Open Access Article
Open Access ArticleNonsilyl bicyclic secondary amine catalyzed Michael addition of nitromethane to β,β-disubstituted α,β-unsaturated aldehydes†
Rohtash
Kumar‡
,
Avinash
Avinash‡
,
Ronak
Mehta
and
Chandrakumar
Appayee
 *
*
Department of Chemistry, Indian Institute of Technology Gandhinagar, Palaj, Gandhinagar, Gujarat 382055, India. E-mail: a.chandra@iitgn.ac.in
First published on 14th April 2025
Abstract
The asymmetric Michael addition of nitroalkanes to β,β-disubstituted α,β-unsaturated aldehydes is a useful method for the construction of all-carbon quaternary stereocenters. Nonsilyl bicyclic secondary amine organocatalysts were employed in reactions involving a wide range of β,β-disubstituted α,β-unsaturated aldehydes with nitroalkanes to achieve products with all-carbon quaternary stereocenters in up to 69% yield and 95% ee. The scalability of this methodology was demonstrated at the 5.1 mmol scale. The synthetic utility of this methodology is showcased through the concise asymmetric synthesis of methsuximide, an anticonvulsant drug.
Introduction
The asymmetric synthesis of molecules bearing all-carbon quaternary stereocenters remains one of the challenging tasks in synthetic organic chemistry.1 Interestingly, this methodology finds potential applications in the total synthesis of natural products and pharmaceutical drugs.2 The Michael addition of carbon nucleophiles to β,β-disubstituted α,β-unsaturated carbonyl compounds is one of the elegant methods to access molecules with all-carbon quaternary stereocenters.3 Nevertheless, the Michael addition of nitromethane to β,β-disubstituted α,β-unsaturated aldehydes has been comparatively underexplored (Scheme 1a).4 Kudo and Akagawa reported an undecapeptide-catalyzed asymmetric Michael addition of nitromethane to β,β-disubstituted α,β-unsaturated aldehydes, affording aldehydes bearing β-quaternary carbon stereocenters.4a Tu and co-workers developed a spiro-pyrrolidine catalyst for the same transformation, followed by reduction using NaBH4, yielding alcohols with all-carbon quaternary stereocenters.4b Although the above two methodologies offer products in moderate to good yields and excellent enantioselectivities, the synthesis of these catalysts involves multistep tedious processes. Hayashi and co-workers demonstrated an asymmetric Michael addition of nitromethane to β,β-disubstituted α,β-unsaturated aldehydes using the Hayashi–Jørgensen catalyst, providing aldehydes with β-quaternary carbon stereocenters.4c,d Although the trimethylsilyl ether group in Hayashi–Jørgensen catalysts plays a crucial role in achieving high enantioselectivity of products, its stability in the presence of acid additives5a,b and reagents5c is a concern. Recently, we reported the synthesis of a nonsilyl bicyclic secondary amine catalyst, derived from 4-hydroxy-L-proline in four steps, which was utilized for the asymmetric transfer hydrogenation of β,β-disubstituted α,β-unsaturated aldehydes.6 Herein, we disclose the nonsilyl bicyclic secondary amine-catalyzed asymmetric Michael addition of nitromethane to β,β-disubstituted α,β-unsaturated aldehydes. Furthermore, we demonstrate the synthetic utility of this methodology for the concise asymmetric synthesis of methsuximide,7 an anticonvulsant drug (Scheme 1b). | ||
| Scheme 1 Asymmetric Michael addition of nitromethane to β,β-disubstituted α,β-unsaturated aldehydes. | ||
Results and discussion
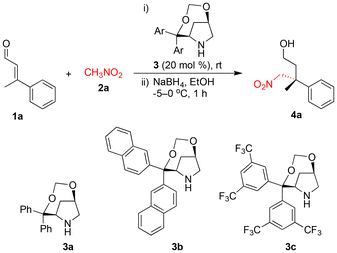
Initially, β-methyl cinnamaldehyde 1a was treated with nitromethane 2a (10 equiv.) in the presence of the nonsilyl bicyclic secondary amine catalyst 3a (20 mol%) under neat reaction conditions at rt for 72 h to afford the Michael adduct. For characterization purposes, the crude product was reduced in situ using NaBH4 in EtOH at −5 to 0 °C, furnishing alcohol 4a in 20% isolated yield with 88% ee (Table 1, entry 1).| Entry | Cat. | Solvent | t (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reactions were performed with 1a (29 mg, 0.2 mmol, 1 equiv.), CH3NO22a (107 μL, 2.0 mmol, 10 equiv.) and catalyst 3 (0.04 mmol, 20 mol%) in solvent (0.2 mL, 1.0 M) at rt followed by reduction to the corresponding alcohol 4a using NaBH4 (76 mg, 2.0 mmol, 10 equiv.) in EtOH (2.5 mL), unless otherwise noted. b Isolated yield after column chromatography. c Determined by HPLC analysis on a chiral stationary phase. d CH3NO2 (53 μL, 1.0 mmol, 5 equiv.) was used. e CH3NO2 (214 μL, 4.0 mmol, 20 equiv.) was used. f Benzoic acid (5 mg, 0.04 mmol, 20 mol%) was added. g Reaction was performed at 0 °C. h Catalyst 3a (6 mg, 0.02 mmol, 10 mol%) was used. IPA = isopropanol; nd = not determined; TFE = trifluoroethanol. | |||||
| 1 | 3a | Neat | 72 | 20 | 88 |
| 2 | 3a | CH2Cl2 | 120 | 21 | 90 |
| 3 | 3a | Toluene | 72 | 25 | 93 |
| 4 | 3a | CH3CN | 120 | 16 | 45 |
| 5 | 3a | MeOH | 24 | 30 | 91 |
| 6 | 3a | EtOH | 54 | 59 | 93 |
| 7 | 3a | IPA | 36 | 69 | 95 |
| 8 | 3a | t-BuOH | 24 | 60 | 95 |
| 9 | 3a | (CH2OH)2 | 30 | 64 | 94 |
| 10 | 3a | Glycerol | 48 | 65 | 94 |
| 11 | 3a | HO(CH2)4OH | 48 | 62 | 91 |
| 12 | 3a | TFE | 24 | 60 | 88 |
| 13d | 3a | IPA | 30 | 40 | 91 |
| 14e | 3a | IPA | 48 | 59 | 93 |
| 15f | 3a | IPA | 92 | 40 | 91 |
| 16g | 3a | IPA | 48 | Trace | nd |
| 17h | 3a | IPA | 96 | 53 | 93 |
| 18 | 3b | IPA | 48 | 35 | 91 |
| 19 | 3c | IPA | 50 | 48 | 91 |
Among the solvents screened (Table 1, entries 2–12), protic solvents were found to be effective, offering shorter reaction times and improved product yields (Table 1, entries 5–12). Notably, the reaction carried out in IPA (isopropanol) was completed in 36 h, affording product 4a in 69% isolated yield with 95% ee (Table 1, entry 7). Attempts to either decrease (Table 1, entry 13) or increase (Table 1, entry 14) the equivalents of nitromethane 2a were not fruitful. The addition of benzoic acid (20 mol%) to the reaction led to a longer reaction time, which in turn resulted in decreased product yield and enantioselectivity (Table 1, entry 15). This decline is attributed to product decomposition occurring over the prolonged reaction time. Traces of product 4a were observed upon lowering the reaction temperature to 0 °C (Table 1, entry 16). The reaction with 10 mol% of catalyst 3a took a longer time (96 h), affording product 4a in 53% yield and 93% ee (Table 1, entry 17). When catalyst 3a was replaced with 3b or 3c under the same reaction conditions, lower product yield and enantioselectivity were observed (Table 1, entries 18 and 19).
After the successful reaction optimization for the asymmetric Michael addition of nitromethane 2a to β-methyl cinnamaldehyde 1a (Table 1, entry 7), the substrate scope was investigated, as shown in Scheme 2. The β-aryl α,β-unsaturated aldehydes 1b–1e containing electron-donating groups (p-OMe, p-Me, p-Cl and p-F) at the para-position of the aryl ring smoothly underwent Michael addition with nitromethane 2a to give the corresponding alcohols 4b–4e in 48–61% yields and 91–95% ees. Similarly, substrates 1f and 1g containing electron-withdrawing groups (p-NO2 and p-CF3, respectively) at the para-position also participated effectively in the reaction, providing alcohols 4f and 4g in 61% yield and 91% ee and in 45% yield and 92% ee, respectively. Substrates 1h and 1k with meta-substituted aryl groups, including electron donating groups (m-OMe or m-Me) and electron-withdrawing groups (m-NO2 or m-CF3), also participated in the transformation efficiently under the optimized reaction conditions to afford the corresponding alcohols 4h and 4k in moderate to good yields and excellent enantioselectivities. β-Aryl α,β-unsaturated aldehydes bearing biphenyl (1l) and 2-naphthyl (1m) substituents at the β-position provided alcohols 4l and 4m in 50% yield and 93% ee and in 52% yield and 94% ee, respectively. Notably, a heteroaromatic substrate bearing a 2-thiophenyl substituent (1n) was well tolerated, affording alcohol 4n in 47% yield and 95% ee. When the β-ethyl β-phenyl α,β-unsaturated aldehyde 1o was studied for the Michael addition of nitromethane 2a, alcohol 4o was obtained in 40% yield and 88% ee. The Michael addition of nitromethane 2a to the 2-oxoindoline-3-ylidene acetaldehyde 1p was not effective under the optimized reaction conditions.8 Interestingly, the use of catalyst 3c (20 mol%) facilitated the reaction, affording alcohol 4p in 51% yield and 87% ee.
This methodology was also applied to the β,β-dialkyl α,β-unsaturated aldehydes 1q–1s to obtain alcohols 4q–4s in 49–61% yields and 58–83% ees. The enantiomeric excesses of alcohols 4r and 4s were determined after converting them to the corresponding carbamate derivatives 6r (85% yield and 68% ee) and 6s (90% yield and 83% ee), respectively, using 4-chlorophenyl isocyanate and Et3N in CH2Cl2 (see the ESI†). The reaction of nitromethane 2a with substrate 1t containing an ester group at the β-position proceeded very slowly in IPA. However, when the reaction was carried out in EtOH, completion was achieved in 18 h, affording aldehyde 5t in 62% yield and 93% ee. The enantiomeric excess of aldehyde 5t was determined after converting it to diester 7t (85% yield and 93% ee) using ethyl(triphenylphosphoranylidene)acetate (see the ESI†). The reaction of nitropropane 2b with β-methyl cinnamaldehyde 1a in the presence of catalyst 3a under neat conditions (as the reaction in IPA was very slow) yielded product 4u as an inseparable mixture of diastereomers in 29% yield with 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 83% ee. The absolute configuration of products 4a–4u was assigned based on the comparison of the optical rotation values of products 4a, 4b, 4f, 4p and 4q with those reported in the literature.4b
1 dr and 83% ee. The absolute configuration of products 4a–4u was assigned based on the comparison of the optical rotation values of products 4a, 4b, 4f, 4p and 4q with those reported in the literature.4b
To showcase the scalability of the methodology, 5.1 mmol of β-methyl cinnamaldehyde 1a was subjected to the optimized reaction conditions, affording alcohol 4a in 66% yield and 95% ee (Scheme 3).
To demonstrate the application of this methodology, a concise asymmetric synthesis of methsuximide, an anticonvulsant drug, was envisaged starting from β-methyl cinnamaldehyde 1a, as shown in Scheme 4. The Michael addition of nitromethane 2a to β-methyl cinnamaldehyde 1a catalyzed by 3a in IPA furnished the corresponding Michael adduct, which was subsequently oxidized to carboxylic acid 8avia Pinnick oxidation (56% yield over two steps). Furthermore, the nitro group in compound 8a was converted to a carboxylic acid using NaNO2 and acetic acid in DMSO, yielding the corresponding dicarboxylic acid. Condensation of this intermediate with methylamine in toluene under reflux conditions afforded methsuximide 9a in 38% yield over two steps and 93% ee.
Based on the reaction outcomes and the observed stereochemistry of the Michael adducts, a possible reaction mechanism is proposed for the Michael addition of nitromethane 2a to β,β-disubstituted α,β-unsaturated aldehydes 1 catalyzed by the nonsilyl bicyclic secondary amine organocatalyst 3a, as shown in Scheme 5. Accordingly, catalyst 3a condenses with the β,β-disubstituted α,β-unsaturated aldehyde 1 to form the corresponding E-iminium ion intermediate A. The nucleophilic nitromethide ion, generated from nitromethane 2a, then attacks the β-position of the iminium ion B from the face opposite to the chiral bulky substituent of the catalyst, leading to the formation of the enamine intermediate C. Upon protonation, the enamine intermediate C forms the iminium intermediate D, which undergoes hydrolysis to yield the Michael adduct 5 along with the regeneration of catalyst 3a.
 | ||
| Scheme 5 Proposed reaction mechanism for the Michael addition of nitromethane 2a to β,β-disubstituted α,β-unsaturated aldehydes 1 catalyzed by 3a. | ||
Conclusions
In summary, the nonsilyl bicyclic secondary amine organocatalysts 3a–3c were investigated for the enantioselective Michael addition of nitromethane 2a to β-methyl cinnamaldehyde 1a. A broad range of β,β-disubstituted α,β-unsaturated aldehydes (1a–1s) were evaluated under the optimized reaction conditions, affording the corresponding products 4a–4s bearing all-carbon quaternary stereocenters in 40–69% yields and 58–95% ees. Substrate 1t, bearing an ester group at the β-position, was successfully converted to the corresponding Michael adduct 5t in 62% yield and 93% ee. Additionally, the use of nitropropane 2b as the nucleophile afforded product 4u in 29% yield with 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and 83% ee. A scale-up reaction was successfully performed at the 5.1 mmol scale using β-methyl cinnamaldehyde 1a, delivering alcohol 4a in 66% yield and 95% ee. Furthermore, the synthetic utility of this methodology was exemplified through a concise asymmetric synthesis of methsuximide, an anticonvulsant drug, starting from β-methyl cinnamaldehyde 1a. Based on the stereochemical outcomes of the Michael adducts, a possible reaction mechanism has been proposed. This study is expected to stimulate further exploration on the use of nonsilyl bicyclic secondary amine organocatalysts for enantioselective Michael addition of various nucleophiles to α,β-unsaturated aldehydes.
1 dr and 83% ee. A scale-up reaction was successfully performed at the 5.1 mmol scale using β-methyl cinnamaldehyde 1a, delivering alcohol 4a in 66% yield and 95% ee. Furthermore, the synthetic utility of this methodology was exemplified through a concise asymmetric synthesis of methsuximide, an anticonvulsant drug, starting from β-methyl cinnamaldehyde 1a. Based on the stereochemical outcomes of the Michael adducts, a possible reaction mechanism has been proposed. This study is expected to stimulate further exploration on the use of nonsilyl bicyclic secondary amine organocatalysts for enantioselective Michael addition of various nucleophiles to α,β-unsaturated aldehydes.
Data availability
Data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Anusandhan National Research Foundation (ANRF), India, (SCP/2022/000823) is gratefully acknowledged. Financial support from the University Grants Commission (UGC), India (fellowship to R. K.) and the Indian Institute of Technology Gandhinagar (fellowship to A. A. and R. M.) is gratefully acknowledged.References
- (a) K. W. Quasdorf and L. E. Overman, Nature, 2014, 516, 181–191 CrossRef CAS PubMed; (b) E. A. Peterson and L. E. Overman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 11943–11948 CrossRef CAS PubMed; (c) M. Büschleb, S. Dorich, S. Hanessian, D. Tao, K. B. Schenthal and L. E. Overman, Angew. Chem., Int. Ed., 2016, 55, 4156–4186 CrossRef PubMed; (d) Y. Jia, K. Zhang, L.-Q. Lu, Y. Cheng and W.-J. Xiao, ACS Catal., 2024, 14, 13550–13556 CrossRef CAS; (e) A. Y. Hong and B. M. Stoltz, Eur. J. Org. Chem., 2013, 2745–2759 CrossRef CAS PubMed; (f) S. Goswami, K. Harada, M. F. El-Mansy, R. Lingampally and R. G. Carter, Angew. Chem., Int. Ed., 2018, 57, 9117–9121 CrossRef CAS PubMed; (g) T. S. Silva and F. Coelho, Beilstein J. Org. Chem., 2021, 17, 1565–1590 CrossRef CAS PubMed; (h) X.-P. Zeng, Z.-Y. Cao, Y.-H. Wang, F. Zhou and J. Zhou, Chem. Rev., 2016, 116, 7330–7396 CrossRef CAS PubMed; (i) T. Ema, Y. Oue, K. Akihara, Y. Miyazaki and T. Sakai, Org. Lett., 2009, 11, 4866–4869 CrossRef CAS PubMed; (j) M. Tanaka, M. Imai, M. Fujio, E. Sakamoto, M. Takahashi, Y. Eto-Kato, X. M. Wu, K. Funakoshi, K. Sakai and H. Suemune, J. Org. Chem., 2000, 65, 5806–5816 CrossRef CAS PubMed.
- (a) C. Li, S. S. Ragab, G. Liu and W. Tang, Nat. Prod. Rep., 2020, 37, 276–292 RSC; (b) Y. Liu, S.-J. Han, W.-B. Liu and B. M. Stoltz, Acc. Chem. Res., 2015, 48, 740–751 CrossRef CAS PubMed; (c) Z. Wang, Org. Chem. Front., 2020, 7, 3815–3841 RSC; (d) T. Ling and F. Rivas, Tetrahedron, 2016, 72, 6729–6777 CrossRef CAS; (e) R. Long, J. Huang, J. Gong and Z. Yang, Nat. Prod. Rep., 2015, 32, 1584–1601 RSC; (f) D. C. Behenna, Y. Liu, T. Yurino, J. Kim, D. E. White, S. C. Virgil and B. M. Stoltz, Nat. Chem., 2012, 4, 130–133 CrossRef CAS PubMed; (g) S.-J. Han, F. Vogt, S. Krishnan, J. A. May, M. Gatti, S. C. Virgil and B. M. Stoltz, Org. Lett., 2014, 16, 3316–3319 CrossRef CAS PubMed; (h) R. M. Lemieux and A. I. Meyers, J. Am. Chem. Soc., 1998, 120, 5453–5457 CrossRef CAS; (i) J. Deng, S. Zhou, W. Zhang, J. Li, R. Li and A. Li, J. Am. Chem. Soc., 2014, 136, 8185–8188 CrossRef CAS PubMed; (j) R. Long, J. Huang, W. Shao, S. Liu, Y. Lan, J. Gong and Z. Yang, Nat. Commun., 2014, 5, 5707 CrossRef CAS PubMed.
- (a) A. G. Csakÿ, G. de la Herrán and M. C. Murcia, Chem. Soc. Rev., 2010, 39, 4080–4102 RSC; (b) C. Hawner and A. Alexakis, Chem. Commun., 2010, 46, 7295–7306 RSC; (c) E. Fillion and A. Wilsily, J. Am. Chem. Soc., 2006, 128, 2774–2775 CrossRef CAS PubMed; (d) A. Wilsily and E. Fillion, Org. Lett., 2008, 10, 2801–2804 CrossRef CAS PubMed; (e) Y. Hamashima, D. Hotta and M. Sodeoka, J. Am. Chem. Soc., 2002, 124, 11240–11241 CrossRef CAS PubMed; (f) C. Hawner, D. Muller, L. Gremaud, A. Felouat, S. Woodward and A. Alexakis, Angew. Chem., Int. Ed., 2010, 49, 7769–7772 CrossRef CAS PubMed; (g) K. Endo, D. Hamada, S. Yakeishi and T. Shibata, Angew. Chem., Int. Ed., 2013, 52, 606–610 CrossRef CAS PubMed; (h) R. Shintani, Y. Tstsumi, M. Nagaosa, T. Nishimura and T. Hayashi, J. Am. Chem. Soc., 2009, 131, 13588–13589 CrossRef CAS PubMed.
- (a) K. Akagawa and K. Kudo, Angew. Chem., Int. Ed., 2012, 51, 12786–12789 CrossRef CAS PubMed; (b) J.-M. Tian, Y.-H. Yuan, Y.-Q. Tu, F.-M. Zhang, X.-B. Zhang, S.-H. Zhang, S.-H. Wang and X.-M. Zhang, Chem. Commun., 2015, 51, 9979–9982 RSC; (c) Y. Hayashi, Y. Kawamoto, M. Honda, D. Okamura, S. Umemiya, Y. Noguchi, T. Mukaiyama and I. Sato, Chem. – Eur. J., 2014, 20, 12072–12082 CrossRef PubMed; (d) T. Mukaiyama, K. Ogata, I. Sato and Y. Hayashi, Chem. – Eur. J., 2014, 20, 13583–13588 CrossRef CAS PubMed.
- (a) M. H. Haindl, M. B. Schmid, K. Zeitler and R. M. Gschwind, RSC Adv., 2012, 2, 5941–5943 RSC; (b) X. Companyó and J. Burés, J. Am. Chem. Soc., 2017, 139, 8432–8435 CrossRef PubMed; (c) G. Hutchinson, C. Alamillo-Ferrer and J. Burés, J. Am. Chem. Soc., 2021, 143, 6805–6809 CrossRef CAS PubMed.
- R. Kumar, V. Maurya, A. Avinash and C. Appayee, J. Org. Chem., 2024, 89, 8586–8600 CrossRef CAS PubMed.
- For the synthesis of methsuximide, see: (a) P.-W. Xu and Z.-X. Huang, Nat. Chem., 2021, 13, 634–642 CrossRef CAS PubMed; (b) Y. Kuroiwa and M. Tamura, Adv. Synth. Catal., 2024, 366, 1996–2002 CrossRef CAS; (c) J. Li, J. Wei, B. Zhu, T. Wang and N. Jiao, Chem. Sci., 2019, 10, 9099–9103 RSC. For the biological activity of methsuximide, see: (d) J. Mifsud, J. S. Millership, P. S. Collier, R. Morrow, J. T. G. Hamilton and W. C. McRoberts, Biopharm. Drug Dispos., 2001, 22, 129–136 CrossRef CAS PubMed; (e) R. J. Porter, J. K. Penry, J. R. Lacy, M. E. Newmark and H. J. Kupferberg, Neurology, 1979, 29, 1509–1513 CrossRef CAS PubMed.
- Under the optimized reaction conditions, product 4p was obtained in 80% yield and 27% ee after 16 h.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00277j |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |