 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of 6-arylpyrimido[4,5-e]indolizine-2,4(1H,3H)-diones through InCl3-catalyzed cycloisomerization†
Ruben Manuel
Figueira de Abreu
,
Alexander
Villinger
 ,
Peter
Ehlers
,
Peter
Ehlers
 and
Peter
Langer
and
Peter
Langer
 *
*
Universität Rostock, Institut für Chemie, Albert-Einstein-Str. 3a, 18059 Rostock, Germany. E-mail: peter.langer@uni-rostock; Fax: +49 381 498 6412; Tel: +49 381 498 6410
First published on 7th May 2025
Abstract
We report the synthesis of hitherto unknown pyrimido[4,5-e]indolizine-2,4(1H,3H)-diones (indolizinouracils) and related polycondensed uracils. The synthetic strategy is based on a chemoselective C–N coupling through an addition–elimination reaction of N,N-dimethyl-5-bromo-6-chlorouracil with N-heterocycles, followed by Sonogashira cross-coupling reaction with alkynes and InCl3-catalysed cycloisomerisation. The methodology allows for the employment of pyrrole and indole as N-heterocyclic building blocks and tolerates various functional groups. The impact of the substitution pattern on the photophysical properties was studied by steady-state UV-Vis and fluorescence spectroscopy, providing new insights into the potential applications of uracil-based polycondensed heterocycles.
Introduction
Pyrimidines and related compounds, such as uracils (pyrimidine-2,4-diones), play key roles in cellular processes and, hence, have evolved as valuable building blocks in modern drug development. They are key components of nucleosides; they form the highly ordered double helix structures of DNA and RNA and hence are fundamental for coding genetic information and ensuring proper functioning of living cells.1,2 Apart from their presence in DNA and RNA, pyrimidines are also found in a variety of natural products, such as xanthines, pteridines, pyrrolopyrimidines and pyridopyrimidines. They possess various biological activities such as anti-bacterial, antiproliferative, antifungal and anti-inflammatory activities.3–5 Hence, there has been considerable interest in the synthesis and properties of fused pyrimidine derivatives, which has led to the discovery of several synthetic drugs, such as pemetrexed (A), immucilin H (B), ganciclovir (C) and ruxolitinib (D) (Fig. 1).1,6Traditionally, fused pyrimidines are obtained using two different approaches. The first synthetic methodology utilizes pre-functionalized heterocycles, and the pyrimidine ring is constructed by employing suitable urea derivatives. The second approach takes advantage of the reactivity of pyrimidine derivatives, and a fused heterocyclic entity is directly constructed on the pyrimidine scaffold.7 Our group has contributed to the latter strategy by employing easily available uracil derivatives followed by sequential functionalisation through substitution and Pd-catalysed cross-coupling reactions.8,9
As an extension of our ongoing interest in the synthesis of heterocyclic fused uracils, we herein report the synthesis of indolizinouracils. While pyrrolo- and pyridouracils are well known and represent intriguing building blocks in medicinal chemistry,1,4,5,10 indolizinouracils have, to the best of our knowledge, not been previously reported in the literature.11 Our synthetic approach is based on a three-step procedure, involving a chemoselective reaction of 6-chloro-5-bromouracil with N-heterocycles, such as pyrrole and indole, followed by a Sonogashira reaction12 and subsequent cycloisomerisation (Scheme 1).
Results and discussion
Synthesis
5-Bromo-6-chlorouracil (1) was prepared as reported in the literature.8,13 Starting material 1 was further functionalized by nucleophilic substitution with pyrrole as the corresponding heteroaromatic nucleus. Initially, NaH was chosen as a base as it allows complete deprotonation of pyrrole. However, TLC monitoring revealed poor conversion of uracil 1, even after prolonged reaction time. Hence, we next employed n-BuLi at −78 °C as the base. Using this base at lower temperature followed by slow warming to room temperature gave the corresponding pyrrole-substituted uracil 2a in 80% yield (Scheme 2). The developed reaction conditions were also applicable to other N-heterocycles, such as imidazole, indole and benzimidazole and gave the corresponding products in good yields. Only the reaction with benzimidazole delivered the corresponding product in only a moderate isolated yield of 40%, which was due to difficulties encountered during its purification by column chromatography. | ||
| Scheme 2 Synthesis of uracils 2a–e. Reaction conditions: (i) n-BuLi, azol, THF, −78 °C → 24 °C, 7 h. Yields of isolated products. | ||
Subsequently, the Sonogashira reaction of 2a with 4-tolylacetylene was studied. As an initial attempt, our previously developed conditions for the Sonogashira reaction of alkynes with uracil derivatives were employed.8 Using Pd(PPh2)Cl2 in the presence of CuI as the co-catalyst and triethylamine as the base in DMSO led to the desired coupling product 3a in 80% yield. Hence, no further optimisation of the reaction conditions was required, and the scope of the reaction was studied next. The reaction of 2a and c–e, containing pyrrole, indole and benzimidazole substituents, with various arylacetylenes afforded products 3a–i (Scheme 3). Products 3a–i were isolated in good to very good yields, ranging from 68–98%. The obtained yields were independent of both the substitution pattern of the employed aryl acetylene and the nature of the heterocycle attached to the uracil ring. Employment of the imidazole-substituted uracil 2b failed to give product 3j. Despite complete conversion of the starting material, we were not able to purify the product from other impurities (which were also not detectable by TLC analysis).
 | ||
| Scheme 3 Synthesis of 3a–i. Reaction conditions: arylacetylene (1.2 equiv.), Pd(PPh3)2Cl2 (5 mol%), CuI (5 mol%), NEt3 (11 equiv.), DMSO, 100 °C, 6 h. Yields of isolated products. | ||
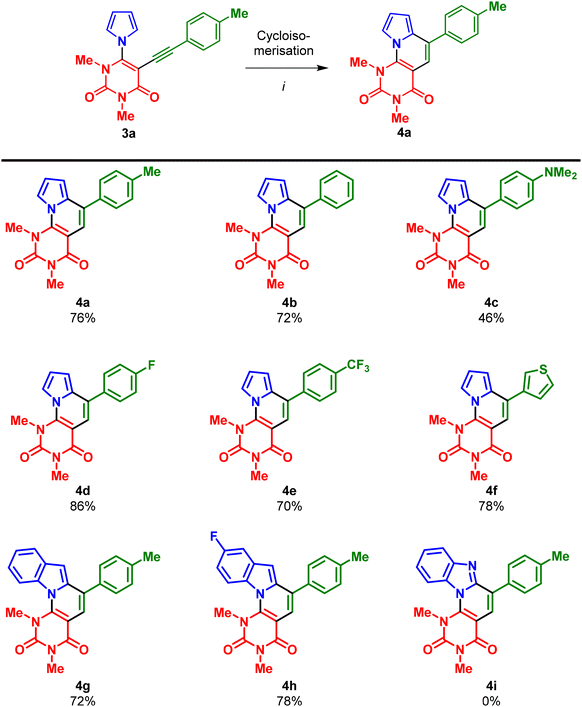
Finally, we studied the cycloisomerisation of 3a as a model substrate to access the desired indolizinouracil 4a (Table 1). As a starting point of our investigation, we used pTsOH·H2O as the Brønsted acid, given its good performance in previously reported transformations.9 Using this acid in toluene at 100 °C gave the desired product, albeit in only 31% yield. Subsequently, we screened different Brønsted acids, such as methanesulfonic acid (MsOH) and trifluoroacetic acid (TFA). Unfortunately, the yields could not be significantly increased. Consequently, we turned our attention to Lewis acids. In the beginning, PtCl2 was employed, which is known to efficiently catalyse the cycloisomerisation of 2-biaryl alkynes due to its π-electron affinity.14,15 However, only complex mixtures, which could not be purified, were obtained. However, employment of catalytic amounts of InCl3 (10 mol%) eventually gave 4a in 76% isolated yield.14,16 Increasing the amount of InCl3 to an equimolar ratio resulted in no further improvement.
| Entry | Acid (equiv.) | Solvent | Temperature (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| 1 | p-TsOH·H2O (15) | Toluene | 100 | 6 | 31 |
| 2 | p-TsOH·H2O (20) | Toluene | 100 | 6 (16) | 30 |
| 3 | MsOH (20) | Toluene | 100 | 6 (16) | 11 |
| 4 | TFA (20) | Toluene | 100 | 6 (16) | 37 |
| 5 | PtCl2 (0.1) | Toluene | 80 | 6 (16) | Mixture |
| 6 | PtCl2 (0.1) | Toluene | 100 | 6 (16) | Mixture |
| 7 | InCl3 (0.05) | Toluene | 80 | 6 (16) | 29 |
| 8 | InCl3 (0.1) | Toluene | 100 | 16 | 76 |
| 9 | InCl3 (1) | Toluene | 100 | 16 | 72 |
With the optimised conditions in hand, InCl3 (0.1 equiv.), toluene, 100 °C, 16 h, the scope of the cycloisomerisation reaction was investigated. The cycloisomerization of 3a–h worked very well and products 4a–h were mostly obtained in good to very good yields (Scheme 4). Only compound 4c, containing a strongly electron-donating NMe2 group, was obtained in a moderate 46% yield. This can be explained by problems during the purification caused by the low solubility of 4c. In the case of benzimidazole-substituted uracil 3i, no conversion was observed and, hence, product 4i could not be obtained, possibly due to the coordination of InCl3 or the formation of imidazolyl cations under acidic conditions.
 | ||
| Scheme 4 Synthesis of 4a–h. Reaction conditions: InCl3 (0.1 equiv.), toluene, 100 °C, 16 h. Yields of isolated products. | ||
The highest yield of 86% was obtained for the fluorine-substituted compound 4d. In general, the reaction conditions do not seem to be affected by the substitution pattern of the arylalkyne moiety, as all compounds (except 4c) were obtained in similar yields.
The structure was unambiguously verified by analysis of the spectroscopic data. The characteristic carbon signals of the triple bond disappeared and a typical vinylic proton signal was observed. Moreover, the loss of symmetry in the pyrrole ring indicated its functionalisation while mass spectroscopy revealed the same molar mass but with different fragmentation. Moreover, the structure of 4a was independently confirmed by X-ray crystallographic analysis. Crystals were obtained by slow evaporation from a mixture of dichloromethane and heptane at room temperature (Fig. 2). Product 4a crystallises in a base-centred monoclinic system with the P21/n space group. The analysis revealed the formation of a planar indolozinouracil core structure. The substituted tolyl ring is twisted out of plane by 47°. Two molecules align in a parallel, slipped head-to-tail orientation with several short CH–π (2.76 Å) and CC–π contacts (3.39 Å), while neighbouring molecules form hydrogen bonds between the carbonyl oxygen and the hydrogen of the adjacent methyl (2.32 Å) and pyrrole ring.
Optical properties
The photophysical properties of selected derivatives were investigated by steady-state absorption and photoluminescence spectroscopy. The influence of the substitution pattern of arylated indolizinouracils on the photophysical properties is shown in Fig. 3. Corresponding photophysical data and quantum yields are given in Table 2.| 4b | 4c | 4e | 4f | 4g | |
|---|---|---|---|---|---|
| a Excitation wavelength λex = 330 nm. b Excitation wavelength λex = 370 nm. c Fluorescence standards: quinine hemi-sulphate in H2SO4 (0.05 M) (Φ = 0.52).17 d Shoulder. | |||||
| λ 1,abs (nm) | 251 | 256 | 264 | 254 | 272 |
| ε λ 1 × 104 (M−1 cm−1) | 2.1 | 1.3 | 2.0 | 2.1 | 2.7 |
| λ 2,abs (nm) | 262 | 332 | 327 | 327 | 293 |
| ε λ 2 × 104 (M−1 cm−1) | 2.0 | 1.6 | 0.94 | 0.97 | 2.3 |
| λ 3,abs (nm) | 324 | 384 | 375 | 353 | |
| ε λ 3 × 104 (M−1 cm−1) | 0.87 | 0.46 | 0.42 | 1.3 | |
| λ 4,abs (nm) | 373 | 369 | |||
| ε λ 4 × 104 (M−1 cm−1) | 0.37 | 1.3 | |||
| λ 1,em (nm) | 386a,d | 419a | 473a | 471a | 539b |
| λ 2,em (nm) | 469a | ||||
| Φ | 4a | 53a | 8a | 4a | 2b |
Compounds 4b, 4e and 4f show very similar absorption and emission properties. All three compounds possess their lowest absorption band (S0 → S1) at approximately 375 nm with comparable extinction coefficients (3700–4600 M−1 cm−1) and emission maxima at ∼470 nm. Interestingly, 4c containing a strongly electron-donating NMe2-group shows a slight bathochromic shift of its absorption features accompanied by enhanced extinction coefficients. In contrast, the emission maximum of 4c is hypsochromically shifted to 419 nm. Moreover, while all measured compounds show low quantum yields ranging from 2–8%, compound 4c showed a distinct enhancement of its quantum yield to 53%, which might be explained by the occurrence of certain donor–acceptor relationships between the electron-rich dimethylaniline moiety and the electron deficient uracil ring.
Compound 4g, representing benzo-indolizinouracil, displayed a red-shifted absorption spectrum, due to the extension of its π-system. The absorption spectrum reveals a certain fine-structure for 4g, with a weak, but broad S0 → S1 transition band at 419 nm. Similarly, the emission spectrum is shifted to 539 nm. However, 4g exhibited the lowest quantum yield among all studied compounds (2%).
Conclusion
In summary, we have developed a novel methodology for the synthesis of indolizinouracils, representing, to the best of our knowledge, a hitherto unknown heterocyclic core structure. The synthetic strategy is based on a chemoselective C–N coupling via an addition–elimination reaction of N,N-dimethyl-5-bromo-6-chlorouracil with N-heterocycles, followed by Sonogashira cross-coupling reaction with alkynes and InCl3-catalysed cycloisomerisation. The obtained products were isolated in moderate to excellent yields under carefully optimised reaction conditions. This methodology is tolerant towards the incorporation of pyrrole and indole as N-heterocyclic entities as well as towards functional groups attached to the aryl ring. Preliminary photophysical properties of selected derivatives have been investigated by steady-state absorption and photoluminescence spectroscopy. The optical properties are strongly altered by the substitution pattern on the indolizinouracil scaffold, with quantum yields ranging from 2 to up to 53%.Experimental section
General information
Nuclear magnetic resonance spectra (1H/13C/19F NMR) were recorded on a Bruker AVANCE 300 III, 250II, or 500. The analysed chemical shifts (δ) are referenced to the residual solvent signals of the deuterated solvents, CDCl3 (δ = 7.26 ppm for 1H-NMR and 77.16 ppm for 13C-NMR). Multiplicities due to spin–spin correlation are reported as follows: s = singlet, d = doublet, dd = double doublet, pt = pseudotriplet, and m = multiplet; they are further described by their coupling constants J. Infrared spectra (IR) were measured as attenuated total reflection (ATR) experiments using a Nicolet 380 FT-IR spectrometer. The signals were characterised by their wavenumbers and the corresponding absorption as very strong (vs), strong (s), medium (m), weak (w) or very weak (vw). UV-vis spectra were recorded on a Cary 60 UV-Vis spectrophotometer, and emission spectra were recorded on an Agilent Cary Eclipse fluorescence spectrophotometer. Basic and high-resolution mass spectra (MS/HRMS) were measured on instruments coupled to a gas chromatograph (GC) or a liquid chromatograph (LC). Samples were ionised by electron impact ionisation (EI) on an Agilent 6890/5973 or Agilent 7890/5977 GC-MS equipped with an HP-5 capillary column using helium carrier gas or by electron spray ionisation (ESI) on an Agilent 1200/6210 Time-of-Flight (TOF) LC-MS. X-ray single-crystal structure analysis was performed on a Bruker Apex Kappa-II CCD diffractometer. The solvent, toluene, was purchased as a dry solvent and used without further purification. Other reagents, catalysts, ligands, acids and bases were used as purchased from commercial suppliers. Column chromatography was performed on Merck Silica gel 60 (particle size 63–200 μm). Solvents for extraction and column chromatography were distilled prior to use.Representative procedure C for the synthesis of 4a–h
A mixture of 3d (312 μmol; 101 mg) and InCl3 (5 mol%; 327 μmol; 72.3 mg) was dissolved in dry toluene (2 mL) and stirred for 16 hours under an argon atmosphere at 100 °C. The reaction was monitored by TLC until completion. The reaction was quenched by the addition of saturated NH4Cl solution (15 mL), the phases were separated, and the aqueous layer was extracted with dichloromethane (3 × 20 mL). The combined organic layers were dried over Na2SO4, concentrated under reduced pressure and purified by column chromatography (heptane/ethyl acetate).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); mp: 203–205 °C. IR (ATR): ṽ [cm−1] = 1702 (s), 1646 (vs), 1607 (s), 1489 (s), 1450 (s), 1364 (s), 1131 (s), 818 (s). 1H NMR (300 MHz, chloroform-d) δ = 7.7 (dd, J = 3.1, 1.3 Hz, 1H), 7.5–7.4 (m, 2H), 7.2 (s, 1H), 7.2 (d, J = 7.8 Hz, 2H), 6.8 (dd, J = 4.0, 3.1 Hz, 1H), 6.6 (dd, J = 4.0, 1.3 Hz, 1H), 3.8 (s, 3H), 3.4 (s, 3H), 2.3 (s, 3H). 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.5, 152.7, 139.1, 138.2, 136.5, 134.9, 129.5, 128.7, 128.2, 116.6, 115.0, 113.7, 102.9, 101.0, 37.0, 28.7, 21.4. MS (EI, 70 eV): m/z (%) = 319 (37, M+), 306 (14), 281 (12), 221 (11), 207 (16), 113 (12). HRMS (EI): calcd for C19H17N3O2 [M]+ 319.13153, found: 319.13163.
2); mp: 203–205 °C. IR (ATR): ṽ [cm−1] = 1702 (s), 1646 (vs), 1607 (s), 1489 (s), 1450 (s), 1364 (s), 1131 (s), 818 (s). 1H NMR (300 MHz, chloroform-d) δ = 7.7 (dd, J = 3.1, 1.3 Hz, 1H), 7.5–7.4 (m, 2H), 7.2 (s, 1H), 7.2 (d, J = 7.8 Hz, 2H), 6.8 (dd, J = 4.0, 3.1 Hz, 1H), 6.6 (dd, J = 4.0, 1.3 Hz, 1H), 3.8 (s, 3H), 3.4 (s, 3H), 2.3 (s, 3H). 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.5, 152.7, 139.1, 138.2, 136.5, 134.9, 129.5, 128.7, 128.2, 116.6, 115.0, 113.7, 102.9, 101.0, 37.0, 28.7, 21.4. MS (EI, 70 eV): m/z (%) = 319 (37, M+), 306 (14), 281 (12), 221 (11), 207 (16), 113 (12). HRMS (EI): calcd for C19H17N3O2 [M]+ 319.13153, found: 319.13163.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); mp: 144–146 °C. IR (ATR): ṽ [cm−1] = 1697 (s), 1640 (vs), 1611 (s), 1479 (s), 1364 (s), 1215 (m), 1195 (m), 756 (s). 1H NMR (300 MHz, chloroform-d) δ = 7.84–7.78 (m, 1H), 7.67–7.61 (m, 2H), 7.51–7.33 (m, 4H), 6.95–6.89 (m, 1H), 6.68 (d, J = 3.8 Hz, 1H), 3.97–3.90 (m, 3H), 3.48 (s, 3H). 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.5, 152.7, 139.3, 137.8, 136.4, 128.8, 128.7, 128.4, 128.3, 116.7, 115.1, 114.0, 102.9, 101.0, 37.0, 28.8. MS (EI, 70 eV): m/z (%) = 305 (43, M+), 292 (14), 281 (14), 248 (10). HRMS (EI): calcd for C18H15N3O2 [M]+ 305.11588, found: 305.11595.
2); mp: 144–146 °C. IR (ATR): ṽ [cm−1] = 1697 (s), 1640 (vs), 1611 (s), 1479 (s), 1364 (s), 1215 (m), 1195 (m), 756 (s). 1H NMR (300 MHz, chloroform-d) δ = 7.84–7.78 (m, 1H), 7.67–7.61 (m, 2H), 7.51–7.33 (m, 4H), 6.95–6.89 (m, 1H), 6.68 (d, J = 3.8 Hz, 1H), 3.97–3.90 (m, 3H), 3.48 (s, 3H). 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.5, 152.7, 139.3, 137.8, 136.4, 128.8, 128.7, 128.4, 128.3, 116.7, 115.1, 114.0, 102.9, 101.0, 37.0, 28.8. MS (EI, 70 eV): m/z (%) = 305 (43, M+), 292 (14), 281 (14), 248 (10). HRMS (EI): calcd for C18H15N3O2 [M]+ 305.11588, found: 305.11595.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); mp: 157–159 °C. IR (ATR): ṽ [cm−1] = 1683 (s), 1650 (vs), 1605 (s), 1514 (s), 1397 (s), 1294 (s), 1205 (s), 1123 (s). 1H NMR (500 MHz, chloroform-d) δ = 7.76 (dd, J = 3.1, 1.3 Hz, 1H), 7.57–7.53 (m, 2H), 7.29 (s, 1H), 6.90 (dd, J = 4.0, 3.1 Hz, 1H), 6.83–6.79 (m, 2H), 6.73 (dd, J = 4.1, 1.3 Hz, 1H), 3.90 (s, 3H), 3.47 (s, 3H), 3.02 (s, 6H). 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.6, 152.7, 150.5, 138.5, 136.6, 129.1, 128.9, 125.5, 116.4, 114.8, 112.5, 112.4, 102.9, 101.2, 40.6, 37.0, 28.7. MS (EI, 70 eV): m/z (%) = 348 (27, M+), 333 (15), 207 (12), 149 (6), 123 (8). HRMS (EI): calcd for C20H20N4O2 [M]+ 34.15808, found: 348.15744.
2); mp: 157–159 °C. IR (ATR): ṽ [cm−1] = 1683 (s), 1650 (vs), 1605 (s), 1514 (s), 1397 (s), 1294 (s), 1205 (s), 1123 (s). 1H NMR (500 MHz, chloroform-d) δ = 7.76 (dd, J = 3.1, 1.3 Hz, 1H), 7.57–7.53 (m, 2H), 7.29 (s, 1H), 6.90 (dd, J = 4.0, 3.1 Hz, 1H), 6.83–6.79 (m, 2H), 6.73 (dd, J = 4.1, 1.3 Hz, 1H), 3.90 (s, 3H), 3.47 (s, 3H), 3.02 (s, 6H). 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.6, 152.7, 150.5, 138.5, 136.6, 129.1, 128.9, 125.5, 116.4, 114.8, 112.5, 112.4, 102.9, 101.2, 40.6, 37.0, 28.7. MS (EI, 70 eV): m/z (%) = 348 (27, M+), 333 (15), 207 (12), 149 (6), 123 (8). HRMS (EI): calcd for C20H20N4O2 [M]+ 34.15808, found: 348.15744.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); mp: 217–219 °C. IR (ATR): ṽ [cm−1] = 1706 (s), 1650 (vs), 1568 (s), 1478 (s), 1357 (s), 1221 (s), 1158 (s), 840 (s). 1H NMR (300 MHz, chloroform-d) δ = 7.82 (dd, J = 3.2, 1.3 Hz, 1H), 7.65–7.56 (m, 2H), 7.31 (s, 1H), 7.20–7.10 (m, 2H), 6.93 (dd, J = 4.0, 3.1 Hz, 1H), 6.62 (dd, J = 4.1, 1.3 Hz, 1H), 3.94 (s, 3H), 3.48 (s, 3H). 19F NMR (282 MHz, chloroform-d) δ = −113.6. 13C {1H} NMR (75 MHz, chloroform-d) δ = 162.8 (d, J = 247.5 Hz), 161.5, 152.7, 139.4, 136.38, 133.8 (d, J = 3.3 Hz), 130.1 (d, J = 8.1 Hz), 127.7, 116.7, 115.8 (d, J = 21.6 Hz), 115.2, 114.1, 102.9, 101.0, 37.1, 28.8. MS (EI, 70 eV): m/z (%) = 323 (100, M+), 266 (24), 251 (28), 237 (15), 223 (20), 211 (19). HRMS (ESI-TOF): calcd for C18H14FN3O2 [M + H]+ 323.10646, found: 323.10613.
2); mp: 217–219 °C. IR (ATR): ṽ [cm−1] = 1706 (s), 1650 (vs), 1568 (s), 1478 (s), 1357 (s), 1221 (s), 1158 (s), 840 (s). 1H NMR (300 MHz, chloroform-d) δ = 7.82 (dd, J = 3.2, 1.3 Hz, 1H), 7.65–7.56 (m, 2H), 7.31 (s, 1H), 7.20–7.10 (m, 2H), 6.93 (dd, J = 4.0, 3.1 Hz, 1H), 6.62 (dd, J = 4.1, 1.3 Hz, 1H), 3.94 (s, 3H), 3.48 (s, 3H). 19F NMR (282 MHz, chloroform-d) δ = −113.6. 13C {1H} NMR (75 MHz, chloroform-d) δ = 162.8 (d, J = 247.5 Hz), 161.5, 152.7, 139.4, 136.38, 133.8 (d, J = 3.3 Hz), 130.1 (d, J = 8.1 Hz), 127.7, 116.7, 115.8 (d, J = 21.6 Hz), 115.2, 114.1, 102.9, 101.0, 37.1, 28.8. MS (EI, 70 eV): m/z (%) = 323 (100, M+), 266 (24), 251 (28), 237 (15), 223 (20), 211 (19). HRMS (ESI-TOF): calcd for C18H14FN3O2 [M + H]+ 323.10646, found: 323.10613.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); mp: 127–128 °C. IR (ATR): ṽ [cm−1] = 1704 (s), 1636 (vs), 1609 (s), 1496 (s), 1320 (vs), 1166 (s), 1120 (vs), 1065 (vs). 1H NMR (300 MHz, chloroform-d) δ = 7.84 (dd, J = 3.1, 1.3 Hz, 1H), 7.80–7.69 (m, 6H), 7.38 (s, 1H), 6.95 (dd, J = 4.0, 3.2 Hz, 2H), 6.64 (dd, J = 4.0, 1.3 Hz, 1H), 3.94 (s, 2H), 3.48 (s, 3H). 19F NMR (282 MHz, chloroform-d) δ = −62.5. 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.3, 152.6, 141.4 (d, J = 1.5 Hz), 139.8, 135.9, 130.3 (q, J = 32.6 Hz), 128.7, 127.2, 125.8 (q, J = 3.7 Hz), 124.2 (q, J = 272.1 Hz), 116.9, 115.4, 114.9, 102.8, 100.8, 37.1, 28.8. MS (EI, 70 eV): m/z (%) = 373 (100, M+), 316 (25), 301 (26), 287 (11), 273 (17). HRMS (EI): calcd for C19H14F3N3O2 [M + H]+ 373.10326, found: 373.10309.
2); mp: 127–128 °C. IR (ATR): ṽ [cm−1] = 1704 (s), 1636 (vs), 1609 (s), 1496 (s), 1320 (vs), 1166 (s), 1120 (vs), 1065 (vs). 1H NMR (300 MHz, chloroform-d) δ = 7.84 (dd, J = 3.1, 1.3 Hz, 1H), 7.80–7.69 (m, 6H), 7.38 (s, 1H), 6.95 (dd, J = 4.0, 3.2 Hz, 2H), 6.64 (dd, J = 4.0, 1.3 Hz, 1H), 3.94 (s, 2H), 3.48 (s, 3H). 19F NMR (282 MHz, chloroform-d) δ = −62.5. 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.3, 152.6, 141.4 (d, J = 1.5 Hz), 139.8, 135.9, 130.3 (q, J = 32.6 Hz), 128.7, 127.2, 125.8 (q, J = 3.7 Hz), 124.2 (q, J = 272.1 Hz), 116.9, 115.4, 114.9, 102.8, 100.8, 37.1, 28.8. MS (EI, 70 eV): m/z (%) = 373 (100, M+), 316 (25), 301 (26), 287 (11), 273 (17). HRMS (EI): calcd for C19H14F3N3O2 [M + H]+ 373.10326, found: 373.10309.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); mp: 200–202 °C. IR (ATR): ṽ [cm−1] = 1697 (s), 1645 (vs), 1487 (s), 1475 (s), 1458 (s), 1339 (s), 783 (s), 72 (s). 1H NMR (300 MHz, chloroform-d) δ = 7.78 (dd, J = 3.1, 1.3 Hz, 1H), 7.59 (dd, J = 2.2 Hz, 1H), 7.46–7.42 (m, 2H), 7.41 (s, 1H), 6.93 (dd, J = 4.0, 3.1 Hz, 1H), 6.79 (dd, J = 4.0, 1.3 Hz, 1H), 3.91 (s, 3H), 3.46 (s, 3H). 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.4, 152.6, 139.1, 138.2, 135.9, 127.6, 126.1, 123.5, 123.0, 116.6, 115.1, 113.6, 102.9, 100.8, 37.1, 28.8. MS (EI, 70 eV): m/z (%) = 311 (100, M+), 300 (87), 296 (22), 273 (35), 269 (29), 254 (57). HRMS (EI): calcd for C16H13N3O2S [M]+ 311.07230, found: 311.07180.
2); mp: 200–202 °C. IR (ATR): ṽ [cm−1] = 1697 (s), 1645 (vs), 1487 (s), 1475 (s), 1458 (s), 1339 (s), 783 (s), 72 (s). 1H NMR (300 MHz, chloroform-d) δ = 7.78 (dd, J = 3.1, 1.3 Hz, 1H), 7.59 (dd, J = 2.2 Hz, 1H), 7.46–7.42 (m, 2H), 7.41 (s, 1H), 6.93 (dd, J = 4.0, 3.1 Hz, 1H), 6.79 (dd, J = 4.0, 1.3 Hz, 1H), 3.91 (s, 3H), 3.46 (s, 3H). 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.4, 152.6, 139.1, 138.2, 135.9, 127.6, 126.1, 123.5, 123.0, 116.6, 115.1, 113.6, 102.9, 100.8, 37.1, 28.8. MS (EI, 70 eV): m/z (%) = 311 (100, M+), 300 (87), 296 (22), 273 (35), 269 (29), 254 (57). HRMS (EI): calcd for C16H13N3O2S [M]+ 311.07230, found: 311.07180.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); mp: 201–202 °C. IR (ATR): ṽ [cm−1] = 1700 (s), 1650 (vs), 1617 (s), 1489 (s), 1446 (vs), 1440 (vs), 1310 (s), 824 (s). 1H NMR (500 MHz, chloroform-d) δ = 7.85–7.81 (m, 1H), 7.76–7.72 (m, 1H), 7.60–7.56 (m, 2H), 7.43–7.39 (m, 2H), 7.36–7.29 (m, 3H), 6.91 (s, 1H), 3.57 (s, 3H), 3.50 (s, 3H), 2.45 (s, 3H). 13C {1H} NMR (126 MHz, chloroform-d) δ = 161.4, 154.1, 142.5, 139.8, 138.4, 134.5, 132.4, 131.7, 129.6, 129.0, 128.1, 124.3, 121.3, 121.1, 116.9, 115.9, 101.1, 98.1, 39.3, 28.6, 21.4. MS (EI, 70 eV): m/z (%) = 369 (100, M+), 326 (10), 311 (12), 297 (10). HRMS (EI): calcd for C23H19N3O2 [M]+ 369.14718, found: 369.14769.
2); mp: 201–202 °C. IR (ATR): ṽ [cm−1] = 1700 (s), 1650 (vs), 1617 (s), 1489 (s), 1446 (vs), 1440 (vs), 1310 (s), 824 (s). 1H NMR (500 MHz, chloroform-d) δ = 7.85–7.81 (m, 1H), 7.76–7.72 (m, 1H), 7.60–7.56 (m, 2H), 7.43–7.39 (m, 2H), 7.36–7.29 (m, 3H), 6.91 (s, 1H), 3.57 (s, 3H), 3.50 (s, 3H), 2.45 (s, 3H). 13C {1H} NMR (126 MHz, chloroform-d) δ = 161.4, 154.1, 142.5, 139.8, 138.4, 134.5, 132.4, 131.7, 129.6, 129.0, 128.1, 124.3, 121.3, 121.1, 116.9, 115.9, 101.1, 98.1, 39.3, 28.6, 21.4. MS (EI, 70 eV): m/z (%) = 369 (100, M+), 326 (10), 311 (12), 297 (10). HRMS (EI): calcd for C23H19N3O2 [M]+ 369.14718, found: 369.14769.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); mp: 193–195 °C. IR (ATR): ṽ [cm−1] = 1710 (s), 1664 (vs), 1446 (s), 1310 (m), 1141 (s), 1028 (m), 848 (s), 828 (s). 1H NMR (300 MHz, chloroform-d) δ = 7.78 (dd, J = 9.2, 4.3 Hz, 1H), 7.59–7.53 (m, 2H), 7.45 (s, 1H), 7.38–7.29 (m, 3H), 7.08 (td, J = 8.9, 2.6 Hz, 1H), 6.87 (s, 1H), 3.57 (s, 3H), 3.50 (s, 3H), 2.45 (s, 3H). 19F NMR (282 MHz, chloroform-d) δ = −117.4. 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.3, 160.1 (d, J = 242.6 Hz), 154.0, 142.2, 141.2, 138.6, 134.3, 132.7 (d, J = 10.6 Hz), 129.7, 128.8, 128.7, 128.1, 117.6, 117.0 (d, J = 9.9 Hz), 109.7 (d, J = 26.7 Hz), 105.8 (d, J = 23.6 Hz), 101.4, 97.9 (d, J = 4.7 Hz), 39.2, 28.6, 21.4 (signals of two carbons are absent, which may relate to signal overlap). MS (EI, 70 eV): m/z (%) = 387 (100, M+), 372 (13), 344 (11), 35 (18), 301 (9), 271 (6). HRMS (ESI-TOF): calcd for C23H19FN3O2 [M + H]+ 388.1461, found: 388.1462.
2); mp: 193–195 °C. IR (ATR): ṽ [cm−1] = 1710 (s), 1664 (vs), 1446 (s), 1310 (m), 1141 (s), 1028 (m), 848 (s), 828 (s). 1H NMR (300 MHz, chloroform-d) δ = 7.78 (dd, J = 9.2, 4.3 Hz, 1H), 7.59–7.53 (m, 2H), 7.45 (s, 1H), 7.38–7.29 (m, 3H), 7.08 (td, J = 8.9, 2.6 Hz, 1H), 6.87 (s, 1H), 3.57 (s, 3H), 3.50 (s, 3H), 2.45 (s, 3H). 19F NMR (282 MHz, chloroform-d) δ = −117.4. 13C {1H} NMR (75 MHz, chloroform-d) δ = 161.3, 160.1 (d, J = 242.6 Hz), 154.0, 142.2, 141.2, 138.6, 134.3, 132.7 (d, J = 10.6 Hz), 129.7, 128.8, 128.7, 128.1, 117.6, 117.0 (d, J = 9.9 Hz), 109.7 (d, J = 26.7 Hz), 105.8 (d, J = 23.6 Hz), 101.4, 97.9 (d, J = 4.7 Hz), 39.2, 28.6, 21.4 (signals of two carbons are absent, which may relate to signal overlap). MS (EI, 70 eV): m/z (%) = 387 (100, M+), 372 (13), 344 (11), 35 (18), 301 (9), 271 (6). HRMS (ESI-TOF): calcd for C23H19FN3O2 [M + H]+ 388.1461, found: 388.1462.
Data availability
All data that support the findings of this study are available in the published article and/or the ESI† of this article.Conflicts of interest
There are no conflicts or financial interest to declare.Acknowledgements
We are grateful for the financial support provided by the State of Mecklenburg-Western Pomerania (Germany) and for the technical and analytical support from the University of Rostock (Germany) and Leibniz Institute for Catalysis (Germany).References
- L. M. de Coen, T. S. A. Heugebaert, D. García and C. V. Stevens, Chem. Rev., 2016, 116, 80–139 CrossRef CAS PubMed.
- M. S. Mohamed, S. A. Ali, D. H. A. Abdelaziz and S. S. Fathallah, BioMed Res. Int., 2014, 2014, 249780 CAS.
- (a) V. O. Iaroshenko, M. Vilches-Herrera, A. Gevorgyan, S. Mkrtchyan, K. Arakelyan, D. Ostrovskyi, M. S. Abbasi, L. Supe, A. Hakobyan, A. Villinger, D. M. Volochnyuk and A. Tolmachev, Tetrahedron, 2013, 69, 1217–1228 CrossRef CAS; (b) M. M. Hammouda, K. M. Elattar, A. Y. El-Khateeb, S. E. Hamed and A. M. A. Osman, Mol. Diversity, 2024, 28, 927–964 CrossRef CAS; (c) P. Seboletswe, P. Awolade and P. Singh, ChemMedChem, 2021, 16, 2050–2067 CrossRef CAS PubMed.
- J. F. Campos, T. Besson and S. Berteina-Raboin, Pharmaceuticals, 2022, 15, 352 CrossRef CAS PubMed.
- F. Buron, J. Y. Mérour, M. Akssira, G. Guillaumet and S. Routier, Eur. J. Med. Chem., 2015, 95, 76–95 CrossRef CAS PubMed.
- (a) L. Baziyar, P. Ahmadi, S. Zare Gheshlaghi, M. Behrouz, M. Emami, M. Saeedi, A. Ebrahimi, L. Emami and S. Khabnadideh, J. Mol. Struct., 2024, 1302, 137435 CrossRef CAS; (b) S. Pathania and R. K. Rawal, Eur. J. Med. Chem., 2018, 157, 503–526 CrossRef CAS.
- (a) J. I. Bardagí and R. A. Rossi, Org. Prep. Proced. Int., 2009, 41, 479–514 CrossRef; (b) W. Zhao-Li, L. An-Di, L. Li and C. Liang, Synlett, 2024, 603–615 Search PubMed; (c) F. Kopp and P. Knochel, Org. Lett., 2007, 9, 1639–1641 CrossRef CAS; (d) N. Boudet and P. Knochel, Org. Lett., 2006, 8, 3737–3740 CrossRef CAS PubMed; (e) A. Pałasz, Monatsh. Chem., 2008, 139, 1397–1404 CrossRef.
- R. M. F. de Abreu, T. Brockmann, A. Villinger, P. Ehlers and P. Langer, Beilstein J. Org. Chem., 2024, 20, 898–911 CrossRef PubMed.
- R. M. F. de Abreu, P. Ehlers and P. Langer, Beilstein J. Org. Chem., 2024, 20, 2708–2719 CrossRef CAS.
- (a) J. Campanini-Salinas, J. Andrades-Lagos, G. Gonzalez Rocha, D. Choquesillo-Lazarte, S. Bollo Dragnic, M. Faúndez, P. Alarcón, F. Silva, R. Vidal, E. Salas-Huenuleo, M. Kogan, J. Mella, G. Recabarren Gajardo and D. Vásquez-Velásquez, Molecules, 2018, 23, 1776 Search PubMed; (b) B. A. Lanman, J. R. Allen, J. G. Allen, A. K. Amegadzie, K. S. Ashton, S. K. Booker, J. J. Chen, N. Chen, M. J. Frohn, G. Goodman, D. J. Kopecky, L. Liu, P. Lopez, J. D. Low, V. Ma, A. E. Minatti, T. T. Nguyen, N. Nishimura, A. J. Pickrell, A. B. Reed, Y. Shin, A. C. Siegmund, N. A. Tamayo, C. M. Tegley, M. C. Walton, H.-L. Wang, R. P. Wurz, M. Xue, K. C. Yang, P. Achanta, M. D. Bartberger, J. Canon, L. S. Hollis, J. D. McCarter, C. Mohr, K. Rex, A. Y. Saiki, T. San Miguel, L. P. Volak, K. H. Wang, D. A. Whittington, S. G. Zech, J. R. Lipford and V. J. Cee, J. Med. Chem., 2020, 63, 52–65 Search PubMed; (c) M. N. Nasr and M. M. Gineinah, Arch. Pharm., 2002, 335, 289 CrossRef CAS; (d) Y. Shen, Q. Xie, Y. Wang, J. Liang, C. Jiang, X. Liu, Y. Wang and C. Hu, Bioorg. Chem., 2023, 141, 106848 CrossRef CAS PubMed.
- T. Inazumi, K. Yamada, Y. Kuroki, A. Kakehi and M. Noguchi, J. Chem. Soc., Perkin Trans. 1, 1994, 557–564 RSC.
- [Der Titel “Gantasala, Fournet et al. 2023 – Accessing spiropiperidines from dihydropyridones” kann nicht dargestellt werden. Die Vorlage “Literaturverzeichnis - Zeitschriftenaufsatz - (Standardvorlage)” beinhaltet nur Felder, welche bei diesem Titel leer sind.].
- W. Pfleiderer and H. Deiss, Isr. J. Chem., 1968, 6, 603–614 CrossRef CAS.
- A. Fürstner and V. Mamane, J. Org. Chem., 2002, 67, 6264–6267 CrossRef.
- B. Martín-Matute, C. Nevado, D. J. Cárdenas and A. M. Echavarren, J. Am. Chem. Soc., 2003, 125, 5757–5766 CrossRef.
- (a) W. Yang, R. R. Kazemi, N. Karunathilake, V. J. Catalano, M. A. Alpuche-Aviles and W. A. Chalifoux, Org. Chem. Front., 2018, 5, 2288–2295 RSC; (b) A. Fürstner, V. Mamane, G. Seidel and D. Laurich, Org. Synth., 2006, 83, 103–110 CrossRef.
- S. R. Meech and D. Phillips, J. Photochem., 1983, 23, 193–217 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2428779. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ob00436e |
| This journal is © The Royal Society of Chemistry 2025 |





