 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Privileged scaffolds on demand: a Passerini-based strategy toward α-ketoamides
Michael
Georgoulakis
,
Ioannis
Angelonidis
,
Konstantinos G.
Froudas
,
Nikolaos
Eleftheriadis
 and
Constantinos G.
Neochoritis
and
Constantinos G.
Neochoritis
 *
*
Department of Chemistry, University of Crete, Heraklion, Greece. E-mail: kneochor@uoc.gr
First published on 14th November 2025
Abstract
We report a scalable Passerini-based method for synthesizing α-ketoamides, key medicinal motifs. Using p-hydroxybenzoic acid and ethanol, Mumm rearrangement was interrupted to yield α-hydroxyamides and then oxidized in one pot. The method tolerates diverse substrates, enables gram-scale synthesis and affords inhibitors of human 15-LOX-1, highlighting its biological and synthetic potential.
α-Ketoamides (also known as α-oxoamides) represent a privileged and versatile scaffold in medicinal chemistry, and are widely exploited for their unique structural and electronic properties.1,2 Structurally, α-ketoamides feature vicinal carbonyl groups: one incorporated into an amide (attached to a nitrogen atom and the molecular backbone) and the other as a ketone (bonded to an alkyl or aryl group).1 The electron-withdrawing nature of the amide enhances the electrophilicity of the adjacent ketone, rendering it particularly reactive toward nucleophiles. By mining the Cambridge Structural Database (CSD),3 we have found 459 single-crystal structures (see the SI) which revealed that the two oxygen atoms are in a trans disposition (in the acyclic compounds), mainly because of the mutual repulsion, and the average length of the C–C bond is 1.54 Å, suggesting no resonance contribution to the interaction between the two carbonyl groups (Fig. 1A). α-Ketoamides are found in a variety of natural products and pharmaceutical agents, exhibiting a broad spectrum of biological activities, most notably as protease inhibitors and immunosuppressants.4–9 Beyond their inherent bioactivity, they have also proven valuable as precursors in diverse functional group transformations and in the synthesis of numerous heterocyclic frameworks, underscoring both their synthetic and therapeutic utility.10–12 Their biochemical significance is exemplified by the FDA-approved hepatitis C drugs boceprevir, telaprevir and narlaprevir, which act as reversible covalent inhibitors of the viral NS3/4A serine protease, through covalent interaction with the enzyme's catalytic serine residue (Fig. 1B).13–15 Compared to other dicarbonyl derivatives, i.e. α-ketoacids and α-ketoesters, α-ketoamides have been shown to possess better pharmacokinetic properties.1
 | ||
| Fig. 1 (A) Structural features of the α-ketoamide motif and (B) its presence in natural products, active pharmaceutical ingredients and bioactive compounds. | ||
As a result, the development of efficient synthetic routes for the preparation of α-ketoamides has attracted considerable attention.2,12 A conventional strategy involves the oxidation of α-hydroxyamides, which are typically synthesized through the condensation of protected α-hydroxy acid derivatives with amines in the presence of coupling agents.16,17 However, this method is often limited by the commercial availability of α-hydroxy acids and the difficulty of their preparation. To overcome these limitations, several alternative approaches based on MCRs have been explored; based on the Passerini reaction (P-3CR), oxalic acid decarbonylation/decarboxylation,18 cinnamaldehyde under basic conditions19 and the stereoconservative Passerini/amine deprotection acyl migration (PADAM) process,20 amongst others, have been employed as well. An alternative strategy is the truncated Passerini reaction, in which a molecule of water replaces the carboxylic acid component, facilitated by Brønsted or Lewis acids (Fig. 2A).21–27 Although this type of reaction proceeds under mild conditions, it often suffers from limited substrate scope, functioning efficiently only with unfunctionalized or minimally functionalized starting materials. Tron et al.28,29 introduced an elegant strategy known as the “sacrificial” Mumm rearrangement, in which an intramolecular nucleophile intercepts the classical rearrangement step of the Passerini reaction. This process generates products through an interrupted pathway, with the carboxylic acid component functioning as a leaving group. Using 2-hydroxymethylbenzoic acid, they demonstrated this concept for the synthesis of α-hydroxyamides (Fig. 2A). In our recent work, we found that vanillic acid could, to a certain extent, exhibit similar behavior in a P-3CR.30 Motivated by this observation, we explored whether other carboxylic acids might play a comparable role and, more importantly, whether an external nucleophile, such as the solvent, could intercept the rearrangement step. We also aimed to integrate a subsequent oxidation step in the same pot, thereby establishing a practical and scalable protocol for the direct synthesis of highly diverse α-ketoamides, without the need to isolate or purify the intermediate α-hydroxyamide. This approach would additionally prevent phthalide formation (Fig. 2B).
Based on the aforementioned findings and subsequent screening (see the SI), we identified p-hydroxybenzoic acid as an inexpensive and readily available substrate that can efficiently be engaged in a sacrificial Passerini reaction. This transformation predominantly furnishes the corresponding α-hydroxyamides 1, which can be further oxidized to α-ketoamides 2 (Scheme 1). Notably, a minor side pathway afforded the classical Passerini adduct (ca. 20%). In contrast, other acids such as 2- and 3-methoxybenzoic acids afforded solely the corresponding classical P-3CR adducts, while 3,4,5-trihydroxybenzoic acid (i.e. gallic acid) gave a complex reaction mixture from which the truncated product could not be identified. Interestingly, under mild conditions, ethanol was found to efficiently intercept the Mumm rearrangement, leading to the formation of α-hydroxyamides. Notably, when the reaction was performed exclusively in dichloromethane (DCM), the solvent commonly used for most Passerini reactions, the P-3CR adduct was predominantly observed, with only trace amounts of the α-hydroxyamide. Conversely, using EtOH, MeOH, trifluoroethanol (TFE) or i-PrOH alone resulted in complex mixtures (see the SI). A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of DCM with various protic polar solvents (EtOH, MeOH) yielded the desired α-hydroxyamides 1 along with the corresponding p-hydroxybenzoate esters. For practical reasons related to product isolation and purification, the EtOH/DCM system was selected as the optimal solvent mixture for further studies. In particular, we refluxed the isolated classical P-3CR adduct in both MeOH and EtOH (see Table S1) and did not observe any transesterification, supporting the proposed interception mechanism.
1 mixture of DCM with various protic polar solvents (EtOH, MeOH) yielded the desired α-hydroxyamides 1 along with the corresponding p-hydroxybenzoate esters. For practical reasons related to product isolation and purification, the EtOH/DCM system was selected as the optimal solvent mixture for further studies. In particular, we refluxed the isolated classical P-3CR adduct in both MeOH and EtOH (see Table S1) and did not observe any transesterification, supporting the proposed interception mechanism.
 | ||
| Scheme 1 Synthetic access to α-hydroxyamides and α-ketoamides via an interrupted Passerini reaction employing p-hydroxybenzoic acid; the single crystal structure of the α-ketoamide 2n (CCDC 1868912)31 and the intermolecular hydrogen bonding of 2.4 Å are shown. aThe corresponding hydroxyamide has been isolated as well. Although the conversion towards the α-hydroxyamides is >75%, their isolation during column chromatography is hampered by the coelution with either the corresponding P-3CR adduct or the p-hydroxybenzoic ethyl ester. | ||
Therefore, the reaction of isobutyraldehyde with p-bromophenyl isocyanide in EtOH/DCM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 50 °C furnished the corresponding α-hydroxyamide 1g in 76% yield, accompanied by a minor amount of the classical Passerini adduct. Subsequent oxidation with PCC led to the formation of the desired α-ketoamide 2a in 89% yield (Scheme 1). In addition, the entire sequence was conducted in a one-pot fashion, without purification of the intermediate α-hydroxyamide, isolating 2a in 74% overall yield. To further demonstrate the sacrificial role of p-hydroxybenzoic acid in EtOH/DCM, we synthesized a small set of additional hydroxy derivatives (1a–1i, 26–76% yields, Scheme 1 and the SI). The methodology exhibits broad substrate scope and excellent functional group tolerance (Scheme 1). It efficiently accommodates both aliphatic (linear and sterically hindered) and aromatic aldehydes, which is a long-standing challenge for most existing synthetic approaches.2 Similarly, a diverse set of isocyanides was successfully employed, including phenyl isocyanides with various substitution patterns, as well as aliphatic and benzyl isocyanides. As expected, the oxidation step proceeded smoothly across all substrates, furnishing the targeted α-ketoamides 2a–2o in 50–78% yields in a one-pot fashion. Importantly, the reaction sequence proved to be scalable, as it was successfully performed on 10 mmol (2k) and 5 mmol (2i) scales without any loss in efficiency or yield. The crystal structure of derivative 2n revealed key spatial features of the α-ketoamide scaffold (Scheme 1).31 The two oxygen atoms adopt a trans disposition with a dihedral angle of approximately 162°, while the plane of the α-keto carbonyl group forms an angle of ∼18° relative to the amide plane (see the SI). Notably, the amide carbonyl participates in an intermolecular hydrogen bond with a neighboring amide NH group, featuring an intermolecular hydrogen bond of 2.4 Å.
1) at 50 °C furnished the corresponding α-hydroxyamide 1g in 76% yield, accompanied by a minor amount of the classical Passerini adduct. Subsequent oxidation with PCC led to the formation of the desired α-ketoamide 2a in 89% yield (Scheme 1). In addition, the entire sequence was conducted in a one-pot fashion, without purification of the intermediate α-hydroxyamide, isolating 2a in 74% overall yield. To further demonstrate the sacrificial role of p-hydroxybenzoic acid in EtOH/DCM, we synthesized a small set of additional hydroxy derivatives (1a–1i, 26–76% yields, Scheme 1 and the SI). The methodology exhibits broad substrate scope and excellent functional group tolerance (Scheme 1). It efficiently accommodates both aliphatic (linear and sterically hindered) and aromatic aldehydes, which is a long-standing challenge for most existing synthetic approaches.2 Similarly, a diverse set of isocyanides was successfully employed, including phenyl isocyanides with various substitution patterns, as well as aliphatic and benzyl isocyanides. As expected, the oxidation step proceeded smoothly across all substrates, furnishing the targeted α-ketoamides 2a–2o in 50–78% yields in a one-pot fashion. Importantly, the reaction sequence proved to be scalable, as it was successfully performed on 10 mmol (2k) and 5 mmol (2i) scales without any loss in efficiency or yield. The crystal structure of derivative 2n revealed key spatial features of the α-ketoamide scaffold (Scheme 1).31 The two oxygen atoms adopt a trans disposition with a dihedral angle of approximately 162°, while the plane of the α-keto carbonyl group forms an angle of ∼18° relative to the amide plane (see the SI). Notably, the amide carbonyl participates in an intermolecular hydrogen bond with a neighboring amide NH group, featuring an intermolecular hydrogen bond of 2.4 Å.
To further demonstrate the utility of our synthetic approach and explore the accessible chemical space, we investigated whether the resulting α-ketoamides could undergo additional functionalization. Accordingly, we subjected compounds 2b, 2k and 2n to a second P-3CR, which successfully afforded the corresponding acyloxyamides (3a–3c) in 42–60% yields (Scheme 2). This verifies the electrophilic character of the α-carbonyl, highlighting the synthetic value and modularity of this union of MCR strategies.32
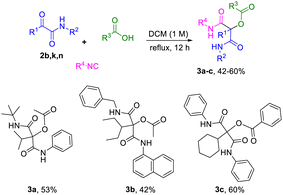 | ||
| Scheme 2 α-Ketoamides as carbonyl components in a P-3CR expanding the chemical space and verifying the electrophilic character of the α-carbonyl. | ||
The distinctive reactivity of α-ketoamides makes them promising candidates for modulating inflammation-related enzymes such as phospholipase A2 (PLA2) and lipoxygenases (LOXs), which play central roles in the release and oxidative metabolism of arachidonic acid.33 While acyl-CoA derivatives and polyphenolic inhibitors have been previously employed to inhibit LOX isoforms,33,34 the application of α-ketoamides as direct inhibitors of human 15-LOX-1 remains underexplored. In our recent paper, we have successfully evolved a fluorescence imaging agent into a potent α-hydroxyamide therapeutic entity, demonstrating a remarkable time dependent inhibitory potency which was attributed to increased binding and iron chelation.30 Building on this observation, we evaluated our newly synthesized derivatives against human 15-LOX-1, following established protocols.30,35–37 In a preliminary inhibitory screen at 50 μM, compounds showing activity were further assessed to determine their IC50 values (Fig. 3 and Fig. S4, S5).
To investigate time-dependent inhibition, the screening was performed after 10 and 30 min preincubations of the compounds with the enzyme.34,38 Our results revealed that ketoamides were more potent than α-hydroxyamides, exhibiting IC50 values below 50 μM (Fig. 3 and Fig. S5). Moreover, the majority of ketoamides exhibited a clear time-dependent inhibitory profile within the 30-minute timeframe (Fig. 3). This trend was confirmed in a detailed time-dependent inhibition assay with two of our most active ketoamide inhibitors, 2j (IC50 at 10 min = 17.8 ± 2.5 μM) and 2f (IC50 at 10 min = 37.1 ± 4.4 μM) (Fig. 3). For comparison, we included ThioLox, a known competitive 15-LOX-1 inhibitor, as a control.39 In these experiments, the enzyme was preincubated with inhibitors for 5–30 min, after which residual activity was measured. 2j and 2f were the only compounds to demonstrate a progressive, time-dependent decline in enzyme activity; in contrast, ThioLox rapidly reached equilibrium inhibition within 5 min and exhibited no further change over 30 min (Fig. 3). Finally, to investigate the inhibition mechanism, we performed a Michaelis–Menten kinetics analysis with 2j. As expected, this compound reduced both Km and Vmax values (Fig. 3; Table S4), indicating an uncompetitive mode of inhibition for 15-LOX-1. Our findings suggest that the inhibitory activity of ketoamides is associated with strong binding to the enzyme, iron chelation, and subsequent enzyme inactivation.
To conclude, we developed a scalable Passerini-based method for direct synthesis of α-ketoamides, key medicinal motifs. Using p-hydroxybenzoic acid and ethanol, we achieved one-pot access to diverse α-ketoamides, confirmed structurally by crystallography. These compounds also served as electrophiles in a second Passerini reaction and showed potent, time-dependent 15-LOX-1 inhibition, highlighting their therapeutic potential.
Author contributions
C. G. N. conceptualized and directed the project. M. G. performed the syntheses and collected the analytical data. I. A. performed the in vitro evaluation. K. G. F. determined the single crystal X-ray structure. C. G. N. and N. E. contributed to manuscript writing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: general procedures, characterization data for products (NMR and MS), CSD data mining and in vitro biological evaluation. See DOI: https://doi.org/10.1039/d5ob01629k.Acknowledgements
We acknowledge (a) the Empeirikeion Foundation, (b) the National Recovery and Resilience Plan Greece 2.0, funded by the European Union-NextGenerationEU (project code: TAEDR-0535850) and (c) the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the Greece 2.0 Basic Research Financing Action “Horizontal support of all sciences” Sub-action 2 (project number: 15511) for providing grants to N. E. We acknowledge Prof. T. Holman (University of California, Santa Cruz) for providing the human 15-LOX-1 plasmid.References
- M. Robello, E. Barresi, E. Baglini, S. Salerno, S. Taliani and F. Da Settimo, J. Med. Chem., 2021, 64, 3508–3545 CrossRef CAS PubMed.
- C. De Risi, G. P. Pollini and V. Zanirato, Chem. Rev., 2016, 116, 3241–3305 CrossRef CAS PubMed.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B:Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- L. Zhang, D. Lin, Y. Kusov, Y. Nian, Q. Ma, J. Wang, A. von Brunn, P. Leyssen, K. Lanko, J. Neyts, A. de Wilde, E. J. Snijder, H. Liu and R. Hilgenfeld, J. Med. Chem., 2020, 63, 4562–4578 CrossRef CAS PubMed.
- X. Xie, X. Yang, T. Wu, Y. Li, M. Li, Q. Tan, X. Wang and B. Tang, Anal. Chem., 2016, 88, 8019–8025 CrossRef CAS PubMed.
- J.-C. Chen, B.-J. Uang, P.-C. Lyu, J.-Y. Chang, K.-J. Liu, C.-C. Kuo, H.-P. Hsieh, H.-C. Wang, C.-S. Cheng, Y.-H. Chang, M. D.-T. Chang, W.-S. W. Chang and C.-C. Lin, J. Med. Chem., 2010, 53, 4545–4549 CrossRef CAS PubMed.
- J. Zhou, E. D. Mock, A. Martella, V. Kantae, X. Di, L. Burggraaff, M. P. Baggelaar, K. Al-Ayed, A. Bakker, B. I. Florea, S. H. Grimm, H. den Dulk, C. T. Li, L. Mulder, H. S. Overkleeft, T. Hankemeier, G. J. P. van Westen and M. van der Stelt, ACS Chem. Biol., 2019, 14, 164–169 CrossRef CAS PubMed.
- D. Stubba, D. Bensinger, J. Steinbacher, L. Proskurjakov, Á. Salcedo Gómez, U. Schmidt, S. Roth, K. Schmitz and B. Schmidt, ChemMedChem, 2019, 14, 2005–2022 CrossRef CAS PubMed.
- A. Chiou, T. Markidis, V. Constantinou-Kokotou, R. Verger and G. Kokotos, Org. Lett., 2000, 2, 347–350 CrossRef CAS PubMed.
- F. R. Bou-Hamdan and J. L. Leighton, Angew. Chem., Int. Ed., 2009, 48, 2403–2406 CrossRef CAS PubMed.
- A. J.-L. Ayitou, J. L. Jesuraj, N. Barooah, A. Ugrinov and J. Sivaguru, J. Am. Chem. Soc., 2009, 131, 11314–11315 CrossRef CAS PubMed.
- M. Bouma, G. Masson and J. Zhu, J. Org. Chem., 2010, 75, 2748–2751 CrossRef CAS PubMed.
- S. J. Matthews and J. W. Lancaster, Clin. Ther., 2012, 34, 1857–1882 CrossRef CAS PubMed.
- F. G. Njoroge, K. X. Chen, N.-Y. Shih and J. J. Piwinski, Acc. Chem. Res., 2008, 41, 50–59 CrossRef CAS PubMed.
- A. Arasappan, F. Bennett, S. L. Bogen, S. Venkatraman, M. Blackman, K. X. Chen, S. Hendrata, Y. Huang, R. M. Huelgas, L. Nair, A. I. Padilla, W. Pan, R. Pike, P. Pinto, S. Ruan, M. Sannigrahi, F. Velazquez, B. Vibulbhan, W. Wu, W. Yang, A. K. Saksena, V. Girijavallabhan, N.-Y. Shih, J. Kong, T. Meng, Y. Jin, J. Wong, P. McNamara, A. Prongay, V. Madison, J. J. Piwinski, K.-C. Cheng, R. Morrison, B. Malcolm, X. Tong, R. Ralston and F. G. Njoroge, ACS Med. Chem. Lett., 2010, 1, 64–69 CrossRef CAS.
- L. J. Lombardo, A. Camuso, J. Clark, K. Fager, J. Gullo-Brown, J. T. Hunt, I. Inigo, D. Kan, B. Koplowitz, F. Lee, K. McGlinchey, L. Qian, C. Ricca, G. Rovnyak, S. Traeger, J. Tokarski, D. K. Williams, L. I. Wu, Y. Zhao, V. Manne and R. S. Bhide, Bioorg. Med. Chem. Lett., 2005, 15, 1895–1899 CrossRef CAS PubMed.
- M. Nakamura, J. Inoue and T. Yamada, Bioorg. Med. Chem. Lett., 2000, 10, 2807–2810 CrossRef CAS PubMed.
- L. A. Martinho, T. P. F. Rosalba and C. K. Z. Andrade, Eur. J. Org. Chem., 2022, 2022, e202201199 CrossRef CAS.
- A. Abdessalem, R. Abderrahim and L. El Kaïm, Synlett, 2015, 2537–2540 Search PubMed.
- T. D. Owens, G.-L. Araldi, R. F. Nutt and J. E. Semple, Tetrahedron Lett., 2001, 42, 6271–6274 CrossRef CAS.
- S. E. Denmark and Y. Fan, J. Am. Chem. Soc., 2003, 125, 7825–7827 CrossRef CAS PubMed.
- T. Soeta, Y. Kojima, Y. Ukaji and K. Inomata, Tetrahedron Lett., 2011, 52, 2557–2559 CrossRef CAS.
- T. Yamada, T. Hirose, S. Ōmura and T. Sunazuka, Eur. J. Org. Chem., 2015, 2015, 296–301 CrossRef CAS.
- Q. Xia and B. Ganem, Org. Lett., 2002, 4, 1631–1634 CrossRef CAS PubMed.
- M. Schiess and D. Seebach, Helv. Chim. Acta, 1983, 66, 1618–1623 CrossRef CAS.
- E. Müller and B. Zeeh, Justus Liebigs Ann. Chem., 1966, 696, 72–80 CrossRef.
- M. Bayat, S. Nasri, H. Hosseini and F. Hassanzadeh, Monatsh. Chem., 2012, 143, 801–804 CrossRef CAS.
- F. La Spisa, A. Feo, R. Mossetti and G. C. Tron, Org. Lett., 2012, 14, 6044–6047 CrossRef CAS PubMed.
- M. Serafini, A. Griglio, E. Oberto, T. Pirali and G. C. Tron, Tetrahedron Lett., 2017, 58, 4786–4789 CrossRef CAS.
- K. S. Adamis, M. Georgoulakis, I. Angelonidis, D. Korovesis, C. Papadopoulos, M. Kapsalis, N. Tavernarakis, N. Eleftheriadis and C. G. Neochoritis, Chem. – Eur. J., 2025, 31, e202501513 CrossRef CAS PubMed.
- L. Lu, X. Pei, Y. Mei, Y. Deng, H. Zhang, L. Zhang and A. Lei, Chem, 2018, 4, 2861–2871 CAS.
- T. Zarganes-Tzitzikas, A. L. Chandgude and A. Dömling, Chem. Rec., 2015, 15, 981–996 CrossRef CAS PubMed.
- H. Sadeghian and A. Jabbari, Expert Opin. Ther. Pat., 2016, 26, 65–88 CrossRef CAS PubMed.
- N. Spacho, M. Casertano, C. Imperatore, C. Papadopoulos, M. Menna and N. Eleftheriadis, Chem. – Eur. J., 2024, 30, e202402279 CrossRef CAS PubMed.
- A. Louka, N. Spacho, D. Korovesis, K. Adamis, C. Papadopoulos, E. Kalaitzaki, N. Tavernarakis, C. G. Neochoritis and N. Eleftheriadis, Angew. Chem., Int. Ed., 2025, 64, e202418291 CrossRef CAS PubMed.
- N. Eleftheriadis, C. G. Neochoritis, N. G. J. Leus, P. E. van der Wouden, A. Dömling and F. J. Dekker, J. Med. Chem., 2015, 58, 7850–7862 CrossRef CAS PubMed.
- N. Eleftheriadis, S. A. Thee, M. R. H. Zwinderman, N. G. J. Leus and F. J. Dekker, Angew. Chem., Int. Ed., 2016, 55, 12300–12305 CrossRef CAS PubMed.
- M. Somaraki, I. Zachilas, E. Tsapinou, G. Boulkou, T. Montagnon, G. Vassilikogiannakis and N. Eleftheriadis, Org. Biomol. Chem., 2025, 23, 9942–9949 RSC.
- N. Eleftheriadis, H. Poelman, N. G. J. Leus, B. Honrath, C. G. Neochoritis, A. Dolga, A. Dömling and F. J. Dekker, Eur. J. Med. Chem., 2016, 122, 786–801 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |


