I2-Catalyzed benzylation of NH-sulfoximines with diarylmethanes and alkylarenes†
Yiyi
Chen
a,
Qisheng
Chen
ab,
Shuangquan
Zhang
ab,
Kun
Feng
a,
Xianqiang
Kong
 *a,
Xiaohui
Chen
*a,
Xiaohui
Chen
 *a,
Wenjuan
Li
*c and
Zhong-Yan
Cao
*a,
Wenjuan
Li
*c and
Zhong-Yan
Cao
 *de
*de
aSchool of Chemical Engineering and Materials, Changzhou Institute of Technology, No. 666 Liaohe Road, Changzhou 213032, China. E-mail: kongxq@czu.cn; Chenxh@czu.cn
bJiangsu Key Laboratory of Advanced Catalytic Materials and Technology, School of Petrochemical Engineering, Changzhou University, Changzhou 213164, China
cHenan Key Laboratory of Biomolecular Recognition and Sensing, College of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu 476000, China. E-mail: liwj0523@126.com
dCollege of Chemistry and Chemical Engineering, Henan University, Kaifeng 475004, China. E-mail: zycao@henu.edu.cn
eHubei Key Laboratory of Natural Products Research and Development, College of Biological and Pharmaceutical Sciences, China Three Gorges University, Yichang, Hubei 443002, P. R. China
First published on 16th January 2025
Abstract
A practical transition metal-free approach for the selective benzylation of NH-sulfoximines has been disclosed by using simple elemental iodine as the catalyst and tert-butyl hydroperoxide (TBHP) as the terminal oxidant. Comparing with known methods for the construction of N-benzylated sulfoximines, our protocol shows broad substrate scope with respect to both diarylmethanes and alkylarenes, and can be conducted in air with good functional group tolerance.
The wide applications of sulfoximines in the fields of pharmaceuticals, agrochemicals, drug discovery and organic synthesis (as chiral substrates, catalysts, ligands and directing groups for C–H bond activation, etc.) have stimulated the development of green and novel methods for their selective construction.1 In all such areas, N-alkylated derivatives have received considerable attention as it has been identified that the nitrogen substituent often leads to significantly improved bioactivities.2 To this end, the development of green and convenient methods for the synthesis of the sulfoximine scaffold with versatile alkyl substituents on the nitrogen atom is of current interest.3 While typical methods rely on Michael addition4 and nucleophilic substitution,5 Eschweiler–Clarke-type methylation,6 Petasis reaction,7 some elegant radical-involved approaches which include cross-dehydrogenative coupling (CDC),8 transition metal-catalyzed alkylation with alkyl (pseudo) halides or peroxides,9 hydroamination or difunctionalization of alkenes,10 and others,11 have also been disclosed. Among them, the sulfoximines N–H/C–H CDC strategy12 with benzylic Csp3–H bonds has received great attention for the synthesis of versatile N-benzyl sulfoximines since 20148 because of the use of simple starting materials in an atom- and step-economical way. For example, Bolm and co-workers pioneered the strategy via Fe catalysis, although only diarylmethanes are suitable (Fig. 1A).8a Inspired by this and by taking advantage of electrosynthesis, our group8c and Wang and Li8e also disclosed the electrochemically driven CDC coupling, although no external metal catalyst is necessary, the scope of alkanes was limited to diarylmethanes and alkylarenes, respectively. Mo and co-workers reported the visible light-induced CDC with toluene (Fig. 1B).8d In these excellent examples, (expensive) metal catalysts or photosensitizer with narrow scope or electrochemical reaction equipment was often necessary. Therefore, the development of a suitable and cheap catalytic system which can make up the shortcomings of the above strategies is of great importance. Herein, continuing with our previous work,8c we are delighted to note that the use of simple elemental iodine as the catalyst13 could promote the efficient CDC reactions of NH-sulfoximines with diarylmethanes and alkylarenes for the first time by using cheap tert-butyl hydroperoxide (TBHP) as the terminal oxidant (Fig. 1C). Our newly developed practical strategy does not need any transition metal and can be conducted in air, with good functional group tolerance as well.
To establish the optimal conditions, simple 1a and diphenylmethane 2a have been chosen as the model substrates. After extensive screening of a variety of reaction parameters which include the iodine source, base and the amounts of reactants (Table 1), we were delighted to find out that the desired N-alkylated sulfoximines 3a could be isolated in 87% yield with a catalytic amount of I2 (0.2 equiv.), Na2CO3 (0.5 equiv.) and tert-butyl hydroperoxide (TBHP, 1 equiv.) under air (entry 1). However, when other iodine sources, such as KI, NaI or n-Bu4NI, were used as the catalyst, the yield of product 3a was decreased (entry 2). The oxidant TBHP is essential as the use of other common oxidants such as (tBuO)2, K2S2O8 or H2O2 delivered no product 3a at all (entry 3). Na2CO3 was the optimal additive for this transformation compared to NaOH, K2CO3, NaHCO3 and CH3CO2Na (entry 4). Changing the amount of I2, Na2CO3, TBHP and 2a only gave inferior yields (entries 5–8). The yield of 3a was dropped obviously by changing the reaction temperature (entry 9). Finally, both I2 and TBHP are essential as the absence of either of them shut down the reactivity (entry 10). Furthermore, our model reaction can be scaled up to 8 mmol, and the desired 3a could be isolated with 78% yield (2.0 gram), demonstrating the practicability of the protocol.
| Entry | Variation from standard conditions | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 1a (0.5 mmol), 2a (10 mmol, 20 equiv.), I2 (0.1 mmol, 0.2 equiv.), Na2CO3 (0.25 mmol, 0.5 equiv.), TBHP (1 equiv.), 60 °C, under air. b Isolated yield. | ||
| 1 | None | 87 |
| 2 | KI, NaI or n-Bu4NI instead of I2 | 31, 55, 32 |
| 3 | (tBuO)2, K2S2O8 or H2O2 instead of TBHP | 0, 0, 0 |
| 4 | NaOH, K2CO3, NaHCO3 or CH3CO2Na instead of Na2CO3 | 34, 46, 29, 35 |
| 5 | 0.3 or 0.5 equiv. instead of 0.2 equiv. I2 | 79, 70 |
| 6 | 1.5 or 0.5 equiv. instead of 1 equiv. TBHP | 42, 53 |
| 7 | 0 or 0.4 equiv. instead of 0.5 equiv. Na2CO3 | 32, 72 |
| 8 | 15 or 25 equiv. instead of 20 equiv. 2a | 58, 41 |
| 9 | 50 °C or 70 °C instead of 60 °C | 42, 50 |
| 10 | No I2 or TBHP | 0, 0 |
| 11 | 8 mmol of 1a scale | 78% |
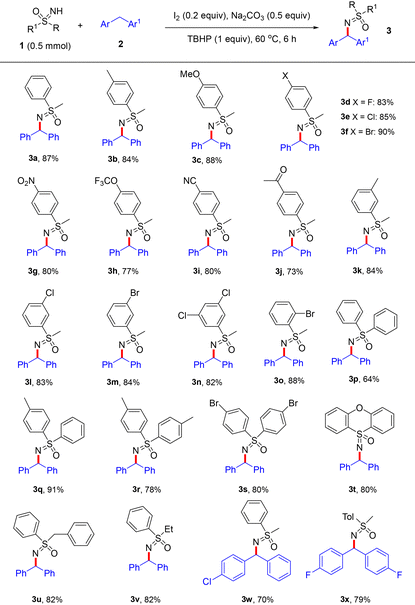
Under the optimized conditions, the scope with respect to various sulfoximines 1 was investigated at first (Table 2). To our delight, the introduction of either electron-donating methyl (1b), methoxy (1c) or electron-withdrawing halogen (1d–1f), nitro (1g), trifluoromethoxy (1h), cyano (1i) and carbonyl (1j) groups at the para position of the phenyl ring of 1 has marginal effect on the yield, and products 3a–3j could be facilely isolated in 73–90% yield. Then, substrates 1 with electron-donating or electron-withdrawing groups at meta- or ortho-positions were also checked, affording the products 3k–3o with good yields too (82–88%). In addition, the reactions between diaryl and heterocyclic sulfoximines 1p–1t and 2a gave 3p–3t in 64–91% yield. More interestingly, besides diarylmethanes, sulfoximines 1u–1v with one phenyl and one alkyl group also proceeded well, delivering products 3u–3v with 82% yield in both cases. Also, substituted diarylmethanes 2b and 2c were applied to this reaction, giving the corresponding products 3w and 3x in 70% and 79% yield.
| a For details, please see the ESI.† |
|---|
|
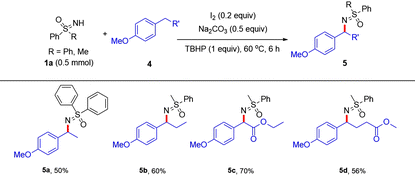
To our delight, compared with previous works that are only suitable for a narrow scope of diarylmethanes or alkylarenes,8 our current catalytic system is compatible with various alkylarenes. As shown in Table 3, ethyl-, propyl-, ester- or long carbon chain-substituted alkylarenes 4a–4d produced 5a–5d in 50–70% yield in our hands. No desired product was detected when using ethylbenzene as the substrate.
| a For details, please see the ESI.† |
|---|
|
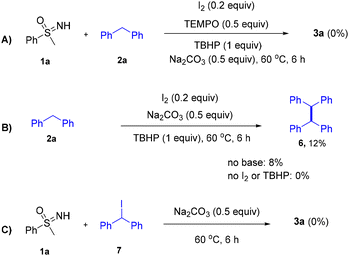
To study the reaction mechanism, a series of control experiments have been conducted (Scheme 1). (1) First of all, the reaction was completely suppressed when the radical scavenger 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) was added under the standard conditions (Scheme 1A). (2) Furthermore, it was noticed that the dimer 6 could be formed in the presence of TBHP and I2 (Scheme 1B), and such species could also be detected in the reaction mixture by GC-MS analysis. These indicate the diphenyl methyl radical might serve as the key intermediate for the transformation. Furthermore, although Patel and Rajbongshi14 have disclosed that N-iodosulfoximine could be obtained by mixing I2, TBHP with 1a, an attempt to detect such intermediate failed in our system, ruling out the possibility of the reaction working through such a species. (3) To test whether the reaction might work through (iodomethylene)dibenzene 7, it has been mixed with 1a and Na2CO3, and no 3a was detected (Scheme 1C).
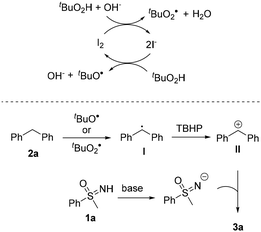
According to the above mechanistic studies and related literature,8a,c,d a plausible mechanism has been proposed (with 1a and 2a as the model substrates). First of all, in the presence of Na2CO3, the I−/I2 redox cycle promotes TBHP to deliver tBuO˙ and tBuOO˙ (Fig. 2).15 After this, either tBuO˙ or tBuOO˙ could abstract hydrogen from 2a, giving rise to diphenyl methyl radical I. Intermediate I could be further oxidized by TBHP to deliver benzylic cation II, which reacts with the anion formed by deprotonation of 1a by base, giving rise to the desired 3a.
Conclusions
In summary, we have developed an efficient method to generate N-alkylated sulfoximines by coupling diarylmethanes or alkylarenes with sulfoximines under transition metal-free and mild conditions. This reaction shows broad tolerance for both sulfoximine and alkylarene substrates. The development of other novel methods for the selective construction of N-substituted sulfoximines is ongoing in our laboratories.Data availability
The data underlying this study are available in the published article and its supplementary information.†Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
Financial support from NSFC (22102012, 22202021, 22272011, 22201062, and 22372015) is gratefully acknowledged. This project is also funded by China Postdoctoral Science Foundation (2024T170222), Postdoctoral Research Grant in Henan Province (HN2024001) and the Opening Funding of Hubei Key Laboratory of Natural Products Research and Development, China Three Gorges University (2022NPRD02), and this support is gratefully acknowledged. Prof. Xianqiang Kong thanks sponsorship by the Qinglan Project of Jiangsu Province of China, and the Changzhou Science and TechnologyPlan Applied Basic Research Project (CJ20235076).References
- (a) V. Bizet, C. M. Hendriks and C. Bolm, Chem. Soc. Rev., 2015, 44, 3378–3390 RSC; (b) C. Sambiagio, D. Schonbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes and M. Schnurch, Chem. Soc. Rev., 2018, 47, 6603–6743 RSC; (c) S. Otocka, M. Kwiatkowska, L. Madalińska and P. Kiełbasiński, Chem. Rev., 2017, 117, 4147–4181 CrossRef CAS PubMed.
- (a) U. Lücking, Angew. Chem., Int. Ed., 2013, 52, 9399–9408 CrossRef PubMed; (b) M. Frings, C. Bolm, A. Blum and C. Gnamm, Eur. J. Med. Chem., 2017, 126, 225–245 CrossRef CAS PubMed; (c) P. Mader and L. Kattner, J. Med. Chem., 2020, 63, 14243–14275 CrossRef PubMed; (d) Y. Han, K. Xing, J. Zhang, T. Tong, Y. Shi, H. Cao, H. Yu, Y. Zhang, D. Liu and L. Zhao, Eur. J. Med. Chem., 2021, 209, 112885 CrossRef CAS PubMed.
- (a) E. Wojaczynska and J. Wojaczynski, Chem. Rev., 2020, 120, 4578–4611 CrossRef CAS PubMed; (b) P. Ghosh, B. Ganguly and S. Das, Asian J. Org. Chem., 2020, 9, 2035–2082 CrossRef CAS; (c) W. Zheng, X. Chen, F. Chen, Z. He and Q. Zeng, Chem. Rec., 2021, 21, 396–416 CrossRef CAS PubMed; (d) M. Andresini, A. Tota, L. Degennaro, J. A. Bull and R. Luisi, Chem. – Eur. J., 2021, 27, 17293–17321 CrossRef CAS PubMed; (e) X. Li, C. Wang and T. Jia, Chin. J. Org. Chem., 2022, 42, 714–731 CrossRef CAS.
- (a) C. R. Johnson, J. J. Rigau, M. Haake, D. McCants Jr., J. E. Keiser and A. Gertsema, Tetrahedron Lett., 1968, 9, 3719–3722 CrossRef; (b) S. G. More, B. D. Rupanawar and G. Suryavanshi, J. Org. Chem., 2021, 86, 10129–10139 CrossRef CAS PubMed.
- (a) N. Furukawa, F. Takahashi, T. Yoshimura, H. Morita and S. Oae, J. Chem. Soc., Perkin Trans. 2, 1981, 432–437 RSC; (b) C. R. Johnson and O. M. Lavergne, J. Org. Chem., 1993, 58, 1922–1923 CrossRef CAS; (c) C. M. M. Hendriks, R. A. Bohmann, M. Bohlem and C. Bolm, Adv. Synth. Catal., 2014, 356, 1847–1852 CrossRef CAS; (d) Z. Li, M. Frings, H. Yu, G. Raabe and C. Bolm, Org. Lett., 2018, 20, 7367–7370 CrossRef CAS PubMed; (e) E. Boulard, V. Zibulski, L. Oertel, P. Lienau, M. Schäfer, U. Ganzer and U. Lecking, Chem. – Eur. J., 2020, 26, 4378–4388 CrossRef CAS PubMed; (f) S. Zhao, D. Zeng, M. Wang and X. Jiang, Nat. Commun., 2024, 15, 727 CrossRef CAS PubMed.
- T. R. Williams, R. E. Booms and D. J. Cram, J. Am. Chem. Soc., 1971, 93, 7338–7340 CrossRef CAS.
- (a) R. Hommelsheim, H. M. N. Ponce, K.-N. Truong, K. Rissanen and C. Bolm, Org. Lett., 2021, 23, 3415–3420 CrossRef CAS PubMed; (b) K. Natarajan, C. P. I. Jesin, A. A. H. Mercy and G. C. Nandi, Org. Biomol. Chem., 2021, 19, 7061–7065 RSC; (c) K. Natarajan, S. Sharma, C. P. I. Jesin, R. Kataria and G. C. Nandi, Org. Biomol. Chem., 2022, 20, 7036–7039 RSC.
- (a) Y. Cheng, W. R. Dong, L. Wang, K. Parthasarathy and C. Bolm, Org. Lett., 2014, 16, 2000–2002 CrossRef CAS PubMed; (b) F. Teng, S. Sun, Y. Jiang, J.-T. Yu and J. Cheng, Chem. Commun., 2015, 51, 5902–5905 RSC; (c) X. Q. Kong, Y. Tian, X. H. Chen, Y. Y. Chen and W. Wang, J. Org. Chem., 2021, 86, 13610–13617 CrossRef CAS PubMed; (d) J. Dong, Q. Su, D. Li and J. Mo, Org. Lett., 2022, 24, 8447–8451 CrossRef CAS PubMed; (e) Q.-R. Zhu, P.-Z. Zhang, X. Sun, H. Gao, P.-L. Wang and H. Li, Green Chem., 2024, 26, 5824–5831 RSC.
- (a) F. Teng, J. Cheng and J.-T. Yu, Org. Biomol. Chem., 2015, 13, 9934–9937 RSC; (b) Y.-F. Zhang, X.-Y. Dong, J.-T. Cheng, N.-Y. Yang, L.-L. Wang, F.-L. Wang, C. Luan, J. Liu, Z.-L. Li, Q.-S. Gu and X.-Y. Liu, J. Am. Chem. Soc., 2021, 143, 15413–15419 CrossRef CAS PubMed; (c) P. Shi, Y. Tu, D. Ma and C. Bolm, Adv. Synth. Catal., 2023, 365, 1613–1617 CrossRef CAS; (d) M. T. Zambri, C. Ho and M. S. Taylor, Org. Lett., 2023, 25, 8274–8278 CrossRef CAS PubMed.
- (a) H. Wang, M. Frings and C. Bolm, Org. Lett., 2016, 18, 2431–2434 CrossRef CAS PubMed; (b) H. Wang, D. Zhang and C. Bolm, Chem. – Eur. J., 2018, 24, 14942–14945 CrossRef CAS PubMed; (c) C. Wang, Y. Tu, D. Ma and C. Bolm, Angew. Chem., Int. Ed., 2020, 59, 14134–14137 CrossRef CAS PubMed; (d) C. Wang, Y. Tu, D. Ma, C. A. Tarint and C. Bolm, Org. Lett., 2021, 23, 6891–6894 CrossRef CAS PubMed; (e) D. Ma, D. Kong, P. Wu, Y. Tu, P. Shi, X. Wang and C. Bolm, Org. Lett., 2022, 24, 2238–2241 CrossRef CAS PubMed; (f) H. Chen, L. Chen, Z. He and Q. Zeng, Green Chem., 2021, 23, 2624–2627 RSC; (g) X.-Y. Cheng, Y.-F. Zhang, J.-H. Wang, Q.-S. Gu, Z.-L. Li and X.-Y. Liu, J. Am. Chem. Soc., 2022, 144, 18081–18089 CrossRef CAS PubMed; (h) C. Wang and T. Jia, Adv. Synth. Catal., 2023, 365, 3666–3673 CrossRef CAS; (i) Y. Y. Li and B. Gao, Org. Lett., 2023, 25, 2756–2760 CrossRef CAS PubMed.
- (a) D. Zhang, H. Wang, H. Cheng, J. G. Hernández and C. Bolm, Adv. Synth. Catal., 2017, 359, 4274–4277 CrossRef CAS; (b) H. Wang, D. Zhang and C. Bolm, Angew. Chem., Int. Ed., 2018, 57, 5863–5866 CrossRef CAS PubMed; (c) S. G. More, B. D. Rupanawar and G. Suryavanshi, J. Org. Chem., 2021, 86, 10129–10139 CrossRef CAS PubMed. For the synthesis of versatile N-substituted sulfoximines, please see: (d) Y. Zou, J. Xiao, Z. Peng, W. Dong and D. An, Chem. Commun., 2015, 51, 14889–14892 RSC; (e) J. Xu and Q. Song, Org. Chem. Front., 2017, 4, 938–942 RSC; (f) A. Wimmer and B. König, Org. Lett., 2019, 21, 2740–2744 CrossRef CAS PubMed; (g) C. Schumacher, H. Fergen, R. Puttreddy, K.-N. Truong, T. Rinesch, K. Rissanen and C. Bolm, Org. Chem. Front., 2020, 7, 3896–3906 RSC; (h) C. Wang, H. Zhang, L. A. Wells, T. Liu, T. Meng, Q. Liu, P. J. Walsh, M. C. Kozlowski and T. Jia, Nat. Commun., 2021, 12, 932 CrossRef CAS PubMed.
- (a) C.-J. Li, Acc. Chem. Res., 2009, 42, 335–344 CrossRef CAS PubMed; (b) S. A. Girard, T. Knauber and C.-J. Li, Angew. Chem., Int. Ed., 2014, 53, 74–100 CrossRef CAS PubMed; (c) Y. Yang, J. Lan and J. You, Chem. Rev., 2017, 117, 8787–8863 CrossRef CAS PubMed.
- (a) J. Zhao, W. Gao, H. Chang, X. Li, Q. Liu and W. Wei, Chin. J. Org. Chem., 2014, 34, 1941–1957 CrossRef CAS; (b) P. M. Jadhav, A. B. Rode, L. Kótai, R. P. Pawar and S. U. Tekale, New J. Chem., 2021, 45, 16389–16425 RSC; (c) D.-S. Yang, X.-L. Chen and A.-X. Wu, Org. Chem. Front., 2024, 11, 2665–2692 RSC.
- P. Rahman, N. Chakraborty, B. K. Patel and K. K. Rajbongshi, J. Org. Chem., 2024, 89, 10472–10484 CrossRef CAS PubMed.
- (a) Z. Liu, J. Zhang, S. Chen, E. Shi, Y. Xu and X. Wan, Angew. Chem., Int. Ed., 2012, 51, 3231–3235 CrossRef CAS PubMed; (b) T. K. Achar and P. Mal, J. Org. Chem., 2015, 80, 666–672 CrossRef CAS PubMed; (c) J. Lai, L. Chang and G. Yuan, Org. Lett., 2016, 18, 3194–3197 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, methods; NMR, and MS data including 1H, 13C NMR spectra. See DOI: https://doi.org/10.1039/d4ob01709a |
| This journal is © The Royal Society of Chemistry 2025 |