Open Access Article
Open Access ArticleParticle-based investigation of excipients stability: the effect of storage conditions on moisture content and swelling†
Isra
Ibrahim
 abc,
Mark
Carroll
ab,
Anas
Almudahka
abc,
Mark
Carroll
ab,
Anas
Almudahka
 abd,
James
Mann
abd,
James
Mann
 e,
Alexander
Abbott
e,
Fredrik
Winge
f,
Adrian
Davis
g,
Bart
Hens
h,
Ibrahim
Khadra
b and
Daniel
Markl
e,
Alexander
Abbott
e,
Fredrik
Winge
f,
Adrian
Davis
g,
Bart
Hens
h,
Ibrahim
Khadra
b and
Daniel
Markl
 *ab
*ab
aCentre for Continuous Manufacturing and Advanced Crystallisation (CMAC), University of Strathclyde, Glasgow, UK. E-mail: daniel.markl@strath.ac.uk
bStrathclyde Institute of Pharmacy & Biomedical Sciences, University of Strathclyde, Glasgow, UK
cDepartment of Pharmaceutics and Pharmaceutical Technology, Yarmouk University, Irbid, Jordan
dDepartment of Pharmaceutics, College of Pharmacy, Kuwait University, Kuwait
eOral Product Development, Pharmaceutical Technology & Development, Operations, AstraZeneca, Macclesfield, UK
fOral Product Development, Pharmaceutical Technology & Development, Operations, AstraZeneca, Gothenburg, Sweden
gAnalytical R&D, Pharmaceutical Sciences Small Molecules Pfizer R&D UK Ltd, Sandwich, UK
hDrug Product Design, Pfizer, Zaventem, Belgium
First published on 27th January 2025
Abstract
Moisture sensitivity poses a challenge in formulating oral dosage forms, particularly when considering disintegrants’ swelling due to prior moisture exposure, impacting performance and physical stability. This study utilises dynamic vapour sorption to simulate real-world storage scenarios, investigating the equilibrium moisture content and dynamics of eight commonly used excipients in oral solid dosage forms. A model was developed to determine the kinetic rate constant of moisture sorption and desorption for different storage conditions. Dynamic vapour sorption tests revealed that excipients with higher moisture-binding capacities showed slower equilibration to the target relative humidity (RH). Elevated temperatures accelerated the moisture sorption/desorption process for all excipients, reducing the equilibrated moisture content for most, except mannitol and lactose. Particle imaging over a 14-day accelerated storage period quantified swelling, indicating approximately 6% increase in particle diameter for croscarmellose sodium (CCS) and sodium starch glycolate (SSG), and a lesser 2.7% for microcrystalline cellulose (MCC), predominantly caused by the humidity. All excipients reached their swelling peak within the first day of storage, with permanent particle size enlargement for CCS and SSG, whereas MCC displayed a partial reversibility post-storage. Enhancing our understanding of excipients’ stability and interaction with moisture and the resulting particle swelling contributes to the rational design of oral solid dosage formulations and promotes a better understanding of their long-term physical stability.
1. Introduction
Excipients are essential components incorporated into pharmaceutical formulations for various purposes, ranging from facilitating manufacturing processes and sustaining product stability to enhancing bioavailability, as well as improving patient acceptability and compliance.1 Although excipients are considered pharmacologically inactive, they are far from inert within a formulation. Instead, they play an active role in the physical aspects of the dosage form as they make up a significant majority of the final formulation. It has become widely recognised that the performance of a dosage form is closely linked to the physical and chemical properties of all its ingredients.2,3 Consequently, excipient properties affect various quality attributes of the drug product, including drug release, friability, tensile strength, appearance, and stability. Among these attributes, stability stands out as a particularly crucial aspect for a drug product to be released to the market.4Advances in particle-based investigation methods have improved our understanding of excipient properties. Techniques like high resolution imaging and single crystal X-ray diffraction enable accurate particle characterisation such as size, surface area, morphology, crystal structure, and presence of polymorphs which are vital to the overall physical, chemical and biological behaviour of pharmaceutical products.5 These particle-based techniques combined with bulk-based methods like dynamic vapour sorption (DVS) allow detailed analysis of individual particles and their interactions with each other and with environmental factors to enhance formulation stability and reduce development costs. As the pharmaceutical industry prioritises efficiency, particle characterisation data are attracting a great attention across development, manufacturing, and quality control processes. Both the API and excipient particle characteristics can influence the stability and efficacy of the drug product, making thorough particle assessment essential. This shift has been influenced by Quality by Design (QbD) principles, which encourage using a data- and risk-based approach to optimize product development leading to more robust pharmaceutical formulations.
Throughout the drug development process, stability testing of pharmacological compounds and drug products is a standard procedure used to investigate the causes or mechanisms of product instability.6 The majority of studies on drug stability have focused on the chemical stability of the drug substance, which can cause a loss in potency. However, it is also crucial to evaluate the physical stability of drugs and excipients within the dosage form to ensure their effectiveness. This includes assessing changes in organoleptic properties, water content, solid-state, tensile strength, disintegration, and dissolution rate, all of which are influenced by the intrinsic physicochemical properties of the individual components such as their particle size, morphology, moisture content, and polymorphic form.7,8 The monographs of each excipient in the pharmacopeia provide “particular tests” that must be followed to indicate their quality and functionality. Additionally, excipient manufacturers and researchers have explored various material properties that can impact tablet performance and stability.9 Numerous factors may influence these properties; however, temperature and water, whether liquid or gaseous as moisture, are considered the primary reactants affecting tablet performance.10
Water plays a crucial role in various stages of pharmaceutical product manufacturing, directly contributing as a component in processes like wet granulation or coating. Environmental moisture during packaging, transportation, storage, and usage also indirectly affects drug product attributes.6 Moisture is adsorbed in the form of monolayers or multilayers, or it may exist as condensed water on the surface. The presence of moisture will not only change the pharmaceutical and mechanical properties of the final product but may also pose a risk of physicochemical instability ultimately affecting the effectiveness of the drug as well.11 The interaction of moisture with different materials is connected to the chemical affinity of their particles, their physical properties such as particle size, specific surface area, and porosity, and on the ambient relative humidity (RH) and temperature of the environment.12–14 Consequently, every element in a formulation possesses a distinct affinity for moisture, so it is crucial to understand their specific interactions with moisture concerning their behaviour at various RH levels and the resulting influence on the performance of the product. In a study conducted by Dalton and Hancock (1997), notable variations in formulations’ water sorption tendencies were observed, primarily due to the considerable differences in how the excipients absorbed water.15 Starch and cellulose excipients, commonly used in oral solid dosage forms, are particularly affected by the hydrogen bonding between water and solid molecules.16
To characterise water associated with these excipients, moisture sorption isotherms are established, which illustrate the relationship between RH and water activity under a constant temperature by establishing a correlation between equilibrium moisture content (EMC) and RH at a fixed temperature. This offers valuable insights into materials’ equilibrium moisture levels and their moisture retention capacities under certain temperature and humidity conditions. Hysteresis, observed with starch and cellulose-based excipients, occurs during desorption where some water molecules remain within the powder and cannot be dissociated at the same equilibrium RH level, attributed to the particles swelling and changes in the conformational structure. This alters the accessibility of binding sites for water molecules and leads to incomplete removal of water molecules during desorption.16–18 As a result, the moisture affinity of a material can be influenced by its prior exposure to moisture or its history of moisture exposure.4 Analysing sorption isotherms can uncover information about water interactions with particles and understand water adsorption and desorption extents under different RH levels. This is of practical importance as it can directly affect drug product performance and stability.
Among the excipients significantly affected by water sorption are disintegrants. The swelling of their particles when they come in contact with a physiological fluid is essential to disrupt interparticulate bonds within the tablet, thereby enhancing tablet disintegration, promoting dissolution, and facilitating drug release.19,20 Common disintegrants include sodium starch glycolate (SSG), croscarmellose sodium (CCS), low-substituted hydroxypropyl cellulose (L-HPC), and crospovidone (XPVP). In addition to disintegrants, other excipients such as microcrystalline cellulose (MCC), typically used as a diluent, filler, or binder, can also experience swelling, thereby also playing a role in the tablet swelling process.21 The swelling of these particles primarily relies on two key factors: the water diffusion coefficient (D) and the maximum absorption ratio (Qmax), which is the ratio of the mass of hydrated particles to dry particles. While Qmax can be experimentally measured through straightforward weighing methods, determining the value of D directly is often more challenging.22 These excipients are considered inert in the dry tablets, but they should cause the tablet matrix to swell and disintegrate rapidly in the patient, accelerating the dissolution of the drug particles. Any prior exposure of these agents to moisture or liquid will impact their moisture affinity and physical properties, potentially leading to a loss of disintegrant power due to the possibility of “pre-activation” of the disintegrant swelling and influencing the performance and stability of the dosage form.23,24
Considerable research efforts have been directed toward quantifying the swelling behaviour of particles in response to liquid water. Soundaranathan et al. (2020) used an optical microscope paired with a custom flow cell and utilising single particle swelling model to quantify the swelling characteristics of different disintegrants (SSG, CCS, and L-HPC) along with five grades of MCC.25 They showed that the swelling behaviour varied among the different excipients, with CCS having the highest diffusion coefficient and SSG having the highest maximum absorption ratio. Zhao and Augsburger (2005) utilised laser diffraction to quantify the intrinsic swelling of disintegrants in a suspension, finding that SSG exhibited a superior swelling capacity compared to CCS.26 Rojas et al. (2012) assessed the swelling value and water uptake ability of SSG, CCS, and MCC powder in simulated gastric and intestinal fluid, revealing that SSG had the highest water uptake ability and swelling value, followed by CCS and MCC.27
Investigations into the behaviour of excipients in response to humidity have predominantly concentrated on the impact of water at or near its saturation point. To the best of the authors’ knowledge, none of these studies have explored the swelling of particles induced by moisture in the gaseous phase during storage. It is noteworthy that the variations in water absorption observed below 96% RH may not align with findings at 99.98% RH.28 This limits the relevance of research outcomes derived from fully saturated conditions for stability studies at lower RH levels. Furthermore, there is also a gap in the literature regarding how moisture affects the particle size of these swelling excipients where the bulk of research efforts have been given to exploring the effects of storage conditions on the tablets containing these excipients.24,29–33
The primary objective of this study is to thoroughly investigate the impact of storage conditions, both moisture and temperature variations, on the intrinsic properties of eight commonly used excipients in oral solid dosage forms. Specifically, this study focuses on the moisture content and moisture sorption kinetics in a manner that reflects the dynamic simulation of moisture sorption in practice, using dynamic vapour sorption (DVS) paired with a mathematical model. The temporal effect of sorbed moisture on the particle size distribution of potential swelling excipients MCC, CCS, and SSG, is also discussed.
2. Materials and methods
2.1. Materials
A full list of excipients studied is detailed in Table 1, including CCS, XPVP, L-HPC, and SSG as disintegrants, as well as MCC, mannitol, lactose, and DCPA as fillers.| Material | Grade | Supplier | Abbreviation |
|---|---|---|---|
| Croscarmellose sodium | AcDiSol | FMC International | CCS |
| Crospovidone | Kollidon® CL | BASF Pharma | XPVP |
| Low-substituted hydroxypropyl cellulose | LH-21 | Shin-Etsu Chemical Co. | L-HPC |
| Sodium starch glycolate | Primojel® | DFE Pharma | SSG |
| Microcrystalline cellulose | Avicel® PH-102 | FMC International | MCC |
| Mannitol | Pearlitol® 200 SD | Roquette | Man |
| Lactose monohydrate | FastFlo® 316 | Foremost Farms USA | Lac |
| Dibasic calcium phosphate anhydrous | Anhydrous Emcompress® | JRS Pharma | DCPA |
For the stability study, sodium chloride and magnesium chloride (Merck Life Science, UK) were used to prepare saturated solutions to maintain humidity levels during storage at 75% and 30%, respectively. Poly vinyl-chloride (PVC) (Sigma-Aldrich, USA) was used as a negative control material due to its non-hygroscopic nature, ensuring that it would not absorb moisture and consequently would not change in size upon exposure to storage conditions.34
The excipients used in this study were the most commonly used in the pharmaceutical industry for manufacturing directly compressed tablets.35 Choosing different materials with a wide range of physicochemical properties was necessary to understand how this would affect their moisture sorption characteristics. MCC is a hygroscopic and insoluble filler, that undergoes swelling upon contact with liquid. DCPA, on the other hand, is a non-hygroscopic and insoluble filler. Both lactose and mannitol serve as soluble fillers.24 Among the disintegrants, CCS, L-HPC, and SSG are classified as swelling disintegrants, while XPVP functions through a shape recovery mechanism.36
2.2. Moisture sorption and desorption analysis
Dynamic vapour sorption apparatus (DVS, Surface Measurement Systems Ltd, London, UK) was used to understand the dynamics of moisture sorption for the studied raw materials during storage, i.e., to measure how quickly and how much moisture is sorbed (adsorbed and absorbed). The device is featured with an SMS UltraBalance with a mass resolution of ±0.1 μg to monitor the intake and loss of vapour gravimetrically and a temperature-controlled incubator to maintain the temperature constant at a ±0.1 °C level.37 10 mg of each powder was placed into the sample pan of the DVS. Then, samples were preconditioned to a constant mass under a stream of dry nitrogen at 0% RH, which was recorded as the sample reference mass. Data was saved every second to help calculate the moisture sorption and desorption rate.Although moisture isotherms are typically constructed by implementing a series of steps across the entire RH range, this approach will impact the EMC and the rate at which EMC is reached due to pre-moisture exposure from the RH steps used. To address this concern, the rate and extent of water uptake in a one-step simulation was assessed, covering the range of 30%–75% RH. This approach simulates more closely real-world storage conditions when a sample intended for specific RH storage is exposed to a single-step adjustment from the baseline humidity, starting at 30% RH as measured in the laboratory to the desired RH level. For instance, to obtain the sorption/desorption isotherm at 75%, the instrument initiated conditioning the sample at 0% RH, then going from 30% to 75% directly as a one, big jump and subsequently returned to 30% RH baseline. Moisture sorption/desorption data were analysed at temperatures of 25 °C and 50 °C. The different conditions studied are summarized in Table 2. A new powder sample was used for each RH point to minimise any moisture or temperature history effects.
| Initial % RH | Target sorption % RH | Target desorption % RH |
|---|---|---|
| 30 | 40 | 30 |
| 30 | 50 | 30 |
| 30 | 60 | 30 |
| 30 | 75 | 30 |
Two equilibrium criteria (stop criteria) were used to consider reaching equilibrium. The first criterion is the rate of change in sample mass with time, dm/dt, which is expressed in the percentage of the reference sample dried mass. The second is the minimum time during which the sample mass remains stable. In this study, equilibrium was assumed to be attained when the weight change was less than or equal to 0.002% of the reference mass over a 10 minute window, which, if not achieved, will keep analysis for a maximum of 6 hours. After that, the equilibrium will be assumed to be completed, and the experiment will proceed to the next programmed humidity stage. The water uptake was expressed as a percentage weight change from the dry weight. However, to simulate the case of storing excipients at humidities over 30% RH (which was considered the baseline), the percentage change in mass was recorded relative to the mass at 30% RH. Fig. 1 shows a DVS profile from (30–75% RH) demonstrating the steps involved in the analysis.
 | ||
| Fig. 1 Example of DVS profile demonstrating the change in mass for MCC particles with time. The DVS profile's sectional analysis reflects the various RH conditions used. | ||
2.3. Sorption and desorption rate constant model
DVS sorption and desorption time data were used to calculate the moisture sorption/desorption rate constant (rsor/des) to understand the dynamics of moisture sorption/desorption and the effect of RH level and temperature on that rate of sorption/desorption. A model was developed to calculate the kinetics rate constants by fitting the temporal sample mass at a specified temperature and humidity:| M(t) = M0e−rsor/dest + Mmax/min(1 − e−rsop/dest) | (1) |
Statistical analysis was conducted through Python 3.9.7 (Python Software Foundation, Virginia, USA) using the SciPy “curve_fit” function. For fitting the model, the statistics reported were the coefficient of determination (R2) and Root mean square of error (RMSE).
2.4. Storage-induced particle size changes
The experimental method was carefully designed to accurately quantify the micro-scale changes to particles stored for a specific time under accelerated humidity and temperature conditions. The main consideration was to ensure that individual particles are exposed to storage conditions rather than the bulk of a powder where the particles close to the surface experience a different exposure to the environment compared to those in the core of the bulk. This study focused on swelling particles only: CCS, SSG, and MCC.| Temperature (°C) | Humidity (% RH) | Time points (days) |
|---|---|---|
| 25 | 75 | 1, 14 |
| 50 | 30 | 1, 14 |
| 50 | 75 | 1, 3, 14 |
A single plate of each powder, along with a vial filled with a saturated salt solution to establish the required humidity level, was placed inside an airtight glass jar which was securely sealed with parafilm after screwing the top lid. Jars were then stored in temperature-controlled ovens at 25 °C or 50 °C for the designated storage duration. Fig. 2 illustrates the experimental set-up employed for storing powder samples throughout the stability study.
Time points for sample collection were of concern because it was expected that storage conditions would affect particles faster than tablets. As observed by Maclean et al. (2022), changes in the physical properties of tablets occurred within the first two weeks of storage before reaching a steady state.24 So, additional time points within the first two weeks of the study would provide more information on the shape of the stability profiles of particle swelling. Particle size distribution analysis was carried out in triplicate for each time point under 50 °C/75% RH condition, indicating that three separate samples were analysed at each time point. In contrast, a single sample was utilised for each time point under 25 °C/75% RH and 50 °C/30% RH.
To study the effect of storage conditions (if any) on the particle size, size distribution, or shape parameters, plates were removed from storage jars at a specific time point to be analysed. For each time point, a new dish was used. Particle size distributions were initially obtained for each excipient before storage, then analysed again after storage while they were on the same plate without touching the particles to ensure no agglomerates formed due to moisture (Fig. 2). This approach facilitated the comparison of the measured particle distribution post-storage with the initial distribution obtained before storage for each dish. Comparing the same sample before and after storage facilitated the tracking of any alterations in particle size distribution attributed to storage, because even with good sampling techniques, differences in size distribution may exist so this method accounts for such variations during the comparison.
Particle imaging was conducted using a 10× magnification lens with z-stacking enabled to take four planes above the initial point of focus to account the changes of the sample in z-direction due to swelling. Z-Stacking also reduced blurring observed around the particles after removal from storage due to moisture diffusion. The threshold of particle detection was adjusted before and after storage to ensure the detection of the whole parts of the particle as well as dark and bright particles. Morphological filtering was applied manually to the raw image data to eliminate partially imaged or overlapping particles. All samples were analysed according to a standard operating procedure (SOP) summarised in Table 4.
| Parameter | Set value |
|---|---|
| Sample volume | 5 mm3 |
| Solid dispersion unit settings | Injection pressure: 1 bar (for CCS and MCC) and 2 bar for SSG |
| Injection time: 20 ms | |
| Settling time: 60 s | |
| Illumination | Diascopic (bottom light) bright field |
| Optics selection | 10× |
| Light intensity | 80% |
| Focus | Manual focus |
| Trash size | 10 pixels |
| Z-Stacking | 4 planes, equivalent to 48.9 μm |
To assess the reliability of this methodology, a careful approach has been followed, incorporating key measures aimed at validating the integrity of the results. Firstly, the experiment was systematically performed on multiple replicates to assess the consistency and reproducibility of findings. Secondly, a controlled material, PVC, characterised by its resistance to moisture-induced swelling, was selected to ensure that variations in particle size are not influenced by external factors other than moisture absorption. Furthermore, a precise adjustment was made to the threshold parameters, creating a controlled variance of ±5%. This precautionary measure helped to ensure the accuracy of the size analysis by confirming that any detected changes in size distribution are exclusively caused by the moisture, and not a consequence of threshold selection and modification. To carefully investigate the reversibility of particle size change following removal from storage, particles were allowed to equilibrate with the controlled laboratory humidity and temperature. The equilibration period lasted between one week and one month, and samples were then analysed again.
This study focused on the circle equivalent diameter (CED), which is the diameter of a circle with the same area as the 2D image of the particle. The CED is calculated by:
 | (2) |
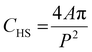 | (3) |
 | (4) |
3. Results and discussion
3.1. Effect of relative humidity level on EMC and kinetic rate constant
Each excipient's moisture sorption/desorption properties were measured using DVS to simulate the conditions used in practice. Fig. 3 illustrates the EMC at various RH levels for the different excipients under investigation. The data is presented as a percentage change in mass relative to the mass recorded at 30% RH. Additionally, the EMC relative to the dry mass of powder at the end of 0% RH is provided in Fig. S1 in the ESI,† with PVC data included as a control.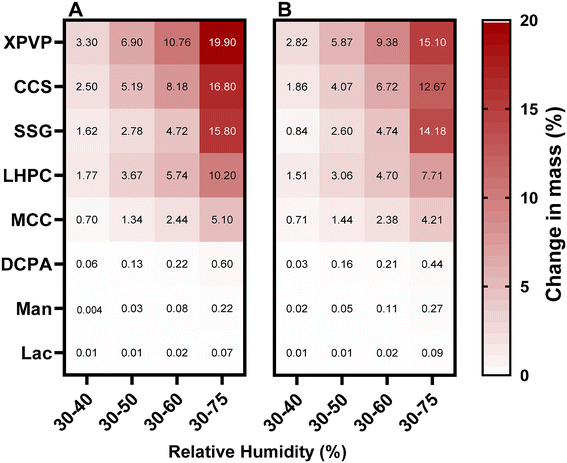 | ||
| Fig. 3 Moisture uptake of excipients at 25 °C (A) and 50 °C (B), presented as the percentage change in mass relative to the mass at 30% RH. | ||
According to the moisture sorption results, lactose, mannitol, and DCPA are non-hygroscopic and do not absorb moisture substantially; even under high RH conditions, the mass change was less than 1%. On the contrary, disintegrants showed very high hygroscopicity (Fig. 3A). CCS and SSG absorb approximately 16% moisture in the 30–75% RH range, while XPVP absorbs nearly 20% at the same %RH at 25 °C. Moisture sorption results relative to the dry mass of powder are consistent with prior findings.13,24,39 The difference in moisture capacities among excipients is attributed to their different interactions with moisture depending on their chemical properties and polarity, as well as on the RH, temperature, particle-size distribution, specific surface area, and porosity of the material.13
Crystalline non-hygroscopic excipients such as lactose and mannitol exhibit limited moisture gain with no monolayer formation; however, delayed multilayering occurs. At low RH, water is adsorbed onto the crystalline material's surface. As RH increases, weak interaction forces, such as van der Waals forces, cause water molecules to attach at the sugar–vapour interface. This limited water uptake behaviour is attributed to their low surface area and stable crystalline lattice supported by stable hydrogen bonding networks, which minimises available binding sites for moisture adsorption of these crystalline sugars.40,41 A tendency for multilayer sorption is expected as RH increases, and at high humidity (generally over 65% RH), solid water-soluble sugar molecules dissolve, forming a saturated sugar solution.12 Our findings show that lactose and mannitol water content remains relatively small at the studied RH ranges. On the other hand, starches and cellulose-based excipients exhibit higher moisture gain which involves the sequential sorption of a single tightly bound water molecule followed by less tightly bound water molecules and additional sorption of free water. They generally demonstrate substantial bulk absorption of water, characterised by a slow diffusion-controlled mechanism.13,42
Increasing humidity levels also resulted in higher moisture uptake, where powders exposed to 75% RH levels exhibited the greatest uptake of moisture compared to other %RH levels. This observed correlation between moisture uptake and relative humidity is consistent with prior findings.43,44
DVS isotherms do not indicate a true affinity for water vapour since the data simply presents the total sorption capacity of each excipient in terms of mass change from the dry mass as a function of humidity with no reference to timescale. As observed from moisture uptake results, there can be similarities in sorption capacity between excipients which suggests that the behaviour of these materials at these humidities towards water vapour is similar while in reality, differences in their performance indicate different interactions. To further classify the excipients in terms of behaviour to water vapour, the DVS sorption time data were used to calculate the rate at which it adsorbs or desorbs moisture. Fig. S2 in the ESI† compares the change in mass as a function of time for CCS and MCC, where the time needed to achieve equilibrium is much higher for CCS compared to MCC despite using the same sample size and the same conditions.
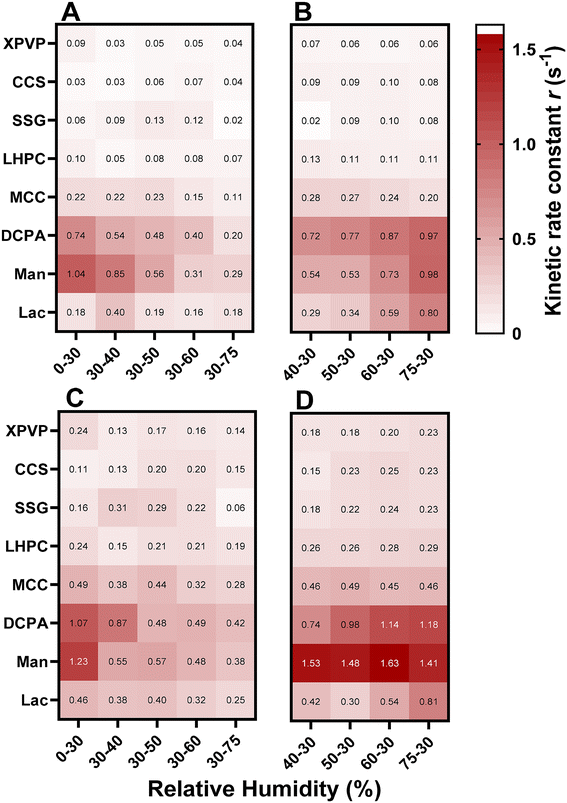
Knowing how quickly a material absorbs moisture is essential for predicting its stability during storage. The moisture sorption kinetic rate constant model was applied to all DVS sorption/desorption time data and compared to the measured data as shown in Fig. S3 in the ESI.† The accurate prediction performance of the moisture kinetics rate constant model is provided in Table S2 in the ESI† which is shown by its high R2 and low RMSE except for mannitol and lactose at low %RH. However, non-hygroscopic excipient data should be interpreted carefully as the readings are more prone to noise in measurement due to very small changes in mass. A summary of the calculated kinetic rate constants extracted from DVS time data for all excipients tested in this work during sorption and desorption is shown in Fig. 4.
Generally, CCS, SSG and XPVP consistently tend to take a longer time to reach equilibrium (Fig. 4A), but they exhibit greater mass sorption under the studied conditions. In contrast, non-hygroscopic excipients such as mannitol, lactose, and DCPA achieve equilibrium more rapidly with a higher rate constant of moisture sorption compared to the hygroscopic excipients. This trend extends to the desorption (Fig. 4B), where CCS, SSG and XPVP exhibit the smallest desorption rate constant indicating a longer time to return to the equilibrium 30% level, while DCPA and mannitol reached the equilibrium humidity level the fastest. It is noteworthy to mention that similar observations have been documented by Heidemann and Jarosz (1991) with different formulations, showing that excipients exhibiting a higher capacity for moisture sorption tend to achieve equilibrium more slowly compared to those with lower moisture affinity.45
At a specific humidity level, desorption rate constants were higher than sorption, suggesting faster moisture loss for the studied excipients compared to moisture gain. Few exceptions were observed with SSG, mannitol, and lactose. Sorption rate constants depend on the target RH, where they are generally higher at lower humidity levels (30% and 40% RH) and smaller humidity increments compared to larger ones (75% RH). This implies that moisture sorption occurred more rapidly at smaller humidity steps and when targeting lower humidity levels, as opposed to larger humidity steps and higher target RH percentages for the tested excipients which require longer time to reach equilibrium. This is attributed to the saturation of available binding sites for moisture sorption, which initiates with the rapid formation of a firmly bound monolayer of water molecules on the surface of dry particles with the entry of the initial water molecules into the system. This process depends on surface binding and diffusional forces. As more water molecules are attached to the surface, moisture-binding sites will be saturated and the diffusional forces start to outweigh the binding forces. The moisture starts diffusing into the bulk, the rate of moisture absorption depends on the difference between the saturation moisture content and the moisture content at a given time. As sorption progresses, the increasing moisture content diminishes the driving force, leading to a gradual reduction in the sorption rate. Multiple layers of moisture that are less tightly bound to the surface or even condensed into the pores, which is associated with swelling in the case of nonrigid porous structures, may happen as well. At higher humidity levels, the amount of unbound water increases, which behaves like pure water that is lightly adsorbed and loosely bound to the surface of the particles.13,46 The process of moisture sorption ceases when the particles reach saturation in moisture content. On the contrary, the desorption rate constants exhibited less sensitivity to both the humidity level and the humidity step size. Nevertheless, it is noticeable that, at higher RH levels, the rate of moisture desorption was high. This can be attributed to the presence of more unbound free water molecules, that could be easily removed from the system. In contrast, at lower %RH levels, the available water molecules tend to be tightly bound in monolayers or condensed within pores, requiring a longer time to be removed. In summary, it's crucial to recognise the pivotal roles that both sorption isotherms and sorption kinetics play in fully understanding and characterising materials and predicting their behaviour to moisture under different storage conditions.
3.2. Effect of temperature on EMC and kinetics rate constant
The effect of temperature is a critical factor that needs to be addressed, especially in the context of storage conditions. Water molecule mobility and the dynamic equilibrium between the vapour and adsorbed phases could both be influenced by temperature. The effect of increasing temperature to 50 °C on moisture uptake is shown in Fig. 3B.At low humidity levels, temperature appears to have minimal impact on excipient moisture content. However, notable differences in EMC emerged at 50 °C under high RH levels. Fig. 5 shows the relative change in moisture content as temperature increased from 25 °C to 50 °C at 30–75% RH. In general, for the studied excipients, maintaining constant humidity levels while elevating the temperature from 25 °C to 50 °C resulted in a notable 24–27% reduction in the fraction of sorbed water for DCPA, XPVP, CCS and L-HPC. SSG exhibited the least sensitivity to increased temperature, showing a 10% decrease in the sorbed moisture. However, mannitol and lactose showed exceptions to this trend, experiencing a 23% and 28% increase in moisture content, respectively. The increase in EMC for lactose and mannitol is small compared to disintegrants. However, it is crucial to note this observation considering that the soluble fillers lactose and mannitol are non-hygroscopic materials; similar effects have been reported in food research for some sugars.47 These differences in EMC are higher when compared to the dry mass of the powder, as shown in Fig. S1 in the ESI,† than from the mass at 30% RH.
The decrease in EMC with an increase in temperature from 25 °C to 50 °C is attributed to the activation of some water molecules at higher temperatures to energies that enable them to separate from their sorption sites, thus reducing the EMC. This observation is consistent with the findings of Al-Muhtaseb et al. (2004) who noted a similar trend in starches under varying temperatures. They attributed the decrease in EMC with rising temperature to a reduction in the number of active sites for water binding due to physical and/or chemical changes induced by heat.48 Palipane and Driscoll (1993) also explained that higher temperatures cause water molecules to reach higher energy levels, making them less stable and more likely to detach from water-binding sites.49 The thermodynamic equation ΔG = ΔH − TΔS explains this necessity, where ΔG is a measure of the spontaneity of a reaction, ΔH is the enthalpy change, T is the temperature in Kelvin, and ΔS is the entropy change. Since ΔG < 0 (indicating that sorption is a spontaneous process) and ΔS < 0 (suggesting reduced freedom for the sorbed water molecule), ΔH must be negative. Consequently, higher temperatures make conditions less favourable for water sorption.50
However, lactose and mannitol deviate from this pattern, becoming more hygroscopic at higher temperatures. This is attributed to their ability to dissolve in adsorbed water vapour, forming a solution that causes a rise in moisture content (Fig. 6). A similar trend is observed with certain sugars (such as glucose), sugar alcohols, and other low molecular weight food constituents (like salt) becoming more hygroscopic at higher temperatures because they can dissolve in water.47 Conversely, Bronlund and Paterson (2004) showed that crystalline lactose exhibits almost no dependence on temperature within the range of 12 °C to 37 °C except for amorphous lactose at 12 °C, which absorbed less moisture than observed at higher temperatures.41
 | ||
| Fig. 6 Illustration of the effect of temperature on the EMC of insoluble particles (left) and soluble particles (right). | ||
Although the temperature increase impacted moisture content, its effect on the kinetics rate constant was more significant (Fig. 4C and D). The sorption and desorption rate constants dramatically increased when temperature increased from 25 °C to 50 °C, particularly for hygroscopic excipients. For example, increasing temperature from 25 °C to 50 °C has resulted in an increased CCS sorption and desorption kinetic rate constants from 0.04 s−1 to 0.15 s−1 and from 0.08 s−1 to 0.23 s−1, respectively. The effect of temperature on the sorption/desorption rate constant is attributed to the fact that at a higher temperature, the kinetic energy of the water molecules increases, causing them to move more quickly and accelerate diffusion, which in turn raises the rate of sorption or desorption. In line with Fick's laws of diffusion, the rate of diffusion is directly proportional to the temperature. It is noteworthy that the effect of temperature on EMC and kinetic rate constant is material and RH-dependent. Fig. 7 illustrates the relative change in sorption and desorption rate constants at 30–75% RH from 25 °C to 50 °C, highlighting that the temperature affected the sorption rate constant more than the desorption, with the exception of XPVP. A substantial increase in the sorption rate constant of approximately 300% for CCS and SSG was observed compared to a 30% increase for mannitol. On the other hand, the desorption rate constant experiences an increase of around 190% for CCS and SSG compared to 42% for mannitol. While this relative change underscores the impact of temperature on the sorption/desorption process, it is essential to incorporate the absolute change for a comprehensive understanding (Fig. S4 in the ESI†). In instances where the relative change may seem very high, such as 300% increase for CCS, the absolute change was 0.11 s−1. Presenting the absolute change becomes crucial for understanding the magnitude of the alterations. This is particularly significant when considering the temporal dynamics of the sorption/desorption processes, as under all conditions, the equilibrium was reached on DVS within a day.
 | ||
| Fig. 7 Relative change in sorption (A) and desorption (B) kinetic rate constants from 25 °C to 50 °C when moving from 30%–75% RH. | ||
3.3. Particle size analysis
| Material | CED (geometric D50) (μm) | HS circularity mean (−) | Convexity mean (−) | Aspect ratio mean (−) |
|---|---|---|---|---|
| CCS | 45 | 0.72 | 0.96 | 0.61 |
| MCC | 94 | 0.76 | 0.98 | 0.61 |
| DCPA | 138 | 0.76 | 0.99 | 0.64 |
| XPVP | 97 | 0.78 | 0.97 | 0.66 |
| Man | 131 | 0.81 | 0.98 | 0.68 |
| SSG | 44 | 0.92 | 0.99 | 0.80 |
| Lac | 111 | 0.79 | 0.98 | 0.71 |
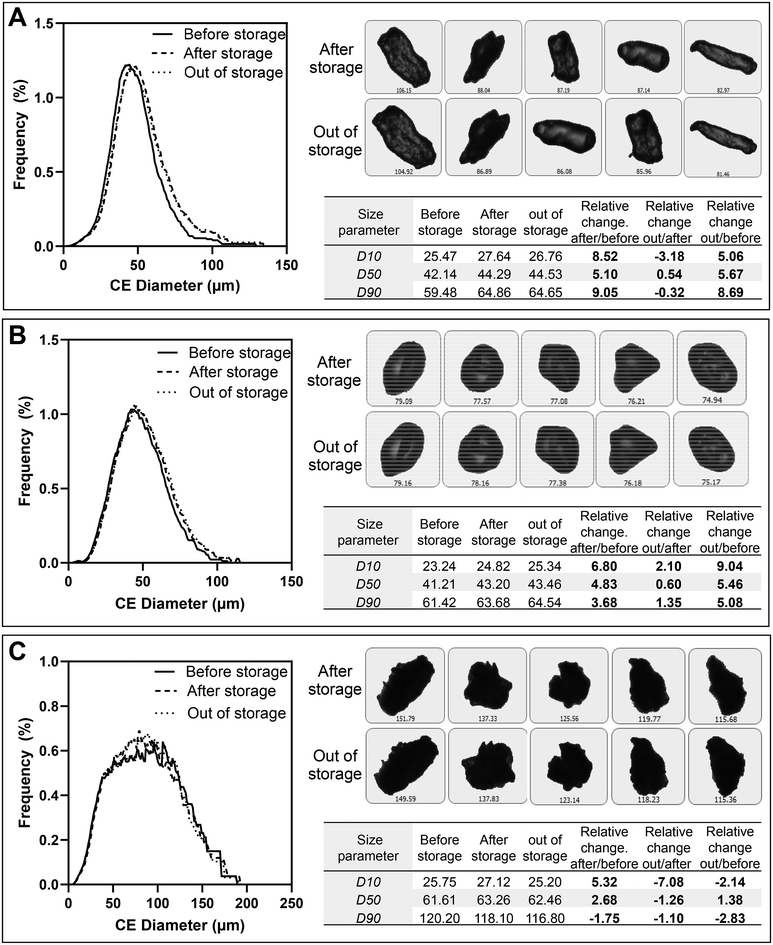
By comparing PSD before and after storage, storing CCS, SSG and MCC particles at 50 °C/75% RH induced a shift in the PSD consistently moving toward a higher particle size across all examined time points (Fig. 9). The PSD before and after storage for the other storage conditions are presented in the ESI (Fig. S7 and S8†). Because of the large number of smaller particles (<10 μm) and to reduce the sensitivity to them, volume-based size distribution has been used to assess the changes in PSD. Changes in the PSD using the number of particles was also used to visualise the change (Fig. S9 in the ESI†). Fig. 10 illustrates the relative changes in various volume-based size parameters for particles after 14 days of storage at 50 °C/75%. The fact that the D10, D50, and D90 all experienced a positive relative change suggests an overall increase in the particle size distribution of the three excipients studied.
 | ||
| Fig. 10 Average relative change in volume-based size parameters after 14 days of storage at 50 °C/75% RH. | ||
It is noteworthy, as shown in Fig. 8, that while the change in particle diameter followed the order of CCS > SSG > MCC, the minimal difference between CCS and SSG implies comparable effects, considering the potential error introduced due to variations in the threshold and errors resulting from it, as observed in Fig. S10 in the ESI.† This agrees with the results from DVS, which showed that CCS and SSG absorb moisture substantially into the porous structure with moisture uptake of 16.8% and 15.8%, respectively, compared to 5.1% for MCC at 50 °C/75% RH. CCS is a crosslinked carboxymethyl cellulose sodium which is a modified form of cellulose with a considerably larger swelling capacity than MCC. The results indicate that SSG and CCS exhibited the highest swelling capability, followed closely by MCC. However, prior findings showed a more pronounced change in volume mean diameter during SSG swelling compared to CCS.25,26,51 SSG, identified as the sodium salt of cross-linked carboxymethylated starch, undergoes modification through two chemical processes; substitution to enhance hydrophilicity and cross-linking to reduce solubility.21,52 The exceptional swelling capacity of SSG is attributed to the generous spacing between cross-linked phosphate groups, enabling effective water penetration, swelling, and gel formation.27 In contrast, CCS, with crosslinking through esterification, lacks significant spacing between polymer chains, resulting in a smaller swelling capacity.53 Soundaranathan et al. (2020) distinguished between the swelling patterns of SSG and CCS due to contact with water and showed that SSG had a more significant overall swelling than CCS, demonstrating a superior capacity to absorb water. At the same time CCS had a greater diffusion coefficient and, thus, a larger diffusion capacity due to its quicker liquid absorption at the surface and quicker initial swelling. The difference observed in our study compared to previous literature can be attributed to various factors. A crucial distinction lies in our specific focus on moisture-induced swelling rather than liquid water, potentially introducing unique considerations that may not precisely align with findings from studies concentrating solely on liquid water-induced swelling. Additionally, the measurement technique used, such as the reliance on circle equivalent diameter, assumes that all particles are spherical, providing the diameter of a circle with the same area as the measured particle. This assumption may not fully capture the complexity of morphological changes and could contribute to differences. Furthermore, the imaging method provides insights into the change in the diameter of the entire particle size distribution, offering a comprehensive overview of the particles’ behaviour. However, because of the polydispersed nature of the particle size distribution, high variability in the measured particle size parameters resulted, as smaller particles may behave differently from larger ones. This approach contrasts with some studies in the literature that focused on examining a few individual particles or compacts potentially overlooking the broader variability within the particle size distribution.
All particles achieved their maximum swelling indicating the attainment of their EMC within the initial day of storage as shown in Fig. 8. This means that the changes resulted due to storage conditions being too fast, and this can be explained by the large surface area of particles exposed to storage conditions which lead the changes to happen faster. Despite the accelerated changes within the first day, the long time required for the required RH level to be achieved inside the jars limited the ability to investigate alterations over shorter durations within this critical period (Fig. S11 in the ESI†). The findings from DVS further supported the prompt moisture equilibrium attainment within a day after exposure to all studied conditions.
The dynamics of particle swelling involve two key mechanisms: moisture uptake at the particle surface and the intrinsic swelling ability of the particle. Soundaranathan et al. (2020) showed that SSG has a relatively gradual initial swelling, indicating lower surface water uptake with hydration primarily originates from the interaction between the anionic carboxyl group and water.26 In contrast, CCS undergoes fast swelling driven by the hydration of the carboxymethyl group.53 However, our results indicated that all studied excipients reached maximum swelling within a day of storage with no differences between them. Taking a closer look at the swelling of these excipients during the first day could reveal more details and differences that might not be evident in the broader day-long observations.
A comprehensive analysis of all possible particle shape descriptors may be just as critical as particle size. Particle shape, however, is often ignored or disregarded. Fig. 11 provides insights into the morphological changes resulting from storage at elevated humidity and temperature. CCS and SSG experience more pronounced alterations in shape parameters compared to MCC, which exhibits the least impact. Specifically, the circularity of CCS particles undergoes the most significant decrease, approximately 9%, in contrast to the 1% decrease observed for MCC. Similarly, convexity decreases up to 5% for CCS particles, while MCC experiences a more modest 1.3% decrease. This consistent reduction in circularity and convexity across all excipients indicates a trend toward less rounded and rougher surface particle morphologies, aligning with the expected behaviour during particle swelling. Simultaneously, there is an increase in aspect ratio, underscoring changes in particle shapes induced by storage conditions. The observed changes were more pronounced for CCS than SSG and MCC suggesting that SSG and MCC swelled in all directions while CCS expanded mainly along the shorter(width) axis implying less elongated particles. This aligns with previous studies suggesting that swelling tends to be omnidirectional, with CCS particles primarily expanding from the shorter axis.21,25,29 It's essential to interpret the AR results cautiously, as these measurements rely on 2D imaging, while the particles’ swelling occurs along all directions. Consequently, capturing the precise direction of swelling poses challenges, and the AR results should be considered as an approximation rather than a definitive representation of the swelling direction.
The reversibility of particle size swelling was also investigated (Fig. 12). A comparison of particle size distribution profiles before and after storage revealed nearly identical PSD profiles for CCS and SSG particles post-storage and after a subsequent month outside of storage at the laboratory (25 °C/30% RH). This implies that there was no obvious shift in the PSD of swollen particles back to their initial state before storage, indicating a persistent alteration in particle size caused by prior exposure to moisture when the particles returned to laboratory baseline conditions. Particle images further supported these findings, indicating a minimal change in particle size, with a shift of approximately 0.5% for both CCS and SSG median diameter. In contrast, partial reversibility in PSD was observed for MCC particles, as evidenced by changes in median diameter decreasing by 1.26% compared to the maximum swelling observed directly after removal from storage (2.68%). This resulted in an overall swelling of 1.38% from the dry particle. However, due to the limited swelling of MCC, these changes should be interpreted cautiously.
Understanding how moisture irreversibly affects the particle's size is crucial to understand how excipients respond to changes during storage conditions, especially when evaluating materials’ stability. The swelling of disintegrants before use, occurring during storage, plays a pivotal role in inducing physical instability, thereby diminishing their effectiveness. This phenomenon directly influences how drugs are released from the dosage forms.23,24 Furthermore, the swelling of these excipients inside the dosage forms due to storage extends beyond affecting the size of the tablets; it also influences porosity and other mechanical properties due to irreversible alteration in their microstructure, thereby impacting their overall physical stability. In a study by Maclean et al. (2022) the impact of storage conditions on different formulations with diverse disintegrants and fillers was compared. Most formulations exhibited an increase in porosity and a decrease in tensile strength after being stored in high humidity, further influencing disintegration time, and attributed to the premature swelling of disintegrants.24 Hersen-Delesalle et al. (2007) similarly observed that an increase in RH resulted in a reduction in tensile strength.30 This was associated with the formation of cracks on the surface and internal structure of tablets containing CCS, SSG, and XPVP disintegrants, likely caused by the premature activation of disintegrants absorbing moisture from the surrounding air.
The properties of the raw materials play a key role in determining how tablets change when they are compressed and exposed to storage conditions. Understanding the swelling behaviour at the particle level provides valuable insights into how these materials respond to environmental factors. This knowledge is essential for anticipating potential changes in tablet properties and performance, such as size, porosity, tensile strength and disintegration, right from the early stages of formulation, contributing to the overall stability and performance of the final tablets.
4. Conclusion
This study focused on investigating the impact of varying humidity and temperature conditions on the moisture uptake rates and extent of commonly used excipients in immediate-release formulations and the resulting changes in their particle size and shape parameters. This is important for further assessing the effect of material attributes on tablets’ performance and physical stability. DVS results indicated that particles exposed to higher humidity levels exhibited increased moisture adsorption, diffusing into the bulk, yet requiring a longer time to reach the EMC. Temperature played a significant role in moisture dynamics, where Increasing temperature increased the sorption/desorption rate constants of all excipients studied, accompanied by a decrease in the EMC, except for mannitol and lactose. Understanding and quantifying the moisture sorption/desorption of particles is pivotal for formulating oral solid dosage forms.The study also highlighted varying swelling behaviour under accelerated conditions, with excipients following the order CCS > SSG > MCC, with minimal difference between CCS and SSG. This swelling was a result of humidity, not temperature effect. However, all of the excipients reached the maximum swelling within the first day of storage. This change in particle size was permanent, exhibiting partial reversibility for MCC and no restoration of initial size for CCS and SSG.
This knowledge aids in predicting and modelling the moisture sorption behaviour of the dosage form, along with other properties influenced by storage moisture, such as swelling and disintegration crucial for drug release. Furthermore, future modelling efforts could focus on linking moisture uptake and particle swelling with drug dissolution performance, exploring how varying levels of moisture affect drug product performance. Moreover, quantifying the swelling attributes of individual particles will enhance the development of formulations, especially for modelling the tablet disintegration process, where particle swelling plays an important role as a key mechanism controlling the performance. These findings could initiate discussions on how regulatory authorities might consider the pre-swelling of particles in future guidelines.
However, predicting moisture behaviour and swelling patterns of powder compact based on particle data requires a deep understanding of the interconnected factors, including excipient interactions, tablet microstructure, liquid absorption kinetics, inter-particle bonding, and potential changes in raw material properties. This approach is of importance for the development of pharmaceutical formulations that exhibit sustained efficacy and stability over time.
Data availability
Data for this article, including the raw data of dynamic vapor sorption and processed data for particle size measurements, are available at University of Strathclyde KnowledgeBase at https://doi.org/10.15129/e9a284a2-8954-463e-9fed-06b5b1893c82.Conflicts of interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Daniel Markl reports a relationship with AstraZeneca PLC that includes: funding grants and non-financial support. Ibrahim Khadra reports a relationship with AstraZeneca PLC that includes: funding grants and nonfinancial support. James Mann reports a relationship with AstraZeneca PLC that includes: employment. Alexander Abbott reports a relationship with AstraZeneca PLC that includes: employment. Fredrik Winge reports a relationship with AstraZeneca PLC that includes: employment. Adrian Davis reports a relationship with Pfizer that includes: employment. Bart Hens reports a relationship with Pfizer that includes: employment.Acknowledgements
The authors would like to acknowledge the Community for Analytical Measurement Science (CAMS) for funding this project (Grant Ref CAMS2021/IPostD/03). The authors would like to acknowledge that this work was carried out in the CMAC National Facility supported by UKRPIF (UK Research Partnership Fund) award from the Higher Education Funding Council for England (HEFCE) (Grant Ref HH13054).References
- M. A. Darji, R. M. Lalge, S. P. Marathe, T. D. Mulay, T. Fatima and A. Alshammari, et al., Excipient Stability in Oral Solid Dosage Forms: A Review, AAPS PharmSciTech, 2018, 19(1), 12–26 CrossRef CAS PubMed.
- S. P. Chamarthy, R. Pinal and M. T. Carvajal, Elucidating Raw Material Variability—Importance of Surface Properties and Functionality in Pharmaceutical Powders, AAPS PharmSciTech, 2009, 10(3), 780 CrossRef PubMed.
- J. M. Viljoen, J. H. Steenekamp, A. F. Marais and A. F. Kotzé, Effect of moisture content, temperature and exposure time on the physical stability of chitosan powder and tablets, Drug Dev. Ind. Pharm., 2014, 40(6), 730–742 CrossRef CAS.
- N. Veronica, P. W. S. Heng and C. V. Liew, Relative Humidity Cycling: Implications on the Stability of Moisture-Sensitive Drugs in Solid Pharmaceutical Products, Mol. Pharmaceutics, 2023, 20(2), 1072–1085 CrossRef CAS PubMed.
- A. J. Hickey and S. Giovagnoli, Physical Properties Characterization, in Pharmaceutical Powder and Particles [Internet], Springer International Publishing, Cham, 2018, pp. 21–28. (AAPS Introductions in the Pharmaceutical Sciences) Search PubMed.
- N. Veronica, P. W. S. Heng and C. V. Liew, Ensuring Product Stability – Choosing the Right Excipients, J. Pharm. Sci., 2022, 111(8), 2158–2171 CrossRef CAS.
- Y. Guo, E. Shalaev and S. Smith, Physical stability of pharmaceutical formulations: solid-state characterization of amorphous dispersions, TrAC, Trends Anal. Chem., 2013, 49, 137–144 CrossRef CAS.
- H. Williams, Predictive Stability Testing Utilizing Accelerated Stability Assessment Program (ASAP) Studies, in Methods for Stability Testing of Pharmaceuticals [Internet], ed. S. Bajaj and S. Singh, Springer New York, New York, NY, 2018, pp. 213–232. (Methods in Pharmacology and Toxicology) Search PubMed.
- A. Berardi, P. H. M. Janssen and B. H. J. Dickhoff, Technical insight into potential functional-related characteristics (FRCs) of sodium starch glycolate, croscarmellose sodium and crospovidone, J. Drug Delivery Sci. Technol., 2022, 70, 103261 CrossRef CAS.
- S. Bajaj, D. Singla and N. Sakhuja, Stability Testing of Pharmaceutical Products, J. Appl. Pharm. Sci., 2012, 2(3), 129–138 Search PubMed.
- A. R. Rajabi-Siahboomi, M. Levina, S. B. Upadhye and J. Teckoe, Excipient Selection in Oral Solid Dosage Formulations Containing Moisture Sensitive Drugs, in Excipient Applications in Formulation Design and Drug Delivery [Internet], ed. A.S Narang and S.HS. Boddu, Springer International Publishing, Cham, 2015, pp. 385–421 Search PubMed.
- C. Ahlneck and G. Zografi, The molecular basis of moisture effects on the physical and chemical stability of drugs in the solid state, Int. J. Pharm., 1990, 62(2–3), 87–95 CrossRef CAS.
- S. Airaksinen, M. Karjalainen, A. Shevchenko, S. Westermarck, E. Leppänen and J. Rantanen, et al., Role of water in the physical stability of solid dosage formulations, J. Pharm. Sci., 2005, 94(10), 2147–2165 CrossRef CAS PubMed.
- J. S. M. Garr and M. H. Rubinstein, The influence of moisture content on the consolidation and compaction properties of paracetamol, Int. J. Pharm., 1992, 81(2–3), 187–192 CrossRef CAS.
- C. R. Dalton and B. C. Hancock, Processing and storage effects on water vapor sorption by some model pharmaceutical solid dosage formulations, Int. J. Pharm., 1997, 156(2), 143–151 CrossRef CAS.
- S. Rajabnezhad, T. Ghafourian, A. Rajabi-Siahboomi, S. Missaghi, M. Naderi and J. P. Salvage, et al., Investigation of water vapour sorption mechanism of starch-based pharmaceutical excipients, Carbohydr. Polym., 2020, 238, 116208 CrossRef CAS.
- M. Caurie, Hysteresis phenomenon in foods, Int. J. Food Sci. Technol., 2007, 42(1), 45–49 CrossRef CAS.
- L. Salmén and P. A. Larsson, On the origin of sorption hysteresis in cellulosic materials, Carbohydr. Polym., 2018, 182, 15–20 CrossRef.
- H. Omidian and K. Park, Swelling agents and devices in oral drug delivery, J. Drug Delivery Sci. Technol., 2008, 18(2), 83–93 CrossRef CAS.
- J. Quodbach and P. Kleinebudde, A critical review on tablet disintegration, Pharm. Dev. Technol., 2016, 21(6), 763–774 CAS.
- P. M. Desai, C. V. Liew and P. W. S. Heng, Review of Disintegrants and the Disintegration Phenomena, J. Pharm. Sci., 2016, 105(9), 2545–2555 CrossRef CAS.
- S. K. De Richter, N. Gaudel, C. Gaiani, A. Pascot, M. Ferrari and M. Jenny, Swelling of couscous grains under saturated conditions, J. Food Eng., 2022, 319, 110910 CrossRef.
- A. Berardi, L. Bisharat, J. Quodbach, S. Abdel Rahim, D. R. Perinelli and M. Cespi, Advancing the understanding of the tablet disintegration phenomenon – An update on recent studies, Int. J. Pharm., 2021, 598, 120390 CrossRef CAS.
- N. Maclean, I. Khadra, J. Mann, H. Williams, A. Abbott and H. Mead, et al., Investigating the role of excipients on the physical stability of directly compressed tablets, Int. J. Pharm., 2022, X, 4 Search PubMed.
- M. Soundaranathan, P. Vivattanaseth, E. Walsh, K. Pitt, B. Johnston and D. Markl, Quantification of swelling characteristics of pharmaceutical particles, Int. J. Pharm., 2020, 590, 119903 CrossRef CAS.
- N. Zhao and L. L. Augsburger, The influence of swelling capacity of superdisintegrants in different pH media on the dissolution of hydrochlorothiazide from directly compressed tablets, AAPS PharmSciTech, 2005, 6(1), 120–126 CrossRef.
- J. Rojas, S. Guisao and V. Ruge, Functional Assessment of Four Types of Disintegrants and their Effect on the Spironolactone Release Properties, AAPS PharmSciTech, 2012, 13(4), 1054–1062 CrossRef CAS PubMed.
- V. A. Lovikka, L. Rautkari and T. C. Maloney, Changes in the hygroscopic behavior of cellulose due to variations in relative humidity, Cellulose, 2018, 25(1), 87–104 CrossRef CAS.
- A. Berardi, L. Bisharat, A. Blaibleh, L. Pavoni and M. Cespi, A Simple and Inexpensive Image Analysis Technique to Study the Effect of Disintegrants Concentration and Diluents Type on Disintegration, J. Pharm. Sci., 2018, 107(10), 2643–2652 CrossRef CAS.
- C. Hersen-Delesalle, B. Leclerc, G. Couarraze, V. Busignies and P. Tchoreloff, The effects of relative humidity and super-disintegrant concentrations on the mechanical properties of pharmaceutical compacts, Drug Dev. Ind. Pharm., 2007, 33(12), 1297–1307 CrossRef CAS PubMed.
- T. N. Hiew, N. A. B. Johan, P. M. Desai, S. M. Chua, Z. H. Loh and P. W. S. Heng, Effect of moisture sorption on the performance of crospovidone, Int. J. Pharm., 2016, 514(1), 322–331 CrossRef CAS.
- J. Quodbach and P. Kleinebudde, Performance of tablet disintegrants: Impact of storage conditions and relative tablet density, Pharm. Dev. Technol., 2015, 20(6), 762–768 CrossRef CAS PubMed.
- M. Sacchetti, R. Teerakapibal, K. Kim and E. J. Elder, Role of Water Sorption in Tablet Crushing Strength, Disintegration, and Dissolution, AAPS PharmSciTech, 2017, 18(6), 2214–2226 CrossRef CAS PubMed.
- S. Suherman, M. Peglow and E. Tsotsas, Measurement and modelling of sorption equilibrium curve of water on pa6, pp, hdpe and pvc by using flory-huggins model, Reaktorn, 2010, 13(2), 89 CrossRef.
- N. Maclean, E. Walsh, M. Soundaranathan, I. Khadra, J. Mann and H. Williams, et al., Exploring the performance-controlling tablet disintegration mechanisms for direct compression formulations, Int. J. Pharm., 2021, 599, 120221 CrossRef CAS PubMed.
- J. Quodbach and P. Kleinebudde, Systematic classification of tablet disintegrants by water uptake and force development kinetics, J. Pharm. Pharmacol., 2014, 66(10), 1429–1438 CrossRef CAS.
- P. Arlabosse, E. Rodier, J. H. Ferrasse, S. Chavez and D. Lecomte, Comparison Between Static and Dynamic Methods for Sorption Isotherm Measurements, Drying Technol., 2003, 21(3), 479–497 CrossRef CAS.
- J. F. Gamble, W. S. Chiu and M. Tobyn, Investigation into the impact of sub-populations of agglomerates on the particle size distribution and flow properties of conventional microcrystalline cellulose grades, Pharm. Dev. Technol., 2011, 16(5), 542–548 CrossRef CAS.
- G. Rowley and L. A. Mackin, The effect of moisture sorption on electrostatic charging of selected pharmaceutical excipient powders, Powder Technol., 2003, 135–136, 50–58 CrossRef CAS.
- B. C. Hancock and S. L. Shamblin, Water vapour sorption by pharmaceutical sugars, Pharm. Sci. Technol. Today, 1998, 1(8), 345–351 CrossRef CAS.
- J. Bronlund and T. Paterson, Moisture sorption isotherms for crystalline, amorphous and predominantly crystalline lactose powders, Int. Dairy J., 2004, 14(3), 247–254 CrossRef CAS.
- G. Zografi and M. J. Kontny, The interactions of water with cellulose- and starch-derived pharmaceutical excipients, Pharm. Res., 1986, 03(4), 187–194 CrossRef CAS.
- L. C. Li, J. Parasrampuria, R. Levans and K. G. Van Scoik, A Study of The Moisture-Uptake Kinetics of a Hygroscopic Pharmaceutical Powder, Drug Dev. Ind. Pharm., 1994, 20(13), 2079–2090 CrossRef CAS.
- N. A. Visalakshi, T. T. Mariappan, H. Bhutani and S. Singh, Behavior of Moisture Gain and Equilibrium Moisture Contents (EMC) of Various Drug Substances and Correlation with Compendial Information on Hygroscopicity and Loss on Drying, Pharm. Dev. Technol., 2005, 10(4), 489–497 CrossRef CAS PubMed.
- D. Heidemann and P. Jarosz, Preformulation Studies Involving Moisture Uptake in Solid Dosage Forms, Pharm. Res., 1991, 08(3), 292–297 CrossRef CAS.
- J. Khazaei, Water absorption characteristics of three wood varieties, Cercetări Agronomice în Moldova, 2008, XLI(2), 134–145 Search PubMed.
- A. H. Al-Muhtaseb, W. A. M. McMinn and T. R. A. Magee, Moisture sorption isotherm characteristics of food products: A review, Food Bioprod. Process., 2002, 80(2), 118–128 CrossRef CAS.
- A. H. Al-Muhtaseb, W. A. M. McMinn and T. R. A. Magee, Water sorption isotherms of starch powders, J. Food Eng., 2004, 61(3), 297–307 CrossRef.
- K. B. Palipane and R. H. Driscoll, Moisture sorption characteristics of in-shell macadamia nuts, J. Food Eng., 1993, 18(1), 63–76 CrossRef.
- J. G. Kapsalis, Influences of Hysteresis and Temperature on Moisture Sorption Isotherms, in Water Activity: Theory and Applications to Food, ed. L. B. Rockland, L. R. Beuchat, Marcel Dekker Inc, New York, USA, 1987, pp. 173–213 Search PubMed.
- P. M. Desai, C. V. Liew and P. W. S. Heng, Understanding Disintegrant Action by Visualization, J. Pharm. Sci., 2012, 101(6), 2155–2164 CrossRef CAS.
- U. Shah and L. Augsburger, Multiple Sources of Sodium Starch Glycolate, NF: Evaluation of Functional Equivalence and Development of Standard Performance Tests, Pharm. Dev. Technol., 2002, 7(3), 345–359 CrossRef CAS.
- P. Zarmpi, T. Flanagan, E. Meehan, J. Mann and N. Fotaki, Biopharmaceutical aspects and implications of excipient variability in drug product performance, Eur. J. Pharm. Biopharm., 2017, 111, 1–15 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4pm00259h |
| This journal is © The Royal Society of Chemistry 2025 |