 (zone 3) | ||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
EXE/LYS 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 165 | 170 | 193.3 | 23.3 |
EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
150 | 165 | 170 | 195.6 | 25.6 |
EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
150 | 165 | 170 | 196.8 | 26.8 |
EXE/MET 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
145 | 170 | 180 | 199.9 | 19.9 |
EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
145 | 175 | 185 | 201.3 | 16.3 |
EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
145 | 175 | 185 | 203.8 | 18.8 |
EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
145 | 170 | 180 | 197.8 | 17.8 |
EXE/VAL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
145 | 170 | 180 | 198.2 | 18.2 |
EXE/VAL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
145 | 170 | 185 | 200.3 | 15.3 |
Differential scanning calorimetry (DSC)
Formation of CAMS from the drug/AA powder mixtures was studied in situ using the DSC204 F1 Phoenix DSC instrument (NETZSCH, Germany). Accurately weighed samples of 3–4 mg were placed in pierced aluminum pans and examined for thermal changes at 10 °C min−1 heating rate under nitrogen gas. Indium was used for instrument calibration. Additionally, DSC was applied to determine the glass forming ability (GFA) of EXE, based on the classification system for crystallization tendency proposed by Baird et al.16 A 1st heating cycle was applied to erase thermal history, followed by fast cooling (at 20 °C min−1) and a 2nd heating cycle to estimate the GFA.In certain cases, modulated DSC (mDSC) was applied to confirm the presence of a single glass transition temperature (Tg). For this measurement, the sample (10–15 mg) was equilibrated at 30 °C for 2 min before ramping at 2 °C min−1 up to 180 °C using a modulation of ±0.212 °C every 40 s. A reverse sample heat flow was processed using the Proteus Analysis® software to confirm the single Tg.
Thermogravimetric analysis (TGA)
The thermal stability of ingredients and drug/AA mixtures was examined over a wide temperature range using a TGA instrument connected to a TA-60-WS controller (Shimadzu Corporation, Kyoto, Japan). Safe HME processing temperature ranges were established to avoid overheating and decomposition during extrusion. For the analysis, 3–4 mg of accurately weighed samples were placed in aluminum pans and heated under a nitrogen atmosphere (N2) at 10 °C min−1. Degradation temperature was determined from the onset temperature according to American Standard Test Method (ASTM E2550)17 specifications. Experiments were conducted in triplicate.Drug/AA miscibility from Hansen solubility parameters (HSPs)
Hansen solubility parameters (HSPs) were computed as a thermodynamic guide to the miscibility of the drug and AA co-formers. It is a simple, direct method to predict miscibility of components and provides a reliable prediction of CAMS formation and physical stability.13,14 The total Hansen solubility parameter (δt) represents attractive intermolecular forces and can be expressed as the square root of the sum of dispersion, polar and hydrogen bonding parameters (HSPs). A difference of Δδt < 5 MPa1/2 is a criterion of miscibility.18–20 HSPs for the drugs and AAs were calculated according to the group contribution method previously described.21Quantification of the drug in the extrudates
Quantification of exemestane (EXE) was undertaken using high pressure liquid chromatography (HPLC). The HPLC system consisted of a pump (LC-10 AD VP), an auto-sampler (SIL-20A HT), and a UV-Vis detector (SPD-10A VP, Shimadzu, Kyoto, Japan). The analytical conditions were adapted from the literature with slight modifications.22 The mobile phase comprised acetonitrile (ACN) and water in a ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v. The stationary phase was a Discovery H C18 column (150 mm, 4.6 mm, 5 μm, Merck KGaA, Darmstadt, Germany) and the flow rate was set to 1.0 mL min−1, with an injection volume of 10 μL. The detection wavelength was 242 nm. The mobile phase was degassed under vacuum (20 min) and sonicated (10 min) before each analysis. Standard samples of the API were analyzed, with concentrations in the range of 9.0–900.0 μg mL−1 (R2 ≥ 0.999).
50 v/v. The stationary phase was a Discovery H C18 column (150 mm, 4.6 mm, 5 μm, Merck KGaA, Darmstadt, Germany) and the flow rate was set to 1.0 mL min−1, with an injection volume of 10 μL. The detection wavelength was 242 nm. The mobile phase was degassed under vacuum (20 min) and sonicated (10 min) before each analysis. Standard samples of the API were analyzed, with concentrations in the range of 9.0–900.0 μg mL−1 (R2 ≥ 0.999).
Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR)
Spectra of the samples were acquired using a Bomem FTIR spectrometer (MB-Series, ABB Bomem Inc., Quebec, QC, Canada) and processed by GRAMS/AI software (version 7.0, Thermo Fisher Scientific, Waltham, MA, USA). The samples were scanned over a wavenumber range from 400 to 4000 cm−1 with a resolution of 4 cm−1. The spectra were averaged from 64 scans.Powder X-ray diffraction (pXRD) of extrudates after preparation and during stability testing
To estimate the crystalline and by difference the amorphous content of unprocessed ingredients, feeds and extruded products, pXRD was applied. 1 g of sample was gently pulverized using a porcelain mortar, and then it was mounted on a 28-position sample plate and analyzed using transmission pXRD (λ = 0.15405 nm, CuKα radiation, Bruker D8 PHASER CRD-diffractometer, Bruker, MA, USA). Data were collected in the range of 5–35° 2θ at 0.02° 2θ step size and 0.5 s step count time. Instrument accuracy was tested against a corundum A26-B29-S reference sample.For the solid-state stability of CAMS in the extrudates, pXRD analysis was applied. The samples were placed in desiccators at 45 °C and 75% RH. To detect and quantify any solid-state changes, pXRD profiles were obtained at day 0, 30 and 90 days, analyzed and compared.
Isothermal microcalorimetry relaxation measurements using a thermal activity monitor
A thermal activity monitor (TAM III, TA Instruments, New Castle, USA) was used to directly measure the relaxation time of amorphous samples by recording the rate of enthalpy relaxation as a function of time during annealing.23 Samples of approximately 200 mg were prepared in 4 mL disposable crimp-sealed ampoules and measured at 25 °C. To minimize the effect of thermal history, freshly prepared samples were collected and loaded into the equilibrium position. The resulting power–time curves were fitted to the derivative of the ‘Modified Stretched Exponential’ (MSE) equation (eqn (1)) to obtain the parameters τ0, τ1, and β.24,25 | (1) |
In eqn (1), P is the power (μW g−1), and t is the measurement (annealing) time (h). The number 277.8 accounts for unit conversions. τ0 and τ1 are relaxation time constants, β represents the distribution of independently relaxing states (0 < β < 1) and ΔHr(∞) is the relaxation enthalpy at infinite time obtained from eqn (2):
| ΔHr(∞) = (Tg − T) × ΔCp | (2) |
T
g is the glass transition temperature, ΔCp is the heat capacity change at Tg, and T is the annealing temperature. The relaxation time ( ) can then be calculated from eqn (3):
) can then be calculated from eqn (3):
 | (3) |
Statistical analyses and fitting of the experimental data to the MSE equation were conducted using Python (version 3.11.7) and the SciPy optimization module (scipy.optimize, version 1.14.1), and Jupyter Notebook IDE (version 7.0.8).
Apparent equilibrium solubility of drugs in aqueous solutions
The apparent equilibrium solubility of EXE was determined both in the crystalline and amorphous drug forms (prepared by quench-cooling) and the corresponding methods are described below separately for each drug form.Precipitation study to evaluate the association tendency of EXE with AAs in solution
To elucidate the re-crystallization tendency of EXE from the CAMS during in vitro dissolution, the impact of amino acids (AAs) on the potential depletion and precipitation of EXE from a pre-supersaturated drug solution was studied by applying the Solvent Shift method.29 Tests were conducted in triplicate on a USP II apparatus (rotating paddle, Pharma Test PTW 2, Hainburg, Germany) at 37 ± 0.5 °C and 50 rpm paddle rotation speed. First, EXE was solubilized in a small volume of dimethyl sulfoxide (100 mg EXE in 20 mL DMSO). This solution was added to 450 mL solution of each AA in deionized water (DMSO and water are miscible), aiming for a sink index (SI) of 0.116 (i.e. 245 mg of EXE in 900 mL of aqueous medium), where SI = crystalline drug solubility over the concentration of completely dissolved drug.29 The amount of each AA pre-dissolved in the deionized water corresponded to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EXE/AA molar ratio in the final combined solution, which was found to be the optimal ratio for CAMS formation. Aliquots were collected over a 120 min time period, filtered through PVDF filters (0.45 μm) and analyzed by HPLC as described in the experimental part.
1 EXE/AA molar ratio in the final combined solution, which was found to be the optimal ratio for CAMS formation. Aliquots were collected over a 120 min time period, filtered through PVDF filters (0.45 μm) and analyzed by HPLC as described in the experimental part.
In vitro dissolution tests
Due to the expected higher solubility of the amorphous drug from the CAMS, the tests were conducted under non-sink conditions using a USP II apparatus (rotating paddle, Pharma Test PTW 2, Hainburg, Germany) at 37 ± 0.5 °C and 50 rpm paddle rotation speed. Samples of extrudates containing 1220 mg of drug each were added to 450 mL of deionized water (SI = 0.0116). Before the test, the medium was degassed to avoid powder floating. 2.5 mL aliquots were automatically withdrawn for analysis and immediately replaced with fresh dissolution medium with the aid of a motorized sampling system consisting of a fraction collector (PTFC-2/8 SP, Pharma Test, Hainburg, Germany) and two syringe pumps (PT-SP6, Pharma Test, Hainburg, Germany). The aliquots were filtered (0.45 μm, PVDF filters) and analyzed by HPLC as described previously. At the end of the test, the precipitants were filtered using a Buchner funnel under vacuum, dried at room temperature for 12 h and analyzed for crystallinity by pXRD (n = 3).Results and discussion
Hot melt extrusion – thermal analysis and processing temperatures
Fig. 1 presents the DSC and TGA thermographs of neat EXE and AA powders. Thermographs of drug/AA crystalline powder mixtures at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratios are presented in Fig. S1 (SI). EXE melts at 198.9 °C (ΔHf = 93.4 J g−1) and decomposes above 205.6 °C (Fig. 1(a)). From the AAs, LYS shows Tm at 204.4 °C (ΔHf = 177.4 J g−1) and Tdec above 173.4 °C with 2.8% weight loss at Tm (Fig. 1(c)), MET shows Tm at 291.5 °C (ΔHf = 908.6 J g−1) and Tdec above 267.8 °C with 2.5% weight loss at Tm (Fig. 1(d)) and VAL shows Tm at 301.2 °C (ΔHf = 863.4 J g−1) and Tdec above 281.5 °C with 1.7% weight loss at Tm (Fig. 1(e)). Fig. S1 presents DSC thermograms of the physical drug/amino acid mixtures. Thermograms taken with sealed pans gave the same melting temperatures as with pierced pans but also multi-peaks near Tdec due to entrapped decomposition compounds.
2 molar ratios are presented in Fig. S1 (SI). EXE melts at 198.9 °C (ΔHf = 93.4 J g−1) and decomposes above 205.6 °C (Fig. 1(a)). From the AAs, LYS shows Tm at 204.4 °C (ΔHf = 177.4 J g−1) and Tdec above 173.4 °C with 2.8% weight loss at Tm (Fig. 1(c)), MET shows Tm at 291.5 °C (ΔHf = 908.6 J g−1) and Tdec above 267.8 °C with 2.5% weight loss at Tm (Fig. 1(d)) and VAL shows Tm at 301.2 °C (ΔHf = 863.4 J g−1) and Tdec above 281.5 °C with 1.7% weight loss at Tm (Fig. 1(e)). Fig. S1 presents DSC thermograms of the physical drug/amino acid mixtures. Thermograms taken with sealed pans gave the same melting temperatures as with pierced pans but also multi-peaks near Tdec due to entrapped decomposition compounds.
 | ||
| Fig. 1 DSC and TGA thermographs for unprocessed materials: (a) exemestane (EXE), (b) L-lysine (LYS), (c) L-methionine (MET) and (d) L-valine (VAL). | ||
Besides the thermal events of individual components, the decomposition temperatures (Tdec) of the drug/AA physical mixtures were determined (Fig. S1). These were used as a guide for the choice of hot-melt extrusion to ensure safe operation below the Tdec, since they were different from the single component Tdec. Accordingly, the TGA weight-loss profiles alongside DSC decomposition peaks were recorded (Fig. 1 and Fig. S1), and the Tdec values are listed in Table 2. They range from 193.3 °C to 203.8 °C, depending on the EXE/AA ratio. By comparing these values with extrusion temperatures (Table 2, highest in zone 3), there is a safety margin of 15.3 °C to 26.8 °C, suggesting low degradation risk. Highest margins (23.3–26.8 °C) are seen for the EXE/LYS combinations. This safety margin is attributed to the feed solvent pretreatment, which imparted plasticization to the mass and reduced extrusion temperatures compared to temperatures needed to extrude the physical mixtures.13
Analysis of the drug content in the extruded products
To confirm that no drug decomposition occurred during extrusion, the extrudates were analyzed by HPLC and the results are presented in Table 1 (last column). The recovered drug ranged from 97.9 to 99.4% w/w of that added in the feeds, indicating negligible loss. Although the coexistence of the drug with AA may decrease the melting temperature and Tdec of EXE, the great reduction in the required extrusion temperatures due to feed pretreatment more than compensates for any Tdec decrease of the drug due to pretreatment.Evaluation of CAMS
The evaluation of the developed CAMS was based on three critical quality attributes (CQAs): (i) co-formability, (ii) physical stability, and (iii) dissolution performance.2Glass-forming ability of drugs. In Fig. 2, DSC thermograms of EXE obtained during heating–cooling–reheating cycles are presented. In the 1st heating cycle, the drug shows a melting endotherm (Tm) at 198.9 °C. No recrystallization peaks are observed upon cooling. In the 2nd heating cycle, Tg is observed at 91.2 °C. Therefore, EXE can be characterized as a class III glass former.16
 | ||
| Fig. 2 DSC thermograms of exemestane during the heating (1st cycle, black)–cooling (red)–heating (2nd cycle, blue) cycle for the determination of its glass forming ability. | ||
Thermodynamic miscibility. The Hansen solubility parameters of the selected AAs and EXE were computed to predict miscibility and the possibility of drug/AA CAMS formation. In Table 3, Hansen solubility parameters together with the number of H-bond acceptor and donor groups for each molecule are presented. It can be seen that in all cases, the total solubility parameter difference (Δδt) between the drug and each AA is well below 4 MPa1/2, indicating good miscibility and a strong possibility for CAMS formation.18–20
| Material | δ d | δ p | δ hb | δ t | Δδt | H-bond acceptor/donor |
|---|---|---|---|---|---|---|
| δ d – dispersion forces; δp – polar forces; δhb – hydrogen-bonding attraction. δt – total solubility, representing all intermolecular attractive forces. | ||||||
| Exemestane | 19.3 | 6.5 | 0.2 | 20.4 | — | 2/0 |
| Lysine | 16.9 | 6.0 | 10.9 | 21.0 | 0.6 | 4/3 |
| Methionine | 17.4 | 13.2 | 10.2 | 24.1 | 3.7 | 4/2 |
| Valine | 18.9 | 13.0 | 7.7 | 24.2 | 3.8 | 3/2 |
Solid-state characterization of extrudates by pXRD. In Fig. 3, pXRD patterns of crystalline (cEXE) and amorphous (aEXE) drugs and of crystalline AA (cLYS, cMET and cVAL) powders are presented. cEXE reflections appear at 10.8°, 14.5°, 15.9°, 16.8°, 18.1°, 19.7°, 21.5°, 22.7°, 23.4°, 24.4°, 26.2°, and 29.2° 2θ.30 In the pXRD patterns of the AAs, the following strong reflections are observed: for cLYS at 5.1°, 10.8°, 17.6°, 24.5°, 31.4°, and 38.4°2θ; for cMET at 5.7°, 23.1°, 29.0°, and 35.1° 2θ; and for cVAL at 7.3°, 14.6°, 21.9°, 29.4°, and 39.9° 2θ.31,32 Therefore, all unprocessed materials (drug and AAs) were crystalline. On the other hand, quench cooling successfully amorphized the drug (aEXE), since its pXRD pattern only shows a halo but no crystalline reflection peaks.
In Fig. 4, pXRD patterns of drug extrudates with the three AAs are presented. Co-amorphization was not achieved for all molar ratios examined. EXE formed CAMS with LYS at drug/AA molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with MET forming CAMS only at a ratio of 1
1, with MET forming CAMS only at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and with VAL only at 2
1 and with VAL only at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. CAMS can be produced at various molar ratios, not just equimolar. Stoichiometry plays an important role in the formation of CAMS, with more than 70% of the reported CAMS prepared at equimolar ratios and 23.1% of the CAMS prepared at other molar ratios.2 This has also been found in our previous studies.13,14 Although co-amorphization was not quantitatively assessed, visual inspection of the pXRD patterns (Fig. 4) suggests that among the different drug/amino acid combinations, EXE/LYS exhibits the highest degree of amorphization. This is indicated by the absence of pronounced diffraction peaks in the pXRD patterns of EXE/LYS compared to EXE/MET and EXE/VAL, and it is attributed to the lower Δδt values (Table 3), implying better miscibility.
1. CAMS can be produced at various molar ratios, not just equimolar. Stoichiometry plays an important role in the formation of CAMS, with more than 70% of the reported CAMS prepared at equimolar ratios and 23.1% of the CAMS prepared at other molar ratios.2 This has also been found in our previous studies.13,14 Although co-amorphization was not quantitatively assessed, visual inspection of the pXRD patterns (Fig. 4) suggests that among the different drug/amino acid combinations, EXE/LYS exhibits the highest degree of amorphization. This is indicated by the absence of pronounced diffraction peaks in the pXRD patterns of EXE/LYS compared to EXE/MET and EXE/VAL, and it is attributed to the lower Δδt values (Table 3), implying better miscibility.
 | ||
| Fig. 4 pXRD patterns of exemestane extruded products with (a) L-lysine (LYS), (b) L-methionine (MET), and (c) L-valine (VAL). | ||
Molecular interactions. Fig. 5 presents ATR-FTIR spectra of the crystalline drug (cEXE), AA crystalline powders (cLYS, cMET and cVAL) and amorphous drug (aEXE). The spectrum of cEXE shows the characteristic drug peaks with stretching vibrations of the –CH2– group at 2937 cm−1, of the cyclopentene ring (D ring) –C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group at 1730 cm−1, of the cyclohexadiene ring (A ring) –C
O group at 1730 cm−1, of the cyclohexadiene ring (A ring) –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group at 1654 cm−1 and of the –C
O group at 1654 cm−1 and of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C– group at 1620 cm−1.33 The aEXE spectrum shows minor changes compared to cEXE, associated with structural rearrangements. More specifically, there is a shifting of the two –C
C– group at 1620 cm−1.33 The aEXE spectrum shows minor changes compared to cEXE, associated with structural rearrangements. More specifically, there is a shifting of the two –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups to higher wavenumbers, from 1730 to 1737 cm−1 and from 1654 to 1656 cm−1, respectively.
O groups to higher wavenumbers, from 1730 to 1737 cm−1 and from 1654 to 1656 cm−1, respectively.
The ATR-FTIR spectra of crystalline amino acids (cLYS, cMET, and cVAL; Fig. 5) show the characteristic peaks of AAs. The wide peak between 2900 and 3000 cm−1 due to the –OH stretching vibrations of the carboxylic group, the peaks at 1650 cm−1 for cLYS, 1652 cm−1 for cMET and 1660 cm−1 for cVAL due to the –NH bending vibrations of the amine group, the peaks between 1550 and 1580 cm−1 (1560 for cLYS, 1567 for cMET and 1574 for cVAL) due to –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the carboxylic group, and the peaks at ca. 1510 cm−1 (1514 for cLYS, 1508 for cMET and 1506 for cVAL) and ca. 1410 cm−1 (1400 for cLYS, 1405 for cMET and 1398 for CVAL) due to C–H stretching vibrations.34,35 The small 2860 cm−1 peak in the spectrum of cLYS is due to the CH2 asymmetric stretching vibrations.
O stretching vibrations of the carboxylic group, and the peaks at ca. 1510 cm−1 (1514 for cLYS, 1508 for cMET and 1506 for cVAL) and ca. 1410 cm−1 (1400 for cLYS, 1405 for cMET and 1398 for CVAL) due to C–H stretching vibrations.34,35 The small 2860 cm−1 peak in the spectrum of cLYS is due to the CH2 asymmetric stretching vibrations.
Fig. 6 shows the ATR-FTIR spectra of EXE/AA extruded CAMS and corresponding physical mixtures of the studied molar ratios in the 1400–1800 cm−1 region of vibration of the two drug carbonyl groups. There are no significant changes between the spectra of extrudates and physical mixtures (PM). A small shift of the drug peak at 1731 cm−1 is seen for all CAMS spectra, and a drop in the intensities of the 1654 and 1731 cm−1 peaks for the EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and EXE/MET 1
2 and EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAMS. However, these differences should not be ascribed to molecular interactions. As already discussed, aEXE shows a small shift of this peak from 1731 to 1737 cm−1 (Fig. 5) compared to cEXE. Therefore, the EXE/AAs extruded CAMS are simply molecular drug/AA associations. The absence of drug/AA molecular interactions partly explains the unsuccessful co-amorphization of EXE/LYS 2
1 CAMS. However, these differences should not be ascribed to molecular interactions. As already discussed, aEXE shows a small shift of this peak from 1731 to 1737 cm−1 (Fig. 5) compared to cEXE. Therefore, the EXE/AAs extruded CAMS are simply molecular drug/AA associations. The absence of drug/AA molecular interactions partly explains the unsuccessful co-amorphization of EXE/LYS 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, EXE/MET 1
1, EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and EXE/VAL 1
1, and EXE/VAL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios, unlike the combinations of griseofulvin with the same AAs, which formed CAMS at all three molar ratios.14
1 ratios, unlike the combinations of griseofulvin with the same AAs, which formed CAMS at all three molar ratios.14
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, EXE/MET 1
1, EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and EXE/VAL 2
1 and EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratios, which produced CAMS.
1 molar ratios, which produced CAMS.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, MET at a ratio of 1
2, MET at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and VAL at a ratio of 2
1 and VAL at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, together with recorded glass transition temperatures (Tgs). A single Tg is seen in all cases, confirming the formation of homogeneous single-phase CAMS. The recorded Tgs (from 90.8 to 91.9 °C, Fig. 7) are close to that of amorphous EXE (91.2 °C, Fig. 2). An increase in the Tg of CAMS over the drug's Tg is generally associated with enhanced intermolecular forces and physical stability.1,2 However, stable drug/AA CAMS have been prepared with Tg close to the drug, signifying that a higher Tg of CAMS compared to the drug is not a prerequisite for stability.13 This is supported by a number of studies, some of which are cited below. Adhikari et al.35 used ceftazidime (Tg 48 °C) with tryptophan (Tg 128 °C) to form CAMS with Tg ∼49 °C (Fig. S2 in their paper). Adhikari et al.37 used ceftazidime (Tg 48 °C) with leucine (Tg theoretical 128 °C) to form CAMS with Tg ∼50 °C. Kasten et al.31 used binary drug/AA combinations of carvedilol (Tg 38 °C), furosemide (Tg 78 °C), indomethacin (Tg 45 °C), and mebendazole (Tg 110 °C) with MET, VAL, and LYS to form CAMS with Tgs close to those of the drugs (Fig. 1 in their paper). Liu et al.2 used budenoside (Tg 89 °C) with arginine (Tg 55 °C) to form CAMS with Tgs between 89 and 93 °C (Table 3 in their paper). Liu et al.24 used simvastatin (Tg 31 °C) with lysine (Tg 68 °C) to form CAMS with Tg 29 °C.
1, together with recorded glass transition temperatures (Tgs). A single Tg is seen in all cases, confirming the formation of homogeneous single-phase CAMS. The recorded Tgs (from 90.8 to 91.9 °C, Fig. 7) are close to that of amorphous EXE (91.2 °C, Fig. 2). An increase in the Tg of CAMS over the drug's Tg is generally associated with enhanced intermolecular forces and physical stability.1,2 However, stable drug/AA CAMS have been prepared with Tg close to the drug, signifying that a higher Tg of CAMS compared to the drug is not a prerequisite for stability.13 This is supported by a number of studies, some of which are cited below. Adhikari et al.35 used ceftazidime (Tg 48 °C) with tryptophan (Tg 128 °C) to form CAMS with Tg ∼49 °C (Fig. S2 in their paper). Adhikari et al.37 used ceftazidime (Tg 48 °C) with leucine (Tg theoretical 128 °C) to form CAMS with Tg ∼50 °C. Kasten et al.31 used binary drug/AA combinations of carvedilol (Tg 38 °C), furosemide (Tg 78 °C), indomethacin (Tg 45 °C), and mebendazole (Tg 110 °C) with MET, VAL, and LYS to form CAMS with Tgs close to those of the drugs (Fig. 1 in their paper). Liu et al.2 used budenoside (Tg 89 °C) with arginine (Tg 55 °C) to form CAMS with Tgs between 89 and 93 °C (Table 3 in their paper). Liu et al.24 used simvastatin (Tg 31 °C) with lysine (Tg 68 °C) to form CAMS with Tg 29 °C.
 | ||
| Fig. 7 Modulated DSC (mDSC) thermograms and recorded Tg values of CAMS of exemestane with L-lysine (EXE/LYS), L-methionine (EXE/MET) and L-valine (EXE/VAL) at molar ratios where CAMS were formed. | ||
The above examples further support the possibility of single-phase drug/AA CAMS with Tg close to that of the drug. This contradicts the Gordon–Taylor model, predicting that the Tg of a single-phase CAMS lies between the Tgs of the two components. By adopting AA values from Borredon et al.36 (37.9 °C for LYS, 8.0 °C for MET and 10.9 °C for VAL), the expected Tgs of the developed CAMS are calculated as 63.0 °C for EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 54.3 °C for EXE/LYS 1
1, 54.3 °C for EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 21.4 °C for EXE/MET 1
2, 21.4 °C for EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 42.1 °C for EXE/VAL 2
1, and 42.1 °C for EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table S1). Since these values are very different from those in Fig. 7, the Gordon–Taylor model is not applicable, signifying that the assumptions of ideal mixing and linear temperature–volume dependence are not met for small molecules.2
1 (Table S1). Since these values are very different from those in Fig. 7, the Gordon–Taylor model is not applicable, signifying that the assumptions of ideal mixing and linear temperature–volume dependence are not met for small molecules.2
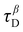 ) was used. Low
) was used. Low  values correspond to high molecular mobility and fast relaxation.
values correspond to high molecular mobility and fast relaxation.
Experimentally recorded relaxation curves of amorphous exemestane (aEXE) and CAMS of the drug with L-lysine (EXE/LYS), L-methionine (EXE/MET) and L-valine (EXE/VAL) are shown in the SI (Fig. S2). Theoretical exponential decay relaxation curves computed using eqn (1) are superimposed on the experimental curves. Fitting eqn (1) to the experimental data is excellent (R2 greater than 0.99037). Relaxation was quantified as the structural relaxation time ( ), which was computed using eqn (1) and (3) as explained in the experimental part. Values of parameters β and
), which was computed using eqn (1) and (3) as explained in the experimental part. Values of parameters β and  are listed in Table 4.
are listed in Table 4.
 in eqn (3) for the four CAMS and for the amorphous drug (aEXE)
in eqn (3) for the four CAMS and for the amorphous drug (aEXE)
For the four CAMS, the  values are 60.4203 for EXE/LYS 1
values are 60.4203 for EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 77.2478 for EXE/LYS 1
1, 77.2478 for EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 43.8475 for EXE/MET 1
2, 43.8475 for EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 13.5792 for EXE/VAL 2
1 and 13.5792 for EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The amorphous drug form (aEXE) has a
1. The amorphous drug form (aEXE) has a  of 20.4117. The higher
of 20.4117. The higher  of CAMS EXE/LYS 1
of CAMS EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, EXE/LYS 1
1, EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and EXE/MET 1
2 and EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 compared to aEXE confirm the stabilizing role of AAs as co-formers in the CAMS. They decrease molecular mobility and thus enhance the physical stability of the drug's amorphous form. On the other hand, EXE/VAL 2
1 compared to aEXE confirm the stabilizing role of AAs as co-formers in the CAMS. They decrease molecular mobility and thus enhance the physical stability of the drug's amorphous form. On the other hand, EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAMS showed a
1 CAMS showed a  value lower than that of aEXE, indicating that VAL did not stabilize the amorphous drug, and hence, although there is coformability at a 2
value lower than that of aEXE, indicating that VAL did not stabilize the amorphous drug, and hence, although there is coformability at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, physical stability appears to be problematic.
1 molar ratio, physical stability appears to be problematic.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, EXE/LYS 1
1, EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, EXE/MET 1
2, EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and EXE/VAL 2
1 and EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAMS taken at the beginning of the stability test and after 30 and 90 days of storage under accelerated conditions (40 °C and 75% RH) are presented. For the equimolar EXE/LYS and EXE/MET and the EXE/LYS 1
1 CAMS taken at the beginning of the stability test and after 30 and 90 days of storage under accelerated conditions (40 °C and 75% RH) are presented. For the equimolar EXE/LYS and EXE/MET and the EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 CAMS, no re-crystallization peaks appeared at any time point (Fig. 8a–c). Therefore, despite the absence of drug–AA chemical interactions, as indicated by the FTIR spectra (Fig. 6), stable CAMS could be formed by sheer molecular mixing. This agrees with previously published results.41–43
2 CAMS, no re-crystallization peaks appeared at any time point (Fig. 8a–c). Therefore, despite the absence of drug–AA chemical interactions, as indicated by the FTIR spectra (Fig. 6), stable CAMS could be formed by sheer molecular mixing. This agrees with previously published results.41–43
Conversely, EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAMS showed poor physical stability, with recrystallization peaks emerging after 90 days of storage (Fig. 8d). It is likely that the excess drug, whose molecular weight and weight proportion are much greater than those of the amino acid, renders the CAMS prone to disruption during the stability test. It is noticed that this recrystallization event tallies with the small relaxation time (
1 CAMS showed poor physical stability, with recrystallization peaks emerging after 90 days of storage (Fig. 8d). It is likely that the excess drug, whose molecular weight and weight proportion are much greater than those of the amino acid, renders the CAMS prone to disruption during the stability test. It is noticed that this recrystallization event tallies with the small relaxation time ( ) of 13.5792 h for EXE/VAL 2
) of 13.5792 h for EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAMS (Fig. S2e), confirming the usefulness of
1 CAMS (Fig. S2e), confirming the usefulness of  as a proxy for CAMS physical stability.
as a proxy for CAMS physical stability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAMS seems to perform slightly better than the other CAMS. It can be concluded that the dissolved AAs slightly elevate the supersaturation levels over that of cEXE. This is not however expected to contribute significantly towards the dissolution improvement of the drug compared to the improvement due to the drug's association with the amino acids in the CAMS.13,14
1 CAMS seems to perform slightly better than the other CAMS. It can be concluded that the dissolved AAs slightly elevate the supersaturation levels over that of cEXE. This is not however expected to contribute significantly towards the dissolution improvement of the drug compared to the improvement due to the drug's association with the amino acids in the CAMS.13,14
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, EXE/LYS 2
1, EXE/LYS 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, EXE/MET 1
1, EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and EXE/VAL 2
1, and EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAMS. Since the release of the amorphous drug is expected to be higher than that of a crystalline drug, the experiments were performed under non-sink conditions (SI = 0.0116). For reference, release data for quench-cooled amorphous EXE (aEXE) and unprocessed crystalline EXE (cEXE) test samples are shown. Dotted lines indicate the equilibrium solubilities of aEXE and cEXE.
1 CAMS. Since the release of the amorphous drug is expected to be higher than that of a crystalline drug, the experiments were performed under non-sink conditions (SI = 0.0116). For reference, release data for quench-cooled amorphous EXE (aEXE) and unprocessed crystalline EXE (cEXE) test samples are shown. Dotted lines indicate the equilibrium solubilities of aEXE and cEXE.
The aEXE alone test sample reaches a plateau close to the crystalline solubility, suggesting rapid recrystallisation when in contact with water. This is demonstrated by the pXRD profiles shown in Fig. S3 (SI) for solids remaining in the vessel at the end of the dissolution test of the developed CAMS EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, EXE/LYS 1
1, EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, EXE/MET 1
2, EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and EXE/VAL 2
1 and EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The pXRD pattern of the recrystallized amorphous drug in the test sample is also shown. The release levels appear to be dependent on the amino acid and the EXE/AA molar ratio. EXE/LYS 1
1. The pXRD pattern of the recrystallized amorphous drug in the test sample is also shown. The release levels appear to be dependent on the amino acid and the EXE/AA molar ratio. EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 delivers the strongest boost. For most of the experimental time, the dissolved concentration remains above the cEXE solubility and, during the first 15 min, even exceeds the aEXE solubility, producing a clear “spring–parachute” profile. It should be noted that the excess solid in the test sample that was used to maintain non-sink conditions remained amorphous throughout, whereas the sample of amorphous drug (aEXE) quickly recrystallized as seen in Fig. S3. The superior performance of EXE/LYS 1
1 delivers the strongest boost. For most of the experimental time, the dissolved concentration remains above the cEXE solubility and, during the first 15 min, even exceeds the aEXE solubility, producing a clear “spring–parachute” profile. It should be noted that the excess solid in the test sample that was used to maintain non-sink conditions remained amorphous throughout, whereas the sample of amorphous drug (aEXE) quickly recrystallized as seen in Fig. S3. The superior performance of EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is consistent with its lower Δδt and better miscibility compared with the EXE/MET and EXE/VAL systems (Table 3). The EXE/VAL 2
1 is consistent with its lower Δδt and better miscibility compared with the EXE/MET and EXE/VAL systems (Table 3). The EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAMS also showed high release levels that were sustained and formed a plateau well above the solubility of cEXE. The release profile is “rise to maximum” instead of “spring–parachute” seen for EXE/LYS 1
1 CAMS also showed high release levels that were sustained and formed a plateau well above the solubility of cEXE. The release profile is “rise to maximum” instead of “spring–parachute” seen for EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which implies low wettability of EXE in this CAMS, resulting in kinetic dissolution delay and masking of the ‘spring and parachute effect’.14 However, VAL is not a preferable conformer due to the poor physical stability of EXE/VAL 2
1, which implies low wettability of EXE in this CAMS, resulting in kinetic dissolution delay and masking of the ‘spring and parachute effect’.14 However, VAL is not a preferable conformer due to the poor physical stability of EXE/VAL 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAMS as was discussed previously.
1 CAMS as was discussed previously.
In contrast, EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and EXE/MET 1
2, and EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAMS did not significantly improve the dissolution profile of EXE, showing similar profiles to that of the aEXE test sample. This behavior has been previously observed for 1
1 CAMS did not significantly improve the dissolution profile of EXE, showing similar profiles to that of the aEXE test sample. This behavior has been previously observed for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar griseofulvin/lysine CAMS and has been attributed to extensive re-crystallization.14 This is demonstrated clearly in Fig. S4 by the pXRD pattern of the remaining EXE/LYS 1
2 molar griseofulvin/lysine CAMS and has been attributed to extensive re-crystallization.14 This is demonstrated clearly in Fig. S4 by the pXRD pattern of the remaining EXE/LYS 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 solids at the end of the dissolution test, showing distinct recrystallization peaks. Also, no dissolution improvement compared to the aEXE amorphous sample is seen in Fig. 10 for EXE/MET 1
2 solids at the end of the dissolution test, showing distinct recrystallization peaks. Also, no dissolution improvement compared to the aEXE amorphous sample is seen in Fig. 10 for EXE/MET 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAMS, although there were no recrystallization events during dissolution (Fig. S3). In this case, the poor dissolution may be attributed to the hydrophobicity of MET, the highest among the three AAs studied.14
1 CAMS, although there were no recrystallization events during dissolution (Fig. S3). In this case, the poor dissolution may be attributed to the hydrophobicity of MET, the highest among the three AAs studied.14
Conclusions
Feed solvent pretreatment hot-melt extrusion (mHME) enabled effective preparation of co-amorphous exemestane/amino acid systems at controlled extrusion temperatures that enabled processing without drug decomposition. Among the three amino acid (AA) co-formers of increasing hydrophobicity studied – L-lysine (LYS), L-valine (VAL) and L-methionine (MET) – L-lysine consistently produced fully amorphous EXE/LYS systems at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratios, delivering superior physical stability (no recrystallization after 90 days at 40 °C/75% RH) and marked dissolution improvement under non-sink conditions. L-methionine co-amorphized only at an equimolar ratio and imparted moderate stabilization but no dissolution improvement, whereas L-valine coamorphized at a 2
2 molar ratios, delivering superior physical stability (no recrystallization after 90 days at 40 °C/75% RH) and marked dissolution improvement under non-sink conditions. L-methionine co-amorphized only at an equimolar ratio and imparted moderate stabilization but no dissolution improvement, whereas L-valine coamorphized at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and yielded CAMS with limited shelf life but improved dissolution. These findings highlight the critical impact of co-former selection on the performance of co-amorphous formulations. Although there may be coformability, physical stability and release may be unsatisfactory. Considered together with the results of previous studies of our group, the mHME approach proved to be a robust, scalable strategy for producing stable CAMS of poorly soluble drugs with amino acids that demonstrated improved dissolution performance compared to the crystalline drug. Modern predictive software and specialized instrumentation could be used to provide more insight into drug–AA interactions at the molecular level and enlighten the formation, stability and dissolution performance of CAMS.
1 ratio and yielded CAMS with limited shelf life but improved dissolution. These findings highlight the critical impact of co-former selection on the performance of co-amorphous formulations. Although there may be coformability, physical stability and release may be unsatisfactory. Considered together with the results of previous studies of our group, the mHME approach proved to be a robust, scalable strategy for producing stable CAMS of poorly soluble drugs with amino acids that demonstrated improved dissolution performance compared to the crystalline drug. Modern predictive software and specialized instrumentation could be used to provide more insight into drug–AA interactions at the molecular level and enlighten the formation, stability and dissolution performance of CAMS.
Author contributions
Ioannis Partheniadis: writing – original draft, methodology, investigation, funding acquisition, formal analysis, data curation, and conceptualization. Maria Tsouka: investigation. Ioannis Nikolakakis: writing – review & editing, methodology, investigation, funding acquisition, formal analysis, data curation, and conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Additional data that support the findings of this study are available on the Open Science Framework (OSF) Repository: https://osf.io/qbrsu/.Supplementary information: DSC, pXRD, ATR-FTIR raw data, and HPLC calibration curves. See DOI: https://doi.org/10.1039/d5pm00146c.
Acknowledgements
This research work was supported by the Hellenic Foundation for Research and Innovation (HFRI) under the 4th Call for HFRI PhD Fellowships (Fellowship Number: 9335). Ioannis Partheniadis gratefully acknowledges the Department of Pharmacy at the University of Copenhagen for hosting him as a visiting PhD researcher under the Erasmus+ Traineeship Program Student Mobility. The authors express their gratitude to Associate Professor Dr Inês C. B. Martins and Research Chair Professor Dr Thomas Rades at the Department of Pharmacy, University of Copenhagen, for generously providing access to the TAM and DMA instruments. Additionally, the authors extend their appreciation to Associate Professor Dr Panagiotis Barmpalexis and Dr Afroditi Kapourani of the Laboratory of Pharmaceutical Technology at the School of Pharmacy of the Aristotle University of Thessaloniki for their kind support in facilitating pXRD measurements.References
- A. Karagianni, K. Kachrimanis and I. Nikolakakis, Pharmaceutics, 2018, 10, 98 CrossRef CAS PubMed.
- J. Liu, H. Grohganz, K. Löbmann, T. Rades and N. J. Hempel, Pharmaceutics, 2021, 13, 389 CrossRef CAS PubMed.
- S. Narala, D. Nyavanandi, P. Srinivasan, P. Mandati, S. Bandari and M. A. Repka, J. Drug Delivery Sci. Technol., 2021, 61, 102209 CrossRef CAS PubMed.
- I. Nikolakakis and I. Partheniadis, Pharmaceutics, 2017, 9, 50 CrossRef PubMed.
- S. J. Dengale, H. Grohganz, T. Rades and K. Löbmann, Adv. Drug Delivery Rev., 2016, 100, 116–125 CrossRef CAS PubMed.
- E. Lenz, K. Löbmann, T. Rades, K. Knop and P. Kleinebudde, J. Pharm. Sci., 2017, 106, 302–312 CrossRef CAS PubMed.
- A. T. Heikkinen, L. DeClerck, K. Löbmann, H. Grohganz, T. Rades and R. Laitinen, Pharmazie, 2015, 70, 452–457 CAS.
- K. T. Jensen, F. H. Larsen, C. Cornett, K. Löbmann, H. Grohganz and T. Rades, Mol. Pharm., 2015, 12, 2484–2492 CrossRef CAS PubMed.
- K. T. Jensen, K. Löbmann, T. Rades and H. Grohganz, Pharmaceutics, 2014, 6, 416–435 CrossRef CAS PubMed.
- K. Kachrimanis and I. Nikolakakis, in Handbook of Polymers for Pharmaceutical Technologies, ed. V. K. Thakur and M. K. Thakur, 2015, pp. 121–149 Search PubMed.
- I. Partheniadis, M. Toskas, F.-M. Stavras, G. Menexes and I. Nikolakakis, Processes, 2020, 8, 1208 CrossRef.
- J. Wesholowski, K. Hoppe, K. Nickel, C. Muehlenfeld and M. Thommes, Eur. J. Pharm. Biopharm., 2019, 142, 396–404 CrossRef CAS PubMed.
- I. Partheniadis and I. Nikolakakis, Int. J. Pharm., 2024, 652, 123824 CrossRef CAS PubMed.
- I. Partheniadis, M. Tsouka and I. Nikolakakis, Int. J. Pharm., 2024, 666, 124818 CrossRef CAS PubMed.
- M. T. França, T. M. Marcos, R. N. Pereira and H. K. Stulzer, Eur. J. Pharm. Sci., 2020, 143, 105178 CrossRef PubMed.
- J. A. Baird, B. Van Eerdenbrugh and L. S. Taylor, J. Pharm. Sci., 2010, 99, 3787–3806 CrossRef CAS PubMed.
- ASTM E2550-17 https://store.astm.org/e2550-17.html.
- X. Chen, I. Partheniadis, I. Nikolakakis and H. Al-Obaidi, Polymers, 2020, 12, 854 CrossRef CAS PubMed.
- A. Forster, J. Hempenstall, I. Tucker and T. Rades, Int. J. Pharm., 2001, 226, 147–161 CrossRef CAS PubMed.
- D. J. Greenhalgh, A. C. Williams, P. Timmins and P. York, J. Pharm. Sci., 1999, 88, 1182–1190 CrossRef CAS PubMed.
- E. Stefanis and C. Panayiotou, Int. J. Thermophys., 2008, 29, 568–585 CrossRef CAS.
- B. Konda, R. N. Tiwari and H. Fegade, J. Chromatogr. Sci., 2011, 49, 634–639 CAS.
- C. Bhugra, R. Shmeis, S. L. Krill and M. J. Pikal, J. Pharm. Sci., 2008, 97, 455–472 CrossRef CAS PubMed.
- J. Liu, D. R. Rigsbee, C. Stotz and M. J. Pikal, J. Pharm. Sci., 2002, 91, 1853–1862 CrossRef CAS PubMed.
- I. C. B. Martins, A. S. Larsen, A. Madsen, O. A. Frederiksen, A. Correia, K. Jensen, H. S. Jeppesen and T. Rades, Chem. Sci., 2023, 14, 11447–11455 RSC.
- A. Kapourani, A. T. Chatzitaki, I. S. Vizirianakis, D. G. Fatouros and P. Barmpalexis, Int. J. Pharm., 2023, 640, 123004 CrossRef CAS PubMed.
- N. S. Trasi and L. S. Taylor, J. Pharm. Sci., 2015, 104, 2583–2593 CrossRef CAS PubMed.
- K. Ueda, S. S. Hate and L. S. Taylor, J. Pharm. Sci., 2020, 109, 2464–2473 CrossRef CAS PubMed.
- T. Yamashita, S. Ozaki and I. Kushida, Int. J. Pharm., 2011, 419, 170–174 CrossRef CAS PubMed.
- A. Singh, Y. R. Neupane, B. Mangla and K. Kohli, J. Pharm. Sci., 2019, 108, 3382–3395 CrossRef CAS PubMed.
- G. Kasten, K. Nouri, H. Grohganz, T. Rades and K. Löbmann, Int. J. Pharm., 2017, 533, 138–144 CrossRef CAS PubMed.
- G. Kasten, K. Löbmann, H. Grohganz and T. Rades, Int. J. Pharm., 2019, 557, 366–373 CrossRef CAS PubMed.
- J. J. Jayapal and S. Dhanaraj, Int. J. Biol. Macromol., 2017, 105, 416–421 CrossRef CAS PubMed.
- M. B. Mary, V. Sasirekha and V. Ramakrishnan, Spectrochim. Acta, Part A, 2006, 65, 955–963 CrossRef PubMed.
- B. R. Adhikari, S. Sinha, K. C. Gordon and S. C. Das, Int. J. Pharm., 2022, 621, 121799 CrossRef CAS PubMed.
- C. Borredon, L. A. Miccio, S. Cerveny and G. A. Schwartz, J. Non-Cryst. Solids:X, 2023, 18, 100185 CAS.
- B. R. Adhikari, S. Sinha, N. Lyons, D. Pletzer, I. Lamont, K. C. Gordon and S. C. Das, Eur. J. Pharm. Biopharm., 2022, 180, 260–268 CrossRef PubMed.
- S. L. Shamblin, B. C. Hancock and M. J. Pikal, Pharm. Res., 2006, 23, 2254–2268 CrossRef CAS PubMed.
- K. Kawakami and Y. Ida, Pharm. Res., 2003, 20, 1430–1436 CrossRef CAS PubMed.
- A. M. Abdul-Fattah, K. M. Dellerman, R. H. Bogner and M. J. Pikal, J. Pharm. Sci., 2007, 96, 1237–1250 CrossRef CAS PubMed.
- S. J. Dengale, O. P. Ranjan, S. S. Hussen, B. S. M. Krishna, P. B. Musmade, G. Gautham Shenoy and K. Bhat, Eur. J. Pharm. Sci., 2014, 62, 57–64 CrossRef CAS PubMed.
- K. Löbmann, H. Grohganz, R. Laitinen, C. Strachan and T. Rades, Eur. J. Pharm. Biopharm., 2013, 85, 873–881 CrossRef PubMed.
- K. Löbmann, R. Laitinen, C. Strachan, T. Rades and H. Grohganz, Eur. J. Pharm. Biopharm., 2013, 85, 882–888 CrossRef.
- J. Bevernage, J. Brouwers, M. E. Brewster and P. Augustijns, Int. J. Pharm., 2013, 453, 25–35 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |







