 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: TBAI-mediated electrochemical oxidative synthesis of quinazolin-4(3H)-ones from 2-aminobenzamides and isothiocyanates
Jingbin
Huang
a,
Yafeng
Liu
c,
Yu
Huang
a,
Xiuli
Wu
a,
Xiao-Bing
Lan
a,
Jian-Qiang
Yu
a,
Wenxue
Li
a,
Ping
Zheng
*a,
Jian
Zhang
*ab and
Zhenyu
An
*a
aKey Laboratory of Protection, Development and Utilization of Medicinal Resources in Liupanshan Area, Ministry of Education, Peptide & Protein Drug Research Center, School of Pharmacy, Ningxia Medical University, Yinchuan 750004, China. E-mail: anzy@nxmu.edu.cn
bMedicinal Chemistry and Bioinformatics Center, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China. E-mail: jian.zhang@sjtu.edu.cn
cSchool of Chemistry and Chemical Engineering, North Minzu University, Yinchuan 750000, Ningxia, China
First published on 28th May 2025
Abstract
Correction for ‘TBAI-mediated electrochemical oxidative synthesis of quinazolin-4(3H)-ones from 2-aminobenzamides and isothiocyanates’ by Jingbin Huang et al., Org. Biomol. Chem., 2025, 23, 4860–4865, https://doi.org/10.1039/d5ob00410a.
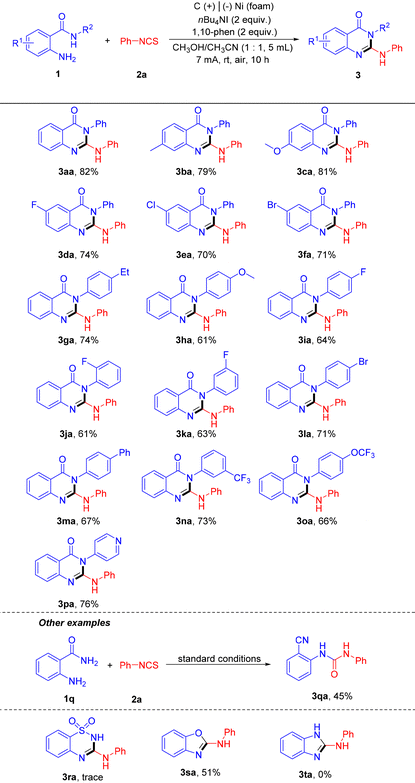
The authors regret that the structure of compound 3qa was incorrectly assigned. The compound is the isomeric o-ureidobenzonitrile and not the intended 2-aminoquinazolinone. The revised structure is shown in the corrected Table 2 below. In addition, updated supplementary information files have been published, which include the revised structure.
a Reaction conditions: 1 (0.2 mmol, 1 equiv.), 2a (0.4 mmol, 2 equiv.), nBu4NI (0.4 mmol, 2 equiv.), 1,10-phen (0.4 mmol, 2 equiv.), and CH3OH/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5 mL) in an undivided cell equipped with carbon rod (Φ 6 mm) as anode and Ni foam (1.0 cm × 1.0 cm × 0.3 cm) as cathode, air, 7 mA (15.9 F mol−1), rt, 10 h, FE = 12.6%. 1, 5 mL) in an undivided cell equipped with carbon rod (Φ 6 mm) as anode and Ni foam (1.0 cm × 1.0 cm × 0.3 cm) as cathode, air, 7 mA (15.9 F mol−1), rt, 10 h, FE = 12.6%.
|
|---|
|
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2025 |
