Open Access Article
Open Access ArticleSynthesis, characterization, genotoxicity assessment and antibacterial applications of Zanthoxylum armatum silver nanoparticles (ZASNPs) with antibiotic efficacy enhancement potential
Nikita
Quadri
 a,
Manganahalli Manjunath
Setty
a,
Manganahalli Manjunath
Setty
 *a,
Anshumali
Awasthi
*b,
Usha
Nayak
c,
Minakshi
Singh
b and
Sharad
Sharma
b
*a,
Anshumali
Awasthi
*b,
Usha
Nayak
c,
Minakshi
Singh
b and
Sharad
Sharma
b
aDepartment of Pharmacognosy, Manipal College of Pharmaceutical Sciences (MCOPS), Manipal Academy of Higher Education (MAHE), Manipal, 576104, Karnataka, India. E-mail: mm.setty@manipal.edu; nikitaquadri@lupin.com
bDrug Safety Assessment (DSA), Novel Drug Discovery and Development (NDDD), Lupin Limited (Research Park), Pune, 412115, Maharashtra, India. E-mail: anshumaliawasthi1@lupin.com
cDepartment of Pharmaceutics, Manipal College of Pharmaceutical Sciences (MCOPS), Manipal Academy of Higher Education (MAHE), Manipal, 576104, Karnataka, India
First published on 9th November 2024
Abstract
This study aimed to develop alternative antibacterial treatments by combining traditional herbal knowledge with modern nanotechnology. This approach targets multiple bacterial strains, combats antibiotic resistance, and offers solutions for treating infections alone or with antibiotics. Phyto-nano synthesis, using plants like Zanthoxylum armatum DC., is highlighted for its safer and stable nanoparticle production. This study successfully synthesized spherical silver nanoparticles (∼6.2 ± 5.1 nm) from Z. armatum, which showed no genotoxicity but effective bactericidal activity against various bacteria. Additionally, these nanoparticles enhanced the antimicrobial effects of several antibiotics, suggesting their potential for more effective treatments with lower doses and reduced side effects.
Introduction
Several bacterial strains have gradually evolved to resist antibiotics, which makes bacterial infections harder to treat and can lead to longer illnesses, prolonged hospital stays and increased medical costs. Herbal medicines validated by scientific research are widely employed to treat infectious diseases. Substantial scientific evidence points towards the effectiveness of many plants in treating diverse types of viral, fungal, bacterial, and parasitic infections.1 One such super herb is Zanthoxylum armatum DC., whose antibacterial properties have been reported by several researchers. The methanolic extracts prepared from the bark, seeds and fruits of this genus have shown strong antibacterial activity against Pseudomonas aeruginosa, Staphylococcus epidermidis, Enterococcus faecalis, Shigella dysenteriae, Bacillus subtilis, Salmonella typhi, Proteus vulgaris, Staphylococcus aureus and its methicillin-resistant strain, and the fruits were reported to have the strongest bactericidal activity in this study.2 Multiple antibiotics are ineffective against the human pathogens Staphylococcus epidermidis and Staphylococcus aureus. The bioactive components from Zanthoxylum armatum DC. fruit and leaf extracts have demonstrated substantial interaction with S. aureus and S. epidermidis bacterial proteins that are responsible for their virulence, and this interaction resulted in bactericidal activity on S. aureus mediated by the methanolic and chloroform extracts of Z. armatum DC. leaf and fruit, respectively. The benzene and methanolic extracts prepared from the leaves and fruits of Z. armatum DC were effective at killing S. epidermidis.3 The primary obstacles to the healing properties of plant-based medicines are the lack of consistency and repeatability in the products made from plants.1 The mechanisms underlying most plant-derived remedies are not well understood, resulting in herbal medicines that lack the specificity and potency required to kill the resistant pathogens. Hence, there is a dire need to develop new treatment schemes capable of combating resistant bacterial strains that are on the rise due to antibiotic overuse. Effective antibacterial properties have been demonstrated by a variety of green method-based silver nanoparticles.4 The antimicrobial potential/sensitivity of silver nanoparticles has been shown to increase with decreasing size, indicating that their antimicrobial activities are size dependent. This property has primarily been attributed to an increase in the surface area to volume ratio that is associated with decreasing sizes.5 With the recent advances in green nanotechnology, Z. armatum too has played a significant role in the successful generation of copper oxide nanoparticles (CuONPs),6,7 iron oxide nanoparticles (Fe3O4 NPs),8 silver nanoparticles (AgNPs)9–11 and zinc oxide nanoparticles (ZnONPs).12 These nanoparticles have shown excellent antibacterial properties against several bacterial strains. Klebsiella pneumonia, Staphylococcus epidermidis, Streptococcus mutans and Staphylococcus pyogenes were sensitive to Zanthoxylum armatum aqueous leaf extract derived CuONPs.6 In another study, Zanthoxylum armatum aqueous leaf extract based CuONPs obtained via microwave-assisted green synthesis were effective at killing E. coli, C. albicans, and B. subtilis.7 The Z. armatum bark and stem-based silver nanoparticles have shown antibacterial efficiency against Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli and Salmonella enteric strains.10,11Escherichia coli, Candida albicans and Staphylococcus aureus were reported to be sensitive towards Z. armatum zinc oxide nanoparticles.12Based on this literature background, the goal of the current research was to synthesize an antibacterial agent to eradicate common human bacterial diseases and combat the alarming rise in resistant bacterial strains worldwide. Our work differs from the studies that have already been published since we suggest a true green synthesis approach without the use of hazardous or toxic reagents. Our technique makes use of the herb's inherent ability to reduce metal salts, thereby producing homogeneous, spherically shaped, and highly stable silver nanoparticles that are capped with the Z. armatum fruit extract. So far, we are the only ones to report the utilization of Z. armatum aqueous fruit extract in the successful synthesis of silver nanoparticles. The current manuscript details all aspects of synthesis, characterization, genotoxicity evaluation, and the application of Zanthoxylum armatum silver nanoparticles as an antibacterial agent with the potential of enhancing the antibacterial efficiency of antibiotics from the common class. Our findings reveal that ZASNPs, averaging ∼6.2 ± 5.1 nm in size, are non-mutagenic and possess strong bactericidal properties against both Gram-negative and Gram-positive bacteria, such as Escherichia coli, Salmonella typhimurium, Bacillus subtilis, and Staphylococcus aureus. Additionally, ZASNPs improve the effectiveness of various antibiotics, indicating a synergistic effect that could result in more efficient treatments for bacterial infections.
Results and discussion
Synthesis
The microwave-based green synthesis of Z. armatum silver nanoparticles was a rapid process. Upon successful formation of silver nanoparticles, the solution often changes colour to a yellowish or brownish hue, serving as an initial visual confirmation (Fig. 1).Characterization
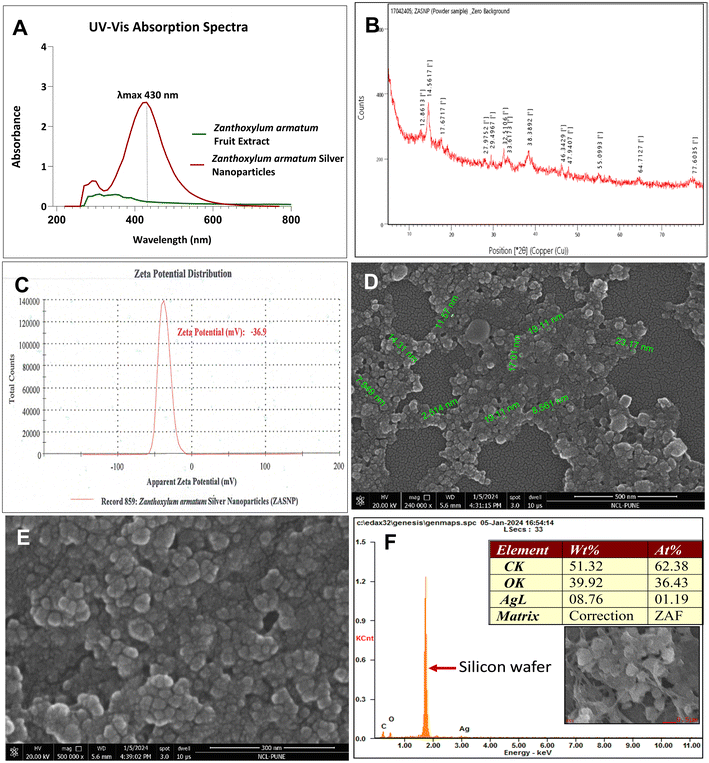
The extract concentration and microwave power played a significant role in Surface Plasmon Resonance (SPR) peak intensities with the utilization of 75% Z. armatum fruit extract and 900 W, yielding better results. The resulting UV-Vis SPR peaks were stably centered at 430 nm (Fig. 2A), which is in line with the available literature reports which state that due to the surface plasmon resonance, the absorption of silver nanoparticles is usually in the range of 400–500 nm and is found to be centered at 430 nm in aqueous medium.Fig. 2B shows 13 diffraction peaks that were observed with varying intensities. However, due to insufficient peak intensities, the crystallite size and structure could not be calculated. As the peaks observed here are like the reported peaks for green synthesized silver nanoparticles, it can be confirmed that the silver nanoparticles were successfully synthesized by utilizing Zanthoxylum armatum fruit extract as a reducing agent.
The synthesized nanoparticles were strongly anionic with a zeta potential of −36.9 (Fig. 2C) and the observed polydispersity index was 0.252 (data not shown). SEM analysis confirmed the successful formation of silver nanoparticles in the size range of ∼2.514 nm to ∼22.59 nm (Fig. 2D). Elemental silver was verified through Energy Dispersive Spectroscopy (EDS), and during analysis, the strongest signal obtained was for the silicone (not assigned to any peak) used to mount the sample on a stub. A signal of ∼3 keV was observed for silver. The detected amounts of C and O could be assigned to the organic compounds derived from the Zanthoxylum armatum fruit extract attached to the silver nanoparticles (Fig. 2F).
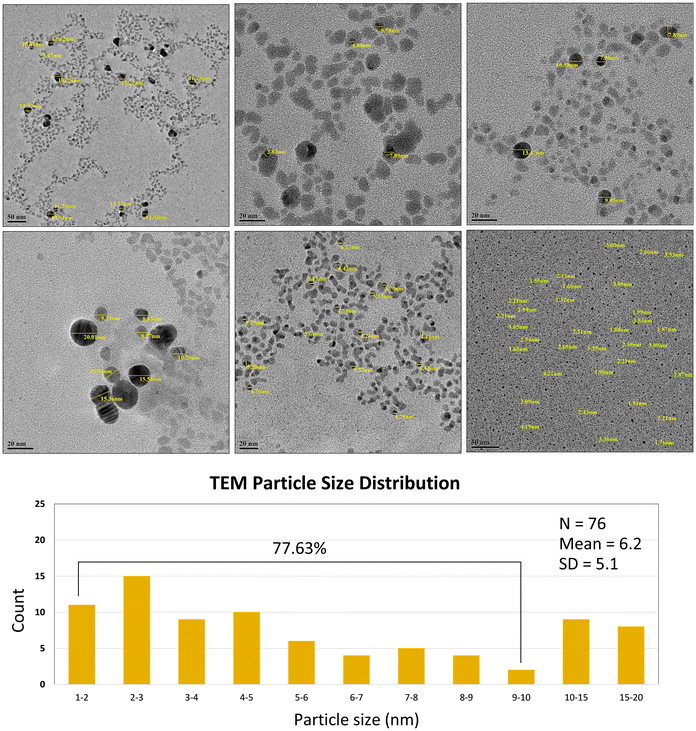
ZASNPs below 10 nm could be resolved with better accuracy in TEM. The nanoparticles formed were majorly below 10 nm in size, accounting for ∼77.63% of the size distribution. The particles were observed to be spherical in shape and uniformly dispersed (Fig. 3).
Genotoxicity evaluation
| Treatment | Concentration (μg ml−1) | Total number of cells scored per culture | Number of aberrant cells | Aberrant cell %a |
|---|---|---|---|---|
| a Numerical aberrations are excluded from the calculation of % aberrant cells. | ||||
| ∼3.5 h treatment (−S9) | Solvent control (media) | 300 | 0 | 0 |
| Positive control mitomycin C – 0.05 | 300 | 33 | 11 | |
| ∼3.5 h treatment (+S9) | Solvent control (media) | 300 | 1 | 0.3 |
| Positive control cyclophosphamide – 25 | 300 | 44 | 14.7 | |
| ∼20 h treatment (−S9) | Solvent control (media) | 300 | 0 | 0 |
| Positive control mitomycin C – 0.2 | 300 | 54 | 18 | |
The ratio of polychromatic erythrocytes (PCEs) to total erythrocytes (TEs) in the ZASNP treated group was comparable to that of the vehicle control group, indicating that there was no evidence of cytotoxicity. The number and percentage of micronucleated polychromatic erythrocytes (PCEs) in animals treated with ZASNP as compared to the control group did not reveal a statistically significant increase, while the positive control animals treated with cyclophosphamide yielded a significantly higher number of micronucleated polychromatic erythrocytes (PCEs) in comparison with the vehicle control group (Fig. 6).
The blood (plasma) biochemistry results revealed that blood urea nitrogen significantly decreased (p = 0.0195) in the ZASNP treated group; however, the plasma biochemistry for creatine kinase, aspartate aminotransferase, albumin, alanine aminotransferase, alkaline phosphatase, total protein and triglycerides was not affected by 14 days of repeated dosing with ZASNPs at the limit dose.
Based on the findings and results obtained under these study conditions, Zanthoxylum armatum silver nanoparticles did not produce micronuclei in mouse immature erythrocytes, when treated once in a day for 14 consecutive days by oral gavage at the limit dose level of 1000 mg kg−1 day−1. It is concluded that Zanthoxylum armatum silver nanoparticles are clearly negative in the mammalian in vivo micronucleus test.
Antibacterial activity
| Bacterial strain | Mean ± SD inhibition zone (mm) | |||
|---|---|---|---|---|
| 0.9% NaCl | ZA extract | Silver nitrate (AgNO3) | ZASNPs | |
| Bacillus subtilis | 0.3 ± 0.6 | 0.2 ± 0.3 | 14 ± 1.0 | 19 ± 1.5 ** |
| Staphylococcus aureus | 0 ± 0.0 | 0 ± 0.0 | 11 ± 1.0 | 21 ± 1.5 *** |
| Salmonella typhimurium | 0.3 ± 0.6 | 0.3 ± 0.6 | 13 ± 1.0 | 20 ± 2.1 ** |
| Escherichia coli | 0 ± 0.0 | 0 ± 0.0 | 15 ± 1.2 | 24 ± 4.6* |
As discs saturated with ZASNPs exhibited significantly larger inhibition zones as compared to AgNO3, only ZASNPs were evaluated in further antimicrobial assays. ZASNPs exhibited good antibacterial efficacy in this method against the selected bacterial strains with ≥19 mm zone of inhibition (ZOI). The observed ZOIs for AgNO3 were significantly smaller, indicating that the natural antibacterial potential of silver was indeed enhanced in the nanoparticle form.
| Bacterial strain | Mean MIC | Mean MBC | MBC/MIC | Result | |
|---|---|---|---|---|---|
| Gram positive | Bacillus subtilis | 0.39 | 1.56 | 4 | Bacteriostatic |
| Staphylococcus aureus | 0.39 | 0.78 | 2 | Bactericidal | |
| Gram negative | Salmonella typhimurium | 0.20 | 0.78 | 3.9 | Bacteriostatic |
| Escherichia coli | 0.78 | 1.56 | 2 | Bactericidal | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Log10), the test item at the evaluated concentration is considered to exhibit bactericidal activity, and if there is no reduction or the reduction is less than 99.9% (<3
Log10), the test item at the evaluated concentration is considered to exhibit bactericidal activity, and if there is no reduction or the reduction is less than 99.9% (<3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Log10), it is concluded to have a bacteriostatic effect on the bacterial strain. ZASNPs showed concentration- and time-dependent antimicrobial activities as observed by the time-kill assay (Fig. 7). Bactericidal effects with ≥3
Log10), it is concluded to have a bacteriostatic effect on the bacterial strain. ZASNPs showed concentration- and time-dependent antimicrobial activities as observed by the time-kill assay (Fig. 7). Bactericidal effects with ≥3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Log10 units i.e. representative of ∼99% reduction in the number of CFU mL−1 were observed for Staphylococcus aureus (MIC, 2xMIC, and 3xMIC concentrations at all time points), Salmonella typhimurium (2xMIC and 3xMIC after 24 h incubation) and Escherichia coli (MIC, 2xMIC, and 3xMIC concentrations after ∼8 h exposure). A bacteriostatic effect was observed with <3
Log10 units i.e. representative of ∼99% reduction in the number of CFU mL−1 were observed for Staphylococcus aureus (MIC, 2xMIC, and 3xMIC concentrations at all time points), Salmonella typhimurium (2xMIC and 3xMIC after 24 h incubation) and Escherichia coli (MIC, 2xMIC, and 3xMIC concentrations after ∼8 h exposure). A bacteriostatic effect was observed with <3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Log10 units’ reduction for Bacillus subtilis.
