Design of coiled-coil N-peptides against HIV-1 based on a CADD strategy†
Yan
Huang‡
a,
Hui
Luo‡
ac,
Yihui
Jin‡
a,
Yuheng
Ma
a,
Yan
Zhao
a,
Xin
Gao
a,
Yuting
Zhao
a,
Xiao
Qi
a,
Guodong
Liang
 *ac,
Lu
Ga
*a,
Gang
Li
*a and
Jie
Yang
*ac,
Lu
Ga
*a,
Gang
Li
*a and
Jie
Yang
 *b
*b
aKey Laboratory for Candidate Drug Design and Screening Based on Chemical Biology, College of Pharmacy, Inner Mongolia Medical University, Hohhot, P.R. China. E-mail: lgd08502214@163.com
bNMPA Key Laboratory for Research and Evaluation of Drug Metabolism, Guangdong Provincial Key Laboratory of New Drug Screening, Guangdong-Hongkong-Macao Joint Laboratory for New Drug Screening, School of Pharmaceutical Sciences, Southern Medical University, Guangzhou, P.R. China. E-mail: yj528@smu.edu.cn
cBeijing Institute of Pharmacology and Toxicology, Beijing, P.R. China
First published on 31st October 2024
Abstract
Human Immunodeficiency Virus (HIV) has continued to endanger human health for decades and has a substantial impact on global health defence. Peptide-based fusion inhibitors, as an integral part of Highly Active Anti-Retroviral Therapy (HAART), are effective in preventing and controlling the AIDS epidemic. Nevertheless, the current market leader, Enfuvirtide, is facing numerous challenges in clinical application. We herein devised a cutting-edge development strategy leveraging SWISS-MODEL and HDOCK, enabling the design of artificial N-peptides. The most active compound, IZNP02QE, surpassed the positive control by demonstrating remarkable nanomolar-level inhibitory activity against HIV-1. Mechanistic investigations unveiled IZNP02QE's ability to disrupt the crucial endogenous 6-helix bundle (6-HB) by forming heteropolymers, underscoring its potential as a novel anti-HIV-1 agent. This work not only pioneers a novel design methodology for N-peptides but also opens up the possibility of a CADD strategy for designing peptide-based fusion inhibitors.
Introduction
The Human Immunodeficiency Virus (HIV), a highly contagious retrovirus, effectively targets CD4+ T-lymphocytes, a crucial component of the body's immune system, ultimately leading to the development of Acquired Immunodeficiency Syndrome (AIDS). AIDS patients are highly susceptible to various pathogens and may develop malignant tumors due to decreased immunity, resulting in a high mortality rate. According to the Joint United Nations Programme on HIV/AIDS (UNAIDS), approximately 39.9 million people worldwide were living with HIV in 2023, and 35.7 million–51.1 million people have died from AIDS-related illnesses since the start of the epidemic (https://www.unaids.org/en/resources/fact-sheet). Fortunately, by the end of December 2023, about 30.7 million people were accessing antiretroviral therapy, up from 7.7 million in 2010.The Food and Drug Administration (FDA) has approved mainly four categories of AIDS therapeutic agents:1,2 Reverse Transcriptase Inhibitors, Protease Inhibitors, Integrase Inhibitors, and Entry Inhibitors. Highly Active Anti-Retroviral Therapy (HAART), commonly known as “cocktail therapy”, involves two or more combinations of the above anti-HIV medications and is currently recognized as the most effective treatment for AIDS.3 The fusion inhibitor Enfuvirtide (also known as T20 in the entry inhibitor classification) is commonly used in cocktail therapies to block the membrane fusion process between HIV and host cells, thus acting in the early lifecycle of the virus. Fusion inhibitors are increasingly prominent in AIDS treatment, offering renewed hope and expanded possibilities.
The membrane fusion process by which HIV enters host cells is mainly dependent on the surface envelope spike, consisting of the envelope glycoprotein gp120 and the transmembrane protein gp41.4 Initially, gp120 mediates the attachment of HIV to the CD4+ receptor on the target cell membrane, triggering a conformational shift that activates gp41 upon binding to either the CXCR4 or CCR5 co-receptors. Subsequently, the fusion peptide portion in gp41 inserts into the target cell membrane, and stretches out to form the pre-hairpin structure. The pre-hairpin stretching-deforms the adjacent N-terminal heptad repeat (NHR) region to create the trimeric core (N-trimer) with widely distributed hydrophobic pockets on the surface, and the three helices in the downstream C-terminal heptad repeat (CHR) region are subsequently inserted into the hydrophobic pockets of the NHR with reverse folding to form the stable six-stranded helix bundle structure (6-HB). 6-HB drives the accomplishment of the virus–host cell membrane fusion process, initiating the entry of viral genetic material into the cell. Fusion inhibitors intervene in the formation of endogenous 6-HB, thereby blocking membrane fusion and exerting antiviral effects.5 Conventionally, peptide-based fusion inhibitors, derived from the constituent 6-HB sequences, are generally categorized into N-peptides targeting the CHR region and C-peptides targeting the NHR region.6 Enfuvirtide, a classic C-peptide inhibitor on the market for years, has encountered clinical resistance and various issues, including a short half-life in vivo and high medication costs.7,8 Therefore, there is an urgent need for more advanced peptide-based fusion inhibitors to effectively solve the abovementioned clinical problems.
N-peptides are emerging peptide-based fusion inhibitors and are expected to overcome the limitations of Enfuvirtide. It is presently assumed that N-peptides need to be spontaneously assembled into an N-trimer-like structure under solution or physiological conditions to exert anti-HIV activity, and Lai and Li et al. have already provided a workaround to construct the N-peptide active conformation through stapled amide bonds (also known as isopeptide bonds) among the helices.9,10 In this paper, we have selected (IZ10N24N)3, a prominent N-peptide, as the lead candidate for further development. Specifically, we innovatively harnessed SWISS-MODEL for homology modeling of N-peptides and HDOCK for docking simulations, providing vital guidance for optimizing the sequence design of our lead compound. Armed with the abovementioned Computer-Aided Drug Design (CADD)-aided rational design approach, we embarked on the synthesis, evaluation of inhibitory efficacy, and elucidation of the mechanistic underpinnings of N-peptides. Ultimately, we obtained IZNP02QE, which showed higher anti-HIV-1 activity than the positive control T20 and demonstrated the anti-HIV-1 mechanism by interrupting natural 6-HB formation. Our findings not only contribute a novel N-peptide inhibitor with a distinct structure and enhanced activity but also pave the way for CADD-aided design of peptide-based fusion inhibitors, addressing a previous gap in this field.
Design
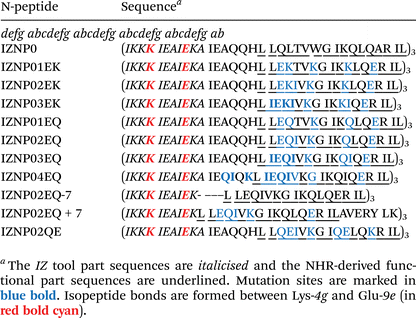
The IZ skeleton has been established as a potent platform for assembling trimers with NHR region-derived peptides, yielding (IZ)N-peptides that exhibit nanomolar-range anti-HIV-1 activity. It has been demonstrated that amino acid residues in the IZ sequence play important roles in trimer construction:11,12 isoleucine residues at the a,d sites in the sequence stack the trimeric core by hydrophobic interaction, while the glutamate residue at the e site of one helix forms a salt bridge with the lysine residue at the g′ site of a neighboring helix to reinforce the trimer. On this basis, Li et al. thioesterified the e site glutamic acid residue and built an amide bond (also known as an isopeptide bond) with the neighboring helix g′ site lysine residue by an acyl transfer reaction (Fig. 1), resulting in novel (IZ)N-peptides with promising inhibitory potency against HIV-1 infection and markedly increased metabolic stability relative to their disulfide-tethered counterpart.10 However, although the researchers obtained the preferred N-peptides, they did not elucidate the interaction mechanism with the target clearly or provide guidance for further structure optimization, and the promising research prospects of N-peptides have not been further extended. Thus, our research will aim to break through the above N-peptide problem and establish practical research strategies. We chose (IZ10N24N)3 as the lead compound from the (IZ)N-peptide series, and carried out the study based on the following three main aspects: (i) the (IZ10N24N)3 sequence contains the functional part and tool part necessary to exert activity, with the functional part covering the key pocket domain sequences, while the tool part is shortened to minimize the impact on binding targets; (ii) the tool part facilitates trimer formation and isopeptide bond creation, enabling the functional domain to form the hydrophobic pocket critical for CHR region binding, which underpins its anti-HIV-1 activity; and (iii) the short sequence, low synthetic difficulty and moderate activity of (IZ10N24N)3 make it suitable for optimization as a candidate structure. | ||
| Fig. 1 The N-peptide design schema. The hydrophobic pocket domain is shown in purple. The isopeptide bond formation site is shown in red. | ||
The realm of CADD has long been instrumental in advancing drug research and development, yet a notable gap exists in the availability of comprehensive, peptide-centric tools tailored for peptide-based fusion inhibitor design. With the increasing maturity of protein/peptide structure modeling tools and docking tools such as HDOCK and ZDOCK for studying the binding between protein/peptide(receptor) and protein/peptide(ligand),13,14 we believe that CADD is more feasible for the development of peptide-based fusion inhibitors. In this context, our approach centers around leveraging SWISS-MODEL for homology modeling of the lead compound (IZ10N24N)3, designated IZNP0, and subsequently harnessing HDOCK for precise docking simulations to assess the potential of N-peptides to bind the CHR target region. This integrated CADD strategy guides the design of novel sequences, using SWISS-MODEL for structural refinement and HDOCK for docking verification, ensuring the rationality and potential efficacy. The new sequences were designed under the above CADD guidance and the design rationality of the sequences was verified cyclically with SWISS-MODEL and HDOCK. The N-peptide design idea is as follows (Fig. 1):
(1) The SWISS-MODEL tool was used to construct a spatial model of IZNP0, and the HDOCK server was used to dock IZNP0 with the target. The results showed that the IZNP0 model was able to create a hydrophobic pocket similar to the natural N36 peptide, and the N-peptide bound to the CHR region in an N-trimer-like structure; macroscopically, the N-peptide hydrophobic pocket matched with the CHR hydrophobic residues, and microscopically, the e,g site amino acid side chains of the N-peptide sequence could interact with the a,d,e site amino acid side chains of the CHR region. Therefore, the b,c,f sites of the N-peptide sequence as non-target binding regions can be further modified. We mutated the sequences at the b,f sites into Glu acid and Lys residues for the formation of an E–K salt bridge at the (i,i + 4) position to enhance N-peptide helicity,15 at the c site into Lys residues to improve the N-peptide solubility, and at the a,d sites into Ile residues that facilitate N-trimer assembly, resulting in the novel N-peptides IZNP01EK, IZNP02EK, and IZNP03EK and the completion of SWISS-MODEL modeling and HDOCK docking.
(2) We mutated the sequences at the c site into Gln residues to improve the N-peptide solubility and exclude the positive charge effect of lysine residues on N-peptides, and at the a,d sites and b,f sites as described above, resulting in the novel N-peptides IZNP01EQ, IZNP02EQ, and IZNP03EQ and the completion of SWISS-MODEL modeling and HDOCK docking.
(3) Based on the sequence design, SWISS-MODEL modeling, and HDOCK docking of the above two groups, the third round of N-peptide design aims to explore the sequence modifiable regularity: ① expanding the mutation region to obtain IZNP04EQ; ② shortening the N-peptide functional region to retain only the hydrophobic pocket sequence to obtain IZNP02EQ-7; ③ the effect of double-pocket domains on N-peptide anti-HIV-1 activity was investigated by introducing both hydrophobic pocket and hydrophobic subpocket sequences to obtain IZNP02EQ + 7; ④ the Ei–Ki+4 salt bridge was replaced with Ei–Ki+3 to investigate the effect of different positions of the salt bridge on N-peptide anti-HIV-1 activity to obtain IZNP02QE. IZNP04EQ, IZNP02EQ-7, IZNP02EQ + 7, IZNP02QE all underwent SWISS-MODEL modeling and HDOCK docking (Table 1).
Result
SWISS-MODEL-based N-peptide homology modeling and the HDOCK server for integrated peptide–target docking
SWISS-MODEL is a fully automated server platform designed for protein/peptide structure homology modeling (https://swissmodel.expasy.org/). Its basic principle is to utilize the known protein structure database to find template structures with high sequence similarity to the target protein/peptide through sequence comparison algorithms, and then predict the 3D conformations of the target molecules based on the templates.16 The N-peptide trimer conformation is a three-stranded homologous helical assembly, and can be modeled using the SWISS-MODEL tool based on existing natural NHR crystal structure data (Fig. S1–S10†). The modeling results of the lead IZNP0 presented a trimeric helical structure with a hydrophobic pocket distributed on the surface similar to the natural NHR region (Fig. 2A), and the SWISS-MODEL-output GMOE (Global Model Quality Estimation) and QMEANDisCo scores were excellent, indicating high model prediction confidence (Table 2).| N-peptides | SWISS-MODEL | HDOCK (C34 as target) | ||
|---|---|---|---|---|
| GMQEa | QMEANDisCob | Docking scorec | Confidence scored | |
| a GMQE is a template-based quality assessment method that mainly considers the relationship between the target protein and the template protein. Its value is between 0 and 1, with values closer to 1 indicating better modelling quality. b QMEANDisCo is a composite scoring function based on global (i.e., whole structure) and local (i.e., per residue) absolute quality estimates for a single model. Its value is between 0 and 1, with values closer to 1 indicating better modelling quality. c Docking Score: the docking scores are calculated using our knowledge-based iterative scoring functions, ITScorePP or ITScorePR. A more negative docking score means a more possible binding model. d Confidence Score: when the confidence score is above 0.7, the two molecules are very likely to bind; when the confidence score is between 0.5 and 0.7, the two molecules are possibly able to bind; when the confidence score is below 0.5, the two molecules are unlikely to bind. Confidence_score = 1.0/[1.0 + e0.02×(Docking_Score+150)]. | ||||
| N36 | — | — | −371.13 (Max) | 0.9881 (Max) |
| IZNP0 | 0.71 | 0.71 ± 0.09 | −203.80 | 0.7457 |
| IZNP01EK | 0.77 | 0.84 ± 0.09 | −166.21 | 0.5803 |
| IZNP02EK | 0.78 | 0.83 ± 0.08 | −217.41 | 0.7938 |
| IZNP03EK | 0.72 | 0.80 ± 0.09 | −202.66 | 0.7414 |
| IZNP01EQ | 0.79 | 0.84 ± 0.09 | −204.38 | 0.7479 |
| IZNP02EQ | 0.69 | 0.69 ± 0.09 | −213.50 | 0.7807 |
| IZNP03EQ | 0.73 | 0.81 ± 0.09 | −207.81 | 0.7606 |
| IZNP04QE | 0.72 | 0.86 ± 0.09 | × | × |
| IZNP02EQ-7 | × | × | × | × |
| IZNP02EQ + 7 | 0.77 | 0.77 ± 0.08 | −196.91 | 0.7187 |
| IZNP02QE | 0.78 | 0.84 ± 0.08 | −241.04 | 0.8607 |
The HDOCK server is a protein/peptide(receptor)–protein/peptide(ligand) docking platform based on a hybrid algorithm of template-based modeling and ab initio free docking (https://hdock.phys.hust.edu.cn/). Within this platform, the Docking Score and Confidence Score are pivotal metrics for evaluating the efficacy of molecular docking. The interaction between N-peptides and the CHR target is essentially a protein–protein interaction, so we used the HDOCK server to assess the ability and details. The output parameters of HDOCK are Docking Score and Confidence Score. The values of −371.13 and 0.988 for natural N36(ligand) and natural C34(receptor) are defined as Docking ScoreMax and Confidence ScoreMax, respectively. The closer the docking score between artificial N-peptide and receptor C34 is to Docking ScoreMax and Confidence ScoreMax, the stronger the binding. As a result, the IZNP0/C34 Docking Score was −203.80, with a Confidence Score higher than 0.7, and IZNP0 could build hydrophobic grooves in space for filling in C34 and its “WWI modif” (Fig. 2B), which indicated that IZNP0 is able to bio-engage with C34 as a reference to the HDOCK parameter standard.17 The SWISS-MODEL model as well as the HDOCK docking results implied that the lead IZNP0 is suitable for further development via CADD.
Next, in the N-peptide designed sequences, all IZNP01EK, IZNP02EK, IZNP03EK, IZNP01EQ, IZNP02EQ and IZNP03EQ could be modeled depending on SWISS-MODEL, and the output scores were higher than or equal to those of IZNP0 (Table 2), which theorizes that regular site-specific mutations in N-peptides did not affect the N-trimer-like structure. In the IZNPnEK series (n = 01, 02, 03), the HDOCK results showed that IZNP01EK exhibited an undesirably low confidence score below 0.7, while the hydrophobic pocket regions of IZNP02EK and IZNP03EK did not match the C34 “WWI modif” (Fig. S11†); presumably all had weak target binding ability. In the IZNPnEQ series (n = 01, 02, 03), the HDOCK output scores are closer to those of IZNP0 (Table 2), with the tangible hydrophobic pockets and feasible C34 matching (Fig. S12†), which may indicate relatively strong target binding ability.
Furthermore, we selected IZNP02EQ (with the lowest score) to investigate N-peptide sequence modifiable regularity and obtained IZNP04EQ, IZNP02EQ-7, IZNP02EQ + 7, and IZNP02QE by sequence mutation. Unfortunately, IZNP02EQ-7 could not be modelled because the sequence was too short without a high similarity template structure, and the docking algorithm result between IZNP04EQ and the receptor was erroneous, presumably due to peptide–target mismatch. Meanwhile, IZNP02EQ + 7 and IZNP02QE could be successfully analysed by SWISS-MODEL and HDOCK, with IZNP02QE showing predicted binding efficacy (Table 2).
Based on the above CADD results, IZNP02EQ-7 was not synthesised due to the inability to assemble a trimer model. The IZNP04EQ intermediate cannot undergo the isopeptide bond reaction, so it was not ultimately obtained. In summary, we ended up synthesising 9 novel N-peptides, which are IZNP0, IZNP01EK, IZNP02EK, IZNP03EK, IZNP01EQ, IZNP02EQ, IZNP03EQ, IZNP02EQ + 7, and IZNP02QE.
N-peptides showed potential inhibitory activity against HIV-1 Env-mediated cell–cell fusion
We evaluated the inhibitory activity of N-peptides via HIV-1 Env-mediated cell–cell fusion, using T20 as the positive control. In Fig. 3A, IZNP01EK, IZNP02EK and IZNP03EK showed slightly lower inhibition than the lead IZNP0, with poor HDOCK docking and confidence scores. Subsequently, we changed the c site in the pocket domain from a Lys residue to a Gln residue, the obtained N-peptide (including IZNP01EQ, IZNP02EQ, and IZNP03EQ) exhibited generally 3- to 5-fold anti-HIV-1 activity compared to lead IZNP0. To our surprise, in the third design round for N-peptides, we were supposed to explore the regularity of the sequence designs, but accidentally created IZNP02QE, which was 3-fold stronger than the positive control T20, with the highest HDOCK docking and confidence scores. It is worth mentioning that we compared IZNP02QE with (3HRN23)3, which was another novel N-peptide with a trimeric helical structure discovered by Lai's team in 2015 and also constructed upon isopeptide bond binding,9 and IZNP02QE presented significantly more potential than (3HRN23)3, offering valuable insights for future N-peptide optimization. In summary, we crafted N-peptides utilizing a coiled-coil trimer assembly strategy and ultimately achieved IZNP02QE, an artificial compound exhibiting remarkable anti-HIV-1 activity, surpassing the positive control. This breakthrough highlights the success of our design approach and IZNP02QE's potential in advancing HIV-1 research.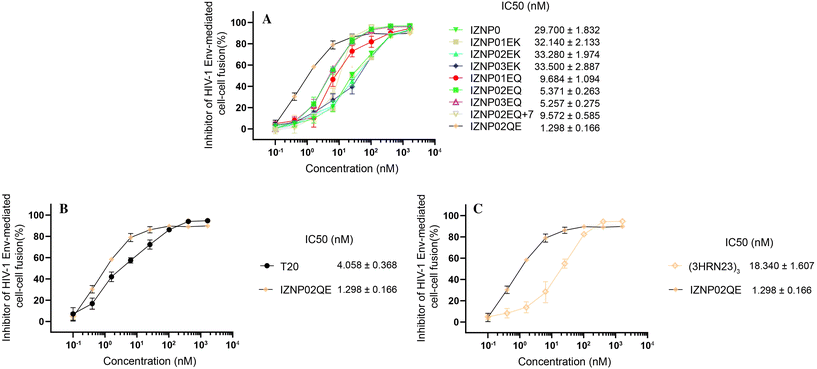 | ||
| Fig. 3 Inhibitory activity of peptides on HIV-1 Env-mediated cell–cell fusion. (A) Inhibition by N-peptides. (B) Inhibition by T20 and IZNP02QE. (C) Inhibition by (3HRN23)3 and IZNP02QE. | ||
IZNP02QE could disrupt the natural 6-HB by forming stable heteropolymers
Fig. 4 demonstrates the circular dichroism (CD) curves of the N-peptide mixed with the 6-HB complex (C34/N36), resulting in a discrepancy between the measured and theoretical values, indicating that the N-peptide could able to interact with the target.18,19Fig. 4A serves as a control where C34/N36 shows a typical 6-HB curve, which is consistent with previously reported data. After incorporating IZNP02QE into C34/N36, the CD spectrogram was significantly changed, |θ|IZNP02QE/C34/N36 < |θ|IZNP02QE+C34+N36, indicating that IZNP02QE was able to disrupt the 6-HB complex formed with C34/N36. However, the specific target entity with which IZNP02QE interacts remains to be elucidated, necessitating further investigation.Under the N-PAGE conditions, the advanced structure of peptides or peptide complexes can be maintained, therefore, with great significance to explore the interaction between the N-peptide and its target.20 The C34, due to its small molecular weight and negative charge, exhibited a single band towards the lower edge (lane 1), while the N36 and IZNP02QE are positively charged and consequently did not show any bands (lane 2 and lane 4). The C34/N36 showed a 6-HB complex band (lane 3). Following the incubation of the IZNP02QE and C34 mixed solution, the C34 band became lighter (lane 5). In lane 6, lane 7, and lane 8, as the increasing concentration of IZNP02QE, the 6-HB complex band got progressively darker (Fig. 5A), indicating that IZNP02QE is known as binding to the 6-HB target and forms “unidentified mixtures”. The SE-HPLC presented experimental findings consistent with N-PAGE (Fig. 5B), and the retention time (RT) of peptides or peptide complexes is only volume-dependent.21 Neither N36 nor IZNP02QE had peaks because of aggregation and deposition in the SE-HPLC. The RTC34 is about 9.5 minutes point, while the RTC34/N36 is about 7.6 minutes point as a larger 6-HB complex. The peaks of IZNP02QE/C34/N36 grew as the concentration multiplicity of IZNP02QE increase, which means that the so-called “unidentified mixtures” indeed formed.
 | ||
| Fig. 5 (A) N-PAGE analysis of IZNP02QE with the target peptide. (B) SE-HPLC analysis of IZNP02QE with the target peptide (IZNP02QE/C34/N36 represents the IZNP02QE, C34 and N36 mixed solution). | ||
Furthermore, we thus applied sedimentation velocity analysis (SVA) to analyze the IZNP02QE/C34/N36 state in PBS solution. In Fig. 6, C34/N36 had a M.W. of 24.1 kDa, whereas IZNP02QE/C34/N36 had M.Ws. of 24.2 kDa and 39.1 kDa. 24.2 kDa is in proximity to M.W.C34/N36, and 39.1 kDa signifies that IZNP02QE/C34/N36 assembles into multimeric complexes (Fig. 6A). We speculate that IZNP02QE can interact with C34/N36 and combine into a multimeric form, thereby preventing the formation of endogenous 6-HB (Fig. 6B).18
 | ||
| Fig. 6 (A) SVA results of IZNP02QE with the target peptide. (B) The plausible interaction mechanisms potentially correlated with N-peptides. | ||
The preceding results conclusively demonstrate that IZNP02QE can definitely interact with the HIV-1 gp41 NHR region, fostering the formation of stable helical multibundles. This process hinders the endogenous assembly of 6-HB complexes, effectively inhibiting HIV-1 replication.
Discussion
SWISS-MODEL could predict the 3D structure of unknown proteins based on known natural structures, which is extremely suitable for the conformation prediction of N-peptide homology trimers. We quickly and successfully obtained the N-peptide spatial conformation via the SWISS-MODEL tool, which not only can validate the rationality of the sequence design, but also lays the modeling foundation for elucidating how the active N-peptide functions with the target.The HDOCK server is a tool for assessing protein/peptide(receptor)–protein/peptide(ligand) interactions, providing docking scores and confidence scores that are capable of assessing the likelihood and the strength between ligand–receptor binding. We docked the N-peptides obtained from SWISS-MODEL modeling as the ligands and the HIV-1 gp41 subunit CHR region as the receptor using the HDOCK server, and screened for the ligand–receptor optimal binding conformations. Based on the docking results between the lead peptide IZNP0 and the target, we boldly modified the N-peptide sequences, verified the possibility of binding to the target using the HDOCK server, and succeeded in obtaining IZNP02QE with optimal anti-HIV-1 activity. It is worth pointing out that the hydrophobic pocket of IZNP02QE did not match the C34 “WWI” modification (Fig. S13†), and perhaps it exhibits potential activity by a novel binding mode to the target, which needs to be further explored in the future!
To date, a standardized CADD blueprint tailored for HIV-1 peptide-based fusion inhibitors remains elusive. We combined SWISS-MODEL with HDOCK in tandem for the development of anti-HIV peptides and proved their feasibility, the first of its kind! In fact, there are many other methods for peptide homology modeling such as AlphaFold, Modeller, YASARA, I-TASSER, etc., and many docking tools for protein/peptide(receptor)–protein/peptide(ligand) docking, such as GalaxyPepDock, MDockPeP, HPEPDOCK, CABS-dock, pepATTRACT, and AutoDock CrankPep (ADCP).22 How to choose the applicable CADD method according to the research needs is a question worth pondering for researchers on their way to the novel peptide-based fusion inhibitors. Still, SWISS-MODEL and HDOCK are currently available to researchers for free, with fast data analysis and highly feasible results, which is the reason why we finally chose them after screening multiple homology modeling and molecular docking tools.
Admittedly, N peptides are still very lagging behind relative to C peptides, with no marketed agents, but there has been a gradual potential to move closer to candidates. The current R&D strategies for N-peptides mainly include site-mutagenesis strategies, self-assembly strategies with chimeric tool peptides, strategies for constructing covalent bonds among coiled-coil helixes (disulfide bonds or isopeptide bonds), and small molecule backbone stapling strategies.6,23–25 Therefore, we need to explore more approaches for facilitating N-peptide R&D and discover more lead compounds with novel structures for N-peptide growth.
Conclusion
Nowadays, Enfuvirtide (T20) and Albuvirtide are available on the global market and in China, respectively, while Sifuvirtide has been approved for Phase III clinical trials in China.26 Peptide-based fusion inhibitors are no longer exclusive to Enfuvirtide. Therefore, abundant lead structures and innovative R&D methods are increasingly required to explore the potential of peptide compounds as candidates for further fusion inhibitor discovery. In this study, we selected N-peptides that exert anti-HIV-1 activity by targeting the CHR region in 6-HB, and finally obtained the novel inhibitor IZNP02QE by structural modification using the SWISS-MODEL and HDOCK tools. Mechanistic investigations showed that IZNP02QE was capable of binding to the CHR region to form more stable heteropolymers, blocking the formation of natural 6-HB, thus exhibiting anti-HIV-1 potential superior to that of the positive control. This article pioneers a novel peptide-based design methodology, the SWISS-MODEL and HDOCK-aided strategy, which enlightens the R&D of CADD-aided peptide-base fusion inhibitors.Experimental section
The homology modelling by the SWISS-MODEL method
The homology modeling by the SWISS-MODEL (https://swissmodel.expasy.org/) is an intuitive and efficient process, particularly suitable for predicting the 3D (three-dimensional) structure of unknown proteins. First, we entered the protein sequence or uploaded the file in FASTA format. Subsequently, templates were selected by alignment by SWISS-MODEL, and the sequences of the input proteins were modeled against the best template with a similar amino acid sequence and known 3D structure. After the model was established, we viewed the generated 3D structure of the N-peptides through the web interface and downloaded the PDB file for further visualization or other intended analyses.The HDOCK method for integrated receptor–ligand docking
The interaction between the N-peptides and the C34 region was predicted using the HDOCK server (https://hdock.phys.hust.edu.cn/). The specific operation is as follows:① Prepare protein: we downloaded the crystal structure of 1AIK from the RCSB Protein Data Bank and the CHR-C34 fraction was extracted. ② Prepare ligands: isopeptide bonds in the N-peptide model generated by SWISS-MODEL were constructed using PyMOL software. ③ Molecular docking: the ligand file and receptor file were uploaded to the HDOCK server for docking. The molecular docking results were analyzed and visualized using PyMOL software.Peptide synthesis
We used the Fmoc-based solid-phase peptide synthesis (SPPS) strategy to synthesise peptides. Fmoc-protected amino acids were obtained from Myriad (Shanghai, China), and the Rink-Amide resins contained 0.53 mmol g−1 loading sample and were purchased from Sunresin New Materials Co., Ltd (Xi'an, China). We alternately ligated each amino acid onto the resin using N,N‘-diisopropylcarbodiimide (DIC) and 1-hydroxybenzotriazole (HOBT) and then removed the Fmoc-protected group with 20% piperidine/N,N-dimethylformamide (DMF) solution. DMF and dichloromethane (DCM) washes were required twice at the end of coupling each amino acid reaction or removing the Fmoc-protected group reaction. An acetic anhydride/N,N-diisopropylethylamine (DIEA) mixed solution (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 4
v = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added to the resin for amino-terminal acetylation of the peptides.
1) was added to the resin for amino-terminal acetylation of the peptides.
The peptide side-chain thioesterified modification and the isopeptide bond cross-linking reaction are performed as follows:10 ①A catalyzer is added to the resin for the O-allyl group removal, which consists of tetrakis (triphenylphosphine) palladium, 5,5-dimethyl-1,3-cyclohexanedione in a DMF/THF mixed solution (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1
v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In this reaction, the nitrogen stream was used to avoid contact with air, and the reaction vessel was wrapped with tin foil to protect it from light. After 4–6 h of reaction, the resin was washed with 0.5% sodium diethyldithiocarbamate trihydrate/DMF solution. Then, 1 eq. of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 1.5 eq. of HOBT, and 4 eq. of benzyl sulfide were added to complete the side-chain thioesterification. Subsequently, the crude peptides were cleaved from the Rink-Amide resin using the above lysis solution. All thioesterified peptides were purified to >90% purity. ②Briefly, the thioesterified peptide was dissolved in PBS (50 mM, pH 7.4)/H2O/CH3CN (v
1). In this reaction, the nitrogen stream was used to avoid contact with air, and the reaction vessel was wrapped with tin foil to protect it from light. After 4–6 h of reaction, the resin was washed with 0.5% sodium diethyldithiocarbamate trihydrate/DMF solution. Then, 1 eq. of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 1.5 eq. of HOBT, and 4 eq. of benzyl sulfide were added to complete the side-chain thioesterification. Subsequently, the crude peptides were cleaved from the Rink-Amide resin using the above lysis solution. All thioesterified peptides were purified to >90% purity. ②Briefly, the thioesterified peptide was dissolved in PBS (50 mM, pH 7.4)/H2O/CH3CN (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v
v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 5
v = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and incubated for 24–72 h at 37 °C. The reaction was completed, acetonitrile was evaporated and the product was purified using RP-HPLC.
1) and incubated for 24–72 h at 37 °C. The reaction was completed, acetonitrile was evaporated and the product was purified using RP-HPLC.
The molecular weight (M.W.) of the pure peptides was confirmed by Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS, Bruker Daltonics, Germany) (Fig. S15–S24†).
HIV-1 Env-mediated cell–cell fusion assay
The inhibitory activity of peptides was determined using the HIV-1 Env-mediated cell–cell fusion assay as described previously:21 TZM-bl cells stably express large amounts of CD4 and CCR5; HL2/3 cells stably express high levels of HIV Gag, Env, Tat, Rev, and Nef proteins. TZM-bl cell suspension was spread on a 96-well cell plate, and incubated in a 37 °C, 5% CO2 incubator for 24 h. HL2/3 cells and peptide samples were added sequentially and incubated at 37 °C for 6–8 h. After adding lysis buffer and allowing it to react for 5 min, the cell lysate was loaded into a 96-well microplate. Cell–cell fusion activity was determined using Spectra Max M5 microplate reader (Molecular Devices, USA) with a fluorescent fusion assay (cat. #E19501, Promega Corporation, USA).Circular dichroism (CD) spectroscopy analysis
The interaction of peptide/peptide was characterized by CD spectroscopy. A JASCO J-1500 spectrometer was used for CD spectrum determinations. The cuvette quartz cell's path length was 1 mm with a wavelength range between 180 and 280 nm at ambient temperature. The peptides were accurately weighed using a Lac part analytical balance. All peptides were diluted in PBS solution (10 mM, pH 7.4) to a final concentration of 10 μM. N-peptides were incubated with an equal molar concentration of N36/C34 at 37 °C for 30 min and diluted in PBS solution (10 mM, pH 7.4) to a final concentration of 10 μM. By comparing the conformational changes of N-peptides before and after binding to the target peptides, the action mechanism was investigated.Native-polyacrylamide gel electrophoresis (N-PAGE)
Peptides at a final concentration of 80 μM, including N-peptides, as well as peptide complexes, were dissolved in PBS (10 mM, pH 7.4) and incubated for 30 min at 37 °C. The same volume of indicator as the peptide sample was added and mixed well, and the sample (20 μL per well) was loaded onto the gel. Gel electrophoresis was carried out at a constant voltage of 90 V at room temperature for 0.5 h and then the voltage was increased to 150 V for an additional 2–3 h. The gel was then stained with Coomassie Brilliant Blue G250. After electrophoresis, the gel was placed in a gel imager to observe the results.Binding assays by size-exclusion chromatography
Peptides at a final concentration of 80 μM, including N-peptides, as well as peptide complexes, were dissolved in PBS (10 mM, pH 7.4) and incubated for 30 min at 37 °C. A Phenomenex BioSep-SEC s2000 column, 300 × 7.8 mm, was used for size-exclusion chromatography. The mobile phase was PBS buffer (10 mM, pH 7.4) run at a flow rate of 0.8 mL min−1. The injection volume was 10 μL, and isocratic elution was performed at a detection wavelength of 210 nm.Sedimentation velocity analysis (SVA)
An analytical ultracentrifuge (Beckman Coulter ProteomeLab XL-A) was used for the SVA experiments. In brief, IZNP02QE was incubated with C34/N36 in PBS (10 mM, pH = 7.4) at 37 °C for 30 min. All samples were prepared at a final concentration of 150 μM and were initially scanned at 3000 rpm for 10 min to identify the appropriate wavelength for data collection. Data were collected at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm at a wavelength of 280 nm. The sedimentation coefficient distribution, c(s), and molecular mass distribution, c(M), were calculated using the SEDFIT program.
000 rpm at a wavelength of 280 nm. The sedimentation coefficient distribution, c(s), and molecular mass distribution, c(M), were calculated using the SEDFIT program.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflict of interest, financial or otherwise.Acknowledgements
We appreciate the financial support from the National Natural Science Foundation of China (No. 82073897, 82373915), the Natural Science Foundation of Inner Mongolia Autonomous Region (No. 2023LHMS08045), the Inner Mongolia Talent Program of China (No. DC2300001713, DC2300001705), the ZHIXUE Talent Project of Inner Mongolia Medical University 2024–2026 (No. ZY20241203), the Laboratory Open Fund Project of Inner Mongolia Medical University (No. 2024GZ17), and the Basic Research and Applied Basic Research Projects in Hohhot (No. 2024- -20).
-20).
The graphical abstract was created using Figdraw (https://www.figdraw.com).
References
- S. Ray, Z. Fatima and A. Saxena, Mini-Rev. Med. Chem., 2010, 10, 147–161 CrossRef CAS PubMed
.
- D. R. Tompa, A. Immanuel, S. Srikanth and S. Kadhirvel, Int. J. Biol. Macromol., 2021, 172, 524–541 CrossRef CAS PubMed
.
- D. Y. Lu, H. Y. Wu, N. S. Yarla, B. Xu, J. Ding and T. R. Lu, Infect. Disord.: Drug Targets, 2018, 18, 15–22 CAS
.
- G. Liang, H. Wang, H. Chong, S. Cheng, X. Jiang, Y. He, C. Wang and K. Liu, Org. Biomol. Chem., 2016, 14, 7875–7882 RSC
.
- J. Pu, J. T. Zhou, P. Liu, F. Yu, X. He, L. Lu and S. Jiang, Curr. Med. Chem., 2022, 29, 700–718 CrossRef CAS PubMed
.
- H. Na, G. Liang and W. Lai, Curr. Pharm. Biotechnol., 2023, 24, 1774–1783 CAS
.
- C. Pan, L. Cai, H. Lu, L. Lu and S. Jiang, J. Biol. Chem., 2011, 286, 28425–28434 CrossRef CAS PubMed
.
- G. Liang, Y. Huang, Y. Tang, L. Ga, C. Huo, Y. Ma, Y. Zhao, H. Na and Z. Meng, Curr. Pharm. Biotechnol., 2024 DOI:10.2174/0113892010297943240325040448
.
- C. Wang, W. Lai, F. Yu, T. Zhang, L. Lu, X. Jiang, Z. Zhang, X. Xu, Y. Bai, S. Jiang and K. Liu, Chem. Sci., 2015, 6, 6505–6509 RSC
.
- C. Wang, X. Li, F. Yu, L. Lu, X. Jiang, X. Xu, H. Wang, W. Lai, T. Zhang, Z. Zhang, L. Ye, S. Jiang and K. Liu, Sci. Rep., 2016, 6, 32161 CrossRef CAS
.
- J. M. Fletcher, A. L. Boyle, M. Bruning, G. J. Bartlett, T. L. Vincent, N. R. Zaccai, C. T. Armstrong, E. H. Bromley, P. J. Booth, R. L. Brady, A. R. Thomson and D. N. Woolfson, ACS Synth. Biol., 2012, 1, 240–250 CrossRef CAS PubMed
.
- D. M. Eckert and P. S. Kim, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 11187–11192 CrossRef CAS
.
- Y. Yan, H. Tao, J. He and S. Y. Huang, Nat. Protoc., 2020, 15, 1829–1852 CrossRef CAS PubMed
.
- B. G. Pierce, K. Wiehe, H. Hwang, B. H. Kim, T. Vreven and Z. Weng, Bioinformatics, 2014, 30, 1771–1773 CrossRef CAS
.
- H. Nishikawa, S. Nakamura, E. Kodama, S. Ito, K. Kajiwara, K. Izumi, Y. Sakagami, S. Oishi, T. Ohkubo, Y. Kobayashi, A. Otaka, N. Fujii and M. Matsuoka, Int. J. Biochem. Cell Biol., 2009, 41, 891–899 CrossRef CAS PubMed
.
- A. Waterhouse, M. Bertoni, S. Bienert, G. Studer, G. Tauriello, R. Gumienny, F. T. Heer, T. A. P. de Beer, C. Rempfer, L. Bordoli, R. Lepore and T. Schwede, Nucleic Acids Res., 2018, 46, W296–W303 CrossRef CAS PubMed
.
- D. C. Chan, C. T. Chutkowski and P. S. Kim, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 15613–15617 CrossRef CAS
.
- H. Luo, Y. Zhao, Y. Ma, G. Liang, L. Ga and Z. Meng, Protein Pept. Lett., 2024, 31, 447–457 CrossRef CAS PubMed
.
- J. Wang, P. Li, R. Li, G. Liang, Y. Ma and H. Na, Curr. Med. Chem., 2024 DOI:10.2174/0109298673291459240328074404
.
- H. Na, H. Luo, J. Wang, L. Sun, X. Gao, G. Liang, Y. Ma and Z. Meng, Bioorg. Med. Chem., 2024, 111, 117865 CrossRef CAS PubMed
.
- W. Lai, C. Wang, F. Yu, L. Lu, Q. Wang, X. Jiang, X. Xu, T. Zhang, S. Wu, X. Zheng, Z. Zhang, F. Dong, S. Jiang and K. Liu, Chem. Sci., 2016, 7, 2145–2150 RSC
.
- G. Weng, J. Gao, Z. Wang, E. Wang, X. Hu, X. Yao, D. Cao and T. Hou, J. Chem. Theory Comput., 2020, 16, 3959–3969 CrossRef CAS PubMed
.
- E. Bianchi, M. Finotto, P. Ingallinella, R. Hrin, A. V. Carella, X. S. Hou, W. A. Schleif, M. D. Miller, R. Geleziunas and A. Pessi, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 12903–12908 CrossRef CAS PubMed
.
- J. J. Dwyer, K. L. Wilson, K. Martin, J. E. Seedorff, A. Hasan, R. J. Medinas, D. K. Davison, M. D. Feese, H. T. Richter, H. Kim, T. J. Matthews and M. K. Delmedico, Protein Sci., 2008, 17, 633–643 CrossRef CAS
.
- C. Wu, I. T. Raheem, D. D. Nahas, M. Citron, P. S. Kim, D. C. Montefiori, E. A. Ottinger, R. W. Hepler, R. Hrin, S. B. Patel, S. M. Soisson and J. G. Joyce, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2317230121 CrossRef CAS
.
- G. Liang, Y. Li, R. Li, Y. Ma and H. Na, Curr. Med. Chem., 2023 DOI:10.2174/0109298673265694231113061842
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob01620c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |