Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reagent-assisted regio-divergent cyclization synthesis of pyrazole†
Mengdi
Pang
ab,
Ali
Ramazani
 c,
Zhengguo
Zhang
*ad and
Guoying
Zhang
c,
Zhengguo
Zhang
*ad and
Guoying
Zhang
 *b
*b
aSchool of Chemistry and Chemical Engineering, North University of China, Taiyuan 030051, P. R. China
bState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, P. R. China. E-mail: zhanggy@sxicc.ac.cn
cThe Organic Chemistry Research Laboratory (OCRL), Department of Chemistry, Faculty of Science, University of Zanjan, Zanjan 45371-38791, lran
dDepartment of Materials Science and Engineering, Shanxi Institute of Technology, Yangquan 045000, P. R. China
First published on 18th February 2025
Abstract
The present study reveals a practical one-pot base-promoted regio-divergent cyclization of hydrazines with alkynyl silane under mild conditions, facilitating the synthesis of diverse silicone-substituted pyrazoles and functionalized pyrazoles in great yields with exceptional selectivity. This protocol is expected to afford a streamlined one-pot approach for the synthesis of multiple compounds in water.
Introduction
Pyrazoles are highly regarded structural patterns that are commonly found in a wide range of biologically active, medically important, and functionally significant compounds.1–6 Moreover, pyrazoles are widely acknowledged as crucial frameworks in numerous reactions for the production of complex and multifunctional compounds (Scheme 1, A).7–9 There are two primary methods for constructing the pyrazole framework: (1) combining hydrazines with unsaturated carbonyl compounds and then annulation,10 and (2) utilizing metal-catalyzed coupling transformations of pyrazoles with halides or similar substances to synthesize the desired target frameworks (Scheme 1, B).11–19 These transformations, however, are often limited by the lack of readily available starting precursors with suitable substitutions and typically require harsh reaction conditions involving complicated catalysts combined with multiple steps. As a result, this inevitably leads to a decrease in atom efficiency due to the generation of unwanted waste byproducts and an increasingly intricate operational process.20 Therefore, it is crucial to develop efficient and practical methodologies for constructing the structural framework of pyrazoles in organic synthesis.Mechanistically, the syntheses of functionalized pyrazoles from hydrazines with corresponding carbonyl-like compounds primarily encompass two different categories of reactions: (a) a highly intricate sequential condensation followed by intramolecular annulation in the presence of a metal catalyst;21,22 (b) a sophisticated intermolecular nucleophilic substitution reaction succeeded by intramolecular annulation guided by refined Lewis's acid catalysis.23 Due to the involvement of these exceptional metal or Lewis acid catalysts, it is frequently observed that these reactions produce products with less-than-optimal chem- and regio-selectivity or unsatisfactory yields.24,25 In recent studies, numerous significant changes have been documented in the process of producing complex compounds through base manipulation (Scheme 1, C).26–30
Results and discussion
These discoveries, along with our advancements in utilizing hydrazines as nucleophiles in coupling reactions facilitated by bases,31–34 have led us to propose that incorporating a bulky leaving group species35–39 could overcome the inherent substrate inhibition (selectivity) observed during base-mediated annulation reactions involving hydrazines. Based on the assumption of this mechanism, we have observed the formation of an intermediate I through the condensation reaction between hydrazine and alkynyl silane. Subsequently, a cyclization process occurs via an intramolecular nucleophilic substitution, resulting in the production of the desired product. However, due to hindrance caused by bulky groups, intermolecular nucleophilic substitution reactions for intermediate III are hindered, not to mention being followed by intramolecular annulation. In this study, we introduce a practical procedure for the successful synthesis of various pyrazoles by combining different hydrazines with alkynyl silane under base-promoted regio-divergent condensation annulation. This innovative approach allows for the efficient formation of silicone-substituted pyrazoles and functionalized pyrazoles with high yields and excellent selectivity, using H2O as the co-solvent in a one-pot fashion under mild reaction conditions.We explored the validity of our hypothesis by investigating the potential for reagent-assisted regio-divergent cyclization transformation of hydrazines with alkynyl silane, resulting in an outstanding yield and high regioselectivity of 1-phenyl-1H-pyrazole (3aa) (Table 1).
| Entry | Variations from optimal conditions | 3aa (%) | 5aa (%) |
|---|---|---|---|
a Reaction conditions: 1a (0.4 mmol), 2a (0.5 mmol), tBuOK (1.1 mmol), H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v, 2.0 mL), 60 °C, 6 h. Yields and ratio were determined via GC with n-dodecane as the internal standard.
b No base. 4, v/v, 2.0 mL), 60 °C, 6 h. Yields and ratio were determined via GC with n-dodecane as the internal standard.
b No base.
|
|||
| 1 | None | >95 | <5 |
| 2 | No base | <5 | >95 |
| 3 | KOH as base | 86 | <5 |
| 4 | K2CO3 as base | <5 | 12 |
| 5 | KHCO3 as base | 0 | <5 |
| 6 | Et3N or DBU as base | 0 | 0 |
| 7 | H2O as solvent | 21 | 0 |
| 8 | EtOH as solvent | 48 | 8 |
| 9 | TFA as solvent | 0 | 0 |
| 10 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) as solvent 2) as solvent |
78 | <5 |
| 11 | 40 °C | 59 | 0 (62)b |
| 12 | O2 atmosphere | 0 | 0 |
This remarkable outcome was achieved through the utilization of tBuOK at 60 °C for a duration of 6 hours (entry 1). In the absence of tBuOK, the desired 1-phenyl-5-(trimethylsilyl)-1H-pyrazole (5aa) was obtained with exceptional yield and remarkable regioselectivity (entry 2). However, when base was substituted by KOH, K2CO3 or KHCO3, this transformation was hindered leading to substantial recovery of the starting materials (entries 3–5). We hypothesize that the weak base modifies the system's pH, thus disrupting the reaction process. It is noteworthy that the successful integration of the trimethylsilyl moiety into the desired product substantiates the feasibility of our strategy. The utilization of alternative organic bases, proved to be unsuitable for this conversion (entry 6). Among the various solvent ratios investigated, a mixture of H2O/ethanol in a ratio of 1/4 exhibited superior effectiveness, yielding 3aa with comparable efficiency also observed at a ratio of 1/2 (entry 10). The desired reactions yielded pyrazoles at a lower reaction temperature with relatively acceptable yields, showcasing their remarkable formation even under milder conditions (entry 11). Under an O2 atmosphere, no desired adducts were observed, and the presence of abundant acid in the reaction mixture was confirmed by GC-MS analysis, showing the absence of cyclization between 2a and 1a (entry 12). Furthermore, through meticulous optimization of various reaction parameters including additives, base type and amount, solvent type, substrate ratio, reaction temperature, and time, we achieved the highest yield of 3aa and 5aa as demonstrated in our ESI.†40
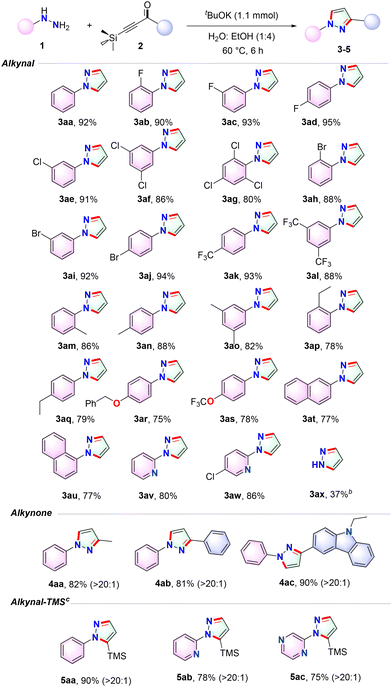
Under optimized reaction conditions, a diverse range of hydrazines were employed in conjunction with 2a to investigate the extent of reagent-assisted regio-divergent cyclization facilitated by base acceleration. The results are presented in Table 2, where various aromatic hydrazines bearing electron-withdrawing or electron-donating substituents on the aromatic ring afforded their respective substituted pyrazoles with excellent high yields.
The yields obtained with aromatic hydrazines containing electron-withdrawing groups (3ab–3al) generally surpass those achieved with aryl hydrazines containing electron-donating groups (3am–3as). Furthermore, in comparison to the results obtained with para- and meta-substituted substrates, no steric hindrance is observed upon substitution at the ortho position of hydrazines (3ab–3ad, 3am–3aq). The halide groups, specifically, display remarkable stability, thereby facilitating the formation of desired 3ab–3aj with exceptional yields. These products can be further employed for synthesizing complicated molecules. It is worth noting that the CF3 and CF3O groups exhibit extraordinary resilience throughout the standard procedure, resulting in the generation of 3ak, 3al and 3as respectively.
The successful synthesis of the annulation products 3at–3au was achieved with satisfactory yields by utilizing either 1- or 2-naphthalenyl hydrazine, respectively, showcasing impressive efficiency. Additionally, the effortless incorporation of diverse heterocyclic moieties such as pyridyl and substituted pyridyl into 3aw–3ax was effortlessly accomplished through the use of their corresponding hydrazines. Despite the utilization of more complex mono-hydrazine as reactants, we were able to successfully obtain 3ax with satisfactory yield. In order to further explore the synthetic potential of this reaction, we investigated a serious of hydrazines while incorporating the more challenging acetylenone silane aliphatic moiety.
The alkyl acetylenone silane also demonstrated remarkable effectiveness as a coupling component, resulting in abundant formation of 4aa with exceptional selectivity towards specific regions. Furthermore, the aromatic acetylenone silane exhibited divergent cyclization, leading to highly efficient production of 4ab. Moreover, by employing an aromatic acetylenone silane containing a carbazolyl group as the coupling partner, the transformation persisted and yielded 4ac with outstanding efficiency and notable selectivity towards specific regions. Additionally, a wide range of pyrazoles that have been modified with silicone exhibited excellent reactivity and successfully produced 5aa–5ac even without the presence of a base. Notably, the trimethylsilyl group remained intact in the resulting compounds.
The comprehension of the mechanism behind the regio-divergent cyclization process assisted by a reagent was further improved through a series of carefully conducted control experiments (Scheme 2). Initially, various reaction conditions were thoroughly investigated using an innovative reaction tube and highly purified substrates (purity >99.99%), resulting in no noticeable impact on the yields of 3aa or 5aa.
Based on ICP-MS analysis, it was determined that the concentration of transition-metal species in the reaction mixture is undetectable, indicating that this transformation does not rely on catalysis facilitated by transition metals (eqn (1)). The inclusion of a complexing agent such as 18-crown-6 led to decreased yields of both products, namely 3aa and 5aa, due to its strong affinity for potassium ions or disruption of the alkali-promoted reaction system. Comparable results were obtained with the incorporation of additives like 15-crown-5 (eqn (2)). The presence of TBC or ethene-1,1-diyldibenzene as radical scavengers led to successful yields of 3aa, suggesting that the reaction may not follow a free radical mechanism (eqn (3)). These findings highlight the crucial role played by tBuOK in facilitating the process rather than relying on the metal catalyst or any impurities it may contain. Moreover, the electron-withdrawing aromatic hydrazines containing groups (3ak) exhibit superior yields compared to aryl hydrazines with electron-donating groups (3an) within a 30-minute timeframe (eqn (4)). The experiment on intermolecular competition vividly demonstrates that the reaction proceeds through condensation cyclization rather than affinity attack, with valuable assistance from Lewis's acid. This certainly confirms the remarkable feasibility of our innovative strategy. Moreover, 5aa can be easily transformed into 3aa in an exceptional yield. However, without base, obtaining 3aa remains challenging while 5aa can be abundantly recovered (eqn (5)).
In addition, the efficient synthesis of 3aa was achieved through a sequential reaction, demonstrating the feasibility of a continuous cascade process involving annulation, cyclization, and desilicification (eqn (6)). These results support the potential involvement of 5aa as an intermediate in this consecutive transformation, while the incorporation of base accelerators effectively speeds up both the annulation and desilicification steps. Based on the obtained results and relevant prior researches,41,42 a preliminary mechanism for this annulation process was proposed. Initially, intermediate I is formed through the condensation of 1a with 2a. Subsequently, an intramolecular nucleophilic substitution cyclization takes place leading to the formation of compound 5aa. Eventually, under base-promoted conditions, desilicification from 5aa′ occurs resulting in the desired product being generated.
To further showcase the robustness of cascade annulation and desilicification transformation, we successfully conducted a gram-scale reaction resulting in an impressive yield of 3aa with 12.2 grams, as elegantly depicted in Fig. 1. Pyrazoles possess exceptional appeal as reactive building blocks that can be effortlessly harnessed for the synthesis of intricate functional compounds. A diverse array of halide-substituted pyrazoles (B1–B3) can be efficiently synthesized through halogenation reactions utilizing NXS compounds, which are widely employed in the realm of organic synthesis. Similarly, a series of reactions between pyrazoles and their respective reagents yield an extensive assortment of functionalized pyrazoles on the phenyl moiety. A comparable outcome was observed in a wide range of reactions between pyrazoles and corresponding reagents, resulting in the formation of a diverse array of exquisitely functionalized pyrazoles on the phenyl ring (B4–B5). Similarly impressive results were obtained in a series of reactions between pyrazoles and corresponding reagents, leading to the formation of an extensive range of functionally modified pyrazoles on the pyrazole ring (B6–B7).
Additionally, through the utilization of the newly developed base-mediated annulation procedure, LQFM032 was synthesized with exceptional efficiency via formylation and subsequent conversions of 3aa. This compound exhibits promising potential as a muscarinic receptor agent for specific sympathoinhibitory, hypotensive, and antihypertensive effects.43 Moreover, 3aa can be employed for further transformations to construct the remarkable LQFM219 compound with notable antinociceptive and anti-inflammatory activity (Fig. 1, middle).44 Furthermore, the newly developed base-mediated products facilitate the synthesis of 3av and 3aw, showcasing their exceptional catalytic activity as diazo ligands in hydrogenation applications (Fig. 1, bottom). The incorporation of bidentate 3av as an auxiliary ligand in the presence of RuCl2(PPh3)2 led to the remarkable yield of Lumefantrine through transfer hydrogenation.45,46
Additionally, utilizing bidentate 3aw as a ligand under a dihydrogen atmosphere in the presence of Ru-catalyst allowed for the excellent production yield of Estradiol.47Lumefantrine, as our first antimalarial drug, can be used to prevent or treat potentially multi-drug resistant malaria. Estradiol is currently the main estrogen that not only monitors women's health, but also shows a strong relationship with inflammation and anti-inflammatory activity.
Conclusions
In summary, we have devised a practical method for the regio-divergent cyclization of substituted pyrazoles with hydrazines and alkynyl silanes under mild conditions. This innovative approach effectively overcomes the hindrance caused by alkynyl silane in a one-pot manner, demonstrating its impressive efficiency. The procedure offers an efficient and direct route to access a wide range of silicone-substituted pyrazoles and functionalized pyrazoles, thereby expanding synthetic possibilities. Moreover, it can be easily scaled up to larger grams quantities, showcasing its versatility on a larger scale. Our carefully designed strategy significantly broadens the scope of base-promoted annulation for synthesizing pyrazole molecules without relying on metal or Lewis's acid catalysts, while cleverly utilizing water as a co-solvent. Currently, our esteemed research group is actively involved in conducting comprehensive studies aimed at gaining a detailed mechanistic understanding of this regio-divergent cyclization and exploring its potential applications in other transformative reactions.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Science and Technology Project of Shanxi Province of China (202303021221256), the Research Project Supported by Shanxi Scholarship Council of China, the International Cooperation Bureau, Chinese Academy of Sciences (040GJHZ2024013MI), the autonomous research project of SKLCC (2024KJT003).References
- P. Zhang, Y. Zhang, Y. Shao, J. Sun and S. Tang, Org. Lett., 2024, 26, 3966–3971 CrossRef CAS PubMed.
- S. Lv, X. Han, J. Y. Wang, M. Wu, Y. Zhou, L. Ma, L. Niu, W. Gao, J. Zhou, W. Hu, Y. Cui and J. Chen, Angew. Chem., Int. Ed., 2020, 59, 11583–11590 CrossRef CAS PubMed.
- P. Zhang, B. Li, L. Niu, L. Wang, G. Zhang, X. Jia, G. Zhang, S. Liu, L. Ma, W. Gao, D. Qin and J. Chen, Adv. Synth. Catal., 2020, 362, 2342–2347 CrossRef CAS.
- R. S. Bhide, J. Neels, L.-Y. Qin, Z. Ruan, S. Stachura, C. Weigelt, J. S. Sack, K. Stefanski, X. Gu, J. H. Xie, C. B. Goldstine, S. Skala, D. L. Pedicord, S. Ruepp, T. G. M. Dhar, P. H. Carter, L. M. Salter-Cid, M. A. Poss and P. Davies, Bioorg. Med. Chem. Lett., 2016, 26, 4256–4260 CrossRef CAS PubMed.
- S. Nordhoff, S. Bulat, S. Cerezo-Gálvez, O. Hill, B. Hoffmann-Enger, M. López-Canet, C. Rosenbaum, C. Rummey, M. Thiemann, V. G. Matassa, P. J. Edwards and A. Feurer, Bioorg. Med. Chem. Lett., 2009, 19, 6340–6345 CrossRef CAS PubMed.
- M. Falorni, G. Giacomelli and A. M. Spanedda, Tetrahedron, 1998, 9, 3039–3046 CrossRef CAS.
- Z. Zhong, T.-K. Ma, A. J. P. White and J. A. Bull, Org. Lett., 2024, 26, 1178–1183 CrossRef CAS PubMed.
- D. M. Wang, D. C. Chen, M. H. Xiu, L. Wang, T. R. Kosten and X. Y. Zhang, Neuropsychopharmacology, 2024, 49, 893–902 CrossRef CAS PubMed.
- C. Ma, P. Wen, J. Li, X. Han, Z. Wu and G. Huang, Adv. Synth. Catal., 2016, 358, 1073–1077 CrossRef CAS.
- V. K. Rao, R. Tiwari, B. S. Chhikara, A. N. Shirazi, K. Parang and A. Kumar, RSC Adv., 2013, 3, 15396–15403 RSC.
- P. P. Sen and S. R. Roy, Org. Lett., 2023, 25, 1895–1900 CrossRef CAS PubMed.
- R. Cano, D. J. Ramon and M. Yus, J. Org. Chem., 2011, 76, 654–660 CrossRef CAS PubMed.
- M. Taillefer, N. Xia and A. Ouali, Angew. Chem., Int. Ed., 2007, 46, 934–936 CrossRef CAS PubMed.
- W. Chen, Y. Zhang, L. Zhu, J. Lan, R. Xie and J. A. You, J. Am. Chem. Soc., 2007, 129, 13879–13886 CrossRef CAS PubMed.
- I. A. El Hassani, K. Rouzi, H. Assila, K. Karrouchi and M. Ansar, Reactions, 2023, 4, 478–504 CrossRef.
- Y. H. Ju and R. S. Varma, J. Org. Chem., 2006, 71, 135–141 CrossRef CAS PubMed.
- Y. H. Ju and R. S. Varma, Tetrahedron Lett., 2005, 46, 6011–6014 CrossRef CAS.
- M. A. Ivonin, O. Y. Bychok, N. V. Safarova and V. V. Sorokin, Russ. J. Gen. Chem., 2017, 87, 2477–2480 CrossRef CAS.
- C. Y. Zhang, Z. Y. Liang, X. F. Jia, M. R. Wang, G. Y. Zhang and M. L. Hu, Chem. Commun., 2020, 56, 14215–14218 RSC.
- T. Norris, R. Colon-Cruz and D. H. B. Ripin, Org. Biomol. Chem., 2005, 3, 1844–1849 RSC.
- Y. Zheng, Y. Long, H. Gong, J. Xu, C. Zhang, H. Fu, X. Zheng, H. Chen and R. Li, Org. Lett., 2022, 24, 3878–3883 CrossRef CAS PubMed.
- Y. Tu, Z. Zhang, T. Wang, J. Ke and J. A. Zhao, Org. Lett., 2017, 19, 3466–3469 CrossRef CAS PubMed.
- M. A. Nasseri, M. Salimi and A. A. Esmaeili, RSC Adv., 2014, 4, 61193–61199 RSC.
- N. Zwettler, A. Dupé, S. Klokić, A. Milinković, D. Rodić, S. Walg, D. Neshchadin, F. Belaj and N. Mösch-Zanetti, Adv. Synth. Catal., 2020, 362, 3170–3182 CrossRef CAS PubMed.
- Y. Xu, J. Cuccui, C. Denman, T. Maharjan, B. W. Wren and G. K. Wagner, Bioorg. Med. Chem., 2018, 26, 2973–2983 CrossRef CAS PubMed.
- T. Hu, Y. Zhang, W. Wang, Q. Li, L. Huang, J. Gao, Y. Kuang, C. Zhao, S. Zhou, L. Gao, Z. Su and Z. Song, J. Am. Chem. Soc., 2024, 146, 23092–23102 CrossRef CAS PubMed.
- C. R. Ai, L. Liu and X. C. Wang, J. Am. Chem. Soc., 2024, 146, 24663–24669 CrossRef CAS PubMed.
- H. Wang, P. Bellotti, X. Zhang, T. O. Paulisch and F. A. Glorius, Chem, 2021, 7, 3412–3424 CAS.
- Z. Liu, G. K. Kole, Y. P. Budiman, Y. Tian, A. Friedrich, X. Luo, S. A. Westcott, U. Radius and T. B. Marder, Angew. Chem., Int. Ed., 2021, 60, 16529–16538 CrossRef CAS PubMed.
- Z. Tao, K. A. Robb, K. Zhao and S. E. Denmark, J. Am. Chem. Soc., 2018, 140, 3569–3573 CrossRef CAS PubMed.
- F. Wang, D. Ding, C. Zhang and G. Zhang, Org. Chem. Front., 2024, 11, 1420–1429 RSC.
- Q. Liang, Y. Cai, W. Jiang, M. Pang, L. Fan and G. Zhang, Chem. Commun., 2024, 60, 1638–1641 RSC.
- W. Hao, S. Gao, H. Cui, D. Ding, S. Jiang, C. Zhang, Y. Ji and G. Zhang, J. Org. Chem., 2024, 89, 2605–2621 CrossRef CAS PubMed.
- C. Zhang, H. Zhao, Z. Li, Z. Liang, S. Qi, M. Cai, S. Zhang, X. Jia, G. Zhang and M. L. Hu, Chem. Commun., 2020, 56, 9521–9524 RSC.
- M. Yoshikawa, Y. Tamura, R. Wakabayashi, M. Tamai, A. Shimojima and K. Kuroda, Angew. Chem., Int. Ed., 2017, 56, 13990–13994 CrossRef CAS PubMed.
- I. S. Odin, K. V. Gordon, R. N. Itakhunov, D. M. Gusev, S. A. Sokov, A. V. Vologzhanina, S. A. Grabovskiy, I. M. Sosnin, A. I. Ukolov, O. I. Orlova, V. A. Lazarenko, P. V. Dorovatovskii, D. D. Darmoroz, A. O. Piven, T. Orlova and A. A. Golovanov, Synthesis, 2024, 56, 243–266 CrossRef.
- A. Simonneau, T. Biberger and M. Oestreich, Organometallics, 2015, 34, 3927–3929 CrossRef CAS.
- E. L. Fisher and T. H. Lambert, Org. Lett., 2009, 11, 4108–4110 CrossRef CAS PubMed.
- W. J. Ye, C. M. Stevens, P. Wen, C. J. Simmons and W. P. Tang, J. Org. Chem., 2020, 85, 16218–16225 CrossRef CAS PubMed.
- See the ESI† for details.
- W. Yao, R. Li, H. Jiang and D. Han, J. Org. Chem., 2018, 83, 2250–2255 CrossRef CAS PubMed.
- C. Eaborn, Pure Appl. Chem., 1969, 19, 375 CrossRef.
- R. Menegatti, F. S. Carvalho, L. M. Liao, B. Villavicencio, H. Verli, A. A. Mourao, C. H. Xavier, C. H. Castro, G. R. Pedrino, O. L. Franco, I. Oliveira-Silva, N. M. Ashpole, O. N. Silva, E. A. Costa and J. O. Fajemiroye, Naunyn-Schmiedeberg's Arch. Pharmacol., 2019, 392, 1071–1083 CrossRef CAS PubMed.
- G. M. Galvao, I. F. Florentino, G. Sanz, B. G. Vaz, L. M. Liao, J. R. Sabino, C. S. Cardoso, D. P. B. da Silva, E. A. Costa, A. L. P. Silva, A. C. G. da Silva, M. C. Valadares, J. A. Leite, S. G. E. de and R. Menegatti, Int. Immunopharmacol., 2020, 88, 106893 CrossRef CAS PubMed.
- Q. Liang, C. Zhang, F. Wang, Z. Luo, W. Yang, G. Zhang, D. Ding and G. Zhang, Sci. China: Chem., 2023, 66, 2028–2036 CrossRef CAS.
- W. Jiang, X. Li, Y. Liu, C. Zhang, G. Zhang and A. Morsali, Org. Chem. Front., 2025 10.1039/d4qo02176b.
- Y. Hu, S. Zhang, J. Xu, Y. Liu, A. Yu, J. Qian and Y. Xie, Angew. Chem., Int. Ed., 2023, e202312564 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00030k |
| This journal is © The Royal Society of Chemistry 2025 |