 Open Access Article
Open Access ArticlePlacental targeted drug delivery: a review of recent progress
Linjian
Wang
a,
Qiuqiu
Mu
c,
Wenjing
Zhang
b,
Weiqian
Zheng
b,
Xiaojun
Zhu
b,
Ying
Yu
b,
YuPeng
Wang
b,
Wenli
Xu
a,
Zhimin
Lu
*b and
Xiujun
Han
 *b
*b
aDepartment of Obstetrics, Haining Maternal and Child Health Hospital, Zhejiang Provincial Clinical Research Center for Obstetrics and Gynecology, No. 309, East Shuiyueting Road, Xiashi Street, Haining, Zhejiang 314400, China
bDepartment of Obstetrics, Women's Hospital, Zhejiang University School of Medicine, Xueshi Road No. 1, Hangzhou, Zhejiang 310006, China. E-mail: luzhimin@zju.edu.cn; hanxiuj@zju.edu.cn
cThird Affliated Hospital of Wenzhou Medical University, WanSong Road No. 108, Ruian, Wenzhou, Zhejiang 325200, China
First published on 12th March 2025
Abstract
The placenta plays a crucial role in mediating nutrient and gas exchange between the mother and fetus during pregnancy. Targeting therapeutic agents to the placenta presents significant opportunities for treating placental disorders and enhancing fetal outcomes. However, the unique structural complexity and selective permeability of the placenta pose substantial challenges for effective drug delivery. This review provides a comprehensive overview of current strategies for placental targeting, including lipid nanoparticle (LNP) delivery systems, targeted peptide modifications, specific antibody targeting of placental receptors, and the use of viral vectors. We critically analyze the advantages and limitations of each approach, emphasizing recent advancements in enhancing targeting specificity and delivery efficiency. By consolidating the latest research developments, this review aims to foster further innovation in placental drug delivery methods and contribute significantly to the advancement of therapeutic strategies for placental disorders, ultimately improving outcomes for both mother and fetus.
1. Introduction
The placenta is increasingly recognized as not only a critical organ for fetal development but also a complex, dynamic interface that integrates maternal and fetal physiology.1,2 The placenta originates from the trophoblast layer, which can be differentiated into distinct cell types. Trophoblast cells give rise to the syncytiotrophoblast, a multinucleated structure forming the outer layer of the placental villi responsible for nutrient and gas exchange between maternal and fetal circulations.3,4 Beneath it, cytotrophoblast cells serve as progenitors for the syncytiotrophoblast and contribute to placental growth.5 Extravillous trophoblast cells invade the maternal decidua, playing a critical role in remodeling maternal blood vessels to ensure adequate placental blood flow.4 The syncytiotrophoblast serves as the primary barrier for drug transfer, while cytotrophoblast and extravillous trophoblast cells influence placental growth and blood flow, potentially affecting the efficiency of drug delivery.6,7 Targeting these specific cell types or utilizing their receptors can enhance the precision and effectiveness of drug transport across the placenta.Recent advancements in placental research have revealed its multifaceted roles in regulating both maternal and fetal health, extending far beyond traditional concepts of nutrient and gas exchange.8–10 It is now understood that the placenta functions as a highly active endocrine organ, orchestrating a range of signaling pathways that impact pregnancy maintenance and fetal development, as well as long-term maternal and fetal health.11 For example, the placenta plays a crucial role in immune modulation, balancing maternal immune tolerance to prevent rejection of the semi-allogeneic fetus, while simultaneously protecting the fetus from potential infections.12,13 Furthermore, emerging research underscores the significant impact of placental dysfunction on fetal programming, with disturbances contributing not only to abnormal fetal growth but also to the development of chronic diseases in adulthood, including cardiovascular disease, diabetes, and neurodevelopmental disorders.14–16 As the placenta is increasingly recognized as a key regulator of fetal health, understanding its molecular and cellular mechanisms has become a focal point for improving prenatal care and advancing the treatment of pregnancy-related complications.
One of the key functions of the placenta is its role in immune modulation.17 It helps maintain maternal immune tolerance, preventing the rejection of the semi-allogeneic fetus while also protecting the fetus from potential infections.18–20 Furthermore, growing evidence emphasizes how placental dysfunction can disrupt fetal development, leading to long-term health consequences.11,21,22 Impaired placental blood flow, hypoxia, and inflammation are factors that contribute to abnormal fetal growth and increase the risk of chronic diseases later in life.16,18,23 As the placenta is recognized as a central regulator of fetal health, understanding its molecular and cellular mechanisms has become crucial for improving prenatal care and addressing pregnancy-related complications.24,25
Research has shown that abnormal placental function is associated with increased risks of chronic diseases in adulthood.26,27 For instance, impaired placental blood flow or gas exchange can have profound effects on fetal immune, metabolic, and neurological systems.28 Factors such as placental hypoxia and chronic inflammation are believed to contribute to developmental delays in the fetus and may increase the likelihood of post-birth health complications.29–31 In addition, the impact of placental diseases extends beyond immediate maternal and fetal health concerns, placing a significant burden on healthcare systems.22 Conditions like preeclampsia, placental abruption, and intrauterine infections lead to higher rates of preterm births, which in turn increase the demand for neonatal intensive care and escalate healthcare costs.32–34
One of the major challenges in treating placental diseases is the difficulty in delivering therapeutics to the placenta without risking harm to the fetus.35 The placenta acts as a selective barrier, limiting the ability of most drugs to reach therapeutic concentrations.36 Additionally, the potential for maternal side effects complicates the safe use of medications during pregnancy. These challenges have spurred the development of placental-targeted drug delivery strategies, which aim to minimize fetal risks. For example, LNPs have shown promise for delivering mRNA to the placenta, and targeting specific receptors or cell types on the placental surface using peptides or small molecules may enhance the specificity of drug delivery.37 Furthermore, liposomes are being explored for encapsulating drugs to improve their delivery to the placenta.38,39 These strategies hold potential for more effective treatments of placental-related diseases by improving the specificity of therapeutic agents, reducing off-target effects, and ultimately improving maternal and fetal health outcomes (Fig. 1).
 | ||
| Fig. 1 Four key strategies for targeted drug delivery to the placenta include LNPs, peptides, antibodies, and viral vectors. | ||
This review focuses on the molecular techniques and nanotechnology employed for placental-targeted therapy in the treatment of various placental disorders (Table 1). We explore the use of specific targeting peptides, LNPs, liposomes, and small biomolecules for targeting the placenta. The review also highlights recent advancements in these strategies and their potential applications in improving treatment outcomes for pregnancy-related complications. Our goal is to provide an overview of the current state of research in placental-targeted therapies, while inspiring further developments in this promising field.
| Delivery strategy | Recent advances | Advantages | Limitations | Ref. |
|---|---|---|---|---|
| Lipid nanoparticles | Enhanced surface modifications of LNPs to improve targeting specificity and delivery efficiency. | High drug loading capacity, excellent biocompatibility, and protection of drugs from degradation. | Potential toxicity issues and delivery efficiency constrained by the placenta's selective permeability. | 47–51 and 53 |
| Targeted peptides | Discovery and optimization of novel placental-targeting peptides to improve stability and modification techniques. | Increased targeting specificity, reduced non-specific distribution, and minimized side effects. | Complex fabrication processes and potential instability or loss of peptide functionality under physiological conditions. | 65–67 and 69–73 |
| Antibodies | Development of novel antibodies and optimization of antibody modification and carrier conjugation techniques. | High specificity, significantly enhancing drug accumulation in the placenta. | High cost of antibodies, potential immunogenicity, and safety concerns. | 93–96, 100 and 102–103 |
| Viral vectors | Design of safer viral vectors with reduced immunogenicity and improved delivery control mechanisms. | High gene delivery efficiency and potential for sustained drug release. | Risks of immune reactions, and the safety and controllability of viral vectors require further validation. | 114–117 and 120–122 |
2. LNP delivery of mRNA for placental disorder treatment
LNPs are nanostructures composed of lipid molecules designed to encapsulate nucleic acids such as mRNA, enabling efficient delivery into cells.40–42 They are primarily used for the delivery of genetic material in therapeutic applications, including mRNA vaccines, gene editing, and gene therapies.43,44 LNPs protect the nucleic acids from degradation, facilitate their cellular uptake, and promote their release into the cytoplasm for translation. Currently, LNPs represent the most advanced non-viral platform for nucleic acid delivery and have been clinically proven in the development of mRNA vaccines for COVID-19, as well as in gene editing therapies for genetic disorders.45,46 Despite their widespread use in these areas, LNP-mediated delivery has been relatively unexplored in the context of pregnancy, particularly for applications targeting the placenta.47,48 Recent studies have begun to investigate the potential of LNPs for placental delivery, with a focus on using mRNA to mediate placental vasodilation in the treatment of pregnancy-related complications such as pre-eclampsia and fetal growth restriction.49,50 These early findings suggest that LNPs could offer a promising strategy for targeted therapies aimed at improving maternal and fetal health during pregnancy.In a recent investigation, Mitchell et al. established a library of ionizable LNPs engineered for the delivery of mRNA to a range of placental cell types,51 including trophoblasts, endothelial cells, and immune cells-key players in maintaining placental function (Fig. 2A). A notable contribution of the study is the development of these LNPs for mediating placental vasodilation, a critical therapeutic goal for addressing pregnancy-related conditions such as pre-eclampsia and fetal growth restriction. The authors demonstrated that the most effective LNP formulation, encapsulating VEGF-mRNA, successfully triggered vasodilation in the placenta, suggesting that LNPs could serve as a promising non-viral delivery vehicle for protein replacement therapies targeting placental dysfunction during pregnancy. The LNPs were formulated by integrating ionizable lipids with essential components, such as cholesterol, phospholipids, and polyethylene glycol-lipids, to enhance particle stability and biocompatibility (Fig. 2B). Cholesterol stabilizes the lipid bilayer, phospholipids help maintain the structural integrity of the particles, and PEG-lipids extend the circulation time in the bloodstream by reducing protein adsorption and evading immune recognition. These ionizable lipids play a crucial role in boosting transfection efficiency, as they undergo protonation in acidic environments, such as within the endosomal compartment, promoting endosomal escape and enabling the release of mRNA into the cytoplasm. As a result, in nonreproductive maternal organs, LNPs tend to accumulate in the spleen, with minimal distribution in other organs and no significant differences observed (Fig. 2C and D). In Fig. 2E and F, the authors focused on the in vivo delivery of mRNA specifically to the uterus, placenta, and fetuses of pregnant mice. The figures show that LNPs effectively deliver mRNA not only to the maternal organs but also to the placenta, with successful transfection observed in these tissues.
 | ||
| Fig. 2 (A) LNPs formulation via microfluidic mixing of ethanol phase (ionizable lipid, phospholipid, cholesterol, lipid-PEG) and aqueous phase (mRNA), synthesis of ionizable lipid A4 via SN2 reaction. (B) Regions of the mouse placenta from maternal to fetal side, including cell types that separate maternal and fetal blood spaces in the labyrinth region. (C) and (D) in vivo delivery of luciferase mRNA via LNPs (0.6 mg kg−1) to non-reproductive maternal organs in nonpregnant and pregnant mice, shown by IVIS imaging and quantification of mRNA distribution in the heart, lungs, liver, kidneys, and spleen. (E) In vivo delivery of luciferase mRNA via lipid nanoparticles (0.6 mg kg−1) to the uterus, placenta, and fetuses in both nonpregnant and pregnant mice, with imaging and measurement of mRNA uptake in these areas (n = 5). (F) IVIS imaging of luciferase mRNA LNP delivery (0.6 mg kg−1) to the placentas of pregnant mice, with normalized flux reported as mean ± SEM for each mouse. Reproduced with permission from ref. 51. Copyright 2023 American Chemical Society. | ||
A key innovation of this study lies in the tailored optimization of LNPs for placental targeting, marking a departure from conventional applications of LNPs, such as in vaccines or gene editing. By focusing on the placenta, the research introduces a novel therapeutic approach aimed at enhancing both maternal and fetal health. However, several challenges remain, including issues related to the specificity and efficiency of LNP targeting, as well as the scalability and long-term safety of such treatments. While the findings show considerable promise, the clinical feasibility and safety of mRNA LNPs for treating placental disorders still require rigorous clinical trials before they can be considered a viable therapeutic option for widespread use.
In 2024, Geisler et al. investigated the development of epidermal growth factor receptor (EGFR) antibody-conjugated lipid nanoparticles (aEGFR-LNPs) to enhance mRNA delivery to the placenta, offering potential therapeutic benefits for pregnancy-related complications.50 By employing SPAAC (strain-promoted alkyne–azide cycloaddition) chemistry, the authors engineered LNPs with varying degrees of EGFR antibody conjugation (Fig. 3A), with the goal of facilitating uptake by placental cells via receptor-mediated endocytosis through the EGFR antibody (Fig. 3B). The authors characterized the particle size of the LNPs and assessed their cytotoxicity, finding that the modification with EGFR antibodies did not affect the particle size or its toxicity (Fig. 3C). The most effective formulation, which featured a moderate level of antibody attachment (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 aEGFR-LNP), exhibited superior luciferase mRNA delivery to murine placentas in vivo, outperforming non-targeted LNPs (A1, Fig. 3D). Notably, this LNP formulation maintained a comparable safety profile to the non-targeted version, with no evidence of fetal luminescence, suggesting no adverse effects on the fetus. Moreover, the study revealed that the 1
5 aEGFR-LNP), exhibited superior luciferase mRNA delivery to murine placentas in vivo, outperforming non-targeted LNPs (A1, Fig. 3D). Notably, this LNP formulation maintained a comparable safety profile to the non-targeted version, with no evidence of fetal luminescence, suggesting no adverse effects on the fetus. Moreover, the study revealed that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 aEGFR-LNPs were preferentially internalized by EGFR-expressing trophoblasts in the placenta, demonstrating roughly double the uptake of non-targeted LNPs. In Fig. 3F, treatment with 1
5 aEGFR-LNPs were preferentially internalized by EGFR-expressing trophoblasts in the placenta, demonstrating roughly double the uptake of non-targeted LNPs. In Fig. 3F, treatment with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 aEGFR-LNPs resulted in a significant increase in the proportion of DiR+ CK7+ trophoblasts (approximately 20%) compared to LNP A1 (10%), indicating that EGFR-targeted LNPs more efficiently internalized in EGFR-expressing trophoblasts. Fig. 6G shows a similar trend in CD45+ immune cells. This suggests that the presence of immune cells at the maternal-fetal interface enhances the targeting and uptake of EGFR-conjugated LNPs in these cell populations. Collectively, these works underscore the promising potential of antibody-conjugated LNPs as a targeted delivery system for mRNA, offering an innovative approach to address obstetric disorders through precise targeting of EGFR-positive cells in the placenta.
5 aEGFR-LNPs resulted in a significant increase in the proportion of DiR+ CK7+ trophoblasts (approximately 20%) compared to LNP A1 (10%), indicating that EGFR-targeted LNPs more efficiently internalized in EGFR-expressing trophoblasts. Fig. 6G shows a similar trend in CD45+ immune cells. This suggests that the presence of immune cells at the maternal-fetal interface enhances the targeting and uptake of EGFR-conjugated LNPs in these cell populations. Collectively, these works underscore the promising potential of antibody-conjugated LNPs as a targeted delivery system for mRNA, offering an innovative approach to address obstetric disorders through precise targeting of EGFR-positive cells in the placenta.
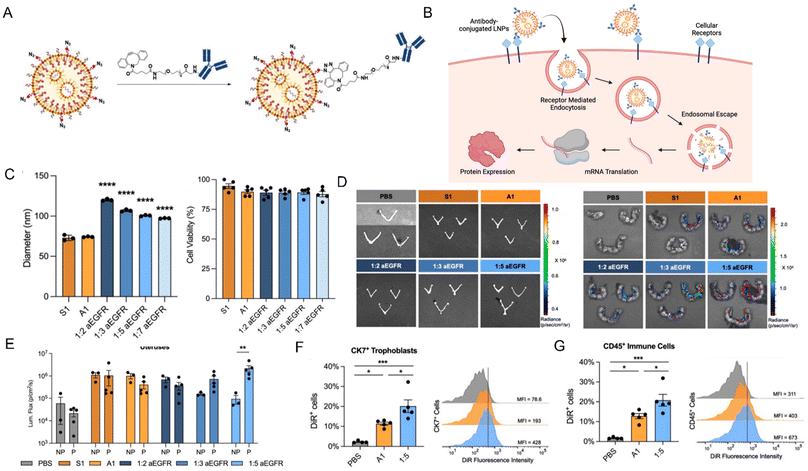 | ||
| Fig. 3 (A) Assembly of antibody-conjugated LNPs and (B) schematic illustrating the receptor-mediated untake of antibody-conjugated LNPs into placental cells. (C) Diameters of LNPs modified with varying ratios of EGFR antibodies and cytotoxicity in JEG-3 trophoblast cells, 24 hours following treatment with non-targeted LNPs. (D) Luciferase imaging of mRNA administration (0.4 mg kg−1) to the uterine tissue in non-pregnant and pregnant mice. (E) Measurement of luminescence intensity in the uterine tissues of non-pregnant (NP) and pregnant (P) mice. Flow cytometric analysis of DiR+ cells in (F) CK7+ trophoblasts and (G) CD45+ immune cells within placental tissues. Reproduced with permission from ref. 50. Copyright 2024 Elsevier. | ||
The authors recognize the importance of antibody selection in the design of antibody-conjugated nanoparticle delivery systems. While they used an off-the-shelf anti-EGFR antibody for simplicity and reproducibility, they acknowledge that optimizing the antibody clone could potentially enhance the targeting efficacy of aEGFR-LNPs. The study also highlights the potential of antibody-conjugated LNPs to target EGFR-expressing cells in the placenta, which could be particularly beneficial for addressing pregnancy complications such as placental dysfunction. However, the authors note that changes in receptor expression during disease progression, such as the upregulation of EGFR in preeclampsia, could further improve the targeting and therapeutic potential of the system. Additionally, the study suggests that engineered antibody fragments, such as ScFvs, may offer advantages over whole antibodies by enhancing safety and reducing immunogenicity, especially in pregnancy. Future research should focus on optimizing antibody clones and exploring the use of aEGFR-LNPs in models of placental dysfunction to treat obstetric complications.
Several other studies have demonstrated the feasibility of LNP-mediated mRNA delivery to the placenta in animal models. For example, the use of different ionizable lipids, phospholipids, and the adjustment of PEG content in LNP formulations can be explored to improve targeting efficacy to trophoblast cells and the placenta.52,53 Additionally, researchers are investigating the use of LNPs to deliver pro-angiogenic factors like vascular endothelial growth factor to improve placental blood flow in cases of placental dysfunction.54
A landmark study by Mitchell et al. developed a placenta-tropic LNP (LNP-55) capable of delivering mRNA with specificity and efficiency.55 Through high-throughput screening of 98 LNP formulations, LNP-55 exhibited a 183-fold higher placental mRNA delivery efficiency compared to FDA-approved LNP benchmarks, attributed to its β2-glycoprotein I (β2-GPI)-mediated endogenous targeting mechanism. In murine models of preeclampsia, a single intravenous injection of VEGF mRNA-loaded LNP-55 normalized maternal hypertension, restored placental vascularization, reduced anti-angiogenic sFlt-1 levels, and improved fetal outcomes, including increased birth weight and placental blood flow. The success of LNP-55 highlights the broader potential of LNP technology beyond vaccines, enabling precise modulation of placental pathophysiology. Furthermore, the study's use of dual preeclampsia models (hypoxia- and inflammation-induced) underscores the robustness of LNP-55 across diverse etiologies, enhancing its translational relevance.
Despite these breakthroughs, challenges remain in translating placental-targeted LNPs to clinical use. Safety concerns, such as off-target effects and immune activation, require rigorous evaluation, particularly given the vulnerability of fetal development. While LNP demonstrated minimal off-target delivery in mice, interspecies differences in placental biology and receptor expression necessitate validation in non-human primates.47,56 Additionally, long-term effects angiogenic factors on placental and fetal development remain unexplored. Scalability and regulatory hurdles, including standardized LNP manufacturing and formulation stability, further complicate clinical translation.57
Future directions may expand LNP applications to other placental disorders, such as intrauterine growth restriction, leveraging modular mRNA payloads to target diverse pathways.58 Collaborative efforts integrating computational lipid design, multi-omics profiling, and advanced imaging could refine placental targeting precision and safety.59 Ultimately, the convergence of LNP innovation and placental biology holds immense potential to redefine maternal-fetal medicine, offering curative rather than palliative interventions for pregnancy complications.60
3. Peptide-based targeted delivery to the placenta
Research on peptide-based targeting of the placenta is a rapidly advancing field, focusing on harnessing the unique physiological and molecular properties of the placenta to enable precise delivery of therapeutic agents or diagnostic probes.61,62 Peptides-based strategies, due to their high specificity, low toxicity, and relatively straightforward synthesis, have emerged as promising candidates for placenta-targeted therapies.63,64 By exploiting differential receptor expression on placental trophoblasts and vasculature, peptides selected through in vivo phage display techniques can be engineered to preferentially home to the placenta. For instance, the VAR2CSA-derived peptide has been shown in preclinical models to exhibit robust placental targeting when conjugated to nanoparticle carriers, thereby improving local drug concentration and reducing off-target distribution.65 Similarly, the tumor-penetrating peptide iRGD,66 which mediates tissue infiltration via integrin and neuropilin-1 interactions, has been repurposed to navigate the highly angiogenic microenvironment of the placenta,67 further demonstrating the versatility of peptide ligands in overcoming biological barriers67,68 (Table 2). Collectively, these findings highlight the inherent advantages of peptide-based delivery systems-notably their high specificity, ease of chemical modification, and capacity for modular design-which render them a promising platform for addressing complex challenges in maternal–fetal medicine.| Peptide | Amino acid sequence | Target site/cell type | Targeting strategy | Disease | Ref. |
|---|---|---|---|---|---|
| VAR2CSA-derived peptide | VAR2CSA | Syncytiotrophoblast surface (binds chondroitin sulfate A) | Conjugated to nanoparticles or drugs for targeted delivery | Preeclampsia, placental insufficiency | 65 |
| CGKRK | CGKRK | Placental vascular endothelial cells and some trophoblasts | Used as a targeting ligand on drug carriers to enhance placental accumulation | Preeclampsia, placental ischemia | 67 and 68 |
| iRGD | CRGDKGPDC | Placental vasculature and stromal cells (via α_v-integrins and neuropilin-1) | Enhances tissue penetration of nanoparticles/drugs across the placental barrier | Placental insufficiency, ischemic conditions | 66, 68 and 78 |
| CNGRC | CNGRC (cyclic) | Placental endothelial cells (via CD13 binding) | Used to decorate nanocarriers for improved targeting of placental vasculature | Abnormal placental angiogenesis, preeclampsia | 66 and 77 |
| PLAP-1 | CSPRRGLC | Syncytiotrophoblasts expressing placental alkaline phosphatase (PLAP) | Employed in imaging or therapeutic delivery systems targeting PLAP-expressing cells | Trophoblastic diseases, placental insufficiency | 79 |
| TPP-1 | CRWYDANAC | Placental vascular endothelium | Directs therapeutic agents specifically to placental blood vessels | Placental ischemia, preeclampsia | 82 |
One of the most studied categories includes trophoblast cell-binding peptides, which interact with specific receptors or proteins expressed on trophoblast cells. For example, peptides that bind to the human placental growth factor receptor (PlGF-R) or integrins such as αvβ3 and αvβ5, which are highly expressed on placental trophoblast cells, have been explored.69–71 Another class includes peptides that target the placental transporters, such as the human placental lactogen receptor (hPLR) or the syncytin-1 receptor, which plays a role in trophoblast fusion and placental function.72,73 Additionally, peptides derived from naturally occurring placental proteins, such as syncytins,74 have been investigated for their potential in targeting trophoblasts, given their inherent ability to bind specific receptors on the placental cell membranes.75–77
Compared to small molecules, peptides can be designed to selectively bind to receptors with high affinity, reducing off-target effects and improving therapeutic outcomes.78,79 Additionally, peptides can be engineered to cross the placental barrier more effectively, ensuring targeted delivery to the fetus in conditions where this is therapeutically beneficial, such as in the case of gene therapies or targeted delivery of anti-cancer agents.61,80 Moreover, peptides are biodegradable and typically exhibit low immunogenicity, making them suitable for long-term use.
Harris et al. investigated the response of the placenta to two tumor-homing peptides, CGKRK and iRGD (Fig. 4A).67 These peptides, as distinct sequences of amino acids, serve as specialized drug delivery vehicles, facilitating the targeted transport of therapeutic agents. In experiments involving pregnant mice, the authors observed that, analogous to the way these peptides effectively target tumors and deliver anticancer drugs to specific sites, they could also be employed to selectively target placental tissue and deliver growth factors to enhance placental function. Notably, the growth hormones had no adverse effects on normal-sized fetuses, while fetuses exhibiting abnormal development due to compromised placental function responded positively to the growth factor treatment. The authors identified membrane-associated calreticulin as a receptor for CGKRK (Fig. 4B and C). Immunofluorescence staining and flow cytometry analysis revealed that calreticulin on the cell membrane is involved in the uptake of the peptide by the placenta (Fig. 4D and E). To develop biocompatible nanocarriers for precise and targeted delivery of therapeutic agents to the placenta, the authors synthesized liposomes functionalized with TAMRA-CGKRK, rhodamine-iRGD, or TAMRA-ARA, and encapsulated them with the fluorescent drug analog carboxyfluorescein. These liposomes were intravenously administered to pregnant mice to evaluate their accumulation at the maternofetal interface (Fig. 4F). Additionally, peptide-functionalized liposomes were incubated with human term placental explants to investigate peptide binding and cellular uptake within human tissue. The findings revealed that the liposomes successfully delivered to the syncytium of placental explants, whereas those functionalized with ARA were ineffective in facilitating efficient delivery.
 | ||
| Fig. 4 (A) Tumor-targeting peptides are enriched in the syncytial layer of human placental explants. Uptake of the peptide (20 mM) by first-trimester or term placental explants (n = 3). Green indicates FAM-labeled peptides. Scale bar, 50 μm. (B) Immunoblot analysis of sequential fractions eluted from the chromatography column. (C) Proteins in mouse placental homogenates were identified by MALDI-TOF, with calreticulin being the most abundant. (D) Immunofluorescence analysis of the binding of FAM-CGKRK to first-trimester placenta. (E) Flow cytometry quantification of the binding rate of FAM-CGKRK to calreticulin. (F) Immunofluorescence analysis of liposomes decorated with tumor-homing peptides for targeted delivery to the placenta. Red, peptide; green, CF cargo; blue, DAPI (nuclei). Scale bar, 50 μm. Reproduced with permission from ref. 67. Copyright 2016 American Association for the Advancement of Science. | ||
A principal limitation highlighted in the study is the efficiency of peptide-mediated drug delivery to the placenta. While the tumor-homing peptides (CGKRK and iRGD) demonstrated selective affinity for the placenta, the capacity to deliver therapeutic payloads effectively and at adequate concentrations in a clinically relevant context remains uncertain. The authors emphasize that, despite strong binding affinity observed in both in vitro and in vivo models, ensuring that these peptides can consistently and efficiently traverse the placental barrier to deliver therapeutic agents at concentrations sufficient for a therapeutic response remains a formidable challenge. This inefficiency could curtail the peptides’ therapeutic potential, particularly in human applications. The study further discusses the inherent variability in placental structure across individuals. The placental surface is not homogenous, with considerable interindividual differences in its architecture, which may influence the efficacy of peptide binding. While the peptides effectively targeted both mouse and human placental tissues, the authors acknowledge that the variability in placental morphology and physiological conditions during pregnancy could affect the consistency of peptide targeting. This variability introduces complexity into the clinical application of these peptides, underscoring the necessity of personalized approaches to optimize therapeutic outcomes.
Moreover, the molecular mechanisms governing peptide binding and their full therapeutic potential remain insufficiently explored. Although the authors identify calreticulin as a pivotal mediator in peptide–placenta interactions, they concede that further research is imperative to unravel the precise molecular pathways through which these peptides modulate placental function. A more comprehensive understanding of these mechanisms is crucial for refining the therapeutic application of peptides and enhancing their clinical efficacy.
In 2018, Fan et al. developed a nanoparticle system for the targeted delivery of therapeutic agents to the placenta by modifying the nanoparticle surface with placental-targeting peptides, plCSA-BP (CSA-binding peptide, EDVKDINFDTKEKFLAGCLIVSFHEGKC) or SCR (EVDNDKKLGLVFEKDKIFTEFACISHCG). The study involved intravenous injection of pICSA-BP-modified nanoparticles (such as those loaded with indocyanine green or methotrexate) into pregnant mice to assess their placental-specific delivery efficiency and potential effects on fetal and placental development.81 They demonstrated that these nanoparticles selectively bound to trophoblast cells and successfully delivered methotrexate to the placenta, significantly affecting placental and fetal development, while showing no evident adverse effects on maternal tissues. This approach offers a strategy for targeted treatment of pregnancy-related complications through placental-specific drug delivery. In detail, Fig. 5A illustrated that, despite the broad tissue distribution of chondroitin sulfate A (CSA) as confirmed by anti-C4S (2B6) staining, the placenta remained uniquely targeted by plCSA-BP. In the synthesis process, lecithin, DSPE-PEG-COOH, and methotrexate (MTX) are used to form a core–shell structure, followed by sonication to create MNPs. The nanoparticles are then functionalized with pICSA for targeted delivery, yielding pICSA-MNPs (Fig. 5B). The cumulative release profiles of free MTX and various types of MNPs (MNPs, SCR-MNPs, and pICSA-MNPs) over 48 hours demonstrate that the release rate of MTX from pICSA-MNPs is more sustained compared to free MTX and the other nanoparticle formulations (Fig. 5C). Furthermore, the size stability of the MNPs over a 6 week period shows that the diameter of pICSA-MNPs remains stable, similar to that of SCR-MNPs and MNPs, indicating that the particles do not undergo significant aggregation or degradation over time (Fig. 5D). TEM images of the MNPs, SCR-MNPs, and pICSA-MNPs reveal their spherical morphology, with the scale bars indicating the size of the nanoparticles and further confirming their uniform size distribution (Fig. 5E). IVIS imaging of the placentas from mice injected with a single dose of plCSA-INP revealed that plCSA-INP exhibited prolonged retention for 48 h (Fig. 5F). Fluorescence staining of the placentas indicated that ICG was primarily co-localized with trophoblast cells, while little or no staining was observed in endothelial cells (Fig. 5G). This confirms that plCSA-INP is capable of targeting and delivering ICG specifically to trophoblast cells.
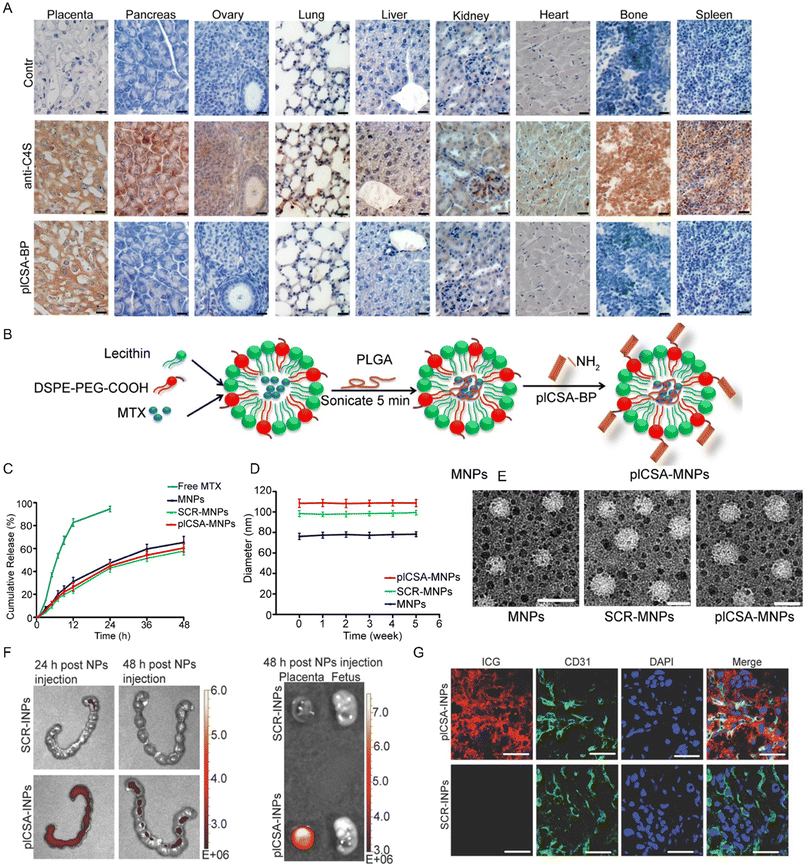 | ||
| Fig. 5 (A) Mouse tissues staining for total CSA in placenta, pancreas, ovary, lung, liver, kidney, heart, bone, and spleen. Scale bar, 20 μm. (B) Schematic illustration showing the sonication process for MNP synthesis and EDC/NHS conjugation with plCSA-BP. (C) Release profile of MTX in MNPs, SCR-MNPs, and plCSA-MNPs. (D) The size stability of nanoparticles in serum over 5 weeks. (E) Transmission electron microscope images of MNPs, SCR-MNPs, and plCSA-MNPs with scale bars of 100 nm. (F) Immunofluorescence staining images of the placenta 48 hours after intravenous injection of SCR-INPs or plCSA-INPs. Scale bar, 50 μm. (G) IVIS imaging shown in vivo delivery of plCSA-MNPs to uteri (n = 5). Reproduced with permission from ref. 81.Copyright 2018 Ivyspring International Publisher. | ||
The utilization of peptides for targeting the placenta in therapeutic applications offers several notable advantages, including high specificity and affinity for placental biomarkers,82 which can enhance the precision of drug delivery and minimize off-target effects.83,84 This targeted approach can lead to increased therapeutic efficacy while reducing systemic side effects, thereby improving overall patient safety. Additionally, peptides can be engineered to facilitate the efficient transport of therapeutic agents across biological barriers, potentially overcoming some of the limitations associated with conventional drug delivery systems.22,85,86 However, there are significant limitations and challenges that must be addressed to realize the full potential of peptide-based placental therapies. One major limitation is the inherent instability of peptides in vivo, as they are prone to rapid degradation by proteases, which can diminish their effectiveness and necessitate frequent dosing or the use of protective delivery systems. Furthermore, the potential immunogenicity of peptide therapeutics poses a risk of adverse immune responses, which could compromise treatment safety and efficacy.87 Another challenge lies in the identification and optimization of peptides with high specificity and binding affinity for placental targets, as off-target interactions could lead to unintended effects on other tissues.88,89 Additionally, the complexity of the placental environment and the dynamic changes that occur during pregnancy make it difficult to predict and control peptide behavior in vivo. Scaling up peptide synthesis for clinical applications also presents logistical and economic challenges, potentially limiting the widespread adoption of such therapies.90 Finally, regulatory hurdles and the need for extensive safety evaluations, particularly in the context of pregnancy, add further layers of complexity to the development and implementation of peptide-targeted placental therapies.22,80 Addressing these limitations and challenges will require multidisciplinary efforts to enhance peptide stability, specificity, and delivery mechanisms, as well as rigorous preclinical and clinical testing to ensure the safety and efficacy of these innovative therapeutic strategies.
By engineering peptides to bind selectively to trophoblast cells, researchers aim to increase both the precision and safety of therapeutic interventions for pregnancy-related complications.61 Notable findings include enhanced drug loading efficiency, improved binding affinity, and reduced systemic toxicity when peptides are conjugated to nanocarriers such as liposomes and polymeric nanoparticles.61,91 These advances have broadened the scope of potential treatments for conditions like preeclampsia and fetal growth restriction, underscoring the value of peptide-based approaches in addressing unmet clinical needs. However, the successful translation of this technology from the laboratory to the clinic faces substantial obstacles. Immunogenicity, peptide stability under physiological conditions, and variability in receptor expression across different gestational stages and patient populations remain key challenges.92 Additionally, the regulatory landscape demands rigorous safety and efficacy evaluations before these peptide-based strategies can be adopted in clinical practice. Despite these hurdles, ongoing efforts to refine peptide design and conjugation methods, alongside an increasing understanding of placental biology, promise to move peptide-targeted placental delivery closer to clinical reality.
4. Antibody-mediated placenta target
Recent studies have highlighted several approaches to enhance the targeted delivery of antibodies or antibody-based molecules to the placenta. One of the most promising strategies involves the use of placenta-specific antigens or receptors to guide antibodies to particular cell types within the placenta.93 For example, the trophoblast cells in the outer layer of the placenta express specific receptors, such as Flt-1 and the syncytiotrophoblast cell-specific receptor, which can be targeted by antibodies or antibody–drug conjugates.94,95 These antibodies, once bound to their targets on the placenta, can mediate the localized delivery of therapeutic agents or even induce therapeutic effects through receptor-mediated endocytosis.One of the key advancements in the field involves the use of monoclonal antibodies (mAbs) and antibody–drug conjugates, which are designed to selectively target the placenta by recognizing specific placental proteins.96 mAbs was used to deliver cytotoxic drugs directly to the placenta, effectively bypassing systemic circulation and minimizing off-target effects.97 These conjugates were shown to bind selectively to trophoblast cells and enhance the localized delivery of therapeutic agents. Further research has focused on the use of antibodies to deliver therapeutic proteins or to block harmful signaling pathways within the placenta.
Hickey et al. investigated how antiphospholipid antibodies (aPL) interact with placental mitochondria to promote oxidative stress and potentially lead to placental dysfunction.98 The researchers used two monoclonal aPL, ID2 and IIC5, as models of pathogenic aPL to assess their effects on first trimester placental explants (Fig. 6A–C). Both ID2 and IIC5 had a higher binding affinity to isolated mitochondria compared to the isotype-matched control antibody. Additionally, the binding of these aPL to placental mitochondria was associated with respiration rate, suggesting that the antibodies specifically interacted with the placental mitochondria, likely through the binding of β2 glycoprotein I (β2GPI) on the mitochondrial membranes (Fig. 6D and E). The study also highlighted that both ID2 and IIC5 are triple-positive aPL, with different epitopes on β2 glycoprotein I, and that ID2 was more potent in affecting mitochondrial function (Fig. 6F).
 | ||
| Fig. 6 (A–C) The IgG, ID2, and IIC5 antibodies specifically bind to placental mitochondria. (D) Comparison of the binding affinities of the three antibodies to mitochondria (white: control IgG, black: ID2, grey: IIC5). (E) Western blot analysis confirmed the presence of the antiphospholipid antibody (aPL) antigen, anti-β2 glycoprotein I (β2GPI), in the mitochondria. (F) Effect of ID2 or IgG antibodies on placental mitochondrial respiration after the addition of different substrates or inhibitors. Reproduced with permission from ref. 98. Copyright 2020 Elsevier. | ||
The study provides important insights into how antiphospholipid antibodies, particularly ID2 and IIC5, target the placental mitochondria and induce reactive oxygen species production, which contributes to oxidative stress and placental dysfunction. However, there are several aspects of the study that could benefit from further investigation and clarification. First, while the research demonstrates that aPL interact with trophoblast mitochondria and increase ROS production, the specific mechanisms by which aPL enter trophoblast cells and bind to mitochondria remain unclear. Although these compounds have shown promise in preclinical models, their use in treating pregnant women with antiphospholipid syndrome requires careful investigation.
Despite these advances, several challenges remain in the development of antibody-based therapies for placental targeting.99 One of the primary obstacles is the difficulty in achieving specific and efficient delivery to the placenta due to the complex nature of placental barriers and the presence of high maternal blood flow.100 Furthermore, while certain antibodies can selectively bind to placental cells, they must also be able to cross the placental barrier to reach their targets.83 This requires careful design of the antibody or conjugate to ensure that it is not only highly specific but also capable of being transported across the placental layers.
The field of antibody targeting to the placenta has seen significant advancements in recent years, driven by the identification of the placenta as a unique and promising therapeutic target due to its distinctive immunological properties and roles in pregnancy-associated diseases.101,102 Researchers have focused on engineering antibodies that can specifically bind to placental antigens, thereby enabling targeted delivery of therapeutic agents to the placenta while minimizing systemic toxicity. Recent studies have explored the use of placenta-specific antibodies to treat conditions such as preeclampsia, placental insufficiency, and placental tumor metastasis.103
Among the most recent innovations, researchers have developed fully humanized monoclonal antibodies, bispecific antibodies, and antibody–drug conjugates optimized for placental targeting. For instance, antibodies targeting placental-specific receptors, such as the chemokine receptors CCR1/CCR2 or the placental growth factor, have shown promise in preclinical models of preeclampsia and placental dysfunction.104,105 Additionally, anti-angiogenic monoclonal antibodies, such as those targeting VEGF, have been investigated for their potential to modulate placental angiogenesis in pathological conditions.55 These advancements have contributed to a deeper understanding of placental biology and have opened new avenues for therapeutic intervention. Recent research findings highlight the potential of placenta-targeted antibodies to improve therapeutic outcomes while reducing systemic side effects. For example, studies using anti-malignant fetal and neonatal collider antibodies have demonstrated the feasibility of targeting placental trophoblasts in cancer metastasis models.106 Furthermore, advancements in antibody engineering, such as the use of placenta-specific antibody fragments or nanobody-based targeting systems, have enhanced the precision and efficacy of placental delivery.107
Despite these promising developments, challenges remain in translating placenta-targeted antibody therapies into clinical practice. Key hurdles include optimizing antibody delivery across the placental barrier, minimizing immune responses, and ensuring fetal safety.108 Additionally, the dynamic and heterogeneous nature of the placenta necessitates a deeper understanding of its temporal and spatial antigen expression patterns. To address these challenges, researchers are exploring novel strategies, such as combination therapies, placenta-specific drug delivery systems, and adaptive immune response modulation.96,109
In summary, antibody-based therapies show great promise for targeting placental dysfunction. The biggest advantage of antibody-based targeting compared to peptide or LNP-based targeting lies in its specificity and versatility.42 Antibodies can be engineered to precisely recognize and bind to specific antigens on the surface of target cells, such as those in the placenta, allowing for a high degree of selectivity. This specificity reduces off-target effects and minimizes damage to non-target tissues, potentially improving the safety profile of the treatment.81 Furthermore, antibodies can be easily conjugated with therapeutic agents, such as small molecules or imaging agents, enhancing their therapeutic efficacy. In contrast, while peptides and LNPs are also effective for targeted delivery, they may have limitations in terms of targeting precision, stability, and immune evasion. Peptides, for example, might be more prone to degradation or immune recognition, whereas LNPs require careful formulation to achieve optimal delivery and release of the payload.84 Antibodies, with their well-characterized structure and ability to bind multiple targets simultaneously, can offer more controlled and adaptable targeting strategies.
5. Viral vectors as delivery tools for placenta delivery
Viral vectors have emerged as effective delivery tools for targeting drugs or genes to the placenta, exhibiting unique characteristics and advantages in this application. Their innate ability to infect cells allows for efficient delivery of exogenous genes or therapeutic agents directly into placental cells.110 Common viral vectors used include adenoviruses, adeno-associated viruses (AAV), retroviruses, and lentiviruses, each offering distinct properties suitable for specific research objectives.111,112A significant feature of viral vectors is their modifiability through genetic engineering. This enables alterations to capsid proteins or viral genomes to enhance specificity toward placental tissue or to reduce immunogenicity.113 Additionally, viral vectors can be designed for controlled gene expression—either transient or sustained—to meet various therapeutic time frames.114,115
The authors infected explants from mid- and late-gestation placentas, as well as early pregnancy chorionic villi, with the Nicaraguan ZIKV strain. ZIKV infected primary placental cells, including trophoblasts, endothelial cells, fibroblasts, Hofbauer cells, amniotic epithelial cells, and trophoblast progenitor cells in the amniochorionic membranes that express viral entry cofactors Axl, Tyro3, and TIM1.116 Compared to late-gestation placentas, mid-gestation amniotic epithelial cells exhibited higher viral titers and induced NS3 and E protein production. Duramycin, a peptide that blocks TIM1 binding, effectively reduced ZIKV infection in placental cells and explants, suggesting that targeting TIM1 may inhibit infection at the uteroplacental interface. Fig. 7A shows that first-trimester chorionic villus explants infected with MR766 or Nica1-16 strains exhibited infected proliferative trophoblasts expressing E and NS3 proteins, suggesting cell-to-cell viral spread. Additionally, second-trimester amniotic epithelial cells infected with MR766 or clinical isolates exhibited 6- to 8-fold higher viral titers at 7 days post-infection, with a 4-fold increase in viral release (Fig. 7B and C). To further explore the transmission pathways of ZIKV, Fig. 7D shows Axl expression in invasive trophoblasts, Hofbauer cells, and amniotic epithelial cells in mid-gestation decidua, placenta, and fetal membranes. Fig. 7E then summarizes the ZIKV transmission pathways, providing further insight into how the virus spreads from the uteroplacental interface to different placental regions and the amniochorionic membranes.
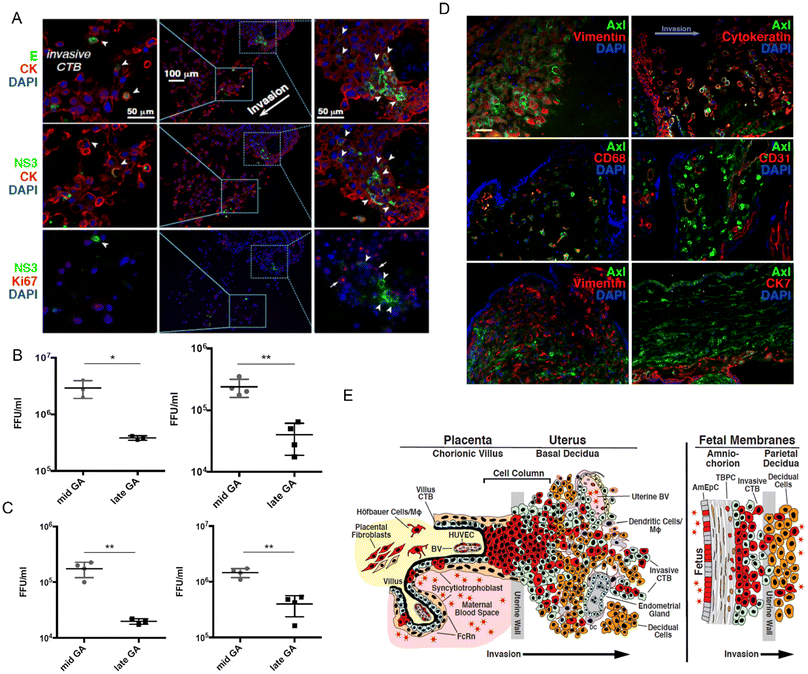 | ||
| Fig. 7 (A) Immunofluorescence images of chorionic villus explants infected with ZIKV MR766 (1 × 106 PFU) and immunostained for envelope protein and cytokeratin. (B and C) Examination of viral titers in ZIKV-infected amniotic epithelial cells during mid- and late gestation. (D) Immunofluorescence analysis of Axl expression at the uterine–placental interface. (E) ZIKV infects diverse cell types, indicating multiple potential transmission pathways. Reproduced with permission from ref. 116. Copyright 2016 Elsevier. | ||
The advantages of using viral vectors for placental targeting are multifaceted. They demonstrate higher transfection efficiencies both in vitro and in vivo compared to non-viral carriers like liposomes or polymeric nanoparticles.115 By engineering viral vectors to target specific cell types within the placenta, off-target effects on non-target tissues can be minimized. Some vectors, such as lentiviruses, have the capability to integrate genes into the host genome, providing long-term gene expression beneficial for sustained therapeutic outcomes.117 Moreover, certain viral vectors like adenoviruses possess large gene-carrying capacities, allowing for the delivery of sizable genes or multiple gene combinations.118
Current research on viral vectors for placental targeting is primarily at the foundational stage, involving cell culture and animal models.119 For example, adenoviral vectors delivering the VEGF-A165 gene in sheep models have shown potential in ameliorating FGR.120 Safety assessments are a critical focus, addressing concerns about the immunogenicity and potential toxicity of viral vectors.121,122 Efforts are underway to develop vectors with reduced immunogenicity and to evaluate their safety profiles in both maternal and fetal contexts.
Enhancements in targeting specificity are being pursued by displaying placenta-specific ligands on viral surfaces or utilizing placenta-specific promoters, thereby increasing the precision of delivery to placental tissues.123 Some viral vectors have progressed to testing in large animal models, laying important groundwork for future human clinical trials. Despite these advancements, several technical challenges persist.66,124 These include avoiding adverse effects on the mother and fetus, controlling the temporal and spatial specificity of gene expression, and navigating potential ethical and regulatory issues associated with gene therapy.
6. Conclusion and discussion
The placenta is a highly specialized, transient organ that serves as the critical interface between the mother and the developing fetus during pregnancy.125,126 It facilitates the exchange of gases, nutrients, and waste products, and plays a pivotal role in hormonal regulation to maintain gestation. The placenta is characterized by its complex structure, comprising fetal chorionic villi that invade the maternal uterine tissue, creating an extensive surface area for efficient exchange while maintaining a selective barrier between maternal and fetal circulations.127,128Targeting the placenta is exceedingly difficult due to several intrinsic and extrinsic factors. Firstly, the placental barrier is designed to be selectively permeable, allowing only specific molecules to pass while restricting others, thereby protecting the fetus from potentially harmful substances.69,129,130 This selective permeability poses a significant challenge for the delivery of therapeutic agents, as many drugs are unable to cross this barrier effectively. Secondly, the placenta exhibits dynamic changes throughout gestation, both structurally and functionally, which complicates the development of universal targeting strategies applicable at all stages of pregnancy.9,30,131 Additionally, the placenta expresses a variety of efflux transporters and metabolic enzymes that actively expel or degrade xenobiotics, including therapeutic compounds, reducing their efficacy. The unique immunological environment of the placenta, which modulates maternal immune responses to tolerate the semi-allogeneic fetus, can also influence the distribution and activity of targeted agents.132 Moreover, the risk of unintended effects on fetal development and maternal health necessitates stringent safety considerations, limiting the types and dosages of agents that can be employed.133 Ethical constraints further restrict experimental interventions during pregnancy, hindering clinical research and the advancement of placenta-targeted therapies.
This review details various methods currently researched for targeting the placenta, including LNP targeting, targeted peptide modifications, specific antibody targeting of placental receptors, and viral vectors. These approaches represent significant advancements in overcoming the challenges associated with delivering therapeutic agents to the placenta, a complex and selectively permeable organ essential for fetal development.18,134
LNPs have gained significant attention as drug delivery systems due to their ability to encapsulate a wide range of therapeutic agents, including nucleic acids, proteins, and small molecules, while offering advantages such as biocompatibility, low immunogenicity, and controlled release profiles.37,71 By modifying the surface of LNPs with placental-targeting ligands, researchers have enhanced their specificity and uptake by placental cells.56 Mechanisms of LNP targeting to the placenta exploiting the natural properties of lipid bilayers and their ability to fuse with cell membranes. For placental targeting, LNPs can be modified with surface ligands that specifically recognize placental receptors, improving cellular uptake and enhancing the specificity of delivery to placental tissues. (1) Receptor-mediated targeting: LNPs can be functionalized with peptides, antibodies, or small molecules that target specific placental receptors such as the transferrin receptor, low-density lipoprotein receptor, or folate receptors.135 These receptors are involved in nutrient and ligand transport across the placenta, providing a mechanism for targeted delivery. (2) Surface modification with PEG or polymers: the surface of LNPs can be coated with polyethylene glycol or other biocompatible polymers to increase circulation time, improve stability, and reduce non-specific interactions with other tissues, enhancing the overall targeting efficiency.136,137 (3) Targeting the trophoblast layer: the trophoblast cells, particularly CTBs and STBs, are key components of the placental barrier and present valuable targets for LNP-mediated drug delivery.138 These cells are accessible through the bloodstream and highly involved in nutrient and immune regulation.
Translating placenta-targeted lipid nanoparticles from the laboratory to clinical applications faces several challenges, including the complexity of the placental barrier, which limits effective delivery. Variability in receptor expression across different pregnancy stages and pathological conditions complicates receptor-targeting strategies.139 Additionally, optimizing the stability, size, and pharmacokinetics of LNPs is crucial to ensure efficient delivery and controlled release of therapeutic agents, while minimizing immune responses.140 Despite these challenges, the potential of LNPs for treating placental disorders is promising. Advances in nanotechnology and receptor targeting offer the possibility of more effective, precise, and safe treatments, with the potential for clinical trials to further assess their efficacy in improving maternal-fetal health.141
Targeted peptide modifications involve designing peptides that specifically bind to receptors or antigens expressed on placental cells.42,83 Current research focuses on identifying peptides that can selectively bind to placental receptors, facilitating the targeted delivery of drugs to placental tissues.96,142 Notable receptors that have been targeted include integrins, folate receptors, and angiogenesis-related receptors such as VEGF.35,121 These receptors are either overexpressed or highly specific to the trophoblast layer, endothelial cells, or other placental structures, making them ideal targets for peptide-mediated delivery systems. Furthermore, peptides are often conjugated to nanoparticles or liposomes to enhance their stability, bioavailability, and efficiency in drug delivery.61,78
Despite the promising potential of peptide-based targeting, several challenges need to be addressed. The complexity of the placental barrier, composed of multiple cellular layers and varying receptor expressions across different pregnancy stages, limits the efficiency of peptide-based drug delivery.61,67 For example, while some peptides may target trophoblasts efficiently, crossing the syncytiotrophoblast layer-an important barrier for maternal-fetal exchange-remains a significant hurdle.143 Additionally, peptides can be susceptible to rapid degradation in vivo, reducing their half-life and limiting their therapeutic potential.67 To improve their clinical efficacy, strategies such as peptide cyclization, incorporation of non-natural amino acids, and the development of protease-resistant peptides are being explored to enhance stability and prolong their circulation time.
There is a lack of comprehensive understanding of receptor dynamics throughout pregnancy and in pathological conditions such as preeclampsia or intrauterine growth restriction.144 The expression of placental receptors may vary across different stages of pregnancy or under various disease conditions, which can complicate the development of universally effective peptide-targeted therapies.95 Therefore, further investigation is needed to map receptor expression patterns at different gestational stages and in distinct placental regions, which could guide the design of more targeted and personalized therapeutic approaches.
Specific antibodies directed against placental receptors offer another avenue for targeted therapy.145 Monoclonal antibodies or antibody fragments can be engineered to recognize and bind to placental antigens with high specificity and affinity. This method holds potential for delivering therapeutics directly to placental tissue, reducing systemic exposure and associated side effects.
Translating placenta-targeted antibodies from the laboratory to the clinic involves a complex interplay of challenges and prospects. The challenges include validation in animal models, which may not always predict human outcomes accurately.81,146 Immunogenicity is another concern, as antibodies from other species might evoke adverse immune responses in humans. Manufacturing these antibodies at a large scale while maintaining consistent quality is technically demanding. Regulatory approval poses significant hurdles due to strict requirements set by FDA. Functional assays are essential to demonstrate the antibodies’ efficacy. Additionally, the unpredictable placental transport can affect their efficacy, as antibodies may be metabolized before reaching their target.147 The diversity of placental antigens among different patients further complicates efficacy and safety profiles.
On the other hand, personalized medicine approaches could enhance the effectiveness of these antibodies by tailoring them to individual patients.148 Synergistic combinations with other therapies, such as cancer treatments or placenta-targeted drugs, could amplify their therapeutic effects. Technological advancements in antibody engineering, including improved stability and bioavailability, could overcome current limitations.149 Enhanced in vitro and in vivo models for placental function could accelerate development processes.150 Ultimately, successful translation could revolutionize the treatment of placental pathologies, improving maternal and fetal health, and serve as a platform for delivering other therapeutic agents.
Viral vectors have been explored for their innate ability to infect cells and deliver genetic material efficiently.113 By engineering viral vectors to display placental-specific ligands or using placental-specific promoters, their specificity for placental cells can be enhanced while reducing immunogenicity.119,151 This approach has shown potential in preclinical models for gene therapy applications aimed at treating placental insufficiencies.
Clinical translation of placental-targeted viral vectors remains in early stages but shows significant potential. Preclinical success includes adenovirus-mediated delivery of miRNA inhibitors (e.g., miR-145) to enhance trophoblast proliferation in fetal growth restriction models. AAV vectors encoding anti-inflammatory cytokines or antioxidant genes have also shown efficacy in mitigating placental oxidative stress in animal studies.151 However, challenges persist, including maternal immune responses to viral capsids, potential fetal exposure, and variability in placental receptor expression across gestational stages. For instance, neutralizing antibodies against common viral serotypes (e.g., Ad5) in mothers may limit repeated dosing.152 To address this, hybrid biomimetic vectors-such as viral particles cloaked in placental cell membranes-are being developed to evade immune detection and enhance tropism.
The future of placental-targeted viral vectors hinges on resolving these barriers through iterative engineering and rigorous safety validation.113,119 Advances in vector design, such as transient expression systems or CRISPR-mediated gene editing, could further enhance precision.151 Clinically, these tools may revolutionize prenatal therapies for genetic disorders, placental dysfunction, and congenital infections, offering interventions that are both localized and minimally invasive.153 However, ethical considerations, stringent regulatory frameworks, and the need for scalable manufacturing processes must be addressed to realize their full translational potential.124
The placenta's selective permeability and dynamic changes throughout gestation complicate the development of universal targeting strategies.8,14,154 Efflux transporters and metabolic enzymes present in the placenta can reduce the efficacy of delivered therapeutics by actively expelling or degrading them.155,156 Additionally, safety concerns are paramount; any therapeutic intervention must avoid unintended effects on fetal development and maternal health. Ethical considerations also limit experimental interventions during pregnancy, posing hurdles for clinical translation.142 Future research should focus on addressing these challenges by improving targeting specificity and delivery efficiency while ensuring safety. Advances in nanotechnology, molecular biology, and a deeper understanding of placental physiology could facilitate the development of more effective targeting strategies.
In conclusion, the development of targeted delivery methods to the placenta holds significant promise for treating placental disorders and improving pregnancy outcomes. While obstacles remain, continued research and innovation in this field are essential. By refining these strategies and addressing safety concerns, it may be possible to develop effective therapies that benefit both mother and fetus, ultimately enhancing neonatal health and long-term developmental outcomes.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We acknowledge support from the General Scientific Research Project of Zhejiang Education Department (Y202353227).References
- E. Maltepe and S. J. Fisher, Placenta: the forgotten organ, Annu. Rev. Cell Dev. Biol., 2015, 31(1), 523–552 CrossRef CAS PubMed.
- A. N. Sferruzzi-Perri and E. J. Camm, The programming power of the placenta, Front. Physiol., 2016, 7, 33 Search PubMed.
- H. Okae, H. Toh, T. Sato, H. Hiura, S. Takahashi and K. Shirane, et al., Derivation of human trophoblast stem cells, Cell Stem Cell, 2018, 22(1), 50–63 CrossRef CAS.
- A. King, L. Thomas and P. Bischof, Cell culture models of trophoblast II: trophoblast cell lines—a workshop report, Placenta, 2000, 21, S113–S1S9 Search PubMed.
- C. H. Graham, T. S. Hawley, R. C. Hawley, J. R. MacDougall, R. S. Kerbel and N. Khoo, et al., Establishment and characterization of first trimester human trophoblast cells with extended lifespan, Exp. Cell Res., 1993, 206(2), 204–211 CrossRef CAS PubMed.
- C. W. Redman, A. C. Staff and J. M. Roberts, Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways, Am. J. Obstet. Gynecol., 2022, 226(2), S907–S927 Search PubMed.
- B. Huppertz, IFPA award in placentology lecture: biology of the placental syncytiotrophoblast-myths and facts, Placenta, 2010, 31, S75–S81 Search PubMed.
- E. Jauniaux, D. Jurkovic, A. M. Hussein and G. J. Burton, New insights into the etiopathology of placenta accreta spectrum, Am. J. Obstet. Gynecol., 2022, 227(3), 384–391 CrossRef.
- G. J. Burton and E. Jauniaux, The human placenta: new perspectives on its formation and function during early pregnancy, Proc. R. Soc. B, 2023, 290(1997), 20230191 Search PubMed.
- M. A. Ortega, O. Fraile-Martínez, C. García-Montero, M. A. Sáez, M. A. Álvarez-Mon and D. Torres-Carranza, et al., The pivotal role of the placenta in normal and pathological pregnancies: a focus on preeclampsia, fetal growth restriction, and maternal chronic venous disease, Cells, 2022, 11(3), 568 CrossRef CAS.
- C. P. Sibley, Treating the dysfunctional placenta, J. Endocrinol., 2017, 234(2), R81–R97 CAS.
- E. Y. Hsiao and P. H. Patterson, Activation of the maternal immune system induces endocrine changes in the placenta via IL-6, Brain, Behav., Immun., 2011, 25(4), 604–615 CrossRef CAS PubMed.
- S. E. Ander, M. S. Diamond and C. B. Coyne, Immune responses at the maternal-fetal interface, Sci. Immunol., 2019, 4(31), eaat6114 CrossRef CAS PubMed.
- S. L. Bronson and T. L. Bale, The placenta as a mediator of stress effects on neurodevelopmental reprogramming, Neuropsychopharmacology, 2016, 41(1), 207–218 CrossRef PubMed.
- J. Huynh, D. Dawson, D. Roberts and R. Bentley-Lewis, A systematic review of placental pathology in maternal diabetes mellitus, Placenta, 2015, 36(2), 101–114 CrossRef CAS PubMed.
- S. Yagel, S. M. Cohen and D. Goldman-Wohl, An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array, Am. J. Obstet. Gynecol., 2022, 226(2), S963–S972 Search PubMed.
- J. Ding, A. Maxwell, N. Adzibolosu, A. Hu, Y. You and A. Liao, et al., Mechanisms of immune regulation by the placenta: Role of type I interferon and interferon–stimulated genes signaling during pregnancy, Immunol. Rev., 2022, 308(1), 9–24 CrossRef CAS PubMed.
- R. Hoo, A. Nakimuli and R. Vento-Tormo, Innate immune mechanisms to protect against infection at the human decidual-placental interface, Front. Immunol., 2020, 11, 2070 CrossRef CAS PubMed.
- E. A. Bordt, L. L. Shook, C. Atyeo, K. M. Pullen, R. M. De Guzman and M.-C. Meinsohn, et al., Maternal SARS-CoV-2 infection elicits sexually dimorphic placental immune responses, Sci. Transl. Med., 2021, 13(617), eabi7428 Search PubMed.
- V. X. Han, S. Patel, H. F. Jones and R. C. Dale, Maternal immune activation and neuroinflammation in human neurodevelopmental disorders, Nat. Rev. Neurol., 2021, 17(9), 564–579 CrossRef PubMed.
- J. J. Adibi, Y. Zhao, H. Koistinen, R. T. Mitchell, E. S. Barrett and R. Miller, et al., Molecular pathways in placental-fetal development and disruption, Mol. Cell. Endocrinol., 2024, 581, 112075 CrossRef CAS PubMed.
- J. D. Aplin, J. E. Myers, K. Timms and M. Westwood, Tracking placental development in health and disease, Nat. Rev. Endocrinol., 2020, 16(9), 479–494 CrossRef CAS PubMed.
- G. J. Burton, T. Cindrova-Davies, H. Yung and E. Jauniaux, Hypoxia and reproductive health: Oxygen and development of the human placenta, Reproduction, 2021, 161(1), F53–F65 CAS.
- E. Obeagu and G. Obeagu, Maternal Hypoxia and Placental Dysfunction: Insights from Molecular Biology, Elite J. Health Sci., 2024, 2(8), 58–69 Search PubMed.
- P. A. Jazwiec, V. S. Patterson, T. A. Ribeiro, E. Yeo, K. M. Kennedy and P. C. Mathias, et al., Paternal obesity induces placental hypoxia and sex-specific impairments in placental vascularization and offspring metabolism, Biol. Reprod., 2022, 107(2), 574–589 CrossRef PubMed.
- M. A. Ortega, O. Fraile-Martínez, C. García-Montero, M. A. Sáez, M. A. Álvarez-Mon and D. Torres-Carranza, et al., The pivotal role of the placenta in normal and pathological pregnancies: a focus on preeclampsia, fetal growth restriction, and maternal chronic venous disease, Cells, 2022, 11(3), 568 CrossRef CAS PubMed.
- T. M. H. Nguyen, S. G. Shin, H. W. Choi and C. W. Bark, Recent advances in self–powered and flexible UVC photodetectors, Exploration, 2022, 2(5), 20210078 CrossRef CAS.
- T. Cindrova-Davies and A. N. Sferruzzi-Perri, Human placental development and function, Semin. Cell Dev. Biol., 2022, 131, 66–77 CrossRef CAS PubMed.
- X. Pan, X. Jin, J. Wang, Q. Hu and B. Dai, Placenta inflammation is closely associated with gestational diabetes mellitus, Am. J. Transl. Res., 2021, 13(5), 4068 CAS.
- J. T. Bangma, H. Hartwell, H. P. Santos Jr, T. M. O'Shea and R. C. Fry, Placental programming, perinatal inflammation, and neurodevelopment impairment among those born extremely preterm, Pediatr. Res., 2021, 89(2), 326–335 CrossRef PubMed.
- E. Musa, E. Salazar-Petres, A. Arowolo, N. Levitt, M. Matjila and A. N. Sferruzzi-Perri, Obesity and gestational diabetes independently and collectively induce specific effects on placental structure, inflammation and endocrine function in a cohort of South African women, J. Physiol., 2023, 601(7), 1287–1306 CrossRef CAS.
- K. Melchiorre, V. Giorgione and B. Thilaganathan, The placenta and preeclampsia: villain or victim?, Am. J. Obstet. Gynecol., 2022, 226(2), S954–S962 Search PubMed.
- M. Cruz-Lemini, J. C. Vázquez, J. Ullmo and E. J. Llurba, Low-molecular-weight heparin for prevention of preeclampsia and other placenta-mediated complications: a systematic review and meta-analysis, Am. J. Obstet. Gynecol., 2022, 226(2), S1126–S1144 CrossRef CAS PubMed.
- H. E. Yong, S.-Y. Chan, A. Chakraborty, G. Rajaraman, S. Ricardo and M. Benharouga, et al., Significance of the placental barrier in antenatal viral infections, Biochim. Biophys. Acta, Mol. Basis Dis., 2021, 1867(12), 166244 Search PubMed.
- E. Ganguly, N. Hula, F. Spaans, C.-L. M. Cooke and S. T. Davidge, Placenta-targeted treatment strategies: An opportunity to impact fetal development and improve offspring health later in life, Pharmacol. Res., 2020, 157, 104836 CrossRef CAS.
- A.-E. Kreuder, A. Bolaños-Rosales, C. Palmer, A. Thomas, M.-A. Geiger and T. Lam, et al., Inspired by the human placenta: a novel 3D bioprinted membrane system to create barrier models, Sci. Rep., 2020, 10(1), 15606 CrossRef CAS.
- H. C. Safford, K. L. Swingle, H. C. Geisler, A. G. Hamilton, A. S. Thatte and A. A. Ghalsasi, et al., Orthogonal design of experiments for engineering of lipid nanoparticles for mRNA delivery to the placenta, Small, 2024, 20(41), 2303568 CAS.
- L. Fliedel, K. Alhareth, J. Seguin, M. El-Khashab, A. Chissey and N. Mignet, et al., Influence of Liposomes’ and Lipoplexes’ Physicochemical Characteristics on Their Uptake Rate and Mechanisms by the Placenta, Int. J. Mol. Sci., 2022, 23(11), 6299 CrossRef CAS PubMed.
- J. Zhao, J. Zhang, Y. Xu, J. Dong, Q. Dong and G. Zhao, et al., Nanotechnological approaches for the treatment of placental dysfunction: recent trends and future perspectives, Nanomedicine, 2023, 18(26), 1961–1978 Search PubMed.
- X. Hou, T. Zaks, R. Langer and Y. Dong, Lipid nanoparticles for mRNA delivery, Nat. Rev. Mater., 2021, 6(12), 1078–1094 CrossRef CAS.
- L. Xu, X. Wang, Y. Liu, G. Yang, R. J. Falconer and C.-X. Zhao, Lipid nanoparticles for drug delivery, Adv. NanoBiomed Res., 2022, 2(2), 2100109 CrossRef CAS.
- J. Pardeike, A. Hommoss and R. H. Müller, Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products, Int. J. Pharm., 2009, 366(1–2), 170–184 Search PubMed.
- H. Peng, F. Yao, J. Zhao, W. Zhang, L. Chen and X. Wang, et al., Unraveling mitochondria–targeting reactive oxygen species modulation and their implementations in cancer therapy by nanomaterials, Exploration, 2023, 3(2), 20220115 CrossRef CAS PubMed.
- E. Souto and R. Müller, Cosmetic features and applications of lipid nanoparticles (SLN®, NLC®), Int. J. Cosmet. Sci., 2008, 30(3), 157–165 CrossRef CAS.
- B. Wilson and K. M. Geetha, Lipid nanoparticles in the development of mRNA vaccines for COVID-19, J. Drug Delivery Sci. Technol., 2022, 74, 103553 CrossRef CAS.
- T. Liu, Y. Tian, A. Zheng and C. Cui, Design strategies for and stability of mRNA–lipid nanoparticle COVID-19 vaccines, Polymers, 2022, 14(19), 4195 CrossRef CAS PubMed.
- R. E. Young, K. M. Nelson, S. I. Hofbauer, T. Vijayakumar, M.-G. Alameh, D. Weissman, et al., Lipid nanoparticle composition drives mRNA delivery to the placenta, bioRxiv, 2022, preprint, DOI:10.1101/2022.12.22.521490.
- K. L. Swingle, H. C. Safford, H. C. Geisler, A. G. Hamilton, A. S. Thatte and M. M. Billingsley, et al., Ionizable lipid nanoparticles for in vivo mRNA delivery to the placenta during pregnancy, J. Am. Chem. Soc., 2023, 145(8), 4691–4706 CrossRef CAS PubMed.
- C. H. Albertsen, J. A. Kulkarni, D. Witzigmann, M. Lind, K. Petersson and J. B. Simonsen, The role of lipid components in lipid nanoparticles for vaccines and gene therapy, Adv. Drug Delivery Rev., 2022, 188, 114416 CrossRef.
- H. C. Geisler, A. A. Ghalsasi, H. C. Safford, K. L. Swingle, A. S. Thatte and A. J. Mukalel, et al., EGFR-targeted ionizable lipid nanoparticles enhance in vivo mRNA delivery to the placenta, J. Controlled Release, 2024, 371, 455–469 CrossRef CAS.
- K. L. Swingle, H. C. Safford, H. C. Geisler, A. G. Hamilton, A. S. Thatte and M. M. Billingsley, et al., Ionizable lipid nanoparticles for in vivo mRNA delivery to the placenta during pregnancy, J. Am. Chem. Soc., 2023, 145(8), 4691–4706 CrossRef CAS PubMed.
- A. Abostait, M. Abdelkarim, Z. Bao, Y. Miyake, W. H. Tse and C. Di Ciano-Oliveir, et al., Optimizing lipid nanoparticles for fetal gene delivery in vitro, ex vivo, and aided with machine learning, J. Controlled Release, 2024, 376, 678–700 CrossRef CAS.
- N. Chaudhary, A. N. Newby, M. L. Arral, S. S. Yerneni, S. T. LoPresti and R. Doerfler, et al., Lipid nanoparticle structure and delivery route during pregnancy dictate mRNA potency, immunogenicity, and maternal and fetal outcomes, Proc. Natl. Acad. Sci. U. S. A., 2024, 121(11), e2307810121 CrossRef CAS PubMed.
- Y. Liu, Q. Zhang, X. Gao and T. Wang, Study on lipid nanomicelles targeting placenta for the treatment of preeclampsia, J. Drug Targeting, 2022, 30(8), 894–909 CrossRef CAS PubMed.
- K. L. Swingle, A. G. Hamilton, H. C. Safford, H. C. Geisler, A. S. Thatte and R. Palanki, et al., Placenta-tropic VEGF mRNA lipid nanoparticles ameliorate murine pre-eclampsia, Nature, 2025, 637(8045), 412–421 CrossRef CAS PubMed.
- N. Chaudhary, A. N. Newby, M. L. Arral, S. S. Yerneni, S. T. LoPresti and R. Doerfler, et al., Lipid nanoparticle structure and delivery route during pregnancy dictate mRNA potency, immunogenicity, and maternal and fetal outcomes, Proc. Natl. Acad. Sci. U. S. A., 2024, 121(11), e2307810121 CrossRef CAS PubMed.
- S. H. Kiaie, N. Majidi Zolbanin, A. Ahmadi, R. Bagherifar, H. Valizadeh and F. Kashanchi, et al., Recent advances in mRNA-LNP therapeutics: immunological and pharmacological aspects, J. Nanobiotechnol., 2022, 20(1), 276 Search PubMed.
- Y. Zong, Y. Lin, T. Wei and Q. Cheng, Lipid nanoparticle (LNP) enables mRNA delivery for cancer therapy, Adv. Mater., 2023, 35(51), 2303261 Search PubMed.
- M. Maugeri, M. Nawaz, A. Papadimitriou, A. Angerfors, A. Camponeschi and M. Na, et al., Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells, Nat. Commun., 2019, 10(1), 4333 CrossRef CAS.
- E. Álvarez-Benedicto, L. Farbiak, M. M. Ramírez, X. Wang, L. T. Johnson and O. Mian, et al., Optimization of phospholipid chemistry for improved lipid nanoparticle (LNP) delivery of messenger RNA (mRNA), Biomater. Sci., 2022, 10(2), 549–559 RSC.
- G. A. Chandorkar, C. Ampasavate, J. F. Stobaugh and K. L. Audus, Peptide transport and metabolism across the placenta, Adv. Drug Delivery Rev., 1999, 38(1), 59–67 CrossRef CAS.
- D. E. Paparini, R. H. Choudhury, D. M. Vota, M. Karolczak-Bayatti, S. Finn-Sell and E. N. Grasso, et al., Vasoactive intestinal peptide shapes first–trimester placenta trophoblast, vascular, and immune cell cooperation, Br. J. Pharmacol., 2019, 176(7), 964–980 CrossRef CAS PubMed.
- J. Huang, Z. Ling, H. Zhong, Y. Yin, Y. Qian and M. Gao, et al., Distinct expression profiles of peptides in placentae from preeclampsia and normal pregnancies, Sci. Rep., 2020, 10(1), 17558 CrossRef CAS PubMed.
- L. Jiang, Y. Zhu, L. Wu, C. Wang, N. Yang and Y. Xu, et al., Comparative peptidomics analysis of preeclamptic placenta and the identification of a novel bioactive SERPINA1 C-terminal peptide, Reprod. Biol., 2024, 24(1), 100858 CrossRef CAS PubMed.
- M. Dahlbäck, L. M. Jørgensen, M. A. Nielsen, T. M. Clausen, S. B. Ditlev and M. Resende, et al., The chondroitin sulfate A-binding site of the VAR2CSA protein involves multiple N-terminal domains, J. Biol. Chem., 2011, 286(18), 15908–15917 CrossRef.
- K. Katayama, R. Furuki, H. Yokoyama, M. Kaneko, M. Tachibana and I. Yoshida, et al., Enhanced in vivo gene transfer into the placenta using RGD fiber-mutant adenovirus vector, Biomaterials, 2011, 32(17), 4185–4193 CrossRef CAS.
- A. King, C. Ndifon, S. Lui, K. Widdows, V. R. Kotamraju and L. Agemy, et al., Tumor-homing peptides as tools for targeted delivery of payloads to the placenta, Sci. Adv., 2016, 2(5), e1600349 CrossRef PubMed.
- Q. Hu, X. Gao, T. Kang, X. Feng, D. Jiang and Y. Tu, et al., CGKRK-modified nanoparticles for dual-targeting drug delivery to tumor cells and angiogenic blood vessels, Biomaterials, 2013, 34(37), 9496–9508 CrossRef CAS.
- R. I. Neuman, M. A. van der Meer, L. Saleh, S. van den Berg, A. van den Meiracker and A. Danser, et al., Copeptin and mid–regional pro–atrial natriuretic peptide in women with suspected or confirmed pre–eclampsia: comparison with sFlt–1/PlGF ratio, Ultrasound Obstet. Gynecol., 2020, 56(6), 872–878 CrossRef CAS PubMed.
- D. C. Hoffmann, S. Willenborg, M. Koch, D. Zwolanek, S. Müller and A.-K. A. Becker, et al., Proteolytic processing regulates placental growth factor activities, J. Biol. Chem., 2013, 288(25), 17976–17989 Search PubMed.
- O. Weitzner, C. Seraya-Bareket, T. Biron-Shental, A. Fishamn, Y. Yagur and K. Tzadikevitch-Geffen, et al., Enhanced expression of αVβ3 integrin in villus and extravillous trophoblasts of placenta accreta, Arch. Gynecol. Obstet., 2021, 303, 1175–1183 Search PubMed.
- S. Handwerger and M. Freemark, The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development, J. Pediatr. Endocrinol. Metab., 2000, 13(4), 343–356 CAS.
- A. Gertler and J. Djiane, Mechanism of ruminant placental lactogen action: molecular and in vivo studies, Mol. Genet. Metab., 2002, 75(3), 189–201 CrossRef CAS.
- R. M. Roberts, T. Ezashi, L. C. Schulz, J. Sugimoto, D. J. Schust and T. Khan, et al., Syncytins expressed in human placental trophoblast, Placenta, 2021, 113, 8–14 CrossRef CAS PubMed.
- J. Tolosa, J. Schjenken, V. Clifton, A. Vargas, B. Barbeau and P. Lowry, et al., The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes, Placenta, 2012, 33(11), 933–941 CrossRef CAS PubMed.
- Q. Huang, J. Li, F. Wang, M. T. Oliver, T. Tipton and Y. Gao, et al., Syncytin-1 modulates placental trophoblast cell proliferation by promoting G1/S transition, Cell. Signalling, 2013, 25(4), 1027–1035 CrossRef CAS PubMed.
- F. Curnis, G. Arrigoni, A. Sacchi, L. Fischetti, W. Arap and R. Pasqualini, et al., Differential binding of drugs containing the NGR motif to CD13 isoforms in tumor vessels, epithelia, and myeloid cells, Cancer Res., 2002, 62(3), 867–874 CAS.
- S. Majumdar and T. J. Siahaan, Peptide–mediated targeted drug delivery, Med. Res. Rev., 2012, 32(3), 637–658 Search PubMed.
- U. Shinde, A. Rao, V. Bansal, D. K. Das, N. H. Balasinor and T. Madan, Isolation of serum-derived placental/amniochorionic extracellular vesicles across pregnancy by immunoaffinity using PLAP and HLA-G, Reproduction, 2024, 167(4), e230215 CAS.
- N. Arumugasaamy, K. D. Rock, C.-Y. Kuo, T. L. Bale and J. P. Fisher, Microphysiological systems of the placental barrier, Adv. Drug Delivery Rev., 2020, 161, 161–175 CrossRef PubMed.
- B. Zhang, L. Tan, Y. Yu, B. Wang, Z. Chen and J. Han, et al., Placenta-specific drug delivery by trophoblast-targeted nanoparticles in mice, Theranostics, 2018, 8(10), 2765 Search PubMed.
- S. A. Sundararaman and A. R. O. John, Prevention of malaria in pregnancy: the threat of sulfadoxine-pyrimethamine resistance, Front. Pediatr., 2022, 10, 966402 Search PubMed.
- B. Zhang, R. Liang, M. Zheng, L. Cai and X. Fan, Surface-functionalized nanoparticles as efficient tools in targeted therapy of pregnancy complications, Int. J. Mol. Sci., 2019, 20(15), 3642 CrossRef CAS PubMed.
- J.-F. Yao, H. Yang, Y.-Z. Zhao and M. Xue, Metabolism of peptide drugs and strategies to improve their metabolic stability, Curr. Drug Metab., 2018, 19(11), 892–901 CrossRef CAS PubMed.
- N. Cureton, I. Korotkova, B. Baker, S. Greenwood, M. Wareing and V. R. Kotamraju, et al., Selective targeting of a novel vasodilator to the uterine vasculature to treat impaired uteroplacental perfusion in pregnancy, Theranostics, 2017, 7(15), 3715 CrossRef CAS PubMed.
- O. Anoshchenko, B. Prasad, N. K. Neradugomma, J. Wang, Q. Mao and J. D. Unadkat, et al., Gestational age–dependent abundance of human placental transporters as determined by quantitative targeted proteomics, Drug Metab. Dispos., 2020, 48(9), 735–741 CrossRef PubMed.
- Y. Yu, Y.-Q. Deng, P. Zou, Q. Wang, Y. Dai and F. Yu, et al., A peptide-based viral inactivator inhibits Zika virus infection in pregnant mice and fetuses, Nat. Commun., 2017, 8(1), 15672 CrossRef CAS PubMed.
- C. Caldwell Jr, C. E. Johnson, V. Balaji, G. A. Balaji, R. D. Hammer and R. Kannan, Identification and validation of a PD-L1 binding peptide for determination of PDL1 expression in tumors, Sci. Rep., 2017, 7(1), 13682 CrossRef PubMed.
- M. Inagaki, M. J. C. Tachikawa and P. Bulletin, Transport characteristics of placenta-derived extracellular vesicles and their relevance to placenta-to-maternal tissue communication, Chem. Pharm. Bull., 2022, 70(5), 324–329 CrossRef CAS PubMed.
- D. Xhindoli, S. Pacor, M. Benincasa, M. Scocchi, R. Gennaro and A. Tossi, The human cathelicidin LL-37—A pore-forming antibacterial peptide and host-cell modulator, Biochim. Biophys. Acta, Biomembr., 2016, 1858(3), 546–566 CrossRef PubMed.
- Y. Zheng, Q. Gu and X. Xu, Inhibition of ocular neovascularization by a novel peptide derived from human placenta growth factor–1, Acta Ophthalmol., 2012, 90(7), e512–e523 Search PubMed.
- F. Beards, L. E. Jones, J. Charnock, K. Forbes and L. K. Harris, Placental homing peptide-microRNA inhibitor conjugates for targeted enhancement of intrinsic placental growth signaling, Theranostics, 2017, 7(11), 2940 CrossRef PubMed.
- M. Tong, C. Viall and L. Chamley, Antiphospholipid antibodies and the placenta: a systematic review of their in vitro effects and modulation by treatment, Hum. Reprod. Update, 2015, 21(1), 97–118 CrossRef PubMed.
- T. Van Bergen, I. Etienne, F. Cunningham, L. Moons, R. O. Schlingemann and J. H. M. Feyen, et al., The role of placental growth factor (PlGF) and its receptor system in retinal vascular diseases, Prog. Retinal Eye Res., 2019, 69, 116–136 CrossRef PubMed.
- Q. D. Nguyen, S. De Falco, F. Behar-Cohen, W. C. Lam, X. Li and N. Reichhart, et al., Placental growth factor and its potential role in diabetic retinopathy and other ocular neovascular diseases, Acta Ophthalmol., 2018, 96(1), e1–e9 CrossRef PubMed.
- M.-R. Nejadmoghaddam, A.-H. Zarnani, R. Ghahremanzadeh, R. Ghods, J. Mahmoudian and M. Yousefi, et al., Placenta-specific1 (PLAC1) is a potential target for antibody-drug conjugate-based prostate cancer immunotherapy, Sci. Rep., 2017, 7(1), 13373 CrossRef PubMed.
- C. Han, E. Perrone, B. Zeybek, S. Bellone, J. Tymon-Rosario and G. Altwerger, et al., In vitro and in vivo activity of sacituzumab govitecan, an antibody-drug conjugate targeting trophoblast cell-surface antigen 2 (Trop-2) in uterine serous carcinoma, Gynecol. Oncol., 2020, 156(2), 430–438 CrossRef CAS PubMed.
- R. Zussman, L. Y. Xu, T. Damani, K. M. Groom, Q. Chen and B. Seers, et al., Antiphospholipid antibodies can specifically target placental mitochondria and induce ROS production, J. Autoimmun., 2020, 111, 102437 CrossRef CAS PubMed.
- M. Aghababaei, K. Hogg, S. Perdu, W. P. Robinson and A. G. Beristain, ADAM12-directed ectodomain shedding of E-cadherin potentiates trophoblast fusion, Cell Death Differ., 2015, 22(12), 1970–1984 CrossRef CAS PubMed.
- W. Zheng, W. Zhao, M. Wu, X. Song, F. Caro and X. Sun, et al., Microbiota-targeted maternal antibodies protect neonates from enteric infection, Nature, 2020, 577(7791), 543–548 CrossRef CAS PubMed.
- K. Abou-Nassar, M. Carrier, T. Ramsay and M. A. Rodger, The association between antiphospholipid antibodies and placenta mediated complications: a systematic review and meta-analysis, Thromb. Res., 2011, 128(1), 77–85 CrossRef CAS PubMed.
- G. W. Wood and C. R. King Jr, Trapping antigen-antibody complexes within the human placenta, Cell. Immunol., 1982, 69(2), 347–362 Search PubMed.
- E. Maidji, G. Nigro, T. Tabata, S. McDonagh, N. Nozawa and S. Shiboski, et al., Antibody treatment promotes compensation for human cytomegalovirus-induced pathogenesis and a hypoxia-like condition in placentas with congenital infection, Am. J. Pathol., 2010, 177(3), 1298–1310 Search PubMed.
- K. M. Lee, G. J. Wilson, M. Pingen, A. Fukuoka, C. A. Hansell and R. Bartolini, et al., Placental chemokine compartmentalisation: A novel mammalian molecular control mechanism, PLoS Biol., 2019, 17(5), e3000287 CrossRef CAS PubMed.
- N. J. Hannan, R. L. Jones, C. A. White and L. A. Salamonsen, The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface, Biol. Reprod., 2006, 74(5), 896–904 Search PubMed.
- R. C. Carter, M. K. Georgieff, K. M. Ennis, N. C. Dodge, H. Wainwright and E. M. Meintjes, et al., Prenatal alcohol-related alterations in maternal, placental, neonatal, and infant iron homeostasis, Am. J. Clin. Nutr., 2021, 114(3), 1107–1122 CrossRef PubMed.
- R. Ghods, M. H. Ghahremani, M. Darzi, A. R. Mahmoudi, O. Yeganeh and A. A. Bayat, et al., Immunohistochemical characterization of novel murine monoclonal antibodies against human placenta–specific, Biotechnol. Appl. Biochem., 2014, 61(3), 363–369 CrossRef CAS PubMed.
- P. Travers and W. J. Bodmer, Preparation and characterization of monoclonal antibodies against placental alkaline phosphatase and other human trophoblast–associated determinants, Int. J. Cancer, 1984, 33(5), 633–641 Search PubMed.
- A. Dupressoir, G. Marceau, C. Vernochet, L. Bénit, C. Kanellopoulos and V. Sapin, et al., Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae, Proc. Natl. Acad. Sci. U. S. A., 2005, 102(3), 725–730 CrossRef CAS PubMed.
- T. Tobita, D. Kiyozumi and M. Ikawa, Placenta-specific gene manipulation using lentiviral vector and its application, Placenta, 2017, 59, S37–S43 CrossRef CAS PubMed.
- N. Abd Ellah, L. Taylor, W. Troja, K. Owens, N. Ayres and G. Pauletti, et al., Development of non-viral, trophoblast-specific gene delivery for placental therapy, PLoS One, 2015, 10(10), e0140879 CrossRef PubMed.
- C. Rossi, M. Lees, V. Mehta, T. Heikura, J. Martin and I. Zachary, et al., Comparison of efficiency and function of vascular endothelial growth factor adenovirus vectors in endothelial cells for gene therapy of placental insufficiency, Hum. Gene Ther., 2020, 31(21–22), 1190–1202 CrossRef CAS PubMed.
- D. J. Platt, A. M. Smith, N. Arora, M. S. Diamond, C. B. Coyne and J. J. Miner, Zika virus–related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice, Sci. Transl. Med., 2018, 10(426), eaao7090 Search PubMed.
- B. Coler, O. Cervantes, M. Li, C. Coler, A. Li and M. Shivakumar, et al., Common pathways targeted by viral hemorrhagic fever viruses to infect the placenta and increase the risk of stillbirth, Placenta, 2023, 141, 2–9 CrossRef CAS PubMed.
- T. Krishnan and A. L. David, Placenta-directed gene therapy for fetal growth restriction, in Seminars in Fetal and Neonatal Medicine, Elsevier, 2017 Search PubMed.
- J. Ding, P. Aldo, C. M. Roberts, P. Stabach, H. Liu and Y. You, et al., Placenta–derived interferon–stimulated gene 20 controls ZIKA virus infection, EMBO Rep., 2021, 22(10), e52450 CrossRef CAS PubMed.
- K. M. Aagaard, A. Lahon, M. A. Suter, R. P. Arya, M. D. Seferovic and M. B. Vogt, et al., Primary human placental trophoblasts are permissive for Zika virus (ZIKV) replication, Sci. Rep., 2017, 7(1), 41389 CrossRef PubMed.
- L. D. Cooke, D. A. Tumbarello, N. C. Harvey, J. K. Sethi, R. M. Lewis and J. K. Cleal, Endocytosis in the placenta: An undervalued mediator of placental transfer, Placenta, 2021, 113, 67–73 CrossRef PubMed.
- H. Koi, J. Zhang and S. Parry, The mechanisms of placental viral infection, Ann. N. Y. Acad. Sci., 2001, 943(1), 148–156 CrossRef PubMed.
- V. Mehta, M. Boyd, H. Barker, A. Avdic-Belltheus, D. Carr and J. Martin, et al., Local administration of AD. VEGF-A165 to the utero-placental circulation reduces brain sparing and may enhance fetal growth in an FGR model of Guinea pig pregnancy, Arch. Dis. Child., 2012, 97(Suppl 1), A8 Search PubMed.
- M. Yamakuchi, M. Okawa, K. Takenouchi, A. Bibek, S. Yamada and K. Inoue, et al., VEGF-A165 is the predominant VEGF-A isoform in platelets, while VEGF-A121 is abundant in serum and plasma from healthy individuals, PLoS One, 2023, 18(4), e0284131 CrossRef PubMed.
- M. M. Karsten, M. H. Beck, A. Rademacher, J. Knabl, J.-U. Blohmer and J. Jückstock, et al., VEGF-A165b levels are reduced in breast cancer patients at primary diagnosis but increase after completion of cancer treatment, Sci. Rep., 2020, 10(1), 3635 CrossRef PubMed.
- D. M. Aronoff, H. Correa, L. M. Rogers, R. Arav-Boger and D. J. Alcendor, Placental pericytes and cytomegalovirus infectivity: Implications for HCMV placental pathology and congenital disease, Am. J. Reprod. Immunol., 2017, 78(3), e12728 CrossRef PubMed.
- E. M. Venceslau, J. P. Guida, E. Amaral, J. L. P. Modena and M. L. Costa, Characterization of placental infection by Zika virus in humans: A review of the literature, Rev. Bras. Ginecol. Obstet., 2020, 42(09), 577–585 CrossRef PubMed.
- G. J. Burton and A. L. Fowden, The placenta: a multifaceted, transient organ, Philos. Trans. R. Soc., B, 2015, 370(1663), 20140066 CrossRef PubMed.
- G. J. Burton and E. Jauniaux, The human placenta: new perspectives on its formation and function during early pregnancy, Proc. R. Soc. B, 2023, 290(1997), 20230191 CrossRef CAS PubMed.
- K. E. Brett, Z. M. Ferraro, J. Yockell-Lelievre, A. Gruslin and K. B. Adamo, Maternal–fetal nutrient transport in pregnancy pathologies: the role of the placenta, Int. J. Mol. Sci., 2014, 15(9), 16153–16185 CrossRef PubMed.
- D. I. Linzer and S. J. Fisher, The placenta and the prolactin family of hormones: regulation of the physiology of pregnancy, Mol. Endocrinol., 1999, 13(6), 837–840 CrossRef CAS.
- J. M. DeSesso, A. L. Williams, A. Ahuja, C. J. Bowman and M. E. Hurtt, The placenta, transfer of immunoglobulins, and safety assessment of biopharmaceuticals in pregnancy, Crit. Rev. Toxicol., 2012, 42(3), 185–210 CrossRef CAS PubMed.
- C. Gedeon and G. Koren, Designing pregnancy centered medications: drugs which do not cross the human placenta, Placenta, 2006, 27(8), 861–868 Search PubMed.
- K. O'Brien and Y. Wang, The placenta: a maternofetal interface, Annu. Rev. Nutr., 2023, 43(1), 301–325 CrossRef.
- F. Olimjanovna, N. Nargiza and J. Osorio, The structure of the placenta in the normal course of pregnancy and in fetoplacental insufficiency, J. Reg. Med. Biol. Res., 2020, 2(6), 1–11 Search PubMed.
- W. A. Grobman, R. Gersnoviez, M. B. Landon, C. Y. Spong, K. J. Leveno and D. J. Rouse, et al., Pregnancy outcomes for women with placenta previa in relation to the number of prior cesarean deliveries, Obstet. Gynecol., 2007, 110(6), 1249–1255 CrossRef PubMed.
- S. Sarker, K. Scholz-Romero, A. Perez, S. E. Illanes, M. D. Mitchell and G. E. Rice, et al., Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy, J. Transl. Med., 2014, 12, 1–19 CrossRef PubMed.
- N. Chaudhary, Engineering immunomodulatory nanoparticles for safe and potent RNA delivery, Carnegie Mellon University, 2022 Search PubMed.
- M. Gautam, A. Jozic, G. L.-N. Su, M. Herrera-Barrera, A. Curtis and S. Arrizabalaga, et al., Lipid nanoparticles with PEG-variant surface modifications mediate genome editing in the mouse retina, Nat. Commun., 2023, 14(1), 6468 CrossRef CAS PubMed.
- T. Suzuki, Y. Suzuki, T. Hihara, K. Kubara, K. Kondo and K. Hyodo, et al., PEG shedding-rate-dependent blood clearance of PEGylated lipid nanoparticles in mice: faster PEG shedding attenuates anti-PEG IgM production, Int. J. Pharm., 2020, 588, 119792 CrossRef CAS.
- C. van Kammen, H. van Hove, D. Kapsokalyvas, R. Greupink, R. Schiffelers and T. Lely, et al., Targeted lipid nanoparticles to prevent trans-placental passage in the ex vivo human placental cotyledon perfusion model, Drug Delivery Transl. Res., 2024, 1–9 Search PubMed.
- J. A. Kulkarni, D. Witzigmann, S. Chen, P. R. Cullis and R. van der Meel, Lipid nanoparticle technology for clinical translation of siRNA therapeutics, Acc. Chem. Res., 2019, 52(9), 2435–2444 CrossRef CAS PubMed.
- D. Wang, Y. Sun, Y. Liu, F. Meng and R. J. Lee, Clinical translation of immunoliposomes for cancer therapy: Recent perspectives, Expert Opin. Drug Delivery, 2018, 15(9), 893–903 CrossRef CAS PubMed.
- L. B. Truong, D. Medina-Cruz and E. Mostafavi, Current state of RNA delivery using lipid nanoparticles to extrahepatic tissues: A review towards clinical translation, Int. J. Biol. Macromol., 2023, 242, 125185 CrossRef CAS.
- A. Cavolo, A. de Boer, L. De Proost, E. Verweij and C. Gastmans, Navigating the ethical landscape of the artificial placenta: A systematic review, Prenatal Diagn., 2025, 45(2), 236–246 CrossRef PubMed.
- C. Ampasavate, G. A. Chandorkar, D. G. V. Velde, J. F. Stobaugh and K. L. Audus, Transport and metabolism of opioid peptides across BeWo cells, an in vitro model of the placental barrier, Int. J. Pharm., 2002, 233(1–2), 85–98 CrossRef CAS PubMed.
- A. Lausman, Intrauterine growth restriction: screening, diagnosis, and management, J. Obstet. Gynaecol. Can., 2013, 35(8), 741–748 CrossRef PubMed.
- J. L. Leach, D. D. Sedmak, J. M. Osborne, B. Rahill, M. D. Lairmore and C. L. Anderson, Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport, J. Immunol., 1996, 157(8), 3317–3322 CrossRef CAS.
- E. Ganguly, F. Spaans, J. S. Morton, R. Kirschenman, M. M. Aljunaidy and T. E. Phillips, et al., Placenta–targeted treatment in hypoxic dams improves maturation and growth of fetal cardiomyocytes in vitro via the release of placental factors, Exp. Physiol., 2020, 105(9), 1507–1514 CrossRef CAS PubMed.
- M. I. de Moraes-Pinto, F. Verhoeff, L. Chimsuku, P. J. Milligan, L. Wesumperuma and R. L. Broadhead, et al., Placental antibody transfer: influence of maternal HIV infection and placental malaria, Arch. Dis. Child., 1998, 79(3), F202–F2F5 CrossRef PubMed.
- E. Trifonova, A. Babovskaya, A. Zarubin, V. Serebrova, M. Gavrilenko and M. Svarovskaya, et al., Transcriptomic Profiling of Placental Cells in Preeclampsia as an Effective Tool for Personalized Medicine, Russ. J. Genet., 2023, 59(12), 1366–1377 CrossRef.
- L. Zhao, S. Wong, J. Sim, J. Zhou, X. Li and C. Wang, Triboelectric nanogenerators and piezoelectric nanogenerators for preventing and treating heart diseases, BMEMat, 2023, 1(2), e12020 CrossRef; D. Keri, M. Walker, I. Singh, K. Nishikawa and F. Garces, Next generation of multispecific antibody engineering, Antibody Ther., 2024, 7(1), 37–52 CrossRef PubMed.
- K. J. Vincent and M. Zurini, Current strategies in antibody engineering: Fc engineering and pH–dependent antigen binding, bispecific antibodies and antibody drug conjugates, Biotechnol. J., 2012, 7(12), 1444–1450 CrossRef PubMed.
- L. P. Villarreal, Viruses and the placenta: the essential virus first view, Apmis, 2016, 124(1–2), 20–30 CrossRef PubMed.
- Y. Chu, H. Liu, G. Lou, Q. Zhang and C. Wu, Human placenta mesenchymal stem cells expressing exogenous kringle1–5 protein by fiber-modified adenovirus suppress angiogenesis, Cancer Gene Ther., 2014, 21(5), 200–208 CrossRef PubMed.
- I. Cardenas, R.E Means, P. Aldo, K. Koga, S.M Lang and C. Booth, et al., Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor, J. Immunol., 2010, 185(2), 1248–1257 CrossRef PubMed.
- T. Braun, L. Ehrlich, W. Henrich, S. Koeppel, I. Lomako and P. Schwabl, et al., Detection of microplastic in human placenta and meconium in a clinical setting, Pharmaceutics, 2021, 13(7), 921 CrossRef PubMed.
- E. D. Watson and J. C. Cross, Development of structures and transport functions in the mouse placenta, Physiology, 2005, 20(3), 180–193 CrossRef PubMed.
- B. D. Elliott, S. Schenker, O. Langer, R. Johnson and T. Prihoda, Comparative placental transport of oral hypoglycemic agents in humans: a model of human placental drug transfer, Am. J. Obstet. Gynecol., 1994, 171(3), 653–660 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |
