 Open Access Article
Open Access ArticleNanoparticle-based drug delivery systems: opportunities and challenges in the treatment of esophageal squamous cell carcinoma (ESCC)
Linjia
Peng
,
Zixuan
Gao
,
Yanfeng
Liang
 ,
Xiaonan
Guo
,
Qiuli
Zhang
and
Daxiang
Cui
,
Xiaonan
Guo
,
Qiuli
Zhang
and
Daxiang
Cui
 *
*
The First Afffliated Hospital of Henan University, N. Jinming Ave, Kaifeng, 475004, China
First published on 7th March 2025
Abstract
Esophageal squamous cell carcinoma (ESCC) is an aggressive malignancy characterized by limited treatment options and poor prognosis. Nanoparticle-based drug delivery systems have emerged as a promising strategy to enhance cancer therapy efficacy by improving drug targeting, reducing toxicity, and enabling multifunctional applications. This review highlights some key types of nanoparticles, including liposomes, polymeric nanoparticles, metallic nanoparticles, dendrimers, and quantum dots, which could effectively improve the delivery of various drugs used in chemotherapy, radiotherapy, and immunotherapy, offering more precise and effective treatment options. With the ability to improve drug stability and overcome biological barriers, nanoparticle-based systems represent a transformative strategy for ESCC treatment. Despite some challenges, such as biocompatibility and scalability, the future of nanoparticle-based drug delivery holds great promise, particularly in the development of personalized nanomedicine and novel therapeutic approaches targeting the tumor microenvironment. With ongoing advancements, nanoparticle-based drug delivery systems hold immense potential to revolutionize ESCC treatment and improve patient outcomes.
1. Introduction
Esophageal squamous cell carcinoma (ESCC) is an aggressive malignancy, which is often diagnosed at advanced stages due to the absence of early symptoms, resulting in a poor prognosis and high mortality rates, particularly in Asia and Africa.1,2 Characterized by unique pathological features, ESCC progresses through a multistep process driven by genetic and epigenetic alterations.3 Environmental factors such as tobacco use,4 heavy alcohol consumption,5,6 consumption of hot beverages,7 and dietary deficiencies8 are some major triggers. Chronic inflammation, such as esophagitis, further predisposes the esophageal lining to malignant transformation.9 Key mutations in tumor suppressor genes like TP53 and oncogenes such as PIK3CA promote uncontrolled cell proliferation and inhibit apoptosis.10–13 The tumor microenvironment, comprising cancer-associated fibroblasts and immune cells, creates barriers to drug penetration and foster tumor progression through various mechanisms.14–17 Additionally, hypoxic conditions within the tumor core reduce sensitivity to radiation and chemotherapy while driving angiogenesis and metastasis.18 The limitations of current treatments, including surgery, chemotherapy, radiotherapy, and immunotherapy still exist, highlighting the urgent need for more precise and less toxic approaches19–22 (Fig. 1).6,23,24 | ||
| Fig. 1 Overview of current treatment approaches for esophageal squamous cell carcinoma. Created with biorender.com. Reprinted with permission from ref. 6, 23 and 24, Copyright © 2023, 2022 and 2021, Frontiers In Immunology, Signal Transduct Target Ther and Elsevier. | ||
Nanoparticle-based drug delivery systems provide an innovative solution to overcome the limitations of conventional cancer therapies. These systems enhance drug delivery by improving the precision, efficiency, and safety of treatment. Engineered nanoparticles specifically target tumor cells, reducing off-target effects and enhancing therapeutic outcomes.25 Their small size and modifiable surface properties allow enhanced permeability and retention (EPR) within tumors, enabling superior accumulation in tumor tissues compared to normal tissues.23,26,27 This property is particularly beneficial for treating difficult-to-reach cancers like ESCC. In ESCC, the challenges of treatment failure are multifactorial, involving factors such as treatment resistance, tumor heterogeneity, recurrence, and inadequate drug penetration. Nanoparticles can address some of these challenges by improving drug delivery and targeting. Moreover, nanoparticles offer multifunctional applications, such as theranostics, which integrates therapy and diagnostics in a single platform. These advanced systems allow for simultaneous drug delivery, imaging, and real-time monitoring of treatment responses, providing a more integrated approach to cancer therapy.28,29 For example, nanoparticles can be loaded with chemotherapeutic agents while being functionalized with contrast agents for imaging,30–32 enabling clinicians to track the drug distribution and efficacy in real-time.
Despite these promising benefits, nanoparticle-based drug delivery systems face challenges, including biocompatibility, potential toxicity, and difficulties in-large-scale manufacturing and quality control. This review explores various types of nanoparticles employed in drug delivery, their applications in ESCC treatment, and the steps needed for successful clinical implementation.
2. Types of nanoparticle-based drug delivery systems
Nanoparticle-based drug delivery systems offer diverse structures and functionalities that enhance the therapeutic potential of anticancer agents. These nanoparticles can be customized in terms of size, shape, surface properties, and composition to suit specific therapeutic needs. The following are some of the most commonly applied types of nanoparticles in drug delivery systems, and Fig. 2 provides an overview of the various types and classifications of nanoparticles.33 | ||
| Fig. 2 Types of nanoparticles in drug delivery. Created by Figdraw. License ID: AerS=e8d2c. Reprinted with permission from ref. 32. Copyright © 2024, ACS Omega. | ||
2.1 Liposomes
Liposomes are composed of phospholipids, such as phosphatidylcholine and cholesterol, forming spherical vesicles with lipid bilayers that can encapsulate hydrophilic drugs in their aqueous core and hydrophobic drugs within the bilayer.34–37 Their mechanism of action involves several key processes: encapsulating drugs to protect them from degradation,38 prolonging circulation time through PEGylation to evade immune clearance,39 and exploiting the Enhanced Permeability and Retention (EPR) effect to passively target tumor tissues by accumulating in their leaky vasculature.40–42 Liposomes can also be engineered for active targeting by attaching ligands or antibodies that bind to cancer-specific receptors, facilitating precise drug delivery to tumors. Liu et al. developed a biomimetic liposome nano-platinum system (nano-Pt/VP@MLipo) that combines the peroxidase-like activity of platinum nanoparticles (nano-Pt), which are encapsulated in the aqueous core of the liposomes, while the photosensitizer verteporfin (VP) is loaded into the lipid bilayer. The liposomes are further hybridized with macrophage cell membranes, imparting biomimetic and targeting properties, which enhance tumor penetration and chemotherapy efficacy.43 Additionally, stimuli-responsive liposomes release their drug payload in response to the acidic tumor microenvironment, ensuring minimal release in healthy tissues. The composition of liposomes can be adjusted to control drug release, enabling sustained delivery over time. This combination of protective encapsulation, targeted delivery, and controlled release makes liposomes highly effective in cancer therapy, improving drug bioavailability while minimizing systemic toxicity. Research has developed pH-sensitive liposomes (IRI&398-s-LPs) for the combined delivery of irinotecan (IRI) and NVP-BGJ398 (398). These liposomes are designed to release both drugs under the acidic conditions of the tumor microenvironment. The compound 398 targets FGFR on the surface of cancer-associated fibroblasts (CAFs) and inhibits their activity, while IRI induces apoptosis in tumor cells. This combination therapy aims to eliminate the “seeds” (tumor cells) and reshape the “soil” (CAFs) to enhance the treatment of colorectal cancer.442.2 Polymeric nanoparticles
Polymeric nanoparticles, made from biocompatible and biodegradable polymers,45,46 such as poly(lactic-co-glycolic acid) (PLGA)47–49 or chitosan,50,51 provide a versatile platform for drug delivery due to their ability to encapsulate drugs and offer controlled, sustained release. These nanoparticles protect drugs from degradation and allow for precise release over time as the polymer matrix degrades.52–54 In cancer therapy, polymeric nanoparticles can be engineered to release drugs in response to the acidic tumor microenvironment, taking advantage of the lower pH to trigger targeted drug release.55 This pH-sensitive design enhances drug accumulation at the tumor site while minimizing release in healthy tissues, reducing systemic toxicity.56 A study has developed polymeric nanoparticles coated with hyaluronic acid (HA), utilizing a double polymer shell made of poly(lactic-co-glycolic acid) (PLGA) and poly(glycolic acid) (PSAR) to target CD44 receptors overexpressed in colorectal cancer cells. The optimized nanoparticles demonstrated enhanced controlled release of the drug, excellent cytotoxicity against the HCT116 cell line, improved pharmacokinetics in vivo, as well as effective targeting and biocompatibility.57 Additionally, the nanoparticles can passively target tumors via the Enhanced Permeability and Retention (EPR) effect and can be further functionalized with ligands or antibodies for active targeting of cancer cell receptors, ensuring higher specificity.58 These combined properties make polymeric nanoparticles particularly effective for cancer treatment by improving drug bioavailability, targeting tumors precisely, and minimizing harmful side effects.2.3 Metallic nanoparticles
Metallic nanoparticles, particularly gold and silver nanoparticles, are composed of metal cores that provide unique optical, electronic, and physical properties due to their high surface area and tunable plasmonic resonance.59–61 These properties enable them to absorb and convert light energy into heat, a key mechanism in photothermal therapy.62–64 In cancer treatment, gold nanoparticles can be engineered to target tumor cells through surface functionalization with ligands or antibodies that bind to specific cancer cell receptors.65,66 Once localized at the tumor site, these nanoparticles are irradiated with near-infrared light, which they absorb and convert into localized heat, leading to the destruction of cancer cells through hyperthermia.67–69 This process selectively kills tumor cells while sparing healthy tissue due to the precise targeting and localized heat generation. Additionally, metallic nanoparticles can be functionalized with chemotherapeutic drugs, allowing for a dual approach in which both chemotherapy and photothermal therapy are employed.70,71 The nanoparticles serve as drug carriers, releasing the chemotherapy agent at the tumor site while simultaneously enhancing the therapeutic effect through heat generation.72–74 This combination of targeted drug delivery and photothermal therapy maximizes tumor destruction and minimizes damage to surrounding healthy tissues, making metallic nanoparticles a powerful tool in cancer treatment.2.4 Dendrimers
Dendrimers are nanoscale, highly branched, tree-like polymers composed of a central core, repeated branching units, and numerous terminal functional groups.75 Their unique, well-defined structure provides a high degree of control over drug loading and release, as each branching point and surface functional group offers multiple attachment sites for therapeutic agents, imaging molecules, or targeting ligands.76 In cancer treatment, dendrimers can be designed to simultaneously carry chemotherapeutic drugs, gene therapies, and even diagnostic agents within a single platform.77,78 Their precise structure allows for controlled and sustained release of these agents, improving drug stability and reducing off-target effects.79 Additionally, the nanoscale size and high surface area-to-volume ratio of dendrimers enable them to penetrate biological barriers, such as the tumor microenvironment and cellular membranes, allowing for enhanced accumulation in tumor tissues.80–83 Functionalization with specific ligands or antibodies further enhances their ability to selectively target cancer cells, ensuring that therapeutic agents are delivered directly to the tumor. This combination of multi-functional drug delivery, precise targeting, and effective penetration makes dendrimers a powerful and versatile tool for cancer treatment, capable of delivering complex, multi-agent therapies while minimizing systemic toxicity and side effects.2.5 Quantum dots and emerging nanocarriers
Quantum dots are nanoscale semiconductor particles, typically composed of materials like cadmium selenide (CdSe) or indium phosphide (InP), that exhibit unique optical properties due to quantum confinement effects.84 These properties allow quantum dots to emit bright, size-tunable fluorescence when excited by light, making them valuable for both imaging and drug delivery applications.85,86 In cancer therapy, quantum dots can be conjugated with therapeutic drugs or targeting ligands on their surface to selectively bind to tumor-specific receptors.87,88 This dual functionality enables real-time imaging of drug distribution while delivering the therapeutic payload to the tumor site.89 Quantum dots can track the movement and accumulation of drugs in tumor tissues, offering precise monitoring of treatment efficacy.90,91 Additionally, their high surface-to-volume ratio allows for the attachment of multiple therapeutic agents, enhancing their versatility in multi-drug delivery systems. In cancer theranostics, quantum dots integrate diagnosis and therapy into a single platform, providing both imaging for diagnosis and targeted treatment.92,93 Alongside quantum dots, other nanocarriers like carbon nanotubes and nanogels are being investigated for their ability to deliver drugs with high precision and efficiency, exploiting their unique structures for enhanced drug loading, controlled release, and targeted delivery, further advancing cancer treatment strategies.94–98These various types of nanoparticles each offer distinct advantages in drug delivery. By carefully selecting the type of nanoparticle based on the desired therapeutic outcome, researchers can optimize drug delivery to improve the effectiveness of ESCC treatment while reducing side effects.
3. Applications of nanoparticle drug delivery systems in ESCC
Nanoparticle-based drug delivery systems significantly enhance the treatment of ESCC by improving drug targeting, therapeutic efficacy, and reducing systemic toxicity. Table 1 provides an overview of nanoparticle drug delivery systems currently utilized in chemotherapy and radiotherapy and Table 2 presents a comprehensive overview of the nanoparticle drug delivery systems that are currently employed in gene therapy and immunotherapy for ESCC.| Type | NP | Assessment | Mechanism | Limitations | Ref. |
|---|---|---|---|---|---|
| Chemotherapy | PEGylated nanoliposome | In vitro and in vivo | -Enhances drug concentration in tumor cells through receptor-mediated uptake, improving efficacy; rapid release of LY294002 inhibits autophagy in tumor cells, increasing their sensitivity to 5-FU | -Oversized particles may affect biodistribution and targeting; in vivo stability and drug release properties lack detailed explanation | 109 |
| FA-M(PTX) | In vitro and in vivo | -The FA-M(PTX) effectively inhibit the growth of ESCC xenografts and extend the survival of tumor-bearing nude mice by inducing apoptosis through the regulation of Bax, Caspase 3, and Bcl2 expression | -The difference in antitumor activity between in vitro and in vivo studies may result from the rapid clearance of free paclitaxel and the “shielding” effect of the nanoparticles | 110 | |
| Hollow carbon spheres (HCSs) | In vitro and in vivo | -Efficiently delivers the chemotherapy drug doxorubicin to tumor sites; exhibits good biocompatibility, evading lysosomal degradation, and extends drug circulation time, altering biodistribution to increase tumor accumulation | -Potential biohazards and health or environmental impacts significantly limit its clinical use; the drug loading capacity needs further improvement | 111 | |
| DSPE-PEG2000 nanoliposomes | In vitro and in vivo | -DOX and ORD induce tumor cell apoptosis by inhibiting the PI3K/AKT/mTOR and Ras/Raf signaling pathways; nano-liposomal carriers enhance the targeting and permeability of the drugs in tumor tissues | The release profile under different pH conditions and the absorption and distribution characteristics in vivo need to be evaluated | 112 | |
| bMED NPs | In vitro and in vivo | -Efficiently co-delivers doxorubicin and β-elemene, releasing them in optimal ratios for enhanced combination therapy; targets tumors with prolonged circulation, increasing drug accumulation; induces apoptosis by upregulating Bax, downregulating Bcl-2, and raising apoptosis rates | -Some toxicity to normal organs remains; tumor accumulation still needs improvement | 113 | |
| TAB-CIS/5-FU LPHNs | In vitro and in vivo | -CIS and 5-FU exhibit synergy in lipid–polymer hybrid nanoparticles (LPHNs); TAB modification boosts nanoparticle uptake by tumor cells; LPHNs allow slow release of CIS and 5-FU, increasing tumor drug concentration | -Release behavior and kinetics need further optimization; optimal drug ratios for synergy require further exploration | 114 | |
| CM-β-CD-PEI-PEG-T7/DTX/CUR (T7-NP-DC) | In vitroIn vitro and in vivo | -Chemotherapy drugs like gemcitabine, doxorubicin, and paclitaxel enhance immune effects by inducing immunogenic cell death and clearing myeloid-derived suppressor cells. Improve cellular uptake and tumor targeting, increasing therapeutic efficacy; these drugs prolong half-life and boost tumor accumulation with pH-responsive release | -Insufficient data on the impact of nanoparticles on the tumor immune microenvironment; only short-term biocompatibility studies were conducted | 115 | |
| RGD-f-PNP/EPI | In vitro and in vivo | -EPI-loaded RGD-f-PNPs enable monitoring of drug delivery and effects via near-infrared fluorescence | -Further studies are needed on biological performance, biodistribution, stability, and targeting in vivo | 116 | |
| PEG-TE 10 @PLGA@DOX-Cur NP (PMPN) | In vitro and in vivo | -Cancer cell membrane-coated PLGA nanoparticles with DOX and Cur target multidrug-resistant esophageal cancer, inhibiting tumor growth in vitro and in vivo | -The preparation is complex, and further animal and clinical trials are required to assess safety and efficacy | 117 | |
| Radiotherapy | PLGA (poly(lactic-co-glycolic acid)) nanoparticles | In vitro and in vivo | -Encapsulating 111In and Ru1 in PLGA nanoparticles enhances DNA damage and cytotoxicity, targeting EGFR-overexpressing tumor cells more effectively | -Toxicity, complex preparation, and in vivo stability, pharmacokinetics, and efficacy need further validation | 118 |
| TADI-COF-Fc | In vitro and in vivo | -Contains iodine for X-ray absorption and a chromium-phenanthroline (Fc) group for disrupting redox balance and inducing ferroptosis; enhance lipid peroxidation and ferroptotic cell death, effective in vitro and in vivo | -The small size (about 110 nm) may impact biodistribution and tumor targeting, and there is inadequate data on in vivo metabolism and toxicity | 119 | |
| GDY–CeO2 nanocomposites | In vitro and in vivo | -miR181a enhances the sensitivity of the ESCC cell line KYSE30 to radiotherapy; induces DNA damage and apoptosis to overcome radioresistance | -CeO2 nanoparticles tend to aggregate, reducing activity; bioavailability and biocompatibility need improvement | 120 | |
| iE-PRNPs | In vitro | -Significant effects in esophageal cancer cells with high and low EGFR through G2/M phase arrest, increased reactive oxygen species, and enhanced DNA double-strand breaks | -The low G2/M phase arrest may relate to slow drug release and IC15 concentration; further research is needed for clinical translation | 121 | |
| MnSe2–lipid | In vitro and in vivo | -Activates the cGAS-STING pathway, promoting the upregulation of p-IRF3, IFN-β, and CXCL10; enhances antitumor immune responses, reduces radiotherapy side effects, and improves treatment efficacy | -The primary mechanism is limited to the tumor environment; biocompatibility and metabolism in normal tissues are needed | 122 | |
| FA-BSA-Au@PTX/CUR | In vitro | -Concentrating ionizing radiation produces more cytotoxic secondary charged particles, maximizing X-ray damage to tumors. The G2/M phase is most sensitive to ionizing radiation; paclitaxel (PTX) induces G2/M arrest and acts as a radiosensitizer | -Further investigation of radiosensitization is needed, along with optimization of complex preparation, large-scale production, and long-term safety | 123 | |
| AuNPs-D-P-DA | In vitro and in vivo | -AuNPs aggregate, enhancing accumulation and improving radiosensitization, DNA damage, apoptosis, and antitumor effects through fatty acid oxidation (FAO) in the acidic tumor microenvironment | -Excessive aggregation may reduce permeability and radiosensitization. Further studies are needed to optimize administration routes, doses, and toxicity | 73 | |
| EBRT and Rhenium-188 (188Re)-liposome | In vitro and in vivo | -EBRT enhances the accumulation of 188Re-liposomes in tumors, liver, and feces, possibly increasing tumor sensitivity to radioactive isotopes | -May harm the lower urinary tract and gastrointestinal system | 124 | |
| Au8(C21H27O2)8 (Au8NC) | In vitro and in vivo | -Generate reactive oxygen species (ROS) under X-ray exposure leads to irreversible apoptosis in cancer cells | -Long-term biosafety needs evaluation; preparation should be optimized for cost, reproducibility, and scalability | 125 |
| Type | NP | Assessment | Mechanism | Limitations | Ref. |
|---|---|---|---|---|---|
| Gene therapy | Thermosensitive gel-nano | In vitro and in vivo | -Use siRNA to inhibit BACH1 and restore T-cell antitumor immunity; REACTIVATE mutant p53 with PRIMA-1 to inhibit tumor growth | -Long-term stability, degradation in vivo, and targeting and efficacy require further study | 126 |
| Lipid nanovector (EYLN) | In vitro and in vivo | -siRNA targeting LPCAT1 (siLPCAT1) is combined with EYLN coated in leukocyte membranes to form mEYLNs-Dox/siLPCAT1, enhancing cell uptake, tumor targeting, and circulation time | -May trigger immune rejection and faces challenges in preparation and large-scale production | 127 | |
| GDY–CeO2 nanocomposites | In vitro and in vivo | -Loading miR181a onto GDY–CeO2 nanozymes enhances radiotherapy by targeting RAD17; PEG-iRGD improves tumor targeting and penetration | -Tend to aggregate, reducing activity; improvements in bioavailability and biocompatibility are needed | 120 | |
| Photoactivated DNA nanodrug (MCD@ TMPyP4@DOX) | In vitro and in vivo | -Target tumor mitochondria using MUC1 and CytC affinity; under near-infrared light, the nanodrug generates ROS, damaging mitochondria, reducing ATP levels, inhibiting P-gp activity, and releasing P-gp DNAzyme to cleave MDR1 mRNA and suppress P-gp expression | -May affect blood cell counts; preparation and administration need optimization for better stability and therapeutic outcomes | 128 | |
| Protamine sulfate–nanodiamond hybrid nanoparticles | In vitro | -PS@ND nanoparticles delivering miR-203 suppressed Ran and DNp63 expression, inhibiting esophageal cancer cell proliferation and migration | -In vivo efficacy and safety are untested, and large-scale production feasibility remains unclear, potentially limiting clinical application | 129 | |
| Four-way junction RNA nanocarrier, 4WJ-EGFRapt-miR-375-PTX | In vitro and in vivo | -Decorated with an EGFR-specific aptamer (EGFRapt), enhances tumor targeting and penetrates 3D ESCC spheroids by promoting endocytosis | -Uniform tumor penetration leads to uneven drug distribution and reduced efficacy | 130 | |
| Immuno-therapy | Lipid Nanoparticles (LNPs) | In vitro and in vivo | -Inhibit PD-L1 expression to boost antitumor immunity via gene silencing; stimulate the immune system and enhance chemotherapy for tumor treatment | -Long-term stability and biocompatibility need evaluation, and drug loading and encapsulation efficiency need improvement | 131 |
| ITFn-Pt(IV) | In vitro and in vivo | -Integrate PD-L1 blockade, chemotherapy, and T-cell activation; enhance T-cell response and tumor infiltration | -Long-term efficacy and safety are needed to evaluate | 132 | |
| OTS964/Ce6@NP | In vitro and in vivo | -Induce significant infiltration of natural killer (NK) cells into the tumor microenvironment, triggering a robust antitumor immune response | -Further studies on other immune cells are needed, and preparation processes and drug loading efficiency require optimization for better in vivo stability and drug release | 133 |
3.1 Advancements in chemotherapy
Current chemotherapy regimens for ESCC typically involve combination therapies to enhance efficacy and minimize resistance. Cisplatin, often paired with fluorouracil (5-FU) or taxanes such as paclitaxel or docetaxel, remains a key component in the first-line treatment.99 These combinations are administered via intravenous infusion and are designed to target cancer cells through multiple mechanisms. Cisplatin exerts its anticancer effects by forming cross-links in DNA, which leads to mispairing of nucleotides and ultimately triggers apoptosis in cancer cells. Similarly, fluorouracil (5-FU) disrupts DNA synthesis by inhibiting thymidylate synthase, while taxanes stabilize microtubules to prevent cell division, contributing to apoptosis.100 Despite the effectiveness of these chemotherapy regimens, resistance remains a significant barrier to successful treatment in ESCC. Despite its effectiveness, cisplatin monotherapy is often hindered by systemic toxicity and the development of drug resistance,101–103 drug resistance in ESCC can be attributed to several factors. One primary factor is the increased efflux of drugs from cancer cells, mediated by the upregulation of ATP-binding cassette (ABC) transporters, which limits the accumulation of therapeutic agents within the cells. Additionally, the overexpression of DNA repair proteins, such as excision repair cross-complementation group 1 (ERCC1), enables cancer cells to effectively repair DNA damage caused by these therapies, thereby preventing apoptosis. Impaired drug uptake is another critical factor contributing to resistance.104 For instance, decreased expression of nucleoside transporters like hENT1 hampers the intracellular accumulation of drugs, while elevated levels of glutathione serve to detoxify and neutralize the therapeutic agents, further diminishing their effectiveness.105,106 Furthermore, alterations in apoptotic pathways and the activation of stress response mechanisms can also complicate treatment outcomes.107,108 Together, these resistance mechanisms present significant challenges to the efficacy of current ESCC chemotherapy regimens, necessitating the exploration of novel therapeutic strategies to overcome these barriers and improve patient outcomes.To address these limitations, nanoparticle-based delivery systems have emerged as a promising solution. Nanomaterials can target resistance genes and enhance the delivery of chemotherapy drugs, acidic environment, thereby improving targeting and intracellular delivery.134 In the context of first-line chemotherapy regimens, combinations such as the FOLFOX (oxaliplatin, 5-FU, leucovorin) and CROSS (carboplatin, paclitaxel) have also been investigated. The addition of nanoparticles to these regimens holds promise for further enhancing treatment outcomes.131 Researchers have also explored chemical modifications of chemotherapy drugs to improve their delivery, targeting functions, and reduce toxicity. A novel four-way junction RNA nanoparticle carrier-4WJ-EGFRapt-miR-375-PTX-was developed to simultaneously deliver miR-375 and the chemotherapy drug paclitaxel (PTX), decorated with epidermal growth factor receptor (EGFR) specific aptamers (EGFRapt). This nanoparticle exhibited good thermal and pH stability, with EGFRapt modification enhancing tumor cell endocytosis and deep penetration into three-dimensional ESCC spheroids. In an ESCC xenograft mouse model, this nanoparticle selectively distributed to tumor sites through EGFRapt-mediated active targeting, showing more effective therapeutic efficacy with co-delivery of miR-375 and PTX while exhibiting lower systemic toxicity130 (Fig. 3a).
 | ||
| Fig. 3 (A) Schematic of the 4WJ-EGFRapt-miR-375-PTX nanoparticle, enhancing tumor targeting and co-delivering miR-375 and paclitaxel (PTX) for improved therapy and reduced toxicity in ESCC. (B) Schematic of ZrRuMn MONs@mem mechanisms in vitro and in vivo. Upon targeting, the nanoparticles demonstrate strong XDT and CO therapy, with enhanced MRI under X-ray irradiation. Reprinted with permission from ref. 126 and 137, Copyright © 2021 and 2022, JNAN and Angew. Chem. Int. Ed. | ||
Overall, the integration of nanoparticles into chemotherapy for ESCC represents a significant advancement. These systems not only enhance drug delivery and efficacy but also mitigate the adverse effects commonly associated with traditional therapies. The successful clinical application of nanoparticle formulations, such as nab-paclitaxel, underscores the potential of this innovative approach in transforming the treatment landscape for ESCC, particularly within first-line chemotherapy regimens, ultimately improving patient outcomes.
3.2 Synergistic approaches with radiotherapy
Currently, radiotherapy (RT) plays a crucial role in the treatment of ESCC, often used in conjunction with chemotherapy to enhance efficacy. These treatment strategies aim to enhance antitumor effects through synergistic actions. The mechanisms of radiotherapy ineffectiveness include the enhanced DNA repair capacity of tumor cells, hypoxic microenvironments that reduce radiation sensitivity, and cell cycle regulation allowing evasion of damage. Tumor cells may neutralize reactive oxygen species (ROS) from radiotherapy by increasing antioxidant enzymes,135 while fibroblasts and immune cells in the tumor microenvironment also impact effectiveness.136,137 Tumor cells can escape immune surveillance by downregulating antigen expression or secreting immunosuppressive factors.138 Cellular heterogeneity results in inconsistent responses to radiotherapy, with some resistant cells surviving treatment and proliferating again.139,140 These mechanisms collectively diminish the efficacy of radiotherapy, highlighting the need for new therapeutic strategies to overcome resistance.To overcome these resistance mechanisms in ESCC, the integration of nanoparticles shows significant potential. Nanoparticles can increase tumor cell sensitivity to radiation, thereby improving therapeutic outcomes. For example, gold nanoparticles can enhance radiation sensitivity through localized thermal effects.125 Additionally, nanoparticles can precisely deliver radioactive isotopes or radiosensitizers, minimizing damage to surrounding healthy tissues. Dai et al. developed a stable and enzyme-like GDY-CeO2 nanocomposite by anchoring and dispersing cerium oxide (CeO2) nanoparticles on graphdiyne (GDY). The GDY–CeO2 nanozymes exhibit excellent catalase-like activity, effectively decomposing hydrogen peroxide into oxygen, thereby alleviating tumor hypoxia and enhancing the sensitivity of radiotherapy. Additionally, the nanocomposite is loaded with the miRNA miR181a, which targets the DNA repair protein RAD17 to increase cellular sensitivity to radiation. To further enhance delivery to tumor sites, the nanocomposite is modified with the tumor-targeting peptide iRGD. This multipronged strategy utilizing GDY–CeO2 nanozymes and miR181a demonstrates improved therapeutic efficacy both in vitro and in vivo141 (Fig. 3b). Moreover, multifunctional nanoparticles with imaging capabilities can enable real-time monitoring of tumor responses to radiotherapy, providing important support for personalized treatment.142 These studies indicate that the strategy of combining nanoparticles with radiotherapy offers new solutions to overcome resistance and enhances overall treatment effectiveness.
3.3 Innovations in gene therapy
Gene therapy represents a promising approach for treating ESCC by targeting genetic mutations or modulating gene expression. However, the effective delivery of nucleic acids, such as siRNA, miRNA, and CRISPR/Cas9 components, poses significant challenges due to their instability in the bloodstream and difficulty penetrating cell membranes.143–145 Nanoparticle-based delivery systems offer a potential solution, protecting nucleic acids from enzymatic degradation and enhancing their cellular uptake. These systems function through various mechanisms, protecting them from enzymatic degradation while improving bioavailability.A key mechanism involves targeted gene delivery via receptor-mediated endocytosis, where nanoparticles are functionalized with ligands or aptamers that bind to overexpressed receptors on ESCC cells, such as EGFR. This enables selective entry into cancer cells, ensuring that gene therapy materials are delivered precisely to the tumor. For instance, the 4WJ-EGFRapt-miR-375-PTX system leverages EGFR-mediated targeting to co-deliver miR-375 and paclitaxel (PTX), effectively inhibiting tumor proliferation, migration, and invasion.130 Another critical mechanism is gene silencing via RNA interference. Nanoparticles are used to deliver small interfering RNA (siRNA) or microRNA (miRNA), which bind to specific mRNA molecules, preventing their translation and leading to gene silencing. In one example, GDY–CeO2 nanoparticles loaded with miR181a target the RAD17 gene, reducing RAD17 protein expression. This impairs the DNA repair capacity of cancer cells, making them more vulnerable to treatments like radiotherapy.120 Nanoparticle systems are also designed for stimuli-responsive gene release, triggered by the tumor microenvironment. These systems respond to specific stimuli, such as the acidic pH of tumors or the presence of overexpressed enzymes like matrix metalloproteinases (MMPs). This targeted release ensures that genetic materials are deployed only where needed. For example, a photo-activated DNA nanomedicine (MCD@TMPyP4@DOX) uses photodynamic therapy (PDT) to generate reactive oxygen species (ROS), which trigger the nanomedicine to self-disassemble, releasing P-gp DNAzyme to silence MDR1 mRNA. This process also inhibits P-glycoprotein (P-gp), which is involved in multidrug resistance (MDR), allowing chemotherapy drugs like doxorubicin (DOX) to accumulate in tumor cells more effectively.128 In addition to gene delivery, nanoparticle systems help overcome drug resistance through combination therapy. For example, lipid nanocarriers such as mEYLNs-Dox/siLPCAT1 co-deliver doxorubicin (DOX) and siRNA targeting the lipid metabolism gene LPCAT1, which is overexpressed in ESCC. This combined approach enhances the suppression of cancer cell proliferation, migration, and metastasis compared to single therapies, showing that combining gene therapy with chemotherapy can significantly improve therapeutic outcomes.127
These mechanisms illustrate how nanoparticle-mediated gene therapy improves treatment efficacy in ESCC by enabling precise targeting, efficient delivery, and controlled release of genetic materials. By integrating these strategies, nanoparticle systems offer a promising future for gene therapy in cancer treatment.
3.4 Enhancements in immunotherapy and immune modulation
ESCC has seen advancements in immunotherapy, with key strategies including immune checkpoint inhibitors, such as pembrolizumab and nivolumab, targeting PD-1/PD-L1 pathways.21,146 These agents have shown promise, especially in patients with advanced ESCC, leading to improved survival rates in some clinical trials.147 Combination therapies, incorporating chemotherapy or radiotherapy with immunotherapy, are also being explored to enhance therapeutic efficacy.148,149 However, immune therapy often encounters challenges leading to treatment failure. One major mechanism of resistance is the upregulation of immune checkpoint proteins, which inhibit T-cell activation and function.150 Additionally, the tumor microenvironment in ESCC can be immunosuppressive, characterized by high levels of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) that impede anti-tumor immunity.151 Moreover, tumor heterogeneity and mutations in antigen presentation machinery can prevent effective recognition and targeting by immune cells.152 These factors contribute to the variable responses seen in patients and highlight the need for novel strategies to enhance immunotherapeutic effectiveness in ESCC.Nanoparticles are emerging as a promising tool in enhancing immunotherapy for ESCC by improving the delivery of immunomodulatory agents and overcoming resistance mechanisms. The combination of the immune checkpoint inhibitor sintilimab with Nab-PTX and platinum-based drugs has shown considerable success as a first-line therapy for metastatic ESCC, achieving an objective response rate of 72.7% and a disease control rate of 90.9%.153 Studies have shown that nanoparticle-based delivery systems can enhance immune responses through various mechanisms. One key mechanism involves the induction of immunogenic cell death (ICD), where nanoparticles are engineered to improve immune cell activation. For example, research has developed a novel nanocomposite material called PBP@CpG, composed of black phosphorus functionalized with dopamine and loaded with the immune adjuvant CpG. This nanocomposite has been shown to trigger tumor cell death via a photoacoustic effect, leading to the release of tumor-associated antigens. These antigens promote dendritic cell maturation, boosting antigen presentation and eliciting a strong adaptive immune response. Additionally, PBP@CpG increases CD8+ T-cell infiltration into tumors and enhances the expression of IFN-γ and Granzyme B, improving the cytotoxic activity of both T-cells and natural killer cells. Furthermore, systemic immune responses are stimulated by this nanocomposite, which increases levels of TNF-α, IL-2, and IL-12 in the bloodstream, further strengthening the antitumor effect154 (Fig. 4). Other studies have highlighted the potential of a thermosensitive gel nanoparticle system, which has been shown to increase T-cell infiltration and enhance immune response within the tumor microenvironment. This system boosts the ratio of CD4+ and CD8+ T-cells within tumor-infiltrating lymphocytes (TILs), while simultaneously reducing the number of T-regulatory cells (Tregs), which are known to suppress immune responses. After treatment with this gel nanoparticle system, IFN-γ levels increase significantly, indicating a potent antitumor immune response. Additionally, the gel system remains in the tumor for extended periods, leading to a prolonged and more effective immune response compared to conventional nanoparticles.126 In terms of fusion immunotherapy systems, studies have introduced the ITFn-Pt(IV) system to enhance the immune response in ESCC by combining PD-L1 blockade, chemotherapy, and T-cell activation. This system uses temperature-regulated drug loading technology to create a smart delivery platform that releases T-cell activating peptides in response to the tumor microenvironment. It also incorporates PD-L1 nanobodies, which target and deliver platinum-based chemotherapy drugs directly to tumor cells, inducing immunogenic cell death and promoting dendritic cell maturation. Preclinical models of ESCC have demonstrated that this system significantly improves T-cell infiltration and antitumor efficacy without causing systemic side effects.132 Finally, lipid–core–shell nanoparticle platforms have been shown to co-deliver the FOLFOX chemotherapy regimen (miriplatin, 5-fluoro-2′-deoxyuridine-5′-monophosphate, and calcium leucovorin) along with siRNA targeting PD-L1. This approach enhances the efficacy of PD-1/PD-L1 immune checkpoint inhibition by blocking PD-L1 expression in tumor cells, overcoming chemoresistance, and boosting the infiltration of CD8+ T-cells and mature dendritic cells into the tumor microenvironment. These mechanisms work synergistically to promote a stronger immune response, significantly improving antitumor outcomes.131
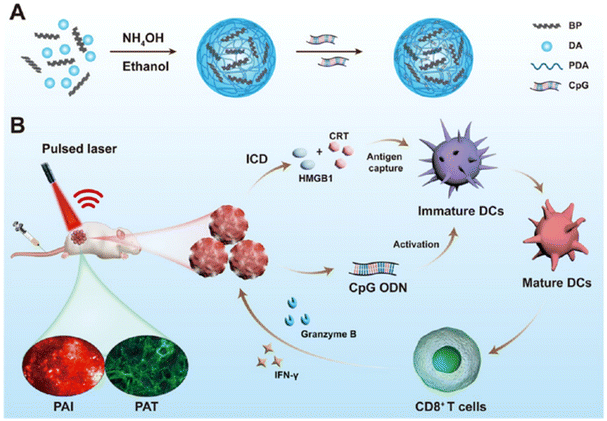 | ||
| Fig. 4 (A) Schematic illustration of the synthesis of PBP@CpG, a multifunctional nanocomposite combining PBP and CpG for enhanced therapeutic effects. (B) PBP@CpG nanoparticles administered intravenously in mice, followed by tumor photoacoustic imaging and immunotherapy, demonstrating their potential for targeted cancer treatment. Reprinted with permission from ref. 150, Copyright © 2023, ACS. | ||
In summary, immunotherapy for ESCC has advanced beyond traditional approaches by incorporating nanoparticle systems that utilize multiple mechanisms to enhance immune responses. These mechanisms include inducing immunogenic cell death, promoting dendritic cell maturation, increasing T-cell infiltration, and targeting immune checkpoints such as PD-L1. Fig. 5 illustrates the immune microenvironment of esophageal squamous cell carcinoma (ESCC) and the role of nanoparticles in enhancing immunotherapy. By integrating these innovative strategies into clinical practice, nanoparticle-based immunotherapy has the potential to significantly improve treatment outcomes for patients with ESCC.
4. Challenges of nanoparticle-based drug delivery systems
While nanoparticle-based drug delivery systems offer promising advantages for treating ESCC, their clinical translation faces several significant challenges. These challenges include biocompatibility, targeting efficiency, manufacturing scalability, and potential toxicity. Addressing these challenges is crucial to fully realizing the therapeutic potential of nanoparticle systems.4.1 Biocompatibility, metabolism, toxicity, and immune response
Ensuring the biocompatibility and safety of nanoparticles is essential for their clinical use. Nanoparticles should ideally be non-toxic, non-immunogenic, and biodegradable to minimize patient harm. Materials such as polymers, lipids, and metals should degrade without generating harmful by-products. However, certain metallic nanoparticles may risk accumulation in tissues, raising long-term toxicity concerns.155,156 Additionally, the body's defense mechanisms, particularly the reticuloendothelial system (RES), can recognize and clear these nanoparticles, diminishing their therapeutic effectiveness. Modifications such as polyethylene glycol (PEG) can help extend circulation time in the bloodstream.Moreover, some nanoparticles, especially those made from inorganic materials like gold or silver, can activate the immune system, leading to inflammation and rapid clearance from the bloodstream. This immune response can reduce the nanoparticles’ therapeutic effectiveness and, in some cases, trigger the generation of antibodies, complicating repeated treatments. Certain nanoparticles may also exhibit cytotoxicity due to their material composition or interactions with biological systems, such as generating reactive oxygen species (ROS), which can cause oxidative stress and cellular damage. These potential risks, including immune responses and toxicity, must be carefully evaluated through preclinical and clinical studies to ensure the safety and effectiveness of nanoparticle-based therapies.
4.2 Efficiency and specificity of drug delivery
Achieving efficient and specific delivery to tumor cells remains a major challenge for nanoparticle systems. Despite the enhanced permeability and retention (EPR) effect that allows nanoparticles to accumulate in tumors, this process is not always consistent or efficient across different tumor types, including ESCC. Tumor heterogeneity, variations in vascular permeability, and the presence of biological barriers, such as dense extracellular matrices, can limit the penetration of nanoparticles into the tumor tissue.157 Additionally, nanoparticles need to be precisely targeted to cancer cells to avoid uptake by healthy tissues, which could lead to off-target effects and toxicity.1584.3 Manufacturing and scalability
The development of nanoparticle drug delivery systems involves complex manufacturing processes that are crucial for their clinical application. While the quest for clinically effective treatments for esophageal squamous cell carcinoma (ESCC) continues, advancements in nanotechnology have enabled the exploration of various production methods.159,160 Large-scale production techniques such as lipid nanoparticles (LNPs) and liposomes are well-established, offering scalable solutions for drug delivery systems.161 Recent breakthroughs in the amplification of extracellular vesicles have contributed to the field, providing novel approaches to nanoparticle production.161 These advancements offer potential for scalable and cost-effective production methods, which are crucial for translating nanotechnologies into clinically viable treatments.162 The focus is on developing methods that ensure consistent quality, safety, and efficacy, which are paramount for regulatory approval and public health protection.163In summary, the scalability of nanoparticle production is driven by the need for innovative and efficient manufacturing processes that can meet the demands of various therapeutic applications. The progress in this field is based on existing facts and advancements in technology, rather than being limited to the clinical outcomes of specific diseases.163,164
The complex immune microenvironment of esophageal squamous cell carcinoma (ESCC), which is characterized by immune suppression, tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and regulatory T cells (Tregs). Nanoparticle-based drug delivery systems are depicted as reversing immune state, preventing PMN formation, inhibiting recurrence and harnessing immune cells. Partly created with biorender.com.
4.4 Ethical challenges
Nanoparticle therapies present ethical concerns, primarily regarding long-term health and environmental impacts, and ensuring equitable access to these treatments.164 Transparency about potential risks and benefits is essential to maintain public trust and informed consent. Ethical use and regulatory oversight are vital to prevent misuse and protect public health.5. Future prospects
The future of nanoparticle-based drug delivery for ESCC treatment is promising, with advancements in nanotechnology and cancer biology offering solutions to current challenges. Novel, biocompatible nanomaterials, including hybrid and “smart” nanoparticles, are being developed for better stability, targeting, and controlled drug release, with the ability to respond to tumor-specific stimuli.Given the advanced stage at which ESCC is often diagnosed, targeting the tumor microenvironment is crucial. Nanoparticles can be engineered to target biomarkers like PD-L1, enhance immune responses, and inhibit tumor migration, reshaping the immune-suppressive microenvironment for improved outcomes such as PD-L1, enhancing immune responses and inhibiting tumor cell migration.165,166
Personalized nanomedicine, tailored to each patient's tumor profile, is another exciting direction.15,167 Advances in genomics will enable the design of nanoparticles that target specific genetic alterations, improving treatment efficacy and reducing side effects.168
Emerging therapies like photodynamic and photothermal therapy, combined with nanoparticle delivery, offer additional strategies to treat ESCC by inducing targeted cell damage, providing new options for challenging cases.169
6. Conclusions
Nanoparticle-based drug delivery systems represent a transformative approach for treating ESCC. By enhancing drug targeting and delivery, these systems can improve therapeutic outcomes while minimizing side effects. However, significant challenges such as biocompatibility, targeted delivery, and regulatory hurdles must be addressed to facilitate clinical adoption. Continued research and innovation in this field will be essential for unlocking the full potential of nanoparticles in cancer therapy.Author contributions
Linjia Peng and Yanfeng Liang conceptualized the article and were responsible for drafting the manuscript and data analysis. Zixuan Gao, Qiuli Zhang, and Xiaonan Guo contributed to the writing of specific sections and conducted a thorough review and revision of the manuscript. Daxiang Cui reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.Data availability
Permission has been acquired for the use of all figures’ copyright in this review.This is a review paper. No primary research results, software or code have been included and no new data were generated or analysed as part of this review. This review cited some data from published papers, and matched data can be obtained from the matched published papers.
Conflicts of interest
All authors declare no conflict of interest.Acknowledgements
This work was supported by Innovation Group Project of National Natural Science Foundation of China (No. 81921002), International cooperation project (No. 82020108017).References
- E. Morgan, I. Soerjomataram, H. Rumgay, H. G. Coleman, A. P. Thrift and J. Vignat, et al., The Global Landscape of Esophageal Squamous Cell Carcinoma and Esophageal Adenocarcinoma Incidence and Mortality in 2020 and Projections to 2040: New Estimates From GLOBOCAN 2020, Gastroenterology, 2022, 163(3), 649–658 CrossRef PubMed.
- R. L. Siegel, A. N. Giaquinto and A. Jemal, Cancer statistics, 2024, CA Cancer J. Clin., 2024, 74(1), 12–49 CrossRef PubMed.
- P. Ahuja, R. Yadav, S. Goyal, C. Yadav, S. Ranga and L. Kadian, Targeting epigenetic deregulations for the management of esophageal carcinoma: recent advances and emerging approaches, Cell Biol. Toxicol., 2023, 39(6), 2437–2465 CrossRef PubMed.
- H. Simba, D. Menya, B. T. Mmbaga, C. Dzamalala, P. Finch and Y. Mlombe, et al., The contribution of smoking and smokeless tobacco to oesophageal squamous cell carcinoma risk in the African oesophageal cancer corridor: Results from the ESCCAPE multicentre case-control studies, Int. J. Cancer, 2023, 152(11), 2269–2282 CrossRef CAS PubMed.
- J. M. Ko, C. Guo, C. Liu, L. Ning, W. Dai and L. Tao, et al., Clonal relationship and alcohol consumption-associated mutational signature in synchronous hypopharyngeal tumours and oesophageal squamous cell carcinoma, Br. J. Cancer, 2022, 127(12), 2166–2174 CrossRef CAS PubMed.
- Z. Fu, S. Li, S. Han, C. Shi and Y. Zhang, Antibody drug conjugate: the “biological missile” for targeted cancer therapy, Signal Transduct. Target. Ther., 2022, 7(1), 93 CrossRef CAS PubMed.
- G. Masukume, B. T. Mmbaga, C. P. Dzamalala, Y. B. Mlombe, P. Finch and G. Nyakunga-Maro, et al., A very-hot food and beverage thermal exposure index and esophageal cancer risk in Malawi and Tanzania: findings from the ESCCAPE case-control studies, Br. J. Cancer, 2022, 127(6), 1106–1115 CrossRef PubMed.
- X. Zhang, X. Zheng, R. Gao, Y. Wang, T. Wei and Z. Zang, et al., Role of diet in the risks of esophageal adenocarcinoma and squamous cell carcinoma: an updated umbrella review, Eur. J. Nutr., 2024, 63(5), 1413–1424 CrossRef PubMed.
- T. Fukuchi, K. Hirasawa, C. Sato, M. Makazu, H. Kaneko and R. Kobayashi, et al., Potential roles of gastroesophageal reflux in patients with superficial esophageal squamous cell carcinoma without major causative risk factors, J. Gastroenterol., 2021, 56(10), 891–902 CrossRef CAS PubMed.
- K. P. Ko, Y. Huang, S. Zhang, G. Zou, B. Kim and J. Zhang, et al., Key Genetic Determinants Driving Esophageal Squamous Cell Carcinoma Initiation and Immune Evasion, Gastroenterology, 2023, 165(3), 613–628 CrossRef CAS PubMed.
- J. Chang, X. Zhao, Y. Wang, T. Liu, C. Zhong and Y. Lao, et al., Genomic alterations driving precancerous to cancerous lesions in esophageal cancer development, Cancer Cell, 2023, 41(12), 2038–2050 CrossRef CAS PubMed.
- L. Zhong, H. Li, W. Chang, Y. Ao, Z. Wen and Y. Chen, TP53 Mutations in Esophageal Squamous Cell Carcinoma, Front. Biosci., 2023, 28(9), 219 CrossRef CAS PubMed.
- M. Liu, Y. Liu, R. Zhou, Z. Liu, C. Guo and R. Xu, et al., Absence of NOTCH1 mutation and presence of CDKN2A deletion predict progression of esophageal lesions, J. Pathol., 2022, 258(1), 38–48 CrossRef CAS PubMed.
- T. Li, L. Xu, J. Teng, Y. Ma, W. Liu and Y. Wang, et al., GADD45G Interacts with E-cadherin to Suppress the Migration and Invasion of Esophageal Squamous Cell Carcinoma, Dig. Dis Sci., 2020, 65(4), 1032–1041 CrossRef CAS PubMed.
- S. Zheng, B. Liu and X. Guan, The Role of Tumor Microenvironment in Invasion and Metastasis of Esophageal Squamous Cell Carcinoma, Front. Oncol., 2022, 12, 911285 CrossRef CAS PubMed.
- F. Zhao, H. Tian, Y. Wang, J. Zhang, F. Liu and L. Fu, LINC01004-SPI1 axis-activated SIGLEC9 in tumor-associated macrophages induces radioresistance and the formation of immunosuppressive tumor microenvironment in esophageal squamous cell carcinoma, Cancer Immunol. Immunother., 2023, 72(6), 1835–1851 CrossRef CAS PubMed.
- S. Nakamura, K. Ohuchida, Y. Ohtsubo, Y. Yamada, C. Tsutsumi and S. Okuda, et al., Single-cell transcriptome analysis reveals functional changes in tumour-infiltrating B lymphocytes after chemotherapy in oesophageal squamous cell carcinoma, Clin. Transl. Med., 2023, 13(1), e1181 CrossRef CAS PubMed.
- H. Yuan, Z. Zhao, J. Xu, R. Zhang, L. Ma and J. Han, et al., Hypoxia-induced TMTC3 expression in esophageal squamous cell carcinoma potentiates tumor angiogenesis through Rho GTPase/STAT3/VEGFA pathway, J. Exp. Clin. Cancer Res., 2023, 42(1), 249 CrossRef CAS PubMed.
- J. A. Ajani, T. A. D'Amico, D. J. Bentrem, D. Cooke, C. Corvera and P. Das, et al., Esophageal and Esophagogastric Junction Cancers, Version 2.2023, NCCN Clinical Practice Guidelines in Oncology, J. Natl. Compr. Cancer Netw., 2023, 21(4), 393–422 Search PubMed.
- L. An, M. Li and Q. Jia, Mechanisms of radiotherapy resistance and radiosensitization strategies for esophageal squamous cell carcinoma, Mol. Cancer, 2023, 22(1), 140 CrossRef CAS PubMed.
- J. Yin, J. Yuan, Y. Li, Y. Fang, R. Wang and H. Jiao, et al., Neoadjuvant adebrelimab in locally advanced resectable esophageal squamous cell carcinoma: a phase 1b trial, Nat. Med., 2023, 29(8), 2068–2078 CrossRef CAS PubMed.
- J. K. Waters and S. I. Reznik, Update on Management of Squamous Cell Esophageal Cancer, Curr. Oncol. Rep., 2022, 24(3), 375–385 CrossRef CAS PubMed.
- Y. Zhang, Z. Li, Y. Huang, B. Zou and Y. Xu, Amplifying cancer treatment: advances in tumor immunotherapy and nanoparticle-based hyperthermia, Front. Immunol., 2023, 14, 1258786 CrossRef CAS PubMed.
- E. Fleming and Y. Luo, Co-delivery of synergistic antioxidants from food sources for the prevention of oxidative stress, J. Agric. Food Res., 2021, 3, 100107 CAS.
- J. Zhang, S. Wang, D. Zhang, X. He, X. Wang and H. Han, et al., Nanoparticle-based drug delivery systems to enhance cancer immunotherapy in solid tumors, Front. Immunol., 2023, 14, 1230893 CrossRef CAS PubMed.
- A. R. Afshari, M. Sanati, H. Mollazadeh, P. Kesharwani, T. P. Johnston and A. Sahebkar, Nanoparticle-based drug delivery systems in cancer: A focus on inflammatory pathways, Semin. Cancer Biol., 2022, 86(Pt 2), 860–872 CrossRef CAS PubMed.
- C. K. Elechalawar, S. K. Gulla, R. V. Roy, N. Means, Y. Zhang and S. Asifa, et al., Biodistribution and therapeutic efficacy of a gold nanoparticle-based targeted drug delivery system against pancreatic cancer, Cancer Lett., 2024, 589, 216810 CrossRef CAS PubMed.
- D. Dhar, S. Ghosh, S. Mukherjee, S. Dhara, J. Chatterjee and S. Das, Assessment of chitosan-coated zinc cobalt ferrite nanoparticle as a multifunctional theranostic platform facilitating pH-sensitive drug delivery and OCT image contrast enhancement, Int. J. Pharm., 2024, 654, 123999 CrossRef CAS PubMed.
- X. Yu, J. Liu, A. Bauer, X. Wei, S. Smith and S. Ning, et al., Enhancing tumor endothelial permeability using MUC18-targeted gold nanorods and mild hyperthermia, J. Colloid Interface Sci., 2024, 676, 101–109 CrossRef CAS PubMed.
- X. Gong, Z. Wang, L. Zhang, W. Dong, R. Wang and Y. Liu, et al., A novel carbon-nanodots-based theranostic nano-drug delivery system for mitochondria-targeted imaging and glutathione-activated delivering camptothecin, Colloids Surf., B, 2022, 218, 112712 CrossRef CAS PubMed.
- N. Vahedi, F. Tabandeh and M. Mahmoudifard, Hyaluronic acid-graphene quantum dot nanocomposite: Potential target drug delivery and cancer cell imaging, Biotechnol. Appl. Biochem., 2022, 69(3), 1068–1079 CrossRef CAS PubMed.
- A. Gheata, G. Gaulier, G. Campargue, J. Vuilleumier, S. Kaiser and I. Gautschi, et al., Photoresponsive Nanocarriers Based on Lithium Niobate Nanoparticles for Harmonic Imaging and On-Demand Release of Anticancer Chemotherapeutics, ACS Nanosci. Au, 2022, 2(4), 355–366 CrossRef CAS PubMed.
- J. Deng, S. Yuan, W. Pan, Q. Li and Z. Chen, Nanotherapy to Reshape the Tumor Microenvironment: A New Strategy for Prostate Cancer Treatment, ACS Omega, 2024, 9(25), 26878–26899 CrossRef CAS PubMed.
- L. Zhang, J. Shi, M. H. Zhu, Y. Huang, Q. Lu and P. Sun, et al., Liposomes-enabled cancer chemoimmunotherapy, Biomaterials, 2025, 313, 122801 CrossRef CAS PubMed.
- M. D. Fulton and W. Najahi-Missaoui, Liposomes in Cancer Therapy: How Did We Start and Where Are We Now, Int. J. Mol. Sci., 2023, 24(7), 1–23 Search PubMed.
- D. N. Moholkar, R. Kandimalla, R. C. Gupta and F. Aqil, Advances in lipid-based carriers for cancer therapeutics: Liposomes, exosomes and hybrid exosomes, Cancer Lett., 2023, 565, 216220 CrossRef CAS PubMed.
- W. Chen, Y. Xu, D. Yang, P. Wang, Y. Xu and J. Zhu, et al., Preparation of Liposomes Coated Superparamagnetic Iron Oxide Nanoparticles for Targeting and Imaging Brain Glioma, Nano Biomed. Eng., 2022, 14(1), 71–80 CAS.
- H. Yu, W. Zhu, C. Lin, M. Jia, X. Tan and Z. Yuan, et al., Stromal and tumor immune microenvironment reprogramming through multifunctional cisplatin-based liposomes boosts the efficacy of anti-PD-1 immunotherapy in pancreatic cancer, Biomater. Sci., 2023, 12(1), 116–133 RSC.
- Z. Gao, J. Zhang, Y. Hou, J. Lu, J. Liang and Y. Gao, et al., Boosting the synergism between cancer ferroptosis and immunotherapy via targeted stimuli-responsive liposomes, Biomaterials, 2024, 305, 122442 CrossRef CAS PubMed.
- J. Zou, Site-specific delivery of cisplatin and paclitaxel mediated by liposomes: A promising approach in cancer chemotherapy, Environ. Res., 2023, 238(Pt 1), 117111 CrossRef CAS PubMed.
- Z. Dong, Q. Zhang, C. Wang, W. Hu, X. Yu and M. Guo, et al., Combined Thermosensitive Gel Co-Loaded with Dermaseptin-PP and PTX Liposomes for Effective Local Chemotherapy, Int. J. Nanomed., 2023, 18, 413–424 CrossRef CAS PubMed.
- X. Li, S. Guan, H. Li, D. Li, D. Liu and J. Wang, et al., Polysialic acid-functionalized liposomes for efficient honokiol delivery to inhibit breast cancer growth and metastasis, Drug Delivery, 2023, 30(1), 2181746 CrossRef PubMed.
- X. L. Liu, X. Dong, S. C. Yang, X. Lai, H. J. Liu and Y. Gao, et al., Biomimetic Liposomal Nanoplatinum for Targeted Cancer Chemophototherapy, Adv. Sci., 2021, 8(8), 2003679 CrossRef CAS PubMed.
- C. Li, Z. Li, X. Gong, J. Liu, T. Zheng and F. Wang, et al., Acidic tumor microenvironment-sensitive liposomes enhance colorectal cancer therapy by acting on both tumor cells and cancer-associated fibroblasts, Nanoscale, 2021, 13(23), 10509–10525 RSC.
- M. A. Beach, U. Nayanathara, Y. Gao, C. Zhang, Y. Xiong and Y. Wang, et al., Polymeric Nanoparticles for Drug Delivery, Chem. Rev., 2024, 124(9), 5505–5616 CrossRef CAS PubMed.
- L. L. Haidar, M. Bilek and B. Akhavan, Surface Bio-engineered Polymeric Nanoparticles, Small, 2024, 20(21), e2310876 CrossRef PubMed.
- I. Rather, N. Shafiq, J. Shukla, G. Kaur, S. Pandey and R. K. Bhandari, et al., Bio-evaluation of poly(lactic-co-glycolic) acid nanoparticles loaded with radiolabelled rifampicin, Br. J. Clin. Pharmacol., 2023, 89(12), 3702–3714 CrossRef CAS PubMed.
- A. Orekhova, C. Palocci, L. Chronopoulou, G. De Angelis, C. Badiali and V. Petruccelli, et al., Poly-(lactic-co-glycolic) Acid Nanoparticles Entrapping Pterostilbene for Targeting Aspergillus Section Nigri, Molecules, 2022, 27(17), 1–13 CrossRef PubMed.
- G. Revilla, N. Al Qtaish, P. Caruana, M. Sainz-Ramos, T. Lopez-Mendez and F. Rodriguez, et al., Lenvatinib-Loaded Poly(lactic-co-glycolic acid) Nanoparticles with Epidermal Growth Factor Receptor Antibody Conjugation as a Preclinical Approach to Therapeutically Improve Thyroid Cancer with Aggressive Behavior, Biomolecules, 2023, 13(11), 1–19 CrossRef PubMed.
- S. Rashki, K. Asgarpour, H. Tarrahimofrad, M. Hashemipour, M. S. Ebrahimi and H. Fathizadeh, et al., Chitosan-based nanoparticles against bacterial infections, Carbohydr. Polym., 2021, 251, 117108 CrossRef CAS PubMed.
- R. Pathak, S. Bhatt, V. D. Punetha and M. Punetha, Chitosan nanoparticles and based composites as a biocompatible vehicle for drug delivery: A review, Int. J. Biol. Macromol., 2023, 253(Pt 7), 127369 CrossRef CAS PubMed.
- T. Kim, H. S. Han, K. Yang, Y. M. Kim, K. Nam and K. H. Park, et al., Nanoengineered Polymeric RNA Nanoparticles for Controlled Biodistribution and Efficient Targeted Cancer Therapy, ACS Nano, 2024, 18(11), 7972–7988 CrossRef CAS PubMed.
- A. M. Itoo, M. Paul, B. Ghosh and S. Biswas, Polymeric graphene oxide nanoparticles loaded with doxorubicin for combined photothermal and chemotherapy in triple negative breast cancer, Biomater. Adv., 2023, 153, 213550 CrossRef CAS PubMed.
- H. N. du Preez and M. Halma, Graphene-based Nanomaterials: Uses, Environmental Fate, and Human Health Hazards, Nano Biomed. Eng., 2024, 16(2), 219–231 CrossRef CAS.
- J. Liu, S. He, Y. Luo, Y. Zhang, X. Du and C. Xu, et al., Tumor-Microenvironment-Activatable Polymer Nano-Immunomodulator for Precision Cancer Photoimmunotherapy, Adv. Mater., 2022, 34(8), e2106654 CrossRef PubMed.
- S. Y. Huang, N. T. Yeh, T. H. Wang, T. C. Hsu, H. Y. Chin and B. S. Tzang, et al., Onion-like doxorubicin-carrying polymeric nanomicelles with tumor acidity-sensitive dePEGylation to expose positively-charged chitosan shell for enhanced cancer chemotherapy, Int. J. Biol. Macromol., 2023, 227, 925–937 CrossRef CAS PubMed.
- N. Phatak, S. Bhattacharya, D. Shah, L. Manthalkar, P. Sreelaya and A. Jain, CD44 targeted delivery of hyaluronic acid-coated polymeric nanoparticles against colorectal cancer, Nanomedicine, 2023, 18(23), 1613–1634 CrossRef CAS PubMed.
- J. Goos, A. Cho, L. M. Carter, T. R. Dilling, M. Davydova and K. Mandleywala, et al., Delivery of polymeric nanostars for molecular imaging and endoradiotherapy through the enhanced permeability and retention (EPR) effect, Theranostics, 2020, 10(2), 567–584 CrossRef CAS PubMed.
- N. A. Gaffar, M. Zahid, A. Asghar, M. F. Shafiq, S. Jelani and F. Rehan, Biosynthesized metallic nanoparticles: A new era in cancer therapy, Arch. Pharm., 2024, 357(7), e2300712 CrossRef PubMed.
- K. Nasiri, S. M. Masoumi, S. Amini, M. Goudarzi, S. M. Tafreshi and A. Bagheri, et al., Recent advances in metal nanoparticles to treat periodontitis, J. Nanobiotechnol., 2023, 21(1), 283 CrossRef PubMed.
- W. Lou, L. Xie, L. Xu, M. Xu, F. Xu and Q. Zhao, et al., Present and future of metal nanoparticles in tumor ablation therapy, Nanoscale, 2023, 15(44), 17698–17726 RSC.
- X. Bi, Q. Bai, M. Liang, D. Yang, S. Li and L. Wang, et al., Silver Peroxide Nanoparticles for Combined Antibacterial Sonodynamic and Photothermal Therapy, Small, 2022, 18(2), e2104160 CrossRef PubMed.
- H. Liu, C. Xu, M. Meng, S. Li, S. Sheng and S. Zhang, et al., Metal-organic framework-mediated multifunctional nanoparticles for combined chemo-photothermal therapy and enhanced immunotherapy against colorectal cancer, Acta Biomater., 2022, 144, 132–141 CrossRef CAS PubMed.
- H. H. Han, S. J. Kim, J. Kim, W. Park, C. Kim and H. Kim, et al., Bimetallic Hyaluronate-Modified Au@Pt Nanoparticles for Noninvasive Photoacoustic Imaging and Photothermal Therapy of Skin Cancer, ACS Appl. Mater. Interfaces, 2023, 15(9), 11609–11620 CrossRef CAS PubMed.
- P. Kesharwani, R. Ma, L. Sang, M. Fatima, A. Sheikh and M. A. S. Abourehab, et al., Gold nanoparticles and gold nanorods in the landscape of cancer therapy, Mol. Cancer, 2023, 22(1), 98 CrossRef CAS PubMed.
- S. Her, D. A. Jaffray and C. Allen, Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements, Adv. Drug Delivery Rev., 2017, 109, 84–101 CrossRef CAS PubMed.
- B. Li, Y. Fu, M. Xie, L. Feng, X. Niu and L. Que, et al., Gold-based nanoparticles realize photothermal and photodynamic synergistic treatment of liver cancer and improve the anaerobic tumor microenvironment under near-infrared light, Front. Bioeng. Biotechnol., 2022, 10, 957349 CrossRef PubMed.
- N. A. Alden, T. J. Yeingst, H. M. Pfeiffer, N. Celik, J. H. Arrizabalaga and A. M. Helton, et al., Near-Infrared Induced miR-34a Delivery from Nanoparticles in Esophageal Cancer Treatment, Adv. Healthc. Mater., 2024, 13(10), e2303593 CrossRef PubMed.
- B. Yin, W. K. H. Ho, X. Xia, C. K. W. Chan, Q. Zhang and Y. M. Ng, et al., A Multilayered Mesoporous Gold Nanoarchitecture for Ultraeffective Near-Infrared Light-Controlled Chemo/Photothermal Therapy for Cancer Guided by SERS Imaging, Small, 2023, 19(6), e2206762 CrossRef PubMed.
- B. Jia, Y. Gao, Z. Ouyang, S. Shen, M. Shen and X. Shi, Diselenide-crosslinked nanogels laden with gold nanoparticles and methotrexate for immunomodulation-enhanced chemotherapy and computed tomography imaging of tumors, J. Mater. Chem. B, 2023, 11(21), 4808–4818 RSC.
- L. Zhao, F. Chang, Y. Tong, J. Yin, J. Xu and H. Li, et al., A Multifunctional Bimetallic Nanoplatform for Synergic Local Hyperthermia and Chemotherapy Targeting HER2-Positive Breast Cancer, Adv. Sci., 2024, 11(16), e2308316 CrossRef PubMed.
- Y. Esmaeili, M. Khavani, A. Bigham, A. Sanati, E. Bidram and L. Shariati, et al., Mesoporous silica@chitosan@gold nanoparticles as “on/off” optical biosensor and pH-sensitive theranostic platform against cancer, Int. J. Biol. Macromol., 2022, 202, 241–255 CrossRef CAS PubMed.
- S. Luan, R. Xie, Y. Yang, X. Xiao, J. Zhou and X. Li, et al., Acid-Responsive Aggregated Gold Nanoparticles for Radiosensitization and Synergistic Chemoradiotherapy in the Treatment of Esophageal Cancer, Small, 2022, 18(19), e2200115 CrossRef PubMed.
- X. Meng, J. Liu, Q. Zheng, S. Li, H. Xiao and J. Huang, et al., Gold-Crowned Bismuth-Based Nanocomposites for Sonodynamic, Photothermal, and Chemotherapeutic Cancer Therapy, ACS Appl. Mater. Interfaces, 2023, 15(50), 58041–58053 CrossRef CAS PubMed.
- A. D. Dey, A. Bigham, Y. Esmaeili, M. Ashrafizadeh, F. D. Moghaddam and S. C. Tan, et al., Dendrimers as nanoscale vectors: Unlocking the bars of cancer therapy, Semin. Cancer Biol., 2022, 86(Pt 2), 396–419 CrossRef PubMed.
- Y. Gao, Z. Ouyang, S. Shen, H. Yu, B. Jia and H. Wang, et al., Manganese Dioxide-Entrapping Dendrimers Co-Deliver Protein and Nucleotide for Magnetic Resonance Imaging-Guided Chemodynamic/Starvation/Immune Therapy of Tumors, ACS Nano, 2023, 17(23), 23889–23902 CrossRef CAS PubMed.
- P. Zhang, Z. Li, W. Cao, J. Tang, Y. Xia and L. Peng, et al., A PD-L1 Antibody-Conjugated PAMAM Dendrimer Nanosystem for Simultaneously Inhibiting Glycolysis and Promoting Immune Response in Fighting Breast Cancer, Adv. Mater., 2023, 35(41), e2305215 CrossRef PubMed.
- S. Pratihar, K. K. Bhagavath and T. Govindaraju, Small molecules and conjugates as theranostic agents, RSC Chem. Biol., 2023, 4(11), 826–849 RSC.
- D. Zhong, X. Hou, D. Pan, Z. Li, Q. Gong and K. Luo, Bioorthogonal In Situ Polymerization of Dendritic Agents for Hijacking Lysosomes and Enhancing Antigen Presentation in Cancer Cells, Adv. Mater., 2024, 36(26), e2403588 CrossRef PubMed.
- Y. Li, Y. Wu, Z. Fang, Y. Zhang, H. Ding and L. Ren, et al., Dendritic Nanomedicine with Boronate Bonds for Augmented Chemo-Immunotherapy via Synergistic Modulation of Tumor Immune Microenvironment, Adv. Mater., 2024, 36(2), e2307263 CrossRef PubMed.
- Y. Hao, Y. Gao, Y. Fan, C. Zhang, M. Zhan and X. Cao, et al., A tumor microenvironment-responsive poly(amidoamine) dendrimer nanoplatform for hypoxia-responsive chemo/chemodynamic therapy, J. Nanobiotechnol., 2022, 20(1), 43 CrossRef CAS PubMed.
- H. Shiba, M. Nishio, M. Sawada, M. Tamaki, M. Michigami and S. Nakai, et al., Carboxy-terminal dendrimers with phenylalanine for a pH-sensitive delivery system into immune cells including T cells, J. Mater. Chem. B, 2022, 10(14), 2463–2470 RSC.
- L. Gu, Z. Duan, X. Chen, X. Li, Q. Luo and A. Bhamra, et al., A Transformable Amphiphilic and Block Polymer-Dendron Conjugate for Enhanced Tumor Penetration and Retention with Cellular Homeostasis Perturbation via Membrane Flow, Adv. Mater., 2022, 34(16), e2200048 CrossRef PubMed.
- J. Sobhanan, J. V. Rival, A. Anas, E. Sidharth Shibu, Y. Takano and V. Biju, Luminescent quantum dots: Synthesis, optical properties, bioimaging and toxicity, Adv. Drug Delivery Rev., 2023, 197, 114830 CrossRef CAS PubMed.
- H. Yukawa, K. Sato and Y. Baba, Theranostics applications of quantum dots in regenerative medicine, cancer medicine, and infectious diseases, Adv. Drug Delivery Rev., 2023, 200, 114863 CrossRef CAS PubMed.
- A. Zarepour, A. Khosravi, N. Yücel Ayten, P. Çakır Hatır, S. Iravani and A. Zarrabi, Innovative approaches for cancer treatment: graphene quantum dots for photodynamic and photothermal therapies, J. Mater. Chem. B, 2024, 12(18), 4307–4334 RSC.
- T. H. Ku, W. T. Shen, C. T. Hsieh, G. S. Chen and W. C. Shia, Specific Forms of Graphene Quantum Dots Induce Apoptosis and Cell Cycle Arrest in Breast Cancer Cells, Int. J. Mol. Sci., 2023, 24(4), 1–14 Search PubMed.
- J. Pilch, P. Kowalik, A. Kowalczyk, P. Bujak, A. Kasprzak and E. Paluszkiewicz, et al., Foliate-Targeting Quantum Dots-β-Cyclodextrin Nanocarrier for Efficient Delivery of Unsymmetrical Bisacridines to Lung and Prostate Cancer Cells, Int. J. Mol. Sci., 2022, 23(3), 1–19 Search PubMed.
- F. Liu, J. Lin, Y. Luo, D. Xie, J. Bian and X. Liu, et al., Sialic acid-targeting multi-functionalized silicon quantum dots for synergistic photodynamic and photothermal cancer therapy, Biomater. Sci., 2023, 11(11), 4009–4021 Search PubMed.
- S. Ostovar, M. Pourmadadi, A. Shamsabadipour and P. Mashayekh, Nanocomposite of chitosan/gelatin/carbon quantum dots as a biocompatible and efficient nanocarrier for improving the Curcumin delivery restrictions to treat brain cancer, Int. J. Biol. Macromol., 2023, 242(Pt 3), 124986 CrossRef CAS PubMed.
- S. Ostovar, M. Pourmadadi and M. A. Zaker, Co-biopolymer of chitosan/carboxymethyl cellulose hydrogel improved by zinc oxide and graphene quantum dots nanoparticles as pH-sensitive nanocomposite for quercetin delivery to brain cancer treatment, Int. J. Biol. Macromol., 2023, 253(Pt 4), 127091 CrossRef CAS PubMed.
- M. Haider, R. Cagliani, J. Jagal, M. N. Jayakumar, B. Fayed and S. B. Shakartalla, et al., Peptide-functionalized graphene oxide quantum dots as colorectal cancer theranostics, J. Colloid Interface Sci., 2023, 630(Pt A), 698–713 CrossRef CAS PubMed.
- H. Yan, Q. Wang, J. Wang, W. Shang, Z. Xiong and L. Zhao, et al., Planted Graphene Quantum Dots for Targeted, Enhanced Tumor Imaging and Long-Term Visualization of Local Pharmacokinetics, Adv. Mater., 2023, 35(15), e2210809 CrossRef PubMed.
- Y. Zhang, S. Chen, J. Ma, X. Zhou, X. Sun and H. Jing, et al., Enzyme-catalyzed electrochemical aptasensor for ultrasensitive detection of soluble PD-L1 in breast cancer based on decorated covalent organic frameworks and carbon nanotubes, Anal. Chim. Acta, 2023, 1282, 341927 CrossRef CAS PubMed.
- P. B. Kasi, V. R. Mallela, F. Ambrozkiewicz, A. Trailin, V. Liška and K. Hemminki, Theranostics Nanomedicine Applications for Colorectal Cancer and Metastasis: Recent Advances, Int. J. Mol. Sci., 2023, 24(9), 1–19 Search PubMed.
- M. Sharma, P. Alessandro, S. Cheriyamundath and M. Lopus, Therapeutic and diagnostic applications of carbon nanotubes in cancer: recent advances and challenges, J. Drug Targeting, 2024, 32(3), 287–299 CrossRef CAS PubMed.
- G. Zhang, M. Zhan, C. Zhang, Z. Wang, H. Sun and Y. Tao, et al., Redox-Responsive Dendrimer Nanogels Enable Ultrasound-Enhanced Chemoimmunotherapy of Pancreatic Cancer via Endoplasmic Reticulum Stress Amplification and Macrophage Polarization, Adv. Sci., 2023, 10(24), e2301759 CrossRef PubMed.
- W. L. Fan, S. Y. Huang, X. J. Yang, F. Bintang Ilhami, J. K. Chen and C. C. Cheng, Hydrogen-bonded cytosine-endowed supramolecular polymeric nanogels: Highly efficient cancer cell targeting and enhanced therapeutic efficacy, J. Colloid Interface Sci., 2024, 665, 329–344 CrossRef CAS PubMed.
- H. Tang, H. Wang, Y. Fang, J. Y. Zhu, J. Yin and Y. X. Shen, et al., Neoadjuvant chemoradiotherapy versus neoadjuvant chemotherapy followed by minimally invasive esophagectomy for locally advanced esophageal squamous cell carcinoma: a prospective multicenter randomized clinical trial, Ann. Oncol., 2023, 34(2), 163–172 CrossRef CAS PubMed.
- C. Zhang, C. Xu, X. Gao and Q. Yao, Platinum-based drugs for cancer therapy and anti-tumor strategies, Theranostics, 2022, 12(5), 2115–2132 CrossRef CAS PubMed.
- D. R. Freyer, P. R. Brock, K. W. Chang, L. L. Dupuis, S. Epelman and K. Knight, et al., Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer: a clinical practice guideline, Lancet Child Adolesc. Health, 2020, 4(2), 141–150 CrossRef CAS PubMed.
- A. M. P. Romani, Cisplatin in cancer treatment, Biochem. Pharmacol., 2022, 206, 115323 CrossRef CAS PubMed.
- J. Chen, D. Zhao, L. Zhang, J. Zhang, Y. Xiao and Q. Wu, et al., Tumor-associated macrophage (TAM)-secreted CCL22 confers cisplatin resistance of esophageal squamous cell carcinoma (ESCC) cells via regulating the activity of diacylglycerol kinase α (DGKα)/NOX4 axis, Drug Resist. Updat., 2024, 73, 101055 CrossRef CAS PubMed.
- R. C. Kiss, F. Xia and S. Acklin, Targeting DNA Damage Response and Repair to Enhance Therapeutic Index in Cisplatin-Based Cancer Treatment, Int. J. Mol. Sci., 2021, 22(15), 1–18 Search PubMed.
- R. K. Hau, S. H. Wright and N. J. Cherrington, Addressing the Clinical Importance of Equilibrative Nucleoside Transporters in Drug Discovery and Development, Clin. Pharmacol. Ther., 2023, 114(4), 780–794 CrossRef CAS PubMed.
- A. K. Eichelmann, G. C. Mayne, K. Chiam, S. L. Due, I. Bastian and F. Butz, et al., Mutant p53 Mediates Sensitivity to Cancer Treatment Agents in Oesophageal Adenocarcinoma Associated with MicroRNA and SLC7A11 Expression, Int. J. Mol. Sci., 2021, 22(11), 1–25 Search PubMed.
- M. H. Dias, A. Friskes, S. Wang, J. M. Fernandes Neto, F. van Gemert and S. Mourragui, et al., Paradoxical Activation of Oncogenic Signaling as a Cancer Treatment Strategy, Cancer Discovery, 2024, 14(7), 1276–1301 CrossRef CAS PubMed.
- S. A. Khales, S. Mozaffari-Jovin, D. Geerts and M. R. Abbaszadegan, TWIST1 activates cancer stem cell marker genes to promote epithelial-mesenchymal transition and tumorigenesis in esophageal squamous cell carcinoma, BMC Cancer, 2022, 22(1), 1272 CrossRef CAS PubMed.
- Z. Yang, P. Zhang, Y. Zhao, R. Guo, J. Hu and Q. Wang, et al., DRD4 promotes chemo-resistance and cancer stem cell-like phenotypes by mediating the activation of the Akt/β-catenin signaling axis in liver cancer, Br. J. Cancer, 2024, 131(7), 1212–1223 CrossRef CAS PubMed.
- W. Wu, Y. Zheng, R. Wang, W. Huang, L. Liu and X. Hu, et al., Antitumor activity of folate-targeted, paclitaxel-loaded polymeric micelles on a human esophageal EC9706 cancer cell line, Int. J. Nanomed., 2012, 7, 3487–3502 CrossRef CAS PubMed.
- L. Zhang, M. Yao, W. Yan, X. Liu, B. Jiang and Z. Qian, et al., Delivery of a chemotherapeutic drug using novel hollow carbon spheres for esophageal cancer treatment, Int. J. Nanomed., 2017, 12, 6759–6769 CrossRef CAS PubMed.
- X. Chen, H. Mao, F. Peng, J. Fan and F. Yang, Novel co-delivery of oridonin and docetaxel nanoliposome for an enhanced antitumor effect on esophageal cancer, J. Gene Med., 2024, 26(8), e3725 CrossRef CAS PubMed.
- W. Zhan, H. Li, Y. Guo, G. Du, Y. Wu and D. Zhang, Construction of Biocompatible Dual-Drug Loaded Complicated Nanoparticles for in vivo Improvement of Synergistic Chemotherapy in Esophageal Cancer, Front. Oncol., 2020, 10, 622 CrossRef PubMed.
- Q. Fu, J. Wang and H. Liu, Chemo-immune synergetic therapy of esophageal carcinoma: trastuzumab modified, cisplatin and fluorouracil co-delivered lipid-polymer hybrid nanoparticles, Drug Delivery, 2020, 27(1), 1535–1543 CrossRef CAS PubMed.
- L. Deng, X. Zhu, Z. Yu, Y. Li, L. Qin and Z. Liu, et al., Novel T7-Modified pH-Responsive Targeted Nanosystem for Co-Delivery of Docetaxel and Curcumin in the Treatment of Esophageal Cancer, Int. J. Nanomed., 2020, 15, 7745–7762 CrossRef CAS PubMed.
- Z. Fan, Y. Chang, C. Cui, L. Sun, D. H. Wang and Z. Pan, et al., Near infrared fluorescent peptide nanoparticles for enhancing esophageal cancer therapeutic efficacy, Nat. Commun., 2018, 9(1), 2605 CrossRef PubMed.
- Y. Gao, Y. Zhu, X. Xu, F. Wang, W. Shen and X. Leng, et al., Surface PEGylated Cancer Cell Membrane-Coated Nanoparticles for Codelivery of Curcumin and Doxorubicin for the Treatment of Multidrug Resistant Esophageal Carcinoma, Front. Cell Dev. Biol., 2021, 9, 688070 CrossRef PubMed.
- M. R. Gill, J. U. Menon, P. J. Jarman, J. Owen, I. Skaripa-Koukelli and S. Able, et al., (111)In-labelled polymeric nanoparticles incorporating a ruthenium-based radiosensitizer for EGFR-targeted combination therapy in oesophageal cancer cells, Nanoscale, 2018, 10(22), 10596–10608 RSC.
- L. L. Zhou, Q. Guan, W. Zhou, J. L. Kan, K. Teng and M. Hu, et al., A Multifunctional Covalent Organic Framework Nanozyme for Promoting Ferroptotic Radiotherapy against Esophageal Cancer, ACS Nano, 2023, 17(20), 20445–20461 CrossRef CAS PubMed.
- X. Zhou, M. You, F. Wang, Z. Wang, X. Gao and C. Jing, et al., Multifunctional Graphdiyne-Cerium Oxide Nanozymes Facilitate MicroRNA Delivery and Attenuate Tumor Hypoxia for Highly Efficient Radiotherapy of Esophageal Cancer, Adv. Mater., 2021, 33(24), e2100556 CrossRef PubMed.
- W. Ren, H. Sha, J. Yan, P. Wu, J. Yang and R. Li, et al., Enhancement of radiotherapeutic efficacy for esophageal cancer by paclitaxel-loaded red blood cell membrane nanoparticles modified by the recombinant protein anti-EGFR-iRGD, J. Biomater. Appl., 2018, 33(5), 707–724 CrossRef CAS PubMed.
- X. Li, H. Liu, W. Gao, Q. Yang, X. Li and X. Zhou, et al., Octadecyl Gallate and Lipid-Modified MnSe(2) Nanoparticles Enhance Radiosensitivity in Esophageal Squamous Cell Carcinoma and Promote Radioprotection in Normal Tissues, Adv. Mater., 2024, 36(23), e2311291 CrossRef PubMed.
- G. Gao, W. Zhou, X. Jiang and J. Ma, Bovine serum albumin and folic acid-modified aurum nanoparticles loaded with paclitaxel and curcumin enhance radiotherapy sensitization for esophageal cancer, Int. J. Radiat. Biol., 2024, 100(3), 411–419 CrossRef CAS PubMed.
- C. H. Chang, S. Y. Liu, C. W. Chi, H. L. Yu, T. J. Chang and T. H. Tsai, et al., External beam radiotherapy synergizes 188Re-liposome against human esophageal cancer xenograft and modulates 188Re-liposome pharmacokinetics, Int. J. Nanomed., 2015, 10, 3641–3649 CAS.
- T. T. Jia, G. Yang, S. J. Mo, Z. Y. Wang, B. J. Li and W. Ma, et al., Atomically Precise Gold-Levonorgestrel Nanocluster as a Radiosensitizer for Enhanced Cancer Therapy, ACS Nano, 2019, 13(7), 8320–8328 CrossRef CAS PubMed.
- K. Gong, J. Lin, X. Chen, Y. Duan, J. Zhang and J. Yu, et al., Thermosensitive gel-nano system against esophageal cancer via restoring p53 activity and boosting T-cell immunity, J. Controlled Release, 2024, 371, 111–125 CrossRef CAS PubMed.
- Y. Jun, Z. Tang, C. Luo, B. Jiang, X. Li and M. Tao, et al., Leukocyte-Mediated Combined Targeted Chemo and Gene Therapy for Esophageal Cancer, ACS Appl. Mater. Interfaces, 2020, 12(42), 47330–47341 CrossRef CAS PubMed.
- D. Wang, H. Yi, S. Geng, C. Jiang, J. Liu and J. Duan, et al., Photoactivated DNA Nanodrugs Damage Mitochondria to Improve Gene Therapy for Reversing Chemoresistance, ACS Nano, 2023, 17(17), 16923–16934 CrossRef CAS PubMed.
- M. Cao, X. Deng, S. Su, F. Zhang, X. Xiao and Q. Hu, et al., Protamine sulfate-nanodiamond hybrid nanoparticles as a vector for MiR-203 restoration in esophageal carcinoma cells, Nanoscale, 2013, 5(24), 12120–12125 RSC.
- X. Li, L. Zhang, X. Guo, F. Xie, C. Shen and Y. Jun, et al., Self-assembled RNA nanocarrier-mediated chemotherapy combined with molecular targeting in the treatment of esophageal squamous cell carcinoma, J. Nanobiotechnol., 2021, 19(1), 388 CrossRef CAS PubMed.
- W. Cao, X. Zhang, R. Li, Z. Li, A. Lu and F. Yu, et al., Lipid core-shell nanoparticles co-deliver FOLFOX regimen and siPD-L1 for synergistic targeted cancer treatment, J. Controlled Release, 2024, 368, 52–65 CrossRef CAS PubMed.
- Q. Xin, D. Wang, S. Wang, L. Zhang, Q. Liang and X. Yan, et al., Tackling Esophageal Squamous Cell Carcinoma with ITFn-Pt(IV): A Novel Fusion of PD-L1 Blockade, Chemotherapy, and T-cell Activation, Adv. Healthc. Mater., 2024, 13(11), e2303623 CrossRef PubMed.
- G. Shi, Y. Cui, J. Zhao, J. Liu, Y. Wang and Y. Yang, et al., Identifying TOPK and Hypoxia Hallmarks in Esophageal Tumors for Photodynamic/Chemo/Immunotherapy and Liver Metastasis Inhibition with Nanocarriers, ACS Nano, 2023, 17(7), 6193–6207 CrossRef CAS PubMed.
- X. Zhang, M. Wang, J. Feng, B. Qin, C. Zhang and C. Zhu, et al., Multifunctional nanoparticles co-loaded with Adriamycin and MDR-targeting siRNAs for treatment of chemotherapy-resistant esophageal cancer, J. Nanobiotechnol., 2022, 20(1), 166 CrossRef CAS PubMed.
- D. Averbeck and C. Rodriguez-Lafrasse, Role of Mitochondria in Radiation Responses: Epigenetic, Metabolic, and Signaling Impacts, Int. J. Mol. Sci., 2021, 22(20), 1–59 Search PubMed.
- Y. Wu, Y. Song, R. Wang and T. Wang, Molecular mechanisms of tumor resistance to radiotherapy, Mol. Cancer, 2023, 22(1), 96 CrossRef CAS PubMed.
- M. Li, M. H. Younis, Y. Zhang, W. Cai and X. Lan, Clinical summary of fibroblast activation protein inhibitor-based radiopharmaceuticals: cancer and beyond, Eur. J. Nucl. Med. Mol. Imaging, 2022, 49(8), 2844–2868 CrossRef CAS PubMed.
- R. C. Hsieh, S. Krishnan, R. C. Wu, A. R. Boda, A. Liu and M. Winkler, et al., ATR-mediated CD47 and PD-L1 up-regulation restricts radiotherapy-induced immune priming and abscopal responses in colorectal cancer, Sci. Immunol., 2022, 7(72), eabl9330 CrossRef CAS PubMed.
- D. Zhao, Y. Mo, M. E. Neganova, Y. Aleksandrova, E. Tse and V. N. Chubarev, et al., Dual effects of radiotherapy on tumor microenvironment and its contribution towards the development of resistance to immunotherapy in gastrointestinal and thoracic cancers, Front. Cell Dev. Biol., 2023, 11, 1266537 CrossRef PubMed.
- V. M. Kho, V. E. Mekers, P. N. Span, J. Bussink and G. J. Adema, Radiotherapy and cGAS/STING signaling: Impact on MDSCs in the tumor microenvironment, Cell. Immunol., 2021, 362, 104298 CrossRef CAS PubMed.
- Q. Dai, L. Wang, E. Ren, H. Chen, X. Gao and H. Cheng, et al., Ruthenium-Based Metal-Organic Nanoradiosensitizers Enhance Radiotherapy by Combining ROS Generation and CO Gas Release, Angew. Chem., Int. Ed., 2022, 61(50), e202211674 CrossRef CAS PubMed.
- Y. Wang, S. Song, T. Lu, Y. Cheng, Y. Song and S. Wang, et al., Oxygen-supplementing mesoporous polydopamine nanosponges with WS(2) QDs-embedded for CT/MSOT/MR imaging and thermoradiotherapy of hypoxic cancer, Biomaterials, 2019, 220, 119405 CrossRef CAS PubMed.
- W. W. Xu, L. Liao, W. Dai, C. C. Zheng, X. P. Tan and Y. He, et al., Genome-wide CRISPR/Cas9 screening identifies a targetable MEST-PURA interaction in cancer metastasis, EBioMedicine, 2023, 92, 104587 CrossRef CAS PubMed.
- R. J. Kelly, B. V. Landon, A. H. Zaidi, D. Singh, J. V. Canzoniero and A. Balan, et al., Neoadjuvant nivolumab or nivolumab plus LAG-3 inhibitor relatlimab in resectable esophageal/gastroesophageal junction cancer: a phase Ib trial and ctDNA analyses, Nat. Med., 2024, 30(4), 1023–1034 CrossRef CAS PubMed.
- Y. M. Yang, P. Hong, W. W. Xu, Q. Y. He and B. Li, Advances in targeted therapy for esophageal cancer, Signal Transduct. Target. Ther., 2020, 5(1), 229 CrossRef CAS PubMed.
- W. Yang, X. Xing, S. J. Yeung, S. Wang, W. Chen and Y. Bao, et al., Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma, J. Immunother. Cancer, 2022, 10(1), 1–11 Search PubMed.
- W. He, C. Wang, C. Li, X. Nie, H. Li and J. Li, et al., The efficacy and safety of neoadjuvant immunotherapy in resectable locally advanced esophageal squamous cell carcinoma: A systematic review and meta-analysis, Front. Immunol., 2023, 14, 1118902 CrossRef CAS PubMed.
- J. Xu, Y. Li, Q. Fan, Y. Shu, L. Yang and T. Cui, et al., Clinical and biomarker analyses of sintilimab versus chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma: a randomized, open-label phase 2 study (ORIENT-2), Nat. Commun., 2022, 13(1), 857 CrossRef CAS PubMed.
- Z. X. Wang, C. Cui, J. Yao, Y. Zhang, M. Li and J. Feng, et al., Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial, Cancer Cell, 2022, 40(3), 277–288 CrossRef CAS PubMed.
- N. Ma, R. Hua, Y. Yang, Z. C. Liu, J. Pan and B. Y. Yu, et al., PES1 reduces CD8(+) T cell infiltration and immunotherapy sensitivity via interrupting ILF3-IL15 complex in esophageal squamous cell carcinoma, J. Biomed. Sci., 2023, 30(1), 20 CrossRef CAS PubMed.
- Y. Baba, D. Nomoto, K. Okadome, T. Ishimoto, M. Iwatsuki and Y. Miyamoto, et al., Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma, Cancer Sci., 2020, 111(9), 3132–3141 CrossRef CAS PubMed.
- Z. Wang, Y. Zhao, Y. Wo, Y. Peng, W. Hu and Z. Wu, et al., The single cell immunogenomic landscape after neoadjuvant immunotherapy combined chemotherapy in esophageal squamous cell carcinoma, Cancer Lett., 2024, 593, 216951 CrossRef CAS PubMed.
- Z. Zhao, M. M. Yin, W. F. Zhao and C. J. Wang, The efficacy and safety of sintilimab combined with chemotherapy as the first-line treatment for metastatic esophageal squamous cell carcinoma, Medicine, 2023, 102(33), e34794 CrossRef CAS PubMed.
- J. He, X. Ouyang, F. Xiao, N. Liu and L. Wen, Imaging-Guided Photoacoustic Immunotherapy Based on the Polydopamine-Functionalized Black Phosphorus Nanocomposites, ACS Appl. Mater. Interfaces, 2023, 15(47), 54322–54334 CrossRef CAS PubMed.
- H. Sajjad, A. Sajjad, R. T. Haya, M. M. Khan and M. Zia, Copper oxide nanoparticles: In vitro and in vivo toxicity, mechanisms of action and factors influencing their toxicology, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2023, 271, 109682 CAS.
- D. Fan, Y. Cao, M. Cao, Y. Wang, Y. Cao and T. Gong, Nanomedicine in cancer therapy, Signal Transduct. Target. Ther., 2023, 8(1), 293 CrossRef PubMed.
- T. Lammers, Nanomedicine Tumor Targeting, Adv. Mater., 2024, 36(26), e2312169 CrossRef PubMed.
- W. Sun, S. Xie, S. F. Liu, X. Hu and D. Xing, Evolving Tumor Characteristics and Smart Nanodrugs for Tumor Immunotherapy, Int. J. Nanomed., 2024, 19, 3919–3942 CrossRef PubMed.
- S. Brezgin, A. Parodi, A. Kostyusheva, N. Ponomareva, A. Lukashev and D. Sokolova, et al., Technological aspects of manufacturing and analytical control of biological nanoparticles, Biotechnol. Adv., 2023, 64, 108122 CrossRef CAS PubMed.
- K. Thanki, D. van Eetvelde, A. Geyer, J. Fraire, R. Hendrix and H. Van Eygen, et al., Mechanistic profiling of the release kinetics of siRNA from lipidoid-polymer hybrid nanoparticles in vitro and in vivo after pulmonary administration, J. Controlled Release, 2019, 310, 82–93 Search PubMed.
- A. Pittiu, M. Pannuzzo, L. Casula, R. Pireddu, D. Valenti and M. C. Cardia, et al., Production of liposomes by microfluidics: The impact of post-manufacturing dilution on drug encapsulation and lipid loss, Int. J. Pharm., 2024, 664, 124641 CrossRef CAS PubMed.
- J. H. Ding, H. R. Zhao and H. B. Yu, A water-based green approach to large-scale production of aqueous compatible graphene nanoplatelets, Sci. Rep., 2018, 8(1), 5567 CrossRef PubMed.
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Engineering precision nanoparticles for drug delivery, Nat. Rev. Drug Discovery, 2021, 20(2), 101–124 CrossRef CAS PubMed.
- E. B. Souto, C. Blanco-Llamero, K. Krambeck, N. S. Kiran, C. Yashaswini and H. Postwala, et al., Regulatory insights into nanomedicine and gene vaccine innovation: Safety assessment, challenges, and regulatory perspectives, Acta Biomater., 2024, 180, 1–17 CrossRef CAS PubMed.
- M. Li, D. Zhao, J. Yan, X. Fu, F. Li and G. Liu, et al., A Redox-Triggered Autophagy-Induced Nanoplatform with PD-L1 Inhibition for Enhancing Combined Chemo-Immunotherapy, ACS Nano, 2024, 18(20), 12870–12884 Search PubMed.
- J. Liu, X. Jiang, Y. Li, K. Yang, R. R. Weichselbaum and W. Lin, Immunogenic Bifunctional Nanoparticle Suppresses Programmed Cell Death-Ligand 1 in Cancer and Dendritic Cells to Enhance Adaptive Immunity and Chemo-Immunotherapy, ACS Nano, 2024, 18(6), 5152–5166 Search PubMed.
- Y. X. Zhao, H. P. Zhao, M. Y. Zhao, Y. Yu, X. Qi and J. H. Wang, et al., Latest insights into the global epidemiological features, screening, early diagnosis and prognosis prediction of esophageal squamous cell carcinoma, World J. Gastroenterol., 2024, 30(20), 2638–2656 Search PubMed.
- H. Q. Dinh, F. Pan, G. Wang, Q. F. Huang, C. E. Olingy and Z. Y. Wu, et al., Integrated single-cell transcriptome analysis reveals heterogeneity of esophageal squamous cell carcinoma microenvironment, Nat. Commun., 2021, 12(1), 7335 Search PubMed.
- Y. Mei, X. Qin, Z. Yang, S. Song, X. Liu and C. Wu, et al., Engineered a dual-targeting HA-TPP/A nanoparticle for combination therapy against KRAS-TP53 co-mutation in gastrointestinal cancers, Bioact. Mater., 2024, 32, 277–291 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |

