 Open Access Article
Open Access ArticlePhenylboronic acid derivatives: advancing glucose-responsive insulin delivery and multifunctional biomedical applications
Kofi Oti
Boakye-Yiadom
,
Debmalya
Roy
,
Hajra
Zafar
and
Faisal
Raza
 *
*
School of Pharmacy, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: faisalraza@sjtu.edu.cn
First published on 25th June 2025
Abstract
Phenylboronic acid (PBA) and its derivatives have emerged as versatile materials with significant implications in biomedical and industrial applications, particularly for glucose-responsive systems. Their unique saccharide-binding properties, dictated by their tunable pKa, enable advanced functionalities in drug delivery and biosensing. In diabetes management, PBA-based systems ranging from bulk hydrogels to micro/nanogels and self-assembled micelles offer precise insulin delivery mechanisms that respond dynamically to glucose levels. These materials are further enhanced by their adaptability to diverse routes of administration, including subcutaneous, transdermal, and oral delivery systems. Beyond insulin delivery, multifunctional PBA derivatives combined with glucose oxidase or polymers have been utilized in diabetic wound healing, biosensing, and environment-sensitive therapeutic applications like siRNA and cancer immunotherapy. The integration of PBA into hybrid and dual-responsive platforms continues to expand its utility, paving the way for innovative solutions in personalized medicine and diagnostics. This review explores the chemistry, applications, and future prospects of PBA derivatives, emphasizing their transformative potential in creating responsive, biocompatible, and multifunctional systems for biomedical use.
Dr Hajra Zafar is a registered pharmacist and an accomplished researcher in the fields of pharmaceutics and nanomedicine. She earned her Ph.D. in Pharmaceutics from the prestigious School of Pharmaceutical Sciences at Shanghai Jiao Tong University (SJTU), China, where she specialized in Pharmaceutics for advanced drug delivery systems. Her research is centered on the development of biomimetic nanomedicine for precision-based therapies, with a primary focus on cancer immunotherapy and targeted drug delivery. She is also working on designing polymer-based nanomedicines for treating infections like H. pylori. Her interdisciplinary work bridges nanotechnology, biomaterials, and pharmaceutical sciences, contributing to innovative therapeutic solutions. |
1. Introduction
PBA derivatives have gained significant attention for their unique chemical properties, enabling diverse applications across scientific and industrial fields.1–3 The pKa of PBA plays a critical role in its functionality, particularly enabling selective interactions with saccharides such as glucose.4–8 This interaction is central to the ability of PBA to form reversible covalent bonds with diols, making it especially valuable in biomedical applications like glucose sensing and insulin delivery systems. The development of advanced PBA derivatives has further expanded their utility by tailoring specific properties such as enhanced glucose responsiveness or biocompatibility.In the context of insulin delivery, PBA-functionalized platforms such as bulk hydrogels,9–14 micro/nanogels,15–19 and self-assembled micelles20–25 have demonstrated remarkable potential. These systems utilize the glucose-binding ability of PBA to enable on-demand insulin release, providing a more physiologically responsive approach for blood glucose regulation for patients with type 1 diabetes (T1D). Beyond insulin delivery, PBA derivatives have demonstrated benefits in diabetic wound healing, where they help modulate the wound microenvironment and support tissue regeneration.26,27 Their utility also extends to biosensing and diagnostic technologies, where PBA-based platforms enable real-time monitoring of glucose and other biological markers.28,29
Emerging applications have pushed the boundaries of PBA use even further. For instance, in cancer immunotherapy and siRNA delivery, PBA-containing platforms are being explored for their ability to respond to environmental cues such as pH changes or oxidative stress.30–37 By incorporating PBA into hybrid systems, researchers have created smart carriers capable of releasing therapeutics in a more targeted and efficient manner, reducing off-target effects and improving treatment.38 These systems contribute to reducing systemic toxicity while enhancing therapeutic efficacy.
Despite this progress, much of the existing literature tends to focus on individual applications of PBA in isolation, be it insulin delivery, wound healing, biosensing, or cancer therapy. What's often missing is a broader, integrative analysis that connects the design principles behind PBA-based materials across these different domains. Overlapping mechanisms such as glucose sensitivity, reactive oxygen species (ROS)-responsiveness, and structural tunability are rarely discussed collectively, even though they offer clear opportunities for cross-application innovation.
This review aims to bridge that gap. Rather than viewing PBA solely as a chemical functional group, we present it as a modular design strategy for creating responsive, multifunctional biomaterials. We explore how the same core chemical interactions, particularly PBA's dynamic bonding with diols, can be adapted for diverse biomedical uses, from glucose-triggered insulin release to ROS-scavenging wound dressings and tumor-targeted drug carriers. By drawing these connections, we aim to provide a more unified framework for future research and design in the field.
The novelty of this work lies in its cross-disciplinary perspective. By examining shared challenges and synergistic design opportunities, we propose a more holistic approach for the development of PBA-based platforms, one that can better support the translation of these materials into clinically relevant technologies.
2. Chemistry of phenylboronic acid
PBA and its derivatives are versatile chemical entities with wide-ranging applications in fields such as drug delivery, sugar sensing, diagnostics, and materials science. The significance of boron-containing compounds was notably recognized in 2010 when the Nobel Prize in Chemistry was awarded for the development of the Suzuki–Miyaura reaction, highlighting the foundational role of organoboron chemistry in modern synthesis.39 Central to the utility of PBAs is their ability to reversibly bind cis-diols via boronate ester formation, a process governed by their Lewis acidity and pKa. The pKa value is a particularly critical parameter, as it dictates the acid–base equilibrium between the neutral trigonal planar (sp2-hybridized) boronic acid and the anionic tetrahedral (sp3-hybridized) boronate form. Diol binding predominantly occurs via the latter, making the pKa a determining factor in the efficiency of saccharide recognition under physiological conditions.40Most unmodified PBA derivatives exhibit pKa values greater than 8.5, limiting their reactivity at physiological pH (∼7.4). Thus, optimization strategies often focus on lowering the pKa to enhance reactivity at near-neutral pH. This is achieved through strategic substitution on the phenyl ring, where electron-withdrawing groups (EWGs) such as –NO2, –CF3, or –CHO stabilize the boronate anion via inductive and resonance effects, thereby reducing the pKa and enhancing Lewis acidity. In contrast, electron-donating groups like –NH2 or –OCH3 destabilize the anionic form and increase the pKa, reducing diol-binding affinity.41,42 This substituent effect can be quantitatively analyzed using Hammett constants (σ), enabling predictive tuning of boronic acid reactivity.
Furthermore, the position of substituents on the phenyl ring, whether ortho, meta, or para, can drastically alter pKa values and binding properties.43,44ortho-Substituted groups, particularly those capable of intramolecular interactions such as >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (in 2-formylphenylboronic acid, 2-FPBA), introduce internal hydrogen bonding or tautomerization that stabilizes the tetrahedral boronate form and lowers the effective pKa. This stabilization not only improves diol affinity but also enhances the hydrolytic stability of the boronate ester. Another ortho-substituted derivative, 2-((dimethylamino)methyl)phenylboronic acid, exhibits a markedly low pKa value (∼5.3), enabling strong diol binding even under mildly acidic conditions.45,46
O (in 2-formylphenylboronic acid, 2-FPBA), introduce internal hydrogen bonding or tautomerization that stabilizes the tetrahedral boronate form and lowers the effective pKa. This stabilization not only improves diol affinity but also enhances the hydrolytic stability of the boronate ester. Another ortho-substituted derivative, 2-((dimethylamino)methyl)phenylboronic acid, exhibits a markedly low pKa value (∼5.3), enabling strong diol binding even under mildly acidic conditions.45,46
A range of PBA derivatives, such as 4-formylphenylboronic acid (4-FPBA), 4-(methylcarbamoyl)phenylboronic acid, and benzoxaborole, have been studied for their binding to biologically active diols like glucose and fructose.45 These derivatives demonstrate pKa values ranging from 5.3 to 8.5, influenced by their substituents. Lower pKa values, as seen in EWGs like 4-FPBA, enhance binding affinity, while higher pKa values, observed in derivatives such as 3-acetamidophenylboronic acid, indicate weaker acidity. Benzoxaborole's cyclic structure offers additional stabilization by relieving ring strain upon ester formation, further reducing the effective pKa.47
Fluoro-substituted PBA molecules, particularly fluoro-2-FPBA analogs, combine strong EWGs at the ortho/meta/para positions with carbonyl functionalities, resulting in dual electronic modulation that dramatically lowers the pKa and enhances diol-binding kinetics. These compounds are especially valuable for protein–sugar interaction studies, where fluorine atoms not only influence acidity but also offer synthetic handles for selective conjugation.48,49 Moreover, the strategic spatial arrangement of substituents next to the boronic acid moiety, especially at positions that allow resonance or steric interplay, can fine-tune both binding strength and selectivity.
Beyond simple arylboronic acids, heterocyclic PBA derivatives such as pyridylboronic acids are emerging as next-generation saccharide binders. The incorporation of nitrogen-containing heterocycles (e.g., pyridine and pyrimidine) introduces additional electronic effects and hydrogen-bonding capabilities that lower pKa and improve water solubility. For example, 3-pyridylboronic acid exhibits strong affinity toward sialic acid and pyrophosphate at near-neutral or mildly acidic pH, owing to the resonance stabilization of its boronate form and its capacity for bidentate interactions.50 Other heterocycles, such as boronopicolinic acid and 5-pyrimidineboronic acid, also exhibit high specificity for nucleoside derivatives and phosphorylated metabolites, showing promise in targeted drug delivery and biosensing.
PBA derivatives play a critical role in stabilizing diols, a property leveraged in prebiotic RNA stabilization and drug delivery. Clinically, boronic acid derivatives like bortezomib serve as proteasome inhibitors for cancer treatment.51 The binding of PBA to polyols, which includes diols in threo and erythro configurations, demonstrates its specificity. threo-1,2-Diols, for instance, provide stable binding due to reduced steric interactions, while erythro-1,2-diols are less stable due to steric hindrance.52 This selectivity underscores the potential of boronic acids in designing biomaterials for targeted applications.
In conclusion, PBA and its derivatives represent a cornerstone of chemical innovation, with their properties dictated by structural modifications and pKa values. Their ability to bind saccharides and interact with diols under specific conditions positions them as indispensable tools in sugar sensing, drug delivery, and beyond. Continued exploration of PBA derivatives holds great promise for advancing both biomedical and industrial applications.
3. PBA-based insulin delivery systems
3.1. Bulk hydrogels
A hydrogel, which is a three-dimensional network retaining substantial volume of water, is stabilized by a chemically or physically crosslinked polymeric framework.9 By incorporating PBA into the polymer network, the hydrogel can exhibit glucose-responsive behavior under specific conditions. When glucose is introduced, it shifts the equilibrium toward boronate ester formation, which alters the interactions within the polymer network or between the polymer and surrounding water molecules.10 This results in increased hydrophilicity, causing the polymer chains to move apart due to steric hindrance, leading to the controlled release of the encapsulated drug.In 2017, a new device was developed by combining a hydrogel formulation with a catheter.53 The catheter had pores aligned perpendicular to its long axis, which acted as channels for the bidirectional flow of insulin and glucose between the internal solution and the surrounding interstitial fluid. The hydrogel coating these openings functioned as a glucose-responsive barrier, controlling the rate of insulin diffusion. When implanted subcutaneously in type 1 diabetic mice, the device lowered their blood glucose levels from around 500 mg dL−1 to 300 mg dL−1 while they maintained a normal diet. A glucose tolerance test conducted on overnight-fasted diabetic mice revealed a surge in serum insulin that coincided with the blood glucose peak, indicating in vivo insulin release triggered by blood glucose levels. However, in spite of the promising performance, the device's clinical translation is limited by its relatively large and invasive design, the need for surgical implantation, and concerns about potential for infection at the implantation site. Additionally, the precision and responsiveness of insulin release under physiological glucose fluctuations remain suboptimal for tight glycemic control.
3.2. Micro/nanogels
Microgels used in glucose-responsive systems are typically synthesized via precipitation or emulsion polymerization techniques. Alternatively, self-assembly of chains can occur, driven by intra- or intermolecular PBA–diol complexation or hydrophobic interactions. Similar to bulk hydrogels, when free glucose binds to PBA, it weakens these forces, leading to the swelling or breakdown of the microgel or nanogel and initiating insulin release.For instance, Zhou and colleagues developed glucose-responsive poly(N-isopropylacrylamide-co-phenylboronic acid) microgels using a post-modification method.5 In this study, 3-aminophenylboronic acid (APBA) was attached onto the poly(N-isopropylacrylamide-co-acrylic acid) backbone of a crosslinked microgel made through precipitation polymerization. Upon glucose exposure (10 mM), the size of the microgel containing 10% APBA increased two-fold at room temperature under basic conditions. Likewise, an alternative team employed a direct polymerization technique to formulate PBA-containing microgels with significant expansion observed at pH 8.5 and 9.5.17 Despite their potential, microgel systems encounter notable challenges. The glucose-responsive swelling often requires alkaline or non-physiological pH conditions (e.g., pH 8.5–9.5), which limits their effectiveness under normal physiological pH (∼7.4). Additionally, post-modification methods can result in uneven or incomplete functionalization of PBA groups, affecting reproducibility and consistency.
3.3. Self-assembled micelles
Introducing PBA into the hydrophobic segment has been utilized to create amphiphilic copolymers exhibiting glucose-responsive properties. For instance, a micelle system made from poly(ethylene glycol)-block-poly(acrylic acid-co-acrylamidophenylboronic acid) demonstrated glucose-triggered insulin release within the physiological glucose range. A follow-up study indicated that the coordination between the carboxylate group and PBA lowered the pKa of PBA, which enhanced its glucose sensitivity at lower pH levels.54 Alternatively, researchers have developed a glucose-sensitive platform by integrating a phenyl borate monomer into the amphiphilic polymer framework.23,55 This copolymer self-assembled into core–shell micelles at pH 7.4. Upon exposure to high glucose concentrations, glucose competed with diols for binding to PBA, resulting in a transition of the polymer from amphiphilic to hydrophilic. In a glucose-rich solution (400 mg dL−1), a significant increase in the release rate of insulin was observed.22 Further investigations have explored the factors affecting glucose-responsiveness and insulin encapsulation efficiency, like the molecular size and the method of drug loading.23 Nevertheless, lengthy and complex copolymerization processes may lead to batch-to-batch variability, hindering reproducibility and posing challenges for large-scale manufacturing.4. Routes of administration
4.1. Subcutaneous injection-based glucose-responsive insulin delivery systems
Subcutaneous injection remains a cornerstone of insulin delivery due to its reliability and ability to provide consistent therapeutic effects. Advances in glucose-responsive insulin delivery systems aim to integrate physiological feedback mechanisms to mimic the function of the pancreas, ensuring optimal glucose regulation. Recent innovations leverage the unique properties of PBA derivatives and their interactions with glucose to develop adaptive delivery platforms. These systems demonstrate significant potential in managing diabetes through enhanced responsiveness, reduced side effects, and prolonged glycemic control.Zhang and colleagues developed a polymeric delivery system that uses a glucose-triggered charge-reversal mechanism to release insulin (Fig. 1a).56 This system is based on a cationic FPBA polymer, which is synthesized by modifying poly(L-lysine) with FPBA. In its unaltered form, the polymer carries a positive charge, which facilitates the efficient loading of gluconic acid-modified insulin, a negatively charged molecule. Under normal blood glucose levels, the polymer maintains its positive charge, resulting in a gradual, sustained release of insulin. However, when blood glucose levels rise during hyperglycemia, the polymer undergoes charge reversal, becoming negatively charged and leading to a rapid release of insulin. In vitro experiments confirmed that the polymer binds glucose and triggers insulin release, while in vivo studies in diabetic mice and minipigs showed that the system was able to regulate blood glucose and maintain normoglycemia for up to a week, demonstrating its potential for long-term diabetes management. Notwithstanding these advantages, limitations remain. The requirement to modify insulin with gluconic acid may complicate large-scale production and regulatory approval.
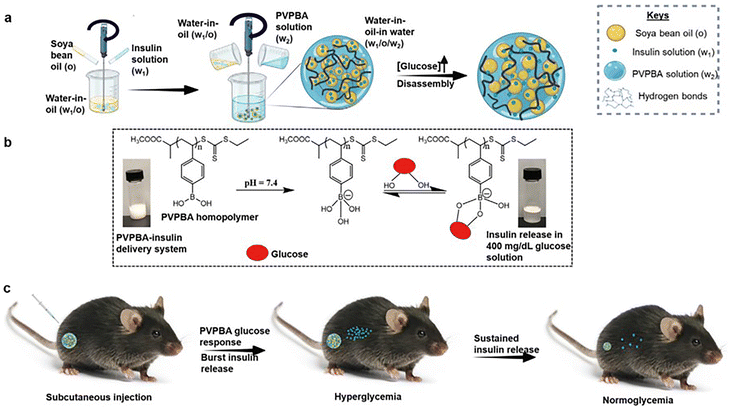 | ||
| Fig. 1 A diagram illustrating the formation of a glucose-responsive gelled emulsion and its application in T1D therapy. (a) Preparation of a gelled multiple emulsion and the release of insulin via a glucose-responsive mechanism. (b) Insulin release from the gelled emulsion upon exposure to a hyperglycemic state. The stability of the emulsion is due to the hydrogen bonds between PBA molecules. In a hyperglycemic environment, these hydrogen bonds are broken down, leading to the destabilization of the emulsion and accelerating insulin release. (c) Treatment regimen of T1D in a streptozotocin-induced diabetes mouse model using the gelled emulsion-based insulin delivery platform. Reproduced with permission from ref. 57. Copyright 2024 Wiley-VCH Verlag GmbH. | ||
Boakye-Yiadom et al. employed RAFT polymerization to synthesize a poly(4-vinylphenylboronic acid) homopolymer with exceptional emulsification and gelling properties (Fig. 1).57 This polymer facilitated the creation of a glucose-responsive gelled water-in-oil-in-water (W/O/W) multiple emulsion for insulin delivery. The system exhibited several advantageous features, including its injectable gel-like scaffold, high glucose responsiveness, and stability under low-glucose conditions. In hyperglycemic environments, the system demonstrated self-accelerating demulsification, enabling rapid insulin release. Furthermore, this platform offers the benefit of simple preparation without requiring chemical modification of insulin, presenting a scalable and adaptive approach for diabetes management. The multistep emulsion fabrication process, while simpler than covalent modification approaches, may still face challenges in achieving batch-to-batch consistency and long-term storage stability. The system's performance in vivo has so far been limited to animal models, and its translational feasibility in humans remains untested, particularly regarding potential immunogenicity, biodegradability, and metabolic clearance of the polymer.
The formation of a boronate ester between PBA and glucose enhances its water solubility and has been investigated as a mechanism for triggering insulin-PBA release from delivery systems. Scientists have investigated the interaction between aliphatic PBA and blood plasma albumin as a potential glucose-responsive approach for insulin delivery.58 This strategy involved modifying insulin with various aliphatic PBA derivatives exhibiting distinct pKa values. In a glucose-rich medium (400 mg dL−1), glucose molecules bound to the PBA moiety, converting it into a boronate ester and reducing the interactions between PBA and albumin, which in turn triggered insulin release. This engineered insulin demonstrated prolonged efficacy similar to insulin detemir and exhibited the ability to dynamically adjust its function in response to changes in blood glucose concentrations. Nonetheless, the covalent modification of insulin raises concerns regarding structural integrity, potential immunogenicity, and altered pharmacokinetics. The variability in binding affinity among different PBA derivatives and their sensitivity to pH fluctuations may also compromise the precision of glucose responsiveness, limiting their immediate clinical translatability.
These subcutaneous injection-based systems represent a leap forward in developing glucose-responsive technologies, offering a promising path toward achieving tighter glycemic control and enhancing the well-being of patients with diabetes.
4.2. Transdermal-based glucose-responsive insulin delivery systems
Transdermal glucose-responsive insulin delivery systems represent an innovative approach for diabetes management, leveraging the competitive dissociation of reversible boronate ester bonds between phenylboronic acid derivatives and diol groups on carrier materials. This mechanism is triggered by elevated glucose levels, leading to crosslinking dissociation and enabling glucose-mediated insulin release.59The pioneering work in this area was conducted by Chen et al., who developed microneedle (MN) patches using hydrogels formed by poly(vinyl alcohol) (PVA) and sodium hyaluronate grafted with 4-((2-aminoethyl)carbamoyl)-3-fluorophenylboronic acid60 or PVA combined with polyallylamine grafted with 3-carboxy-4-fluorophenylboronic acid.61 These hydrogels were crosslinked through boronate ester bonds. In hyperglycemic conditions, glucose was expected to reduce crosslinking density, thereby facilitating a glucose-dependent insulin release. However, in vivo studies in type 1 diabetic rats primarily demonstrated prolonged insulin release without evidencing differential glucose responsiveness due to the absence of a non-responsive insulin-loaded MN group. While glucose-dependent release has been demonstrated in vitro, these MN patch systems show limited in vivo evidence of true glucose responsiveness due to the lack of appropriate non-responsive control groups.
Alternatively, Fu et al. developed a dissolving MN patch incorporating insulin-loaded mesoporous silica nanoparticles capped with zinc oxide nanoparticles (Fig. 2).62 Dynamic boronic ester bonds between gluconamide diols and phenylboronic acid derivatives regulated insulin release through an on/off gating mechanism. Under hyperglycemic conditions, glucose cleaved the bonds, uncapping the mesopores and releasing insulin. In diabetic mice, these patches maintained normoglycemia for approximately three hours longer than subcutaneous insulin. Additionally, glucose tolerance tests and hypoglycemia assessments confirmed their glucose sensitivity, outperforming subcutaneous insulin in both efficacy and safety. Although the system shows improved glucose sensitivity and extended glycemic control in diabetic mice, challenges remain regarding the complexity of nanoparticle fabrication and the stability of dynamic boronic ester bonds under physiological conditions. The on/off gating mechanism may face variability in response times and insulin release rates in vivo due to fluctuating glucose levels and potential interference from other biomolecules.
 | ||
| Fig. 2 Schematic illustration of a dissolving microneedle patch (SGRM patch) containing mesoporous silica nanoparticles (G-MSN@Insulin@ZnO-PBA-2) for transdermal delivery of insulin. Reproduced with permission from ref. 62. Copyright 2022 Elsevier. | ||
Subsequent efforts focused on developing synthetic polymer hydrogels modified with pyridiniumboronic acid motifs, aiming for reversible glucose-triggered insulin release.63 While these designs showed promise, many studies failed to demonstrate a clear glucose-specific response, primarily due to the absence of a proper non-responsive control group. However, recent advancements have addressed this gap, enhancing the reliability of these glucose-responsive systems. Chen et al. developed a core–shell MN patch where the shell comprised PVA and ε-polylysine modified with 4-carboxy-3-fluorophenylboronic acid, crosslinked through boronate ester bonds.64 Under hyperglycemic conditions, glucose competitively dissociated the bonds, reducing shell density and allowing insulin to diffuse from the core. This system demonstrated glucose-dependent release in vitro and effectively managed daily glucose fluctuations in diabetic rats, with a reduced risk of hypoglycemia under normoglycemic conditions. However, the absence of comprehensive in vivo validation limits the understanding of its therapeutic efficacy and safety under physiologically dynamic conditions. Mechanical robustness and consistent skin penetration across varying skin types remain critical concerns, particularly for the dual-layer architecture. Moreover, the long-term biocompatibility and metabolic clearance of fluorophenylboronic acid-modified ε-polylysine require thorough toxicological assessment.
Together, these advancements highlight how transdermal glucose-responsive systems could pave the way for safer and more effective insulin delivery in diabetes care. That said, incorporating non-responsive controls in future studies will be essential to confidently confirm that the observed effects are truly driven by glucose responsiveness.
4.3. Oral-based glucose-responsive insulin delivery systems
Since the discovery of insulin, oral administration has been a long-standing goal.65 Nevertheless, insulin is rapidly broken down by enzymes in the stomach and intestinal tract, and its transport across the intestinal epithelium remains problematic.66 Recently, Gu and colleagues designed an oral insulin delivery system aimed at regulating blood glucose levels after meals. This system involved encapsulating insulin within liposomes designed to target the neonatal Fc receptor (FcRn), which were subsequently coated with a glucose-responsive hyaluronic acid-phenylboronic acid (HA-PBA) shell.67 Upon oral administration, the HA shell disassociated from the liposomes due to elevated blood glucose concentration in the intestine following meal ingestion. This allowed the exposed Fc portion on the surface of the liposome to bind with FcRn receptors on the small intestine, promoting the absorption of insulin across the epithelium. In vitro tests established that glucose-triggered exposure contributed to an increase in zeta potential, and in vivo studies demonstrated effective blood glucose regulation following oral insulin administration. Despite these promising results, several limitations exist. The complex structure and multi-component nature of the system raise concerns about reproducibility and scalability for large-scale manufacturing. Additionally, the efficiency of FcRn-mediated transcytosis in humans remains to be fully validated, and the stability of the HA-PBA coating in the harsh gastrointestinal environment poses a risk to the consistency of glucose-triggered release. Furthermore, variability in intestinal glucose levels among patients could affect the responsiveness and reliability of the system in clinical use.5. Multifunctional PBA derivatives
5.1. Hybrid materials: combining PBA with polymers for dual or enhanced functionalities
Hybrid materials that integrate PBA with polymers have emerged as a promising avenue in biomedical engineering, offering dual or enhanced functionalities for various applications.68,69 These materials utilize the unique properties of PBA, particularly its dynamic boronate ester bonding with diols, and combine them with the structural and functional versatility of polymers. Two key innovations in this field focus on developing pH-sensitive systems and biodegradable systems, each designed to address specific challenges in drug delivery and therapeutic interventions.One significant advancement lies in the development of pH-sensitive systems that simultaneously respond to glucose and pH variations. These systems leverage the pH-dependent binding affinity of PBA to glucose, enabling precise control over drug or insulin release under specific environmental conditions. For example, in acidic microenvironments, such as inflamed tissues or tumors, the altered pH modulates the PBA–glucose interaction, triggering the release of encapsulated therapeutic agents.70,71 This dual responsiveness enhances the efficiency and specificity of drug delivery, making these systems particularly valuable in managing conditions like diabetes and cancer. In the case of glucose-responsive insulin delivery, such materials can ensure insulin release under specific pH conditions characteristic of the target environment in response to elevated glucose levels, thereby improving glycemic control and reducing side effects.72,73 In parallel, biodegradable systems incorporating PBA with polymers have demonstrated immense potential for improving biocompatibility. By conjugating PBA to biodegradable polymers such as poly(L-lysine),56,72 polycaprolactone,71,74 or natural polymers like chitosan,75,76 researchers have created environmentally responsive materials that safely degrade within the body after delivering their therapeutic payload. This degradability not only minimizes long-term toxicity but also aligns with clinical and regulatory standards, making these systems ideal for sustained drug delivery and temporary scaffolding in regenerative medicine. For instance, glucose-responsive hydrogels formulated with biodegradable polymers provide a platform for prolonged insulin delivery, degrading gradually after the drug is released. Similarly, PBA-functionalized biodegradable nanoparticles have been developed for cancer immunotherapy, targeting tumor cells while decomposing into nontoxic byproducts post-treatment.
In summary, hybrid materials combining PBA with polymers offer a unique blend of functionality and safety, addressing critical needs in targeted and controlled drug delivery. The integration of pH-sensitive and biodegradable properties enables these materials to adapt to complex physiological environments, ensuring precision, efficacy, and reduced side effects. As research continues, these innovations are poised to transform the landscape of therapeutic interventions, fostering the development for more effective and user-friendly solutions.
5.2 Combining GOx and PBA responsive systems
Tong et al. designed a dual-responsive transdermal insulin delivery system integrating glucose oxidase and phenylboronic acid functionalities (Fig. 3).77 This system used dissolvable MN patches made from polyvinylpyrrolidone and PVA, which encapsulated polymersomes loaded with insulin and glucose oxidase. The polymersomes were synthesized from an amphiphilic triblock copolymer consisting of polyethylene glycol (PEG), poly(phenylboronic acid pinacol ester), and poly(3-acrylamidophenylboronic acid), enabling two distinct glucose-responsive mechanisms. | ||
| Fig. 3 Diagram illustration of fabricated MN patches and transdermal drug delivery in diabetic rats. Reproduced with permission from ref. 77. Copyright 2018 American Chemical Society. | ||
First, the phenylboronic acid pinacol ester groups hydrolyze in the presence of hydrogen peroxide, produced during glucose oxidase-catalyzed glucose oxidation. This reaction makes the copolymer water-soluble, triggering polymersome disassembly and insulin release. Second, the 3-acrylamidophenylboronic acid groups gain a negative charge upon glucose binding under hyperglycemic conditions, causing polymer swelling. This swelling reduces the interaction between the polymer and insulin, enhancing insulin release.
The performance of the system was evaluated in diabetic rats divided into four groups: a control group using MN patches with empty polymersomes, a group receiving subcutaneous insulin injections (20 IU kg−1), a group with glucose-responsive polymersomes containing insulin only (40 IU kg−1), and a group with dual-responsive polymersomes containing both insulin (40 IU kg−1) and glucose oxidase.
Results showed that the dual-responsive MN patches sustained blood glucose levels below 200 mg dL−1 for up to four hours, compared to only two hours for MN patches without glucose oxidase. Subcutaneous insulin injections caused a rapid drop in glucose to 80 mg dL−1 but failed to maintain control beyond an hour.
This study demonstrates that incorporating glucose oxidase enhances glucose-triggered insulin release, achieving more prolonged and effective glycemic control compared to single-mechanism systems. However, limitations remain, including the short duration of glycemic control (only four hours), the relatively high insulin dose required (40 IU kg−1), and concerns about long-term stability and activity of glucose oxidase within the microneedles. Moreover, repeated use of GOx may induce oxidative stress or immune responses, and large-scale manufacturing of such complex triblock copolymers with consistent quality could present translational challenges.
6. Applications beyond insulin delivery
6.1 Diabetic wound healing
Diabetic wounds present significant challenges in clinical practice, primarily due to prolonged inflammation, excessive oxidative stress, impaired tissue regeneration, and susceptibility to infections. These chronic wounds, especially in individuals with uncontrolled diabetes, often fail to progress through the normal stages of wound healing, leading to complications such as ulcers, infections, and in severe cases, amputations. Therefore, addressing the unique needs of diabetic wounds requires innovative therapeutic strategies. One of the promising approaches gaining attention involves the use of PBA-based polymers in hydrogel formulations. PBA polymers, known for their ability to form dynamic boronate ester bonds, have been widely explored for glucose-responsive insulin delivery, but their potential extends far beyond this application, offering new possibilities in diabetic wound healing.PBA-based hydrogels offer an advanced platform for localized drug delivery in response to specific microenvironmental stimuli, which is essential for managing the complex dynamics of diabetic wound healing. In particular, their ability to respond to both glucose and ROS provides a unique advantage. The diabetic wound environment is characterized by elevated glucose levels and increased oxidative stress, which not only impedes healing but also exacerbates inflammation. By utilizing the dual-responsive nature of PBA, these hydrogels can adapt to the fluctuating levels of glucose and ROS, releasing therapeutic agents precisely when and where they are needed.
Additionally, a hydrogel system incorporating polyboronic acid-modified carboxymethyl chitosan and rosmarinic acid (RA) has been developed, demonstrating not only antioxidant and antibacterial properties but also the ability to promote the migration of key wound-healing cells.79 The RAgel system, as it is called, enhances macrophage polarization to the M2 phenotype, which is crucial for resolving inflammation and promoting tissue repair. In a diabetic wound model, this hydrogel facilitated improved tissue regeneration by promoting collagen deposition and accelerating wound closure (Fig. 4a).
 | ||
| Fig. 4 (a) Schematic representation of RAgel, a polyboronic acid-modified carboxymethyl chitosan hydrogel loaded with rosmarinic acid, demonstrating its antioxidant, antibacterial, and wound healing properties in diabetic wound management. Reproduced with permission from ref. 79. Copyright 2024 Elsevier. (b) Schematic of a pH/ROS-responsive hydrogel dressing composed of HA-PBA and 4A-PEG-Dopa, loaded with antimicrobial peptides and quercetin-loaded micelles, designed for sequential antibacterial, antioxidant, and anti-inflammatory release in diabetic wound healing. Reproduced with permission from ref. 80. Copyright 2024 American Chemical Society. | ||
Moreover, the ability of PBA-based hydrogels to scavenge ROS plays a crucial role in preventing the oxidative stress that impedes wound healing in diabetic patients. A noteworthy innovation in this regard is the development of a hydrogel system that integrates polyphenolic compounds like epigallocatechin gallate (EGCG) with PBA-modified gelatin. This hydrogel demonstrates glucose-responsive ROS-scavenging activity, effectively reducing oxidative stress in the wound environment and promoting tissue regeneration. Through the dissociation of boronate ester bonds, more EGCG is released as glucose levels rise, ensuring that the healing process is supported when it is most needed.
Similarly, hydrogels designed with HA-PBA and fulvic acid (FA) have been proposed for diabetic wound healing.82 This system is glucose-responsive, enabling the localized and controlled release of EN106, a compound that targets the FEM1b-FNIP1 axis. EN106 plays a role in regulating inflammation and promoting tissue repair by modulating macrophage behavior. The combination of FA as a crosslinker and an anti-inflammatory agent, alongside the role of EN106 in targeting specific molecular pathways, underscores the versatility of PBA-based hydrogels in addressing both the biochemical and physiological barriers to wound healing.
In conclusion, PBA-based polymers offer a versatile and innovative solution for advancing diabetic wound healing beyond insulin delivery. Unlike conventional wound dressings and hydrogel systems, PBA-functionalized materials provide a unique combination of glucose/ROS responsiveness, dynamic drug release, and the ability to modulate inflammation and oxidative stress in real time. By capitalizing on the distinctive properties of PBA, researchers are paving the way for smart, self-regulating wound care systems that can precisely address the underlying causes of impaired healing in diabetic patients. With continued innovation and comparative performance validation against existing biomaterials, these hydrogels hold the potential to revolutionize the treatment of diabetic wounds, providing targeted, effective, and patient-centric therapies for one of the most challenging aspects of diabetes management (Table 1).
| Application | System type | PBA-based systems | Conventional systems | Current limitations & future considerations |
|---|---|---|---|---|
| Diabetic wound healing | Drug-responsive hydrogel | Glucose-/ROS-responsive hydrogels enable on-demand release, modulate oxidative stress and inflammation | Passive or standard hydrogels lack stimuli-responsiveness and precise drug release control | - Comprehensive long-term biocompatibility studies |
| - Tailoring responsiveness to chronic wound physiology | ||||
| - Scalable manufacturing for clinical translation | ||||
| Glucose monitoring | Biosensor technology | Non-enzymatic PBA-modified sensors offer reversible glucose recognition, enhanced stability, and reuse | Enzyme-based sensors (e.g., glucose oxidase) suffer from instability, narrow pH tolerance, and degradation | - Enhancing glucose specificity in complex biofluids |
| - Integration into miniaturized or wearable devices | ||||
| - Validation under real-world physiological conditions | ||||
| Cancer immunotherapy applications | Targeted delivery systems | PBA-enabled nanocarriers allow glycan-selective targeting, microenvironment-responsive release, and increased antibody stability | Traditional or immunotherapies face nonspecific distribution, systemic toxicity, and low tumor accumulation | - Optimizing tumor-selective ligand design |
| - Evaluating immunogenicity and metabolic fate of PBA-based materials | ||||
| - Bridging preclinical efficacy with regulatory readiness |
6.2 Biosensing and diagnostics
The use of PBA-modified sensors in glucose detection has revolutionized biosensing technology by exploiting the reversible interaction of PBA with diols, such as glucose and glycoproteins. These sensors have become indispensable tools for biomedical diagnostics, offering significant advancements in sensitivity, selectivity, and functional adaptability. Unlike traditional enzymatic glucose sensors that depend on glucose oxidase, which are often limited by enzyme instability, narrow pH operating ranges, and reduced activity over time, PBA-based sensors provide enhanced chemical stability, reusability, and tunable glucose affinity under physiological conditions. Their non-enzymatic nature eliminates the need for cofactors and reduces susceptibility to biological degradation (Table 1). Beyond voltammetric platforms, the development of potentiometric sensors and fluoride-functionalized systems demonstrates the growing versatility and robustness of this approach. As such, PBA-modified sensors not only expand the landscape of glucose biosensing but are also superior to conventional systems in terms of stability, responsiveness, and long-term applicability.Similarly, bis-PBA derivatives immobilized on gold (Au) electrodes and polymer-coated electrodes using 3-hydroxyphenylboronic acid have provided selective glucose detection.84 In these systems, sensitivity and selectivity are finely tuned through the combination of PBA specificity and the conductive properties of the electrode material. These designs enable glucose monitoring across physiologically relevant ranges, a critical requirement for clinical applications.
Ion-sensitive field-effect transistors (FETs) modified with PBA88,89 or fluorinated PBA90 demonstrated precise glucose detection under physiological conditions. Polymeric liquid membranes with quaternary ammonium salts exhibited glucose-selective responses, particularly with bis-PBA. Advanced materials like ferrocene boronic acid derivatives91–96 and tetrathiafulvalene-bridged bis-PBA97 highlighted the versatility of PBA-based sensors for sugar detection, including applications in enzymatic glucose isomerization monitoring and electrochemical sugar detection.
Additionally, PBA-based sensors have been employed for detecting various glycoproteins, including bovine serum albumin and avidin.101 For BSA, MIP-based sensors on Au nanoparticles displayed voltammetric responses in the range of 1 × 10−11 to 1.0 × 10−5 g mL−1, while avidin detection using 4MPBA-modified Au nanoparticles relied on binding affinity through boronate ester bonds. Displacement sensors utilizing glycan-coated electrodes and redox markers like FcBA showed reduced signals upon binding to Con A or E. coli, indicating their utility in biosensing applications.102
Additionally, PBA-based sensors have been developed for bleomycin detection using oligonucleotide-modified electrodes. The sensors exhibited impedimetric and voltammetric responses due to the cleavage of oligonucleotide chains by bleomycin.107 PBA-modified Au nanoparticles and graphene-based electrodes demonstrated redox responses, with detection limits as low as 0.2 × 10−6 M.108
Furthermore, PBA-modified electrodes have been used for acetyl cholinesterase-based sensors, detecting organophosphorus and carbamate insecticides at low ppb levels through enzymatic oxidation of thiocholine. Studies on FcBA derivatives have shown their potential in constructing reagentless sensors for compounds like salicylic acid,109 fructosyl valine,110 catechin,111 and hypochlorite ions112 based on changes in electrochemical properties.
In conclusion, the integration of PBA-modified sensors across voltammetric, potentiometric, glycoprotein, and miscellaneous platforms underscore their versatility and potential in biosensing. These systems leverage the reversible and specific binding of PBA to diols and saccharides, enabling real-time detection of biomolecules with high sensitivity and selectivity. As a result, they play a key role in advancing biomedical diagnostics and real-time health monitoring.
6.3 Drug delivery for comorbid conditions
Recent advancements in CI include cytokine therapy, immune checkpoint blockade (ICB), and dendritic cell therapy. ICB, particularly, has revolutionized cancer treatment by targeting co-inhibitory agents such as programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1) and CTLA-4.116 This strategy has demonstrated remarkable success against cancers like melanoma, breast cancer, lung cancer, and bladder cancer.117 Another significant approach is adoptive cell transfer, which utilizes receptor-modified T cells and CAR-T or CAR-NK cells for targeted and efficient elimination of cancer cells.118–120 Despite the promising benefits of CI, challenges like immune evasion, antigenic variation, and reduced cell persistence persist, limiting its efficacy in clinical trials.
To address these challenges, researchers have integrated advanced materials like nanoparticles and biomaterials with immune cells. Among these, PBA-mediated materials have gained significant attention. The reversible boronate ester bonds of PBA allow for controlled binding and release of cells, enhancing the targeting precision in cancer immunotherapy. However, the oxidative instability of PBA under physiological conditions poses challenges. To mitigate this, derivatives such as oxaborolones and PBA complexes with citrate, cysteine, and PEG have been developed, offering improved oxidative stability while retaining their ability for dynamic covalent bonding with glycans and amino acid residues.121 These innovations make PBA-based materials valuable for applications in drug delivery, cancer immunotherapy, and cell spheroid formation.
PBA-mediated polymers and nanoparticles have emerged as effective drug carriers due to their stability, biocompatibility, and long circulation times.122,123 These materials enhance immune responses while reducing systemic adverse effects.124 For instance, in pancreatic cancer, where myeloid-derived suppressor cells (MDSCs) significantly contribute to tumor immunosuppression, PBA-functionalized polymeric nanoparticles formulated with chemotherapy drugs have been engineered.125 These nanoparticles inhibit the recruitment of MDSCs by targeting the P-selectin/PSGL-1 pathway, effectively reducing tumor progression. Biocompatibility studies in healthy mice further confirmed the safety of these nanoparticles, highlighting their potential in CI.
One of the most significant breakthroughs in CI involves targeting immune checkpoints like programmed death-1 and programmed death-ligand 1 (PD-L1). PD-L1, a critical player in cancer immune evasion, has become a key therapeutic target in solid cancers.126,127 While antibodies targeting PD-L1 have shown success, their limitations include poor intra-tumoral accumulation and rapid clearance.128 To address these challenges, PBA-based nanocomplexes have been developed to enhance antibody stability and delivery. These complexes protect antibodies from degradation, prolong their circulation time, and facilitate tumor-specific accumulation. In vivo studies demonstrated that PBA–antibody nanocomplexes significantly increased T cell infiltration into tumors, enhancing antitumor immune responses.129
PBA-based materials have also been employed in gene-editing approaches for cancer therapy. For example, PBA-functionalized tecto dendrimers combined with gold nanoparticles were used for CRISPR/Cas9-mediated PD-L1 blockade.130 This approach demonstrated superior tumor suppression compared to commercial anti-PD-L1 therapies and activated robust T-cell-mediated immune responses. Moreover, PBA-based micelle-like nanoparticles loaded with chemotherapeutic agents like doxorubicin have shown promise in remodeling the tumor immune microenvironment, further enhancing chemoimmunotherapy outcomes.70
In conclusion, cancer immunotherapy continues to advance steadily, opening new avenues for effective and personalized cancer treatment. Among emerging technologies, PBA-mediated materials stand out due to their unique chemical reactivity, reversible diol binding, and structural tunability. These properties confer distinct advantages over traditional immunotherapeutic platforms, such as enhanced antibody stability, improved delivery precision, and responsive modulation of the tumor microenvironment. Unlike conventional methods that often face limitations in targeting efficiency and systemic toxicity, PBA-based systems offer superior control, selectivity, and adaptability under physiological conditions (Table 1). As a result, they present a transformative approach for overcoming major obstacles in current immunotherapy. Continued research and development in this area are essential for harnessing the full clinical potential of PBA-mediated strategies, ultimately leading to more effective and durable outcomes for cancer patients worldwide.
Traditional approaches for addressing this challenge include covalent conjugation of siRNA to homing polymers,134–138 the incorporation of hydrophobic moieties to reinforce core aggregation,139,140 and disulfide cross-linking of the micelle core.141,142 Although these methods are effective, they often lead to highly complex structures and preparation processes, which can limit their practicality and scalability.
An innovative solution has emerged using PBA functionalities, which provide a sophisticated yet simplified method for stabilizing PIC micelles.143 This approach exploits the unique characteristics of PBA, which enables chemical conjugation with the ribose at the 3′-ends of siRNA strands. This interaction not only stabilizes the micelle but also facilitates intermolecular cross-linking through the bis-bidentate ribose arrangement, enhancing the structural integrity of the complex. Moreover, PBA exhibits reversible changes in hydrophobicity depending on the degree of acid dissociation144 and ribose concentration, allowing the micelle stability to be finely tuned for extracellular and intracellular conditions.
To demonstrate this approach, researchers developed poly(ethylene glycol)-block-poly(L-lysine) (PEG-b-PLys) polymers, which were modified with 3-fluoro-4-carboxyphenylboronic acid (FPBA) to varying extents. The PEG segment had a weight-average molecular weight of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, while the poly(L-lysine) block had a mean degree of polymerization of 42 (Fig. 5). Systematic optimization of the degree of PBA modification and the polymer-to-siRNA mixing ratio resulted in PIC micelles that were stable under extracellular conditions but disrupted in response to intracellular adenosine triphosphate concentrations, facilitating siRNA release.
000, while the poly(L-lysine) block had a mean degree of polymerization of 42 (Fig. 5). Systematic optimization of the degree of PBA modification and the polymer-to-siRNA mixing ratio resulted in PIC micelles that were stable under extracellular conditions but disrupted in response to intracellular adenosine triphosphate concentrations, facilitating siRNA release.
 | ||
| Fig. 5 Schematic illustration of the phenylboronic acid modified block copolymer assemblies for ATP-responsive siRNA delivery. Reproduced with permission from ref. 143. Copyright 2012 Wiley-VCH Verlag GmbH. | ||
Preliminary experiments revealed that these PBA-functionalized micelles could effectively silence the proto-oncogene polo-like kinase 1 in human renal carcinoma cells. The micelles exhibited a dose-dependent silencing effect with minimal cytotoxicity, demonstrating their potential for selective siRNA delivery. By integrating stabilization and controlled release mechanisms into a single, straightforward system, this PBA-based approach holds significant promise for advancing siRNA therapeutics.
6.4 Stability considerations across PBA-based applications
The stability of PBA-based platforms is a critical factor influencing their performance in a range of biomedical applications, including glucose-responsive insulin delivery, diabetic wound healing, and cancer drug delivery. In glucose-responsive insulin delivery systems, it is essential to maintain the structural integrity and appropriate mechanical properties of hydrogels145 or nanoparticles146 to ensure controlled insulin release and prevent premature leakage. Rheological studies offer valuable insights into the viscoelastic behavior and mechanical robustness of these hydrogels under physiological conditions, guiding formulation optimization. Similarly, the surface charge and colloidal stability of PBA-functionalized nanoparticles, commonly assessed through zeta potential analysis, are pivotal in determining their dispersion stability, cellular interactions, and circulation time in vivo. Furthermore, cell viability assays such as MTT or XTT are widely employed to evaluate the cytocompatibility of PBA-based hydrogels and nanoparticles, ensuring their safety for biomedical use.147 Notably, these materials often exhibit a storage modulus (G′) greater than the loss modulus (G′′), indicating a predominantly elastic and stable gel network.148 They also typically exhibit zeta potential values exceeding ±30 mV, indicating strong electrostatic repulsion and stable colloidal behavior, as well as excellent cytocompatibility.149,150 These characteristics collectively support their suitability for in vivo applications.However, the dynamic nature of boronate ester bonds, which respond to glucose and pH fluctuations, can lead to undesired dissociation and degradation of the materials under physiological conditions.151 To address this, researchers have developed multi-crosslinking strategies that combine reversible boronate ester linkages with more stable covalent or hydrogen bonds.152–155 The incorporation of robust polymeric backbones, such as PEG156 or HA derivatives,157 further enhances mechanical strength and enzymatic resistance, thereby extending the functional lifespan of these delivery systems.
In diabetic wound healing, PBA-based hydrogels must function effectively in a challenging microenvironment characterized by fluctuating pH, elevated glucose levels, ROS, and heightened enzymatic activity.158–160 To maintain both responsiveness and structural integrity under these conditions, multi-stimuli-responsive designs are often employed, integrating ROS-sensitive components coupled with boronate ester moieties.161,162 Hybrid crosslinking networks that combine dynamic and covalent bonds further enhance mechanical stability, enabling prolonged drug release and sustained wound coverage.162 Rheological characterization in this context is particularly important to ensure the hydrogel retains sufficient mechanical strength and injectability throughout the treatment duration.
For cancer drug delivery, materials must remain stable in systemic circulation while enabling selective drug release within the acidic and enzyme-rich tumor microenvironment. PBA-based micelles and nanoparticles typically utilize hydrophobic cores to shield boronate ester bonds and encapsulated drugs from premature degradation.163,164 Their surfaces are often modified with hydrophilic polymers such as PEG to enhance colloidal stability and extend circulation time.50,165 Zeta potential analysis plays a critical role here as well, confirming favorable surface charge profiles that minimize aggregation and improve biocompatibility. Multi-responsive systems capable of responding to pH, ROS, and glucose stimuli enable precise, site-specific drug release while preserving carrier integrity.
Overall, achieving a balance between responsiveness and structural stability is essential for optimizing PBA-based platforms. Advanced material designs that integrate dual or multiple crosslinking mechanisms, employ robust polymer backbones, and incorporate multi-stimuli sensitivity represent promising strategies to address stability challenges and improve the clinical translation of PBA-based biomedical applications.
7. Conclusion and future perspectives
In conclusion, PBA derivatives have demonstrated remarkable potential across a broad spectrum of scientific and biomedical applications. Their ability to selectively bind with saccharides, particularly glucose, has positioned PBA as a key player in the development of glucose-responsive insulin delivery systems, contributing significantly to diabetes management. From bulk hydrogels to micro/nanogels and self-assembled micelles, PBA-based materials have shown promising results in achieving controlled and efficient insulin release, enhancing glycemic regulation, and offering a potential solution for long-term diabetes care.Looking ahead, the future of PBA-based systems holds immense promise for further innovation and broader applications. The continued exploration of PBA derivatives in drug delivery, particularly for comorbid conditions like cancer immunotherapy, suggests new avenues for targeted therapies with reduced side effects. Additionally, the integration of PBA with polymers in biosensing and diagnostic platforms may lead to the development of more sensitive, accurate, and real-time monitoring systems for various health conditions. The potential for environment-sensitive siRNA delivery based on PBA highlights its application in personalized medicine, offering precise therapeutic interventions tailored to specific biological environments.
Moreover, future research should focus on refining PBA-based platforms to improve biocompatibility, minimize toxicity, and enhance responsiveness under diverse physiological conditions. In parallel, immunological implications warrant careful attention, especially for applications involving chronic or repeated use. While many studies demonstrate short-term safety, the potential for long-term immune activation, foreign body responses, or adaptive immunogenicity remains insufficiently explored. Thus, future studies should prioritize comprehensive immunotoxicity assessments and long-term in vivo evaluations to ensure the sustained safety of PBA-based systems. Integrating PBA with other responsive agents, such as glucose oxidase or smart polymers, offers new avenues for developing multifunctional, adaptable platforms. As PBA technologies continue to mature, their reach will likely expand well beyond insulin delivery into wound healing, biosensing, and complex drug delivery applications, paving the way for more effective, personalized, and responsive therapies.
Ultimately, PBA derivatives hold transformative potential, advancing biomedical research and offering innovative solutions to complex clinical challenges. Continued interdisciplinary research and development will ensure that PBA-based systems lead the way in biomedical development, fostering more effective, personalized, and responsive therapeutic strategies.
Conflicts of interest
The authors declare no conflict of interest.Data availability
Data will be available upon request from the corresponding author.References
- S. Gao, Y. Zhang and R. Zhou, et al., Boronic acid-assisted detection of bacterial pathogens: Applications and perspectives, Coord. Chem. Rev., 2024, 518, 216082 CrossRef CAS.
- B. C. Das, P. Chokkalingam and P. Masilamani, et al., Stimuli-responsive boron-based materials in drug delivery, Int. J. Mol. Sci., 2023, 24(3), 2757 CrossRef CAS PubMed.
- A. Matsumoto and Y. Miyahara, ‘Borono-lectin'based engineering as a versatile platform for biomedical applications, Sci. Technol. Adv. Mater., 2018, 19(1), 18–30 CrossRef CAS PubMed.
- K. Kataoka, H. Miyazaki and M. Bunya, et al., Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on− off regulation of insulin release, J. Am. Chem. Soc., 1998, 120(48), 12694–12695 CrossRef CAS.
- Y. Zhang, Y. Guan and S. Zhou, Synthesis and volume phase transitions of glucose-sensitive microgels, Biomacromolecules, 2006, 7(11), 3196–3201 CrossRef CAS PubMed.
- T. Hoare and R. Pelton, Engineering glucose swelling responses in poly (N-isopropylacrylamide)-based microgels, Macromolecules, 2007, 40(3), 670–678 CrossRef CAS.
- T. Hoare and R. Pelton, Charge-switching, amphoteric glucose-responsive microgels with physiological swelling activity, Biomacromolecules, 2008, 9(2), 733–740 CrossRef CAS PubMed.
- A. Matsumoto, T. Kurata and D. Shiino, et al., Swelling and shrinking kinetics of totally synthetic, glucose-responsive polymer gel bearing phenylborate derivative as a glucose-sensing moiety, Macromolecules, 2004, 37(4), 1502–1510 CrossRef CAS.
- D. Kumarasamy, M. K. Ghosh and T. K. Giri, Polymer-based responsive hydrogel for drug delivery, Polymer Gels: Perspectives and Applications, 2018, pp. 1–25 Search PubMed.
- J. N. Cambre and B. S. Sumerlin, Biomedical applications of boronic acid polymers, Polymer, 2011, 52(21), 4631–4643 CrossRef CAS.
- S. Kitano, K. Kataoka and Y. Koyama, et al., Glucose–responsive complex formation between poly (vinyl alcohol) and poly (N–vinyl–2−pyrrolidone) with pendent phenylboronic acid moieties, Macromol. Chem. Rapid Commun., 1991, 12(4), 227–233 CrossRef CAS.
- S. Kitano, Y. Koyama and K. Kataoka, et al., A novel drug delivery system utilizing a glucose responsive polymer complex between poly (vinyl alcohol) and poly (N-vinyl-2-pyrrolidone) with a phenylboronic acid moiety, J. Controlled Release, 1992, 19(1–3), 161–170 CrossRef CAS.
- S. Kitano, I. Hisamitsu and Y. Koyama, et al., Effect of the incorporation of amino groups in a glucose–responsive polymer complex having phenylboronic acid moieties, Polym. Adv. Technol., 1991, 2(5), 261–264 CrossRef CAS.
- I. Hisamitsu, K. Kataoka and T. Okano, et al., Glucose-responsive gel from phenylborate polymer and poly (vinyl alcohol): prompt response at physiological pH through the interaction of borate with amino group in the gel, Pharm. Res., 1997, 14, 289–293 CrossRef CAS PubMed.
- Y. Kotsuchibashi, R. V. C. Agustin and J.-Y. Lu, et al., Temperature, pH, and glucose responsive gels via simple mixing of boroxole-and glyco-based polymers, ACS Macro Lett., 2013, 2(3), 260–264 CrossRef CAS PubMed.
- H. Ge, Y. Ding and C. Ma, et al., Temperature-controlled release of diols from N-isopropylacrylamide-co-acrylamidophenylboronic acid microgels, J. Phys. Chem. B, 2006, 110(41), 20635–20639 CrossRef CAS PubMed.
- V. Lapeyre, I. Gosse and S. Chevreux, et al., Monodispersed glucose-responsive microgels operating at physiological salinity, Biomacromolecules, 2006, 7(12), 3356–3363 CrossRef CAS PubMed.
- V. Lapeyre, C. Ancla and B. Catargi, et al., Glucose-responsive microgels with a core–shell structure, J. Colloid Interface Sci., 2008, 327(2), 316–323 CrossRef CAS PubMed.
- G. Zenkl, T. Mayr and I. Klimant, Sugar–responsive fluorescent nanospheres, Macromol. Biosci., 2008, 8(2), 146–152 CrossRef CAS.
- D. Roy, J. N. Cambre and B. S. Sumerlin, Sugar-responsive block copolymers by direct RAFT polymerization of unprotected boronic acid monomers, Chem. Commun., 2008, 21, 2477–2479 RSC.
- D. Roy and B. S. Sumerlin, Glucose-sensitivity of boronic acid block copolymers at physiological pH, ACS Macro Lett., 2012, 1(5), 529–532 CrossRef CAS.
- Y. Yao, X. Wang and T. Tan, et al., A facile strategy for polymers to achieve glucose-responsive behavior at neutral pH, Soft Matter, 2011, 7(18), 7948–7951 RSC.
- Y. Yao, L. Zhao and J. Yang, et al., Glucose-responsive vehicles containing phenylborate ester for controlled insulin release at neutral pH, Biomacromolecules, 2012, 13(6), 1837–1844 CrossRef CAS PubMed.
- J. N. Cambre, D. Roy and B. S. Sumerlin, Tuning the sugar–response of boronic acid block copolymers, J. Polym. Sci., Part A: Polym. Chem., 2012, 50(16), 3373–3382 CrossRef CAS.
- L. Zhao, J. Ding and C. Xiao, et al., Glucose-sensitive polypeptide micelles for self-regulated insulin release at physiological pH, J. Mater. Chem., 2012, 22(24), 12319–12328 RSC.
- W. Zhang, Z. Yang and M. Zhang, et al., A hybrid hydrogel constructed using drug loaded mesoporous silica and multiple response copolymer as an intelligent dressing for wound healing of diabetic foot ulcers, J. Mater. Chem. B, 2023, 11(22), 4922–4933 RSC.
- Y. Liu, T. Liu and Z. Zhu, et al., An advanced hydrogel dressing system with progressive delivery and layer-to-layer response for diabetic wound healing, Acta Biomater., 2024, 190, 79–94 CrossRef CAS PubMed.
- Q. Wang, I. Kaminska and J. Niedziolka-Jonsson, et al., Sensitive sugar detection using 4-aminophenylboronic acid modified graphene, Biosens. Bioelectron., 2013, 50, 331–337 CrossRef CAS.
- M. Darvishi, F. Tosan and P. Nakhaei, et al., Recent progress in cancer immunotherapy: Overview of current status and challenges, Pathol., Res. Pract., 2023, 241, 154241 CrossRef CAS PubMed.
- C.-C. Song, R. Ji and F.-S. Du, et al., Oxidation-accelerated hydrolysis of the ortho ester-containing acid-labile polymers, ACS Macro Lett., 2013, 2(3), 273–277 CrossRef CAS PubMed.
- H. He, Y. Chen and Y. Li, et al., Effective and selective anti–cancer protein delivery via all–functions–in–one nanocarriers coupled with visible light–responsive, reversible protein engineering, Adv. Funct. Mater., 2018, 28(14), 1706710 CrossRef.
- T. T. Hoang, T. P. Smith and R. T. Raines, A Boronic Acid Conjugate of Angiogenin that Shows ROS–Responsive Neuroprotective Activity, Angew. Chem., 2017, 129(10), 2663–2666 CrossRef.
- M. Piest and J. F. Engbersen, Role of boronic acid moieties in poly (amido amine) s for gene delivery, J. Controlled Release, 2011, 155(2), 331–340 CrossRef CAS PubMed.
- X. Liu, J. Xiang and D. Zhu, et al., Fusogenic reactive oxygen species triggered charge–reversal vector for effective gene delivery, Adv. Mater., 2016, 28(9), 1743–1752 CrossRef CAS PubMed.
- J. Xiang, X. Liu and Z. Zhou, et al., Reactive oxygen species (ROS)-responsive charge-switchable nanocarriers for gene therapy of metastatic cancer, ACS Appl. Mater. Interfaces, 2018, 10(50), 43352–43362 CrossRef CAS PubMed.
- D. Zhu, H. Yan and X. Liu, et al., Intracellularly disintegratable polysulfoniums for efficient gene delivery, Adv. Funct. Mater., 2017, 27(16), 1606826 CrossRef.
- Z. Zhang, Q. Wang and Q. Liu, et al., Dual–locking nanoparticles disrupt the PD–1/PD–L1 pathway for efficient cancer immunotherapy, Adv. Mater., 2019, 31(51), 1905751 CrossRef CAS PubMed.
- M. Naito, T. Ishii and A. Matsumoto, et al., A phenylboronate–functionalized polyion complex micelle for ATP–triggered release of siRNA, Angew. Chem., Int. Ed., 2012, 51(43), 10751–10755 CrossRef CAS.
- I. P. Beletskaya, F. Alonso and V. Tyurin, The Suzuki-Miyaura reaction after the Nobel prize, Coord. Chem. Rev., 2019, 385, 137–173 CrossRef CAS.
- K. A. Kurnia, W. Setyaningsih and N. Darmawan, et al., A comprehensive study on the impact of the substituent on pKa of phenylboronic acid in aqueous and non-aqueous solutions: A computational approach, J. Mol. Liq., 2021, 326, 115321 CrossRef CAS.
- M. Dowlut and D. G. Hall, An improved class of sugar-binding boronic acids, soluble and capable of complexing glycosides in neutral water, J. Am. Chem. Soc., 2006, 128(13), 4226–4227 CrossRef CAS PubMed.
- J. Yan, G. Springsteen and S. Deeter, et al., The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—it is not as simple as it appears, Tetrahedron, 2004, 60(49), 11205–11209 CrossRef CAS.
- S. A. Valenzuela, J. R. Howard and H. M. Park, et al., 11B NMR spectroscopy: structural analysis of the acidity and reactivity of phenyl boronic acid–diol condensations, J. Org. Chem., 2022, 87(22), 15071–15076 CrossRef CAS PubMed.
- S. E. Bodman, C. Breen and F. Plasser, et al., Impact of varying the phenylboronic acid position in macrocyclic Eu (iii) complexes on the recognition of adenosine monophosphate, Org. Chem. Front., 2022, 9(20), 5494–5504 RSC.
- W. L. Brooks, C. C. Deng and B. S. Sumerlin, Structure–reactivity relationships in boronic acid–diol complexation, ACS Omega, 2018, 3(12), 17863–17870 CrossRef CAS PubMed.
- J. Jian, R. Hammink and C. J. Mckenzie, et al., Probing the Lewis Acidity of Boronic Acids through Interactions with Arene Substituents, Chem. – Eur. J., 2022, 28(9), e202104044 CrossRef CAS.
- A. Adamczyk-Woźniak and A. Sporzyński, The influence of ortho-substituents on the properties of phenylboronic acids [J, J. Organomet. Chem., 2020, 913, 121202 CrossRef.
- Q. Li, C. Lü and Z. Liu, Preparation and characterization of fluorophenylboronic acid-functionalized monolithic columns for high affinity capture of cis-diol containing compounds [J, J. Chromatogr., A, 2013, 1305, 123–130 CrossRef CAS PubMed.
- A. Adamczyk-Woźniak, M. K. Cabaj and P. M. Dominiak, et al., The influence of fluorine position on the properties of fluorobenzoxaboroles, Bioorg. Chem., 2015, 60, 130–135 CrossRef.
- A. K. Jangid and K. Kim, Phenylboronic acid-functionalized biomaterials for improved cancer immunotherapy via sialic acid targeting, Adv. Colloid Interface Sci., 2024, 103301 CrossRef CAS PubMed.
- R. Scorei, Is boron a prebiotic element? A mini-review of the essentiality of boron for the appearance of life on earth, Origins Life Evol. Biospheres, 2012, 42, 3–17 CrossRef CAS PubMed.
- J. A. Peters, Interactions between boric acid derivatives and saccharides in aqueous media: Structures and stabilities of resulting esters, Coord. Chem. Rev., 2014, 268, 1–22 CrossRef CAS.
- A. Matsumoto, M. Tanaka and H. Matsumoto, et al., Synthetic “smart gel” provides glucose-responsive insulin delivery in diabetic mice, Sci. Adv., 2017, 3(11), eaaq0723 CrossRef PubMed.
- B. Wang, R. Ma and G. Liu, et al., Effect of Coordination on the Glucose–Responsiveness of PEG–b–(PAA–co–PAAPBA) Micelles, Macromol. Rapid Commun., 2010, 31(18), 1628–1634 CrossRef PubMed.
- W. Yuan, L. Li and H. Zou, Thermo-and glucose-responsive micelles self-assembled from phenylborate ester-containing brush block copolymer for controlled release of insulin at physiological pH, RSC Adv., 2015, 5(98), 80264–80268 RSC.
- J. Zhang, X. Wei and W. Liu, et al., Week-long normoglycaemia in diabetic mice and minipigs via a subcutaneous dose of a glucose-responsive insulin complex, Nat. Biomed. Eng., 2024, 8(10), 1214–1225 CrossRef CAS PubMed.
- K. O. Boakye-Yiadom, Q. Chen and Y. Teng, et al., Injectable Gelled Multiple Emulsion for Glucose–Responsive Insulin Delivery, Adv. Healthcare Mater., 2024, 13(26), 2304195 CrossRef CAS PubMed.
- D. H.-C. Chou, M. J. Webber and B. C. Tang, et al., Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(8), 2401–2406 CrossRef CAS.
- Y. Lu, H. Yu and L. Wang, et al., Recent advances in the smart insulin delivery systems for the treatment of diabetes, Eur. Polym. J., 2021, 161, 110829 CrossRef CAS.
- X. Chen, H. Yu and L. Wang, et al., Microneedle patch prepared from a hydrogel by a mild method for insulin delivery, ChemNanoMat, 2021, 7(11), 1230–1240 CrossRef CAS.
- X. Chen, H. Yu and L. Wang, et al., Cross-linking-density-changeable microneedle patch prepared from a glucose-responsive hydrogel for insulin delivery, ACS Biomater. Sci. Eng., 2021, 7(10), 4870–4882 CrossRef CAS.
- Y. Fu, P. Liu and M. Chen, et al., On-demand transdermal insulin delivery system for type 1 diabetes therapy with no hypoglycemia risks, J. Colloid Interface Sci., 2022, 605, 582–591 CrossRef CAS PubMed.
- Z. Ye, Y. Xiang and T. Monroe, et al., Polymeric microneedle arrays with glucose-sensing dynamic-covalent bonding for insulin delivery, Biomacromolecules, 2022, 23(10), 4401–4411 CrossRef CAS PubMed.
- X. Chen, H. Yu and L. Wang, et al., Glucose–Responsive Microneedle Patch With Variable Crosslinking Density and Flexible Core–Shell Structure for Insulin Delivery, Adv. Mater. Technol., 2023, 8(13), 2202124 CrossRef CAS.
- G. P. Carino and E. Mathiowitz, Oral insulin delivery, Adv. Drug Delivery Rev., 1999, 35(2–3), 249–257 CrossRef CAS PubMed.
- E. M. Pridgen, F. Alexis and T. T. Kuo, et al., Transepithelial transport of Fc-targeted nanoparticles by the neonatal fc receptor for oral delivery, Sci. Transl. Med., 2013, 5(213), 213ra167–213ra167 Search PubMed.
- J. Yu, Y. Zhang and J. Wang, et al., Glucose-responsive oral insulin delivery for postprandial glycemic regulation, Nano Res., 2019, 12, 1539–1545 CrossRef CAS.
- Y. Xiong, M. Li and G. Qing, Biomolecule–responsive polymers and their bio–applications, Interdiscip. Mater., 2024, 3, 865–896 CAS.
- J. Wang, Z. Wang and J. Yu, et al., Glucose–responsive insulin and delivery systems: innovation and translation, Adv. Mater., 2020, 32(13), 1902004 CrossRef CAS PubMed.
- L. Wang, S. He and R. Liu, et al., A pH/ROS dual-responsive system for effective chemoimmunotherapy against melanoma via remodeling tumor immune microenvironment, Acta Pharm. Sin. B, 2024, 14(5), 2263–2280 CrossRef CAS PubMed.
- Y. Wang, L. Zhang and X. Zhang, et al., Precise polymerization of a highly tumor microenvironment-responsive nanoplatform for strongly enhanced intracellular drug release, ACS Appl. Mater. Interfaces, 2016, 8(9), 5833–5846 CrossRef CAS PubMed.
- J. Wang, Z. Wang and G. Chen, et al., Injectable biodegradable polymeric complex for glucose-responsive insulin delivery, ACS Nano, 2021, 15(3), 4294–4304 CrossRef CAS PubMed.
- J. Wang, J. Yu and Y. Zhang, et al., Charge-switchable polymeric complex for glucose-responsive insulin delivery in mice and pigs, Sci. Adv., 2019, 5(7), eaaw4357 CrossRef CAS PubMed.
- W. Yin, Y. Wang and Y. Xiao, et al., Phenylboronic acid conjugated mPEG-b-PCL micelles as DOX carriers for enhanced drug encapsulation and controlled drug release, Eur. Polym. J., 2022, 173, 111235 CrossRef CAS.
- J. Wang, L. G. Liu and W.-Q. Jiao, et al., Phenylboronic acid-conjugated chitosan nanoparticles for high loading and efficient delivery of curcumin, Carbohydr. Polym., 2021, 256, 117497 CrossRef CAS PubMed.
- X. Wang, H. Tang and C. Wang, et al., Phenylboronic acid-mediated tumor targeting of chitosan nanoparticles, Theranostics, 2016, 6(9), 1378 CrossRef CAS.
- Z. Tong, J. Zhou and J. Zhong, et al., Glucose-and H2O2-responsive polymeric vesicles integrated with microneedle patches for glucose-sensitive transcutaneous delivery of insulin in diabetic rats, ACS Appl. Mater. Interfaces, 2018, 10(23), 20014–20024 CrossRef CAS.
- B. Zhao, S. Zhu and Y. Liu, et al., Enriching and Smart Releasing Curcumin via Phenylboronic Acid–Anchored Bioinspired Hydrogel for Diabetic Wound Healing, Adv. NanoBiomed Res., 2023, 3(5), 2200177 CrossRef CAS.
- S. Xiong, X. Ding and L. Zhou, et al., An antibacterial and antioxidant rosmarinic acid hydrogel normalizes macrophage polarization to expedite diabetic wound healing, J. Colloid Interface Sci., 2024, 683, 357–371 CrossRef PubMed.
- Y. Li, H. Gong and T. Gan, et al., Smart Hydrogel Dressing Enhances the Healing of Chronic Infectious Diabetic Wounds through Dual-Barrier Drug Delivery Action, Biomacromolecules, 2024, 25(10), 6814–6829 CrossRef CAS.
- F. Chen, J. Qin and P. Wu, et al., Glucose–Responsive Antioxidant Hydrogel Accelerates Diabetic Wound Healing, Adv. Healthcare Mater., 2023, 12(21), 2300074 CrossRef CAS.
- W. Zhang, K. Zha and Y. Xiong, et al., Glucose-responsive, antioxidative HA-PBA-FA/EN106 hydrogel enhanced diabetic wound healing through modulation of FEM1b-FNIP1 axis and promoting angiogenesis, Bioact. Mater., 2023, 30, 29–45 CAS.
- S. Takahashi and J.-I. Anzai, Phenylboronic acid monolayer-modified electrodes sensitive to sugars, Langmuir, 2005, 21(11), 5102–5107 CrossRef CAS.
- H.-C. Wang, H. Zhou and B. Chen, et al., A bis-boronic acid modified electrode for the sensitive and selective determination of glucose concentrations, Analyst, 2013, 138(23), 7146–7151 RSC.
- E. Shoji and M. S. Freund, Potentiometric saccharide detection based on the p K a changes of poly (aniline boronic acid), J. Am. Chem. Soc., 2002, 124(42), 12486–12493 CrossRef CAS PubMed.
- S. Aytaç, F. Kuralay and İ.H Boyacı, et al., A novel polypyrrole–phenylboronic acid based electrochemical saccharide sensor, Sens. Actuators, B, 2011, 160(1), 405–411 CrossRef.
- H. Çiftçi and U. Tamer, Functional gold nanorod particles on conducting polymer poly (3-octylthiophene) as non-enzymatic glucose sensor, React. Funct. Polym., 2012, 72(2), 127–132 CrossRef.
- A. Matsumoto, N. Sato and Y. Miyahara, Label free carbohydrate detection by using phenylboronic acid gate-modified field effect transistor, Curr. Appl. Phys., 2009, 9(4), e214–e2e7 CrossRef.
- A. Matsumoto, N. Sato and T. Sakata, et al., Glucose-sensitive field effect transistor using totally synthetic compounds, J. Solid State Electrochem., 2009, 13, 165–170 CrossRef CAS.
- A. Matsumoto, H. Matsumoto and Y. Maeda, et al., Simple and robust strategy for potentiometric detection of glucose using fluorinated phenylboronic acid self-assembled monolayer, Biochim. Biophys. Acta, Gen. Subj., 2013, 1830(9), 4359–4364 CrossRef CAS PubMed.
- S. Takahashi, N. Abiko and N. Haraguchi, et al., Voltammetric response of ferroceneboronic acid to diol and phenolic compounds as possible pollutants, J. Environ. Sci., 2011, 23(6), 1027–1032 CrossRef CAS.
- K. Lacina, J. Novotný and Z. Moravec, et al., Interaction of ferroceneboronic acid with diols at aqueous and non-aqueous conditions-signalling and binding abilities of an electrochemical probe for saccharides, Electrochim. Acta, 2015, 153, 280–286 CrossRef CAS.
- L. Liu, N. Xia and J. Du, et al., A simple and rapid method for probing of isomerization of glucose to fructose with ferroceneboronic acid, Int. J. Electrochem. Sci., 2013, 8(7), 9163–9170 CrossRef CAS.
- X. Wang, L. Zhang and F. Gu, et al., Investigation of the interaction between ferroceneboronic acid and sugars and its application in probing of enzyme activity, Int. J. Electrochem. Sci., 2013, 8(12), 12557–12565 CrossRef CAS.
- J. Li, Y.-Q. Sun and Y.-M. Wei, et al., Phenylboronic acid and dopamine as probe set for electrochemical detection of saccharides, Chin. Chem. Lett., 2013, 24(4), 291–294 CrossRef CAS.
- M. Saleem, H. Yu and L. Wang, et al., Study on synthesis of ferrocene-based boronic acid derivatives and their saccharides sensing properties, J. Electroanal. Chem., 2016, 763, 71–78 CrossRef CAS.
- M. Shao and Y. Zhao, Phenylboronic acid-functionalized TTFAQ: modular synthesis and electrochemical recognition for saccharides, Tetrahedron Lett., 2010, 51(18), 2508–2511 CrossRef.
- E. Lenters-Westra, R. K. Schindhelm and H. J. Bilo, et al., Haemoglobin A1c: Historical overview and current concepts, Diabetes Res. Clin. Pract., 2013, 99(2), 75–84 CrossRef CAS PubMed.
- A. Chopra, S. Rawat and V. Bhalla, et al., Point–of–Care Amperometric Testing of Diabetic Marker (HbA1c) Using Specific Electroactive Antibodies, Electroanalysis, 2014, 26(3), 469–472 CrossRef CAS.
- Y. Zhou, H. Dong and L. Liu, et al., Fabrication of electrochemical interface based on boronic acid-modified pyrroloquinoline quinine/reduced graphene oxide composites for voltammetric determination of glycated hemoglobin, Biosens. Bioelectron., 2015, 64, 442–448 CrossRef CAS PubMed.
- Y. Xing, L. Liu and D. Zhao, et al., Synthesis of water-dispersed ferrecene/phenylboronic acid-modified bifunctional gold nanoparticles and the application in biosensing, Materials, 2014, 7(8), 5554–5564 CrossRef PubMed.
- D. Dechtrirat, N. Gajovic-Eichelmann and F. Wojcik, et al., Electrochemical displacement sensor based on ferrocene boronic acid tracer and immobilized glycan for saccharide binding proteins and E. coli, Biosens. Bioelectron., 2014, 58, 1–8 CrossRef CAS PubMed.
- F. K. Sartain, X. Yang and C. R. Lowe, Holographic lactate sensor, Anal. Chem., 2006, 78(16), 5664–5670 CrossRef CAS PubMed.
- L. Frullano, J. Rohovec and S. Aime, et al., Towards targeted MRI: new MRI contrast agents for sialic acid detection, Chem. – Eur. J., 2004, 10(20), 5205–5217 CrossRef CAS PubMed.
- M. Zhong, Y. Teng and S. Pang, et al., Pyrrole–phenylboronic acid: A novel monomer for dopamine recognition and detection based on imprinted electrochemical sensor, Biosens. Bioelectron., 2015, 64, 212–218 CrossRef CAS PubMed.
- Z. Iskierko, M. Sosnowska and P. S. Sharma, et al., Extended-gate field-effect transistor (EG-FET) with molecularly imprinted polymer (MIP) film for selective inosine determination, Biosens. Bioelectron., 2015, 74, 526–533 CrossRef CAS.
- B. M. Zeglis, V. C. Pierre and J. K. Barton, Metallo-intercalators and metallo-insertors, Chem. Commun., 2007, 44, 4565–4579 RSC.
- X. He, W. Liu and X. Zhang, et al., Electrochemical determination of bleomycins based on dual-amplification of 4-mercaptophenyl boronic acid-capped gold nanoparticles and dopamine-capped gold nanoparticles, Anal. Methods, 2014, 6(17), 6893–6899 RSC.
- S. Takahashi, N. Haraguchi and N. Abiko, et al., Voltammetric determination of salicylic acid and derivatives based on ferroceneboronic acid, Sens. Lett., 2011, 9(5), 1845–1848 CrossRef CAS.
- H. C. Chien and T. C. Chou, A Nonenzymatic Amperometric Method for Fructosyl–Valine Sensing Using Ferroceneboronic Acid, Electroanalysis, 2011, 23(2), 402–408 CrossRef CAS.
- W. Shi, J. Wang and L. Zhu, et al., Electrochemical determination of catechin, protocatechuic acid, and L-lactic acid based on voltammetric response of ferroceneboronic acid, J. AOAC Int., 2014, 97(6), 1742–1745 CrossRef CAS PubMed.
- B. Wang and J.-I. Anzai, A facile electrochemical detection of hypochlorite ion based on ferrocene compounds [J, Int. J. Electrochem. Sci., 2015, 10(4), 3260–3268 CrossRef CAS.
- L. L. Cao and J. C. Kagan, Targeting innate immune pathways for cancer immunotherapy, Immunity, 2023, 56(10), 2206–2217 CrossRef CAS PubMed.
- S. Kim, S. Li and M. Gajendiran, et al., Lipid anchor-mediated NK cell surface engineering for enhanced cancer immunotherapy, Chem. Eng. J., 2023, 473, 145211 CrossRef CAS.
- A. K. Jangid, S. Kim and H. J. Kim, et al., Biomaterial-mediated exogenous facile coating of natural killer cells for enhancing anticancer efficacy toward hepatocellular carcinoma, Bioconjugate Chem., 2023, 34(10), 1789–1801 CrossRef CAS PubMed.
- Y. Takeuchi and H. Nishikawa, Roles of regulatory T cells in cancer immunity, Int. Immunol., 2016, 28(8), 401–409 CrossRef CAS PubMed.
- T. Powles, J. P. Eder and G. D. Fine, et al., MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer, Nature, 2014, 515(7528), 558–562 CrossRef CAS PubMed.
- M. Peipp, K. Klausz and A. S. Boje, et al., Immunotherapeutic targeting of activating natural killer cell receptors and their ligands in cancer, Clin. Exp. Immunol., 2022, 209(1), 22–32 CrossRef.
- F. Fang, W. Xiao and Z. Tian, NK cell-based immunotherapy for cancer; proceedings of the Seminars in immunology, 2017, 31, 37–54 CAS.
- S. Guedan, H. Calderon and A. D. Posey, et al., Engineering and design of chimeric antigen receptors, Mol. Ther.-Methods Clin. Dev., 2019, 12, 145–156 CrossRef CAS PubMed.
- B. J. Graham, I. W. Windsor and B. Gold, et al., Boronic acid with high oxidative stability and utility in biological contexts, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(10), e2013691118 CrossRef CAS PubMed.
- A. Matsumoto and S. Chen, A boronate gel-based synthetic platform for closed-loop insulin delivery systems, Polym. J., 2021, 53(12), 1305–1314 CrossRef CAS.
- Y. Li, G. Feng and J. Liu, et al., Progress in Glucose–Sensitive Hydrogels for Biomedical Applications, Macromol. Chem. Phys., 2023, 224(23), 2300257 CrossRef CAS.
- S. Hosseini, J. Mohammadnejad and S. Salamat, et al., Theranostic polymeric nanoparticles as a new approach in cancer therapy and diagnosis: a review, Mater. Today Chem., 2023, 29, 101400 CrossRef CAS.
- Y. Wu, M. Yi and M. Niu, et al., Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy, Mol. Cancer, 2022, 21(1), 184 CrossRef PubMed.
- A. Akinleye and Z. Rasool, Immune checkpoint inhibitors of PD-L1 as cancer therapeutics, J. Hematol. Oncol., 2019, 12(1), 92 CrossRef PubMed.
- A. De Sousa Linhares, C. Battin and S. Jutz, et al., Therapeutic PD-L1 antibodies are more effective than PD-1 antibodies in blocking PD-1/PD-L1 signaling, Sci. Rep., 2019, 9(1), 11472 CrossRef.
- J. Naidoo, D. Page and B. T. Li, et al., Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies, Ann. Oncol., 2015, 26(12), 2375–2391 CrossRef CAS PubMed.
- J. Lim, J. Lee and S. Jung, et al., Phenylboronic-acid-based nanocomplex as a feasible delivery platform of immune checkpoint inhibitor for potent cancer immunotherapy, J. Controlled Release, 2021, 330, 1168–1177 CrossRef CAS PubMed.
- J. Liu, G. Li and H. Guo, et al., Dual-responsive core–shell tecto dendrimers enable efficient gene editing of cancer cells to boost immune checkpoint blockade therapy, ACS Appl. Mater. Interfaces, 2023, 15(10), 12809–12821 CrossRef CAS.
- S. M. Elbashir, J. Harborth and W. Lendeckel, et al., Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells, Nature, 2001, 411(6836), 494–498 CrossRef CAS PubMed.
- K. A. Whitehead, R. Langer and D. G. Anderson, Knocking down barriers: advances in siRNA delivery, Nat. Rev. Drug Discovery, 2009, 8(2), 129–138 CrossRef CAS PubMed.
- N. Nishiyama and K. Kataoka, Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery, Pharmacol. Ther., 2006, 112(3), 630–648 CrossRef CAS PubMed.
- M. Oishi, Y. Nagasaki and K. Itaka, et al., Lactosylated poly (ethylene glycol)-siRNA conjugate through acid-labile β-thiopropionate linkage to construct pH-sensitive polyion complex micelles achieving enhanced gene silencing in hepatoma cells, J. Am. Chem. Soc., 2005, 127(6), 1624–1625 CrossRef CAS PubMed.
- D. B. Rozema, D. L. Lewis and D. H. Wakefield, et al., Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes, Proc. Natl. Acad. Sci. U. S. A., 2007, 104(32), 12982–12987 CrossRef CAS PubMed.
- M. Meyer, C. Dohmen and A. Philipp, et al., Synthesis and biological evaluation of a bioresponsive and endosomolytic siRNA− polymer conjugate, Mol. Pharm., 2009, 6(3), 752–762 CrossRef CAS PubMed.
- H. Mok, S. H. Lee and J. W. Park, et al., Multimeric small interfering ribonucleic acid for highly efficient sequence-specific gene silencing, Nat. Mater., 2010, 9(3), 272–278 CrossRef CAS PubMed.
- H. Takemoto, A. Ishii and K. Miyata, et al., Polyion complex stability and gene silencing efficiency with a siRNA-grafted polymer delivery system, Biomaterials, 2010, 31(31), 8097–8105 CrossRef PubMed.
- A. Alshamsan, A. Haddadi and V. Incani, et al., Formulation and delivery of siRNA by oleic acid and stearic acid modified polyethylenimine, Mol. Pharm., 2009, 6(1), 121–133 CrossRef CAS PubMed.
- W. J. Kim, L. V. Christensen and S. Jo, et al., Cholesteryl oligoarginine delivering vascular endothelial growth factor siRNA effectively inhibits tumor growth in colon adenocarcinoma, Mol. Ther., 2006, 14(3), 343–350 CrossRef CAS PubMed.
- S. Matsumoto, R. J. Christie and N. Nishiyama, et al., Environment-responsive block copolymer micelles with a disulfide cross-linked core for enhanced siRNA delivery, Biomacromolecules, 2009, 10(1), 119–127 CrossRef CAS PubMed.
- R. J. Christie, K. Miyata and Y. Matsumoto, et al., Effect of polymer structure on micelles formed between siRNA and cationic block copolymer comprising thiols and amidines, Biomacromolecules, 2011, 12(9), 3174–3185 CrossRef CAS PubMed.
- M. Naito, T. Ishii and A. Matsumoto, et al., A phenylboronate–functionalized polyion complex micelle for ATP–triggered release of siRNA, Angew. Chem., Int. Ed., 2012, 43(124), 10909–10913 CrossRef.
- A. Matsumoto, S. Ikeda and A. Harada, et al., Glucose-responsive polymer bearing a novel phenylborate derivative as a glucose-sensing moiety operating at physiological pH conditions, Biomacromolecules, 2003, 4(5), 1410–1416 CrossRef CAS PubMed.
- Y. Lu, H. Yu and L. Wang, et al., Preparation of phenylboronic acid–based glucose-responsive hydrogels and microneedles for regulated delivery of insulin, Eur. Polym. J., 2023, 192, 112061 CrossRef CAS.
- G. Cui, K. Zhao and K. You, et al., Synthesis and characterization of phenylboronic acid-containing polymer for glucose-triggered drug delivery+, Sci. Technol. Adv. Mater., 2020, 21(1), 1–10 CrossRef CAS PubMed.
- Y. Zhang, M. Wu and W. Dai, et al., Gold nanoclusters for controlled insulin release and glucose regulation in diabetes, Nanoscale, 2019, 11(13), 6471–6479 RSC.
- A. R. Mohanty, A. Ravikumar and N. A. Peppas, Recent advances in glucose-responsive insulin delivery systems: novel hydrogels and future applications, Regener. Biomater., 2022, 9, rbac056 CrossRef CAS PubMed.
- Y. Liu, Y. Wang and Y. Yao, et al., Glucose–responsive charge–switchable lipid nanoparticles for insulin delivery, Angew. Chem., Int. Ed., 2023, 62(20), e202303097 CrossRef CAS PubMed.
- R. Xu, S. K. Bhangu and K. C. Sourris, et al., An engineered nanosugar enables rapid and sustained glucose–responsive insulin delivery in diabetic mice, Adv. Mater., 2023, 35(21), 2210392 CrossRef CAS PubMed.
- S. Cho, S. Y. Hwang and D. X. Oh, et al., Recent progress in self-healing polymers and hydrogels based on reversible dynamic B–O bonds: boronic/boronate esters, borax, and benzoxaborole, J. Mater. Chem. A, 2021, 9(26), 14630–14655 RSC.
- F. Yu, X. Cao and J. Du, et al., Multifunctional hydrogel with good structure integrity, self-healing, and tissue-adhesive property formed by combining Diels–Alder click reaction and acylhydrazone bond, ACS Appl. Mater. Interfaces, 2015, 7(43), 24023–24031 CrossRef CAS PubMed.
- Z. Wei, J. H. Yang and Z. Q. Liu, et al., Novel biocompatible polysaccharide–based self–healing hydrogel, Adv. Funct. Mater., 2015, 25(9), 1352–1359 CrossRef CAS.
- Z. Jiang, A. Bhaskaran and H. M. Aitken, et al., Using synergistic multiple dynamic bonds to construct polymers with engineered properties, Macromol. Rapid Commun., 2019, 40(10), 1900038 CrossRef PubMed.
- S. Mukherjee, W. L. Brooks and Y. Dai, et al., Doubly-dynamic-covalent polymers composed of oxime and oxanorbornene links, Polym. Chem., 2016, 7(10), 1971–1978 RSC.
- V. Yesilyurt, M. J. Webber and E. A. Appel, et al., Injectable self-healing glucose-responsive hydrogels with pH-regulated mechanical properties, Adv. Mater., 2015, 28(1), 86 CrossRef PubMed.
- W. Shi, B. Hass and M. A. Kuss, et al., Fabrication of versatile dynamic hyaluronic acid-based hydrogels, Carbohydr. Polym., 2020, 233, 115803 CrossRef CAS PubMed.
- Y. Zhao, Y. Zhao and B. Xu, et al., Microenvironmental dynamics of diabetic wounds and insights for hydrogel-based therapeutics, J. Tissue Eng., 2024, 15, 1–26 Search PubMed.
- X. Du, B. Jia and W. Wang, et al., pH-switchable nanozyme cascade catalysis: a strategy for spatial–temporal modulation of pathological wound microenvironment to rescue stalled healing in diabetic ulcer, J. Nanobiotechnol., 2022, 20(1), 12 CrossRef CAS PubMed.
- Z. Shao, T. Yin and J. Jiang, et al., Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing, Bioact. Mater., 2023, 20, 561–573 CAS.
- Z. Xu, G. Liu and J. Huang, et al., Novel glucose-responsive antioxidant hybrid hydrogel for enhanced diabetic wound repair, ACS Appl. Mater. Interfaces, 2022, 14(6), 7680–7689 CrossRef CAS PubMed.
- Z. Xu, G. Liu and Q. Li, et al., A novel hydrogel with glucose-responsive hyperglycemia regulation and antioxidant activity for enhanced diabetic wound repair, Nano Res., 2022, 15(6), 5305–5315 CrossRef CAS.
- F. Lu, H. Zhang and W. Pan, et al., Delivery nanoplatforms based on dynamic covalent chemistry, Chem. Commun., 2021, 57(58), 7067–7082 RSC.
- S. Zhou, L. Dai and L. Pan, et al., Phenylboronic acid-modified nanoparticles for cancer treatment, Chem. Commun., 2025, 61(24), 4595–4605 RSC.
- S. Tiwari, J. Sarolia and V. Kansara, et al., Synthesis, colloidal characterization and targetability of phenylboronic acid functionalized α-tocopheryl polyethylene glycol succinate in cancer cells, Polymers, 2020, 12(10), 2258 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |



