Open Access Article
Open Access ArticleAdvances in targeted therapies and emerging strategies for blood cancer treatment
Samson A.
Adeyemi
 *a,
Lindokuhle M.
Ngema
a and
Yahya E.
Choonara
*a,
Lindokuhle M.
Ngema
a and
Yahya E.
Choonara
 *ab
*ab
aWits Advanced Drug Delivery Platform Research Unit, Department of Pharmacy and Pharmacology, School of Therapeutic Sciences, Faculty of Health Sciences, University of the Witwatersrand, 7 York Road, Parktown, Johannesburg 2193, South Africa. E-mail: Samson.adeyemi@wits.ac.za; yahya.choonara@wits.ac.za
bInfectious Diseases and Oncology Research Institute (IDORI), Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
First published on 1st July 2025
Abstract
Blood cancers, including leukemia, lymphoma, and multiple myeloma, originate within the bone marrow, where the intricate microenvironment presents considerable challenges for conventional therapies such as chemotherapy, immunotherapy, radiotherapy, and hematopoietic stem cell transplantation. These approaches often suffer from poor specificity, low bioavailability, and systemic toxicity, resulting in suboptimal treatment outcomes. In response, significant advances in targeted drug delivery systems, including liposomes, pegylated formulations, and polymeric nanoparticles have been developed to enhance drug stability, prolong circulation time, and improve tumor accumulation while reducing off-target effects. This review provides a comprehensive overview of recent innovations in ligand-directed drug delivery systems for blood cancers. Emphasis is placed on systems functionalized with antibodies, peptides, aptamers, and proteins designed to overcome the barriers of the bone marrow niche and enable selective delivery to malignant cells. Notably, leukemia has emerged as a key model for evaluating these technologies, with promising preclinical and clinical results. However, despite technological progress, critical translational challenges remain. These include biological heterogeneity, variability in target receptor expression, immunogenicity of nanoparticles, and the complexity of scaling multifunctional delivery systems under clinical conditions. Furthermore, current in vitro and in vivo models fail to accurately recapitulate the bone marrow's dynamic physiology, underscoring the need for improved predictive systems. Future perspectives suggest the integration of personalized nanomedicine approaches that adapt to patient-specific genetic profiles and disease states. Additionally, artificial intelligence (AI) and big data analytics are expected to revolutionize delivery optimization, biomarker discovery, and therapy customization. Ultimately, interdisciplinary collaboration is required to bridge the gap between bench and bedside. By addressing current limitations and embracing innovation, the field moves closer to realizing safe, precise, and effective therapies for patients with hematologic malignancies.
1. Introduction
Blood cancer is a type of cancer caused by mutations in the deoxyribonucleic acid (DNA) complex within blood cells causing abnormalities.1 Contrary to other cancers that manifest in other organs as solid tumors, blood cancer manifests as liquid tumors in the lymphatic system or bone marrow.2 Leukemia, lymphoma, and multiple myeloma are the most common forms of blood cancer (Fig. 1), each characterized by distinct symptoms, treatments, and prognoses.1 Leukemia emanates from the uncontrollable proliferation of mutant progenitors, caused by a hematopoiesis dysfunction, leading to the generation of large quantities of abnormal leukocytes in peripheral blood, bone marrow, and other organs.3 Moreover, leukemia is further classified into various subtypes depending on the mutant progenitor cell type (i.e., lymphoid or myeloid) and the disease onset (i.e., acute or chronic).1,3 | ||
| Fig. 1 Graphical depiction of the most common types of blood cancer. Adapted with permission from.11 | ||
The common subtypes of leukemia include acute lymphoblastic leukemia, chronic lymphoblastic leukemia, acute myeloid leukemia, and chronic myeloid leukemia.3 Ac-cordingly, approximately 2.5% of all cancer cases are attributed to leukemia, with more than 470![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 patients diagnosed globally in 2020.4 Meanwhile, another type of blood cancer, multiple myeloma (MM) is characterized as a hematological malignancy of plasma cells localized in the bone marrow. This form of blood cancer is the second most prev-alent hematological malignancy, and is considered incurable, with relapse rates of more than 90%.5 Various risk factors have been implicated in MM, including age, sex, ethnicity, and family history, with multiple related complications such as bone defects, kidney problems, and anemia.6 More than 34
000 patients diagnosed globally in 2020.4 Meanwhile, another type of blood cancer, multiple myeloma (MM) is characterized as a hematological malignancy of plasma cells localized in the bone marrow. This form of blood cancer is the second most prev-alent hematological malignancy, and is considered incurable, with relapse rates of more than 90%.5 Various risk factors have been implicated in MM, including age, sex, ethnicity, and family history, with multiple related complications such as bone defects, kidney problems, and anemia.6 More than 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new cases and 12
000 new cases and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths related to MM were reported in 2022.1
000 deaths related to MM were reported in 2022.1
Additionally, lymphoma which are heterogenous lymphoid tumors emanating from a malignant transformation of peripheral lymphocytes during differentiation.7 Malignant lymphomas can be classified as Hodgkin lymphomas (HL), which are relatively rare, or non-Hodgkin lymphomas (NHL), which are the most prevalent. NHL further comprise various subtypes such as diffuse large B-cell lymphoma, mantle cell lymphoma, follicular lymphoma, and marginal zone lymphoma.8 In 2022, about 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 new cases and 21
910 new cases and 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 lymphoma-related deaths were reported.1
000 lymphoma-related deaths were reported.1
Current treatments for blood cancer remain unsatisfactory due to off-target effects and drug resistance.9 Chemotherapy, immunotherapy, radiotherapy, and bone marrow transplantation are the current treatment modalities for blood cancer. However, these modalities present with limited therapeutic efficacy and mostly accompanied by treat-ment-associated toxicity.10 Although, various chemotherapeutic drugs are currently in use, their therapeutic efficacy is compromised by dose-related cytotoxicity and lack of tumor cell specificity.1,11 Moreover, the poor bioavailability and non-specificity of current chemotherapeutics require frequent administration of high dosages of the drugs to achieve the required therapeutic levels in bone marrow or the lymphatic system, thus causing in-creased adverse effects and patient intolerability.1,7
Moreover, it is challenging to achieve adequate therapeutic dosages of anticancer therapeutics at tumor sites within the lymphatic system and bone marrow using conventional systematic administration.12 Additionally, the bone marrow microenvironment presents with high numbers of progenitor cells which are resistant to chemotherapeutics, and promote disease relapse.1,12 As such, there have been extensive efforts made in developing advanced and clinically effective drug delivery systems to deliver anticancer drugs with improved therapeutic efficacy, bioavailability, and minimal cytotoxicity. This review provides a comprehensive synthesis of current and emerging targeted drug delivery strategies for blood cancer, highlighting a critical shift from conventional systemic therapies toward more refined, ligand-directed nanocarrier approaches. The key novel insight lies in its focused examination of how targeted delivery systems—including liposomes, pegylated drugs, and polymeric nanoparticles functionalized with ligands like antibodies, peptides, and aptamers—are designed to overcome the intrinsic barriers of the bone marrow microenvironment, which remains a major site of therapeutic resistance. Furthermore, the review contributes to the field by identifying and addressing several unmet needs and translational challenges including clinical translation, barriers to commercialization, unmet research needs and personalized and adaptive delivery systems. While numerous ligand-targeted nanocarriers have demonstrated promising preclinical efficacy, very few have successfully transitioned into routine clinical practice.13 This is largely due to the biological complexity of haematological malignancies and the heterogeneity in receptor expression, which limits the universal applicability of single ligand targeting strategies. Also, the logistical and regulatory hurdles associated with manufacturing and scaling advanced delivery systems, particularly polymeric nanoparticles and multifunctional platforms, which require rigorous quality control, reproducibility, and stability under clinical conditions are highlighted. A significant gap identified is the limited understanding of how the dynamic interactions within the bone marrow niche—including immune suppression, hypoxia, and stromal support—affect nanoparticle transport, cellular uptake, and drug release. Furthermore, current models do not adequately simulate the physiological complexity of the human bone marrow, suggesting a need for improved in vitro and in vivo systems that better predict clinical outcomes. The review calls attention to the need for customizable platforms that adapt to disease stage, patient-specific biomarkers, and resistance mechanisms. There is also a compelling need for theranostic systems that combine diagnostic imaging and therapy for real-time monitoring of treatment response in hematologic cancers. In a nutshell, the review advances the field by synthesizing recent innovations in blood cancer drug delivery while clearly articulating the biological, technological, and translational barriers that must be addressed to realize the full therapeutic potential of these approaches. It serves as a roadmap for researchers and clinicians aiming to bridge the bench-to-bedside gap in blood cancer therapy.
2. Blood cancer variations and related complications
2.1 Leukemia
Leukemia is primarily characterized by the uncontrollable proliferation of mutant progenitors as a result of hematopoiesis dysfunction, generating a large number of ab-normal leukocytes which accumulate in peripheral blood, bone marrow, and spleen.3 Essentially, leukemia halts the production of normal blood cells, leading to anemia, coag-ulation disorders, as well as high risk of infection.3,14 The current traditional treatment modalities for leukemia include chemotherapy, radiotherapy, and immunotherapy.3 Unfortunately, these traditional treatments have been shown to be unsatisfactory, particularly for the acute forms in adults, with the 5-year survival rate of acute leukemia often recorded at 30–50%, with a decrease to 17% for acute lymphoblastic leukemia, and 7% for acute myeloid leukemia.3,15The unsatisfactory prognosis is mainly related to treatment-aligned toxicity and dis-ease relapse.3,15 Essentially, the majority of drugs used to treat leukemia suffer from non-specificity, leading to off-target complications and drug resistance.3 On the other hand, the prevalence of minimal residual disease (MRD) during therapy often leads to higher chances of relapse in both adults and children, with around 56–100% 5-year re-lapse rate for MRD-positive patients.15 As such, targeted therapies offer a promising avenue for attaining deeper first remissions, while preventing relapse.15
2.2 Multiple myeloma
Multiple myeloma (MM) is a clonal plasma cell neoplasm, characterized by hypercalcemia, renal insufficiency, and bone destruction.6 It is localized in the bone marrow, and remains incurable with over 90% relapse rates.5 The complexity of MM, perpetuated by disease heterogeneity, drug resistance, and relapse, continues to affect the treatment outcomes from current therapies.16 MM is a highly heterogeneous disease, and the intra and inter-patient heterogeneity complicates treatment approaches and fosters the survival of MRD, leading to relapse. The majority of MM patients experience relapse from the emergence of resistant myeloma clones, which evolve to escape the cytotoxic drugs and render current treatments ineffective.6,16 As such, most patients often experience relapse even after achieving remission. Moreover, the microenvironmental influence by the bone marrow microenvironment plays a significant role in the growth and survival of myeloma cells.16 Particularly, the bone marrow microenvironment can also stimulate angiogenesis while inhibiting immune responses, resulting in therapies being ineffective and contribute to resistance.162.3 Lymphoma
Lymphomas represent the most prevalent form of hematological malignancies which arise from a malignant transformation of precursor or peripheral lymphocytes when undergoing different states of differentiation.7 Lymphomas consist of two subtypes; Hodgkin lymphoma, which are rare aggressive malignancies of B-cell origin, and non-Hodgkin lymphomas, which represent the most common subtype of lymphomas.7,8 The front-line therapy for lymphomas remains chemotherapy, with the use of genotoxic cytostatic drugs, such as alkylating agents, nucleoside analogs, anthracyclines, topoisomerase inhibitors, as well as vinca alkaloids.7 However, lymphomas still present a number of challenges in treatment, contributing to the limitations and ineffectiveness of current therapies.17 Despite advancements in treatment protocols, several key issues remain unresolved, leading to suboptimal outcomes for many patients.17 One of the most significant complications in lymphoma treatment is the development of resistance to therapies. Lymphoma cells can develop mutations or alter their signalling pathways, enabling them to evade destruction by chemotherapy and radiation.6,173. Current therapeutic approaches to treat blood cancers
Therapies that are currently available for the treatment of blood cancer include chemotherapy, immunotherapy, radiotherapy, as well as hematopoietic stem cell trans-plantation. These have undergone relatively notable advancements over the years, although still presenting with some clinical limitations such as toxicity and side effects, rapid clearance, as well as interference with bone marrow.10 The specific limitations from the current therapeutic approaches are discussed in the sections below. Moreover, leukemia, lymphoma, and multiple myeloma possess significant challenges when it comes to treatment, owing to heterogeneity and the complex interplay of genetic and mo-lecular factors.1,10,18 Accordingly, although the current conventional therapies have been a crucial component of the blood cancer treatment arsenal, providing strategies to control blood cancer severity, alleviate symptoms, and improve the patients’ overall quality of life,3 more advanced drug delivery strategies are still required to improve the efficacy of these therapies.3.1 Chemotherapy
Chemotherapy is one of the earliest interventions of blood cancer, with mustargen first approved in 1949 by the U.S. Food and Drug Administration (FDA) as a chemotherapeutic drug for the treatment of leukemia, lymphosarcoma, and Hodgkin's disease.3 Subsequently, more chemotherapy drugs have been approved and prescribed as first-line drugs in the treatment of blood cancers. Presented in Table 1 is a summary of the FDA-approved chemotherapeutic drugs for application in blood cancer treatment. The dominance of chemotherapy in blood cancer management has been evident for over 40 years, but the severe adverse effects caused by the indiscriminate toxicity of the drugs to both cancer cells and healthy tissue remains a daunting drawback.19 In addition, the pathophysiology of bone marrow in blood cancer patients, particularly leukemia, may induce chemoresistance of leukemia cells via specific cellular interactions, weakening the cytotoxicity of chemotherapeutic drugs.3,19 Thus, the development of advanced drug delivery systems for improving targeting ability and limiting drug resistance remains crucial in blood cancer treatment.| Drug | Mechanisms of action | Type of blood cancer | Delivery route(s) | Challenge(s) | Ref. |
|---|---|---|---|---|---|
| Imatinib (Gleevec) | Tyrosine kinase inhibitor | Chronic myeloid leukemia | Oral | Delayed liver toxicity | 20–22 |
| Dasatinib (Sprycel) | Tyrosine kinase inhibitor | Chronic myeloid leukemia | Oral | Drug resistance/intolerance | 20 and 23 |
| Acute lymphoblastic leukemia | |||||
| Nilotinib (Tasigna) | Tyrosine kinase inhibitor | Chronic myeloid leukemia | Oral | Adverse drug reactions | 20 and 24 |
| Bosutinib (Bosulif) | Tyrosine kinase inhibitor | Chronic myeloid leukemia | Oral | Adverse drug reactions | 20 and 25 |
| Ponatinib (Iclusig) | Tyrosine kinase inhibitor | Chronic myeloid leukemia | Oral | Reduced selectivity | 20 |
| Acute lymphoblastic leukemia | |||||
| Cyclophosphamide (Cytoxan) | Alkylating agent | Leukemia | Oral | Bladder and gonodal toxicity | 26 and 27 |
| Lymphoma | Intravenous | ||||
| Multiple myeloma | |||||
| Doxorubicin (Adriamycin) | Intercalating agent | Leukemia | Intravenous | Drug resistance and cardiotoxicity | 26 and 28 |
| Lymphoma | |||||
| Multiple myeloma | |||||
| Methotrexate | Antimetabolite | Leukemia | Oral | Drug resistance | |
| Lymphoma | Subcutaneous | ||||
| Intravenous | |||||
| Fludarabine (Fludara) | Antimetabolite | Chronic lymphocytic leukemia | Oral | Non-specificty | 29–31 |
| Intravenous | |||||
| Bendamustine (Treanda) | Alkylating agent | Non-Hodgkin lymphoma | Intravenous | Adverse toxicity and opportunistic infections | 32 and 33 |
| Chronic lymphocytic lymphoma | |||||
| Daunorubicin (Cerubidine) | Intercalating agent | Acute myeloid leukemia | Intravenous | Adverse drug reactions and non-specificity | 34 and 35 |
| Cytarabine (Cytosar-U) | DNA/RNA synthesis inhibitor | Acute myeloid leukemia | Intravenous | Adverse drug reactions | 36 |
| Subcutaneous | |||||
| Intrathecal |
3.2 Immunotherapy
Over the years, immunotherapy has undoubtedly gained importance in the treatment of blood cancers.37 Advanced immunotherapeutic approaches involving checkpoint inhibitors, cell-based therapies, and vaccines have been used and successfully integrated into standard regimen. These immunotherapies harness the immune system to eradicate blood cancers, and prevent the spread of cancerous cells beyond the primary site.37 Checkpoint inhibitors are a common immunotherapeutic strategy that blocks the immune suppression pathways used by cancer cells to evade the attack by the cytotoxic T-cells.38 Particularly, it has been shown that Reed-Sternberg cells of Hodgkin lymphoma make use of the programmed death 1 (PD-1) checkpoint to escape immune detection, and thus anti-PD-1 checkpoint inhibitors have been used in therapy for patients with Hodgkin lymphoma. Also, cytotoxic T-lymphocyte antigen (CTLA-4) checkpoint inhibitors are currently clinically investigated, with promising results.37,38On the other hand, cell-based therapies have shown potential as a promising therapy for blood cancers, and chimeric antigen receptor (CAR) T cells have garnered a massive interest.37 The first CAR T cell-based therapies were approved in 2017 for the treatment of advanced lymphoma in adults and acute lymphocytic leukemia in children.38,39 The two FDA-approved cell-based therapies for acute lymphocytic leukemia, tisagenlecleucel and brexucabtagene autoleucel are CD-19 directed, and the former is also approved for treatment of diffuse large B cell non-Hodgkin lymphoma.39 More antigen receptors, including CD-17, CD-13, and CD-70-specific are being investigated, and significant efforts have been made to improve the design and therapeutic efficacy of CAR T cell-based therapies.40 Likewise, cell-based vaccines have been devised for multiple myeloma, categorized as either vaccines that use antigen-presenting cells generated ex vivo or vaccines that use whole tumor cells.41 Accordingly, the application of GVAX vaccine combined with lenalidomide in myeloma patients in near complete remission yield a robust immunity with a durable disease control.42
3.3 Radiotherapy
Radiotherapy is one of the conventional treatment modalities for blood cancers, primarily utilized for localized disease control or as part of the comprehensive conditioning regimen.10 Essentially, the precise application of radiotherapy is dependent on the type and stage of the blood cancer, and the overall intervention plan.43 Particularly, in Hodgkin lymphoma, radiotherapy is often utilized to precisely deliver radiation to areas where cancerous cells are concentrated, consequently eradicating tumors, alleviating symptoms, and achieving remission.44 Importantly, radiotherapy has proven to be more beneficial in cases where the cancer is confined to a limited region of the body. Moreover, radiotherapy may be applied in combination with other interventions for beneficial outcomes. Accordingly, during stem cell transplantation, radiotherapy may be administered to destroy cancerous cells and suppress the immune system to make it receptive to the transplanted stem cells.43,44 This further minimizes the risk of graft rejection, while increasing the chances of a successful transplant.443.4 Hematopoietic stem cell transplantation
Hematopoietic stem cell transplantation (HSCT), generally known as bone marrow transplantation is an available treatment option for lymphoma, leukemia, and multiple myeloma.45 HSCT involves intravenous infusion of normal hematopoietic stem cells, responsible for blood cells production, to repair the damaged hematopoiesis and immune functionality.3 HSCT can be categorized as allogeneic or autologous stem cell transplantation, with the former being the most widely used form involving donors which are usually relatives with matching bone marrow types, meanwhile the latter involves the use of stem cells derived from the patient's own bone marrow.46 Fundamentally, HSCT presents a vital approach for completely curing certain types of blood cancers, such as leukemia, with reported 5-year survival rates of more than 70% and complete remission.47 However, there exists some drawbacks from HSCT, including high risk of graft-versus-host disease occurrence, and severe immune disorders from graft rejection.34. Current drug delivery systems for improved blood cancer treatment
Certain drug delivery systems have been devised to enhance the bioavailabilty of the drugs and the pharmacokinetics thereof, to improve the treatment outcomes in blood cancer.48 These systems are capable of shielding the drugs from rapid metabolism and elimination by the liver and kidneys, while increasing blood circulation time and biodistribution48 (Table 2). The currently available drug delivery systems include liposomes, pegylated drug systems, and polymeric nanoparticles, and these are undoubtedly the fastest developed in the translation pipeline.3 Highlighted in Table 2 are FDA-approved and clinical trial product representatives of these systems.| System | Product name | Drug | Status |
|---|---|---|---|
| Liposome | Vyxeos | Daunorubicin | Approved |
| Cytarabine | |||
| Liposome | Marqibo kit | Vincristine | Approved |
| Liposome | Doxil | Doxorubicin | Phase II |
| Liposome | DepoCyte | Cytarabine | Phase II |
| Liposome | BP1001 | Grb2 antisense oligonucleotide | Phase II |
| Liposome | — | Annamycin | Phase II |
| Liposome | — | Mitoxantrone | Phase I |
| Pegylated drug | Asparlas | Asparaginase homotetramer | Approved |
| Pegylated drug | Oncaspar | Pegaspargase | Approved |
| Pegylated drug | — | Interferon-alpha | Phase III |
| Nanoparticle | — | Barasertib | Phase I |
4.1 Liposomes
Liposomal drug delivery systems stand out amongst the other types of current drug delivery systems, owing to their desirable biological properties and advantageous technological aspects3 (Table 3 and Fig. 2). Accordingly, various liposomal drug delivery systems for numerous anticancer drugs used in blood cancer intervention, including doxorubicin, vincristine, annamycin, daunorubicin, and cytarabine, have been formulated and demonstrate enhanced in vivo pharmacokinetics and therapeutic efficacy.8,49 One representative system that stands out is the liposomal formulation (CPX-351) co-loaded with daunorubicin and cytarabine approved in 2017.50 The system yielded a prolonged circulation time of the loaded drugs with improved accumulation in bone marrow, and maximally enhanced synergistic antitumor efficiency. Moreover, the liposomal system significantly increased the terminal half-life of daunorubicin (18.5 h) and cytarabine (1–3 h) to 31.5 h and 40.4 h, respectively.50 Interestingly, the overall survival from the treatment with CPX-351 was found to be 9.6 months, higher than the 6.0 months reported from the standard chemotherapy. The system further alleviated the side effects of the drugs, such as gastrointestinal complications and hair loss.50 | ||
| Fig. 2 Mechanisms of Action of Liposomal drug delivery systems in the Treatment of Acute Myeloid Leukemia (AML). Reprinted with permission from.53 | ||
| Delivery strategy | Efficacy | Scalability | Limitations | Translational potential |
|---|---|---|---|---|
| Liposomes | Moderate to high: enables encapsulation of hydrophilic and hydrophobic drugs; improves drug stability and circulation time | High: scalable via established industrial processes (e.g., extrusion, microfluidics) | Prone to leakage and fusion; rapid clearance by reticuloendothelial system (RES); limited specificity | Several FDA-approved formulations (e.g., Doxil); proven clinical translation |
| Polymeric nanoparticles | High: controlled and sustained release; tunable size and surface properties enhance targeting | Moderate: scalable using nanoprecipitation and emulsion methods, but batch consistency is a concern | Potential cytotoxicity from degradation products; complex synthesis | Strong preclinical data; increasing entry into clinical trials |
| Antibody-mediated systems | Very high: high specificity through antigen recognition; ideal for targeted delivery of cytotoxins (e.g., ADCs) | Low to moderate: production is expensive and requires complex biologics manufacturing | Immunogenicity concerns; target antigen heterogeneity; off-target toxicity | Several approved ADCs (e.g., Gemtuzumab ozogamicin for AML); growing clinical success |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio, an optimal ratio established through preclinical studies to maximize antileukemic efficacy while minimizing systemic toxicity.51 The liposomal delivery system plays a crucial role in enhancing the pharmacological performance of the drug combination. It ensures prolonged circulation time, targeted accumulation in the bone marrow, where leukemic cells predominantly reside, and controlled, sustained drug release. This improves therapeutic selectivity and reduces exposure to healthy tissues, thereby limiting adverse effects often associated with conventional chemotherapy.
5 molar ratio, an optimal ratio established through preclinical studies to maximize antileukemic efficacy while minimizing systemic toxicity.51 The liposomal delivery system plays a crucial role in enhancing the pharmacological performance of the drug combination. It ensures prolonged circulation time, targeted accumulation in the bone marrow, where leukemic cells predominantly reside, and controlled, sustained drug release. This improves therapeutic selectivity and reduces exposure to healthy tissues, thereby limiting adverse effects often associated with conventional chemotherapy.
Within the liposome, daunorubicin, an anthracycline antibiotic, intercalates into DNA and inhibits topoisomerase II, resulting in the formation of double-strand DNA breaks and subsequent induction of apoptosis (Fig. 2). It also contributes to cellular damage by generating reactive oxygen species (ROS). Cytarabine, on the other hand, is a cytidine analog that undergoes intracellular phosphorylation to its active triphosphate form (Ara-CTP). This active metabolite competes with natural nucleotides during DNA replication, leading to premature chain termination and inhibition of DNA polymerase, thus inducing cell cycle arrest in the S-phase and promoting cell death.52 The fixed molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (daunorubicin to cytarabine) within the liposome allows for a synergistic interaction, significantly enhancing DNA damage and cytotoxicity in leukemic cells compared to traditional 7 + 3 regimens (seven days of cytarabine plus three days of daunorubicin). Overall, Vyxeos® represents a novel paradigm in AML treatment by integrating optimized drug ratios with targeted, nanocarrier-based delivery to improve efficacy and patient outcomes.
5 (daunorubicin to cytarabine) within the liposome allows for a synergistic interaction, significantly enhancing DNA damage and cytotoxicity in leukemic cells compared to traditional 7 + 3 regimens (seven days of cytarabine plus three days of daunorubicin). Overall, Vyxeos® represents a novel paradigm in AML treatment by integrating optimized drug ratios with targeted, nanocarrier-based delivery to improve efficacy and patient outcomes.
4.2 Pegylated drug systems
The pegylation strategy entails the modification of molecules by polyethylene glycol (PEG) chains to shield the linked drug from recognition by the immune systems while increasing the body-residence time.54,55 Commonly, therapeutic proteins including the Escherichia coli-derived asparaginase (approved for acute lymphoblastic leukemia), are affected by short half-life and adverse allergies which limit their in vivo efficacy.56 However, through pegylation, the elimination half-life of asparaginase could be significantly enhanced, elevating it from 26–30 h to 5.5–7 days, resulting in reduced dosage and administration frequency.3 The pegylation technique has also been applied to avert the rapid clearance of naïve IFN-® by modification with monomethoxy polyethylene glycol (mPEG) chains, leading to improve in vivo activity and pharmacokinetics. Currently, pegylated IFN-® (Pegasys) is in Phase III clinical trial (NCT02736721) for the treatment of chronic myeloid leukemia.534.3 Polymeric nanoparticles
The therapeutic potency of nanoparticle-based delivery systems in blood cancer treatment is governed by their exclusive advantages, such as improved stability and biocompatibility, high permeability, enhanced retention and precise targeting, and reduced drug toxicity (Fig. 3) (Table 2).57 Accordingly, in addition to current liposomal and pegylated drug delivery systems, there is a polymeric nanoparticle-based barasertib (AZD2811) delivery system currently in clinical trial (NCT03217838) as a potential blood cancer nanomedicine.58,59 Essentially, barasertib is an Aurora kinase B inhibitor capable of arresting the cell cycle and inducing chromosome misalignments, leading to cell death.60 As such, in this formulated nanoparticle delivery system, AZD2811 is encapsulated in poly(D,L-lactide)-poly(ethylene glycol) (PLGA-PEG) nanoparticles using an in situ ion pairing technique. The system reportedly exhibited a sustained delivery of AZD2811 for more than one week, and 93% tumor regression at 19 days, in preclinical models of acute myeloid leukemia.58 Currently, more studies exploiting the specific properties of polymeric nanoparticles for drug delivery in cancer treatment are underway, and envisaged to yield positive outcomes.57 | ||
| Fig. 3 Outline of specific advantages of nanoparticle-based drug delivery systems for blood cancer treatment. Adapted with permission from,57 published under a Creative Commons License. | ||
5. New drug delivery systems developed for blood cancer treatment
Various novel targeted drug delivery systems are being developed and investigated for blood cancer treatment to enhance accumulation in the tumor site (i.e., bone marrow and lymphatic system) and improve the efficacy and safety of the drugs.1 Although, the bone marrow is the most complex system, its microenvironment comprises different cell types that can be targeted either passively or actively.1 Essentially, passive targeting is achieved through leaky vasculatures, allowing drug accumulation at tumor sites, mean-while active targeting depends on the specific surface biomarkers expressed by blood cancer cells which bind the ligands from the drug delivery system.61 Accordingly, the latter has been the most widely explored technique for developing targeted drug delivery systems for blood cancer treatment. Amongst these systems are antibody-modified, pep-tide-mediated, aptamer-mediated, and protein-mediated targeting systems1 (Table 4).| Delivery system | Targeting mechanism | Key components | Clinical status | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| Antibody-modified nanosystems | Monoclonal antibodies recognize and bind specific antigens on cancer cells | Liposomes, polymeric nanoparticles + antibodies (e.g., anti-CD33, anti-CD19) | Some in clinical use (e.g., gemtuzumab ozogamicin for AML) | High specificity-proven efficacy in hematological cancers-established in clinical oncology | High cost-potential immunogenicity-limited tumor penetration | 57 |
| Peptide-mediated targeted systems | Short peptide ligands bind to overexpressed receptors on malignant cells | SLNs, liposomes, dendrimers + targeting peptides (e.g., RGD, NGR) | Preclinical to early-phase trials | Easier synthesis and modification-lower immunogenicity-good tissue penetration | Less stable than antibodies-susceptible to enzymatic degradation | 1 and 62 |
| Aptamer-mediated targeted systems | DNA/RNA aptamers bind to specific cell surface markers via 3D structures | Polymeric nanoparticles, lipid carriers + aptamers (e.g., AS1411) | Mostly preclinical | High binding affinity-chemically synthesized-lower batch-to-batch variation | Susceptible to nuclease degradation-requires chemical stabilization | 63 |
| Protein-mediated targeted systems | Use of functional proteins that naturally bind cancer cell receptors or markers | Nanogels, micelles, exosomes + proteins (e.g., transferrin, lactoferrin) | Preclinical to exploratory clinical phases | Biocompatible-can leverage natural transport pathways-multifunctional capabilities | Complex purification and formulation-risk of immune response in some cases | 64 and 65 |
5.1 Antibody-modified nanosystems
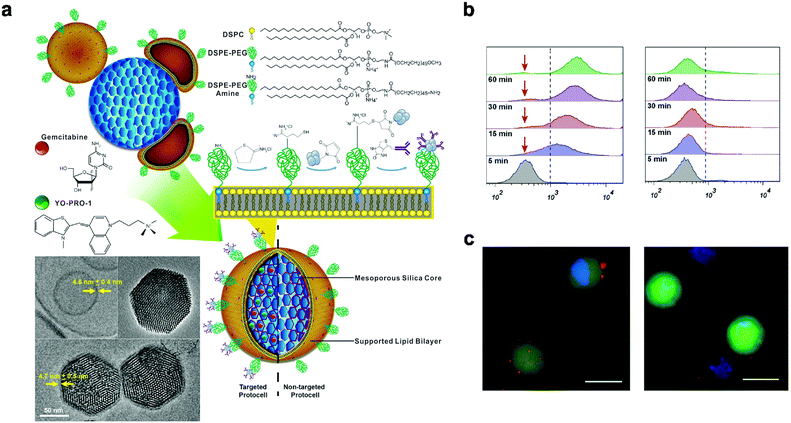
Antibody-drug conjugates hold great promise in targeted drug delivery, owing to the high specificity effects of antibodies coupled with the antitumor efficacy of the cytotoxic drugs.66 Consequently, the decoration of drug nanocarriers with antibodies presents an efficient drug delivery approach in blood cancer treatment. A drug delivery system for active targeting of leukemia cells was developed by Durfee et al., (2016), comprising mesoporous silica nanoparticles loaded with gemcitabine and grafted with epidermal growth factor receptor (EGFR) antibody-modified lipid bilayers (Fig. 4).67 This drug delivery nanosystem exhibited high stability in blood circulation, and a great targetability of leukemia cells via antibody-mediated interactions between the cells and the nanocarrier. Moreover, the system showed faster and enhanced internalization into EGFR-positive cells, with only minimal interaction with EGFR-negative cells, demonstrating specificity to targeted cells.67 These results suggested the promising application of this antibody-mediated nanosytem in targeted therapy for leukemia. Various antigens, including CD19, CD20, CD22, CD33, and CD44 have since been proven to be over-expressed in leukemia cells, serving as potential targets in lekemia treatment.5.2 Peptide-mediated targeted systems
Various studies have investigated the potential application of cell penetrating peptides with the ability to penetrate cellular membranes via internalization and enhance the antitumor efficacy of the drugs, for targeted treatment of blood cancers.68 CPP44 is one of the peptides that has been shown to bind the M160 receptor on the acute myeloid leukemia cell surface, and has been used to formulate CP44-decorated bifunctional dendrimers containing P16INK4a payload (cytotoxic compound), as an anti-acute myeloid leukemia therapeutic system.69 The system exhibited significantly higher anti-acute myeloid leukemia activity compared to the cytotoxic compound alone.69 Besides cell penetrating peptides, other normal peptides can also be exploited as targeting moieties in drug delivery carriers in blood cancer. A typical example is the peptide AA13 which targets and binds low density lipoprotein receptor, overexpressed in certain types of leukemia cells.705.3 Aptamer-mediated targeted systems
Various Nucleic acid aptamers present with numerous advantages for application as targeting moieties in anticancer drug delivery systems. The advantages include, but not limited to smaller size, chemical structure stability, target specificity, lack of immunogenicity, and are easily chemically modified.71 As such, these artificially synthesized DNA/RNA molecules have sparked great interest as new promising targeting ligands. The As1411 is one of the therapeutic DNA aptamers with specific recognition for the nucleolin protein, currently explored for combination with cytarabine for the treatment of acute myeloid leukemia.72 Essentially, the combination of anticancer drugs with aptamers can improve the targeting ability and minimize the drugs’ side effects. Interestingly, Sgc8 which specifically binds protein tyrosine kinase 7 (PTK7) is the first aptamer that could be covalently linked to doxorubicin to achieve targeted cell binding for treatment of leukemia.73 Moreover, the site-specific release of doxorubicin for targeted killing was observed, due to the hydrazone bond breakage in the acidic tumor microenvironment.73 In another work, a DNA aptamer targeting CD177 was developed for combination with methotrexate, yielding an aptamer-drug conjugate with significant inhibition of acute myeloid leukemia cell proliferation.745.4 Protein-mediated targeted systems
Proteins are also potential candidates for application as targeting moieties in targeted drug delivery systems.3 Particularly, ferritin and transferrin, which are blood proteins containing iron have been investigated for application in protein-mediated targeting delivery systems in blood cancer.75 Ferritin has been used to develop a ferritin-based trivalent arsenic nanomedicine for targeting overexpressed CD71 receptors of leukemia cells. It was discovered that the ferritin-arsenic nanocomplex resulted in notable anti-leukemia effects in different leukemia types, further exhibiting enhanced antitumor efficacy and minimal side effects compared to pristine arsenic trioxide.75 Likewise, transferrin has been decorated onto different drug delivery systems to enable specific targeting of highly expressed tumor transferrin receptors (TfR) for selective uptake by cancerous cells in blood cancer treatment.75 Accordingly, a TfR-targeted liposomal carrier was proposed for delivering Bcl-2-specific antisense oligodeoxyribonucleotide (G3139) for Blc-2 protein downregulation in leukemia.75 Additionally, more other proteins, including high-density lipoproteins are under investigations for tumor cell targeting in blood cancer treatment.36. Conclusions and future perspectives
The treatment of blood cancers such as leukemia, lymphoma, and multiple myeloma continues to face substantial challenges, primarily due to the non-specificity, poor bioavailability, and systemic toxicity of conventional therapies. Standard approaches, chemotherapy, radiotherapy, immunotherapy, and stem cell transplantation, have demonstrated limited efficacy in effectively targeting malignant cells within the bone marrow's complex and protective microenvironment. To overcome these barriers, various advanced drug delivery system, including liposomes, pegylated formulations, and polymeric nanoparticles, have been developed to improve drug stability, circulation time, and tumor retention while minimizing off-target effects. These innovations have not only optimized pharmacokinetics and therapeutic index but have also provided a basis for site-specific drug delivery, thereby enhancing clinical outcomes. Notably, some of these systems have already gained regulatory approval, while others are in advanced clinical trial phases, demonstrating real translational potential.Moreover, the integration of targeting ligands such as antibodies, peptides, aptamers, and proteins into these delivery systems has opened new pathways for precise and personalized therapeutic intervention. These ligand-directed systems enable specific recognition of cell-surface markers overexpressed on malignant cells, promoting receptor-mediated endocytosis and enhanced intracellular drug delivery. Leukemia has emerged as a key model in evaluating these strategies, with preclinical models showing significant improvements in both specificity and therapeutic efficacy.
Despite these promising advances, several translational hurdles remain. Regulatory approval, manufacturing scalability, long-term biocompatibility, and cost-effective production are critical challenges that must be addressed to accelerate the clinical translation of emerging targeted delivery systems. Additionally, variability in patient response, immune clearance of nanoparticles, and heterogeneity of tumor markers further complicate widespread application.
Personalized nanomedicine offers a promising frontier for blood cancer treatment. By tailoring delivery systems based on patient-specific biomarkers, disease subtypes, and genetic profiles, therapies can be customized to maximize effectiveness and reduce adverse effects. The future of blood cancer therapy may increasingly rely on integrating such precision medicine approaches with advanced delivery technologies.
Furthermore, the incorporation of artificial intelligence (AI) and big data analytics holds transformative potential in optimizing targeted drug delivery. AI algorithms can assist in identifying optimal drug–ligand combinations, predicting pharmacokinetics, and simulating tumor-drug interactions. Machine learning models trained on large clinical datasets may guide real-time treatment decisions, improve patient stratification, and support adaptive therapy designs.
In summary, while current progress in targeted drug delivery for blood cancers is promising, continued interdisciplinary research, merging nanotechnology, systems biology, computational science, and clinical oncology, is essential to fully realize the potential of these innovations. A future defined by personalized, AI-driven, and precisely targeted therapies is within reach, offering renewed hope for patients battling hematologic malignancies.
Author contributions
Samson A. Adeyemi: conceptualization, literature survey, writing original draft, review & editing; Lindokuhle M. Ngema: conceptualization, literature survey, writing original draft, review & editing; Yahya E. Choonara: conceptualization, review, editing, validation, and supervision.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results have been included, and no new data were generated as part of this review.Acknowledgements
The authors acknowledge the financial support of the National Research Foundation (NRF) of South Africa under the SARChI Chair program (Grant No. PPNT230823145247), awarded to Prof. Yahya Choonara.References
- Y. Jiang, W. Lin and L. Zhu, Targeted Drug Delivery for the Treatment of Blood Cancers, Molecules, 2022, 27(4), 1310 CrossRef CAS PubMed.
- S. A. Tuazon, L. A. Holmberg, O. Nadeem and P. G. Richardson, A clinical perspective on plasma cell leukemia; current status and future directions, Blood Cancer J., 2021, 11(2), 23 Search PubMed.
- T. Ci, W. Zhang, Y. Qiao, H. Li, J. Zang and H. Li, et al., Delivery strategies in treatments of leukemia, Chem. Soc. Rev., 2022, 51(6), 2121–2144 Search PubMed.
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram and A. Jemal, et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, CA Cancer J. Clin., 2021, 71(3), 209–249 Search PubMed.
- P. de la Puente and A. K. Azab, Nanoparticle delivery systems, general approaches, and their implementation in multiple myeloma, Eur. J. Haematol., 2017, 98(6), 529–541 CrossRef CAS PubMed.
- R. Chakraborty and N. S. Majhail, Treatment and disease-related complications in multiple myeloma: Implications for survivorship, Am. J. Hematol., 2020, 95(6), 672–690 Search PubMed.
- T. Etrych, A. Braunova, D. Zogala, L. Lambert, N. Renesova and P. Klener, Targeted Drug Delivery and Theranostic Strategies in Malignant Lymphomas, Cancers, 2022, 14(3), 626 Search PubMed.
- S. H. Swerdlow and J. R. Cook, As the world turns, evolving lymphoma classifications-past, present and future, Hum. Pathol., 2020, 95, 55–77 CrossRef PubMed.
- N. L. Crossnohere, D. R. Richardson, C. Reinhart, B. O'Donoghue, S. M. Love and B. D. Smith, et al., Side effects from acute myeloid leukemia treatment: results from a national survey, Curr. Med. Res. Opin., 2019, 35(11), 1965–1970 Search PubMed.
- D. T. Debela, S. G. Muzazu, K. D. Heraro, M. T. Ndalama, B. W. Mesele and D. C. Haile, et al., New approaches and procedures for cancer treatment: Current perspectives, SAGE Open Med., 2021, 9, 20503121211034366 CrossRef PubMed.
- Advanced Drug Delivery Systems in the Management of Cancer [Internet]. 2021 [cited 2025 May 21]. Available from: https://shop.elsevier.com/books/advanced-drug-delivery-systems-in-the-management-of-cancer/dua/978-0-323-85503-7.
- M. R. Reagan, J. Lian, C. J. Rosen and G. Stein, A Perspective on Malignancy in the Marrow, J. Cell Physiol., 2017, 232(12), 3218–3220 Search PubMed.
- M. Roostaee, A. Derakhshani, H. Mirhosseini, E. B. Mofakham, S. Fathi-Karkan and S. Mirinejad, et al., Composition, preparation methods, and applications of nanoniosomes as codelivery systems: a review of emerging therapies with emphasis on cancer, Nanoscale, 2024, 16(6), 2713–2746 Search PubMed.
- E. Jabbour and H. Kantarjian, Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring, Am. J. Hematol., 2020, 95(6), 691–709 Search PubMed.
- K. A. Hodby and D. I. Marks, Recent Advances in the Management of Acute Lymphoblastic Leukaemia, Curr Treat Options Oncol., 2020, 21(3), 23 CrossRef PubMed.
- J. N. Vo, Y.-M. Wu, J. Mishler, S. Hall, R. Mannan, L. Wang, et al., The genetic heterogeneity and drug resistance mechanisms of relapsed refractory multiple myeloma, - Abstract - Europe PMC, [cited 2025 May 21], Available from: https://europepmc.org/article/pmc/pmc9243087.
- Q. L. Ekpa, P. C. Akahara, A. M. Anderson, O. O. Adekoya, O. O. Ajayi and P. O. Alabi, et al., A Review of Acute Lymphocytic Leukemia (ALL) in the Pediatric Population: Evaluating Current Trends and Changes in Guidelines in the Past Decade, Cureus, 2023, 15(12), e49930 Search PubMed.
- C. C. Kumar, Genetic abnormalities and challenges in the treatment of acute myeloid leukemia, Genes Cancer, 2011, 2(2), 95–107 CrossRef CAS PubMed.
- A. Wang and H. Zhong, Roles of the bone marrow niche in hematopoiesis, leukemogenesis, and chemotherapy resistance in acute myeloid leukemia, Hematology, 2018, 23(10), 729–739 CrossRef CAS PubMed.
- R. Roskoski, Properties of FDA-approved small molecule protein kinase inhibitors, Pharmacol. Res., 2019, 144, 19–50 CrossRef CAS PubMed.
- M. L. Hensley and J. M. Ford, Imatinib treatment: specific issues related to safety, fertility, and pregnancy, Semin. Hematol., 2003, 40(2 Suppl 2), 21–25 CrossRef CAS PubMed.
- A. E. G. Osman and M. W. Deininger, Chronic Myeloid Leukemia: Modern therapies, current challenges and future directions, Blood Rev., 2021, 49, 100825 CrossRef CAS PubMed.
- N. P. Shah, P. Rousselot, C. Schiffer, D. Rea, J. E. Cortes and J. Milone, et al., Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034, Am. J. Hematol., 2016, 91(9), 869–874 CrossRef CAS PubMed.
- S. Y. Ahn, S. K. Son, G. H. Lee, I. Kim, J. W. Cheong and W. S. Lee, et al., Safety and efficacy of nilotinib in adult patients with chronic myeloid leukemia: a post-marketing surveillance study in Korea, Blood Res., 2022, 57(2), 144–151 CrossRef CAS PubMed.
- J. H. Lipton, T. H. Brümmendorf, K. Sweet, J. F. Apperley and J. E. Cortes, Practical considerations in the management of patients treated with bosutinib for chronic myeloid leukemia, Ann. Hematol., 2024, 103(9), 3429–3442 CrossRef PubMed.
- E. Derenzini, A. Rossi and D. Treré, Treating hematological malignancies with drugs inhibiting ribosome biogenesis: when and why, J. Hematol. Oncol., 2018, 11(1), 75 CrossRef PubMed.
- D. Dan, R. Fischer, S. Adler, F. Förger and P. M. Villiger, Cyclophosphamide: As bad as its reputation? Long-term single centre experience of cyclophosphamide side effects in the treatment of systemic autoimmune diseases, Swiss Med. Wkly., 2014, 144, w14030 Search PubMed.
- J. Lee, M. K. Choi and I. S. Song, Recent Advances in Doxorubicin Formulation to Enhance Pharmacokinetics and Tumor Targeting, Pharmaceuticals, 2023, 16(6), 802 CrossRef CAS PubMed.
- M. Javad Javid-Naderi, N. Valizadeh, B. Banimohamad-Shotorbani, M. Shahgolzari, F. Shayegh and R. Maleki-baladi, et al., Exploring the biomedical potential of iron vanadate Nanoparticles: A comprehensive review, Inorg. Chem. Commun., 2023, 157, 111423 CrossRef CAS.
- M. Pourmadadi, H. M. Dehaghi, A. Ghaemi, H. Maleki, F. Yazdian and A. Rahdar, et al., Polymeric nanoparticles as delivery vehicles for targeted delivery of chemotherapy drug fludarabine to treat hematological cancers, Inorg. Chem. Commun., 2024, 167, 112819 CrossRef CAS.
- M. Mukhtar, A. L. Ezra Manicum, M. Shojaei Barjouei, R. Eshaghi Malekshah, R. Behzadmehr and A. Rahdar, et al., Nanocarriers for methotrexate delivery/codelivery in the frame of cancer diagnostics and treatment: a review, Front. Biomater. Sci., 2023, 2, 1200670 CrossRef.
- L. M. Leoni, B. Bailey, J. Reifert, H. H. Bendall, R. W. Zeller and J. Corbeil, et al., Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents, Clin. Cancer Res., 2008, 14(1), 309–317 CrossRef CAS PubMed.
- R. Shotton, R. Broadbent, A. Alchawaf, M. B. Mohamed, A. Gibb and N. Martinez-Calle, et al., Safety of bendamustine for the treatment of indolent non-Hodgkin lymphoma: a UK real-world experience, Blood Adv., 2024, 8(4), 878–888 CrossRef CAS PubMed.
- D. A. Gewirtz, A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin, Biochem. Pharmacol., 1999, 57(7), 727–741 CrossRef CAS PubMed.
- M. Pourmadadi, A. Ghaemi, A. Shamsabadipour, M. Rajabzadeh-Khosroshahi, M. Shaghaghi and A. Rahdar, et al., Nanoparticles loaded with Daunorubicin as an advanced tool for cancer therapy, Eur. J. Med. Chem., 2023, 258, 115547 CrossRef CAS PubMed.
- C. Schneider, T. Oellerich, H. M. Baldauf, S. M. Schwarz, D. Thomas and R. Flick, et al., SAMHD1 is a biomarker for cytarabine response and a therapeutic target in acute myeloid leukemia, Nat. Med., 2017, 23(2), 250–255 CrossRef CAS PubMed.
- O. L. Lanier, E. Pérez-Herrero, A. P. D. Andrea, K. Bahrami, E. Lee and D. M. Ward, et al., Immunotherapy approaches for hematological cancers, iScience, 2022, 25(11), 105326 CrossRef PubMed.
- L. Chen and D. B. Flies, Molecular mechanisms of T cell co-stimulation and co-inhibition, Nat. Rev. Immunol., 2013, 13(4), 227–242 CrossRef PubMed.
- H. Rafei, R. S. Mehta and K. Rezvani, Editorial: Cellular Therapies in Cancer, Front. Immunol., 2019, 10, 2788 CrossRef CAS PubMed.
- D. Gomes-Silva, E. Atilla, P. A. Atilla, F. Mo, H. Tashiro and M. Srinivasan, et al., CD7 CAR T Cells for the Therapy of Acute Myeloid Leukemia, Mol. Ther., 2019, 27(1), 272–280 CrossRef CAS PubMed.
- D. Avigan and J. Rosenblatt, Vaccine therapy in hematologic malignancies, Blood, 2018, 131(24), 2640–2650 CrossRef CAS PubMed.
- L. Biavati, C. A. Huff, A. Ferguson, A. Sidorski, M. A. Stevens and L. Rudraraju, et al., An Allogeneic Multiple Myeloma GM-CSF–Secreting Vaccine with Lenalidomide Induces Long-term Immunity and Durable Clinical Responses in Patients in Near Complete Remission, Clin. Cancer Res., 2021, 27(24), 6696–6708 CrossRef CAS PubMed.
- H. H. W. Chen and M. T. Kuo, Improving radiotherapy in cancer treatment: Promises and challenges, Oncotarget, 2017, 8(37), 62742–62758 CrossRef PubMed.
- M. Witkowska, A. Majchrzak and P. Smolewski, The role of radiotherapy in Hodgkin's lymphoma: what has been achieved during the last 50 years?, BioMed Res. Int., 2015, 2015, 485071 Search PubMed.
- I. L. Weissman, Translating stem and progenitor cell biology to the clinic: barriers and opportunities, Science, 2000, 287(5457), 1442–1446 CrossRef CAS PubMed.
- C. Chabannon, J. Kuball, A. Bondanza, F. Dazzi, P. Pedrazzoli and A. Toubert, et al., Hematopoietic stem cell transplantation in its 60s: A platform for cellular therapies, Sci. Transl. Med., 2018, 10(436), eaap9630 CrossRef PubMed.
- E. A. Copelan, Hematopoietic stem-cell transplantation, N. Engl. J. Med., 2006, 354(17), 1813–1826 CrossRef CAS PubMed.
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Cancer nanomedicine: progress, challenges and opportunities, Nat. Rev. Cancer, 2017, 17(1), 20–37 CrossRef CAS PubMed.
- P. Tardi, S. Johnstone, N. Harasym, S. Xie, T. Harasym and N. Zisman, et al., In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy, Leuk. Res., 2009, 33(1), 129–139 CrossRef CAS PubMed.
- J. E. Lancet, G. L. Uy, J. E. Cortes, L. F. Newell, T. L. Lin and E. K. Ritchie, et al., CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia, J. Clin. Oncol., 2018, 36(26), 2684–2692 CrossRef CAS PubMed.
- M. Molica, S. Perrone, C. Mazzone, L. Cesini, M. Canichella and P. de Fabritiis, CPX-351: An Old Scheme with a New Formulation in the Treatment of High-Risk AML, Cancers, 2022, 14(12), 2843 CrossRef CAS PubMed.
- L. Pagano, R. Danesi, E. Benedetti, R. Morgagni, L. Romani and A. Venditti, The Role of CPX-351 in the Acute Myeloid Leukemia Treatment Landscape: Mechanism of Action, Efficacy, and Safety, Drugs, 2025, 85, 855–866 CrossRef PubMed.
- M. Talpaz, A. Rakhit, K. Rittweger, S. O'Brien, J. Cortes and S. Fettner, et al., Phase I evaluation of a 40 kDa branched-chain long-acting pegylated IFN-alpha-2a with and without cytarabine in patients with chronic myelogenous leukemia, Clin. Cancer Res., 2005, 11(17), 6247–6255 CrossRef CAS PubMed.
- M. Pourmadadi, S. E. Gerami, N. Ajalli, F. Yazdian, A. Rahdar and S. Fathi-karkan, et al., Novel pH-responsive hybrid hydrogels for controlled delivery of curcumin: Overcoming conventional constraints and enhancing cytotoxicity in MCF-7 cells, Hybrid Adv., 2024, 6, 100210 CrossRef.
- G. Pasut and F. M. Veronese, State of the art in PEGylation: the great versatility achieved after forty years of research, J. Controlled Release, 2012, 161(2), 461–472 CrossRef CAS PubMed.
- P. A. Dinndorf, J. Gootenberg, M. H. Cohen, P. Keegan and R. Pazdur, FDA drug approval summary: pegaspargase (oncaspar) for the first-line treatment of children with acute lymphoblastic leukemia (ALL), Oncologist, 2007, 12(8), 991–998 CrossRef CAS PubMed.
- J. Wang, L. Sheng, Y. Lai and Z. Xu, An overview on therapeutic efficacy and challenges of nanoparticles in blood cancer therapy, J. King Saud Univ., Sci., 2022, 34(6), 102182 CrossRef.
- S. Ashton, Y. H. Song, J. Nolan, E. Cadogan, J. Murray and R. Odedra, et al., Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo, Sci. Transl. Med., 2016, 8(325), 325ra17 Search PubMed.
- S. Bakhshi, A. Shoari, P. Alibolandi, M. Ganji, E. Ghazy and A. Rahdar, et al., Emerging innovations in vincristine-encapsulated nanoparticles: Pioneering a new era in oncological therapeutics, J. Drug Delivery Sci. Technol., 2024, 91, 105270 CrossRef CAS.
- R. W. Wilkinson, R. Odedra, S. P. Heaton, S. R. Wedge, N. J. Keen and C. Crafter, et al., AZD1152, a selective inhibitor of Aurora B kinase, inhibits human tumor xenograft growth by inducing apoptosis, Clin. Cancer Res., 2007, 13(12), 3682–3688 CrossRef CAS PubMed.
- S. Hirsjärvi, C. Passirani and J. P. Benoit, Passive and active tumour targeting with nanocarriers, Curr. Drug Discovery Technol., 2011, 8(3), 188–196 CrossRef PubMed.
- L. Wang, N. Wang, W. Zhang, X. Cheng, Z. Yan and G. Shao, et al., Therapeutic peptides: current applications and future directions, Signal Transduction Targeted Ther., 2022, 7(1), 1–27 CrossRef PubMed.
- F. Mahmoudian, A. Ahmari, S. Shabani, B. Sadeghi, S. Fahimirad and F. Fattahi, Aptamers as an approach to targeted cancer therapy, Cancer Cell Int., 2024, 24(1), 108 CrossRef PubMed.
- H. Han and H. A. Santos, Nano- and Micro-Platforms in Therapeutic Proteins Delivery for Cancer Therapy: Materials and Strategies, Adv. Mater., 2024, 36(45), 2409522 CrossRef CAS PubMed.
- M. Kędzierska and M. Bańkosz, Role of Proteins in Oncology: Advances in Cancer Diagnosis, Prognosis, and Targeted Therapy—A, Narr. Rev. J. Clin. Med., 2024, 13(23), 7131 Search PubMed.
- R. V. J. Chari, M. L. Miller and W. C. Widdison, Antibody-drug conjugates: an emerging concept in cancer therapy, Angew. Chem., Int. Ed., 2014, 53(15), 3796–3827 CrossRef CAS PubMed.
- P. N. Durfee, Y. S. Lin, D. R. Dunphy, A. J. Muñiz, K. S. Butler and K. R. Humphrey, et al., Mesoporous Silica Nanoparticle-Supported Lipid Bilayers (Protocells) for Active Targeting and Delivery to Individual Leukemia Cells, ACS Nano, 2016, 10(9), 8325–8345 CrossRef CAS PubMed.
- N. Habibi, N. Kamaly, A. Memic and H. Shafiee, Self-assembled peptide-based nanostructures: Smart nanomaterials toward targeted drug delivery, Nano Today, 2016, 11(1), 41–60 CrossRef CAS PubMed.
- C. Kojima, K. Saito and E. Kondo, Design of peptide–dendrimer conjugates with tumor homing and antitumor effects, Res. Chem. Intermed., 2018, 44(8), 4685–4695 CrossRef CAS.
- J. J. Sonju, A. Dahal, S. S. Singh and S. D. Jois, Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment, J. Controlled Release, 2021, 329, 624–644 CrossRef CAS PubMed.
- R. Yazdian-Robati, A. Arab, M. Ramezani, K. Abnous and S. M. Taghdisi, Application of aptamers in treatment and diagnosis of leukemia, Int. J. Pharm., 2017, 529(1–2), 44–54 CrossRef CAS PubMed.
- P. J. Bates, D. A. Laber, D. M. Miller, S. D. Thomas and J. O. Trent, Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer, Exp. Mol. Pathol., 2009, 86(3), 151–164 CrossRef CAS PubMed.
- D. Shangguan, Y. Li, Z. Tang, Z. C. Cao, H. W. Chen and P. Mallikaratchy, et al., Aptamers evolved from live cells as effective molecular probes for cancer study, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(32), 11838–11843 CrossRef CAS PubMed.
- N. Zhao, S. N. Pei, J. Qi, Z. Zeng, S. P. Iyer and P. Lin, et al., Oligonucleotide aptamer-drug conjugates for targeted therapy of acute myeloid leukemia, Biomaterials, 2015, 67, 42–51 CrossRef CAS PubMed.
- T. R. Daniels, T. Delgado, J. A. Rodriguez, G. Helguera and M. L. Penichet, The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer, Clin. Immunol., 2006, 121(2), 144–158 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |