Open Access Article
Open Access ArticleDual palladium-organophotoredox catalyzed C–H olefination–annulation of aryl carboxylic acids†
A Anagha
Varma
and
Purushothaman
Gopinath
 *
*
Department of Chemistry, Indian Institute of Science Education and Research (IISER) Tirupati, Tirupati 517619, India. E-mail: gopi@iisertirupati.ac.in
First published on 1st April 2025
Abstract
Herein, we report a dual palladium-photoredox mediated tandem C–H olefination–cyclization of aryl carboxylic acids with both terminal and internal alkenes using molecular oxygen as a green oxidant. This method offers a mild and simple route for the synthesis of various isobenzofuranone derivatives. The synthetic utility of the method was demonstrated by late stage functionalization of various carboxylic acid containing drugs. Further derivatizations of the final isobenzofuranones were performed to access other important molecular scaffolds. Mechanistic studies indicated that the final cyclization step involves an oxopalladation–protodemetallation mechanism rather than a simple oxa-Michael addition commonly employed in other methodologies at elevated temperatures.
Introduction
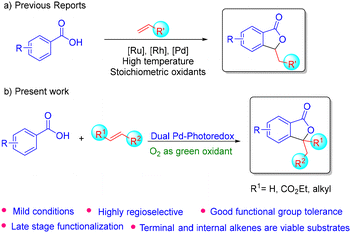
Transition metal catalysed C–H olefination comprises an important reaction in organic synthesis.1–10 The pioneering work of Fujiwara and Moritani on C–H olefination attracted much attention and stimulated the progress in this reaction,11 which led to tandem C–H olefination–annulation of arenes becoming another powerful strategy for directly accessing carbocycles and heterocycles in a step- and atom-economical manner. In this context, C–H olefination–annulation of weakly coordinating aryl carboxylic acids is a highly desirable transformation as it affords biologically relevant isobenzofuranones in a single step. Also, since the carboxylate group can act as an inherent directing group, additional steps required for pre-functionalization of the substrate can be eliminated.A few groups have demonstrated the synthesis of isobenzofuranones via transition metal catalysed C–H olefination–oxa-Michael addition of aryl carboxylic acids.12–16 For example, Miura and co-workers in 1998 reported a Pd(II) catalysed oxidative cross-coupling of aryl sulfonamides and benzoic acids with acrylates affording annulated products.12 In 2011, Ackermann's group reported a Ru(II) catalysed alkenylative annulation of benzoic acids.13,14 In 2022, Ji's group reported a ligand assisted Pd(II)-catalysed alkenylative annulation of benzoic acids and phenylacetic acids.16 Most of these methods employ harsh reaction conditions such as high temperatures, the use of stoichiometric or super-stoichiometric amounts of external oxidants and additives, etc. Also, the scope of acrylates is often limited to terminal alkenes with no examples of internal or di-substituted alkenes. The final cyclization step involves an oxa-Michael addition type mechanism, which generally requires higher temperature, thereby making this overall process less efficient.
Recently, dual transition metal-photoredox catalysis has gained much attention among the scientific community for C–H functionalization of various substrates.17–20 In this direction, simple C–H olefination of various arene derivatives was reported, wherein the photocatalyst in combination with molecular oxygen functions as an oxidant to regenerate the active transition metal catalyst, which obviates the use of stoichiometric external oxidants and the toxic by-products.21–23 Continuing this, Baidya et al. developed a dual Ru(II) and dye-sensitized TiO2 photocatalyzed C–H olefination–annulation of aryl carboxylic acids in a single step.24 Unfortunately, the substrate scope was mostly limited to simple vinyl sulfones with limited examples of acrylates and again no scope for internal or di-substituted alkenes. The yield of this reaction was generally moderate to low with some substrates requiring higher reaction temperature. Herein, we have demonstrated a dual palladium-organophotoredox catalysed C–H olefination–annulation of aryl carboxylic acids with various acrylates including terminal as well as internal and di-substituted alkenes for accessing 3-alkyl/dialkyl substituted isobenzofuranones. We have also showcased the broad applicability of the methodology via late stage functionalization of various carboxylic acid containing drugs. Finally, our mechanistic studies for the final cyclization step indicated an oxopalladation–protodemetallation mechanism rather than a usual oxa-Michael addition (Scheme 1).
Results and discussion
We started our investigation with commercially available p-anisic acid and ethyl acrylate using K2CO3 as the base, N-Ac-Gly as the MPAA ligand, Pd(OAc)2 as the transition metal catalyst, and fluorescein as the photocatalyst in HFIP as solvent. Under blue light irradiation in the presence of an O2 atmosphere, the desired product was obtained in 20% yield. In order to improve the yield further, we screened a variety of organic and inorganic bases in various equivalences. Finally, 3 equiv. of K2CO3 afforded the desired lactone in 69% yield (Table S1, ESI†). Similarly, palladium acetate (10 mol%) and fluorescein (5 mol%) seemed to be the best transition metal catalyst and photocatalyst, respectively (Tables S2 and S3, ESI†). Furthermore, various mono N-protected amino acid ligands were screened and 30 mol% of N-Ac-Val was found to be the most effective ligand (Table S4, ESI†), affording the desired lactone in 79% yield in HFIP solvent using molecular O2 as the oxidant.With the optimized reaction conditions in hand, we next focused our efforts to demonstrate the substrate scope. Accordingly, the reaction of p-anisic acid with a variety of acrylates under the optimized conditions afforded the corresponding lactones in moderate to good yields (3a–3t). Acrylates containing sterically hindered substituents afforded the resultant lactone products (3e, 3h and 3o–3r) in good to moderate yields. Interestingly, diethyl vinylphosphonate and phenyl vinyl sulfone also afforded the desired products (3s and 3t), albeit in moderate yields. However, the reaction with acrylamide failed to give the corresponding product under the standard conditions.
Next the scope of various aryl carboxylic acid derivatives was demonstrated. Accordingly, electron-donating substituents were well tolerated under the reaction conditions, affording the products in good to moderate yields (5b–5e). Similarly, moderately electron-withdrawing substituents such as –Cl, –Br and –F were found to be suitable candidates for this transformation, giving the corresponding lactones in good yields (5f, 5h and 5i). Interestingly, benzoic acids containing various other functional groups such as vinyl, formyl, acetyl, and benzoyl afforded the corresponding products (5m–5p) in good yields, demonstrating the chemoselectivity of the protocol. The reaction with disubstituted benzoic acids also afforded the lactone products (5q and 5r) in moderate yields. Next, in order to demonstrate the regioselectivity of the protocol, various meta-substituted aryl carboxylic acids were studied. In all the cases, C–H olefination occurred regioselectively at the sterically less hindered position, followed by annulation. The reaction with 1-naphthoic acid (5v) and 2-naphthoic acid (5u) also afforded the corresponding products regioselectively in good yields. Similarly, the reaction of 3,4-dimethoxy benzoic acid (5w) also afforded the corresponding lactone regioselectively on the less hindered side (Scheme 2).
Inspired by these results, we then extended our investigation to demonstrate the scope of various α,β-disubstituted acrylates, which were not previously studied. Interestingly, disubstituted internal olefins such as diethyl fumarate, ethyl crotonate, etc., reacted well under these conditions with various carboxylic acid derivatives and afforded the corresponding isobenzofuranones (7a–7g) in good yields. However, cyclic acrylates such as maleic anhydride and maleimide failed to give any desired products (Scheme 3).
Next, we focused our efforts towards late-stage functionalization of various drug molecules containing the carboxylic acid functional group. Accordingly, late-stage functionalization of acedoben, adapalene, tranilast, ataluren and eudesmic acid derivatives afforded the corresponding products in moderate yields under the standard conditions (Scheme 4a). We further extended our investigation to study the synthetic utility and scalability of the present methodology. Accordingly, a gram-scale reaction under the standard conditions afforded the corresponding isobenzofuranone derivative in 75% yield (Scheme 4b). Next, we demonstrated various downstream synthetic transformations of the isobenzofuranones. First, selective hydrolysis of the ester moiety was carried out in the presence of 6 M HCl to form the corresponding carboxylic acid 12a in 72% yield. Then, reduction with LiAlH4 led to a triol derivative, 12b, in 60% yield. We also achieved the synthesis of a hydroxy lactone derivative, 12c, via selective reduction of an ester functional group with NaBH4, in the presence of a cyclic lactone (Scheme 4c).
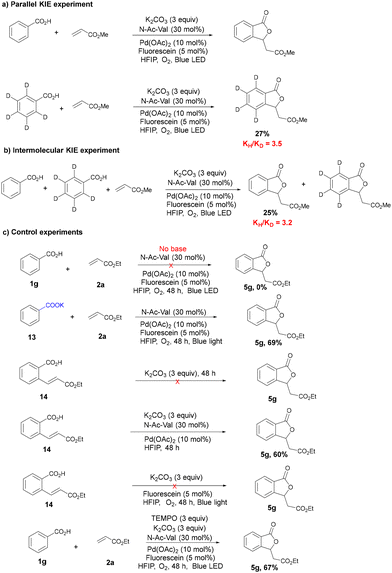
In order to gain insights into the reaction mechanism, we performed kinetic studies as well as some control experiments. Accordingly, both parallel and intermolecular kinetic isotope effect (KIE) experiments with benzoic acid and its deuterated analogue afforded KH/KD values of 3.5 and 3.2, respectively. This high value of KH/KD indicates that C–H activation is the kinetically relevant step. Next, we performed a few control experiments to understand the mechanism of this reaction. Reactions in the absence of either ligand, photocatalyst, O2 or light completely failed to afford the desired product, indicating that all are essential for the transformation (Tables S2 and S4, ESI†). Similarly, the reaction in the absence of a base did not afford any product. On the other hand, the reaction with the potassium salt of the carboxylic acid without any base under the standard conditions afforded the product in 69% yield, which shows that the role of the base was to generate the corresponding carboxylate salt.
Next, in order to understand the mechanism of the cyclization, olefinated benzoic acid was directly treated with potassium carbonate (3 equiv.). Surprisingly, the reaction did not afford any cyclized product, ruling out the possibility of a simple oxa-Michael addition type mechanism. On the other hand, when olefinated benzoic acid was treated with Pd(OAc)2, N-Ac-Val and K2CO3, the corresponding lactone was obtained. However, the reaction of olefinated benzoic acid with a base, fluorescein and O2 in the presence of light did not afford any desired product. These results indicate that the cyclization step is catalysed by palladium and it is not a simple photo-mediated cyclization process (Scheme 5). In order to check whether a single electron transfer is involved in the reaction, we carried out the reaction with TEMPO. The reaction afforded 67% yield of the final isobenzofuranone, thereby ruling out the possibility of a radical mechanism (Scheme 5). Stern–Volmer studies showed that the excited photocatalyst is effectively quenched by the Pd(0) species (see the ESI†).
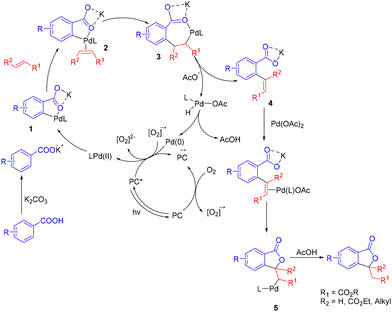
Based on the previous reports and the control experiments, we propose the following mechanism. At first, deprotonated carboxylic acid coordinates with a Pd(II) complex to form a 5-membered palladacycle intermediate 1. Next, olefin coordinates with this palladacycle, followed by migratory insertion to form a 7-membered palladacycle 3. This intermediate then undergoes β-hydride elimination to afford an ortho olefinated product, 4, and a Pd(II) intermediate, which then undergoes reductive elimination to form the Pd(0) species. The Pd(0) species is then reoxidized to its active Pd(II) form by the synergistic effect of the photocatalyst and O2. The olefinated product on the other hand again coordinates with the Pd(II) catalyst, which undergoes oxopalladation25 to form intermediate 5. Intermediate 5 finally undergoes protodemetallation to afford 3-alkyl/dialkyl substituted isobenzofuranones (Scheme 6).
Conclusions
In summary, we have successfully developed a dual palladium-organophotoredox catalyzed one pot C–H olefination–annulation reaction of aryl carboxylic acids. A broad range of aryl carboxylic acids and acrylates reacted well under the standard conditions, affording the corresponding 3-alkyl/dialkyl substituted isobenzofuranone derivatives in good yields and in high regioselectivity. The reaction proceeded smoothly with terminal as well as internal or disubstituted alkenes to afford the products in moderate yields. Late stage functionalization of drugs containing the carboxylic acid moiety was also successfully achieved. The practical applicability of the method was shown by carrying out a gram-scale reaction and via synthetic diversifications of the final lactone product. Mechanistic studies for the cyclization step indicated an oxopalladation–protodemetallation mechanism instead of a simple intramolecular oxa-Michael addition type mechanism commonly reported in other similar reactions.Data availability
The data supporting this article have been included as part of the ESI.†Crystallographic data for compounds 3e and 5u have been deposited at the CCDC under CCDC numbers 2376512 and 2376513.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. G. thanks the DST-Nano Mission (DST/NM/TUE/EE-03/2019-1G-IISERTp) for financial support. A. A. V. thanks the CSIR for financial support. The authors thank Vaibhav Singh and Saumya Singh for their help and support in crystallographic experiments.References
- M. Wasa, K. M. Engle and J. Q. Yu, Pd(II)-Catalyzed Olefination of sp3 C–H Bonds, J. Am. Chem. Soc., 2010, 132, 3680–3681 CrossRef CAS.
- A. Deb, S. Bag, R. Kancherla and D. Maiti, Palladium-Catalyzed Aryl C–H Olefination with Unactivated, Aliphatic Alkenes, J. Am. Chem. Soc., 2014, 136, 13602–13605 CrossRef CAS PubMed.
- B. Li, J. Ma, N. Wang, H. Feng, S. Xu and B. Wang, Ruthenium-Catalyzed Oxidative C–H Bond Olefination of N-Methoxybenzamides Using an Oxidizing Directing Group, Org. Lett., 2012, 14, 736–739 CrossRef CAS PubMed.
- S. I. Kozhushkov and L. Ackermann, Ruthenium-catalyzed direct oxidative alkenylation of arenes through twofold C–H bond functionalization, Chem. Sci., 2013, 4, 886–896 RSC.
- F. W. Patureau and F. Glorius, Rh Catalyzed Olefination and Vinylation of Unactivated Acetanilides, J. Am. Chem. Soc., 2010, 132, 9982–9983 CrossRef CAS PubMed.
- S. H. Park, J. Y. Kim and S. Chang, Rhodium-Catalyzed Selective Olefination of Arene Esters via C−H Bond Activation, Org. Lett., 2011, 13, 2372–2375 CrossRef CAS.
- S. Maity, E. Hoque, U. Dhawa and D. Maiti, Palladium catalyzed selective distal C–H olefination of biaryl systems, Chem. Commun., 2016, 52, 14003–14006 RSC.
- K. Navid, Ligand-Accelerated ortho-C-H Olefination of Phenylacetic Acids, Org. Synth., 2015, 92, 58–75 CrossRef PubMed.
- K. M. Engle, D. Wang and J. Yu, Constructing Multiply Substituted Arenes Using Sequential Palladium(II)-Catalyzed C□H Olefination, Angew. Chem., Int. Ed., 2010, 49, 6169–6173 CrossRef CAS PubMed.
- S. Panja, S. Ahsan, T. Pal, S. Kolb, W. Ali, S. Sharma, C. Das, J. Grover, A. Dutta, D. B. Werz, A. Paul and D. Maiti, Non-directed Pd-catalysed electrooxidative olefination of arenes, Chem. Sci., 2022, 13, 9432–9439 RSC.
- I. Moritani and Y. Fujiwara, Aromatic substitution of styrene-palladium chloride complex, Tetrahedron Lett., 1967, 8, 1119–1122 CrossRef.
- M. Miura, T. Tsuda, T. Satoh, S. Pivsa-Art and M. Nomura, Oxidative Cross-Coupling of N-(2′-Phenylphenyl)benzene- sulfonamides or Benzoic and Naphthoic Acids with Alkenes Using a Palladium−Copper Catalyst System under Air, J. Org. Chem., 1998, 63, 5211–5215 CrossRef CAS.
- L. Ackermann and J. Pospech, Ruthenium-Catalyzed Oxidative C–H Bond Alkenylations in Water: Expedient Synthesis of Annulated Lactones, Org. Lett., 2011, 13, 4153–4155 Search PubMed.
- A. Bechtoldt, C. Tirler, K. Raghuvanshi, S. Warratz, C. Kornhaaß and L. Ackermann, Ruthenium Oxidase Catalysis for Site-Selective C–H Alkenylations with Ambient O2 as the Sole Oxidant, Angew. Chem., Int. Ed., 2016, 55, 264–267 Search PubMed.
- Q. Jiang, C. Zhu, H. Zhao and W. Su, Rh(III)-Catalyzed C−H Olefination of Benzoic Acids under Mild Conditions using Oxygen as the Sole Oxidant, Chem. – Asian J., 2016, 11, 356–359 CrossRef CAS PubMed.
- Y. Wang, X. Xu, G. Wu, B. Pang, S. Liao and Y. Ji, Ligand-Enabled C–H Olefination and Lactonization of Benzoic Acids and Phenylacetic Acids via Palladium Catalyst, Org. Lett., 2022, 24, 799–982 CrossRef PubMed.
- S. S. Babu, M. Shahid and P. Gopinath, Dual palladium–photoredox catalyzed chemoselective C–H arylation of phenylureas, Chem. Commun., 2020, 56, 5985–5988 RSC.
- M. Shahid, A. J. Punnya, S. S. Babu, S. Sarkar and P. Gopinath, Dual palladium-photoredox-mediated regioselective acylation of carbazoles and Indolines, J. Org. Chem., 2023, 88, 13686–13698 CrossRef CAS PubMed.
- M. Shahid, P. Muthuraja and P. Gopinath, Substituent-controlled regioselective arylation of carbazoles using dual catalysis, Org. Biomol. Chem., 2024, 22, 753–758 RSC.
- A. Y. Chan, I. B. Perry, N. B. Bissonnette, B. F. Buksh, G. A. Edwards, L. I. Frye, O. L. Garry, M. N. Lavagnino, B. X. Li, Y. Liang, E. Mao, A. Millet, J. V. Oakley, N. L. Reed, H. A. Sakai, C. P. Seath and D. W. C. MacMillan, Metallaphotoredox: The Merger of Photoredox and Transition Metal Catalysis, Chem. Rev., 2022, 122, 1485–1542 CrossRef CAS.
- D. C. Fabry, J. Zoller, S. Raja and M. Rueping, Combining rhodium and photoredox catalysis for C-H functionalizations of arenes: oxidative Heck reactions with visible light, Angew. Chem., Int. Ed., 2014, 53, 10228–10231 CrossRef CAS PubMed.
- D. C. Fabry and M. Rueping, Merging Visible Light Photoredox Catalysis with Metal Catalyzed C–H Activations: On the Role of Oxygen and Superoxide Ions as Oxidants, Acc. Chem. Res., 2016, 49, 1969–1979 CrossRef CAS PubMed.
- A. Saha, S. Guin, W. Ali, T. Bhattacharya, S. Sasmal, N. Goswami, G. Prakash, S. K. Sinha, H. B. Chandrashekar, S. Panda, S. S. Anjana and D. Maiti, Photoinduced Regioselective Olefination of Arenes at Proximal and Distal Sites, J. Am. Chem. Soc., 2022, 144, 1929–1940 CrossRef CAS.
- S. Dana, P. Dey, S. A. Patil and M. Baidya, Enhancing Ru(II)–Catalysis with Visible–Light–Mediated Dye–Sensitized TiO2 Photocatalysis for Oxidative C−H Olefination of Arene Carboxylic Acids at Room Temperature, Chem. – Asian J., 2020, 15, 564–567 CrossRef CAS.
- R. M. Trend, Y. K. Ramtohul and B. M. Stoltz, Oxidative Cyclizations in a Nonpolar Solvent Using Molecular Oxygen and Studies on the Stereochemistry of Oxypalladation, J. Am. Chem. Soc., 2005, 127, 17778–17788 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2376512 and 2376513. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ob00275c |
| This journal is © The Royal Society of Chemistry 2025 |