Open Access Article
Open Access ArticleCatalyst-free microwave-assisted azo-Povarov reaction of N-carbonyl aryldiazenes with trans-cyclooctene to access ring-fused cinnoline derivatives†
Xabier
Jiménez-Aberásturi
 ,
Gurutze
Padrones
,
Javier
Vicario
,
Gurutze
Padrones
,
Javier
Vicario
 and
Jesús M.
de los Santos
and
Jesús M.
de los Santos
 *
*
Department of Organic Chemistry I, Faculty of Pharmacy and Lascaray Research Center, University of the Basque Country (UPV/EHU), Paseo de la Universidad 7, 01006 Vitoria, Spain. E-mail: jesus.delossantos@ehu.eus
First published on 4th June 2025
Abstract
A previously unprecedented azo-Povarov reaction between N-carbonyl aryldiazenes and trans-cyclooctene derivatives has been developed. The participation of these aryldiazenes in the uncatalyzed [4 + 2] cycloaddition reaction has enabled the construction of a variety of appealing fused cinnoline derivatives, with yields ranging from 34% to 91% across a broad substrate scope. The starting materials are cost-effective and readily accessible, while the reaction conditions and procedures are straightforward, requiring no external catalysts. Moreover, the synthetic significance of this methodology has been demonstrated through a gram-scale azo-Povarov reaction and further derivatizations of the resulting N-containing heterocycles.
Introduction
Cinnolines exhibit a broad range of pharmacological activities, including antitumor, anti-inflammatory, analgesic, antibacterial, anticonvulsant, antihypertensive and antifungal properties.1 Representative drug candidates featuring cinnoline scaffolds are outlined in Fig. 1. For instance, compounds I and II can act as selective GABAA receptors allosteric modulators for the treatment of anxiety and other psychiatric disorders.2 Meanwhile, the cinnoline-isoxazole hybrid compound III presents greater in vitro antibacterial potency against both Gram-positive and Gram-negative bacteria compared to the standard drug norfloxacin.3 Alternatively, cinnoline derivatives have been designed as promising candidates for anticancer drugs. In this context, cinnoline derivative IV, an inhibitor of colony-stimulating factor 1 receptor (CSF-1R) tyrosine kinase, plays a significant role in both inflammation processes and cancer.4 Whereas, ARC-31 V exhibits a greater ability to trigger DNA cleavage in the presence of Topoisomerase I (TOP1).5 In addition, cinnoline carboxamide VI is a highly potent and selective ataxia telangiectasia mutated (ATM) kinase inhibitor, has demonstrated tumor regression in a colorectal cancer cell line and is currently undergoing preclinical evaluation.6As a result, considerable focus has been directed to the efficient construction of the cinnoline scaffold. Despite this, the conventional methods for accessing cinnoline derivatives, such as intermolecular annulation reactions requiring prefunctionalization of nitriles,2 aryl hydrazines,7 and aryl hydrazones,8 or cyclization of phenyldiazonium ions with highly active triazenes ortho to a terminal phenylacetylene,9 generally exhibit a limited synthetic applicability and involve complex multi-step reaction sequences, making them unsuitable as general synthetic approaches. Moreover, other improved methods previously described for the synthesis of heterocycles, particularly those involving transition-metal-catalyzed C–H bond activation,10 have emerged as a powerful tool for constructing the valuable cinnoline skeleton. In recent years, C–H activation and functionalization reactions catalyzed by stable rhodium(III) complexes have experienced a rapid development. The Rh-catalyzed cascade annulation reaction of azobenzenes with terminal alkynes for the preparation of indolo[1,2-b]cinnolines, developed by Yuan et al.;11 the annulation of N-phenylindazoles and diazo compounds for the synthesis of indazolo[2,1-a]cinnolines;12 and the construction of pyrazolo[1,2-a]cinnolines through Rh-catalyzed annulation of pyrazoline derivatives with sulfoxonium ylides, improved by Liu, Wang et al.,13 are only a few representative examples that have been recently reported.14 Iridium is another noble metal used with success in metal-catalyzed C–H activation reactions for the synthesis of cinnoline derivatives.14c,15 However, alternative cost-effective transition metals, such as Pd16 or Ru,12,17 have emerged as appealing catalysts for C–H bond activation to construct the cinnoline backbone.
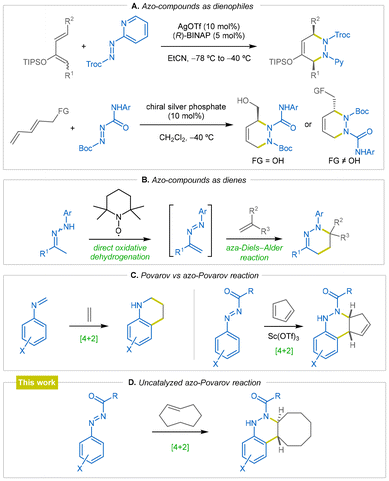
Additionally, azo compounds have found extensive applications in cycloaddition reactions with diverse partners for the preparation of a number of nitrogen-containing heterocyclic compounds. For instance, in 2006, Yamamoto et al. reported a highly regio-, diastereo- and enantioselective azo-Diels–Alder reaction as an efficient synthetic route to a series of chiral 1,4-diamines (Scheme 1A).18 Similarly, chiral silver phosphate species effectively catalyze a highly regio- and enantioselective azo hetero-Diels–Alder reaction of diazenes, affording high product yields with excellent ee values (Scheme 1A).19 Furthermore, azo compounds have been also widely used as dienes in [4 + 2] cycloaddition reactions. Accordingly, the synthesis of tetrahydropyridazines has been achieved via azo-Diels–Alder reaction of olefins with azoalkenes, which were previously generated through the direct oxidative dehydrogenation of ketohydrazones using TEMPO (Scheme 1B).20 In this research field, in the past, we have demonstrated the value of phosphorus-substituted azoalkenes in the synthesis of functionalized mercapto diketones,21 α-amino phosphonates,22 and various hetereocyclic compounds, such as pyrazine derivatives23,24 and quinoxalines.24
On the other hand, the Povarov reaction25 between aldimines and an olefinic or acetylenic component represents a powerful approach for the construction of substances containing N-heterocyclic frameworks, providing access to tetrahydroquinolines, quinolines and julolidines in a single step (Scheme 1C). Despite the typical advantages offered by C–H bond activation reactions for the preparation of cinnoline scaffolds,11–17 such as a high regioselectivity, atom economy, and fewer reaction steps, we have recently accomplished the first azo-version of the Povarov reaction (azo-Povarov reaction). This involves a Sc(OTf)3-catalyzed [4 + 2] cycloaddition reaction of cyclopentadiene with N-carbonyl aryldiazenes, that act as 4π-electron donors26 (Scheme 1C).
Inspired by our previous studies on the chemistry of azoalkenes, herein we report a practical, microwave-assisted, and catalyst-free method for synthesizing cinnoline scaffolds. More precisely, our novel method consists of a [4 + 2] cycloaddition reaction (azo-Povarov reaction) between N-carbonyl aryldiazenes and trans-cyclooctene derivatives (Scheme 1D).
Results and discussion
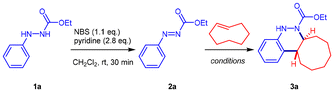
Following our first example using cyclopendadiene as the dienophile component, we attempted to expand the scope of the azo-Povarov reaction using a broader variety of alkenes. However, substrates such as cyclopentene, indene, styrene, norbornene, phenylacetylene, buta-1,3-diene, enamines, enol ethers, and cis-cyclooctene all failed to deliver the desired cinnoline derivatives when reacted with aryldiazenes under catalyzed azo-Povarov conditions.26 Consequently, strained alkenes were selected as dienophiles for the azo-Provarov reaction.Our initial efforts were focused on optimizing the reaction conditions, using aryldiazene carboxylate 2a and trans-cyclooctene as model substrates (Table 1). The starting aryldiazene carboxylates 2 can be straightforwardly synthesized through the selective oxidation of aromatic hydrazines 1 using N-bromosuccinimide (NBS)/Py.27 According to our previous work,26 we began our studies by exploring the [4 + 2] cycloaddition reaction of 2a (0.5 mmol) with trans-cyclooctene (0.75 mmol) in the presence of Sc(OTf)3 (1.2 equiv.) in chloroform at room temperature. To our delight, after 0.5 h, octahydrocycloocta[c]cinnoline 3a was isolated in 56% yield (Table 1, entry 1). As previously reported for the Sc(OTf)3-catalyzed [4 + 2] cycloaddition of aryldiazene carboxylates with cyclopentadiene,26 when exploring the catalyst loading, the use of 1.2 equiv. of the catalyst appeared to be essential in the current reaction. A catalyst loading of 20 mol% led to a significant decrease in the reaction yield and required a longer reaction time (Table 1, entry 2). Considering the high reactivity expected in the strained alkene bond of trans-cyclooctene, we then directed our efforts towards the uncatalyzed version of the azo-Povarov reaction, which has not been described thus far. However, no reaction occurred when aryldiazene 2a was treated with trans-cyclooctene at room temperature. Interestingly, when using refluxing chloroform as the solvent, cinnoline derivative 3a was achieved in very high yield (98%), although a time-consuming reaction of 10 days was required for full conversion (Table 1, entry 3).
| Entrya | Solvent | Conditions | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Unless otherwise noted, reactions were conducted on a 0.5 mmol scale and 3 mL of the corresponding solvent. b Isolated yields. c Some starting materials were observed in the crude reaction. | ||||
| 1 | CHCl3 | Sc(OTf)3 (1.2 equiv.), rt | 0.5 | 56 |
| 2 | CHCl3 | Sc(OTf)3 (0.2 equiv.), rt | 72 | 19 |
| 3 | CHCl3 | 61 °C | 240 | 98 |
| 4 | CHCl3 | 86 °C, MW | 16 | 89 |
| 5 | CHCl 3 | 111 °C, MW | 4 | 84 |
| 6 | DCE | 133 °C, MW | 2 | 74c |
| 7 | H2O | 150 °C, MW | 1.5 | 26 |
| 8 | H2O | 125 °C, MW, TBAB (0.1 mol%) | 2.5 | 72 |
| 9 | H2O | 40 °C, TBAB (0.1 mol%) | 216 | 48c |
| 10 | PBS | 40 °C | 408 | 44c |
Microwave-assisted synthesis has emerged as an important tool for the synthesis of heterocycles in an eco-friendly and energy-efficient manner.28 It offers several advantages, such as mild reaction conditions, short reaction times, high yields, homogeneous heat distribution leading to lower side reaction, better control of reaction temperature, and good functional group tolerance. Recently, significant progress has been made in the use of microwaves for the synthesis of heterocycles.29 For this reason, we next explored the uncatalyzed-azo-Povarov reaction assisted by microwaves. A mixture of N-carbonyl aryldiazene 2a and trans-cyclooctene was subjected to microwave heating at 86 °C (200 W) and, after 16 h, a very good yield (89%) of compound 3a was obtained under moderate reaction conditions (Table 1, entry 4). In view of the acceleration observed under microwave irradiation, we then optimized the diverse reaction parameters using microwaves. Notably, by increasing the temperature to 111 °C, we reduced the reaction time from 16 h to 4 h, while maintaining a similar chemical yield (84%) of 3a, as shown in Entry 5.
Next, we investigated the influence of different solvents and the reaction temperatures on the reaction yield. It was observed that compound 3a was attained with a slightly lower yield (74%) when 1,2-dichloroethane (DCE) was used as the solvent under microwave irradiation at 133 °C during the cycloaddition reaction (Table 1, entry 6).
The use of environmentally friendly solvents in organic reactions is highly desirable.30 For this reason, we next tested water as the solvent in the model [4 + 2] cycloaddition reaction. However, the use of water at 150 °C reduced the reaction time but led to a significant decrease in the chemical yield (see entry 7). Nevertheless, the addition of tetrabutylammonium bromide (TBAB, 0.1 mol%) as an additive in water promoted the azo-Povarov process, yielding 3a in 72% with a reduced reaction time of 2.5 h (Table 1, entry 8). It should be noted that under identical reaction conditions without microwave irradiation, a considerable increase in the reaction time was required, obtaining only a 48% yield of 3a (see entry 9), thus demonstrating the benefits of using microwaves in this process.
In addition, bioorthogonal chemistry encompasses a class of highly efficient chemical reactions that occur rapidly and selectively in biological environments, without interfering with endogenous functional groups through side reactions.31 In this context, we attempted to evaluate the tolerance of this procedure in biological media. As reported in Table 1, entry 10, we were pleased to observe that under phosphate-buffered saline medium (PBS, pH = 7.2), the azo-Povarov reaction of aryldiazene 2a and trans-cyclooctene at 40 °C was moderately efficient, yielding 44% of 3a after 17 days.
Considering that the preliminary studies suggested chloroform as the optimal solvent and 111 °C as the ideal temperature for the microwave-assisted [4 + 2] cycloaddition, we adopted these reaction conditions for further investigations. Accordingly, the substrate scope of aryldiazene carboxylates was studied as shown in Table 2. N-Aryldiazenes bearing activating groups (Me, OMe, OCF3, 2b–2d), deactivating groups (CF3, 2e), or halogen-substituted groups (Br, F, 2f–2g) at the para position of the phenyl ring produced the target cinnoline derivatives 3b–3g in yields ranging from 34% to 91%. The 4-trifluoromethyl-substituted derivative 3e was achieved with the highest yield (91%). In terms of electronic and steric effects, no significant influence was observed in the case of ortho- or meta-substituted compounds. For example, compound 2h bearing a fluorine atom at the ortho-position of the phenyl ring (R1 = F, R2 = R3 = H), reacted with trans-cyclooctene to afford substrate 3h in good yield (Table 2). In addition, a separable mixture of cinnoline derivatives 3i1 (30% yield) and 3i2 (48% yield) was obtained in the cycloaddition reaction of meta-F-substituted N-aryldiazene 2i with trans-cyclooctene. As evidenced from the scope of the reaction, halogen substituents were found to be suitable, making the synthetic approach useful in organic synthesis due to the potential modifications at the halogenated positions. Finally, the use of 1-naphthyl derived N-aryldiazene 2j was also well tolerated in this transformation, affording the corresponding octahydrocycloocta[c]cinnoline 3j in good yield (Table 2). These results showed that the electron density of substituents or their position on the benzene ring (2-, 3-, or 4-position) does not significantly influence the efficiency of this reaction.
| a Reaction conditions: 2 (0.5 mmol), trans-cyclooctene (0.75 mmol) in CHCl3 (1 mL) at 111 °C and 200 W, under microwave irradiation. b Isolated yield. c See ESI† for experimental details. d Some starting material 2b was observed in the crude reaction. e Some reduced starting material (functionalized hydrazine 1g) was observed in the crude reaction. |
|---|

|
Cinnoline derivatives 3, resulting from the [4 + 2] cycloaddition reaction, were characterized based on their spectroscopic data and HRMS (see ESI† for details). The most characteristic chemical shifts for compound 3a in the 1H NMR spectrum are the two well-resolved double triplets at δH = 4.52 and 2.84 ppm, corresponding to H6a and H12a, respectively, with a reciprocal coupling constant of 3JHH = 11.4 Hz, characteristic of a trans-fused ring. The NH group of the ring in 3a appears as broad singlet at δH = 6.31 ppm and, as expected, it exchanges with D2O. In the 13C NMR spectrum, the formation of 3a is evident from the presence of two signals corresponding to the tertiary carbons of the ring junctions, C6a and C12a, which appear at δC = 55.8 and 34.9 ppm, respectively. The carbonyl group shows a chemical shift at δC = 155.6 ppm, while the quaternary carbon corresponding to C4a resonates at δC = 146.2 ppm. The multiplicity of all the signals in the 13C NMR spectrum were confirmed by DEPT experiments. Moreover, the structure of 3a has been unequivocally established through X-ray crystallography. The CIF data is provided in ESI,† and the ORTEP drawing of 3a is depicted in Table 2.
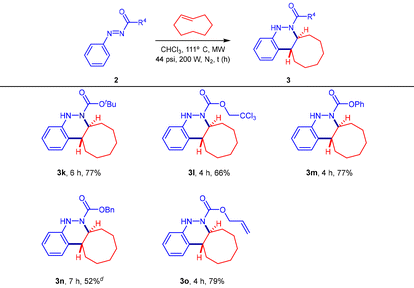
Motivated by the aforementioned results obtained in the uncatalyzed [4 + 2] cycloaddition reaction between aryldiazene carboxylates 2 and trans-cyclooctene, we next proceeded to explore the substrate scope by varying the functional group (R4) at the nitrogen atom of the aryldiazene 2 (Table 3). In this regard, a selection of several protecting groups at the nitrogen atom of the N-aryldiazene was well tolerated in this transformation, affording the corresponding octahydrocycloocta[c]cinnolines (3k–3o) in good yields. For instance, using microwave irradiation under the optimized reaction conditions, the [4 + 2] cycloaddition reaction of N-Boc aryldiazene 2k (R4 = OtBu) with trans-cyclooctene afforded cycloadduct 3k in 77% yield. However, a slight drop in the reaction yield was observed for N-Troc-derivative 3l (66%) or N-Cbz-cinnoline derivative 3n (52%) when using aryldiazene carboxylates 2l or 2n, bearing R4 = 2,2,2-trichloro-ethoxy or OBn, respectively. Furthermore, the cycloaddition reactions of other aryldiazene carboxylates (2m, 2o) with functional groups such as R4 = OPh and Oallyl (N-Alloc) were also successful, yielding cinnoline derivatives 3m and 3o in good yields (Table 3).
In addition, we also examined the scope of the reaction using N-carbonyl aryldiazenes 2p (R4 = Me) and 2q (R4 = Ph) as substrates, as shown in Scheme 2. Under microwave irradiation and adopting similar reaction conditions as before, N-acetyl aryldiazene 2p (R4 = Me), derived from N′-phenylacetohydrazide, reacted with trans-cyclooctene to afford, in only 0.5 h, hexahydrocycloocta[c]cinnoline 4a in 70% yield (based in the amount of compound 4a formed and the functionalized hydrazine 1p recovered). The oxidation of octahydrocycloocta[c]cinnoline 3p to yield the cinnoline derivative 4a was accompanied by the formation of some N′-phenylacetohydrazide 1p, resulting from the reduction of the starting N-acetyl aryldiazene 2p. The same behavior was observed when N-benzoyl aryldiazene 2q (R4 = Ph), derived from N′-phenylbenzohydrazide, was used in the reaction, yielding the same product 4a in 42% yield (Scheme 2). When the reaction was performed using conventional heating (refluxing chloroform for 30 h), 98% of 4a was obtained after treatment of diazene 2p with trans-cyclooctene.
Encouraged by the satisfactory results using trans-cyclooctene, we attempted to extend the substrate scope to other trans-cyclooctene derivatives. The optimal reaction conditions were applied to the uncatalyzed [4 + 2] cycloaddition reaction between aryldiazene carboxylates 2 and 5-acetyl-substituted trans-cyclooctene, as shown in Scheme 3. In the case of aryldiazene carboxylate 2a, 87% yield of cinnoline derivative 5a was obtained. However, the cycloaddition reaction was not regioselective, leading to a mixture of two regioisomers of 5a. Additionally, for each regioisomer, two different diasteroisomers were formed, distinguished by the stereochemistry of the acetyl substituent in trans-cyclooctene. The formation of these diastereoisomers in the final product highlights the complexity of the reaction, resulting from the lack of regioselectivity and the formation of multiple diastereoisomers. Notably, the bromo group demonstrates good tolerance, as compound 5f can be obtained in 74% yield as a mixture of regio- and diastereoisomers.
In order to investigate whether other trans-cyclooctene derivatives could also undergo this azo-Povarov reaction with aryldiazenes 2, we explored a range of trans-cyclooctene derivatives (Fig. 2). To our disappointment, our studies revealed that (1E,5E)-cycloocta-1,5-diene or (1Z,3E)-cycloocta-1,3-diene failed to deliver the desired cinnoline derivative when reacted with aryldiazene 2a. Only the starting material and the functionalized hydrazine 1a, resulting from the reduction of the aryldiazene carboxylate 2a, were observed. Additionally, the more sterically hindered methyl-substituted trans-cyclooctene, featuring a methyl substituent at the double bond, was also tested in this reaction, but no conversion was observed at all. As in the previous cases, only a mixture of diazene 2a and functionalized hydrazine 1a was recovered.
Finally, to explore if the new procedure could be extended to strained dienophiles beyond trans-cyclooctene, we examined cyclooctyne as substrate in the uncatalyzed [4 + 2] cycloaddition reaction (Scheme 4). Therefore, under microwave irradiation and applying the previously established reaction conditions, aryldiazene carboxylate 2a reacted with cyclooctyne, yielding in this case a mixture of tetrahydrocycloocta[c]cinnoline 6a, cinnoline derivative 4a, and functionalized hydrazine 1a in 24%, 30% and 30% yields, respectively, after 8 h. A similar outcome was obtained when employing aryldiazene carboxylate 2e in the reaction. This led to the formation of a mixture of products 6e/4e/1e in 18%, 33% and 36% yields, respectively (Scheme 4). Conversely, N-acetyl aryldiazene 2p (R4 = Me) reacted with cyclooctyne to afford, after 2.5 h, compound 4a in 26% yield together with N′-phenylacetohydrazide 1p.
As outlined in Scheme 5, the process is proposed to begin with an uncatalyzed [4 + 2] cycloaddition reaction of N-carbonyl aryldiazenes 2, serving as 4π-electron donors, and cyclooctyne. The resulting [4 + 2] intermediate VII can follow two possible pathways: (1) undergoing hydrogen loss to yield tetrahydrocycloocta[c]cinnoline 6 (green pathway), or (2) undergoing oxidation of intermediate VIII, leading to the formation of aromatic cinnoline derivative 4 along with compound 1 (black pathway).
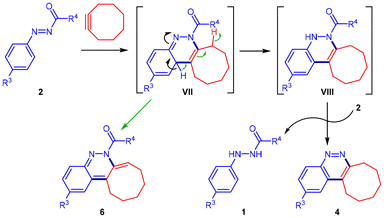 | ||
| Scheme 5 Postulated mechanism for the azo-Povarov reaction between N-carbonyl aryldiazenes and cyclooctyne. | ||
Currently, there is an ongoing discussion about the intricacies of the Povarov reaction mechanism. While some authors suggest a concerted aza-Diels–Alder [4 + 2] cycloaddition process,32 many others assert evidences supporting an ionic mechanism consisting of a Mannich-type addition of an electron-rich alkene to an activated imine, followed by a subsequent cyclization via an intramolecular Friedel–Crafts reaction (stepwise mechanism).33 However, in recent years, the ionic mechanism has gained greater acceptance for the Povarov reaction. This shift is supported by significant experimental evidence favoring a stepwise process rather than a concerted aza-Diels–Alder reaction. Conversely, the trans-stereochemistry detected in isolated cinnoline derivatives 3 strongly supports the idea of a concerted mechanism in the uncatalyzed synthesis of cinnoline derivatives via the azo-Povarov reaction, effectively supporting a concerted process.
To highlight the applicability and robustness of this methodology, a gram-scale experiment was performed using N-aryldiazene 2a (0.71 g, 4 mmol) and trans-cyclooctene, yielding 0.75 g of 3a in 65% yield (Scheme 6).
In order to demonstrate the practical value of the substrates obtained through the microwave-assisted azo-Povarov reaction, we next focused our efforts on some potential synthetic modifications of compounds 3 (Scheme 7). In particular, N-acetyl cinnoline carboxylate 7 was obtained in 81% yield when compound 3a reacted with acetyl chloride in the presence of a base (TEA) in chloroform. Additionally, NBS-mediated bromination/dehydrogenation34 with the concomitant deprotection of the N-protecting group in compound 3a under mild reaction conditions afforded product 8 in 43% yield.
Conclusions
A direct method for the construction of cinnoline derivatives has been developed through a catalyst-free, microwave-assisted [4 + 2] cycloaddition reaction of N-carbonyl aryldiazenes with trans-cyclooctene derivatives or cyclooctyne. N-Aryldiazenes bearing activating groups (Me, OMe, OCF3), deactivating groups (CF3), or even halogen-substituted groups (Br, F) at the para position of the phenyl ring are well tolerated and produce cycloocta[c]cinnolines selectively in moderate to excellent yields by using microwave irradiation under the optimized reaction conditions. The electron density of substituents or the position of substitutions on the benzene ring (2-, 3-, or 4-position) did not significantly influence the efficiency of this reaction, providing a general synthetic methodology for the construction of the cinnoline scafold. This example, together with the previous contributions of other authors in this field, may contribute to shed some light into the understanding of the real mechanisms involved on the azo-Povarov reaction. Although previous results suggest a stepwise mechanism, in our case, the use of a non-activated 2π system and the total stereoselectivity of the bonds formed, point to a concerted [4 + 2] mechanism. The concrete machinery of the process seems to be dependent of the electronic nature of the substrates and, probably, both options, stepwise and concerted, are implicated when nucleophilic 2π-systems are employed, while an exclusive concerted mechanism is the driving force if non-activated alkenes are used as 2π-partners. As far as we are concerned, this report represents the first example of an uncatalyzed-azo-Povarov reaction.Author contributions
X. J.-A.: formal analysis, investigation, methodology, visualization, writing – review and editing. G. P.: formal analysis, investigation. J. V.: funding acquisition, project administration, resources, supervision, visualization, writing – review and editing. J. M. S.: funding acquisition, methodology, project administration, resources, supervision, visualization, writing – original draft, writing – review and editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support PID2021-122558OB-I00 funded by the Ministerio de Ciencia, Innovación y Universidades MICIU/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”, and by Gobierno Vasco (GV, IT1701-22; UPV-EHU) is gratefully acknowledged. X. J.-A. thanks the Basque Country Government for the granted pre-doctoral fellowship. The authors thank technical and human support provided by SGIker (UPV/EHU/ERDF, EU).References
- (a) M. Szumilak and A. Stanczak, Cinnoline Scaffold—A Molecular Heart of Medicinal Chemistry?, Molecules, 2019, 24, 2271 CrossRef CAS; (b) Y. T. Han, J.-W. Jung and N.-J. Kim, Recent Advances in the Synthesis of Biologically Active Cinnoline, Phthalazine and Quinoxaline Derivatives, Curr. Org. Chem., 2017, 21, 1265–1291 CrossRef CAS; (c) W. Lewgowd and A. Stanczak, Cinnoline Derivatives with Biological Activity, Arch. Pharm., 2007, 340, 65–80 CrossRef CAS.
- C. Alhambra, C. Becker, T. Blake, A. (H.-F.) Chang, J. R. Damewood Jr., T. Daniels, B. T. Dembofsky, D. A. Gurley, J. E. Hall, K. J. Herzog, C. L. Horchler, C. J. Ohnmacht, R. J. Schmiesing, A. Dudley, M. D. Ribadeneira, K. S. Knappenberger, C. Maciag, M. M. Stein, M. Chopra, X. F. Liu, E. P. Christian, J. L. Arriza and M. J. Chapdelaine, Development and SAR of functionally selective allosteric modulators of GABAA receptors, Bioorg. Med. Chem., 2011, 19, 2927–2938 CrossRef CAS.
- M. B. Bommagani, J. R. Yerrabelly, M. Chitneni, G. Thalari, N. R. Vadiyala, S. K. Boda and P. R. Chitneni, Synthesis and antibacterial activity of novel cinnoline-isoxazole derivatives, Chem. Data Collect., 2021, 31, 100629 CrossRef CAS.
- E. D. Awad, M. M. El-Abadelah, S. Matar, M. A. Zihlif, R. G. Naffa, E. Q. Al-Momani and M. S. Mubarak, Synthesis and Biological Activity of Some 3-(4-(Substituted)-piperazin-1-yl)cinnolines, Molecules, 2012, 17, 227–239 CrossRef CAS PubMed.
- A. L. Ruchelman, S. K. Singh, A. Ray, X. Wu, J.-M. Yang, N. Zhou, A. Liu, L. F. Liu and E. J. LaVoie, 11H-Isoquino[4,3-c]cinnolin-12-ones: novel anticancer agents with potent topoisomerase I-targeting activity and cytotoxicity, Bioorg. Med. Chem., 2004, 12, 795–806 CrossRef CAS.
- B. Barlaam, E. Cadogan, A. Campbell, N. Colclough, A. Dishington, S. Durant, K. Goldberg, L. A. Hassall, G. D. Hughes, P. A. MacFaul, T. M. McGuire, M. Pass, A. Patel, S. Pearson, J. Petersen, K. G. Pike, G. Robb, N. Stratton, G. Xin and B. Zhai, Discovery of a Series of 3-Cinnoline Carboxamides as Orally Bioavailable, Highly Potent, and Selective ATM Inhibitors, ACS Med. Chem. Lett., 2018, 9, 809–814 CrossRef CAS.
- I. D. Jurberg and F. Gagosz, Formation of cinnoline derivatives by a gold(I)-catalyzed hydroarylation of N-propargyl-N′-arylhydrazines, J. Organomet. Chem., 2011, 696, 37–41 CrossRef CAS.
- (a) M. A.-M. Gomaa, An efficient and facile synthesis of substituted cinnoline and benzo[h]cinnoline derivatives, Tetrahedron Lett., 2003, 44, 3493–3496 CrossRef CAS; (b) M. S. Shvartsberg and I. D. Ivanchikova, An unknown route of cyclocondensation of peri-acetylenylquinones with hydrazine, Tetrahedron Lett., 2000, 41, 771–773 CrossRef CAS; (c) A. S. Kiselyov and C. Dominguez, A novel synthesis of 3,4-disubstituted cinnolines from o-trifluorophenyl hydrazones, Tetrahedron Lett., 1999, 40, 5111–5114 CrossRef CAS; (d) C. B. Kanner and U. K. Pandit, Reaction of β-amino-α, β-unsaturated esters and amides with aryl diazonium salts: Synthesis of cinnoline derivatives, Tetrahedron, 1981, 37, 3513–3518 CrossRef CAS.
- D. B. Kimball, A. G. Hayes and M. M. Haley, Thermal Cyclization of (2-Ethynylphenyl)triazenes: Facile Synthesis of Substituted Cinnolines and Isoindazoles, Org. Lett., 2000, 2, 3825–3827 CrossRef CAS PubMed.
- For reviews on C–H bond activation for the synthesis of heterocycles, see: (a) B. Nie, W. Wu, Y. Zhang, H. Jiang and J. Zhang, Recent advances in the synthesis of bridgehead (or ring-junction) nitrogen heterocycles via transition metal-catalyzed C–H bond activation and functionalization, Org. Chem. Front., 2020, 7, 3067–3099 RSC; (b) A. Baccalini, G. Faita, G. Zanoni and D. Maiti, Transition Metal Promoted Cascade Heterocycle Synthesis through C−H Functionalization, Chem. – Eur. J., 2020, 26, 9749–9783 CrossRef CAS PubMed; (c) T. H. L. Nguyen, N. Gigant and D. Joseph, Advances in Direct Metal-Catalyzed Functionalization of Azobenzenes, ACS Catal., 2018, 8, 1546–1579 CrossRef CAS.
- S. Zhang, B. Wang, X. Jia and Y. Yuan, Rhodium-Catalyzed Cascade Annulation Reaction via C−H Activation of Azobenzenes with Terminal Alkynes: A Synthesis of Indolo[1,2-b]cinnolines, Adv. Synth. Catal., 2019, 361, 451–455 CrossRef CAS.
- X. Zhang, R. Bai, H. Xiong, H. Xu and W. Hou, Meeting organometallic chemistry with drug discovery: C–H activation enabled discovery of a new ring system of 12H-Indazolo[2,1-a]cinnolin-12-ones with anti-proliferation activity, Bioorg. Med. Chem. Lett., 2020, 30, 126916 CrossRef CAS.
- S. Hu, X. Han, X. Xie, F. Fang, Y. Wang, A. Saidahmatov, H. Liu and J. Wang, Synthesis of Pyrazolo[1,2-a]cinnolines via Rhodium(III)-Catalyzed [4 + 2] Annulation Reactions of Pyrazolidinones with Sulfoxonium Ylides, Adv. Synth. Catal., 2021, 363, 3311–3317 CrossRef CAS.
- For other examples of C–H activation catalyzed by rhodium(III) complexes for the preparation of cinnoline derivatives, see: (a) C. Pan, C. Yuan, D. Chen, Y. Chen and J.-T. Yu, Rh(III)-Catalyzed C−H Activation/Annulation of N-Methyl Arylhydrazines with Iodonium Ylides toward Ring-fused Cinnolines, Asian J. Org. Chem., 2022, 11, e202100809 CrossRef CAS; (b) S. Kim, H. K. Park, J. Y. Kang, N. K. Mishra and I. S. Kim, Assembly of the Hydroxycinnoline Core via Hydrazide-Assisted Rh(III)-Catalyzed C–H Functionalization and Annulation, Synthesis, 2022, 4461–4471 CrossRef CAS; (c) Y.-C. Zheng, B. Shu, Y.-F. Zeng, S.-Y. Chen, J.-L. Song, Y.-Z. Liu, L. Xiao, X.-G. Liu, X. Zhang and S.-S. Zhang, A cascade indazolone-directed Ir(III)- and Rh(III)-catalyzed C(sp2)–H functionalization/[4 + 2] annulation of 1-arylindazolones with sulfoxonium ylides to access chemically divergent 8H-indazolo [1,2-a]cinnolines, Org. Chem. Front., 2022, 9, 5185–5190 RSC; (d) C.-Y. Lin, W.-W. Huang, Y.-T. Huang, S. Dhole and C.-M. Sun, Rhodium-Catalyzed [4 + 2] Annulation of N-Aryl Pyrazolones with Diazo Compounds To Access Pyrazolone-Fused Cinnolines, Eur. J. Org. Chem., 2021, 4984–4992 CrossRef CAS; (e) M. S. Park, K. Moon, H. Oh, J. Y. Lee, P. Ghosh, J. Y. Kang, J. S. Park, N. K. Mishra and I. S. Kim, Synthesis of (2H)-Indazoles and Dihydrocinnolinones through Annulation of Azobenzenes with Vinylene Carbonate under Rh(III) Catalysis, Org. Lett., 2021, 23, 5518–5522 CrossRef CAS.
- C.-F. Liu, M. Liu and L. Dong, Iridium(III)-Catalyzed Tandem Annulation Synthesis of Pyrazolo[1,2-α]cinnolines from Pyrazolones and Sulfoxonium Ylides, J. Org. Chem., 2019, 84, 409–416 CrossRef CAS.
- (a) W.-J. Chiu, T.-Y. Chu, I. J. Barve and C.-M. Sun, Parallel Synthesis of Pyrazolone-Fused Cinnolines by the Palladium-Catalyzed [4 + 2] Annulation of Pyrazol-3-ones with Substituted Allenoates, J. Org. Chem., 2024, 89, 395–401 CrossRef CAS PubMed; (b) C. K. Mahesha, S. Naharwal, N. D. Kharat, S. K. Mandal and R. Sakhuja, Regiodivergent synthesis of cinnoline-fused indazolones through Pd-catalyzed annulation of 1-arylindazolones with allenoates, J. Org. Chem., 2022, 87, 3701–3706 CrossRef CAS PubMed; (c) H. Li, J. Zhao, S. Yi, K. Hu and P. Feng, Consequent Construction of C–C and C–N Bonds via Palladium-Catalyzed Dual C–H Activation: Synthesis of Benzo[c]cinnoline Derivatives, Organometallics, 2021, 40, 880–889 CrossRef CAS.
- C. Pan, C. Yuan and J.-T. Yu, Ruthenium-catalyzed C−H Functionalization/Annulation of N-Aryl Indazoles/Phthalazines with Sulfoxonium Ylides to access Tetracyclic-fused Cinnolines, Asian J. Org. Chem., 2022, 11, e202200346 CrossRef CAS.
- M. Kawasaki and H. Yamamoto, Catalytic Enantioselective Hetero-Diels−Alder Reactions of an Azo Compound, J. Am. Chem. Soc., 2006, 128, 16482–16483 CrossRef CAS.
- B. Liu, T.-Y. Liu, S.-W. Luo and L.-Z. Gong, Asymmetric Hetero-Diels–Alder Reaction of Diazenes Catalyzed by Chiral Silver Phosphate: Water Participates in the Catalysis and Stereocontrol, Org. Lett., 2014, 16, 6164–6167 CrossRef CAS.
- X.-L. Yang, X.-X. Peng, F. Chen and B. Han, TEMPO-Mediated Aza-Diels–Alder Reaction: Synthesis of Tetrahydropyridazines Using Ketohydrazones and Olefins, Org. Lett., 2016, 18, 2070–2073 CrossRef CAS PubMed.
- O. A. Attanasi, S. Lillini, F. Mantellini, J. M. de los Santos, R. Ignacio and F. Palacios, Domino Reaction for the Construction of New 2-Oxo[1,2,4]triazolo[5,1-c,][1,4]thiazines, Synlett, 2009, 735–738 CAS.
- (a) F. Palacios, D. Aparicio, Y. López and J. M. de los Santos, Synthesis of functionalized α-amino-phosphine oxides and -phosphonates by addition of amines and aminoesters to 4-phosphinyl- and 4-phosphonyl-1,2-diaza-1,3-butadienes, Tetrahedron, 2005, 61, 2815–2830 CrossRef CAS; (b) F. Palacios, D. Aparicio and Y. López, J. M. de los Santos. Addition of amine derivatives to phosphorylated 1,2-diaza-1,3-butadienes. Synthesis of α-aminophosphonates, Tetrahedron Lett., 2004, 45, 4345–4348 CrossRef CAS.
- F. Palacios, D. Aparicio, Y. López, J. M. de los Santos and C. Alonso, Cycloaddition Reactions of Phosphorylated 1,2-Diaza-1,3-butadienes with Olefins: Regioselective Synthesis of Pyridazine Derivatives, Eur. J. Org. Chem., 2005, 1142–1147 CrossRef CAS.
- D. Aparicio, O. A. Attanasi, P. Filippone, R. Ignacio, S. Lillini, F. Mantellini, F. Palacios and J. M. de los Santos, Straightforward Access to Pyrazines, Piperazinones, and Quinoxalines by Reactions of 1,2-Diaza-1,3-butadienes with 1,2-Diamines under Solution, Solvent-Free, or Solid-Phase Conditions, J. Org. Chem., 2006, 71, 5897–5905 CrossRef CAS PubMed.
- (a) J. Polkaehn, R. Thom, P. Ehlers, A. Villinger and P. Langer, π-Expanded azaullazines: synthesis of quinolino-azaullazines by Povarov reaction and cycloisomerisation, Org. Biomol. Chem., 2024, 22, 2027–2042 RSC; (b) S.-G. Lei, Y. Zhou, L.-S. Wang, Z.-C. Yu, T. Chen, Y.-D. Wu, M. Gao and A.-X. Wu, One Stone, Three Birds: One-Pot Synthesis of Pyrido[3,2-a]phenoxazin-5-one Derivatives from o-Aminophenols with Triple Roles, Paraformaldehyde, and Enaminones via the Povarov Reaction, J. Org. Chem., 2023, 88, 11150–11160 CrossRef CAS PubMed; (c) W. Liu, T. Qin, W. Xie, J. Zhou, Z. Ye and X. Yang, Enantioselective Synthesis of Azahelicenes through Organocatalyzed Multicomponent Reactions, Angew. Chem., Int. Ed., 2023, 62, e202303430 CrossRef CAS PubMed; (d) C. Masdeu, J. M. de los Santos, F. Palacios and C. Alonso, The Intramolecular Povarov Tool in the Construction of Fused Nitrogen-Containing Heterocycles, Top. Curr. Chem., 2023, 381, 20 CrossRef CAS; (e) X.-R. Ren, B. Bai, Q. Zhang, L.-J. Wan, D. Wang, Q. Hao and Y. Guo, Constructing Stable Chromenoquinoline-Based Covalent Organic Frameworks via Intramolecular Povarov Reaction, J. Am. Chem. Soc., 2022, 144, 2488–2494 Search PubMed; (f) N.-F. Mo, Y. Zhang and Z.-H. Guan, Highly Enantioselective Three-Component Povarov Reaction for Direct Construction of Azaspirocycles, Org. Lett., 2022, 24, 6397–6401 CrossRef CAS PubMed; (g) O. Ghashghaei, C. Masdeu, C. Alonso, F. Palacios and R. Lavilla, Recent advances of the Povarov reaction in medicinal Chemistry, Drug Discovery Today, 2018, 29, 71–79 CrossRef PubMed; (h) D. Bello, R. Ramon and R. Lavilla, Mechanistic Variations of the Povarov Multicomponent Reaction and Related Processes, Curr. Org. Chem., 2010, 14, 332–356 CrossRef; (i) H. Ishitani and S. Kobayashi, Catalytic asymmetric aza Diels-Alder reactions using a chiral lanthanide Lewis acid. Enantioselective synthesis of tetrahydroquinoline derivatives using a catalytic amount of a chiral source, Tetrahedron Lett., 1996, 37, 7357–7360 CrossRef; (j) M. A. McCarrick, Y. D. Wu and K. N. Houk, Hetero-Diels-Alder reaction transition structures: reactivity, stereoselectivity, catalysis, solvent effects, and the exo-lone-pair effect, J. Org. Chem., 1993, 58, 3330–3343 CrossRef; (k) L. S. Povarov, α, β-Unsaturated Ethers and Their analogues in Reactions of Diene Synthesis, Russ. Chem. Rev., 1967, 36, 656–670 CrossRef.
- X. Jiménez-Aberásturi, F. Palacios and J. M. de los Santos, Sc(OTf)3-Mediated [4 + 2] Annulations of N-Carbonyl Aryldiazenes with Cyclopentadiene to Construct Cinnoline Derivatives: Azo-Povarov Reaction, J. Org. Chem., 2022, 87, 11583–11592 CrossRef PubMed.
- H. Bock, G. Rudolph, E. Baltin and J. Kroner, Farbe und Konstitution bei Azoverbindungen, Angew. Chem., Int. Ed. Engl., 1965, 77, 469–484 CrossRef.
- (a) D. Dallinger and C. O. Kappe, Microwave-Assisted Synthesis in Water as Solvent, Chem. Rev., 2007, 107, 2563–2591 CrossRef; (b) B. A. Roberts and C. R. Strauss, Toward Rapid, “Green”, Predictable Microwave-Assisted Synthesis, Acc. Chem. Res., 2005, 38, 653–661 CrossRef; (c) Microwave Assisted Organic Synthesis, ed. J. P. Tierney and P. Lidström, Wiley-Blackwell, 2005 Search PubMed; (d) M. Nüchter, B. Ondruschka, W. Bonrath and A. Gum, Microwave assisted synthesis – a critical technology overview, Green Chem., 2004, 6, 128–141 RSC.
- For reviews on microwave-assisted synthesis of heterocycles, see: (a) C. Prakash and R. Singh, Microwave-assisted Synthesis of Fluorinated Heterocycles, Curr. Green Chem., 2022, 9, 145–161 CrossRef CAS; (b) M. Henary, C. Kananda, L. Rotolo, B. Savino, E. A. Owens and G. Cravotto, Benefits and applications of microwave-assisted synthesis of nitrogen containing heterocycles in medicinal chemistry, RSC Adv., 2020, 10, 14170–14197 RSC; (c) E. Suna and I. Mutule, Microwave-assisted Heterocyclic Chemistry, Top. Curr. Chem., 2006, 266, 49–101 CrossRef CAS.
- (a) T. Kitanosono, K. Masuda, P. Xu and S. Kobayasi, Catalytic Organic Reactions in Water toward Sustainable Society, Chem. Rev., 2018, 118, 679–746 CrossRef CAS PubMed; (b) W. Leitner, P. G. Jessop, P. Wasserscheid and A. Stark, in Green Solvents, Vol. 5: Reactions in Water, ed. A. Stark, C.-J. Li, P. T. Anastas, P. Wasserscheid, P. G. Jessop and W. Leitner, Wiley, 2010 Search PubMed.
- (a) E. Kozma and P. Kele, Bioorthogonal Reactions in Bioimaging, Top. Curr. Chem., 2024, 382, 7 CrossRef; (b) Q. Fu, S. Shen, P. Sun, Z. Gu, Y. Bai, X. Wang and Z. Liu, Bioorthogonal chemistry for prodrug activation in vivo, Chem. Soc. Rev., 2023, 52, 7737–7772 RSC; (c) R. D. Row and J. A. Prescher, Constructing New Bioorthogonal Reagents and Reactions, Acc. Chem. Res., 2018, 51, 1073–1081 CrossRef; (d) P. Shieh and C. R. Bertozzi, Design strategies for bioorthogonal smart probes, Org. Biomol. Chem., 2014, 12, 9307–9320 RSC; (e) E. M. Sletten and C. R. Bertozzi, Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality, Angew. Chem., Int. Ed., 2009, 48, 6974–6998 CrossRef.
- H. Xu, S. J. Zuend, M. G. Woll, Y. Tao and E. N. Jacobsen, Asymmetric Cooperative Catalysis of Strong Brønsted Acid–Promoted Reactions Using Chiral Ureas, Science, 2010, 327, 986–990 CrossRef CAS PubMed.
- G. Dagousset, J. Zhu and G. Masson, Chiral Phosphoric Acid-Catalyzed Enantioselective Three-Component Povarov Reaction Using Enecarbamates as Dienophiles: Highly Diastereo- and Enantioselective Synthesis of Substituted 4-Aminotetrahydroquinolines, J. Am. Chem. Soc., 2011, 133, 14804–14813 CrossRef CAS.
- R. Yang, Y. Xiong, S. Deng, J. Bai, X.-R. Song and Q. Xiao, NBS-mediated bromination and dehydrogenation of tetrahydro-quinoline in one pot: scope and mechanistic study, RSC Adv., 2023, 13, 33495–33499 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2343772. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ob00508f |
| This journal is © The Royal Society of Chemistry 2025 |