 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective hydrogen isotope exchange on sulfonamides, sulfilimides and sulfoximines by electrochemically generated bases†
Carla Marie
Stork
 a,
Elisabeth
Glöckler
a,
Volker
Derdau
a,
Elisabeth
Glöckler
a,
Volker
Derdau
 b,
Philipp
Schnieders
c and
Siegfried R.
Waldvogel
b,
Philipp
Schnieders
c and
Siegfried R.
Waldvogel
 *ad
*ad
aMax Planck Institute for Chemical Energy Conversion, Stiftstraße 34-36, 45470 Mülheim an der Ruhr, Germany. E-mail: siegfried.waldvogel@cec.mpg.de
bSanofi-Aventis Deutschland GmbH, Integrated Drug Discovery, Industriepark Höchst, 65926 Frankfurt am Main, Germany
cDeutero GmbH, Am Ring 29, 56288 Kastellaun, Germany
dKarlsruhe Institute of Technology, Institute of Biological and Chemical Systems – Functional Molecular Systems (IBCS FMS), 76131 Karlsruhe, Germany
First published on 10th June 2025
Abstract
We present a mild, metal-free electrochemical method to selectively add deuterium to the position α of the sulfur atom in sulfonamides, sulfilimides, and sulfoximines using a simple two-electrode setup under galvanostatic conditions. Our method is based on readily available NMR solvent DMSO-d6 as the deuterium source and reusable glassy carbon electrodes. A low current density ensures functional group tolerance and enables selective incorporation of deuterium into pharmaceutically relevant moieties. With deuterium incorporation up to 97% the method stands out as a new possibility to label molecules electrochemically without the use of toxic and expensive transition-metal catalysts.
Introduction
Isotopic labelling of molecules is a powerful tool in life sciences and a well-known technique in chemistry.1–6 The hydrogen isotope deuterium has a prominent role in isotopic chemistry.7–12 Moreover, during the last decade the labelling of drug candidates with deuterium has been used to create improved pharmacological properties.13–15 With deutetrabenazine as the first deuterated drug to be approved by the Food and Drug Administration (FDA) in 2017, showing enhanced metabolic stability, a new era in drug development was activated.15,16 Lately, deuruxolitinib was also approved by the FDA, with this drug possessing not only better metabolic features but also possessing enhanced inhibition of the respective kinase, which further displays the emerging opportunities related to stable isotope labelling of drug candidates with deuterium.17,18 Electrosynthesis as a technique has undergone a renaissance over the last two decades.19–26 Using electrochemistry as a method in chemical reactions is widely shown to open the possibilities to avoid, for example, transition and rare metals, and toxic or expensive reagents. Additionally, electrochemistry is reported to be an inherently safe method.23,27,28 Exploiting chemical modifications regarding the pharmacological properties of a potential drug candidate by deuteration requires selective incorporation into the molecule. Several approaches for selective hydrogen isotope exchange (HIE) reactions have been established.11,14,29–31 Although there are HIE reaction techniques known using acid or base catalysis,12,32–41 transition-metal catalysis42–50 is often employed. Sulfur-containing molecules represent a class of substances with broad application in drug research and chemical synthesis.51–54 Initially developed as antibiotics,55–57 scaffolds with a sulfonamide functionality are nowadays found in, for example, anti-cancer agents,58–60 or in treatments for Alzheimer's disease61–63 or migraine.64–66 Additionally, sulfoximines have emerged as a highly promising functional group with growing significance in academia as well as in the pharmaceutical and agrochemical industry.67–69 Sulfilimides are widely utilized in organic chemistry.70–72 Furthermore, they can serve as key synthetic intermediates forming the respective tetravalent sulfur species.73–75 However, the ease of oxidation presents challenges in the HIE reaction of these molecules. Some reports of HIE with sulfur-containing molecules have already been reported (see Fig. 1). Chen demonstrated HIE of sulfones and sulfoxides by applying the base barium oxide (BaO) and deuterium water (D2O),12 whereas Hevia reported recently the labelling of activated positions in molecules, including in position α to sulfoxide and dithiane units, by using sodium hexamethyldisilazane (NaHMDS) in DMSO-d6.76 Recently, Sawama described the α-deuteration of thioethers by using photochemistry.77 Kerr established a method using an Ir(I) catalyst for the deuterium incorporation at the methyl functionality of sulfoximines.31 Nevertheless, we were motivated to find a general electrochemical method without the use of transition-metals for a widespread scope of sulfur containing molecules. | ||
| Fig. 1 Previous work on base-catalysed hydrogen isotope exchange (HIE) reaction on sulfur-involving compounds,12 by using transition-metal catalysis31 and our electrochemical method to deuterate sulfonamides, sulfilimides and sulfoximines. | ||
Results and discussion
Optimisation of the deuterium incorporation into sulfonamide 1
The screening and optimisation experiments were conducted in 5 mL glass cells, allowing a quick electrochemical study.78–80 Electrochemical studies demonstrated that the position α to the sulfur atom in a simple sulfonamide (1) can be activated and subsequently deuterated during electrolysis, using D2O (200 eq.) as the deuterium source. This process occurs in a non-deuterated polar solvent such as dimethylacetamide (DMAc-h9, 81%D, Table 1, entry 2), dimethylformamide (DMF-h7, 60%D, Table 1, entry 3), or dimethylsulfoxide (DMSO-h6, 58%D, Table 1 entry 4). Interestingly, HIE reactions in D2O alone did not show any deuteration (Table 1, entry 5). After screening different polar aprotic solvents, DMSO-h6 was chosen as the solvent due to its reduced toxicity compared to the other solvents and availability from biogenic origin (Kraft pulping process).81,82 However, reactions in non-deuterated DMSO-h6 provided suboptimal results, likely due to partial deuteration of the solvent. Consequently, we opted to switch to the readily available and cost-effective deuterated solvent DMSO-d6. We initially evaluated electrochemical parameters, including current density and the amount of applied charge (Fig. 2A). As the current density was increased (ranging from 2.5 to 50 mA cm−2), deuterium incorporation slightly decreased, while the 1H NMR yield remained constant. The highest deuterium incorporation (75%D) was observed at 2.5 mA cm−2. However, due to a shortened reaction time, we chose to proceed with 5 mA cm−2 (73%D). Subsequently, the amount of applied charge was assessed, ranging from 1 to 16 F. Deuterium incorporation increased up to 12 F (90%D). Next, the concentration of the supporting electrolyte NEt4BF4 was studied (Fig. 2B). Using 0.05 M NEt4BF4, corresponding to one equivalent of substrate 1, did not significantly affect deuterium incorporation compared to the previously used 0.1 M NEt4BF4. Lowering the supporting electrolyte concentration further resulted in slightly lower deuterium incorporation, so 0.05 M NEt4BF4 was selected. Additionally, we discovered, that adding small amounts of D2O and MeOD-d4 increased deuterium incorporation (Table 1, entries 1 and 7). D2O was chosen as an attractive additive due to the low cost. The effect of D2O concentration was then evaluated. Increasing the amount of D2O from 1 vol% (96%D) to 5–10 vol% (90–91%D) led to a slight decrease in deuterium incorporation, as did reducing the additive concentration to 0.1 vol% (89%D). Various carbon-based electrode materials were tested (Table 1, entries 8–10). While other carbon-based materials such as isostatic graphite (94%D, Table 1, entry 8) or graphite felt (93%D, Table 1, entry 10) performed as good as glassy carbon, corrosion of the electrodes was visible after the reaction for isostatic graphite, carbon felt and graphite foil. DMSO-d6 was shown to be recyclable with a small decrease in deuterium incorporation (77%D, Table 1, entry 11). The optimised reaction parameters for the synthetic scope were established as follows: 12 F of applied charge, 5 mA cm−2 current density, glassy carbon electrodes, an electrolyte composed of 5 mL DMSO-d6, 50 μL D2O as an additive, and 0.05 M NEt4BF4 as the supporting electrolyte. For all optimisation and scope experiments, the same set of glassy carbon electrodes were used, cleaned with solvents in between of experiments, demonstrating the excellent reusability of this metal-free electrode material.| Entry | Deviation from optimised conditions | Deuterium incorporation (%D) |
|---|---|---|
| Reaction conditions unless indicated otherwise: 0.25 mmol 1, 0.25 mmol Et4NBF4 in 5 mL DMSO-d6 and 50 μL D2O as the solvent and deuterium source. Electrochemical reactions were performed using glassy carbon electrodes as anode and cathode, 5 mA cm−2, 12 F. After confirmation of the position of deuterium incorporation via1H NMR the incorporation was determined via UPLC-MS in accordance with the 1H NMR data.a 0.1 M NEt4BF4 used.b In the experiments corrosion of the cathode was visible (see Fig. S2†). | ||
| 1 | None | 96 |
| 2a | DMAc-h9 (instead of DMSO-d6), D2O (200 eq.) | 81 |
| 3a | DMF-h7 (instead of DMSO-d6), D2O (200 eq.), 4 F | 60 |
| 4a | DMSO-h6, D2O (200 eq.) | 58 |
| 5a | D2O (no DMSO-d6, 5 mL) | — |
| 6a | No D2O | 90 |
| 7a | Addition of 1 vol% MeOD-d4 | 95 |
| 8b | Graphite electrodes | 94 |
| 9b | Graphite foil electrodes | 7 |
| 10b | Graphite felt electrodes | 93 |
| 11a | Recycled DMSO-d6 | 77 |
| 12a | Stirring at doubled speed | 39 |
| 13a | No electricity | — |
Mechanistical discussions
An aim of our investigation was to gain a deeper understanding of the mechanisms underlying the deuteration reaction by studying 1 through cyclic voltammetry experiments, using DMAc as the solvent and NEt4BF4 as the supporting electrolyte. However, the results showed no electrochemical activity of 1 within the electrochemical window of the electrolyte. Instead, the experiments revealed electrochemical activity attributed solely to the electrolyte in the absence of 1 (see ESI†). Electrochemical base generation has been studied by several groups.83–90 Therefore, we used this basis as a starting point for mechanistical investigation of our reaction. Experiments conducted using D2O as the solvent (Table 1, entry 5) showed no deuteration, highlighting the necessity of DMSO, DMAc or DMF for the reaction. We also observed a decreased deuterium incorporation with increased stirring rate (Table 1, entry 12), suggesting that the reaction likely involves a locally generated base species facilitating the deuteration. To further investigate the mechanism, we conducted studies in an on–off setup (Fig. 2C). When 2 F of current was applied, 1 underwent deuteration in both the DMAc/D2O (upper graph) and DMSO-d6 (lower graph) system. After additional stirring for the equivalent time of 2 F without any current applied, no further deuterium incorporation was observed. Subsequent experiments involving two additional cycles produced consistent results regarding deuterium incorporation. These findings, showing deuteration only during application of electrical current, emphasise the requirement for electricity in the reaction. Based on this, we propose an electricity-driven, base-catalysed mechanism occurring in the vicinity of the electrode.Scope and limitations
We selected further C–H active compounds to study the scope and limitations of our method. In addition to methylsulfonamides with functional modifications in the heterocyclic ring, we also screened benzylsulfonamides with diverse modifications in both the heterocyclic ring and the aryl group. Moreover, sulfilimides and sulfoximines were chosen as substrates due to their significance in the chemical and pharmaceutical industry. Several sulfonamides 1–11 were deuterated with good to excellent deuterium incorporations. The methylsulfonamides 1–3 showed deuterium incorporation of >94%D while the benzylsulfonamides 4–10 were deuterated with good to very good deuterium incorporation (67–95%D). Interestingly, the deuterium incorporation increased from pyrrolidine 4 and piperazine 5 (75%D) to morpholine 6 (91%D) indicating the influence of the heterocycle to the reaction. However, the isolated yields of 4–6 need to be improved in the future. For 4 we observed degradation of the compound in the 1H NMR spectrum of the crude reaction mixture. However, comparing these results to monobrominated 7 we observed a higher deuterium incorporation (93%D) while the yield was quantitative by a simple extraction. Polyhalogenated electron-poor aromatics were predominantly deuterated in benzylic positions (8: 95%D, 9: 80%D) beside the aromatic ring (8: 26%D, 9: 9%D) with much lower efficiency. Compound 9 demonstrates that open-chain sulfonamides and free alcohols are compatible with this method, achieving an isolated yield of 82%. In the more complex structure 10 deuterium incorporation of 67%D could be reached while maintaining the stereoinformation. When comparing alkyl functionalities in disulfonamide 11 we observed a deuteration both at the methyl and the butyl moiety, although the deuterium incorporation preferably occurred at the methyl (see ESI†). Interestingly, sulfilimide 20 displayed more deuterium incorporation onto the benzylic position (83%D) than at the methyl moiety (52%D). Furthermore, we expanded the scope to sulfilimides and sulfoximines. Compound 18 showed very good deuterium incorporation (95%D) with moderate isolated yields (40%) using the DMAc/D2O system described in the optimisation. Sulfoximine 22 was tested both for the DMSO-d6/D2O system as well as for the DMAc/D2O system. Both systems achieved good deuterium incorporation (90%D) while the isolated yield was better in the DMAc/D2O system. Replacement of the tosyl protecting group for a nitrile group (24) achieved slightly higher deuterium incorporation (93%D) most likely because of the electron-withdrawing effect of the nitrile. However, a drawback in isolated yield was visible which could be attributed to the electrochemical reaction of the nitrile.91–93 An electron-donating methoxy functionality at the para-position of the aryl group showed no decrease in deuterium incorporation for the respective sulfoximine (23, 91%D) and a slight decrease in deuterium incorporation for the sulfilimide (19, 83%D). Compound 21, a benzodithiazol, was also successfully deuterated (81%D). With the commercially available insecticide sulfoxaflor 25 a complex agrochemical was deuterated in high efficiency in both the benzylic position (97%D) and methyl moiety (94%D) while a nitrile and trifluoromethyl moiety were tolerated. Limitations of the method are displayed with 12–17. Deuterium incorporation into the ethyl functionalities of 12–14 was inferior to the methyl compounds. Other alkyl functionalities even showed no incorporation (15–17) (Fig. 3).Conclusions
A simple and mild electrochemical method to label sulfonamides, sulfilimides and sulfoximines in position α to the sulfur is established. Readily available and inexpensive DMSO-d6 was used as the source of deuterium and proven to be recyclable. The whole protocol is metal-free avoiding metal contaminations. Noteworthy, DMSO is a side-stream of the Kraft pulping process and therefore of biogenic origin. Employment of reusable glassy carbon electrodes thereby avoiding transition metal catalysts for the HIE reactions is very advantageous in terms of sustainability. The simple and quite common galvanostatic two-electrode setup represents a versatile and practical method for general applications. Mechanistical discussions revealed the electrochemical generation of a base that enables the deuteration and the importance of a polar aprotic solvent such as DMSO, DMAc or DMF.Author contributions
Carla Marie Stork: Conceptualization (leading), investigation (leading), methodology (leading), supervision (supporting), project administration (supporting), writing – original draft preparation (leading). Elisabeth Glöckler: Investigation (supporting), methodology (supporting). Dr Volker Derdau: Conceptualization (supporting), supervision (supporting) writing – original draft preparation (supporting). Dr Philipp Schnieders: Conceptualization (supporting). Prof. Dr Siegfried R. Waldvogel: Conceptualization (leading), supervision (leading), project administration (leading), funding acquisition (leading), writing – original draft preparation (supporting).Data availability
The data supporting this article have been included as part of the ESI.† All data therein are detailed and will allow an easy reproduction of the results.Conflicts of interest
V. D. is employee of Sanofi Germany and may hold stocks and options of the company.Acknowledgements
The authors acknowledge financial support from the BMBF Initiative Clusters4future ETOS–Electrifying Technical Organic Synthesis (03ZU1205HC). The authors gratefully acknowledge technical support from the mechanical workshops MPI CEC. Open Access funding provided by the Max Planck Society.References
- T. Kurioka, Y. Lu, J. Jones, X. Wang, D. Hernández-Valdés, A. Genady, R. M. van Dam, S. Inagi and S. Sadeghi, J. Fluorine Chem., 2024, 279, 110350 CrossRef CAS.
- C. M. Waldmann, A. Lebedev, N. Allison and S. Sadeghi, Appl. Radiat. Isot., 2017, 127, 245 CrossRef CAS PubMed.
- M. G. J. Doyle, B. A. Mair, A. Sib, O. Bsharat, M. Munch, V. Derdau, B. H. Rotstein and R. J. Lundgren, Nat. Protoc., 2024, 19, 2147 CrossRef CAS PubMed.
- N. Grankvist, J. D. Watrous, K. A. Lagerborg, Y. Lyutvinskiy, M. Jain and R. Nilsson, Cell Chem. Biol., 2018, 25, 1419 CrossRef CAS PubMed.
- T. S. Collier, P. Sarkar, W. L. Franck, B. M. Rao, R. A. Dean and D. C. Muddiman, Anal. Chem., 2010, 82, 8696 CrossRef CAS PubMed.
- C. Opitz, S. Isogai and S. Grzesiek, J. Biomol. NMR, 2015, 62, 373 CrossRef CAS PubMed.
- R. C. A. Schellekens, F. Stellaard, H. J. Woerdenbag, H. W. Frijlink and J. G. W. Kosterink, Br. J. Clin. Pharmacol., 2011, 72, 879 CrossRef CAS PubMed.
- C. S. Elmore and R. A. Bragg, Bioorg. Med. Chem. Lett., 2015, 25, 167 CrossRef CAS PubMed.
- R. A. Totah and A. E. Rettie, Med. Chem. Res., 2023, 32, 2048 CrossRef CAS.
- W. Li, J. Rabeah, F. Bourriquen, D. Yang, C. Kreyenschulte, N. Rockstroh, H. Lund, S. Bartling, A.-E. Surkus, K. Junge, A. Brückner, A. Lei and M. Beller, Nat. Chem., 2022, 14, 334 CrossRef CAS PubMed.
- S. Kopf, F. Bourriquen, W. Li, H. Neumann, K. Junge and M. Beller, Chem. Rev., 2022, 122, 6634 CrossRef CAS PubMed.
- H.-K. Fan, S. Yang, J.-H. Li, Q.-Q. Teng and M. Chen, Eur. J. Org. Chem., 2022, e202201218 CrossRef CAS.
- J. Atzrodt, V. Derdau, W. J. Kerr and M. Reid, Angew. Chem., Int. Ed., 2018, 57, 1758 CrossRef CAS PubMed.
- J. Atzrodt, V. Derdau, W. J. Kerr and M. Reid, Angew. Chem., Int. Ed., 2018, 57, 3022 CrossRef CAS PubMed.
- R. M. C. Di Martino, B. D. Maxwell and T. Pirali, Nat. Rev. Drug Discovery, 2023, 22, 562 CrossRef CAS PubMed.
- C. Schmidt, Nat. Biotechnol., 2017, 35, 493 CrossRef CAS PubMed.
- E. L. Jolkovsky and D. J. Goldberg, Dermatol. Rev., 2024, 5, e70008 CrossRef.
- B. King, M. M. Senna, N. A. Mesinkovska, C. Lynde, M. Zirwas, C. Maari, V. H. Prajapati, S. Sapra, P. Brzewski, L. Osman, S. Hanna, M. C. Wiseman, C. Hamilton and J. Cassella, J. Am. Acad. Dermatol., 2024, 91, 880 CrossRef CAS PubMed.
- H. Lund, J. Electrochem. Soc., 2002, 149, S21 CrossRef CAS.
- D. Pollok and S. R. Waldvogel, Chem. Sci., 2020, 11, 12386 RSC.
- H.-C. Xu and K. D. Moeller, J. Org. Chem., 2021, 86, 15845 CrossRef CAS PubMed.
- R. D. Little and K. D. Moeller, Chem. Rev., 2018, 118, 4483 CrossRef CAS PubMed.
- A. Wiebe, T. Gieshoff, S. Möhle, E. Rodrigo, M. Zirbes and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57, 5594 CrossRef CAS PubMed.
- S. Möhle, M. Zirbes, E. Rodrigo, T. Gieshoff, A. Wiebe and S. R. Waldvogel, Angew. Chem., Int. Ed., 2018, 57, 6018 CrossRef PubMed.
- S. Ning, C. Wu, L. Zheng, M. Liu, Y. Zhang, X. Che and J. Xiang, Green Chem., 2023, 25, 9993 RSC.
- Z. Zhao, R. Zhang, Y. Liu, Z. Zhu, Q. Wang and Y. Qiu, Nat. Commun., 2024, 15, 3832 CrossRef CAS PubMed.
- B. A. Frontana-Uribe, R. D. Little, J. G. Ibanez, A. Palma and R. Vasquez-Medrano, Green Chem., 2010, 12, 2099 RSC.
- J. Seidler, J. Strugatchi, T. Gärtner and S. R. Waldvogel, MRS Energy Sustainability, 2020, 7, E42 CrossRef.
- H. Li, M. Shabbir, W. Li and A. Lei, Chin. J. Chem., 2024, 42, 1145 CrossRef CAS.
- P. L. Norcott, Chem. Commun., 2022, 58, 2944 RSC.
- B. I. P. Smith, N. M. L. Knight, G. J. Knox, D. M. Lindsay, L. C. Paterson, J. Bergare, C. S. Elmore, R. A. Bragg and W. J. Kerr, Angew. Chem., Int. Ed., 2025, 64, e202417179 CrossRef CAS PubMed.
- A. Tortajada and E. Hevia, J. Am. Chem. Soc., 2022, 144, 20237 CrossRef CAS PubMed.
- S. J. Rozze and M. J. Fray, J. Labelled Compd. Radiopharm., 2009, 52, 435 CrossRef CAS.
- A. Miyazawa, H. Shimodaira and Y. Kawanishi, Bull. Chem. Soc. Jpn., 2011, 84, 1368 CrossRef CAS.
- K. Müller and A. Seubert, Isot. Environ. Health Stud., 2014, 50, 88 CrossRef PubMed.
- F. Li, Q. Chen, C.-C. Liu, Y.-H. Wu, X.-P. Liu and G.-F. Yang, Appl. Magn. Reson., 2012, 42, 169 CrossRef CAS.
- A. Martins and M. Lautens, Org. Lett., 2008, 10, 4351 CrossRef CAS PubMed.
- Z. Zhao, Q. Tian, Y. Chen, S. Wen, Y. Zhang and G. Cheng, J. Org. Chem., 2021, 86, 10407 CrossRef CAS PubMed.
- T. V. Omelian, A. V. Dobrydnev, O. Y. Utchenko, E. N. Ostapchuk, I. S. Konovalova and Y. M. Volovenko, Monatsh. Chem., 2020, 151, 1759 CrossRef CAS.
- K. Wojciechowski and S. Kosinski, Eur. J. Org. Chem., 2002, 947 CrossRef CAS.
- J. Redondo, C. Jaime and A. Marqués, J. Pharm. Biomed. Anal., 2016, 131, 454 CrossRef CAS PubMed.
- H. Kramp, R. Weck, M. Sandvoss, A. Sib, G. Mencia, P.-F. Fazzini, B. Chaudret and V. Derdau, Angew. Chem., Int. Ed., 2023, 62, e202308983 CrossRef CAS PubMed.
- Q.-K. Kang, Y. Li, K. Chen, H. Zhu, W.-Q. Wu, Y. Lin and H. Shi, Angew. Chem., Int. Ed., 2022, 61, e202117381 CrossRef CAS PubMed.
- A. R. Cochrane, A. R. Kennedy, W. J. Kerr, D. M. Lindsay, M. Reid and T. Tuttle, Catalysts, 2020, 10, 161 CrossRef CAS.
- J. A. Brown, A. R. Cochrane, S. Irvine, W. J. Kerr, B. Mondal, J. A. Parkinson, L. C. Paterson, M. Reid, T. Tuttle, S. Andersson and G. N. Nilsson, Adv. Synth. Catal., 2014, 356, 3551 CrossRef CAS.
- Y. Y. Loh, K. Nagao, A. J. Hoover, D. Hesk, N. R. Rivera, S. L. Colletti, I. W. Davies and D. W. C. MacMillan, Science, 2017, 358, 1182 CrossRef CAS PubMed.
- W. Li, R. Qu, W. Liu, F. Bourriquen, S. Bartling, N. Rockstroh, K. Junge and M. Beller, Chem. Sci., 2021, 12, 14033 RSC.
- F. Bourriquen, K. Junge and M. Beller, Synlett, 2023, 332 CAS.
- E. Martinelli, M. Spiller, R. Weck, P. Llompart, C. Minoletti, S. Güssregen, A. Sib and V. Derdau, Chem. – Eur. J., 2024, 30, e202402038 CrossRef CAS PubMed.
- C. M. Stork, R. Weck, M. Valero, H. Kramp, S. Gussregen, S. R. Waldvogel, A. Sib and V. Derdau, Angew. Chem., Int. Ed., 2023, 62, e202301512 CrossRef CAS PubMed.
- P. Zhao and Q. Zeng, Chem. – Eur. J., 2023, 29, e202302059 CrossRef CAS PubMed.
- W. Zafar, S. H. Sumrra, A. U. Hassan and Z. H. Chohan, J. Coord. Chem., 2023, 76, 546 CrossRef CAS.
- U. Lücking, Chem. – Eur. J., 2022, 28, e202201993 CrossRef PubMed.
- S. Liang, K. Hofman, M. Friedrich, J. Keller and G. Manolikakes, ChemSusChem, 2021, 14, 4878 CrossRef CAS PubMed.
- G. Domagk, Dtsch. Med. Wochenschr., 1935, 61, 250 CrossRef CAS.
- G. Domagk, Klin. Wochenschr., 1937, 16, 1412 CrossRef CAS.
- G. Domagk, Dtsch. Med. Wochenschr., 1947, 72, 6 CrossRef CAS.
- H. I. Gul, C. Yamali, H. Sakagami, A. Angeli, J. Leitans, A. Kazaks, K. Tars, D. O. Ozgun and C. T. Supuran, Bioorg. Chem., 2018, 77, 411 CrossRef CAS PubMed.
- T. Lu, C. A. Laughton, S. Wang and T. D. Bradshaw, Mol. Pharmacol., 2015, 87, 18 CrossRef PubMed.
- T. J. Van Mouwerik, P. M. Caines and R. Ballentine, Drug Intell. Clin. Pharm., 1987, 21, 330 CAS.
- S. Yahiaoui, K. Hamidouche, C. Ballandonne, A. Davis, J. S. de Oliveira Santos, T. Freret, M. Boulouard, C. Rochais and P. Dallemagne, Eur. J. Med. Chem., 2016, 121, 283 CrossRef CAS PubMed.
- D. Knez, N. Coquelle, A. Pišlar, S. Žakelj, M. Jukič, M. Sova, J. Mravljak, F. Nachon, X. Brazzolotto, J. Kos, J.-P. Colletier and S. Gobec, Eur. J. Med. Chem., 2018, 156, 598 CrossRef CAS PubMed.
- S. Bowers, G. D. Probst, A. P. Truong, R. K. Hom, A. W. Konradi, H. L. Sham, A. W. Garofalo, K. Wong, E. Goldbach, K. P. Quinn, J.-M. Sauer, W. Wallace, L. Nguyen, S. S. Hemphill, M. P. Bova and G. S. Basi, Bioorg. Med. Chem. Lett., 2009, 19, 6952 CrossRef CAS PubMed.
- J. Bou, T. Domènech, J. Puig, A. Heredia, J. Gras, D. Fernández-Forner, J. Beleta and J. M. Palacios, Eur. J. Pharmacol., 2000, 410, 33 CrossRef CAS PubMed.
- M. D. Ferrari and P. R. S. Saxena, Psychiatr., Neurol., Neurochir., 1992, 94, 73 Search PubMed.
- M. Ferrari, E. M. Bayliss, S. Ludlow and A. J. Pilgrim, Cephalalgia, 1989, 9, 348 CrossRef.
- G. B. Watson, M. W. Siebert, N. X. Wang, M. R. Loso and T. C. Sparks, Pestic. Biochem. Physiol., 2021, 178, 104924 CrossRef CAS PubMed.
- Y. Zhu, M. R. Loso, G. B. Watson, T. C. Sparks, R. B. Rogers, J. X. Huang, B. C. Gerwick, J. M. Babcock, D. Kelley, V. B. Hegde, B. M. Nugent, J. M. Renga, I. Denholm, K. Gorman, G. J. DeBoer, J. Hasler, T. Meade and J. D. Thomas, J. Agric. Food Chem., 2011, 59, 2950 CrossRef CAS PubMed.
- A. C. Barnes, P. W. Hairsine, P. J. Ramm and J. Bodenham Taylor, D. PatentamtDE2530466, Germany, 1975 Search PubMed.
- X. Tian, L. Song and A. S. K. Hashmi, Chem. – Eur. J., 2020, 26, 3197 CrossRef CAS PubMed.
- V. Desikan, Y. Liu, J. P. Toscano and W. S. Jenks, J. Org. Chem., 2007, 72, 6848 CrossRef CAS PubMed.
- C. R. Johnson, K. Mori and A. Nakanishi, J. Org. Chem., 1979, 44, 2065 CrossRef CAS.
- M. Klein and S. R. Waldvogel, Angew. Chem., Int. Ed., 2021, 60, 23197 CrossRef CAS PubMed.
- M. Klein, D. L. Troglauer and S. R. Waldvogel, JACS Au, 2023, 3, 575 CrossRef CAS PubMed.
- O. García Mancheño, O. Bistri and C. Bolm, Org. Lett., 2007, 9, 3809 CrossRef PubMed.
- M. S. Tschopp, A. Tortajada and E. Hevia, Angew. Chem., Int. Ed., 2025, 64, e202421736 CrossRef CAS PubMed.
- R. Ogasahara, M. Mae, Y. Itabashi, K. Ohkubo, K. Matsuura, H. Shimizu, K. Ban, M. Togami, T. Udagawa, H. Fujioka, M. Kamiya, S. Akai and Y. Sawama, J. Am. Chem. Soc., 2025, 15499–15509 CrossRef CAS PubMed.
- S. B. Beil, D. Pollok and S. R. Waldvogel, Angew. Chem., Int. Ed., 2021, 60, 14750 CrossRef CAS PubMed.
- M. Dörr, M. M. Hielscher, J. Proppe and S. R. Waldvogel, ChemElectroChem, 2021, 8, 2621 CrossRef.
- M. Klein and S. R. Waldvogel, Angew. Chem., Int. Ed., 2022, 61, e202204140 CrossRef CAS PubMed.
- M. Breiner, J. Strugatchi and S. R. Waldvogel, GIT Labor-Fachz., 2021, 1 Search PubMed.
- F. G. Calvo-Flores and J. A. Dobado, ChemSusChem, 2010, 3, 1227 CrossRef CAS PubMed.
- H. Lund, J. Electroanal. Chem., 2005, 584, 174 CrossRef CAS.
- H. Lund, H. Svith, S. U. Pedersen and K. Daasbjerg, Electrochim. Acta, 2005, 51, 655 CrossRef CAS.
- T. Shono, S. Kashimura and H. Nogusa, J. Org. Chem., 1984, 49, 2043 CrossRef CAS.
- K. Matsumoto, H. Shimazaki, Y. Miyamoto, K. Shimada, F. Haga, Y. Yamada, H. Miyazawa, K. Nishiwaki and S. Kashimura, J. Oleo Sci., 2014, 63, 539 CrossRef CAS PubMed.
- T. Caruso, M. Feroci, A. Inesi, M. Orsini, A. Scettri and L. Palombi, Adv. Synth. Catal., 2006, 348, 1942 CrossRef CAS.
- Z. I. Niazimbetova, D. H. Evans, L. M. Liable-Sands and A. L. Rheingold, J. Electrochem. Soc., 2000, 147, 256 CrossRef CAS.
- L. Palombi, M. Feroci, M. Orsini, L. Rossi and A. Inesi, Tetrahedron Lett., 2002, 43, 2881 CrossRef CAS.
- L. Palombi, M. Feroci, M. Orsini and A. Inesi, Tetrahedron: Asymmetry, 2002, 13, 2311 CrossRef CAS.
- A. M. Romanin, A. Gennaro and E. Vianello, J. Electroanal. Chem. Interfacial Electrochem., 1978, 88, 175 CrossRef CAS.
- R. Narobe, M. N. Perner, M. d. J. Gálvez-Vázquez, C. Kuhwald, M. Klein, P. Broekmann, S. Rösler, B. Cezanne and S. R. Waldvogel, Green Chem., 2024, 26, 10567 RSC.
- R. Xia, D. Tian, S. Kattel, B. Hasa, H. Shin, X. Ma, J. G. Chen and F. Jiao, Nat. Commun., 2021, 12, 1949 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00799b |
| This journal is © The Royal Society of Chemistry 2025 |


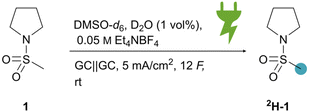

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yields representing 1H NMR yields using mesitylene (13.7 mg, 0.11 mmol) as an internal standard.
Yields representing 1H NMR yields using mesitylene (13.7 mg, 0.11 mmol) as an internal standard.