Log10 units’ reduction for Bacillus subtilis.
Synergism of Zanthoxylum armatum silver nanoparticles with antibiotics
The mean zone of inhibition observed for E. coli and Staphylococcus aureus treated with 10 μl ZASNPs/discs was 21 mm. No zone of inhibition was observed for both strains when treated with 10 μl of 0.9% NaCl. Zanthoxylum armatum silver nanoparticles showed a synergistic effect with antibiotics from the main classes. The lowest synergism i.e. a 0.8-fold increase was observed with ciprofloxacin tested against E. coli while the highest synergism i.e. a fold increase of 3 was observed with gentamicin tested against Staphylococcus aureus (Fig. 8). The synergism observed between the evaluated antibiotics and the ZASNPs was found to be statistically significant (Fig. 9), indicating that the antibacterial activity of these antibiotics was indeed substantially enhanced upon the addition of ZASNPs. | ||
| Fig. 9 Statistical analysis using an unpaired t-test between the antibiotic alone and combination treatments was found to be statistically significant for each of the evaluated combinations. | ||
The bactericidal effect of silver nanoparticles involves several mechanisms, although the precise mechanism is yet to be fully understood. Owing to their small size, AgNPs can readily penetrate the microbial cell wall and induce the generation of reactive oxygen species (ROS) and free radicals. The production of ROS leads to the oxidative damage of DNA, proteins and lipids ultimately leading to apoptosis.15 The silver ions (Ag+) released from silver nanoparticles disrupt the bacterial cell wall and damage the bacterial proteins and enzymes. Interaction of silver ions with bacterial DNA hinders replication and transcription; silver nanoparticles are also known to interfere with essential metabolic processes leading to bacterial cell death.
Experimental
Materials
Dried fruits of Zanthoxylum armatum DC commonly known as Timur or Nepali dhania were bought from Kathmandu, Nepal. Resazurin, silver nitrate, acridine mutagen ICR 191, methyl methanesulfonate, 2-nitrofluorene, sodium azide and 2-aminoanthracene were procured from Sigma Aldrich. The antibiotic discs penicillin-G (Cat. # SD028-1VL), ciprofloxacin (Cat. # SD060-1VL), tetracycline (Cat. # SD037-1VL), azithromycin (Cat. # SD204-1VL), gentamicin (Cat. # SD016-1VL), cefdinir (Cat. # SD218-1VL), zone scale (PW096), sterile filter discs (SD067-1VL), Müeller–Hinton agar and broth No. 2 were purchased from HiMedia.Bacterial strains and animals
Ames bacterial tester strains TA1537, TA1535, TA100, TA98, and WP2uvrA and the post-mitochondrial supernatant (S9) were purchased from MOLTOX. Bacillus subtilis (ATCC 35021), Escherichia coli (ATCC 25922), Salmonella typhimurium (ATCC 14028) and Staphylococcus aureus (ATCC 25923) were bought from ATCC. The mouse strain CD-1 was issued against the IAEC approved protocol No. IAEC/TOX/1086.Synthesis
Zanthoxylum armatum DC fruits were authenticated by microscopic morphological analysis at ACME Research Solution with a Plant Authentication Certificate Reference No. ACME/PA/11058. The synthesis of Zanthoxylum armatum silver nanoparticles was conducted using the bottom-up approach utilizing microwaves to expedite the process and produce uniform nanoparticles. Briefly, as depicted in Fig. 10, the reaction mixture consisting of 75% of Zanthoxylum armatum fruit extract prepared at 1 mg mL−1 concentration and 25% of 5 mM silver nitrate solution was microwaved at 900 W for ∼12 min. A visible change in colour of the reaction mixture marked the fulfillment of the preliminary criteria for the evaluation of nanoparticle synthesis.Characterization
Further characterization of the synthesized nanoparticles was done by UV-Vis spectroscopy. The absorption spectra were recorded in the UV-Vis range of 190–800 nm. A complete reaction mixture at 0 h was used as the reference sample (blank). For X-ray diffraction (XRD), powdered ZASNPs were prepared by oven drying at 60 °C until complete liquid evaporation. The resulting sticky film was submerged in absolute alcohol to ease scraping followed by drying at 60 °C until complete evaporation of alcohol. For Field Emission Scanning Electron Microscopy (FESEM) along with Energy Dispersive Spectroscopy (EDS) analysis, the samples were prepared by diluting ZASNPs by 2-fold in Milli-Q water followed by sonication for 10 min. ∼20 μL of sample was mounted on a metal stub by spreading on a silicon wafer and allowed to air dry. The specimen was sputter coated with gold as a conductive material. ZASNPs were diluted to ∼10-fold in Milli-Q water and sonicated for 10 minutes for Transmission Electron Microscopy (TEM). A drop of diluted ZASNP solution was placed on a standard copper grid, which was left overnight for air drying completely. Zeta potential and PDI (polydispersity index) analysis was conducted on 5-fold diluted ZASNPs. For PDI analysis the following parameters were set: temperature 25 °C, material refractive index 1.55, dispersant RI 1.330, viscosity (cP) 0.8872, material absorption 0.100 and the dispersant used was water.Genotoxicity evaluation
Many silver nanoparticle genotoxicity assessments have produced a negative Ames test result. According to some experts, the Ames test may not have produced the desired results because of increased particle sizes or bacterial resistance to particle uptake. In the comet and micronucleus assay, smaller nanoparticles have been found to be more genotoxic.16 However, AgNPs with a size of 5 nm were found to be negative in the Ames test.17Bacterial reverse mutation assay (AMES test)
A bacterial reverse mutation assay (Ames test) was performed to evaluate the potential of synthesized Zanthoxylum armatum silver nanoparticles (ZASNPs) to induce reverse mutation at the histidine and tryptophan loci of the genome of selected tester strains of Salmonella typhimurium and Escherichia coli, respectively, with and without an exogenous mammalian metabolic activation system (S9 mix). Salmonella typhimurium tester strains TA98, TA100, TA1535, and TA1537 and the Escherichia coli strain WP2uvrA were used in this assay. The assay was conducted employing the plate incorporation method with and without metabolic activation originally described by Maron and Ames,18 Mortelmans and Riccio19 and Mortelmans and Zeiger.20The bacterial tester strains were treated at a range of six different ZASNP concentrations viz. 0.03, 0.1, 0.3, 1, 3 and a limit dose of 5 μl per plate (ref. 21) in the absence and presence of a rat exogenous metabolic activation system (10% S9). 5 μl per plate (limit dose) of the fruit extract was incorporated to rule out the probable potential (if any) of the extract mediated genetic toxicity. Deionized (DI) water was used as the solvent control and strain-specific positive controls were employed in the assay (Table 4).
| Tester strain(s) | S9 | Positive control |
|---|---|---|
| TA98 | − | 2-Nitrofluorene |
| TA100, TA1535 | − | Sodium azide |
| TA1537 | − | Acridine Mutagen ICR 191 |
| WP2uvrA | − | Methyl methanesulfonate |
| TA98, TA100, TA1535, TA1537, WP2uvrA | + | 2-Aminoanthracene |
In vitro mammalian chromosomal aberration test
This test was performed to identify if ZASNPs were capable of causing structural chromosomal aberrations in the cultured Chinese Hamster Ovary (CHO) cells. 0.5 mg mL−1 was selected as the starting dose based on the recommendation of OECD Test No. 473.22 Furthermore, starting at 0.256 mg mL−1, six lower concentrations were selected at a 2-fold interval, viz. 0.128, 0.064, 0.032, 0.016, 0.008 and 0.004 mg mL−1. Treatment with 0.5 mg mL−1 of the fruit extract was included to evaluate if the aqueous fruit extracts of Zanthoxylum armatum could induce chromosomal aberrations. The required number of T25 flasks (2 × 3 per concentration) prepared with 5 × 105 cells per 5 mL per flask and were incubated for ∼24 hours at 37 ± 1 °C with 90–100% humidity and 5% CO2. 50 μL of the solvent control (deionised water), the positive control and ZASNPs were added to the respective flasks. One set of flasks were incubated for ∼3–6 hours (short treatment) and for the other set the incubation was continued for ∼20 hours until harvest time. 2–2.5 hours prior to harvest, 37.5 μL colchicine was added to each culture flask. Cells were trypsinised and post-treatment counts were noted down. After a wash with PBS the cells were fixed with a chilled methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid fixative. Slides were prepared by dropping from a height about 2–3 drops of fixed cell suspension onto pre-cleaned glass slides. After drying for ∼24 hours the slides were stained with 5% Giemsa stain and mounted with the DPX mountant post drying. For evaluation of chromosomal aberrations, at least 300 analysable metaphases were scored for each concentration by dividing them equally among the duplicates.
acetic acid fixative. Slides were prepared by dropping from a height about 2–3 drops of fixed cell suspension onto pre-cleaned glass slides. After drying for ∼24 hours the slides were stained with 5% Giemsa stain and mounted with the DPX mountant post drying. For evaluation of chromosomal aberrations, at least 300 analysable metaphases were scored for each concentration by dividing them equally among the duplicates.
In vivo micronucleus test
A mammalian in vivo micronucleus test13 was conducted with the purpose of assessing the potential genotoxicity that could arise due to the in vivo metabolism of ZASNPs when given by oral gavage to mice (CD-1) for a period of at least 14 consecutive days. As the bone marrow of young rodents is the site for erythrocyte production, it was selected as the target tissue for assessing genetic damage.All the animal care procedures and standards in this study were in compliance with the regulations of the Committee for Control and Supervision of Experiments on Animals (CCSEA), Government of India, and the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC), USA. All the experimental procedures were duly approved by the Institutional Animal Ethics Committee (IAEC). Animals were housed up to 3 animals per cage in polycarbonate cages covered with a stainless steel grid top with corn cobs as the bedding material. Diet and water were provided ad libitum to study animals. Environmental controls for the animal room were set to maintain 19–25 °C, a relative humidity of 40–70%, a minimum of 12 air changes per hour, and a 12-hour light/12-hour dark cycle. Animals were given cage-enrichment material. Animals were acclimatized for at least five days prior to the start of the dosing and were randomized using the body weight stratification method. Each animal assigned was within ±20% of the mean body weight viz. 37.3 ± 7.5. 18 male mice were assigned to groups and the doses were administered as specified in Table 5.
Based on the recommendation of OECD Test No. 474![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 the limit dose of 1000 mg kg−1 day−1 was selected as the starting dose. As the limit dose was well tolerated by all animals, further lower doses were not evaluated. Animals were dosed at a volume of 10 mL kg−1 by oral gavage once daily for at least 14 days consecutively. The doses were based on the most recently recorded scheduled body weight. Blood samples were collected from fasted animals from G1 and G2 for haematology and clinical chemistry evaluation. ∼1.5 mL of blood sample was collected in heparinized tubes for clinical chemistry evaluation and ∼0.5 mL of blood was collected for haematology analysis in K2 EDTA tubes. Peripheral blood was collected from the retro-orbital venous plexus for each mouse after ∼ 24 hours of last dose administration i.e. day 15. Animals were sacrificed (euthanized via carbon dioxide inhalation and exsanguinated) following 14 consecutive days of dosing, ∼24 hours after dosing cessation i.e., on day 15. The proportion of immature erythrocytes among total (immature + mature) erythrocytes was analysed by observing the slides under the 100× oil immersion objective of a light microscope. At least 500 total erythrocytes were scored for each animal. To assess the incidence of Micronucleated Immature Erythrocytes (PCEs) the slides were evaluated under a light microscope using the 100× oil immersion objective, at least 4000 polychromatic (immature) erythrocytes (PCEs) were collected from each animal. The micronucleated PCE count for each animal was recorded in the data sheet.
13 the limit dose of 1000 mg kg−1 day−1 was selected as the starting dose. As the limit dose was well tolerated by all animals, further lower doses were not evaluated. Animals were dosed at a volume of 10 mL kg−1 by oral gavage once daily for at least 14 days consecutively. The doses were based on the most recently recorded scheduled body weight. Blood samples were collected from fasted animals from G1 and G2 for haematology and clinical chemistry evaluation. ∼1.5 mL of blood sample was collected in heparinized tubes for clinical chemistry evaluation and ∼0.5 mL of blood was collected for haematology analysis in K2 EDTA tubes. Peripheral blood was collected from the retro-orbital venous plexus for each mouse after ∼ 24 hours of last dose administration i.e. day 15. Animals were sacrificed (euthanized via carbon dioxide inhalation and exsanguinated) following 14 consecutive days of dosing, ∼24 hours after dosing cessation i.e., on day 15. The proportion of immature erythrocytes among total (immature + mature) erythrocytes was analysed by observing the slides under the 100× oil immersion objective of a light microscope. At least 500 total erythrocytes were scored for each animal. To assess the incidence of Micronucleated Immature Erythrocytes (PCEs) the slides were evaluated under a light microscope using the 100× oil immersion objective, at least 4000 polychromatic (immature) erythrocytes (PCEs) were collected from each animal. The micronucleated PCE count for each animal was recorded in the data sheet.
Antibacterial activity
The antimicrobial efficacy of the Zanthoxylum armatum fruit extract and synthesized silver nanoparticles (ZASNPs) was evaluated by the CLSI recommended methods like disc diffusion, minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and time kill curve against Gram-negative bacteria such as Escherichia coli and Salmonella typhimurium and Gram-positive bacteria such as Bacillus subtilis and Staphylococcus aureus. Several agents have been tested for their antibacterial efficacy using the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC), two commonly used metrics. According to reports, the action is bacteriostatic when the ratio of MBC/MIC = 4 or 16 and bactericidal when MBC/MIC = 1 or 2.23 A bacteriostatic effect has been observed for an MBC to MIC ratio of >4, which has been considered by certain studies.24Disc diffusion method
The antibacterial efficacy of the synthesized silver nanoparticles was evaluated using the Kirby–Bauer disk diffusion method. A single colony of each strain was grown overnight in Müller–Hinton broth on a rotary shaker (200 rpm) at 35 °C. If necessary, the overnight culture was diluted with 0.9% NaCl to a 0.5 McFarland standard measured on a densitometer. The cultured cells were then applied onto Müller–Hinton agar plates (5 ± 1 mm in depth) in triplicate by streaking in 3 planes evenly with a sterile cotton swab. The plates were air dried for a few minutes. 4 sterile discs saturated with 10 μl of 0.9% NaCl, ZA extract, AgNO3 and ZASNPs respectively were placed on the agar surface equidistantly, allowed to dry for a few minutes and then incubated at 35 °C for 24–48 hours. After incubation, the inhibition zones were measured using the zone scale. The assays were performed in triplicate. For each bacterial strain, 4 test discs were used viz. sterile discs saturated with 10 μl of 0.9% NaCl (to serve as the negative control), sterile discs saturated with 10 μl ZA extract, sterile discs saturated with 10 μl AgNO3 and sterile disc saturated with 10 μl ZASNPs.Minimum inhibitory concentration (MIC) by the broth microdilution method
0.5 McFarland suspension of overnight grown cultures was diluted 100× to a density of 106 CFU mL−1. 50 μL Müller–Hinton broth was added to columns 2 to 11, 100 μL to column 12 of a 96-well plate (blank), and 100 μL of ZASNPs was added to column 1. Serial two-fold dilutions of ZASNPs were prepared by transferring 50 μL from column 1 to 2, 2 to 3 etc. up to column 10. The final concentrations of 25, 12.5, 6.25, 3.13, 1.56, 0.78, 0.39, 0.20, 0.10 and 0.05 μL ZASNPs per well were obtained. 50 μl from the adjusted bacterial suspension was then added to all wells except column 12, resulting in approx. 5 × 105 CFU ml−1. The plates were incubated for 24 h at 37 °C. 30 μl of 0.015% resazurin was added to all wells and further incubated for 2–4 h for colour development. The columns showing no colour change (blue) were scored as concentrations above the MIC value.Minimum bactericidal concentration (MBC)
The contents of representative 3 wells having concentrations greater than the MIC value were directly plated onto MHA plates and incubated for 24 h for determining the MBC. Representative 3 blank and 3 control well contents were also plated on MHA plates to serve as the lowest and highest bacterial counts, respectively. The lowest concentration of ZASNPs that resulted in 99.9% killing of the final inoculum after incubation for 24 h was scored as the MBC.Time kill curve
All bacterial inoculum suspensions were diluted to ∼106 CFU mL−1. ZASNPs were diluted in MHB containing bacterial suspension to obtain the final concentrations of MIC, 2xMIC and 3xMIC (1 mL final volume). One tube per culture was maintained untreated to serve as the control. All tubes were incubated at 37 °C with 150–200 rpm agitation. At pre-determined time points (0, 2, 4, 6, 8 and 24 h), 100 μL aliquots were serially diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in 1% phosphate buffered saline (PBS), plated onto MHA and then incubated for 24 hours in a CO2 incubator at 37 °C. After incubation at 37 °C for 24 h, a colony count was performed followed by the calculation of CFU mL−1 using formula (1). The time-kill curve was performed thrice. The graph of mean Log10 CFU mL−1 values versus time was plotted.
100 in 1% phosphate buffered saline (PBS), plated onto MHA and then incubated for 24 hours in a CO2 incubator at 37 °C. After incubation at 37 °C for 24 h, a colony count was performed followed by the calculation of CFU mL−1 using formula (1). The time-kill curve was performed thrice. The graph of mean Log10 CFU mL−1 values versus time was plotted. | (1) |
Synergism of Zanthoxylum armatum silver nanoparticles with antibiotics
The synergistic effect of ZASNPs with antibiotics from the main classes was assessed by the disc diffusion method, performed in triplicate. A single colony of each strain (Staphylococcus aureus and Escherichia coli) was cultured overnight in Müller–Hinton broth on a rotary shaker (200 rpm) at 35 °C. The overnight culture was diluted with 0.9% NaCl to a 0.5 McFarland standard, which was then applied onto Müller–Hinton agar plates in triplicate. The plates were air dried for a few minutes. Standard antibiotic discs, standard antibiotic discs saturated with 10 μl of ZASNPs and sterile discs saturated with 10 μl of 0.9% NaCl (to serve as the negative control) were placed on the agar surface equidistantly. After incubation at 35 °C for 24–48 hours, the inhibition zones were measured using the zone scale. The synergism represented as a fold increase was evaluated using the following formula:| Fold increase = (b2 − a2)/a2 | (2) |
Conclusions
In current research, we successfully synthesized silver nanoparticles using the Zanthoxylum armatum aqueous fruit extract, demonstrating a green and environmentally friendly approach towards nanotechnology. The synthesized nanoparticles were thoroughly characterized, revealing spherical nanoparticles with a size average of ∼6.2 ± 5.1 nm, strongly anionic having a zeta potential of −36.9, indicating high stability in solution. The genotoxicity assessments confirmed the biocompatibility of these nanoparticles, ensuring their safety for potential biomedical applications.The efficacy of ZASNPs against various pathogenic bacteria underscores their potential as a powerful antimicrobial agent. As demonstrated through the study, ZASNPs positively enhanced the efficacy of commonly used antibiotics, appearing to be a suitable alternative to treat antibiotic-resistant strains. This study not only highlights the significance of green synthesis in producing effective and safe nanoparticles but also opens new avenues for their application in medical and environmental fields. Potential clinical applications of ZASNPs include developing new antibacterial treatments, enhancing antibiotic therapies, and incorporating them into wound dressings, medical device coatings, and dental materials. They could also be used in pharmaceutical formulations, cancer therapy, and diagnostic tools. Continued research is essential to scale up the synthesis process, explore a broader range of applications, and understand their interactions with biological systems to fully realize their potential.
These applications highlight the versatility and potential of ZASNPs in various clinical settings. Continued research and development are essential to fully realize these benefits and ensure their safe and effective use in medical practice. Future research should focus on scaling up the synthesis process, exploring the full spectrum of applications for these promising nanoparticles and investigating their interactions with different biological systems to further understand their mechanisms of action.
Author contributions
N. Q.: conceptualization, formal analysis, data curation, and writing – original draft. M. M. S. and A. A.: supervision, guidance on drafting and revisions of the manuscript. All authors reviewed the draft manuscript, read, and approved the final manuscript.Data availability
The data that support the findings of this study are included in this manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge that this work has been done in collaboration with Manipal Academy of Higher Education (MAHE) and Lupin Limited (Pune). The authors are also grateful to Lupin Limited (Pune) for providing the facility and resources to conduct the experiments.References
- M. I. Ionescu, Are herbal products an alternative to antibiotics?. InBacterial Pathogenesis and Antibacterial Control, IntechOpen, 2017 Search PubMed.
- N. Phuyal, P. K. Jha, P. P. Raturi and S. Rajbhandary, In vitro antibacterial activities of methanolic extracts of fruits, seeds, and bark of Zanthoxylum armatum DC, J. Trop. Med., 2020, 2020(1), 2803063 Search PubMed.
- M. Mukhtar, H. A. Khan and S. Naz, Antibacterial Profiling of Zanthoxylum armatum Extracts: A Comprehensive Computational and Experimental Study, Nat. Prod. Commun., 2024, 19(3), 1934578X241237911 CrossRef CAS.
- Y. Y. Loo, Y. Rukayadi, M. A. Nor-Khaizura, C. H. Kuan, B. W. Chieng, M. Nishibuchi and S. Radu, In vitro antimicrobial activity of green synthesized silver nanoparticles against selected gram-negative foodborne pathogens, Front. Microbiol., 2018, 9, 1555 CrossRef PubMed.
- S. Agnihotri, S. Mukherji and S. Mukherji, Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy, RSC Adv., 2014, 4(8), 3974–3983 RSC.
- A. U. Mirza, M. S. Khan, S. A. Nami, A. Kareem, S. Rehman, S. A. Bhat and N. Nishat, Copper oxide nanomaterials Derived from Zanthoxylum armatum DC. and Berberis lycium Royle plant species: Characterization, assessment of free radical scavenging and antibacterial activity, Chem. Biodiversity, 2019, 16(8), e1900145 CrossRef PubMed.
- A. Chauhan, S. Kumari, R. Verma, V. Dutta, S. Ghotekar, M. Kaur, S. Kulshrestha, K. Singh, K. Y. Lin and R. Kumar, Fabrication of copper oxide nanoparticles via microwave and green approaches and their antimicrobial potential, Chem. Pap., 2022, 76(11), 7147–7162 CrossRef CAS.
- A. V. Ramesh, D. Rama Devi, S. Mohan Botsa and K. Basavaiah, Facile green synthesis of Fe3O4 nanoparticles using aqueous leaf extract of Zanthoxylum armatum DC. for efficient adsorption of methylene blue, J. Asian Ceram. Soc., 2018, 6(2), 145–155 CrossRef.
- K. Jyoti and A. Singh, Green synthesis of nanostructured silver particles and their catalytic application in dye degradation, J. Genet. Eng. Biotechnol., 2016, 14(2), 311–317 CrossRef PubMed.
- M. Riaz, M. Altaf, P. Ahmad, M. U. Khandaker, H. Osman, E. M. Eed and Y. Shakir, Biogenic synthesis of ag nanoparticles of 18.27 nm by Zanthozylum armatum and determination of biological potentials, Molecules, 2022, 27(4), 1166 CrossRef CAS PubMed.
- U. Habib, A. Ahmad Khan, T. U. Rahman, M. A. Zeb and W. Liaqat, Green synthesis, characterization, and antibacterial activity of silver nanoparticles using stem extract of Zanthoxylum armatum, Microsc. Res. Tech., 2022, 85(12), 3830–3837 CrossRef CAS PubMed.
- R. Verma, A. Chauhan, S. Kumari, R. Jasrotia, A. Ali, C. Gopalakrishnan, R. Kumar and S. Ghotekar, Green synthesis of ZnO NPs using Timur (Zanthoxylum armatum DC.) plant extract for antimicrobial and dye degradation applications, Chem. Pap., 2023, 77(9), 5587–5597 CrossRef CAS.
- Organisation for Economic Co-operation and Development, Test No. 474: Mammalian Erythrocyte Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, 2016. DOI:10.1787/9789264264762-en.
- National Committee for Clinical Laboratory Standards, Methods for determining bactericidal activity of antimicrobial agents; Approved guideline. NCCLS document M26-A [ISBN 1-56238-384-1], 1999 Search PubMed.
- P. Prasher, M. Singh and H. Mudila, Oligodynamic effect of silver nanoparticles: a review, BioNanoScience, 2018, 8, 951–962 CrossRef.
- K. S. Butler, D. J. Peeler, B. J. Casey, B. J. Dair and R. K. Elespuru, Silver nanoparticles: correlating nanoparticle size and cellular uptake with genotoxicity, Mutagenesis, 2015, 30(4), 577–591 CrossRef CAS PubMed.
- Y. Li, D. H. Chen, J. Yan, Y. Chen, R. A. Mittelstaedt, Y. Zhang, A. S. Biris, R. H. Heflich and T. Chen, Genotoxicity of silver nanoparticles evaluated using the Ames test and in vitro micronucleus assay, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2012, 745(1–2), 4–10 CrossRef CAS PubMed.
- D. M. Maron and B. N. Ames, Revised methods for the Salmonella mutagenicity test, Mutat. Res., Environ. Mutagen. Relat. Subj., 1983, 113(3–4), 173–215 CAS.
- K. Mortelmans and E. S. Riccio, The bacterial tryptophan reverse mutation assay with Escherichia coli WP2, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2000, 455(1–2), 61–69 CrossRef CAS PubMed.
- K. Mortelmans and E. Zeiger, The Ames Salmonella/microsome mutagenicity assay, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2000, 455(1–2), 29–60 CrossRef CAS PubMed.
- Organisation for Economic Co-operation and Development, Test No. 471: Bacterial Reverse Mutation Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, 2020. DOI:10.1787/9789264071247-en.
- Organisation for Economic Co-operation and Development, Test No. 473: In Vitro Mammalian Chromosomal Aberration Test, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, 2016. DOI:10.1787/9789264264649-en.
- K. Konaté, J. F. Mavoungou, A. N. Lepengué, R. R. Aworet-Samseny, A. Hilou, A. Souza, M. H. Dicko and B. M'Batchi, Antibacterial activity against β-lactamase producing Methicillin and Ampicillin-resistants Staphylococcus aureus: Fractional Inhibitory Concentration Index (FICI) determination, Ann. Clin. Microbiol., 2012, 11, 1–2 Search PubMed.
- G. A. Pankey and L. D. Sabath, Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections, Clin. Infect. Dis., 2004, 38(6), 864–870 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |