Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of S-allylic sulfinamides by the catalytic nucleophilic allylation of N-sulfinylamines
Shamim
Fanai-Danesh
ab,
Daniel F.
Moseley
c,
Kevin M.
Foote
c,
Kieran D.
Jones
 ab,
Stephen P.
Argent
ab,
Stephen P.
Argent
 b and
Hon Wai
Lam
b and
Hon Wai
Lam
 *ab
*ab
aThe GlaxoSmithKline Carbon Neutral Laboratories for Sustainable Chemistry, University of Nottingham, Jubilee Campus, Triumph Road, Nottingham, NG7 2TU, UK. E-mail: hon.lam@nottingham.ac.uk
bSchool of Chemistry, University of Nottingham, University Park, Nottingham, NG7 2RD, UK
cPharmaron, West Hill Innovation Park, Hertford Road, Hoddesdon, Hertfordshire EN11 9FH, UK
First published on 29th October 2025
Abstract
Sulfinamides are important compounds in organic synthesis and biologically active molecules, but S-allylic sulfinamides have received scant attention. Herein, we describe the synthesis of S-allylic sulfinamides by indium- or (thio)urea-catalyzed additions of allylboron reagents to N-sulfinylamines.
Introduction
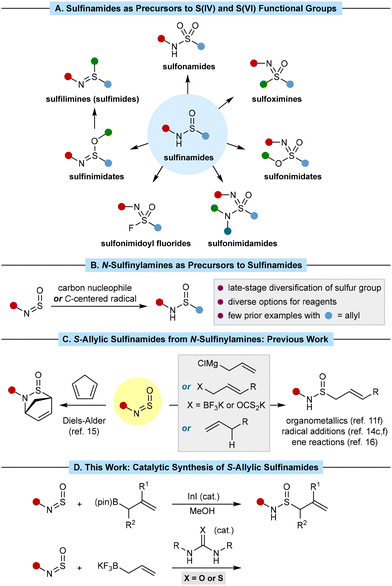
Sulfinamides are important compounds in organic synthesis.1 They are used as amide bioisosteres,2 chiral auxiliaries,3 chiral ligands,4 and chiral organocatalysts.5 Sulfinamides are also versatile precursors to a range of other sulfur(IV)- or sulfur(VI)-containing functional groups,6,7 some of which are of significant interest for developing new pharmaceuticals and agrochemicals (Scheme 1A).8Given the widespread interest in sulfinamides, developing new methods for their synthesis is an important goal. An alternative to the common approach of using substrates with pre-installed carbon–sulfur linkages is the addition of non-sulfur-containing carbon reagents to N-sulfinylamines, which exist as the Z-isomers9,10 (Scheme 1B). This strategy is advantageous if a library of sulfinamides differing at the carbon substituent attached to sulfur is required, as this group is introduced at a late stage and numerous reagents can be employed. Highly reactive organolithium or Grignard reagents can be used but they require rigorous exclusion of air and moisture and can present issues of functional group incompatibility.11 Recent research has focused on milder methods such as catalytic additions of aryl halides,12,13c alkyl halides,13e alkenyl triflates,12 propargyl acetates,13f arylboron reagents,7e,13a,b,d or radicals7f,14 to N-sulfinylamines, which also include asymmetric variants.13 Collectively, these methods have greatly expanded the options available to access diverse sulfinamides.
S-Allylic sulfinamides are potentially useful building blocks because both sulfinamide and allyl groups are useful functional handles for further manipulations. Cyclic S-allylic sulfinamides can be prepared from Diels–Alder reactions of N-sulfinylamines,15 whereas acyclic S-allylic sulfinamides can be prepared from N-sulfinylamines by the addition of allylmagnesium chloride11f or allylic radicals,14c,f and by ene reactions of alkenes (Scheme 1C).16 However, new methods that: (i) exhibit broader scope; (ii) are conducted under mild conditions, and (iii) employ more stable, more functional-group- tolerant reagents would be valuable and allow access to a greater range of S-allylic sulfinamides. Herein, we describe the synthesis of S-allylic sulfinamides by indium- or (thio)urea-catalyzed additions of allylboron reagents to N-sulfinylamines (Scheme 1D).
Results and discussion
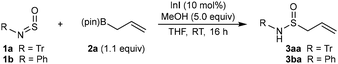
This study began with the reaction of allyl pinacolboronate (2a) with N-sulfinyltritylamine (1a)17 or commercial N-sulfinylaniline (1b) (Table 1). Inspired by previous indium-catalyzed reactions of allylboron reagents with ketones,18aN-acylhydrazones,18b and N,O-aminals,18c we found that reaction of 1a with 2a (1.1 equiv.) in the presence of InI (10 mol%) and MeOH (5.0 equiv.) in THF at room temperature for 16 h gave S-allylic sulfinamide 3aa in 73% isolated yield (entry 1). A reaction conducted without MeOH led to consumption of allylboronate 2a but N-sulfinylamine 1a remained and none of 3aa was detected (entry 2). A reaction conducted without InI led to complete recovery of starting materials with no 3aa detected (entry 3). The use of N-sulfinylaniline (1b) in place of 1a led to no allylation (entry 4), and significant decomposition of 1b to aniline was observed. Given the known sensitivity of N-sulfinylamines to moisture,9 the instability of 1b towards MeOH is not surprising.Using the conditions of Table 1, entry 1, the scope of this process was explored using other N-sulfinylamines and allylboronates (Scheme 2). As well as the successful formation of 3aa in 73% yield, allyl pinacolboronate (2a) reacted with tert-octylsulfinylamine to give 3ca in 46% yield. As with N-sulfinyl aniline (1b) (Table 1, entry 4), N-sulfinylamines with other N-aryl groups such as 2-methoxyphenyl, 4-cyanophenyl, or 3-carbomethoxyphenyl are unsuitable substrates under these conditions and undergo decomposition to the corresponding aniline. However, N-sulfinylamines containing sterically hindering N-aryl groups such as 2,6-diisopropylphenyl or mesityl are more stable to decomposition and reacted smoothly with allyl pinacolboronate to give S-allylic sulfinamides 3da and 3ea in reasonable yields. 2-Methylallyl pinacolboronate (2b) is also a competent pronucleophile and reacted with a range of N-sulfinylamines to give allylation products 3ab, 3db, and 3eb in 56–63% yield.
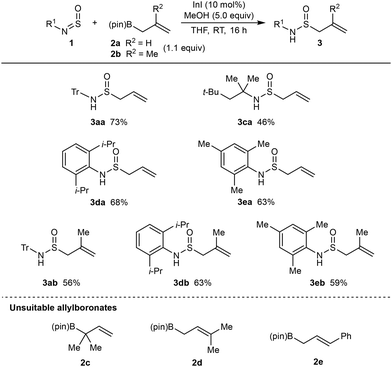 | ||
| Scheme 2 Scope of the indium-catalyzed nucleophilic allylation of N-sulfinylamines with allylboronates. | ||
Reactions of sterically more hindered allylboronates 2c–2e with N-sulfinyltritylamine (1a) were unsuccessful; although the allylboronate was partially consumed, 1a did not react and was recovered unchanged. However, the reaction of racemic allylboronate rac-2f with 1a gave S-allylic sulfinamide 3af with two stereocenters in 44% yield and >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, as determined by 1H NMR analysis of the crude reaction mixture (Scheme 3). The relative configuration of 3af was determined by X-ray crystallography.
1 dr, as determined by 1H NMR analysis of the crude reaction mixture (Scheme 3). The relative configuration of 3af was determined by X-ray crystallography.
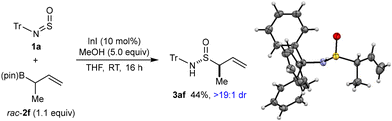 | ||
| Scheme 3 Formation of S-allylic sulfinamide 3af with two stereocenters and X-ray crystal structure with ORTEP with ellipsoid probabilities at 50%. | ||
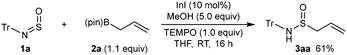
Based upon literature precedent,18 we believe these reactions occur via allylindium species formed by transmetalation from the allylboronates. However, to rule out alternative mechanisms involving radical intermediates, the reaction of 1a with 2a was conducted in the presence of a stoichiometric quantity of the radical scavenger TEMPO (Scheme 4). This reaction gave 3aa in 61% yield, which suggests that radical species are less likely to be involved.
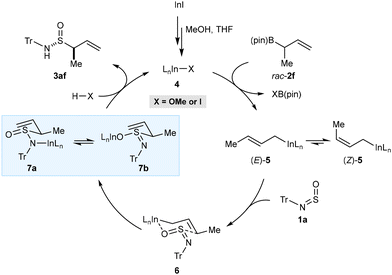
A tentative catalytic cycle for these reactions, using 1a and rac-2f as representative substrates, is shown in Scheme 5. First, transmetalation of rac-2f with an indium(I) species 4 derived from InI gives the E-crotylindium species (E)-5, which could be in equilibrium with the less thermodynamically stable Z-crotyl isomer (Z)-5via σ–π–σ isomerization.18b Reaction of (E)-5 with 1a in a six-membered chair-like transition state 6, in which indium is coordinated to the oxygen atom of 1a, and the S = NTr moiety occupies a less-hindered pseudoequatorial position, would give 7a and/or 7b. Protonolysis of 7a and/or 7b with HX (X = OMe or I), would release the product 3af and regenerate the indium(I) species 4. However, alternative models (involving coordination of indium to sulfur or nitrogen, or involving open transition states) cannot be ruled out. More studies, such as DFT calculations, will be required to shed more light on the mechanism and stereochemical model.
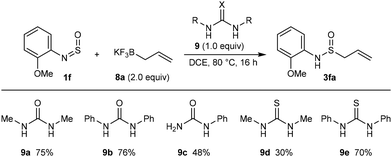
The requirement for MeOH in these reactions restricts the scope of N-sulfinylamines to those that are more stable to MeOH-induced decomposition to the corresponding aniline. Therefore, we investigated whether other conditions can be used. Our preliminary experiments used potassium allyltrifluoroborate (8a) and N-sulfinylamine 1f, which does not react with allyl pinacolboronate (2a) using the indium-catalyzed conditions shown in Scheme 2 but instead undergoes decomposition to 2-methoxyaniline (see Scheme 6 for the structure of 1f). No reaction occurred when 1f and 8a were heated together in various solvents.
Next, additives were screened to try and promote reaction, and ureas or thioureas were selected based on two hypotheses (Scheme 6). First, Batey and co-workers have shown that potassium allyltrifluoroborates engage in the nucleophilic allylation of aldehydes19 in the presence of BF3·OEt2,19a,b presumably through the in situ-generation of reactive allylboron difluorides by Lewis acid-promoted removal of fluoride ions.20 Since (thio)ureas have also been shown to bind fluoride ions through hydrogen bonding,21 we believed they could be effective in the reaction of 1f and 8a. Second, (thio)ureas are well-known hydrogen bonding catalysts,22 and we considered it possible they could activate N-sulfinylamines towards nucleophilic allylation through hydrogen bonding to the oxygen or nitrogen atom. We were pleased to find that heating a mixture of 1f, 8a (2.0 equiv.), and N,N’-dimethylurea (9a, 1.0 equiv.) in DCE at 80 °C for 16 h led to the formation of allylation product 3fa in 75% NMR yield. Similar results were obtained using N,N’-diphenylurea (9b) and N,N’-diphenylthiourea (9e) but lower yields were obtained with N-phenylurea (9c) and N,N’-dimethylthiourea (9d). N,N’-Dimethylurea (9a) was selected for further experiments based on its performance and low molecular weight. It should be noted that allylboronate 2a did not react with N-sulfinylamines under (thio)urea catalysis. Also, potassium allyltrifluoroborate (8a) did not react with N-sulfinylamines under the indium-catalyzed conditions shown in Scheme 2.
Pleasingly, we found that in a preparative experiment, the quantity of 9a can be reduced to 20 mol% without detriment as shown by the formation of 3fa in 70% isolated yield (Scheme 7) compared to 75% NMR yield using stoichiometric 9a (Scheme 6). Under these conditions, allylation products containing phenyl (3ba), 4-cyanophenyl (3ga), or 4-chlorophenyl groups (3ha) were obtained in 48–66% yield from the corresponding N-sulfinylamines (Scheme 7). Despite performing only modestly in the allylation of N-sulfinylamine 1f (Scheme 6), N,N’-dimethylthiourea (9d) gave better yields than N,N’-dimethylurea (9a) in allylations of N-sulfinylamines with 2-bromophenyl (3ja), 3-carbomethoxyphenyl (3ka), or 4-fluorophenyl groups (3la). N-Sulfinyltritylamine (1a) was unreactive under these conditions using either 9a or 9d. This reaction appears to be limited to the use of potassium allyltrifluoroborate (8a) as attempted reactions of N-sulfinylaniline (1b) with substituted allyltrifluoroborates 8b and 8c led to no allylation products, with only partial decomposition of 1b into aniline observed.
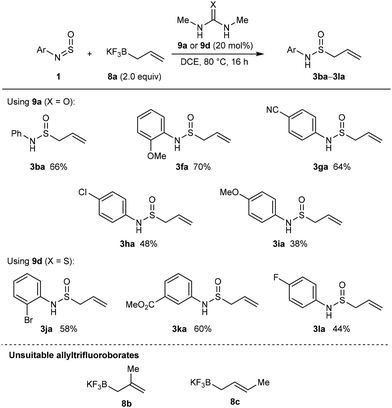 | ||
| Scheme 7 Scope of (thio)urea-catalyzed nucleophilic allylations of N-sulfinylamines with potassium allyltrifluoroborate (8a). | ||
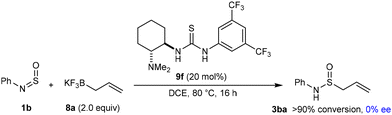
The reaction of N-sulfinylamine 1b with 8a in the presence of a chiral thiourea catalyst 9f gave 3ba but with no enantioselectivity (Scheme 8). This observation may suggest these reactions do not operate through hydrogen bonding of the (thio)urea to the N-sulfinylamine,21 but rather through fluoride abstraction from potassium allyltrifluoroborate to generate allylboron difluoride.19a,b However, more experiments are required for greater insight.
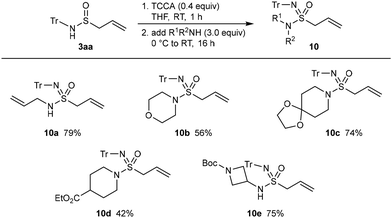
Further reactions were conducted on product 3aa to demonstrate the synthetic utility of these compounds. N-Sulfinamides are versatile precursors to other sulfur(IV)- or sulfur(VI)-containing functional groups,6,7 but it was of interest to determine whether the alkene in our allylation products is compatible with the reagents used in these reactions. As a representative transformation, the conversion of 3aa into sulfonimidamides was investigated. Pleasingly, treatment of 3aa with trichloroisocyanuric acid (TCCA, 0.4 equiv.) gave a sulfonimidoyl chloride that was not isolated but reacted immediately with various amines (3.0 equiv.) to give sulfonimidamides 10a–10e in 42–79% yield, where the alkene remain unchanged (Scheme 9).7e The amines included allylamine (10a), morpholine (10b), piperidine derivatives (10c and 10d), and a Boc-protected 4-aminoazetidine (10e).
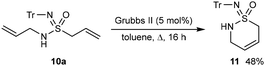
Finally, cyclization of 10a by ring-closing metathesis using the second-generation Grubbs catalyst (5 mol%) in toluene at reflux gave cyclic sulfonimidamide 11 in 48% yield (Scheme 10).
Conclusions
In summary, we have described the catalytic nucleophilic allylation of N-sulfinylamines with allylboron reagents. N-Sulfinylamines containing sterically hindering N-substituents react with allyl pinacolboronates using catalytic indium(I) iodide in the presence of MeOH, whereas other N-sulfinylamines react with potassium allyltrifluoroborate using a (thio)urea catalyst. These reactions give ready access to S-allylic sulfinamides, which have been comparatively underexplored. Future work will focus on enantioselective variants of these reactions.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included in the supplementary information (SI) and the Nottingham Research Data Management Repository at: https://doi.org/10.17639/nott.7611. Supplementary information: experimental procedures, full spectroscopic data for new compounds, and crystallographic data. See DOI: https://doi.org/10.1039/d5ob01562f.CCDC 2475361 (3af) contains the supplementary crystallographic data for this paper.23
Acknowledgements
This work was supported by Pharmaron and the Engineering and Physical Sciences Research Council (EP/W524402/1).References
- S. Das, A. Dhibar and B. Sahoo, ACS Org. Inorg. Au, 2025, 5, 1–12 Search PubMed.
- (a) W. J. Moree, L. C. van Gent, G. A. van der Marel and R. M. J. Liskamp, Tetrahedron, 1993, 49, 1133–1150 CrossRef; (b) W. J. Moree, G. A. van der Marel and R. J. Liskamp, J. Org. Chem., 1995, 60, 5157–5169 CrossRef.
- (a) M. T. Robak, M. A. Herbage and J. A. Ellman, Chem. Rev., 2010, 110, 3600–3740 Search PubMed; (b) M.-C. Huang, Y.-W. Chao, Y.-M. Lin, B.-S. Wu, C.-T. Chou, H.-S. Chen and C.-C. Tsai, Tetrahedron Lett., 2024, 148, 155243 Search PubMed.
- S. Otocka, M. Kwiatkowska, L. Madalińska and P. Kiełbasiński, Chem. Rev., 2017, 117, 4147–4181 Search PubMed.
- Selected examples: (a) D. Pei, Z. Wang, S. Wei, Y. Zhang and J. Sun, Org. Lett., 2006, 8, 5913–5915 Search PubMed; (b) M. T. Robak, M. Trincado and J. A. Ellman, J. Am. Chem. Soc., 2007, 129, 15110–15111 CrossRef PubMed; (c) K. L. Kimmel, M. T. Robak and J. A. Ellman, J. Am. Chem. Soc., 2009, 131, 8754–8755 CrossRef PubMed; (d) H. Xu, S. J. Zuend, M. G. Woll, Y. Tao and E. N. Jacobsen, Science, 2010, 327, 986–990 CrossRef PubMed; (e) K. L. Kimmel, J. D. Weaver and J. A. Ellman, Chem. Sci., 2012, 3, 121–125 RSC; (f) K. C. Forbes and E. N. Jacobsen, Science, 2022, 376, 1230–1236 CrossRef PubMed.
- (a) E. Wojaczyńska and J. Wojaczyński, Chem. Rev., 2020, 120, 4578–4611 CrossRef PubMed; (b) X. Zhang, F. Wang and C.-H. Tan, JACS Au, 2023, 3, 700–714 CrossRef PubMed.
- Selected examples: (a) D. Leca, L. Fensterbank, E. Lacôte and M. Malacria, Org. Lett., 2002, 4, 4093–4095 CrossRef PubMed; (b) D. Leca, K. Song, M. Amatore, L. Fensterbank, E. Lacôte and M. Malacria, Chem. – Eur. J., 2004, 10, 906–916 CrossRef PubMed; (c) P. M. Matos, W. Lewis, J. C. Moore and R. A. Stockman, Org. Lett., 2018, 20, 3674–3677 CrossRef PubMed; (d) Y. Aota, T. Kano and K. Maruoka, Angew. Chem., Int. Ed., 2019, 58, 17661–17665 CrossRef PubMed; (e) P. K. T. Lo and M. C. Willis, J. Am. Chem. Soc., 2021, 143, 15576–15581 CrossRef PubMed; (f) J. A. Andrews, J. Kalepu, C. F. Palmer, D. L. Poole, K. E. Christensen and M. C. Willis, J. Am. Chem. Soc., 2023, 145, 21623–21629 CrossRef PubMed; (g) S. Tsuzuki and T. Kano, Angew. Chem., Int. Ed., 2023, 62, e202300637 CrossRef PubMed.
- (a) P. K. Chinthakindi, T. Naicker, N. Thota, T. Govender, H. G. Kruger and P. I. Arvidsson, Angew. Chem., Int. Ed., 2017, 56, 4100–4109 CrossRef PubMed; (b) U. Lücking, Org. Chem. Front., 2019, 6, 1319–1324 RSC; (c) P. Mäder and L. Kattner, J. Med. Chem., 2020, 63, 14243–14275 CrossRef PubMed; (d) A. Ovung and J. Bhattacharyya, Biophys. Rev., 2021, 13, 259–272 CrossRef PubMed; (e) G. B. Watson, M. W. Siebert, N. X. Wang, M. R. Loso and T. C. Sparks, Pestic. Biochem. Physiol., 2021, 178, 104924 CrossRef CAS PubMed; (f) M. J. Tilby and M. C. Willis, Expert Opin. Drug Discovery, 2021, 16, 1227–1231 CrossRef; (g) U. Lücking, Chem. – Eur. J., 2022, 28, e202201993 CrossRef.
- T. Q. Davies and M. C. Willis, Chem. – Eur. J., 2021, 27, 8918–8927 CrossRef CAS.
- R. M. Romano and C. O. Della Védova, J. Mol. Struct., 2000, 522, 1–26 CrossRef CAS.
- Representative examples: (a) H. Gilman and H. L. Morris, J. Am. Chem. Soc., 1926, 48, 2399–2404 CrossRef CAS; (b) J.-B. Baudin and S. A. Julia, Tetrahedron Lett., 1986, 27, 837–840 CrossRef CAS; (c) I. Sen, D. P. Kloer, R. G. Hall and S. Pal, Synthesis, 2013, 3018–3028 CAS; (d) M. Malik, R. Senatore, D. Castiglione, A. Roller-Prado and V. Pace, Chem. Commun., 2023, 59, 11065–11068 RSC; (e) D. Austrup and F. Saito, Angew. Chem., Int. Ed., 2023, 62, e202315123 CrossRef CAS PubMed; (f) B. C. Haas, N.-K. Lim, J. Jermaks, E. Gaster, M. C. Guo, T. C. Malig, J. Werth, H. Zhang, F. D. Toste, F. Gosselin, S. J. Miller and M. S. Sigman, J. Am. Chem. Soc., 2024, 146, 8536–8546 CrossRef CAS.
- M.-K. Wei, D. F. Moseley, R. M. Bär, Y. Sempere and M. C. Willis, J. Am. Chem. Soc., 2024, 146, 19690–19695 CrossRef CAS PubMed.
- For catalytic enantioselective arylations, alkenylations, alkylations, or propargylations of N-sulfinylamines, see: (a) Y. Shi, Y. Yuan, J. Li, J. Yang and J. Zhang, J. Am. Chem. Soc., 2024, 146, 17580–17586 CrossRef CAS; (b) L. Xi, X. Fang, M. Wang and Z. Shi, J. Am. Chem. Soc., 2024, 146, 17587–17594 CrossRef PubMed; (c) X. Fang, L. Xi, M. Wang, J. Xiao, Y. Zhao, M. C. Willis and Z. Shi, Nat. Commun., 2025, 16, 2547 CrossRef; (d) Y. Yuan, X. Tian, H. Zheng, Y. Li, J. Zhang and J. Yang, Angew. Chem., Int. Ed., 2025, 64, e202506243 CrossRef; (e) N. Liu, H. Xia, Y. Shi, K. Xu, Z. Yu, X. Wu, G. Huang, J. Qu and Y. Chen, J. Am. Chem. Soc., 2025, 147, 26786–26796 CrossRef; (f) L. Ding, J. Chen, H. Lu and Z. Shi, J. Am. Chem. Soc., 2025, 147, 28389–28398 CrossRef PubMed; (g) Y. Wang, Z. Zhang, Q. Chong and F. Meng, ACS Catal., 2025, 15, 15857–15866 CrossRef.
- (a) L. Li, S.-q. Zhang, Y. Chen, X. Cui, G. Zhao, Z. Tang and G.-x. Li, ACS Catal., 2022, 12, 15334–15340 CrossRef; (b) H. T. Dang, A. Porey, S. Nand, R. Trevino, P. Manning-Lorino, W. B. Hughes, S. O. Fremin, W. T. Thompson, S. K. Dhakal, H. D. Arman and O. V. Larionov, Chem. Sci., 2023, 14, 13384–13391 RSC; (c) M. Yan, S.-f. Wang, Y.-p. Zhang, J.-z. Zhao, Z. Tang and G.-x. Li, Org. Biomol. Chem., 2024, 22, 348–352 RSC; (d) G.-D. Xia, R. Li, L. Zhang, Y. Wei and X.-Q. Hu, Org. Lett., 2024, 26, 3703–3708 CrossRef PubMed; (e) S. Das, P. P. Mondal, A. Dhibar, A. Ruth and B. Sahoo, Org. Lett., 2024, 26, 3679–3684 CrossRef PubMed; (f) X. Zhang, S.-C. Chien and X. Feng, CN119100956A, 2024; (g) S. Jian, J. Wang, X. Feng, M. Bao and X. Zhang, Org. Lett., 2025, 27, 7212–7217 CrossRef PubMed; (h) X. Zhu, J. Wu, J. Zhang and J. Yang, RSC Adv., 2025, 15, 4532–4535 RSC; (i) M. Southern, C. Pearce and M. C. Willis, Org. Lett., 2025, 27, 10325–10329 CrossRef.
- Selected examples: (a) G. Kresze, A. Maschke, R. Albrecht, K. Bederke, H. P. Patzschke, H. Smalla and A. Trede, Angew. Chem., Int. Ed. Engl., 1962, 1, 89–98 CrossRef; (b) S. W. Remiszewski, J. Yang and S. M. Weinreb, Tetrahedron Lett., 1986, 27, 1853–1856 CrossRef; (c) Y. Zhang and C. J. Flann, J. Org. Chem., 1998, 63, 1372–1378 CrossRef; (d) D. Stien, G. T. Anderson, C. E. Chase, Y.-h. Koh and S. M. Weinreb, J. Am. Chem. Soc., 1999, 121, 9574–9579 CrossRef; (e) A. Bayer, M. M. Endeshaw and O. R. Gautun, J. Org. Chem., 2004, 69, 7198–7205 CrossRef PubMed; (f) M. M. Endeshaw, L. K. Hansen and O. R. Gautun, J. Heterocycl. Chem., 2008, 45, 149–154 CrossRef.
- Selected examples: (a) R. Bussas and G. Kresze, Angew. Chem., Int. Ed. Engl., 1980, 19, 732–737 CrossRef; (b) G. Kresze and R. Bussas, Liebigs Ann. Chem., 1980, 843–857 CrossRef; (c) R. Bussas, H. Muenster and G. Kresze, J. Org. Chem., 1983, 48, 2828–2832 CrossRef; (d) W. Starflinger, G. Kresze and K. Huss, J. Org. Chem., 1986, 51, 37–40 CrossRef; (e) G. Deleris, J. Dunogues and A. Gadras, Tetrahedron, 1988, 44, 4243–4258 CrossRef; (f) J. K. Whitesell, J. F. Carpenter, H. K. Yaser and T. Machajewski, J. Am. Chem. Soc., 1990, 112, 7653–7659 CrossRef; (g) L. Bayeh, P. Q. Le and U. K. Tambar, Nature, 2017, 547, 196–200 CrossRef PubMed.
- T. Q. Davies, A. Hall and M. C. Willis, Angew. Chem., Int. Ed., 2017, 56, 14937–14941 CrossRef PubMed.
- (a) U. Schneider, M. Ueno and S. Kobayashi, J. Am. Chem. Soc., 2008, 130, 13824–13825 CrossRef PubMed; (b) A. Chakrabarti, H. Konishi, M. Yamaguchi, U. Schneider and S. Kobayashi, Angew. Chem., Int. Ed., 2010, 49, 1838–1841 CrossRef PubMed; (c) Y.-Y. Huang, A. Chakrabarti, N. Morita, U. Schneider and S. Kobayashi, Angew. Chem., Int. Ed., 2011, 50, 11121–11124 CrossRef.
- (a) R. A. Batey, A. N. Thadani and D. V. Smil, Tetrahedron Lett., 1999, 40, 4289–4292 CrossRef; (b) R. A. Batey, A. N. Thadani, D. V. Smil and A. J. Lough, Synthesis, 2000, 990–998 CrossRef; (c) A. N. Thadani and R. A. Batey, Org. Lett., 2002, 4, 3827–3830 CrossRef.
- K. Omoto and H. Fujimoto, J. Org. Chem., 1998, 63, 8331–8336 CrossRef.
- Selected examples: (a) J. A. Birrell, J.-N. Desrosiers and E. N. Jacobsen, J. Am. Chem. Soc., 2011, 133, 13872–13875 CrossRef PubMed; (b) G. Pupo, F. Ibba, D. M. H. Ascough, A. C. Vicini, P. Ricci, K. E. Christensen, L. Pfeifer, J. R. Morphy, J. M. Brown, R. S. Paton and V. Gouverneur, Science, 2018, 360, 638–642 CrossRef; (c) G. Pupo, A. C. Vicini, D. M. H. Ascough, F. Ibba, K. E. Christensen, A. L. Thompson, J. M. Brown, R. S. Paton and V. Gouverneur, J. Am. Chem. Soc., 2019, 141, 2878–2883 CrossRef PubMed; (d) C. Dooley, F. Ibba, B. B. Botlik, C. Palladino, C. A. Goult, Y. Gao, A. Lister, R. S. Paton, G. C. Lloyd-Jones and V. Gouverneur, Nat. Catal., 2025, 8, 107–115 CrossRef PubMed.
- Selected reviews: (a) S. J. Connon, Chem. Commun., 2008, 2499–2510 RSC; (b) Y. Takemoto, Chem. Pharm. Bull., 2010, 58, 593–601 CrossRef; (c) D. Limnios and C. G. Kokotos, in Sustainable Catalysis: Without Metals or Other Endangered Elements, Part 2, ed. M. North and M. North, The Royal Society of Chemistry, 2015, pp. 196–255 Search PubMed; (d) F. Steppeler, D. Iwan, E. Wojaczyńska and J. Wojaczyński, Molecules, 2020, 25, 401 CrossRef.
- CCDC 2475361: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p2tcz.
| This journal is © The Royal Society of Chemistry 2025 |