Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparison of macromolecule permeation through extracellular matrix and hyaluronic acid to inform in vitro testing of subcutaneous therapies
Jana
Javorovic
a,
Belal I.
Hanafy
 b,
Frans
Franek
bc and
Driton
Vllasaliu
b,
Frans
Franek
bc and
Driton
Vllasaliu
 *a
*a
aInstitute of Pharmaceutical Science, School of Cancer and Pharmaceutical Science, King's College London, London SE1 9NH, UK. E-mail: driton.vllasaliu@kcl.ac.uk; Tel: +2078481728
bAdvanced Drug Delivery, Pharmaceutical Sciences, R&D, AstraZeneca, Cambridge, UK
cAdvanced Drug Delivery, Pharmaceutical Sciences, R&D, AstraZeneca, Gothenburg, Sweden
First published on 26th February 2025
Abstract
Subcutaneous injection is a widely used route of drug administration, but biopredictive in vitro tools for predicting in vivo bioavailability are not widely established. One such system, the subcutaneous injection site simulator (SCISSOR), incorporates hyaluronic acid (HA) as a model of the subcutaneous extracellular matrix (ECM), which dictates the diffusion of test compounds. However, the native ECM found is markedly more complex. Here for the first time, we compared the permeation of macromolecules with different physicochemical properties (molecular weight and charge) and model biological molecules across the HA hydrogel (used in SCISSOR) and an animal-derived basement membrane extract (BME), an ECM. We coated tissue culture inserts with these matrices as a simple experimental set up to test the permeation. The results show that the two matrices displayed similarities and some notable differences in their performance as barriers for macromolecules of different properties, suggesting that a simple experimental setup utilising biologically derived ECM may act as an inexpensive and accessible tool to predict the in vivo performance of biotherapeutics for SC administration.
1. Introduction
Subcutaneous (SC) injection is an established and well-tolerated route of drug administration which is becoming more commonly used due to the ongoing proliferation of biological therapies. The SC route can offer important advantages over intravenous administration, including the convenience of self-administration (therefore reduced healthcare burden), improved patient experience and an improved safety profile. Although SC administration of biotherapeutics is well-established, there remain important gaps in knowledge around basic aspects of SC delivery of biotherapeutics and development of associated drug delivery technologies. A key gap in knowledge relates to the understanding of the key factors affecting the fate and systemic bioavailability of different types of biologics and formulations following SC injection.1 An important contributing factor to this knowledge gap is the lack of predictive and accessible laboratory (preclinical) models for prediction of the rate and extent of drug absorption following SC injection.1The lack of in vitro models for predicting the fate and bioavailability of subcutaneously administered therapies means that the development of new therapies for SC administration relies on in vivo models. This is an issue for several reasons, including the questionable predictive value of different animal models for SC bioavailability prediction,2,3 as well as the unnecessary use of animals in research. Predictive in vitro methods that are accessible to researchers would accelerate the development of new therapies for SC administration, while reducing costs and the need for animal experiments.
The SC tissue is a complex mixture of cells (e.g. fibroblasts, macrophages and adipose cells), and extracellular matrix (ECM). Following SC injection, the drug or drug formulation is in close contact with the ECM, which will dictate the diffusion and absorption profile of the drug.4 It is generally accepted that understanding the interaction of the drug and/or formulation with the ECM is essential to the understanding of the biological performance of the subcutaneously administered therapies.4 Therefore, in vitro tools used to predict the in vivo performance of subcutaneously administered drugs should accurately emulate the interaction between the drug and formulation with the ECM.
There is significant interest in biopredictive in vitro tools for predicting clinical outcomes of subcutaneously injected drugs and formulations. These, for example, include the subcutaneous injection site simulator (SCISSOR),5,6 and the emulator of subcutaneous absorption and release (ESCAR).7 SCISSOR has been used in conjunction with principal component analysis and Partial Least Square modelling (PLS) to estimate the in vivo bioavailability of SC monoclonal antibodies (mABs)8 and as a screening tool in to assess the stability of mABs.9 Apparatus such as SCISSOR are therefore clearly proving to be useful tools capable of predicting the clinical outcomes following SC injection.
A key component of this system is a matrix-filled injection chamber or cartridge into which test compounds diffuse across following injection. The originally developed matrix component of SCISSOR consists of a simple hyaluronic acid (HA) hydrogel, although there are recent attempts to modify this simple hydrogel to between simulate the native in vivo ECM. To this end, a recent study by Gomes et al.10 have described new crosslinked format of HA (‘HA-XR’) that is more amenable for longer-term monitoring of drug release, in addition to hydrogels where a mixture of collagens or chondroitin sulphate have been incorporated.
Compared to the SCISSOR matrices, the native biological ECM is significantly more complex, consisting of structural proteins such as collagen and elastin, and a gel-like phase comprised of glycosaminoglycans, such as hyaluronan and proteoglycans.11,12 Collagen and hyaluronan are components that significantly contribute to the barrier properties of ECM through interstitial volume exclusion.13 The major components of ECM, collagen and hyaluronan, have opposing positive and negative charges, respectively; however, negatively charged proteoglycans result in the overall net charge of ECM being negative.14 Diffusion of biologics across ECM is expected to not only be size-dependent but also charge-dependent.13 Therefore, in contrast to the biological ECM, which is a highly complex gel composed of a large number of structural components of ECM, as well as soluble factors, the HA-based matrices are simple systems. Given this difference, it is reasonable to assume that the interaction of drugs with the matrix components will be different.
In this work we therefore set out to compare the permeation of model biologics across the HA-based matrix utilised in SCISSOR and an animal-derived ECM. We used a commercially available animal derived ECM in this work since human alternatives or fully synthetic versions that accurately model its complexity are currently not available. We employed a simple experimental set up of placing the gels on tissue culture inserts (Transwells) to determine the permeability of several model macromolecules, including those having different molecular weights and charges. The work therefore aimed to both compare the HA hydrogel with potentially a more physiologically-relevant matrix (ECM) and establish whether a simple experimental setup utilising biologically-derived ECM may act as an inexpensive and accessible tool to predict the in vivo performance of subcutaneously administered biotherapeutics.
2. Materials and methods
2.1 Materials
Hank's Balanced Salt Solution modified with sodium bicarbonate, fluorescein isothiocyanate (FITC)-labelled dextrans of average molecular weights of 10, 40, 59–77, 150 kDa, bovine FITC-albumin, FITC-labelled recombinant human insulin and FITC-IgG (from human serum) were purchased from Sigma-Aldrich (St Louis, MO, USA). Anionic FITC-dextran of average molecular weight of 10 kDa was acquired from Thermo Fisher Scientific (Waltham, MA, US). Cationic FITC-dextran of average molecular weight 10 kDa was obtained from TdB Labs AB (Uppsala, Sweden). ECM in the form of Basement Membrane Extract (BME, commercially known as Cultrex® Ultimatrix) was purchased from Bio-Techne (Abingdon, UK). This commercially available BME is a soluble form of basement membrane purified from murine Engelbreth-Holm-Swarm tumour and its major components include laminin, collagen IV, entactin, and heparin sulfate proteoglycan.Hyaluronic acid (HA) gel was obtained from Pion Inc. (Bellerica, MA). Ambion™ Cy3-labeled Negative Control siRNA was purchased from Thermo Fisher Scientific (Waltham, MA, US). Transwell® polycarbonate permeable inserts of 6.5 mm diameter and 0.4 μm pore size were purchased from Corning (Corning, NY, USA) and black 96-well plates were acquired from Thermofisher Scientific (Bleiswijk, Netherlands).
2.2 Methods
The acceptor chamber of Transwells® was filled with 500 μL of HBSS solution pre-warmed to 34 °C. Permeability studies were conducted at this temperature, in line with studies of this nature, to model the average temperature of the subcutaneous tissue. 20 μL of solutions of model drugs were applied to matrix-coated inserts. The solution from the Transwell® acceptor chambers was sampled regularly (and replaced with equal volumes to maintain sink conditions and a constant volume). Model drugs were quantified using a fluorescence plate reader (Tecan Infinite M200 Pro, Tecan Life Sciences, Switzerland). The experiments were repeated three times. For FITC-labelled materials, fluorescence intensity was assessed at excitation and emission wavelengths of 485 nm and 535 nm, respectively; for Cy3-labelled siRNA, excitation and emission wavelengths of 520 nm and 563 nm, respectively, were used. Model drugs were quantified using calibration curves (fluorescence intensity versus compound concentration) generated for each individual compound dissolved in HBSS (n = 3).
For FITC-dextrans, the gradient of cumulative mass transport of the compound into the basolateral chamber during the initial 60 min of the experiment was determined by fitting linear regression to cumulative diffusion data used to calculate flux values according to eqn (1).
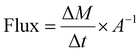 | (1) |
For FITC-albumin, FITC-insulin, FITC-IgG and siRNA, the % of dose that had permeated at four hours was used to compare the permeation to account for the differences in the applied concentrations.
2.4 Statistical analysis
GraphPad Prism 10 software was used to perform two-way ANOVA statistical analyses in all cases. Bonferroni's post-hoc test was used to compare effects of matrix on flux values of dextran. Tukey's post-hoc test was used to compare the effects of molecular weight on permeation of dextran through each matrix and to assess the effect of charge on dextran permeation through HA and BME matrices. Bonferroni's post-hoc test was used to compare the permeation of IgG, albumin, insulin and siRNA through HA and BME matrices. To assess the suitability of data for ANOVA analysis, visual linearity of QQ plot and Shapiro–Wilk test (p > 0.05) were used to assess normality of ANOVA residuals.3. Results
3.1 Permeation of dextrans with different molecular weights
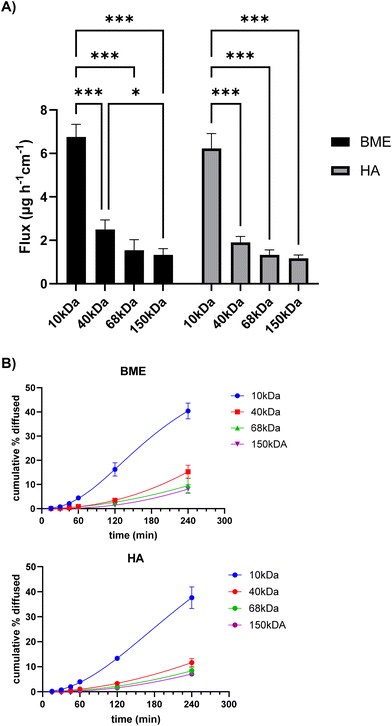
FITC-dextrans of varying molecular weights were utilised to compare the two matrices (BME vs. HA) and determine whether they are capable of discriminating based on molecular weight. To this end, the flux during the initial 60 minutes of the experiment, as well as the total quantity of the compounds permeating within 4 h were quantified. Fig. 1 shows a trend of decrease in the rate of permeation with increasing molecular weight. However, no statistically significant differences in flux could be determined between 40–150 kDa dextrans in the study; only the flux of 10 kDa dextran is significantly different to the next largest counterpart of 40 kDa. The flux values for both BME and HA matrices appeared to follow a similar trend, with no statistically significant differences between the two matrices observed with any FITC-dextrans.3.2 Permeation of dextrans with different charges
To test the potential influence of molecular charge of macromolecules on their permeation across BME and HA, we compared the permeation of neutral, cationic and anionic FITC-dextrans in the matrices. Fig. 2 demonstrates that charge influenced the rate of flux, with uncharged FITC-dextran associated with the highest flux value, followed by cationic dextran, which is lastly followed by the anionic dextran. A similar trend was observed in both matrices, although a statistically significant difference between anionic and cationic dextran was only observed in BME. At the same time, considering the comparison between neutral and cationic dextrans, a significant difference was only observed in the HA matrix.To establish whether the simple ‘ECM-in-a-transwell’ set up described here may serve as a tool to predict the in vivo performance of subcutaneously administered biotherapeutics, we compared the in vitro diffusion rate of the four exemplar biologics with their reported time to reach peak plasma concentration (Tmax) following subcutaneous injection in vivo in humans. Specifically, using the SC Tmax values of four days for IgG,15 48 hours for albumin,16 1.5 hours for insulin17 and 4 hours for siRNA (inclisiran),18 a relationship depicted in Fig. 4 between in vitro permeation in the two matrices and in vivo absorption can be derived. This analysis shows an inverse correlation between the in vitro permeation in two matrices and in vivo Tmax values, with the ECM data showing a markedly higher degree of correlation (R2 = 0.95) than the HA matrix (R2 = 0.24).
4. Discussion
Subcutaneous (SC) injection is a widely used route of drug administration, which is commonly utilised for biological therapies such as monoclonal antibodies. However, the field of SC drug delivery is currently lacking widely established biopredictive in vitro tools for predicting clinical outcomes. One such system, which has been developed relatively recently and is primarily being used by the pharmaceutical industry, is the subcutaneous injection site simulator (SCISSOR). A critical component of this instrument is a cartridge filled with an artificial, hyaluronic acid (HA)-based extracellular matrix (ECM), which dictates the diffusion of test compounds. However, compared to the simple HA hydrogel matrix, the native biological ECM found in the SC tissue in vivo is significantly more complex. In this work we therefore aimed to compare the permeation of macromolecules and model biologics across HA hydrogel (used in SCISSOR) and an animal-derived basement membrane extract (BME), an ECM extracted from Engelbreth-Holm-Swarm mouse sarcoma. This material was used as to our knowledge it is the only commercially available ECM and human ECM or animal-free alternatives that accurately model the complexity of human ECM are currently not available. However, the composition of this BME is thought to closely match that of SC human ECM, comprising of a combination of laminins, collagen IV, entactin and heparin sulfate.19We employed matrix-coated tissue culture inserts as a simple experimental set up to test the permeation of model macromolecules with different molecular weights and charges, as well as a panel of biological macromolecules (siRNA, insulin, albumin and IgG). Such a simple Transwell set-up is potentially beneficial considering the cost and footprint of the SCISSOR system. In fact, there have been previous attempts to develop simple and inexpensive in vitro models to predict the performance of SC drugs and formulations. Leung et al. described a simple set-up using a conventional quartz cuvette, with the drug and formulation embedded within an agarose gel phase at the bottom of the cuvette and a second agarose gel layer present above this, separating from a sink reservoir consisting of PBS. The authors showed how this model can be used as a performance differentiator for different formulations of both large and small molecules.4 Schöner et al.20 developed an in vitro system based on a 12-well plate, which utilises a special collagen-hyaluronic acid hydrogel. The system could discriminate regular human insulin and rapid acting insulin formulations. Finally, Hakim et al.21 documented that a simple in vitro Transwell assay recapitulates important drug/matrix interactions and, as one might expect, the properties of ECM and permeating compound determines its movement through the matrix.
The results show that there were no significant differences in permeation across the two matrices observed for dextran macromolecules of different molecular weight. Despite the differences in the complexity of the composition of BME and HA matrixes, dextran flux values in both matrixes appeared to follow a similar trend (Fig. 1A), with no significant differences observed with change in the molecular weight of dextrans. Similarly, both matrices overall displayed a charge-dependent barrier to the permeation of neutral and differently charged dextrans, although only BME provided a statistically significant discrimination between anionic and cationic dextrans.
Considering the comparison between HA and BME with respect to the permeation of biological molecules, three of the tested compounds, namely albumin, insulin and siRNA, showed a higher permeation in BME than HA. It is currently unclear why this observation was apparent. In the SC tissue, collagen and HA are two key components that influence mass transport. Collagen, which is absent in the HA matrix but present in BME is the most abundant ECM protein and can take part in electrostatic interaction with diffusing molecules.21 It is impossible to predict the effect on the diffusive barrier of the matrix that the presence of collagen (in addition to HA) would have since while collagen can interact with the diffusing molecule, the presence of this molecule, as well as that of a multitude of other ECM proteins, would undoubtedly influence the overall 3D structure and other properties of the matrix, including its net charge.
Interestingly, a preliminary analysis comparing the in vitro permeability in the two matrices observed here and the in vivo Tmax data reported in the literature shows a notable higher correlation for BME compared to HA (Fig. 4). However, this comparison should be interpreted cautiously and future work should confirm this preliminary finding by focusing on a more detailed comparison between the two matrices, achieved for example by testing the permeation of a larger number of compounds and formulations (e.g. for insulin).
The work overall shows that there are similarities and some notable difference in the performance of the HA hydrogel and an animal-derived ECM as a key component of the SC space which dictates the absorption profile of the drug. This work is valuable since for the first time provides a comparison between the HA matrix which is increasingly being used to predict the clinical outcomes of SC drug products and a potentially more physiologically relevant ECM. At the same time, we also show that a simple Transwell experimental setup using this biologically derived ECM may act as a convenient, inexpensive and accessible tool for this purpose. The simplicity of this method may allow for quick screening of ECM-mimicking hydrogels, including non-animal alternatives for which there is a significant need, to outline potential sensitivities/mechanisms/excipients important for assessing the compounds and/or formulations of interest.
5. Conclusion
This study compares the permeation of a range of macromolecules with different physicochemical properties in HA hydrogel and an animal-derived ECM. The permeation of model macromolecules in BME and HA demonstrated similarities, but also some notable differences providing early indications of the value of utilising native ECM in these studies. It is therefore hoped that this work will inspire future studies to compare the performance of these two matrices against in vivo pharmacokinetic data and confirm whether the Transwell setup with a biologically derived ECM may in the future serve as a simple biopredictive tool for in vitro testing of SC therapies.Data availability
The authors confirm that the data supporting the findings of this study are available within the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by an Innovate UK ‘Innovation Scholars’ secondment, in collaboration with Astra Zeneca (project reference 75319).References
- M. Sanchez-Felix, M. Burke, H. H. Chen, C. Patterson and S. Mittal, Predicting bioavailability of monoclonal antibodies after subcutaneous administration: Open innovation challenge, Adv. Drug Delivery Rev., 2020, 167, 66–77 CrossRef CAS PubMed.
- D. N. McLennan, C. J. Porter and S. A. Charman, Subcutaneous drug delivery and the role of the lymphatics, Drug Discovery Today: Technol., 2005, 2(1), 89–96 CrossRef CAS PubMed.
- W. F. Richter and B. Jacobsen, Subcutaneous absorption of biotherapeutics: knowns and unknowns, Drug Metab. Dispos., 2014, 42(11), 1881–1889 CrossRef PubMed.
- D. H. Leung, Y. Kapoor, C. Alleyne, E. Walsh, A. Leithead, B. Habulihaz, G. M. Salituro, A. Bak and T. Rhodes, Development of a Convenient In Vitro Gel Diffusion Model for Predicting the In Vivo Performance of Subcutaneous Parenteral Formulations of Large and Small Molecules, AAPS PharmSciTech, 2017, 18(6), 2203–2213 CrossRef CAS PubMed.
- H. M. Kinnunen, V. Sharma, L. R. Contreras-Rojas, Y. Yu, C. Alleman, A. Sreedhara, S. Fischer, L. Khawli, S. T. Yohe, D. Bumbaca, T. W. Patapoff, A. L. Daugherty and R. J. Mrsny, A novel in vitro method to model the fate of subcutaneously administered biopharmaceuticals and associated formulation components, J. Controlled Release, 2015, 214, 94–102 CrossRef CAS PubMed.
- H. Lou, C. Berkland and M. J. Hageman, Simulating particle movement inside subcutaneous injection site simulator (SCISSOR) using Monte-Carlo method, Int. J. Pharm., 2021, 605, 120824 CrossRef CAS PubMed.
- H. Lou and M. J. Hageman, Development of Drug Release Model for Suspensions in ESCAR (Emulator of SubCutaneous Absorption and Release), AAPS J., 2023, 25(3), 29 CrossRef CAS PubMed.
- H. K. Bown, C. Bonn, S. Yohe, D. B. Yadav, T. W. Patapoff, A. Daugherty and R. J. Mrsny, In vitro model for predicting bioavailability of subcutaneously injected monoclonal antibodies, J. Controlled Release, 2018, 273, 13–20 CrossRef CAS PubMed.
- C. M. Jogdeo, D. S. Bhattacharya, V. Lin, P. Kolhe and A. Badkar, Assessing Physicochemical Stability of Monoclonal Antibodies in a Simulated Subcutaneous Environment, J. Pharm. Sci., 2024, 113(7), 1854–1864 CrossRef CAS PubMed.
- C. Gomes, K. Gridley, I. Anastasiou, B. Sinko and R. J. Mrsny, Hydrogel formats to model potential drug interactions occurring at the subcutaneous injection site, Eur. J. Pharm. Biopharm., 2024, 199, 114308 CrossRef CAS PubMed.
- C. Walker, E. Mojares and A. Del Rio Hernandez, Role of Extracellular Matrix in Development and Cancer Progression, Int. J. Mol. Sci., 2018, 19(10), 3028 CrossRef PubMed.
- A. D. Theocharis, D. Manou and N. K. Karamanos, The extracellular matrix as a multitasking player in disease, FEBS J., 2019, 286(15), 2830–2869 CrossRef CAS PubMed.
- A. D. Theocharis, S. S. Skandalis, C. Gialeli and N. K. Karamanos, Extracellular matrix structure, Adv. Drug Delivery Rev., 2016, 97, 4–27 CrossRef CAS PubMed.
- R. P. Mohanty, X. Liu and D. Ghosh, Electrostatic driven transport enhances penetration of positively charged peptide surfaces through tumor extracellular matrix, Acta Biomater., 2020, 113, 240–251 CrossRef CAS PubMed.
- F. A. Bonilla, Pharmacokinetics of immunoglobulin administered via intravenous or subcutaneous routes, Immunol. Allergy Clin. North Am., 2008, 28(4), 803–819 CrossRef PubMed , ix.
- W. Hollander, P. Reilly and B. A. Burrows, Lymphatic flow in human subjects as indicated by the disappearance of 1–131-labeled albumin from the subcutaneous tissue, J. Clin. Invest., 1961, 40(2), 222–233 CrossRef CAS PubMed.
- E. Potocka, R. A. Baughman and H. Derendorf, Population pharmacokinetic model of human insulin following different routes of administration, J. Clin. Pharmacol., 2011, 51(7), 1015–1024 CrossRef CAS PubMed.
- D. Kallend, R. Stoekenbroek, Y. He, P. F. Smith and P. Wijngaard, Pharmacokinetics and pharmacodynamics of inclisiran, a small interfering RNA therapy, in patients with hepatic impairment, J. Clin. Lipidol., 2022, 16(2), 208–219 CrossRef PubMed.
- rndsystems https://www.rndsystems.com/products/cultrex-basement-membrane-extract-pathclear_3432-010-01 (accessed 15/08/2024).
- T. A. Schöner, V. Vogel, M. Venczel, K. Knoth, W. Kamm, T. Paehler, G. Louit, I. T. Teran, P. Mundinger, A. Marker, P. Loos, M. Hittinger and C. M. Lehr, Biorelevant subcutaneous in vitro test predicts the release of human and fast acting insulin formulations, Int. J. Pharm., 2024, 655, 123995 CrossRef PubMed.
- M. H. Hakim, B. H. Jun, A. Ahmadzadegan, P. M. Babiak, Q. Xu, K. P. Buno, J. C. Liu, A. M. Ardekani, P. P. Vlachos and L. Solorio, Investigation of macromolecular transport through tunable collagen hyaluronic acid matrices, Colloids Surf., B, 2023, 222, 113123 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |