Surface engineering of NIR-II luminescent gold nanoclusters for brain glioma-targeted imaging†
Shufen
Tong
a,
Jie
Liu
a,
Yonghui
Chen
a,
Xinyun
Xiao
b,
Shihua
Li
*b,
Xiaorong
Song
 *ac and
Huanghao
Yang
*ac and
Huanghao
Yang
 ac
ac
aMOE Key Laboratory for Analytical Science of Food Safety and Biology, College of Chemistry, Fuzhou University, Fuzhou, Fujian 350116, China. E-mail: xrsong@fzu.edu.cn
bSchool of Medical Imaging, Fujian Medical University, Fuzhou, Fujian 350122, China. E-mail: lishihua@fjmu.edu.cn
cFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou 350108, China
First published on 21st March 2025
Abstract
Ultrasmall gold nanoclusters (AuNCs) with photoluminescence in the second near-infrared region (NIR-II) have emerged as promising probes for in vivo biomedical applications. However, it remains a challenge to utilize NIR-II-emitting AuNCs for imaging brain glioblastoma (GBM), which is highly lethal and hard to diagnose in time. Herein, we have presented systematic investigations on the brain delivery and GBM targeting efficacies of NIR-II-emitting AuNCs protected by different ligands. We first synthesized four types of AuNCs with surface coatings of small thiolated ligands and proteins, and then studied their in vitro penetration capability into the blood–brain barrier (BBB) and in vivo GBM targeting performances. It was found that the BBB permeability of AuNCs determined by the in vitro transwell model was not evidently affected by the surface ligands. Significantly, AuNCs protected by albumin exhibited notably extended blood circulation and less skull binding compared to those protected by small ligands, enabling superior in vivo brain GBM-targeted NIR-II PL imaging. We also modified the albumin–AuNCs with targeting peptides to improve in vivo imaging contrast. Additionally, AuNCs had negligible toxic effects on major organs as well as brain tissues and neurons, corroborating their good biocompatibility. This study examined the surface engineering of NIR-II luminescent AuNCs for brain GBM targeting, which may offer insights into the future design of AuNCs for bioapplications.
Introduction
Primary glioblastoma (GBM) is one of the most common and lethal intracranial malignant tumors, due to its high mortality and poor prognosis.1–3 At present, the clinical diagnosis of GBM is generally based on the use of brain computed tomography (CT) and magnetic resonance imaging (MRI), which however may encounter low spatial resolution, insensitivity, or radiation risk.4 To satisfy the practical requirements of precise surgery and therapeutic monitoring, imaging techniques with real-time and high-contrast imaging capabilities are always desired.5–7 In recent years, investigators have paid increasing attention to in vivo photoluminescence (PL) imaging techniques in the second near-infrared (NIR-II) region,8–11 which has significant advantages in terms of imaging sensitivity and resolution as well as light penetration depth, compared to the ultraviolet–visible12,13 and first NIR (NIR-I) region.14–17 Through-skull NIR-II PL imaging is therefore considered promising for the diagnosis and therapeutic monitoring of GBM and other brain diseases.18–20In order to achieve high-quality NIR-II PL imaging of GBM, it is necessary to select appropriate luminescent probes with low toxicity to the brain. As a class of generally recognized low-toxicity materials, gold materials with unique physicochemical properties have been widely studied in bioimaging and therapy fields.21–24 For example, gold complexes are utilized in the clinic for the treatment of arthritis, tumors, neurological disorders, etc.25–29 In this scenario, the emerging NIR-II luminescent ultrasmall gold nanoclusters (AuNCs) might be a solution for in vivo imaging of brain GBM. However, it is essential to consider whether they can effectively traverse the blood–brain barrier (BBB), which can prevent exogenous substances from entering the brain while ensuring nutrient supply and substance exchange.30,31 Although there are some reports on the brain-related applications of visible/NIR-I-emitting AuNCs, the brain delivery efficiencies are still not enough and such traditional AuNCs may differ from NIR-II AuNCs in material compositions and surface properties as well as the related biofate and brain delivery capabilities.7,32 Notably, there have been no reports on brain GBM imaging using NIR-II luminescent AuNCs so far.
Herein, we demonstrate the rational engineering of NIR-II luminescent AuNCs for achieving high-efficiency brain GBM-targeted imaging.33–35 We first synthesized AuNCs with surface coatings of glutathione (GSH), cyclodextrin (CD), N-acetyl cysteine (NAC), and bovine serum albumin (BSA), which are hereafter denoted as GNCs, CNCs, NNCs, and BNCs, respectively. These four kinds of AuNCs possessed NIR-II PL at around 1050 nm and high water dispersibility. By using the classical transwell model,36,37 we found that the penetration rates and tumor cell uptake amounts of the four AuNCs differed only slightly, as revealed by in vitro NIR-II PL imaging and metal content analysis. This indicated the plausible weak correlation between surface coatings and in vitro BBB permeability of the ultrasmall AuNCs. Significantly, BNCs enabled more efficient GBM targeting and NIR-II PL imaging in orthotopic mouse brain GBM models, compared to GNCs, CNCs, and NNCs. The enhanced brain GBM targeting of BNCs was found to be caused by their much longer blood circulation and lower skull accumulation attributes. Moreover, we can additionally decorate BNCs with targeting peptides,38–40 achieving approximately 2-fold contrast improvement in NIR-II PL imaging of GBM in vivo, relative to that in healthy mice. Besides, we systematically studied the brain biocompatibility of BNCs and confirmed their negligible toxicity effects on neurological functions and brain tissues. This work unravels the surface coating effects of NIR-II luminescent AuNCs on their brain delivery and GBM targeting efficiencies, which could offer insights into brain-related imaging and therapy research.
Results and discussion
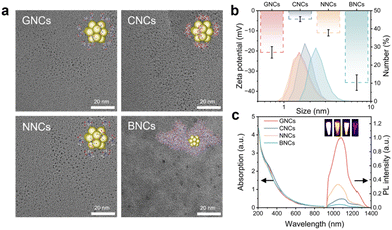
In view of the fact that the biofate of AuNCs is closely related to their surface properties, we tried to synthesize AuNCs with varying surface ligands, including GSH, CD, NAC, and BSA. The AuNCs were synthesized through a sodium borohydride mediated one-pot reduction method in alkaline environments.41–43 Transmission electron microscopy (TEM, Fig. 1a) indicated that these clusters had ultrasmall sizes of approximately 2 nm. The hydrodynamic diameters (HDs) were slightly increased in the AuNCs carrying larger-sized ligands, and BNCs had the largest HDs of about 2.5 nm (Fig. 1b). Zeta potentials of these AuNCs were all negative due to the presence of carboxyl and/or hydroxyl groups in the ligands, which also endowed them with good water dispersibility (Fig. S1†). We also tried to examine the molecular information about AuNCs by performing electrospray ionization mass spectrometry (ESI-MS) or matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). As illustrated in Fig. S2† (measured by ESI-MS), GNCs had three prominent sets of MS peaks with charges of 6−, 5−, and 4−, respectively (Fig. S2a†). In each charge state, four main components can be observed, accompanied by some other weak peaks. Taking the sets of charge 4− as an example, the peaks at m/z = 1727.28, 1754.56, 1803.20, and 2055.70 can be assigned to [Au18(SG)11-4H]4−, [Au17(SG)12-4H]4−, [Au18(SG)12-4H]4−, and [Au20(SG)14-4H]4−, respectively (Fig. S2b–e†). Also, the simulated isotope patterns matched the experimental data well. Thus, the synthesized GNCs may be described as Au17–20(SG)11–14.44–46 As for the ESI-MS data of NNCs (Fig. S3†), two prominent sets of peaks with charges of 6− and 5− were observed, each of which included four main components. Through analyzing charge 6−, the peaks at m/z = ∼1786.35, 1841.02, 1900.86, and 1986.50 can be assigned to [Au36(NAC)22 + 2Na-8H]6−, [Au36(NAC)24 + 2Na-8H]6−, [Au37(NAC)25 + 2Na-8H]6−, and [Au38(NAC)27 + 2Na-8H]6−, respectively. Therefore, NNCs can be approximately described as Au36–38(NAC)22–27. We also attempted to determine the atomic number of CNCs by ESI-MS analysis. However, it is hard to clearly figure out the charge states and molecular weights. Also, we have failed to obtain precise atomic information about BNCs from MALDI-TOF MS. This could be due to the fact that it is often challenging to obtain the atomic number of AuNCs protected by macromolecular ligands/proteins. Further studies of atomically precise synthesis methods may be needed.In addition, the absorption spectra showed that all AuNCs exhibited broad absorption in visible and NIR regions (300–900 nm), differentiating them from large-sized (e.g., >10 nm) Au nanoparticles with typical surface plasmon resonance absorption peaks (Fig. 1c). These AuNCs also exhibited NIR-II PL emission at around 1050 nm upon 808 nm excitation. The PL quantum yields (QYs) of GNCs, CNCs, NNCs, and BNCs were found to be approximately 0.55%, 0.17%, 0.30%, and 0.05%, respectively, by using IR-26 dye (QY = 0.05% in dimethyl sulfoxide) as a reference. The NIR-II PL imaging capabilities of these AuNCs were also verified, indicating their good bioimaging potential (inset, Fig. 1c).
To evaluate the BBB permeability of the obtained AuNCs, we established a transwell-based in vitro BBB model by culturing mouse brain microvascular endothelial cells (bEnd.3) and glioma cells (GL261) in the upper and bottom chambers, respectively (Fig. 2a). To confirm the construction of in vitro transwell BBB models, the trans-endothelial electrical resistance (TEER) value was monitored. The TEER value is considered as a vital indicator of cell layer integrity and permeability, and a high TEER value (e.g., >150 Ω cm2) can indicate the formation of an intact cell layer with proper intercellular tight junctions.47–49 We then cultured the cells until TEER values reached up to >300 Ω cm2 to guarantee successful construction of transwell models (Fig. S4†).37,50 In addition, we used confocal laser scanning microscopy (CLSM) to examine the cell integrity. Through the co-staining of cell nuclei, lysosomes, and membranes, we can see that the cells were well cultured in the upper and bottom chambers (Fig. 2a and S5†). After culturing AuNCs with the cells in the upper chamber, we conducted NIR-II PL imaging to survey the BBB permeability. NIR-II PL images of the culture media in the bottom chamber brightened with prolonged incubation time, indicative of their gradual penetration through the cell layer (Fig. 2b). Moreover, we quantitatively detected the penetration rates by using inductively coupled plasma mass spectrometry (ICP-MS). Results showed that the four AuNCs have almost similar penetration rates of ∼50% after 48 h of incubation (Fig. 2c). Besides, GL261 cells showed no obvious difference in the uptake of AuNCs, showing around 3% of the added dose (Fig. 2d). This suggests that the in vitro BBB permeability might not be significantly related to the surface coatings of AuNCs. Because the tight junctions (TJs) between endothelial cells (ECs) of the BBB have a gap of 4–6 nm,51,52 the ultrasmall AuNCs (<2 nm) may be able to cross the BBB in a passive diffusion manner, resulting in weak effects of surface coatings on their BBB penetration efficiencies.53,54 Furthermore, we evaluated the cytotoxicity of AuNCs by using cell counting kit-8 (CCK-8). Both bEnd.3 cells and GL261 cells had high cell viabilities, larger than 80% even after incubation with AuNCs at concentrations of 500 μM (on the basis of the Au element), indicating their low cytotoxicity (Fig. S6†).
We subsequently tried to figure out whether surface ligands on AuNCs could affect in vivo brain delivery and GBM targeting efficacies. To this end, we constructed in situ brain GBM models by utilizing an automatic stereotaxic instrument. All animal procedures were performed in accordance with the relevant laws and institutional guidelines and were approved by the Animal Ethics Committee of Fuzhou University. The successful construction of the GBM models was revealed by brain tissue examinations and bioluminescence (BL) imaging (Fig. S7 and S8†). Experimentally, the orthotopic brain GBM models can be well obtained at 21 d post inoculation. Healthy mice and brain GBM-bearing mice were then intravenously injected with different AuNCs, respectively. At desired time points after injection, the mice were imaged by using an InGaAs camera under 808 nm excitation (200 mW cm−2) (Fig. 3a). It can be seen that GNCs, CNCs, and NNCs were rapidly excreted into the bladder in a few minutes after injection into healthy and tumor-bearing mice, along with very low accumulation in the liver and other major organs (Fig. S9 and S10†). Particularly, strong NIR-II PL signals were observed in the mouse skull after injection of the three AuNCs, indicating their strong skull binding (Fig. 3b). This leads to low GBM targeting and poor diagnosis capability for brain GBM.55 In contrast, BNCs showed a prolonged blood circulation time after the intravenous injection, allowing high-contrast in vivo blood vessel imaging within a few hours (Fig. S9 and S10†). More significantly, BNCs had low skull accumulation and the NIR-II PL signals in the healthy mouse brain gradually decreased as time prolonged.56,57 The NIR-II PL signals in the brain regions of tumor-bearing mice gradually increased within 12 h (Fig. 3c). At 24 h post injection, about a 7-fold increase of NIR-II PL signals can be observed in the brain (relative to that before the injection of BNCs), as supported by NIR-II PL images of ex vivo tumor tissues (Fig. 3d and e). The gold content detected by ICP-MS also supported the enhanced accumulation (about 10 fold) in the brain GBM in BNC groups compared to that in the other three groups (Fig. 3f). Besides, BNCs obviously accumulated in major organs of mice (e.g., liver and kidneys), consistent with the previous findings in BSA-based materials (Fig. S11†).58 Therefore, BNCs facilitated enhanced in vivo brain GBM targeting compared to GNCs, CNCs, and NNCs. This could be mainly attributed to the prolonged blood circulation capability of BNCs (Fig. S12 and S13†), which permits their brain tumor accumulation after BBB penetration in vivo. The BBB penetration of BNCs was also verified by NIR-II PL imaging of ex vivo brain tissues, which showcased obvious signal enhancement after injection of BNCs (Fig. S14†). However, we also acknowledge that the NIR-II PL signals in brain tissues were not very high, probably owing to the limitations in the PL efficiency and BBB penetration capability of BNCs.
To further improve in vivo GBM targeting efficacy, we next modified BNCs with arginine–glycine–aspartic acid (RGD) targeting peptides that can actively target tumor cells expressing integrin.38,59 The peptide was designed with a free thiol end to form a stable gold–sulfur bond on AuNCs,39 resulting in RGD-modified BNCs (denoted as BNCs-RGD) (Fig. 4a).58 By using an ultra-performance liquid chromatography mass spectrometer (see the Experimental section in the ESI† for details), the molar ratio of albumin/RGD was determined to be ∼0.5 (Fig. S15†).60 The particle size showed negligible changes, while the surface negative charge was slightly decreased after RGD modification (Fig. 4b and S16†). Meanwhile, the PL intensity of BNCs was increased 2.5 times after RGD modification, likely due to the presence of electron-rich atoms and groups (e.g., carboxyl and amide groups) in the RGD ligand that could facilitate PL.61–64 The tumor targeting effect of BNCs-RGD was then studied in vitro. Based on NIR-II PL imaging of cells, the uptake amount of BNCs-RGD in GL261 cells was found to be 1.5 times that of the BNC group, suggesting enhanced tumor cell uptake after RGD modification (Fig. 4c and d). We then injected BNCs-RGD into healthy mice and brain GBM-bearing mice via the tail vein for in vivo NIR-II PL imaging (Fig. S17 and S18†). Significantly, enhanced NIR-II PL signals can be observed in brain GBM-bearing mice, showing a maximum PL increase of ∼9 fold at 24 h post injection, while only weak PL signals were observed in the brain of healthy mice (Fig. 4e and f). Also, commercial indocyanine green (ICG) was intravenously injected into GBM-bearing mice and negligible NIR-II PL signals were detected in the brain (Fig. S19†).65,66 These results thereby implied the good brain tumor targeting efficacy of BNCs-RGD.
In addition, we systematically evaluated the biocompatibility and safety of these AuNCs in mice. The routine blood tests were first conducted to examine the blood compatibility and toxicity to metabolism-related organs (e.g., liver and kidneys). As shown in Fig. S20,† these AuNCs had no obvious cytotoxic effects on the blood and did not affect the renal and liver functions. Histological analysis of tissues (collected at 24 h post injection) confirmed the low toxicity and high tissue biocompatibility of these AuNCs (Fig. S21†). Considering the critical roles of the central nervous system, we also investigated the potential brain toxicity of BNCs by performing histological analyses of the brain tissues and the detection of brain function-related biomarkers.67,68 As illustrated by hematoxylin and eosin (H&E) staining, there were no signs of pathological damage to the brain tissues after BNC treatment (Fig. 5a and b). Additionally, Fluor-Jade B (FJB) staining, which can specifically bind to degenerating neurons, showed negligible existence of green fluorescence in the brain tissues of BNC-treated mice, suggesting negligible toxic effects of BNCs on the neurons. We then determined the levels of the brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) in mice, which are important biomarkers associated with learning, memory, and others.69,70 24 h after the injection of AuNCs, the levels of these biomarkers in the isolated brain tissues were all within the normal ranges, indicating no evident toxic effects on the central nervous system (Fig. 5c and d). As essential antioxidant enzymes, superoxide dismutase (SOD) and catalase (CAT) are vital for maintaining the cerebral health and function.71 No significant changes in the enzymatic activities were observed in the isolated brain tissues of AuNC-injected mice, in comparison with those in the PBS-treated mice (Fig. 5e and f). To sum up, these results indicated that AuNCs had low toxicity to the brain and BNCs could be promising as imaging probes for brain applications.
Conclusions
In summary, we have systematically studied in vitro BBB permeability and in vivo brain GBM targeting efficacy of surface-engineered AuNCs, developing BNCs for effective NIR-II PL imaging of brain GBM. Four kinds of NIR-II-emitting AuNCs were synthesized by using small thiolated ligands and proteins. We found that the BBB permeability of AuNCs was not evidently affected by the surface ligands plausibly due to their ultrasmall sizes. In comparison with AuNCs protected by small ligands, BNCs had notably extended blood circulation and less skull binding features, enabling superior in vivo brain GBM-targeted NIR-II PL imaging. We also successfully modified BNCs with targeting peptides to further improve the targeting efficacy and imaging contrast. Besides, we revealed negligible toxic effects of AuNCs on major organs as well as brain tissues and neurons, which corroborates their good biocompatibility. This study is therefore anticipated to provoke further research interest in designing AuNCs for brain-related bioimaging and therapy applications.Author contributions
This project was supervised by S.L., X.S., and H.Y.; S.T., S.L., and X.S. designed experiments, S.T. and J.L. conducted experiments and analyzed the data, Y.C. and X.X. helped with data recording and analysis, S.L., X.S., and H.Y. revised the manuscript. All authors took part in the discussion of results and manuscript preparation.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (No. 22027805, 22274024, and 22404022), the Major Project of Science and Technology of Fujian Province (No. 2020HZ06006), the Natural Science Foundation of Fujian Province (No. 2024J01701), and the Start-up Fund for Scientific Research of Fujian Medical University (No. XRCZX2024008).References
- S. B. Rikan, A. S. Azar, A. Naemi, J. B. Mohasefi, H. Pirnejad and U. K. Wiil, Sci. Rep., 2024, 14, 2371 Search PubMed.
- E. I. Styliara, L. G. Astrakas, G. Alexiou, V. G. Xydis, A. Zikou, G. Kafritsas, S. Voulgaris and M. I. Argyropoulou, Curr. Oncol., 2024, 31, 2233–2243 Search PubMed.
- R. Mathur, Q. Wang, P. G. Schupp, A. Nikolic, S. Hilz, C. Hong, N. R. Grishanina, D. Kwok, N. O. Stevers, Q. Jin, M. W. Youngblood, L. A. Stasiak, Y. Hou, J. Wang, T. N. Yamaguchi, M. Lafontaine, A. Shai, I. V. Smirnov, D. A. Solomon, S. M. Chang, S. L. Hervey-Jumper, M. S. Berger, J. M. Lupo, H. Okada, J. J. Phillips, P. C. Boutros, M. Gallo, M. C. Oldham, F. Yue and J. F. Costello, Cell, 2024, 187, 446–463 CrossRef CAS PubMed.
- K. Connor, E. Conroy, K. White, L. P. Shiels, S. Keek, A. Ibrahim, W. M. Gallagher, K. J. Sweeney, J. Clerkin, D. O'Brien, J. B. Cryan, P. J. O'Halloran, J. Heffernan, F. Brett, P. Lambin, H. C. Woodruff and A. T. Byrne, Sci. Rep., 2024, 14, 2720 CAS.
- Z. Yu, B. Musnier, K. D. Wegner, M. Henry, B. Chovelon, A. Desroches-Castan, A. Fertin, U. Resch-Genger, S. Bailly, J.-L. Coll, Y. Usson, V. Josserand and X. Le Guével, ACS Nano, 2020, 14, 4973–4981 Search PubMed.
- F. Ren, Z. Jiang, M. Han, H. Zhang, B. Yun, H. Zhu and Z. Li, View, 2021, 2, 20200128 CrossRef.
- R. Thangam, R. Paulmurugan and H. Kang, Nanomaterials, 2021, 12, 18 CrossRef PubMed.
- K. Zhang, F. R. Chen, L. Wang and J. Hu, Small, 2023, 19, 2206044 CrossRef CAS PubMed.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, X. Mu, J. Wang, W. Mi, J. Luo, J. Xie and X. D. Zhang, Adv. Mater., 2019, 31, 1901015 CrossRef CAS PubMed.
- S. Ni, Y. Liu, S. Tong, S. Li and X. Song, J. Anal. Test., 2023, 7, 260–271 CrossRef.
- S. Liang, D. Hu, G. Li, D. Gao, F. Li, H. Zheng, M. Pan and Z. Sheng, Sci. Bull., 2022, 67, 2316–2326 CrossRef CAS PubMed.
- K. Yang, Y. Yang, D. Sun, S. Li, X. Song and H. Yang, Sci. China Mater., 2023, 66, 4090–4099 CrossRef CAS.
- X. Song, M. Li, S. Ni, K. Yang, S. Li, R. Li, W. Zheng, D. Tu, X. Chen and H. Yang, Nano Lett., 2023, 23, 1878–1887 CrossRef CAS.
- A. Yahia-Ammar, D. Sierra, F. Mérola, N. Hildebrandt and X. Le Guével, ACS Nano, 2016, 10, 2591–2599 CAS.
- T. Chen, H. Lin, Y. Cao, Q. Yao and J. Xie, Adv. Mater., 2021, 34, 2103918 Search PubMed.
- L. Liang, J. Chen, K. Shao, X. Qin, Z. Pan and X. Liu, Nat. Mater., 2023, 22, 289–304 CAS.
- H. Chen, B. Ding, P. A. Ma and J. Lin, Adv. Drug Delivery Rev., 2022, 188, 114414 CAS.
- Y. Du, S. Ni, Q. Ma, X. Song and H. Yang, Coord. Chem. Rev., 2023, 496, 215401 CAS.
- D. Huang, Q. Wang, Y. Cao, H. Yang, M. Li, F. Wu, Y. Zhang, G. Chen and Q. Wang, ACS Nano, 2023, 17, 5033–5046 CAS.
- N. Yin, Y. Wang, Y. Cao, Y. Huang, L. Jin, S. Zhang, J. Liu, T. Zhang, Z. Lv, Y. Liu, S. Song, D. Wang and H. Zhang, Nano Today, 2022, 46, 101619 CAS.
- J. Yang, F. Yang, C. Zhang, X. He and R. Jin, ACS Mater. Lett., 2022, 4, 1279–1296 CAS.
- E. Porret, X. Le Guével and J.-L. Coll, J. Mater. Chem. B, 2020, 8, 2216–2232 CAS.
- L. Shang, J. Xu and G. U. Nienhaus, Nano Today, 2019, 28, 100767 Search PubMed.
- D. Sang, X. Luo and J. Liu, Nano-Micro Lett., 2023, 16, 44 Search PubMed.
- G. Yang, K. Liu, Y. Wang, X. Pan, J. Ye, Y. Li, F. Du, T. Feng and X. Yuan, Aggregate, 2023, 5, e435 Search PubMed.
- F. Xiao, Y. Chen, J. Qi, Q. Yao, J. Xie and X. Jiang, Adv. Mater., 2023, 35, 2210412 CrossRef CAS.
- G. Yang, X. Mu, X. Pan, Y. Tang, Q. Yao, Y. Wang, F. Jiang, F. Du, J. Xie, X. Zhou and X. Yuan, Chem. Sci., 2023, 14, 4308–4318 RSC.
- W. Cheng, Y.-L. Su, H.-H. Hsu, Y.-H. Lin, L.-A. Chu, W.-C. Huang, Y.-J. Lu, C.-S. Chiang and S.-H. Hu, ACS Nano, 2022, 16, 4014–4027 CrossRef CAS PubMed.
- N. Perets, O. Betzer, R. Shapira, S. Brenstein, A. Angel, T. Sadan, U. Ashery, R. Popovtzer and D. Offen, Nano Lett., 2019, 19, 3422–3431 CrossRef CAS.
- J. Li, M. Zheng, O. Shimoni, W. A. Banks, A. I. Bush, J. R. Gamble and B. Shi, Adv. Sci., 2021, 8, 2101090 CrossRef CAS.
- B. A. Buil, C. Tackenberg and R. Rust, Brain, 2023, 146, 823–841 CrossRef.
- Y. Cheng, Q. Dai, R. A. Morshed, X. Fan, M. L. Wegscheid, D. A. Wainwright, Y. Han, L. Zhang, B. Auffinger, A. L. Tobias, E. Rincón, B. Thaci, A. U. Ahmed, P. C. Warnke, C. He and M. S. Lesniak, Small, 2014, 10, 5137–5150 CAS.
- S. Li, Q. Ma, C. Wang, K. Yang, Z. Hong, Q. Chen, J. Song, X. Song and H. Yang, Anal. Chem., 2022, 94, 2641–2647 CAS.
- X. Song, W. Zhu, X. Ge, R. Li, S. Li, X. Chen, J. Song, J. Xie, X. Chen and H. Yang, Angew. Chem., Int. Ed., 2020, 60, 1306–1312 Search PubMed.
- Y. Yu, Z. Luo, C. S. Teo, Y. N. Tan and J. Xie, Chem. Commun., 2013, 49, 9740–9742 CAS.
- D. Qi, H. Lin, B. Hu and Y. Wei, J. Controlled Release, 2023, 358, 78–97 CAS.
- A. Wolff, M. Antfolk, B. Brodin and M. Tenje, J. Pharm. Sci., 2015, 104, 2727–2746 CAS.
- H. Javid, M. A. Oryani, N. Rezagholinejad, A. Esparham, M. Tajaldini and M. Karimi-Shahri, Cancer Med., 2024, 13, e6800 CAS.
- G. Liang, X. Jin, S. Zhang and D. Xing, Biomaterials, 2017, 144, 95–104 CAS.
- Y. Wu, D. Hu, D. Gao, C. Liu, H. Zheng and Z. Sheng, Adv. Healthcare Mater., 2022, 11, 2202379 CAS.
- X. Yuan, B. Zhang, Z. Luo, Q. Yao, D. T. Leong, N. Yan and J. Xie, Angew. Chem., Int. Ed., 2014, 53, 4623–4627 CAS.
- Q. Dan, Z. Yuan, S. Zheng, H. Ma, W. Luo, L. Zhang, N. Su, D. Hu, Z. Sheng and Y. Li, Pharmaceutics, 2022, 14, 1645 CAS.
- L. Tang, X. Zeng, H. Zhou, C. Gui, Q. Luo, W. Zhou, J. Wu, Q. Li, Y. Li and Y. Xiao, Chem. Res. Chin. Univ., 2021, 37, 934–942 CAS.
- Q. Yao, M. Zhu, Z. Yang, X. Song, X. Yuan, Z. Zhang, W. Hu and J. Xie, Nat. Rev. Mater., 2024, 10, 89–108 Search PubMed.
- H. Shan, J. Shi, T. Chen, Y. Cao, Q. Yao, H. An, Z. Yang, Z. Wu, Z. Jiang and J. Xie, ACS Nano, 2023, 17, 2368–2377 CrossRef CAS.
- Z. Luo, X. Yuan, Y. Yu, Q. Zhang, D. T. Leong, J. Y. Lee and J. Xie, J. Am. Chem. Soc., 2012, 134, 16662–16670 CAS.
- X. Ma, L. Kuang, Y. Yin, L. Tang, Y. Zhang, Q. Fan, B. Wang, Z. Dong, W. Wang, T. Yin and Y. Wang, ACS Nano, 2023, 17, 2341–2355 CrossRef CAS PubMed.
- S. I. Ahn, Y. J. Sei, H.-J. Park, J. Kim, Y. Ryu, J. J. Choi, H.-J. Sung, T. J. MacDonald, A. I. Levey and Y. Kim, Nat. Commun., 2020, 11, 175 CrossRef CAS PubMed.
- K. B. Johnsen, M. Bak, P. J. Kempen, F. Melander, A. Burkhart, M. S. Thomsen, M. S. Nielsen, T. Moos and T. L. Andresen, Theranostics, 2018, 8, 3416–3436 CrossRef CAS PubMed.
- H. Chen, J. Ji, L. Zhang, C. Luo, T. Chen, Y. Zhang, C. Ma, Y. Ke and J. Wang, Small, 2024, 20, 2306714 CrossRef CAS PubMed.
- E. Nance, S. H. Pun, R. Saigal and D. L. Sellers, Nat. Rev. Mater., 2021, 7, 314–331 CrossRef PubMed.
- S. Zha, H. Liu, H. Li, H. Li, K.-L. Wong and A. H. All, ACS Nano, 2024, 18, 1820–1845 CrossRef CAS PubMed.
- J. Zhang, T. Yang, W. Huang, Y. Yu and T. Sun, Curr. Med. Chem., 2022, 29, 6063–6083 CrossRef CAS PubMed.
- H. Sela, H. Cohen, P. Elia, R. Zach, Z. Karpas and Y. Zeiri, J. Nanobiotechnol., 2015, 13, 1–9 CrossRef PubMed.
- D. Li, Q. Liu, Q. Qi, H. Shi, E. C. Hsu, W. Chen, W. Yuan, Y. Wu, S. Lin, Y. Zeng, Z. Xiao, L. Xu, Y. Zhang, T. Stoyanova, W. Jia and Z. Cheng, Small, 2020, 16, 2003851 CrossRef CAS PubMed.
- S. Lee, C. Lee, S. Park, K. Lim, S. S. Kim, J. O. Kim, E. S. Lee, K. T. Oh, H.-G. Choi and Y. S. Youn, Int. J. Pharm., 2018, 553, 363–374 CrossRef CAS PubMed.
- D.-H. Hu, Z.-H. Sheng, P.-F. Zhang, D.-Z. Yang, S.-H. Liu, P. Gong, D.-Y. Gao, S.-T. Fang, Y.-F. Ma and L.-T. Cai, Nanoscale, 2013, 5, 1624–1628 RSC.
- C. Zhang, N. Zhang, W. Niu, R. Li, Y. Liu, Y. Mu, C. Xu, Q. Yao and X. Gao, Nano Today, 2024, 55, 102195 CrossRef CAS.
- J. Mo, L. He, B. Ma and T. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 6811–6825 CrossRef CAS PubMed.
- Q. Luo, H. Zhang, Y. Zhou, Z. Liu and Z. Cai, Rapid Commun. Mass Spectrom., 2021, 35, e9117 CrossRef CAS PubMed.
- W. Wang, Y. Kong, J. Jiang, Q. Xie, Y. Huang, G. Li, D. Wu, H. Zheng, M. Gao, S. Xu, Y. Pan, W. Li, R. Ma, M. X. Wu, X. Li, H. Zuilhof, X. Cai and R. Li, Angew. Chem., Int. Ed., 2020, 59, 22431–22435 CrossRef CAS PubMed.
- F. Bertorelle, K. D. Wegner, M. P. Bakulić, H. Fakhouri, C. Comby-Zerbino, A. Sagar, P. Bernadó, U. Resch-Genger, V. Bonačić-Koutecký, X. Le Guével and R. Antoine, Chem. – Eur. J., 2022, 28, e202200570 CrossRef CAS PubMed.
- M. Guo, G. Zhang, R. Zhao, H. Ma, Y. Yan, S. Yang, J. Meng, Y. Huang, X.-D. Zhang, H. Wang and R. Zhang, ACS Appl. Nano Mater., 2023, 6, 15945–15958 CrossRef CAS.
- Z. Wu and R. Jin, Nano Lett., 2010, 10, 2568–2573 CrossRef CAS PubMed.
- D. Zhang, S. Tian, Y. Liu, M. Zheng, X. Yang, Y. Zou, B. Shi and L. Luo, Nat. Commun., 2022, 13, 6835 CrossRef CAS PubMed.
- M. Zhu, Z. Sheng, Y. Jia, D. Hu, X. Liu, X. Xia, C. Liu, P. Wang, X. Wang and H. Zheng, ACS Appl. Mater. Interfaces, 2017, 9, 39249–39258 CrossRef CAS PubMed.
- M. Miranda, J. F. Morici, M. B. Zanoni and P. Bekinschtein, Front. Cell. Neurosci., 2019, 13, 472800 Search PubMed.
- S.-K. Sims, B. Wilken-Resman, C. J. Smith, A. Mitchell, L. McGonegal, C. Sims-Robinson and G. Delevati Colpo, Neural Plast., 2022, 2022, 1–15 CrossRef PubMed.
- M. Irfan, J. H. Kim, R. E. Druzinsky, S. Ravindran and S. Chung, Sci. Rep., 2022, 12, 2042 CrossRef CAS PubMed.
- M. Irfan and S. Chung, Sci. Rep., 2023, 13, 74 CrossRef CAS PubMed.
- G. K. Ferreira, E. Cardoso, F. S. Vuolo, L. S. Galant, M. Michels, C. L. Gonçalves, G. T. Rezin, F. Dal-Pizzol, R. Benavides, G. Alonso-Núñez, V. M. Andrade, E. L. Streck and M. M. S. Paula, Mater. Sci. Eng., C, 2017, 79, 748–755 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr05158k |
| This journal is © The Royal Society of Chemistry 2025 |