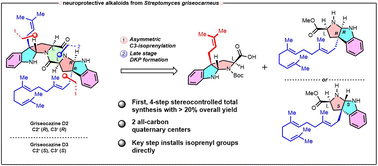
The first stereoselective total synthesis of (−)-griseocazine D2 and D3 is reported. (−)-Griseocazine D2 and D3 are multiprenylated pyrroloindoline-DKP alkaloids biogenetically related to several bioactive natural products. These alkaloids are neuroprotective agents as recently shown through a glutamate-induced excitotoxicity model by Pei-Yuan Qian et al. Structural challenges include a 7-annulated-ring system with 6 stereocenters among which two are all-carbon quaternary centers. Our concise and enantiospecific route accesses (−)-griseocazine D2 and D3 in four steps and 24% and 32% overall yields, respectively. L-Tryptophan is used as a common precursor for the regio- and stereoselective C3-isoprenylation step to afford complete diastereocontrol across the pyrroloindolines.