Open Access Article
Open Access ArticleNatural products in antiparasitic drug discovery: advances, opportunities and challenges
Xiaofei
Shang
 *abe,
Lixia
Dai
a,
Xinyuan
Cao
c,
Yudong
Ma
a,
Ilgekbayeva
Gulnaz
d,
Xiaolou
Miao
*ae,
Xiuhui
Li
*b and
Xiaorong
Yang
*abe,
Lixia
Dai
a,
Xinyuan
Cao
c,
Yudong
Ma
a,
Ilgekbayeva
Gulnaz
d,
Xiaolou
Miao
*ae,
Xiuhui
Li
*b and
Xiaorong
Yang
 *ae
*ae
aKey Laboratory of Veterinary Pharmaceutical Development of Ministry of Agriculture, Key Laboratory of New Animal Drug Project, Gansu Province, Lanzhou Institute of Husbandry and Pharmaceutical Sciences, Chinese Academy of Agricultural Sciences, Lanzhou, China. E-mail: yangxiaorong01@caas.cn; shangxiaofei@caas.cn; miaoxiaolou@caas.cn
bBeijing Youan Hospital, Capital Medical University, Beijing, China. E-mail: lixiuhui@sohu.com
cPeople's Hospital of Ningxia Hui Autonomous Region, Ningxia Medical University, Yinchuan, China
dDepartment of Biological Safety, Kazakh National Agrarian Research University, Almaty, Kazakhstan
eChina-Kazakh Joint Research Center for Natural Veterinary Drug, Lanzhou, China
First published on 12th June 2025
Abstract
Covering: up to 2024.
Parasites infect hundreds of millions of people, result in significant disability rates and mortality and lead to devastating social and economic consequences, especially in developing countries and regions. Traditional medicines have been used for centuries to treat parasitic diseases. Some natural products (NPs) and their derivatives have been derived from these medicines and applied in clinical settings, attracting the attention of the scientific community throughout history. With the development and application of revolutionized technologies over the past few years, more promising compounds have been found from natural resources and provided new possibilities for the development of novel antiparasitic drugs. In this review, we aimed to discuss the strategies used for developing drugs from natural resources and mainly describe the causative pathogens, epidemiology and current treatment of parasitic diseases. Promising NPs and their derivatives are listed, and their effectiveness, potential mechanism and structural optimization are described. Subsequently, the advantages and limitations of the drug development process and the role of technologies in this process are discussed. A prospective analysis of research on and development of antiparasitic drugs based on NPs is presented. The high attrition rates, accessibility, sustainable supply, IP constraints and other problems still hinder the development of NPs; however, the therapeutic significance and broad clinical utilization of approved natural product-derived drugs, exemplified by quinine, artemisinin, and ivermectin in treating parasitic diseases, underscore that natural products remain a highly promising reservoir of chemical agents. Their exceptional structural diversity and marked bioactivities continue to stimulate scientific interest in novel antiparasitic drug discovery. In combination with the recent development and application of revolutionized technologies, NPs will provide a stronger basis for drug discovery and will continue to provide major contributions to human and veterinary health.
1 Introduction
Parasites are a group of eukaryotic pathogens that include protozoa, helminths and arthropods, which partially or entirely complete their life cycle within their host organisms. Parasites infect hundreds of millions of people, resulting in significant mortality with devastating social and economic consequences,1,2 especially in tropical and subtropical regions of the world. Most parasitic diseases are neglected tropical diseases (NTDs) and spread through various modes of transmission, including surfaces (scabies and psoroptes), infected vectors (Chagas disease, lymphatic filariasis, etc.), and contaminated food and/or water (echinococcosis).3,4 Although some vaccine candidates, including ChAd63-KH for leishmaniasis,5 the PfSPZ vaccine and ChAd63 for malaria,6 and recombinant proteins of Tc24 with Th1 adjuvants for African trypanosomiasis, have been investigated,3 only chicken coccidiosis vaccines have been used.7 Today, there are no vaccines available against major parasitic infections in humans, and medication is still used only for control and prevention of parasitic diseases. However, owing to the poor compliance of individuals and the poor safety, low efficacy, resistance and high cost of drugs, the utility of conventional drugs is limited and decreased.2 The management of parasitic diseases is a great challenge.8 Therefore, it is necessary to find new therapeutic agents against parasitic infections.For centuries, traditional medicines have been used to treat parasitic diseases. Some prominent plants such as Cinchona and Artemisia annua have been screened and used by humans for a long time and are applied in clinical settings today to treat malaria in Africa and China, respectively. Currently, 70–95% of the population in developing countries continue to rely on medicinal plants for their primary Pharmacopeia,9 and 80% of 122 plant-derived drugs were discovered from traditional medicines.10 Considering that the plants present good features such as good safety, strong effects, affordability and long clinical practice in humans and animals, the WHO has established the use of plants as a traditional medicine strategy.11 Natural product platforms derived from traditional medicines have shown potential for the management of many parasitic diseases.
Natural products (NPs) derived from plants, animals and microorganisms have played an important role in drug discovery since the early days of medicine, especially for treating infectious diseases.12 Compared with the synthetic counterparts, NPs with a high degree of bioavailability not only provide remarkable prototype drugs such as artemisinin, avermectin, morphine, berberine, taxol, quinine, and camptothecin for treating diseases, but also offer special features with enormous scaffolds and diverse structures for synthesizing new chemical entities (NCEs), such as avermectin.13 Currently, more than 1000 NCEs derived from natural sources are approved for clinical use, and in international databases, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 patents are authorized by governments.14 During 1970–1980, the investigation of NPs peaked in the Western pharmaceutical industry. Newman & Cragg15 found that among all FDA-proven drugs (1881) between 1981 and 2019, 49.44% (930) were directly and indirectly derived from NPs. For parasitic diseases, approximately 60% of drugs are derived from NPs.
000 patents are authorized by governments.14 During 1970–1980, the investigation of NPs peaked in the Western pharmaceutical industry. Newman & Cragg15 found that among all FDA-proven drugs (1881) between 1981 and 2019, 49.44% (930) were directly and indirectly derived from NPs. For parasitic diseases, approximately 60% of drugs are derived from NPs.
With an estimated 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 500
000 to 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 plant species and approximately 2 million lower-level organisms worldwide, these resources provide a chemotherapeutic pool for finding novel compounds.16 According to the Medicinal Plant Names Services (MPNS), approximately 28
000 plant species and approximately 2 million lower-level organisms worldwide, these resources provide a chemotherapeutic pool for finding novel compounds.16 According to the Medicinal Plant Names Services (MPNS), approximately 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 187 species of plants are utilized in medicine, accounting for nearly 7.5% of all plant life.17,18 They may provide insights into the efficacy and safety of novel compounds. However, considering the time-consuming and labor- and cost-intensive nature of traditional extraction, isolation and identification, many groups and pharmaceutical companies have experienced a slow decline in the discovery of novel NPs over the past two decades, and only small parts of natural resources have been exploited. Promisingly, a range of revolutionized technologies (including genomics, metagenomics, proteomics and metabolomics) are providing an unprecedented opportunity and new welcome impetus for researchers and pharmaceutical companies to discover antiparasitic drugs from natural resources.11 The WHO Program of Tropical Diseases has declared that NPs are still a crucial priority for the management of parasitic diseases.3 In this paper, we review the opportunities and challenges encountered during the discovery of antiparasitic drugs from natural resources (Table 1).
187 species of plants are utilized in medicine, accounting for nearly 7.5% of all plant life.17,18 They may provide insights into the efficacy and safety of novel compounds. However, considering the time-consuming and labor- and cost-intensive nature of traditional extraction, isolation and identification, many groups and pharmaceutical companies have experienced a slow decline in the discovery of novel NPs over the past two decades, and only small parts of natural resources have been exploited. Promisingly, a range of revolutionized technologies (including genomics, metagenomics, proteomics and metabolomics) are providing an unprecedented opportunity and new welcome impetus for researchers and pharmaceutical companies to discover antiparasitic drugs from natural resources.11 The WHO Program of Tropical Diseases has declared that NPs are still a crucial priority for the management of parasitic diseases.3 In this paper, we review the opportunities and challenges encountered during the discovery of antiparasitic drugs from natural resources (Table 1).
| Disease | Causative pathogen | Treatment | Promising natural products and their analogs | |
|---|---|---|---|---|
| Current drugs | Disadvantage | |||
| a Represent the antiparasitic drugs were derived from NPs. b Represent the antiparasitic drugs were NPs. | ||||
| Protozoal disease | ||||
| Leishmaniasis | Leishmania donovani, L. infantum for VL; L. tropica and other species for MCL and CL | Pentamidine, pentavalent antimonials, amphotericin Ba, miltefosine | Resistance for pentamidine and antimonials; high cost for amphotericin B; the contraindicated in pregnancy of miltefosine; toxic effects and parenteral route of all drugs | Arnica montana tincture; jacoumaric acid, corosolic acid |
| Chagas's disease | Trypanosoma cruzi | Nifurtimox; benznidazole | Benznidazole is not effective against the chronic phases; long treatment course; adverse effects | Ascosalipyrrolidinone A; valinomycin; terpinen-4-ol |
| African trypanosomiasis | T. brucei rhodesiense; T. gambiense | Suramin, pentamidine, melarsoprol, eflornithine | Pentamidine and suramin are effective only on the early haemolymphatic stage; pentamidine needs parenteral administration; poorly tolerated | 7,8-Dihydroxyflavone; 1,12-dehydro-13-oxo-plakortide Q; manadoperoxides I and B; 2,7-dibro-mocryptolepine; 12-isomanadoperoxide B |
| Malaria | Plasmodium spp. | Quinineb, chloroquinea, primaquinea, mefloquinea, artemisininb, atovaquonea | Resistance, low compliance, cost and toxin of these drugs | Dioncophyllines F; dehydroantofne; tylophoridicine; tsitsikammamine C; puberulic acid; fortunilide A |
| Toxoplasmosis | Toxoplasma gondii | Dihydrofolate reductase inhibitors (pyrimethamine, trimethoprim), dihydropteroate synthetase inhibitors (sulfadiazine, sulfamethoxazole, sulfadoxine) | Most drugs acts only against the tachyzoite, didn't affect the cysts and cross the blood–brain barrier; the undesirable side-effects and the resistance of pyrimethamine were appeared widely | Trametes versicolor (Turkey tail) methanol extract; propolis and wheat germ oil; abscisic acid; manzamine A; sigmosceptrellin-B; plakortide |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Helminths | ||||
| Schistosomiasis | Schistosoma mansoni, S. haematobium, S. japonica | Oxamniquine and praziquantel | Oxamniquine only effective against S. mansoni, and praziquantel does not kill immature worms. The resistance to praziquantel was appeared | Ectracts of Agave lophantha, Furcraea selloa and Solanum elaeagnifolium; artemisinin (artesunate or artemether); 4-nerolidylcatechol; menadione |
| Lymphatic filariases and onchocerciasis | Brugia malayi, Wuchereria bancrofti, and Onchocerca volvulus | Diethylcarbamazine or ivermectinb in combination with albendazole; moxidectin | Diethylcarbamazine cannot be used in O. volvulus-endemic areas, albendazole only used in combination therapy, and ivermectin does not eliminate adult worms; the resistance, high cost, and adverse effects are appeared | Anthraquinone K; 7-fluoro-6-oxybenzoxaborole; A-1574083 (tylosin A analog); doxycycline |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Ectoparasites | ||||
| Scabies | Sarcoptes scabiei | Permethrina, ivermectinb | The poor or limited ovicidal action of permethrin, and the resistance are appeared | Tinospora cordifolia lotion; tea oil; clove oils; eugenol, juglone, octadecanoic acid-tetrahydrofuran-3,4-diylester |
| Ticks | Ornithodoros spp., Otobius spp., Hyalomma spp., Ixodes spp., Dermacentor spp., Amblyomma spp., Boophilus spp., Rhipicephalus spp. | Permethrina, ivermectina, fluralaner, etc. | The resistance, high cost, and adverse effects are appeared | Some plant essential oils, such as MyggA® Natural (Bioglan, Lund, Sweden) and Citriodiol® |
2 Current control strategies for parasitic diseases
2.1 Protozoa
Protozoa are eukaryotic unicellular organisms that are free-living in water or other organisms, and protozoan parasite infection leads to high rates of mortality and morbidity worldwide. In this review, we focus on the current control strategies against Leishmania spp., Trypanosoma spp., Plasmodium spp., and Toxoplasma spp.Malaria caused by five species of Plasmodium remains a massive problem in many regions of the world,19 and there were 247 million cases of malaria and 619![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths in 2022 worldwide.20,21 Although malaria vaccine pilots were launched in 2019 by the Ministries of Health of some Africa countries and organizations, a 5-month-old girl has received the world's first malaria vaccine recently (RTS, S/AS01 or RTS, S). In 1820, quinine (1), the first antimalarial agent, was isolated from Cinchona tree bark and is widely used to treat malaria,22 and classic amino alcohols and 4-aminoquinolines (2–4) were developed over the last century. Tafenoquine (5) and primaquine (6) from the 8-aminoquinoline scaffold are recommended in combination with other antimalarials to prevent relapse of P. vivax and P. ovale infections23 (Fig. 1A). In addition, artemisinin's derivative, dihydroartemisinin (8), as well as artesunate (9) and artemether-lumefantrine (10), was approved by the WHO20 (Fig. 1B). Currently, chemoprevention strategies, based on mass drug administration (MDA), remain the most important and efficient methods for controlling malaria. The history of chemotherapy for controlling malaria is intimately linked with the history of herbal medicinal products. Over the past few decades, significant progress has been made in new approaches to control and eliminate malaria, including the identification of new druggable targets, promising drug candidates, and several new therapies. Despite this measurable progress for screening NPs, no NCEs have been licensed. Of course, the annual number of malaria cases still persists, highlighting the vital need for new medicines.24
000 deaths in 2022 worldwide.20,21 Although malaria vaccine pilots were launched in 2019 by the Ministries of Health of some Africa countries and organizations, a 5-month-old girl has received the world's first malaria vaccine recently (RTS, S/AS01 or RTS, S). In 1820, quinine (1), the first antimalarial agent, was isolated from Cinchona tree bark and is widely used to treat malaria,22 and classic amino alcohols and 4-aminoquinolines (2–4) were developed over the last century. Tafenoquine (5) and primaquine (6) from the 8-aminoquinoline scaffold are recommended in combination with other antimalarials to prevent relapse of P. vivax and P. ovale infections23 (Fig. 1A). In addition, artemisinin's derivative, dihydroartemisinin (8), as well as artesunate (9) and artemether-lumefantrine (10), was approved by the WHO20 (Fig. 1B). Currently, chemoprevention strategies, based on mass drug administration (MDA), remain the most important and efficient methods for controlling malaria. The history of chemotherapy for controlling malaria is intimately linked with the history of herbal medicinal products. Over the past few decades, significant progress has been made in new approaches to control and eliminate malaria, including the identification of new druggable targets, promising drug candidates, and several new therapies. Despite this measurable progress for screening NPs, no NCEs have been licensed. Of course, the annual number of malaria cases still persists, highlighting the vital need for new medicines.24
Leishmaniasis is a disease caused by an obligate intracellular parasite that contains over 20 Leishmania species. The main forms of the disease are cutaneous leishmaniasis (CL), which is characterized by self-healing ulcers; mucocutaneous leishmaniasis (MCL), which involves progressive nasopharyngeal infections; and visceral leishmaniasis (VL), which is also known as kala-azar,25,26 and over 90% of VL occur in poor rural and suburban areas of Bangladesh, Brazil, Ethiopia, Sudan, India and South Sudan.27,28 According to the Global Burden of Disease (GBD) study conducted in 2019, it was estimated that between 498![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 862
000 and 862![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 new cases of leishmaniasis occur worldwide each year.29 Currently, there is no perfect vaccine or suitable drug to eradicate leishmaniasis completely,30 and the treatment of leishmaniasis mainly relies on pentavalent antimonials (11), paromomycin (12), miltefosine (13), amphotericin B (14) and other drugs. Among these, amphotericin B (14) is a breakthrough antibiotic against leishmaniasis and was isolated from Streptomyces spp.31 (Fig. 2A). Due to the development of extensive resistance to pentavalent antimonials (11) and the toxic effects, parenteral route and high cost of other drugs, these drugs are gradually being limited. Although some NCEs have progressed into phase I clinical research, including the CRK12 inhibitor GSK3186899/DDD853651 (15) and proteasome inhibitor LXE408 (16),32 no new drugs have been approved in recent decades (Fig. 2B).
000 new cases of leishmaniasis occur worldwide each year.29 Currently, there is no perfect vaccine or suitable drug to eradicate leishmaniasis completely,30 and the treatment of leishmaniasis mainly relies on pentavalent antimonials (11), paromomycin (12), miltefosine (13), amphotericin B (14) and other drugs. Among these, amphotericin B (14) is a breakthrough antibiotic against leishmaniasis and was isolated from Streptomyces spp.31 (Fig. 2A). Due to the development of extensive resistance to pentavalent antimonials (11) and the toxic effects, parenteral route and high cost of other drugs, these drugs are gradually being limited. Although some NCEs have progressed into phase I clinical research, including the CRK12 inhibitor GSK3186899/DDD853651 (15) and proteasome inhibitor LXE408 (16),32 no new drugs have been approved in recent decades (Fig. 2B).
 | ||
| Fig. 2 Current chemicals or antibiotics used to treat leishmaniasis (A). Drug candidates for the treatment of leishmaniasis (B). | ||
Trypanosoma brucei and T. cruzi are the pathogens causing human African trypanosomiasis (sleeping sickness) and American trypanosomiasis (Chagas disease), respectively. These diseases are prevalent in low- and middle-income countries and cause a large number of deaths (approximately 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people per year).33–35 Significant progress has been achieved in treating sleeping sickness; however, drugs and developmental pipelines for Chagas disease urgently need to be investigated, although the novel drug nifurtimox (22) invented by Bayer was approved by the FDA in Q3 2020.36 Moreover, the curative effect of current drugs is limited; pentamidine (18) and suramin (17) are effective only against the early hemolymphatic stage, and benzimidazole (20) is effective only in children aged 2–12 years whose recent infection is in the acute phase of Chagas disease but not against the chronic stage.37 Pentamidine (18) requires parenteral administration, and some drugs are poorly tolerated and difficult to administer. Hence, drugs with novel structures and mechanisms of action different from those of current drugs (17–23) are needed38 (Fig. 3A). However, due to their unsuitable structural and pharmacokinetic properties, numerous drug candidates have failed to advance to the later stages of clinical development. Some traditional medicines and NPs have presented clinical efficacy in treating complementary neurological disorders associated with sleeping sickness and other disease sites in adipose tissue, skin, cardiac muscle, etc., and have attracted the interest of many researchers.39
000 people per year).33–35 Significant progress has been achieved in treating sleeping sickness; however, drugs and developmental pipelines for Chagas disease urgently need to be investigated, although the novel drug nifurtimox (22) invented by Bayer was approved by the FDA in Q3 2020.36 Moreover, the curative effect of current drugs is limited; pentamidine (18) and suramin (17) are effective only against the early hemolymphatic stage, and benzimidazole (20) is effective only in children aged 2–12 years whose recent infection is in the acute phase of Chagas disease but not against the chronic stage.37 Pentamidine (18) requires parenteral administration, and some drugs are poorly tolerated and difficult to administer. Hence, drugs with novel structures and mechanisms of action different from those of current drugs (17–23) are needed38 (Fig. 3A). However, due to their unsuitable structural and pharmacokinetic properties, numerous drug candidates have failed to advance to the later stages of clinical development. Some traditional medicines and NPs have presented clinical efficacy in treating complementary neurological disorders associated with sleeping sickness and other disease sites in adipose tissue, skin, cardiac muscle, etc., and have attracted the interest of many researchers.39
 | ||
| Fig. 3 Current chemicals or antibiotics used to treat American trypanosomiasis and human African trypanosomiasis (A) and toxoplasmosis (B). | ||
Toxoplasma gondii is an apicomplexan obligate intracellular parasite.40 The parasite occurs in nature as oocysts, bradyzoites, and replicating tachyzoites, and the last form is the hallmark of active disease.41,42 According to the GBD estimate, the disease affects about one-third of the population, and causes 1.2 million disability-adjusted life years (DALYs) for >190![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 annual cases.43 In particular, chronic infections frequently become reactivated in individuals infected with HIV who have advanced to AIDS due to immunocompromise, leading to a significantly elevated risk of mortality.44 Treatment of toxoplasmosis usually involves a combination of two enzyme inhibitors to block folate synthesis, including dihydropterate synthetase inhibitors, such as sulfadoxine (24), sulfadiazine (25), and sulfamethoxazole (26), and dihydrofolate reductase inhibitors, such as trimethoprim (27) and pyrimethamine (28). Among them, pyrimethamine is one of the most effective drugs against T. gondii.42 However, most drugs act only against the tachyzoite and do not affect cysts; undesirable side effects and resistance to pyrimethamine also appear widely45 (Fig. 3B).
000 annual cases.43 In particular, chronic infections frequently become reactivated in individuals infected with HIV who have advanced to AIDS due to immunocompromise, leading to a significantly elevated risk of mortality.44 Treatment of toxoplasmosis usually involves a combination of two enzyme inhibitors to block folate synthesis, including dihydropterate synthetase inhibitors, such as sulfadoxine (24), sulfadiazine (25), and sulfamethoxazole (26), and dihydrofolate reductase inhibitors, such as trimethoprim (27) and pyrimethamine (28). Among them, pyrimethamine is one of the most effective drugs against T. gondii.42 However, most drugs act only against the tachyzoite and do not affect cysts; undesirable side effects and resistance to pyrimethamine also appear widely45 (Fig. 3B).
Considering that T. gondii may cross the blood–brain barrier and then establish persistent infection in a drug-resistant bradyzoite stage,46,47 an ideal agent should achieve therapeutic, systemic, brain and eye concentrations that are effective in the organs and active against the acute replicating tachyzoite and latent bradyzoite stages.48 Anyway, although many NPs have been investigated and explored, no suitable leads or drug candidates have been found.
2.2 Helminths
Helminths, multicellular invertebrates, comprise nematodes or round worms, cestodes or tapeworms, and trematodes or flat worms, which are among the most persistent public health problems caused by human and veterinary infections.49,50 More than 1 billion humans are infected with helminths annually worldwide; helminths are present in various parts of the body system, and can lead to serious pathological outcomes.51,52 At present, anthelmintic drugs, which are used to eliminate helminth parasites in host organisms, have solved a major problem in animal husbandry and made an important contribution to the problem of human malnutrition and disease.26 However, drugs that treat the parasitic worms after the bloodstream, lymphatics or other tissues are infected are limited, and drugs that treat resident adult worms causing diseases such as river blindness and lymphatic filariasis are still needed. In addition, the increasing prevalence and severity of resistance of human and animal pathogenic helminths have become global phenomena, necessitating the search for new bioactive agents.53,54Schistosomes (Schistosoma mansoni, S. haematobium, and S. japonica) infect over 200 million people and cause more than 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths globally every year.55 Schistosomes are multicellular pathogens with complex life cycles and biological properties causing schistosomiasis, the treatment of which relies on the chemicals oxamniquine (29) and praziquantel (30);2 however, oxamniquine (29) is only effective against S. mansoni, and praziquantel (30) does not kill immature worms (Fig. 4A). Moreover, possible resistance to praziquantel has been reported; thus, relying on praziquantel monotherapy is risky.56 Due to the complex life cycle of schistosomes, the development of new safe and effective drugs to target parasites at all stages is still hard.
000 deaths globally every year.55 Schistosomes are multicellular pathogens with complex life cycles and biological properties causing schistosomiasis, the treatment of which relies on the chemicals oxamniquine (29) and praziquantel (30);2 however, oxamniquine (29) is only effective against S. mansoni, and praziquantel (30) does not kill immature worms (Fig. 4A). Moreover, possible resistance to praziquantel has been reported; thus, relying on praziquantel monotherapy is risky.56 Due to the complex life cycle of schistosomes, the development of new safe and effective drugs to target parasites at all stages is still hard.
 | ||
| Fig. 4 Current chemicals or antibiotics used to treat schistosomiasis (A) and lymphatic filariases and onchocerciasis (B). | ||
Lymphatic filariases and onchocerciasis are caused by Brugia malayi, Wuchereria bancrofti, and Onchocerca volvulus, respectively.57,58B. malayi infection has spread to southeast Asia, W. bancrofti has the estimated 120 million cases in 83 countries, and O. volvulus affects nearly 37 million people in 34 countries, with small foci in southern and central America.59 In 1987, Merck Laboratories registered the first formulation of ivermectin (32), and then as the only drug used in a control program for annual or semi-annual dosing, it was officially approved by the FDA in 1996 for the treatment of onchocerciasis and strongyloidiasis.60 In 2018, moxidectin (31) was approved by the FDA for the management of onchocerciasis in patients over 12 years old, which was repositioned as a veterinary medicine.61,62 Along with the recurrent donations and MDA campaigns of ivermectin, the spread of lymphatic filariases and onchocerciasis has been partly controlled in some endemic regions over the past few decades. The combination of diethylcarbamazine (33) or ivermectin with albendazole (34) has become the basis of a global program for the elimination of lymphatic filariasis (Fig. 4B). However, due to the risk of adverse effects, diethylcarbamazine and ivermectin could not be used to eliminate O. volvulus and adult worms, respectively. Albendazole can only be used in combination therapy.2 Moreover, the development of resistance to these drugs affects their clinical application. Hence, a new chemical class with slow action, oral use, and low cost (ideally a single oral dose) is needed against all parasite stages in humans.4
2.3 Ectoparasites
Ectoparasites (such as fleas, ticks, lice, flies, or mites) and vector-borne diseases have a major impact on the health and productivity of hosts; in addition, ectoparasites cause a constant risk for the survival and well-being of the public and animals worldwide by transmitting diseases such as Crimean-Congo hemorrhagic fever, Q fever caused by Coxiella, babesiosis and hymenolepiasis caused by Hymenolepis.63–66Currently, the vast majority of human and animal ectoparasites are still arthropods. Mites can live freely in the environment and can also parasitize plants, humans or domestic animals. Scabies is an important parasitic disease of the skin caused by the ectoparasite Sarcoptes scabiei var. hominis, which is associated with debilitating itch and major morbidity worldwide and leads to severe bacterial infection and immune-mediated diseases in humans.67 It can occur across all age groups, and affect 150–200 million people yearly (Fig. 5A). In 2017, scabies was added to the WHO list of NTDs;68 it was responsible for 0.21% of DALYs studied by the GBD in 2015 worldwide. In tropical regions, the burdens of scabies in children, elderly people and adolescents are greater69 (Fig. 5A). As the first-line treatment used topically, permethrin (35) (5%) derived from natural pyrethrins from Tanacetum cinerariifolium, which is efficient after 1 week repeating, indicates poor or limited ovicidal action (Fig. 5B). In addition, due to emerging resistance, scabies is becoming less sensitive to permethrin therapy,70 and the efficacy of permethrin has been reduced in Australia for the past 20 years.71 Ivermectin (32) (Fig. 5C), the only oral drug currently available for the treatment of scabies, is primarily prescribed for patients with severe crusted scabies and patients with intercurrent infections or eczematous skin lesions in scabies-endemic areas or in facilities where large-scale use of effective drugs is required to control outbreaks.72 However, along with ivermectin-based MDA strategy is widely used to control scabies in clinic, the resistance appears inevitable.73 Since the first documented case from human infestations in Australia in 1994,74 there have been reports on the resistance of Sarcoptes scabiei to ivermectin in vitro and in vivo. In 1997, mites grown in vitro in the presence of ivermectin survived for 1 hour; however in 2006, this time increased to 2 hours.75 Moreover, considering that ivermectin is poorly metabolized in both humans and animals, after the large-scale use in human and livestock, ivermectin and its metabolites are constantly released into the environment mainly via feces (90%), the potential ecotoxicity in freshwater systems from agriculture or latrines and others should be given more attention,76 and the environmental fate should be taken into account. Of course, some neurotoxins that selectively target the arthropod nervous system were also used for controlling ectoparasite infections.77 Considering that permethrin and ivermectin are all derived from natural resources, NPs have attracted great interest. Our group analyzed and reviewed the global research profile of anti-ectoparasitic agents for animals from Jan. 2015 to Jun. 2020. Among 284 papers published by international journals, 204 papers (71.83%) aimed to obtain active NPs, and more than 16% papers focused on investigating acaricidal activity of NPs against Sarcoptes and Psoriasis mites.78
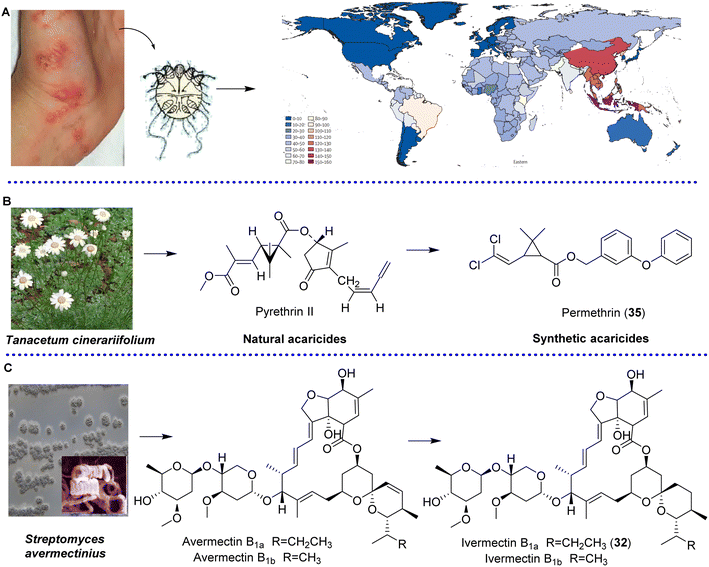 | ||
| Fig. 5 Epidemiology of scabies (A),69 and history of permethrin (B) and ivermectin development (C) in clinical applications. | ||
Among blood-sucking arthropods, ticks are second only to mosquitoes in causing harm from a public health and veterinary viewpoint.79 Due to the severity of tick-borne diseases, many livestock breeders spend a significant portion of their annual input costs on managing and controlling ticks and the diseases they transmit.80 According to statistics, the annual loss was estimated at $720 million in Africa, $100 million in Australia, and up to $1 billion per year in South America.81 In Brazil, the cattle tick (Rhipicephalus microplus) alone has caused $3.24 billion economic losses,82 and other tropical and subtropical regions are likely to face similarly severe impacts. Currently, many commercially available chemicals including macrocyclic lactones, organophosphates, pyrethroids, carbamates and others were widely used to control ticks.83 The long-term, high-scale use of these chemical drugs has led to problems such as drug resistance and drug residues, which seriously affect the treatment of the disease. At present, the use of botanicals for the control of domestic animal parasites has received renewed attention due to their safety, effectiveness and low price. Pavela et al.84 have recently stated that more than 200 plant species were used as herbal preparations to repel ticks from livestock by traditional communities worldwide. Additional studies have recommended that an integrated strategy is used to control ticks based on the rotation and combinations of acaricides, immunization and biological control, and practices against ticks.85 House management, pasture alternation and/or rotation, and nutritional management should also be focused on.86
3 Strategies for developing drugs from natural resources
Typically, a successful drug discovery campaign spans 10–15 years, and high attrition rates and costs always hinder antiparasitic drug discovery. Hence, the number of new compounds in clinical development is very low and clinical needs are unlikely to be met. To improve the success rate of drug discovery by relatively few working organizations, people have tried to find new agents from natural resources over the past century, and some strategies for drug development have been applied (Fig. 6).3.1 Isolation and identification of NPs from natural sources
The use of a bioassay-guided isolation method or a direct phytochemical isolation method to find active NPs from medicinal plants, marine microorganisms, and other natural resources remains the main strategy or approach. Some active NPs could be used as antiparasitic agents directly. Although the traditional use of plants and animals for hundreds of years ensures that isolated NPs exhibit bioactivities, the ability to isolate and purify active compounds remains limited. In addition, this workflow is time-consuming and labor- and cost-intensive; some known compounds are found repeatedly; and more promising or target compounds with low contents may be missed. Due to the presence of synergistic effects, compounds with better activity may not necessarily be obtained in many active crude extracts.In the past decade, novel extraction technologies have been developed to improve extraction yields and downstream detection efficiency, such as high-speed countercurrent chromatography,87 semipreparative liquid chromatography, supercritical fluid extraction,88 deep eutectic extraction,89 and high-intensity pulsed electric fields combined with semibionic extraction.90 An idealized system coupled with online affinity screening, separation and identification was developed to improve the efficiency of discovering active ingredients from extracts. Through this approach, bioactive compounds can be detected at an early stage; thus, the bioactivity-guided isolation process is shortened and unnecessary efforts, costs, and working times are reduced.91 The application of metabolomics in NP research combines two concepts, i.e., metabolic profiling with the introduction of photodiode arrays and HRFTMS detectors that were coupled with HPLC, NMR spectroscopy and LC-MS.92 It could identify active components at the early fractionation step and predict which structures might be bioactive.93,94 For example, a family of triterpenoid compounds from Psidium guajava leaves were found with antileishmanial activity in the amastigotes stage, including corosolic acid (36, IC50 = 0.0021 μM) and jacoumaric acid (37, IC50 = 0.0021 μM), using metabolomics (Fig. 7A).92 In addition, a number of prefractionation strategies coupled with sensitive NMR technology have been reported for separation, which addressed isolation and structure-elucidation bottlenecks. When combined with a high-throughput screening (HTS) strategy, higher hit rates and faster screen speeds were realized (Fig. 7B).95 For example, through high-throughput screening, the topoisomerase I inhibitor niranthin (38)96 was discovered to alter the DNA topology of Leishmania promastigotes with an EC50 value of 1.26 μM; inhibiting the polyamine pathway can kill Leishmania, and targeting this pathway, betulin (39)97 was efficiently screened out through high-throughput screening.
 | ||
| Fig. 7 Main strategies for finding active NPs from natural sources based on phytochemical analysis (A) and high-throughput screening (B). | ||
The development of sequencing technologies has accelerated the process of discovering drugs from NPs. Genome mining techniques have emerged as a powerful approach to discover and identify potential interesting products98,99 or novel compounds from bacteria and fungi, specifically through the identification of secondary metabolites derived from biosynthetic gene clusters encoding novel bioactive metabolites, such as the polyphenolic polyketide antibiotic clostrubin, which was identified from Clostridium beijerinckii using this technology.100 Since the 1960s, mass spectrometry has been extensively utilized to characterize small molecules and NPs through their fragments.101 In parallel, the proteomics have been applied to expedite the identification process of small-molecule agents. Moreover, chemical proteomics with the affinity principle between compounds and targets has been widely used to find the potential targets of NPs and then to design novel agents.102,103 In addition, the combination of cell membrane-coated technology and sequencing technology can more effectively screen the active ingredients in NPs. The biomimetic environment created by cell membranes greatly enhances the accuracy and efficiency of the screening process.104
NPs retain their pivotal role in drug discovery, particularly in pioneering therapeutics with novel mechanisms of action. Although escalating challenges in identifying natural compounds with superior bioactivity and cost-efficiency have diminished pharmaceutical industry investment in this field over recent decades, the convergence of interdisciplinary technological advancements now presents two strategic pathways: rational development of existing natural product frameworks, or systematic discovery of new bioactive candidates. Throughout these processes, methodical observation, critical analysis, and transformative innovation constitute essential determinants of successful outcomes.105
3.2 New application of existing drugs
Considering that NP-based drug discovery represents a complex endeavor and hard work that requires an integrated interdisciplinary approach, many NP groups have been eliminated in most large pharmaceutical companies in the U.S. Under this condition, methods to extend the label or activity of existing NP treatments for other human ailments have gained the interest of many people and companies. This strategy may enable pharmaceutical companies to reduce the economic cost of discovering new drugs and increase the new application and economic value of existing products.3 Historically, many antiparasitic drugs were developed for other purposes. For instance, moxidectin (31), a veterinary anthelminthic agent that is an analog of ivermectin, has progressed to phase II clinical trials for controlling lymphatic filariasis and onchocerciasis.106,107 Ivermectin is used not only for filariasis/onchocerciasis but also for ectoparasites.108 In addition, some commercial drugs with anti-inflammatory, antiviral or other activities present antiparasitic activity, such as auranofin approved by the FDA for the treatment of rheumatoid arthritis, which exhibits activity against Toxoplasma gondii in vitro and in vivo.109 During the past three decades, the pharmaceutical industry has provided limited support for specific drug discovery programs. This approach would reduce the cost and time to market; for example, artemisinin and its derivatives with antimalarial activity were also used to treat schistosomiasis in clinical trials.3.3 NP library and HTS
Employing automation tools, miniaturized assay formats, and large-scale data analysis, the HTS strategy enables the screening of approximately one million compounds daily from the NP library, enabling the rapid detection of biological activity within a minimal time frame.109 Compared with the traditional approach, advances in these technologies would decrease drug screening time, shorten drug discovery timelines and enhance hit-to-lead development.110 However, for the HTS approach, a high-quality and abundant NP library is a crucial prerequisite for achieving effective screening. Currently, a corresponding trend is developing lead compounds from NP libraries or libraries of NP hybrids, NP analogs and NP-inspired molecules using HTS technology, such as the Dictionary of NPs, Drug Discovery Portal, Chinese Natural Product Database, iSMART, AfroDb, NuBBe, MarinLit and Super Natural II111–122 (Table 2). Among them, the Dictionary of NPs containing 270![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds was considered as authoritative and comprehensive data in the NP field.122,123
000 compounds was considered as authoritative and comprehensive data in the NP field.122,123
| Database | Number of entries | Additional information | Ref. |
|---|---|---|---|
| Super Natural 3.0 | 355![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2D structure; vendor information for over 449![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 005 compounds 005 compounds |
42 |
| Universal Natural Product Database | 197![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 201 201 |
3D structures assembled from Chinese database | 43 |
| Chinese Natural Product Database | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Has been used in a virtual screen for PPAR-γ agonists | 44 |
| Drug Discovery Portal | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
All based on available samples | 45 |
| iSMART | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Compounds from traditional Chinese medicines | 46 |
| Database from historical medicinal plants, DIOS | 6702 | It has been used in several virtual screening campaigns | 47 |
| AfroDb | 1000 | Compounds from medicinal African plants | 48 |
| NuBBE | 640 | Compounds from Brazilian sources | 49 |
| MarinLit | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542 542 |
Marine natural products research | 50 |
| Microbial Natural Products Database | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Compounds from microbial | 51 |
| The Natural Products Atlas (https://www.npatlas.org/) | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 372 372 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 068 compounds from bacterial and 20 068 compounds from bacterial and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 304 from fungal sources 304 from fungal sources |
52 |
| Dictionary of Natural Products | 270![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Some of them are considered vital components of many modern drugs | 53 |
Due to the complex life cycle and difficult culture conditions of parasites compared with other pathogens, it is difficult to screen active NPs with low concentrations in vitro or in vivo. The strategy based on target-based HTS and medium-throughput screening (MTS) in whole-parasite assays against specific proteins and whole parasites has been developed and used to identify NCEs from NP libraries. With the continuous development of HTS technologies, some impedance-based methods based on cell monitoring products or custom “in-house” systems for the target application have been developed to assess anti-schistosomiasis activity based on their mobility measurements.124,125 These methods will ultimately screen NPs beyond small research laboratory-based proof-of-principle studies, and more lead compounds may be found. Furthermore, chemoinformatic methodologies are now being integrated with genomics, in silico screening, and the cocrystallization of proteins with small molecules, to accelerate the discovery of antiparasitic drugs.126,127 From heterogeneous cell-based screens, a machine learning approach for defining antimalarial drug was developed.128 Although a large gap remains between enzyme inhibitors and antiparasitic agents, some encouraging results have been obtained.129,130
3.4 Optimization of the NP structure
Lead optimization is a key process in which medicinal chemistry is used to optimize the structure of NPs and find new compounds; this is the most crucial process in drug discovery stage to address several problems, including bioavailability, metabolism and toxicity. Between 1981 and 2019, 43.2% of FDA-approved commercial drugs were indirectly derived from NPs, which were optimized.131 Chemical scaffolds of NPs have historically been sources of inspiration for the development of novel molecules132 that can retain the antiparasitic activities of parent NPs, which are beneficial in this aspect; thus, the use of parent NPs to design and synthesize novel agents has received much attention. Apart from NPs such as paclitaxel that can be directly used in clinical applications, most bioactive NPs with excellent biological activity often suffer from limitations such as poor stability, low solubility, toxicity, and inadequate bioavailability. Implementing simple structural modifications to address these application challenges represents a highly effective optimization strategy.133 Additionally, chemical modifications through approaches such as bioisosterism, active substructure hybridization, and local modification, as well as constructing a series of derivatives based on core scaffolds, serve as a crucial means for discovering potential drug candidate molecules.134Moreover, the advancement of Computer-Aided Drug Design (CADD) technologies has enabled the use of computational tools to predict and optimize interactions between lead compounds and their biological targets, significantly accelerating both the discovery and optimization processes of lead compounds.135 Fragment-Based Drug Design (FBDD) has emerged as another robust approach, where small molecular fragments demonstrating target binding affinity can be systematically developed into more potent compounds through structure elaboration.136
Given the significant contributions and application potential of NPs in the field of antiparasitics, the advancement of these technologies has once again reignited enthusiasm for developing natural product-based antiparasitic drugs. Recently, public-private partnership (PPP) has been established to promote antiparasitic drug discovery, which will enhance the development of an active compound into a drug candidate together with interdisciplinary expertise.137,138 More medicinal chemists, parasitologists, biologists, pharmacologists, botanists and companies have participated in this project to develop novel antiparasitic drugs.
3.5 Development of adjuvant therapy drugs from natural resources
An ideal medicine will not only directly kill the pathogens but also indirectly alleviate the inflammatory and immune responses caused by these pathogens. For antiparasitic agents, adjuvant therapy is very important because it inhibits the inflammatory response and oxidant stress of the body induced by parasites and enhances the immunity of humans and animals to improve the quality of life.139 Compared to direct antiparasitic therapy, adjuvant therapy with traditional medicines and NPs is more feasible for this disease in the current situation. For instance, ruxolitinib (40) could be combined with antimalarial therapy to regulate the long-term immune response of the host (Fig. 8).24 In the natural drug discovery field, finding the adjuvant therapy drugs from natural resource has been paid more attention.T. gondii infection causes multiple organ or tissue injury and central nervous system disease and leads to the death of animals or humans;44 thus, recovering injured tissues is very important for controlling the development of toxoplasmosis. Resveratrol (41),140,141 coixol (42),142 and ginsenoside Rh2 (43)143 ameliorate Toxoplasma gondii infection-induced liver, lung and neuronal injuries by inhibiting the inflammatory response (Fig. 8). Considering that elevated IgE levels occurred in 96% of patients with crusted scabies, the upper limit of normal for 17 times, an immunotherapy strategy or natural immunomodulatory drugs were applied in the clinic combined with ivermectin.144 As an adjuvant (Fig. 8), picroliv (44) has been proposed to enhance the efficacy of anti-leishmania drugs and has shown therapeutic index in phase I and phase II clinical trials.145 For schistosomes, some NPs not only decreased worm burden and egg production but also demonstrated antifibrotic and immunomodulatory activities, which improved the status of the human body. Hence, adjuvant therapy is an important strategy for treating parasitic diseases. However, further research is still needed to investigate its mechanism of action and evaluate its clinical efficacy, so as to provide scientific and valuable treatment strategies for parasitic diseases.
Besides having broad application prospects in adjuvant therapy, NPs can also enhance drug activity, improve treatment efficacy, and overcome drug resistance when used in combination with pharmaceuticals, representing an effective strategy for disease treatment that has been widely studied in the context of cancer and infectious diseases.146–149 In particular, formulating NPs with drugs into new dosage forms, such as nanoparticles, has significantly enhanced the application potential of this therapeutic strategy. Some active compounds also demonstrated this activity, such as chitosan with amphotericin B (45), contributing to a therapeutic approach for the control of leishmaniasis.150 In addition, a number of studies have demonstrated that NPs could contain drugs that overcome the multidrug resistance (MDR) of parasites.151 The 1,4-dihydropyridine family of compounds containing oxazolo[3,2-α]pyridines units that are enantiomers 20S (46) and 20R (47) reversed the resistance to daunomycin and miltefosine in the L. tropica strain with 6.7-fold and 8.7-fold reversion indices, respectively (Fig. 8).152 Although the above-mentioned strategies have been studied in the treatment of parasitic diseases, related research remains limited due to the specific characteristics of parasitic research.
Of course, while many NPs may possess various unique and promising potentials in anti-parasitic applications, not all are suitable for clinical use. Some NPs may have issues such as toxicity, low bioavailability, or unclear mechanisms of action. Therefore, adequate preclinical and clinical studies are essential to ensure their safety and efficacy.
4 Natural products and current natural drugs against parasitic diseases
In history, many plants are used as medicinal herbs for the treatment of human and animal parasitic diseases, and play an important role in ensuring public health security worldwide. Accompanied by the development of modern chemical techniques for isolating and identifying NPs, in particular the discovery of substances with anti-malarial activity, a new era was opened in the discovery of active NPs from plants. Especially since 2010, the WHO has insisted that NPs should be developed as alternatives for the treatment of CL;153,154 NPs, such as quinones, alkaloids, flavonoids, terpenes, lignans, and some marine microorganism secondary metabolites, have found direct medicinal application as pharmaceutical entities because they exhibit fewer side effects and effective properties. In this section, we summarize the history of research and development of natural product-based antiparasitic drugs and recent research progress of promising NPs based on different sources of NPs (plants, microorganisms, and marine organisms) up to 2024. According to the guide of the Drugs for Neglected Diseases Initiative (DNDi) and other criteria, we only described and listed compounds with good activity and high safety (e.g. for Leishmania spp. and P. falciparum strains, IC50 ≤ 0.1 μM and/or SI > 100). According to the type of compounds, a list of taxonomic groups including alkaloids, quinones and flavonoids, terpenes, essential oils and others is supplemented (Tables 3–6).| Compounds | Parasitic | Species | Activity | Ref. |
|---|---|---|---|---|
| 7-Oxostaurosporine | Leishmania sp. | Streptomyces sanyensis | IC50 = 0.0075 μM against L. amanzonensis promastigotes; 0.0012 μM against L. donovani promastigotes; 0.0002 against L. amanzonensis, amastigotes | 269 |
| 40-Demethyl-40-oxostaurosporine | Leishmania sp. | Streptomyces sanyensis | IC50 = 0.037 μM against L. amanzonensis promastigotes; >0.089 μM against L. donovani promastigotes; 0.005 against L. amanzonensis, amastigotes | 269 |
| Staurosporine | Leishmania sp. | Streptomyces sanyensis | IC50 = 0.00017 μM against L. amanzonensis promastigotes; 0.0045 μM against L. donovani promastigotes; 0.0224 μM against L. amanzonensis, amastigotes | 269 |
| Streptocarbazole B | Leishmania sp. | Streptomyces sanyensis | IC50 = 0.0224 μM against L. amanzonensis promastigotes; >0.089 μM against L. donovani promastigotes | 269 |
| Renieramycin A | Leishmania sp. | Neopetrosia species | IC50 = 0.35 μM against L. amanzonensis | 271 |
| Dihydrocorynantheine | Leishmania sp. | Corynanthe pachyceras | IC50 = 3 μM against L. major | 166 |
| Corynantheine | Leishmania sp. | Corynanthe pachyceras | IC50 = 3 μM against L. major | 166 |
| Corynantheidine | Leishmania sp. | Corynanthe pachyceras | IC50 = 3 μM against L. major | 166 |
| Buchtienine | Leishmania sp. | Kopsia griffithii | IC50 < 3.15 μM against L. donovani promastigotes | 167 |
| Duguetine β-N-oxide | Leishmania sp. | Duguetia furfuracea | IC50 = 0.11 μM | 168 |
| Dicentrinone | Leishmania sp. | Duguetia furfuracea | IC50 = 0.01 μM | 168 |
| Viridamide A | Leishmania sp. | Oscillatoria nigroviridis | EC50 = 1.5 μM against L. mexicana | 168 |
| Ancistectorine N-methyl A1 | P. falciparum | Ancistrocladus tectorius | IC50 = 0.08 μM (against K1 strain), SI = 646 (against L6 cell) | 160 |
| Ancistectorine N-methyl A2 | P. falciparum | Ancistrocladus tectorius | IC50 = 0.08 μM (against K1 strain), SI = 705 (against L6 cell) | 160 |
| Ancistectorine 5-epi-A2 | P. falciparum | Ancistrocladus tectorius | IC50 = 0.03 μM (against K1 strain), SI = 3340 (against L6 cell) | 160 |
| Dioncophyllines F | P. falciparum | Ancistrocladus ileboensis | IC50 = 0.045 and 0.09 μM (against K1 and NF54 strains), SI = 3340 (against L6 cell) | 161 |
| Dehydroantofne | P. falciparum | Ficus septica | IC50 = 0.028 μM (against 3D7 strain), SI > 1964 (against L929 cell) | 162 |
| Tylophoridicine | P. falciparum | Ficus septica | IC50 = 0.058 μM (against 3D7 strain), SI > 966 (against L929 cell) | 163 |
| Dimethylisoborreverine | P. falciparum | Flindersia amboinensis | IC50 = 0.06 and 0.02 μM (against K1 and FCR3 strains), SI = 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 265 (against HEK 293 and HeLa cells) 265 (against HEK 293 and HeLa cells) |
163 |
| Tsitsikammamine C | P. falciparum | Marine sponge Zyzzya sp. | IC50 = 0.013 and 0.018 μM (against 3D7 and Dd2 strains), SI = 276![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (against HEK 293 cells) 200 (against HEK 293 cells) |
164 |
| Thiaplakortone A | P. falciparum | Marine sponge Plakortis lita | IC50 = 0.051 and 0.0066 μM (against 3D7 and Dd2 strains), SI = 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 591 (against HEK 293 cells) 591 (against HEK 293 cells) |
165 |
| Berberine, piperine | R. microplus | — | EC50 values were 6.76 mM and 6.04 mM, respectively, with larvicidal activity | 232 |
| Compounds | Parasitic | Species | Activity | Ref. |
|---|---|---|---|---|
| Plumbagin | Leishmania sp. | Plumbago species | IC50 = 2.24 μM against L. donovani amastigotes and 5.87 μM against L. amazonensis amastigotes | 185 |
| 2-Methyl-5-(30-methyl-but-20-enyloxy)-[1,4]naphthoquinone | Leishmania sp. | Plumbago zeylanica | EC50 = 1.9 and 3.46 μM against promastigote and amastigote forms of L. donovani | 179 |
| Burmanin A | Leishmania sp. | Diospyros burmanica | IC50 = 0.053 μM against L. major | 183 |
| Joziknipholones A | P. falciparum | Bulbine frutescens | IC50 = 0.14 μM against K1 | 301 |
| Joziknipholones B | P. falciparum | Bulbine frutescens | IC50 = 0.23 μM against K1 | 184 |
| Rufigallol | P. falciparum | — | IC50 = 35 nM against D6 Pf strain | 184 |
| Gambogic acid derivative | P. falciparum | Original compound from Garcinia resin | IC50 = 0.0102 and 0.0123 μM (against Dd2 and 3D7 strain), SI = 142, 118 (against HEK293 cell) | 302 |
| Menadione | S. mansoni | — | At oral dose of 40 mg kg−1, menadione reduced the worm burden (48.57%) in female BALB/c mice infected with S. mansoni, and reduced the number of eggs in the liver of infected mice by 53.57% | 303 |
| Casticin | Helminths | — | Mice treated with 20 mg per kg per day casticin for 14 consecutive days reduced worm burden and presented antifibrotic activity | 304 |
| Compounds | Parasitic | Species | Activity | Ref. |
|---|---|---|---|---|
| Avarone | Leishmania sp. | Dysidea avara | IC50 = 28.21 μM against L. infantum promastigotes; 20.28 μM against L. tropica promastigotes  |
270 |
| Avarol | Leishmania sp. | Dysidea avara | IC50 = 7.42 μM against L. infantum promastigotes; 7.08 μM against L. tropica promastigotes; 3.19 μM against L. infantum amastigote | 123 |
| Linalool | Leishmania sp. | Croton cajucara | IC50 = 28 nM and 143 nM against promastigotes and intracellular amastigotes of L. amazonensis by destroying kinetoplastid and mitochondrial swelling followed by cell lysis in parasite | 305 and 306 |
| Isoiguesterin | Leishmania sp. | Salacia madagascariensis | IC50 = 0.198 and 0.082 μM against L. donovani and L. mexicana, respectively | 307 |
| 20-Epi-isoiguesterinol | Leishmania sp. | Salacia madagascariensis | IC50 = 0.079 μM against L. donovani | 307 |
| Mesabalide III | Leishmania sp. | Maesa balansae | IC50 = 5 nM against L. infantum intracellular amastigotes | 200 |
| Mesabalide IV | Leishmania sp. | Maesa balansae | IC50 = 9 nM against L. infantum intracellular amastigotes | 308 |
| Fortunilide A | P. falciparum | Chloranthus species | IC50 = 0.0052 μM (against Dd2 strain), SI = 1700 (against WI38 cell) | 196 |
| Fortunilide B | P. falciparum | Chloranthus species | IC50 = 0.019 μM (against Dd2 strain), SI = 163 (against WI38 cell) | 196 |
| Dichapetalin A | S. hematobium | Dichapetalum crassifolium | The dichapetalin A presented in vitro antischistosomal activity against clinical isolates of S. hematobium with IC50 of 151.1 μg mL−1 | 204 |
| Hederacochiside C | S. hematobium | Pulsatilla chinensis Regel | It showed potential antischistosomal and immunomodulatory effect | 309 |
| β-Cyclocitral | R. appendiculatus | Gynandropsis gynandra | It has more than 90.0% repellence rate at 0.1% at 5 min | 231 |
| α-Ionone | R. appendiculatus | Gynandropsis gynandra | It has more than 90.0% repellence rate at 0.1% at 5 min | 231 |
| Cedrene | R. appendiculatus | Gynandropsis gynandra | It has more than 86.7% repellence rate at 0.1% at 5 min | 231 |
| 1-α-Terpineol | R. appendiculatus | Gynandropsis gynandra | It has more than 89.9% repellence rate at 0.1% at 5 min | 231 |
| Extracts or compounds | Parasitic | Species or major compositions | Activity | Ref. |
|---|---|---|---|---|
| Palstimolide (macrolide) | Leishmania sp. | Marine Cyanobacteria | IC50 = 4.67 μM against L. infantum amastigote | 310 |
| 2-[(3,4-Dihydroxyphenyl)methylene]-6-hydroxybenzofuran-3(2H)-one (phenolics) | Leishmania sp. | — | EC50 = 0.33–0.40 μM and 4.58 μM against promastigotes of Leishmania spp. and amastigotes of L. donovani | 311 |
| Asuarinin (phenolics) | Leishmania sp. | Punica granatum, Casuarina | EC50 = 0.52 μM against L. donovani | 312 |
| Orthidine F derivative | P. falciparum | Aplidium orthium | IC50 = 0.0086 μM (against K1 strain), SI > 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (against L6 cell) 000 (against L6 cell) |
313 |
| Carmaphycin B derivative (cyclodepsipeptides) | P. falciparum | Symploca sp. | IC50 = 0.0033 μM (against Dd2 strain), SI = 379 (against HepG2 cell) | 314 |
| Puberulic acid | P. falciparum | Culture broth of the Penicillium sp. FKI-4410 fungus | IC50 = 0.0547 and 0.0547 μM (against K1 and FCR3 strains), SI = 5720 (against MCR5 cell) | 262 |
| Divaricatic acid | S. mansoni | Canoparmelia texana | IC50 was 100.6 μM in vitro. It could cause death, motile changes and ultrastructural damage to worms | 263 |
| Brazilian red propolis | Helminths | Bee | It 25 μg mL−1 caused 100% mortality of adult parasites ex vivo, and reduced worm burden and egg production in early and chronic S. mansoni infection | 315 |
| (−)-6,6′-Dinitrohinokinin | Helminths | Piper cubeba | IC50 was 103.9 μM at 24 h against adult worms in vitro. It also displayed moderate activity against the juvenile liver parasite, (LC50 179.5 μM at 72 h) by reducing the number of egg | 316 |
| 4-Nerolidylcatechol | Helminths | Pothomorphe umbellata | The compound presented in vitro activity with EC50 of 2.9 μM (0.91 μg mL−1) and SI of 68 against Vero cells. In S. mansoni infection, the oral treatment decreased worm burden and egg production in 52.1% and 52.3%, respectively | 317 |
| Hydroalcoholic extract | Helminths | Arctium lappa | The extract (400, 200, and 100 μg mL−1) caused 100% mortality and reduction on motor activity of all adult worms of S. mansoni | 318 |
| Ethyl acetate extract | Helminths | Dichapetalum | The ethyl acetate extract presented in vitro antischistosomal activity against clinical isolates of S. hematobium with IC50 of 248.6 μg mL−1 | 204 |
| 2-Methylcardol diene | Helminths | Anacardium occidentale | It was active against S. mansoni adult worms in vitro, with LC50 values of 14.5 μM and SI of 21.2 | 317 |
| Usnic acid potassium salt | Helminths | — | It presented schistosomicidal property against couples of adult worms of S. mansoni, changed in motility and mortality of schistosomules and young worms | 319 |
| Nerolidol | Helminths | — | It at concentrations of 31.2 and 62.5 μM reduced the worm motor activity and caused the death of male and female schistosomes, respectively | 320 |
| Gomphoside monoacetate and uscharin | Helminths | — | They (10 mg kg−1) showed suitable therapeutic indices in vivo against a chronic S. mansoni infection mice | 321 |
| Methanol extract of Aegle marmelos leaves | H. bispinosa; R.(B.) microplus | Aeglemarmelosine, alkaloids, coumarins | It caused 100% mortality against two ticks at 2 mg mL−1 at 24 h | 221 |
| Methanol extract of Andrographis paniculata leaves | H. bispinosa; R.(B.) microplus | Tannins, flavonoids, carbohydrates | It caused 100% mortality against H. bispinosa at 3 mg mL−1 and larvicidal activity against R. (B.) microplus at 2 mg mL−1, respectively, at 24 h | 222 |
| Acetone and methanol extract of Anisomeles | H. bispinosa | Alkaloids, saponins, protein, gum, mucilage | Two extracts caused 100% acaricidal activity against this ticks at 3 mg mL−1 at 24 h | 223 |
| Hexane extract of Calea serrata aerial parts | R. (B.) microplus, R. sanguineus | Eupatoriochromene, precocene II | It at 6.25 mg mL−1 caused 100% larvicidal mortality rate of both tick species at 48 h post treatment | 224 |
| Hexane extract of Piper tuberculatum flower | R. (B.) microplus | Piplartine, dihydropiplartine, 3,4,5-tri-methoxydihydrocinnamic acid | It (0.12 mg mL−1) showed 100% larvicidal mortality at 24 h post treatment, 100% oviposition reduction and acaricidal efficiency | 225 |
| Water extract of Solanum trilobatum leaves | Hyalomma anatolicum (a.) anatolicum | Carbohydrates, saponins, phytosterols, tannins | It caused 100% larvicidal mortality rate at 10 mg L−1 | 226 |
| Artemisia herbaalba essential oils | Ixodes ricinus | Piperitone (26%) | It has 84.2% repellence rate at 0.015 mg cm−2 | 227 |
| Calendula officinalis essential oils | Ixodes ricinus | α-Cadinol (21%), carvone (18%) | It has 82.0% repellence rate at 0.015 mg cm−2 | 227 |
| Amyris balsamifera essential oils | Ixodes ricinus, Amblyomma americanum | — | EC50 were 0.003 and 0.009 mg cm−2, respectively, at 15 min | 228 |
| Conyza dioscoridis essential oils | Ixodes ricinus | α-Cadinol (10%), hexadecanoic acid (10%) | It has 94% repellence rate at 0.015 mg cm−2 | 227 |
| Mentha spicata essential oils | Ixodes ricinus | Carvone (55%), pulegone (14%) | It has 93.2% and 59.4% repellence at 0.015 and 0.0075 mg cm−2, respectively | 229 |
| Origanum onites essential oils | Ixodes ricinus | 4-Terpineol (55.6%) | It has 84.3% repellence rate at 0.015 mg cm−2 | 229 |
| Tagetes minuta essential oils | Amblyomma americanum | cis-Ocimene, dihydrotagetone, piperitenone | EC50 was 0.002 mg cm−2 | 230 |
| Rosmarinus officinalis essential oils | Ixodes ricinus | 1,8-Cineole, borneol | It has 100.0% repellence rate at 0.015 mg cm−2 | 227 |
| Carvacrol | Ixodes ricinus | — | It has more than 89.9% repellence rate at 0.1% at 5 min | 231 |
| Linalool | R. appendiculatus | — | It has more than 85.0% repellence rate at 0.1% at 5 min | 231 |
| m-Cymene | R. appendiculatus | — | It has more than 90.0% repellence rate at 0.1% at 5 min | 231 |
| Methyl salicylate | R. appendiculatus | — | It has more than 87.7% repellence rate at 0.1% at 5 min | 231 |
| Nerol and nerolidol | R. appendiculatus | — | It has more than 90.0% and 100% repellence rate at 0.1% at 5 min, respectively | 231 |
| Phenyl acetonitrile and phenyl acetaldehyde | R. appendiculatus | — | It has more than 84.9% and 87.9% repellence rate at 0.1% at 5 min, respectively | 231 |
| trans-Geraniol and trans-geranyl acetone | R. appendiculatus | — | It has more than 90.0 and 90.0% repellence rate at 0.1% at 5 min, respectively | 231 |
4.1 Plants
Considering the promising prospects of NPs to find novel anti-malarial agents, great efforts have been dedicated to this field. From 2010 to 2017, a total of 1524 compounds were assayed against Plasmodium.157 Among these, 39% (594) were described as new NPs and 29% (442) had IC50 ≤ 3.0 μM. Some of these NPs including isoquinoline, quinoline, quinazoline, and indole alkaloids have the potential to be developed into anti-malarial drugs by targeting gametocytes (Fig. 9A), such as cryptolepine (48) from Cryptolepis sanguinolenta,158 strictosamide (49) from Nauclea pobeguinii,159 protopine (50), allocryptopine (51), and berberine (52) from Argemone mexicana,160,161 and febrifugine (53) from Dichroa febrifuga,162,163 which presented antimalarial effects in preclinical or clinical tests.164 In Table 3, the compounds with IC50 ≤ 0.1 μM and SI > 100 are listed.165–170 Although research into NPs with the potential to block the transmission of malaria remains in its infancy and needs to be vigorously pursued, it still has broad research prospects and merits.157
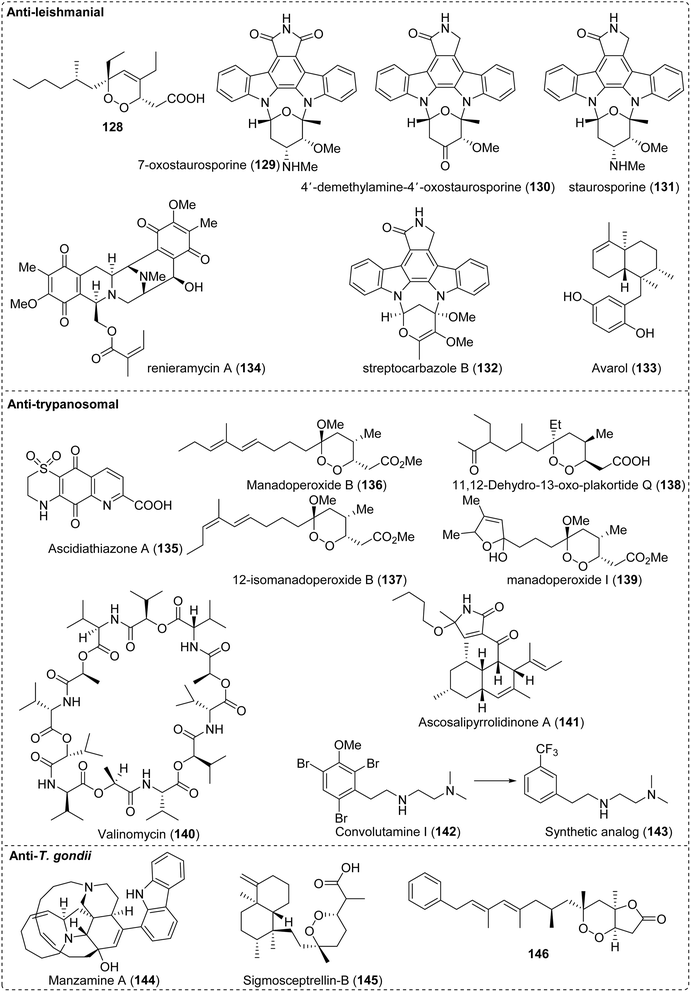
Moreover, a small number of NPs present antileishmanial activities (Fig. 9B). Corynantheine (54), dihydrocorynantheine (55) and corynantheidine (56) with the indole core from the bark of Corynanthe pachyceras exhibited inhibitory activity (IC50 3 μM) against L. major by inhibiting its ETC,171 and buchtienine (57) from Kopsia griffithii showed the remarkable activity (IC50 < 3.15 μM) against L. donovani promastigotes.172 Aporphine alkaloids, duguetine β-N-oxide (58) and dicentrinone (59), isolated from Duguetia furfuracea, exhibited antileishmanial activity, with IC50 values of 0.11 μM and 0.01 μM, respectively.173 Structural optimization using the framework of NPs with strong biological activity as the core is a crucial strategy for identifying lead compounds with enhanced efficacy and broader application prospects. Istanbullu et al.174 evaluated derivatives of thiazolopyrimidine and found that the derivatives (65) exhibited the most promising antipromastigote activity in vitro against L. tropica and L. infantum, with IC50 values of 0.04 μM and 0.042 μM, respectively (Fig. 10A). Quinazoline alkaloids are important natural sources for antileishmanial agents, and their derivatives present good activity. In 2022, Seifu et al.175 reported that 3-aryl-2-styryl substituted-4(3H)-quinazolinone derivatives (66) displayed the most promising antileishmanial activity against L. donovani promastigotes (IC50 0.0212 μM), which was 2 and 150 times more potent than the reference drugs amphotericin B deoxycholate (IC50 0.0460 μM) and miltefosine (IC50 3.1911 μM) (Fig. 10B).
Besides the antileishmanial activity, indole alkaloids such as 2,7-dibromocryptolepine (60) have a strong activity against the T. brucei bloodstream form (IC50 0.0029 μM) with an exceptional SI value of 2083 and inhibited parasitemia by oral, im, and iv administration.176,177 Two pyridoacridone alkaloid derivatives (61–62) presented a stronger trypanocidal activity than that of the parent compound ascididemin, with IC50 values of 0.007 and 0.018 μM, respectively; in contrast, the IC50 value for melarsoprol (positive control) was 0.003 μM, and the mechanisms of action involved DNA intercalation and DNA oxidative damage178 (Fig. 9C). The tetrahydrofuran lignan scaffold derivatives (67) presented strong and synergistic effects in combination with benznidazole132 (Fig. 10C).
Alkaloids and their derivatives presented anti-T. gondii activity (Fig. 9D). A quinoline-related compound, PPQ-8 (63), presented an anti-T. gondii effect in a mouse model of acute and chronic toxoplasmosis.179 It reduced the parasite load of the liver and spleen and improved the pathology of the liver and spleen in acute infection. In chronic toxoplasmosis, PPQ-8 caused degeneration and reduction of brain cysts without stimulating a devastating inflammatory response within the brain, thereby prolonging the survival of mice.180 In addition, from a series of tetrahydroquinolone derivatives, JAG21 (64) significantly reduced T. gondii tachyzoites and encysted bradyzoites in primary and chronic murine infections. After oral administration of JAG21 at 2.5 mg kg−1 or 3 days of treatment at a reduced dose (0.625 mg per kg per day), causal prophylaxis and radical cure were achieved after P. berghei sporozoite infection. The drug could eliminate parasitemia and lead to 100% survival. Hence, it could be used as a preclinical candidate for controlling toxoplasmosis and malaria.181
Alkaloid NPs are a class of compounds with diverse structures and a large variety. We have found that alkaloid NPs with rigid skeletal structures such as quinoline, isoquinoline, quinazoline, and indole seem to exhibit good biological activity against parasites, while the anti-parasitic activity of other alkaloid NPs appears to be generally weaker. In general, molecules with rigid structures can better maintain their three-dimensional shapes, thereby enhancing the binding affinity with target proteins. The specificity of this binding can reduce the off-target effects of drugs and decrease side effects, which may be one of the reasons for the differences in the anti-parasitic activities of alkaloid NPs with different structures.182,183 In addition, it is unclear whether the “proton sponge effect”, which is one of the mechanisms of action of chloroquine, is a contributing factor to this result. However, due to numerous contradictory reports to date, the scientific community has not reached a consensus on the validity of this hypothesis, and there is also a lack of corresponding experimental evidence.184 Therefore, it is necessary to conduct further in-depth studies on the anti-parasitic mechanisms of action of alkaloid NPs to summarize and explain this phenomenon, which has important scientific value for the research and development of anti-parasitic drugs.
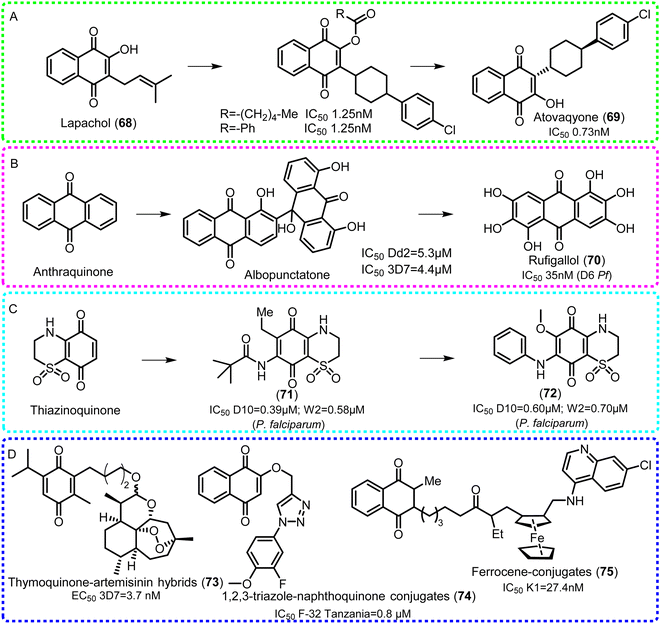 | ||
| Fig. 11 Structural optimization of benzoquinone and naphthoquinone (A), anthraquinone (B), thiazinoquinone (C) and some hybrids and conjugates of compounds (D) against Plasmodium sp. | ||
Fournet et al.193 found that plumbagin (76) and 2-methyl-5-(3′-methyl-but-2′-enyloxy)-[1,4]naphthoquinone (77) isolated from Plumbago species presented activity against amastigotes of L. donovani and L. amazonensis with EC50 values of 2.24 and 1.9 μM, and 3.46 and 5.87 μM in vitro, respectively, which were better than the positive control miltefosine. Moreover, at doses of 2.5 and 5 mg per kg per day plumbagin also showed in vivo activity. For L. major, burmanin A (78), identified from Diospyros burmanica, presented stronger activity with an IC50 of 0.053 μM.194 In addition, seven anthraquinone-2-carbaldehydes (79–85) from Morinda lucida distributed in certain West African countries presented promising activity against promastigotes of L. major, and chloroquine-susceptible (3D7) and chloroquine-resistant (Dd2) strains of P. falciparum in vitro. These results indicated that anthraquinones have remarkable inhibitory effects on Leishmania parasites, and an aldehyde group at C-2 and a phenolic hydroxy group at C-3 could be beneficial for this activity, as revealed by structure–activity relationship (SAR) analysis195 (Fig. 12).
To find promising quinones, Stoppani, Cruz and Docampo studied the effect of lapachol (68), β-lapachone (86) and their derivatives on T. cruzi. Although no active natural compounds were found, the derivative (CG9-442)196 (87) presented inhibitory activity against epimastigote proliferation, and then caused damage to the mitochondrion, chromatin and cellular membranes197 (Fig. 12). Dhananjeyan et al. reported198 that the anthraquinone analog anthraquinone K (88) at 0.0185 μM showed 100% mortality within 1, 5, and 3 days against microfilarial and adult worms of B. malayi by affecting intrauterine embryos (Fig. 12).
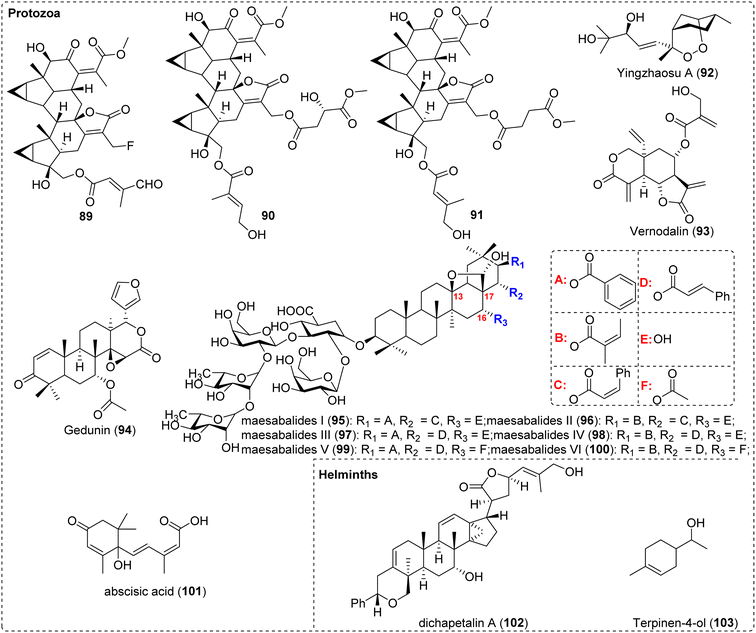
In addition, some terpenoid compounds have the potential to be developed into anti-malarial drugs by targeting gametocytes, such as yingzhaosu A (92) from Artabotrys uncinatus,205 vernodalin (93) from Vernonia amygdalina,206 and gedunin (94) from Azadirachta indica,207 which presented antimalarial effects in preclinical or clinical tests (Fig. 13).
From the leaves of the Vietnamese medicinal plant Maesa balansae, six triterpenoid saponins with potent and specific antileishmanial activity, namely maesabalides I–VI (95–100), were isolated and identified, exhibiting in vitro IC50 values ranging from 0.0046 nM to 0.029 nM (Fig. 13). Among them, maesabalide III (97) was the most active compound, demonstrating remarkable in vivo efficacy as well, with a single subcutaneous injection of 0.2 mg kg−1 reducing the liver amastigote burden by over 90%. Through the construction of natural product derivatives, a preliminary structure–activity relationship analysis was performed, revealing that both the ester moiety and the sugar moiety of the compound molecules are essential for activity. The hydroxyl group at the C-16 position has a more pronounced effect on the activity, and the oxygen bridge between C-13 and C-17 may also play a crucial role in the activity.208 Besides, abscisic acid (101) can control calcium-dependent egress and development in T. gondii.209
Artemisinin and its derivatives not only presented antimalarial activity but also demonstrated efficacy against Schistosoma species. The administration of artemisinin (500 mg kg−1) to mice with 30-day Schistosoma mansoni infection elicited distinct morphological alterations in the parasites, including tegumental erosion and desquamation, ultrastructural damage to sensory tubercles, and the formation of membrane-bound vesicles.210 To prove the clinical efficacy of artemisinin derivatives in S. japonicum infection for a patent, 24 randomized controlled trials (RCTs) were performed. After administrating artesunate (6 mg kg−1, p.o.) at 1 week intervals in 5 trials and at 2 week intervals for up to 13 doses in 11 trials, the protective efficiencies were 96% and 85%, respectively. When artemether was administrated once every 2 weeks for up to 12 doses in other 8 trials, the efficacy was up to 86%.211
Recently, from the stems and roots of D. crassifolium, unique tetracyclic and pentacyclic triterpenoids were separated and discovered, exhibiting promising antiparasitic activity. Especially, dammarane-type tetracyclic triterpenoid dichapetalin A (102), which can currently only be separated from the roots of D. crassifolium, showed in vitro antischistosomal activity (IC50) of 0.26 μM. In contrast, the activity of the currently clinically used standard drug praziquantel is 0.05 μM. This provides new lead compounds for the search in combating various neglected tropical diseases caused by parasitic pathogens.212 Besides, terpinen-4-ol (103) exhibited potential antitrypanosomal activity with the IC50 and SI values of 0.13 μM and 1000 (ref. 213) (Fig. 13).
As described above, the incorporation of natural bioactive components into synthetic drugs is a novel aspect that should be explored from the perspective of medicinal chemistry. Reports have shown that natural chalcones such as licochalcone A (107) and dihydrochalcone derivatives (108) display good antileishmanial activity.217 When these types of compounds were optimized (Fig. 14), a promising synthesized scaffold compound (109) emerged as the lead derivative with an IC50 value of 0.03 μM.218
Considering that unique enzymes act as signaling molecules in metabolic pathways and are essential for survival, more herbal-based target inhibitors were found along with the introduction of HTS against molecular targets.219 As topoisomerase II inhibitors, luteolin220 (110) alter the topology of DNA with an IC50 value of 45.5 μM, against promastigote; genistein (111) would inhibit the RTK pathway to kill Leishmania221 (Fig. 14). Although there is a large gap between these inhibitors and commercial drugs, the timeline from hit NP identification to hit-to-lead development and drug discovery is shortened rapidly.
In recent decades, our group and other international groups have found a series of active compounds for controlling T. gondii via different mechanisms (Fig. 14). Wu et al. discovered some chalcone derivatives (112–114), which also showed better anti-T. gondii effects than acetylspiramycin (https://pubchem.ncbi.nlm.nih.gov/compound/acetylspiramycin) in vivo, with proliferation inhibition rates of more than 82.2% at a dose of 20 mg kg−1, as well as liver-protecting effects, and the Michael acceptor is very important for their activity.222 Abugri et al.223 found that combining taxifolin (115) from fructus Polygoni orientalis with pyrimethamine (28) may offer a promising tool for toxoplasmosis treatment with an IC50p of 0.0046 μM, which presented activity by inhibiting the calcium-dependent protein kinase activity of parasites.
In recent decades, many plant extracts and essential oils have been demonstrated to be efficient against ticks,229–240 and some commercial formulations based on essential oils such as MyggA® Natural, and Citriodiol® have been used to control ticks in some countries.241 At high concentrations, nicotine-rich extracts (Tobacco) presented the promising anti-tick activity by killing all ticks, regardless of what stage ticks are at.242 Fang et al.243 studied and compared the efficacy of ten oils against scabies; clove oils were best, followed by oils from tea tree, lavender, geranium, bitter orange, palmarosa, Japanese cedar, and others. In Australia, tea tree oil extracted from Melaleuca alternifolia has shown higher activity than ivermectin in killing mites, and has been used by the Royal Darwin Hospital as an adjunct to scabies.244
From 2013 to now, some new extracts or agents were found, but only a small number of them presented promising activity (Table 6). de Castro et al.250 found that many NPs including extracts presented activity against Schistosoma species in vivo and in vitro. However, according to the criteria involving hit activity against helminths (100% inhibition of motility in S. mansoni adults at 5 μg mL−1), most NPs fail. Among 346 plant methanol extracts, only three extracts presented strong in vitro schistosomicidal activity, including extracts of Agave lophantha (LC50 of 8.2 μg mL−1), Furcraea selloa (LC50 of 7.10 μg mL−1), and Solanum elaeagnifolium (LC50 of 6.0 μg mL−1),251 and the acetonitrile extract of Jatropha curcas had an LC90 value of 6.0 μg mL−1.252 In an in vivo study, Balanites aegyptiaca fruit aqueous extract (200 mg kg−1),253Chenopodium ambrosioides hydroalcoholic extract (50 mg kg−1),254Baccharis trimera (200–400 mg kg−1),255 and blue green algae (200 mg kg−1)256 presented anthelmintic activity by killing immature and adult worms of S. mansoni and exhibited immunomodulatory, hepatoprotective and antioxidant activities.
Moreover, some phenolic compounds, lignans, coumarins, and other NPs and their derivatives also exhibit good anti-parasitic activity. For example, curcumin (116) from Curcuma longa159 presented antimalarial effects in preclinical or clinical tests; myrislignan (117) induces oxidation–reduction to lead to autophagy in T. gondii;257 4-nerolidylcatechol (118) presented significant in vitro schistosomicidal activity with an EC50 value of 0.0029 μM and SI of 68 against Vero cells, and oral treatment decreased worm burden and egg production by 52.1% and 52.3%, respectively258 (Fig. 15A).
 | ||
| Fig. 15 Other promising active NPs (A), and the two examples of promising acaricidals from medicinal plants ((B) for Azadirachta indica oil and (C) for clove oil). | ||
Ying and her group found that octadecanoic acid-tetrahydrofuran-3,4-diylester (119) isolated from Azadirachta indica essential oil presented acaricidal activity against S. scabiei var. cuniculi larvae with an LC50 value of 3.06 μM in vitro; however, after optimizing the chemical structure, benzyloxy-2-benzoic acid-3,4-tetrahydrofuran diester (120) was obtained with an LC50 value of 1.08 μM259,260 (Fig. 15B). The main component of clove oil, eugenol (121), as well as its analogs acetyleugenol and isoeugenol, presented a strong acaricidal activity within an hour of contact261 (Fig. 15C). Our group found that it inhibited complex I activity in the mitochondrial respiratory chain by binding to NADH dehydrogenase chain 2 (MTND2) and then resulted in the death of mites,262 and for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of eugenol and ivermectin, the LC50 value against mites will decrease 23 times compared with ivermectin only in vitro (unpublished data). 4-Methoxycoumarin with significant acaricidal activity were also found.263 In a single-blind RCT test, after four weeks of treatment with Tinospora cordifolia lotion, significant mean global evaluation scores were achieved, and the clinical cure rates were similar to those of permethrin.264
1 mixture of eugenol and ivermectin, the LC50 value against mites will decrease 23 times compared with ivermectin only in vitro (unpublished data). 4-Methoxycoumarin with significant acaricidal activity were also found.263 In a single-blind RCT test, after four weeks of treatment with Tinospora cordifolia lotion, significant mean global evaluation scores were achieved, and the clinical cure rates were similar to those of permethrin.264
The research into extracting NPs from plants has seen significant advancements, uncovering numerous compounds with therapeutic promise and offering key insights for the development of novel pharmaceuticals. In particular, NPs have made a crucial impact in the realm of treating parasitic infections. A comprehensive review reveals that the most extensive research has been conducted on protozoa, such as Plasmodium, Leishmania, and Trypanosome, with significant drug potential identified in NPs ranging from alkaloids and quinones to terpenes and flavonoids. Illustrations of this include the transformative discovery and use of quinine and artemisinin in malaria treatment, as well as the broader application of derivatives such as atovaquone and ivermectin in antiparasitic therapies. However, research on helminths, such as schistosomes, and ectoparasites, including scabies mites and ticks, remains relatively underexplored. Tropical diseases such as schistosomiasis, scabies, lymphatic filariasis, and onchocerciasis, categorized as NTDs continue to face a shortage of effective and safe treatments. These diseases predominantly affect populations in impoverished regions, yet the lack of research investment and limited market returns have hindered the depth of basic research and the progress of drug development, leaving the actual needs unmet. Consequently, there is an urgent call to intensify research efforts directed at these parasitic diseases.
More importantly, the analysis of biological outcomes for flavonoids and polyphenols should also be conducted with great caution, with attention to screening for Pan-assay interference compounds (PAINS). These compounds do not operate by binding to specific biological targets, but interfere with assay methods via various non-specific mechanisms, leading to false-positive results,265 such as the widely bioactive natural product curcumin.266 Therefore, identifying and excluding PAINS is crucial for drug discovery, as they not only lead to the wastage of time and resources but may also result in erroneous judgments about potential drugs. Although publicly available filters can help identify potential PAINS, these filters cannot provide a comprehensive conclusion as to whether these suspect compounds are “bad” or innocent.267 They may inappropriately flag effective compounds as PAINS. Chen et al. believe that employing a “fair testing strategy” to identify interesting molecules among PAINS suspect compounds can provide certain structural-functional insights for the development of multi-target-directed ligands (MTDLs).268
Of course, this problem is not exclusive to flavonoid and polyphenolic NPs. Similar possibilities may also exist in other NPs.269 It is necessary to pay special attention to this possibility when screening anti-parasitic NPs.270 Although PAINS can cause interference, not all compounds with PAINS characteristics are necessarily harmful. Some compounds may indeed possess activity, but it is necessary to carefully evaluate their mechanisms of action to rule out the possibility of detection interference. Moreover, modifying the compounds may eliminate their PAINS properties while retaining or enhancing their activity.271
For essential oils and crude extracts in NPs, they may not be sufficient to be developed as anti-parasitic drugs. However, they may have unique advantages in the adjuvant treatment of parasitic diseases. Promoting research in this area can not only extend the lifespan of existing clinical drugs but also achieve more desirable therapeutic effects when treating parasitic diseases clinically.
4.2 Microorganisms and animal
The compound boron-pleuromutilin AN11251 (124) (50 mg kg−1) reduced Wolbachia by >99% in a Litomosides sigmodontis mouse model, and thus, it was considered as a lead candidate. In addition, good pharmacokinetic and physicochemical properties were also proved.274 In 2005, Taylor and colleagues reported275 that doxycycline (125) administered orally at 200 mg per day for 8 weeks shows potential anti-Wolbachia activity. The drug is readily available, inexpensive and safe to use in adult nonpregnant patients (Fig. 16C). Then, this group found a promising macrolide veterinary antibiotic tylosin A (122) analog, A-1574083 (123), that presented remarkable anti-Wolbachia macrolide activity with high safety profile as a short-term oral drug course for treating lymphatic filariasis and onchocerciasis. A 1 or 2 week course of oral administration of this compound would provide >90% Wolbachia depletion from nematodes in infected animals276 (Fig. 16B).
Puberulic acid (126), a compound isolated from the culture broth of Penicillium FKI-4410, exhibits potent anti-malarial activity (Fig. 16C). The in vitro IC50 values against both chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum were 0.05 nM, and it showed weak cytotoxicity with an IC50 value of 0.29 μM against human MRC-5 cells. Compared with currently used anti-malarial drugs, puberulic acid demonstrated significant therapeutic effects in vivo. Moreover, the hydroxyl group at the C-7 position of puberulic acid seems to be an important part of its anti-malarial activity, while the carboxyl group at the C-4 position appears to be important for selectivity. This provided an important reference for subsequent structural optimization and the development of new anti-malarial drugs.277
Divaricatic acid (127), a depside class natural product isolated from Canoparmelia texana, was capable of killing Schistosoma mansoni by altering motor capacity and causing ultrastructural damage, with an in vitro IC50 value of 100.6 μM. Moreover, it exhibited no cytotoxicity to human peripheral blood mononuclear cells at effective concentrations (Fig. 16C).278
4.3 Marine organisms
Marine microorganisms provide vast biodiversity as well as significant chemical diversity, and some NPs with significant anti-leishmaniasis property have been identified and show great promise (Fig. 17). For example, a peroxide (128) produced by the sponge Plakortis angulospiculatus is active against L. mexicana by causing lysis of the cell membrane with an LD50 value of 0.97 nM, and the positive ketoconazole was 0.11 nM.283 From Streptomyces sanyensis, 7-oxostaurosporine (129), 4′-demethylamine-4′-oxostaurosporine (130), staurosporine (131), and streptocarbazole B (132) were isolated, all of which presented significant activity against L. amanzonensis promastigotes, L. donovani promastigotes, and L. amanzonensis amastigotes.284 Avarol (133) from Dysidea avara has activity against L. infantum promastigotes, L. tropica promastigotes, and L. infantum amastigotes, with IC50 values of 7.42, 7.08 and 3.19 μM, respectively.285 Renieramycin A (134) from Neopetrosia species showed antileishmanial activity against L. amazonensis with an IC50 value of 0.35 μM.286In 2013, Lam et al.234 found that ascidiathiazone A (135) from the tunicate Aplidium sp. presented activity against the T. b. rhodesiense bloodstream form via generating reactive oxygen species (ROS) and inhibiting mitochondrial function, and the EC50 and SI values were 3.1 μM and 50 against L6 cells, respectively.287 The endoperoxide motif exemplifies the diverse three-dimensional structural nature of bioactive NPs. Manadoperoxide B (136) and 12-isomanadoperoxide B (137), all isolated from the sponge Plakortis cf. lita (EC50 0.0088 μM and 0.032 μM, respectively), exhibited anti-T. b. rhodesiense bloodstream form activity, with SI values >3000 and >350 compared with HMEC cells and L6 cells, respectively.288,289 11,12-Dehydro-13-oxo-plakortide Q (138) and manadoperoxide I (139) significantly inhibited T. b. brucei and T. b. rhodesiense bloodstream form parasites.290 A cyclic peptide, valinomycin (140), presented potential property with an EC50 value of 0.0032 μM and high selectivity with an SI value of 3500 (ref. 291) (Fig. 17). In addition, an unusual tetramic acid metabolite ascosalipyrrolidinone A (141) from the marine fungus Ascochyta salicorniae showed activity against T. cruzi (EC50 1.1 μg mL−1), whereas the positive drug benznidazole was 0.12 μM.292 As mentioned above, the chemical scaffold of NPs can retain the biological activity of the parent NPs, which is very important for the development of lead compounds with better activity and drugability. After chemical optimization, a derivative (143) of convolutamine I (142), against Trypanosoma brucei natural product isolated from the bryozoan Amathia tortusa, showed better efficacy than the prototype, with an EC50 value of 0.5 μM, and its pharmacokinetic properties were also significantly improved.293
Some active alkaloids identified from marine sponges presented remarkable activity against T. gondii. Manzamines with a unique group of polycyclic alkaloids presented anti-T. gondii activity, which were isolated from the Okinawan sponge genus Haliclona in 1986.294 Manzamine A (144) (0.098 nM) achieved 70% inhibition of the T. gondii parasite without causing cell toxic effects and prolonged the survival of Swiss Webster mice to 20 days after the administration of 8 mg kg−1 for 8 consecutive days (i.p.) compared to the 16 days observed with untreated controls.295 Sigmosceptrellin-B (145) (0.099 nM) from Diacarnus erythraeanus exhibits potent activity against T. gondii in human diploid fibroblasts with inhibition rates of 84–99%.296 Plakortolide (146) with a cyclic peroxylactone from the sponge Plakinastrella onkodes exhibited activity against T. gondii with an IC50 value of 64 nM in vitro.297
5 Advantages and limitations of NPs in the drug development process
As the main resource for drugs, NPs not only exhibit advantages but also pose challenges during the drug discovery process (Fig. 18):(i) Traditional medicinal plants contain a large number of secondary metabolites with diverse chemical structures and various functions. These bioactive compounds such as alkaloids, flavonoids, and terpenoids after a long period of evolutionary screening often possess unique pharmacological activities and relatively low toxic and side effects.298 Moreover, traditional medical systems such as Traditional Chinese Medicine and Indian Ayurvedic medicine have accumulated thousands of years of medicinal experience, documenting extensive knowledge about the therapeutic properties and clinical applications of various plants.299 These traditional knowledge systems provide invaluable clues for modern drug discovery, which can significantly enhance the success rate of translating NPs into viable pharmaceutical agents. By further exploring and organizing traditional medical knowledge and establishing a complete database and knowledge base, it is possible to provide a more reliable basis for drug discovery.300,301 Meanwhile, strengthening cooperation with traditional medicine practitioners and jointly conducting research and development on medicinal plants can also improve the success rate of drug research and development. Of course, with the rapid development of multi-omics technologies such as genomics, proteomics, and metabolomics, as well as technologies such as CADD, high-throughput screening (HTS), and high-content screening (HCS), researchers are now able to gain a deeper understanding of the gene expression, metabolic pathways, and bioactive components of medicinal plants. This accelerates the screening and optimization process of lead compounds and provides new strategies for drug discovery based on traditional medicinal plants.302
(ii) NPs are the result of the evolution of organisms in nature, and they exhibit great diversity in terms of chemical structure and biological activity.303,304 Moreover, due to factors such as environmental adaptation and species interaction, organisms in different geographical environments will produce NPs with different biological functions, even if they belong to similar taxonomic categories.305 With the continuous progress of synthetic biology and computational biology, as well as the ongoing development of technologies such as gene editing and multi-omics, it has become possible to regulate the evolution of plants and microorganisms.306,307 Through means such as metabolic engineering transformation, combinatorial biosynthesis, and environmental regulation, we can not only obtain NPs with potential medicinal value more efficiently but also discover NPs with new biological functions, providing more diverse raw materials for drug discovery.308–312
(iii) Recently, along with the development of AI and reasonable molecular design technologies, targets were considered to be the “source power” to discover new drugs. For example, the Plasmodium genome contains about 5000 genes, and it has been estimated that about 200 (4%) might encode suitable drug targets. About 30 genes could be used as potential targets for drug discovery, which were not similar to any human genes.313 Moreover, due to the complexity and diversity of chemical structures, as molecular probes, some modified NPs were adopted to capture their targets, and then, they were identified by mass spectrometry or selectively isolated, such as using photoaffinity labeling chemistry technology FoF1-ATP synthase.314 NPs with novel targets may present synergistic effects with conventional drugs to combat diseases by decreasing resistance and increasing efficacy.
(iv) In recent years, an increasing number of orally administered drugs defying Lipinski's Rule of Five have gained regulatory approval.315 By virtue of their distinctive structural complexity and bioactive profiles, NPs demonstrate high binding affinity and selectivity toward target proteins, have emerged as a critical source for developing novel oral therapeutics, and exhibit unique advantages in transcending conventional pharmaceutical guidelines.316,317 NPs can further serve as lead compounds that undergo structural modification through chemical synthesis or biosynthetic engineering, thereby optimizing their physicochemical properties and pharmacokinetic profiles to enhance compatibility with oral administration criteria. The rapid development of drug delivery systems has also provided new strategies for the oral administration of NPs. For example, solid lipid nanoparticles (SLNs) can be used to encapsulate NPs, improve their stability and bioavailability, and achieve targeted delivery.318 Other delivery systems such as microparticles, liposomes, and polymer micelles have also been widely studied for the purpose of enhancing the oral absorption of NPs.
However, NPs still involve limitations:
(i) The isolation and identification of bioactive compounds from natural resources remain challenges.316 Traditional methods for the isolation of NPs still rely on techniques such as solvent extraction combined with chromatography, mass spectrometry, and membrane separation. The complicated operations and low efficiency limit the discovery of bioactive compounds, and the extensive use of organic reagents may also have a negative impact on the environment.319 Although high-efficiency green extraction and separation technologies such as deep eutectic extraction have been studied,319,320 there is still a significant gap between laboratory research and practical application, and these technologies have not been widely applied so far. By combining various technical strategies such as natural product databases, liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR), efficient natural product dereplication methods have significantly reduced the waste of time and resources. Moreover, with the continuous development of analytical techniques and computational resources, the efficiency and accuracy of natural product dereplication will keep improving.321,322 However, due to the fact that the content of NPs in living organisms is usually low, it is still a very difficult task to isolate and purify a single active ingredient and clearly elucidate its complex chemical structure.
(ii) From 1981 to 2019, although approximately 45% of FDA-approved drugs were derived from NPs, only 3.8% of prototypes were used directly as drugs, and more chemicals should be optimized comprehensively. Low ADME properties, potential toxicity and several other drawbacks of NPs have led pharmaceutical companies to reduce drug discovery programs, especially antiparasitic agents, and the difficult total synthesis of active compounds also seriously affects the development prospects. For example, to find new hits or lead compounds for fighting this disease, researchers have focused on medicinal plants and NPs over the past three decades. Approximately 400 species belonging to almost 100 families have been evaluated for activity, especially for Anacardiaceae, Annonaceae, Asteraceae, Euphorbiaceae, Fabaceae, Lamiaceae, Malpighiaceae, and Phytolaccaceae, and more than 200 compounds with anti-trypanosomatid activity were found.34,323 However, according to the guide of the Drugs for Neglected Diseases initiative (DNDi),22 a small portion of compounds are promising to develop further.
(iii) Gaining intellectual property (IP) rights for NPs exhibiting relevant bioactivities is hard, since naturally occurring compounds in their original form may not always be patented.324 An additional layer of complexity results from regulations that define the need to share with countries where the biological material originated, framed in the Convention on Biological Diversity of UN approved in 1992 and the Nagoya Protocol in 2014,325 as well as recent developments concerning benefit sharing linked to the use of marine resources.
(iv) The inherent challenges in isolating and characterizing bioactive compounds, compounded by the potential low natural abundance and structural complexity of active NPs, have engendered significant obstacles in achieving standardized and scalable manufacturing processes for NP-based drug development. Particularly, the intricate molecular architectures characteristic of bioactive NPs often render synthetic modification technically demanding, thereby constraining pharmaceutical development and optimization endeavors (Fig. 18).
Drug discovery and development are long and complex processes that need experts from multiple disciplines to work closely from the beginning to develop sound strategies that guide the entire process, such as physicists, chemists, biologists, computer scientists and pharmacists. The identification and validation of target is an ideal beginning for the drug discovery. Recently, progress has been achieved to increase knowledge on parasite-specific drug targets, especially after using the CRISPR/Cas system and other technologies for structural and functional studies, and more targets have been found. Based on novel targets, computer-aided predictions and design and high-throughput drug screening will help us find promising hits against parasites.326,327
6 Prospective analysis
Parasitic diseases seriously threaten human and animal health and have a negative impact on public health security and socioeconomic development. In history, approximately US 300–500 million is needed for research expenditure to develop and release a new product to the market.2,92 Because these diseases are mainly prevalent in low-income regions, market forces are enough to drive the discovery and development of new agents, and the number of global organizations and investment in antiparasitic drug discovery are all lower than those for other common diseases. However, there have been good opportunities in the past few years, and several welcome developments have provided new impetus. The WHO, European Union, China, US National Institute of Health, Canadian Institute for Health Research and Health Sector in the African Region paid more attention to parasitic diseases. The establishment of PPP that focuses on tropical diseases, at least to some extent, stimulated the enthusiasm of many pharmaceutical companies to become involved in antiparasitic drug discovery, including the DNDi, the Medicines for Malaria Venture (MMV), and the Institute for One World Health (IOWH). These PPPs disease-specific knowledge and experience of public health care organizations to discovery and develop drug will increase the success rate of candidate drugs in clinical trials.24,328 The WHO Special Programme for Research and Training in Tropical Diseases (TDR), the World Bank, and the United Nations Education of Scientific and Cultural Organization (UNESCO) promote the development of antiparasitic drugs. More funds were injected into the area of antiparasitic research and provided a large impact, such as the Wellcome Trust and the Bill and Melinda Gates Foundation.In the past hundreds, people have focused more on exploring NPs from plants for (i) the validation of traditional NPs use in endemic populations and (ii) the use of NPs as sources of new potential antiparasitic compounds. According to the ClinicalTrials.gov (http://ClinicalTrials.gov) database, nearly 15% of the drug interventions are derived from plants, with 60% drugs isolated from only 10 taxonomic families.329,330 Most plant species have not been studied comprehensively for discovering novel drugs. During their long evolutionary process, plants have developed a series of complex defense mechanisms to resist various biological stresses, including parasitic invasions. These mechanisms have led to the production of a diverse array of secondary metabolites responsible for the survival of the organisms, as well as for defending against competitors and invaders.331,332 Therefore, for a long time, NPs have been an important source of new drugs for parasitic diseases. Due to their remarkable chemical diversity, biochemical specificity, and other molecular characteristics, NPs are favorable lead structures for drug discovery. Especially with the establishment of natural product (NP) libraries and the development and application of new technologies such as high-throughput screening (HTS), the time required for drug discovery will be significantly shortened, which has already promoted the progress of drug research and development for parasitic diseases such as malaria and cryptosporidiosis.333
In the past 40 years, more than 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 novel chemicals, with hundreds of new compounds still being discovered from non-traditional sources of marine bacteria and cyanobacteria every year, provide a diverse array of NPs, primarily from invertebrates (e.g. sponges and tunicates). More than 32
000 novel chemicals, with hundreds of new compounds still being discovered from non-traditional sources of marine bacteria and cyanobacteria every year, provide a diverse array of NPs, primarily from invertebrates (e.g. sponges and tunicates). More than 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 compounds are listed in the database of marine NPs.119 About 30 compounds belong to the marine pharmaceutical clinical pipeline, which comprises 7 FDA-approved drugs and 22 drug candidates for the development of drugs.334,335 Some of them presented antiparasitic and drug-resistant parasitic activities and have been selected as promising leads for extended preclinical assessment. For example, haliclonacyclamine A from the marine sponge Haliclona spp. presented promising activity against chloroquine-sensitive and -resistant strains of P. falciparum.159 Considering that the world's oceans have played an important role in controlling the global infectious disease burden,336 the identification of more novel drug leads and even drugs from ocean resources will be realized.
000 compounds are listed in the database of marine NPs.119 About 30 compounds belong to the marine pharmaceutical clinical pipeline, which comprises 7 FDA-approved drugs and 22 drug candidates for the development of drugs.334,335 Some of them presented antiparasitic and drug-resistant parasitic activities and have been selected as promising leads for extended preclinical assessment. For example, haliclonacyclamine A from the marine sponge Haliclona spp. presented promising activity against chloroquine-sensitive and -resistant strains of P. falciparum.159 Considering that the world's oceans have played an important role in controlling the global infectious disease burden,336 the identification of more novel drug leads and even drugs from ocean resources will be realized.
Drug discovery remains a high-risk process. The drug development is still a challenge and is hindered by high fail rates, high costs and economic pressures, multidisciplinary collaboration, drug's accessibility and IP constraints. Although this process is time-consuming and requires a significant amount of work, a rational selection of research strategies, along with active NPs that always possess favorable suboptimal pharmacological effects or ADMET characteristics, provides more possibilities and a higher success rate for the development from “hits” to “leads,” and ultimately to new drugs. Of course, chemical modification may be needed for most of the NPs, and total chemical synthesis, semisynthesis and active fragment assembly will play a more important role in this condition. Moreover, with breakthroughs in various emerging technologies, drug development has progressed from relying solely on phenotype-based drug discovery to developing new strategies based on target-based drug discovery.337 For NPs, the phenotypic drug discovery strategy remains crucial at present, as it enables the discovery of drugs with complex mechanisms of action. Recently, the “evolution”-based drug discovery strategy has also attracted the attention of researchers.338 In addition, as we have previously proposed, drug repositioning is a strategy that can significantly shorten drug development time and reduce costs, as the safety data of approved drugs are usually known. Similarly, exploring the potential of NPs in the adjunctive treatment of parasitic diseases can provide new breakthroughs in clinical treatment efficacy and regimens.
The development and application of various technologies have led to a renewed emphasis on natural product-based drug screening in the field of drug discovery. Nowadays, data science and artificial intelligence (AI) are increasingly becoming key forces driving its vigorous development, as their ability to process complex data offers tremendous assistance in the discovery of new drugs.339–341 AI can predict which proteins may serve as drug targets by analyzing a large amount of genetic data. After combining with the analysis of a vast amount of bioactivity data, it is also capable of predicting the interactions between NPs and target proteins, thus accelerating the screening process of NPs. Wang et al.342 described a large-scale RNAi screen in adult S. mansoni that examined the function of 2216 genes. Among them, TAO and STK25 have the potential, which could change the muscle-specific messenger RNA transcription, and then loss of either of these kinases results in paralysis and worm death. Alkaloids, such as tryptamines, protoberberines and aporphines, also presented potential activity via regulating Sm.5HTRL.343 Moreover, advanced machine learning techniques can obtain models through multi-dimensional data learning that are capable of predicting the potential activities of untested chemical structures. Additionally, these techniques can design natural product analogs that may possess specific biological activities, which is helpful for expanding the molecular space of drug development and discovering entirely new drug lead compounds.341,344 Although AI methods have opened up new possibilities for the design, synthesis, and biological analysis of existing and new small molecules, at the core of these methods are public databases of bioactivity data for a large number of (protein) targets and chemical structures.345 However, the existing natural product data are multimodal, imbalanced, unstandardized, and scattered across many data repositories. This makes it difficult to use natural product data together with existing deep learning architectures, as these architectures require fairly standardized, and usually non-relational, data. This also hinders models from learning the overall patterns in natural product science.341 Therefore, data science technologies for constructing and managing large-scale natural product databases still require further research.346 For example, the TCMs-CFA platform integrates traditional Chinese medicine knowledge bases, chemomics data, and high-content screening data, and is used to predict active compounds and their potential mechanisms.301
Currently, factors limiting the discovery of antiparasitic drugs include not only the identification of active compounds but also the capability to establish animal models, which is a significant constraint. Parasite animal infection models play a crucial role in the research and development of antiparasitic drugs. They serve as a key bridge between in vitro experiments and clinical trials, enabling the assessment of a drug's efficacy, toxicity, and pharmacokinetic properties.347 However, due to the diverse and highly complex life cycles and parasitic states of parasites, most diseases require testing in several animal models at different stages. These animal models do not always replicate the human infection process and disease pathology. Compared with the primary model related to the acute stage, more important and complex chronic or resistant models are needed for screening the active compounds. Such compounds presenting the efficacy against Chagas disease in both primary model and secondary infection model were considered as lead compounds. Furthermore, there are still many parasites for which predictive preclinical models are lacking, such as P. vivax malaria, Chagas disease, and cryptosporidiosis. This has become a research gap that the scientific community urgently needs to address in order to advance the development of antiparasitic drugs.333
Drug discovery and development are essentially multidisciplinary areas where chemists, pharmacists, physicists, biologists, computer scientists, and so on must work together even from the beginning, delineating the rationale that will guide the whole process.
7 Conclusion
In summary, the high attrition rates, accessibility, sustainable supply, IP constraints and some problems still hinder the development of NPs; however, based on quinine, artemisinin, ivermectin and other approved antiparasitic drugs derived from NPs, NPs remain a promising pool with high structural diversity and remarkable activities and NPs are the center of attention for the scientific community and exploration of novel antiparasitic drugs. In combination with the recent development and application of revolutionized technologies, NPs will provide a stronger basis for drug discovery and will continue to provide major contributions to human and veterinary health.8 Conflicts of interest
The authors declare no competing financial interests.9 Acknowledgements
This work was financed by the National Natural Science Foundation of China (32373055; 32302924), the Outstanding Youth Fund of Gansu Province (23JRRA563), the Natural Science Foundation of Gansu Province (25JRRA454), the Innovation Project of the Chinese Academy of Agricultural Sciences (No. CAAS-ASTIP-2015-LIHPS), and the Central Public-interest Scientific Institution Basal Research Fund (No. 1610322024013), Chinese Academy of Agricultural Sciences.10 References
- Anisuzzaman, M. S. Hossain, T. Hatta, S. S. Labony, K. D. Kwofie, H. Kawada, N. Tsuji and M. A. Alim, Adv. Parasitol., 2023, 120, 87–136 CrossRef CAS.
- R. Pink, A. Hudson, M. A. Mouriès and M. Bendig, Nat. Rev. Drug Discovery, 2005, 4, 727–740 CrossRef CAS PubMed.
- O. Adegboye, M. A. Field, A. Kupz, S. Pai, D. Sharma, M. J. Smout, P. Wangchuk, Y. Wong and C. Loiseau, Clin. Microbiol. Rev., 2021, 34, e0034820 CrossRef PubMed.
- A. M. S. Mayer, A. D. Rodriguez, O. Taglialatela-Scafati and N. Fusetani, Mar. Drugs, 2017, 15, 273 CrossRef PubMed.
- M. Osman, A. Mistry, A. Keding, R. Gabe, E. Cook, S. Forrester, R. Wiggins, S. Di Marco, S. Colloca, L. Siani, R. Cortese, D. F. Smith, T. Aebischer, P. M. Kaye and C. J. Lacey, PLoS Neglected Trop. Dis., 2017, 11, e0005527 CrossRef PubMed.
- H. de Graaf, R. O. Payne, I. Taylor, K. Miura, C. A. Long, S. C. Elias, M. Zaric, A. M. Minassian, S. E. Silk, L. Li, I. D. Poulton, M. Baker, S. J. Draper, D. Gbesemete, N. J. Brendish, F. Martins, A. Marini, D. Mekhaiel, N. J. Edwards, R. Roberts, J. Vekemans, S. Moyle, S. N. Faust, E. Berrie, A. M. Lawrie, F. Hill, A. V. S. Hill and S. Biswas, Front. Immunol., 2021, 12, 694759 CrossRef CAS PubMed.
- T. Zaheer, R. Z. Abbas, M. Imran, A. Abbas, A. Butt, S. Aslam and J. Ahmad, Parasitol. Res., 2022, 121, 2749–2763 CrossRef PubMed.
- F. T. Akinsolu, P. O. Nemieboka, D. W. Njuguna, M. N. Ahadji, D. Dezso and O. Varga, Int. J. Environ. Res. Public Health, 2019, 16, 1925 CrossRef PubMed.
- R. B. G. Kew and K. J. Willis, State of the World's Plants, Royal Botanic Gardens, Kew, London, England, 2017 Search PubMed.
- D. S. Fabricant and N. R. Farnsworth, Environ. Health Perspect., 2001, 109, 69–75 CAS.
- A. L. Harvey, R. Edrada-Ebel and R. J. Quinn, Nat. Rev. Drug Discovery, 2015, 14, 111–129 CrossRef CAS PubMed.
- K. Dzobo, Comprehen. Pharmacol., 2022, 408–422 Search PubMed.
- C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 1997, 23, 3–25 CrossRef CAS.
- D. J. Newman and G. M. Cragg, Planta Med., 2016, 82, 775–789 CrossRef CAS PubMed.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2020, 83, 770–803 CrossRef CAS PubMed.
- L. Bohlin, U. Goeransson, C. Alsmark, C. Weden and A. Backlund, Phytochem. Rev., 2010, 9, 279–301 CrossRef CAS PubMed.
- G. Porras, F. Chassagne, J. T. Lyles, L. Marquez, M. Dettweiler, A. M. Salam, T. Samarakoon, S. Shabih, D. R. Farrokhi and C. L. Quave, Chem. Rev., 2021, 121, 3495–3560 CrossRef CAS PubMed.
- MNPS, Medicinal Plant Names Services (MNPS), 2020, https://mpns.science.kew.org/mpns-portal/, accessed on October 14, 2020 Search PubMed.
- L. B. Arendse, S. Wyllie, K. Chibale and I. H. Gilbert, ACS Infect. Dis., 2021, 7, 518–534 CrossRef CAS PubMed.
- WHO, Malaria chemoprevention Preferred product characteristics, 2023, https://www.who.int/publications/i/item/9789240070967 Search PubMed.
- A. Dicko, M. Konare, D. Traore, J. Testa, R. Salamon, O. Doumbo and C. Rogier, Malar. J., 2012, 11, 73 CrossRef CAS PubMed.
- J. Achan, A. O. Talisuna, A. Erhart, A. Yeka, J. K. Tibenderana, F. N. Baliraine, P. J. Rosenthal and U. D'Alessandro, Malar. J., 2011, 10, 144 CrossRef CAS PubMed.
- Y. A. Ebstie, S. M. Abay, W. T. Tadesse and D. A. Ejigu, Drug Des., Dev. Ther., 2016, 10, 2387–2399 CrossRef CAS PubMed.
- J. L. Siqueira-Neto, K. J. Wicht, K. Chibale, J. N. Burrows, D. A. Fidock and E. A. Winzeler, Nat. Rev. Drug Discovery, 2023, 22, 807–826 CrossRef CAS PubMed.
- T. Simonart, J. C. Noel, E. De Clercq and R. Snoeck, Clin. Infect. Dis., 1998, 27, 1562 CrossRef CAS PubMed.
- M. Donia and M. T. Hamann, Lancet Infect. Dis., 2003, 3, 338–348 CrossRef CAS PubMed.
- I. Okwor and J. Uzonna, Am. J. Trop. Med. Hyg., 2016, 94, 489–493 Search PubMed.
- J. Alvar, I. D. Velez, C. Bern, M. Herrero, P. Desjeux, J. Cano, J. Jannin, M. den Boer and WHOLC Team, PLoS One, 2012, 7, e35671 CrossRef CAS PubMed.
- R. S. Yadav and S. Jain, Control of Neglected Tropical Diseases of WHO, Operational Manual on Leishmaniasis Vector Control, Surveillance, Monitoring and Evaluation, 2023, https://www.who.int/publications/i/item/9789240060340 Search PubMed.
- O. Kayser, A. F. Kiderlen and S. L. Croft, Parasitol. Res., 2003, 90, S55–S62 CrossRef PubMed.
- M. Ghorbani and R. Farhoudi, Drug Des., Dev. Ther., 2018, 12, 25–40 CrossRef CAS PubMed.
- M. G. Thomas, M. De Rycker, M. Ajakane, S. Albrecht, A. Isabel Alvarez-Pedraglio, M. Boesche, S. Brand, L. Campbell, J. Cantizani-Perez, L. A. T. Cleghorn, R. C. B. Copley, S. D. Crouch, A. Daugan, G. Drewes, S. Ferrer, S. Ghidelli-Disse, S. Gonzalez, S. L. Gresham, A. P. Hail, S. J. Hindley, R. M. Lowe, C. J. MacKenzie, L. MacLean, S. Manthri, F. Martin, J. Miguel-Siles, N. Van Loc, S. Norval, M. Osuna-Cabello, A. Woodland, S. Patterson, I. Pena, M. Teresa Quesada-Campos, I. H. Reid, C. Revill, J. Riley, J. Ramon Ruiz-Gomez, Y. Shishikura, F. R. C. Simeons, A. Smith, V. C. Smith, D. Spinks, L. Stojanovski, J. Thomas, S. Thompson, T. Underwood, D. W. Gray, J. M. Fiandor, I. H. Gilbert, P. G. Wyatt, K. D. Read and T. J. Miles, J. Med. Chem., 2019, 62, 1180–1202 CrossRef CAS PubMed.
- M. C. Field, D. Horn, A. H. Fairlamb, M. A. J. Ferguson, D. W. Gray, K. D. Read, M. De Rycker, L. S. Torrie, P. G. Wyatt, S. Wyllie and I. H. Gilbert, Nat. Rev. Microbiol., 2018, 16, 714 CrossRef CAS PubMed.
- E. Izumi, T. Ueda-Nakamura, B. P. Dias Filho, V. F. Veiga Junior and C. V. Nakamura, Nat. Prod. Rep., 2011, 28, 809–823 RSC.
- WHO, World Chagas Disease Day, 2023, https://www.who.int/campaigns/world-chagas-disease-day/2023 Search PubMed.
- L. Urquhart, Nat. Rev. Drug Discovery, 2020, 19, 746 CrossRef CAS PubMed.
- J. D. Alpern, R. Lopez-Velez and W. M. Stauffer, PLoS Neglected Trop. Dis., 2017, 11, e0005794 CrossRef.
- B. M. Mony, P. MacGregor, A. Ivens, F. Rojas, A. Cowton, J. Young, D. Horn and K. Matthews, Nature, 2014, 505, 681 CrossRef CAS PubMed.
- M. De Rycker, S. Wyllie, D. Horn, K. D. Read and I. H. Gilbert, Nat. Rev. Microbiol., 2022, 20, 702 CrossRef CAS PubMed.
- E. K. Elmahallawy, H. A. M. El Fadaly, A. H. Soror, F. A. Z. Ali, K. A. A. El-Razik, Y. A. Soliman, A. A. M. Alkhaldi, N. K. A. Albezrah and A. M. Barakat, Biomed. Pharmacother., 2022, 156, 113811 CrossRef CAS PubMed.
- J. G. Montoya and O. Liesenfeld, Lancet, 2004, 363, 1965–1976 CrossRef CAS PubMed.
- I. R. Dunay, K. Gajurel, R. Dhakal, O. Liesenfeld and J. G. Montoya, Clin. Microbiol. Rev., 2018, 31, e00057 CrossRef CAS PubMed.
- P. R. Torgerson and P. Mastroiacovo, Bull. W. H. O., 2013, 91, 501–508 CrossRef PubMed.
- S. K. Matta, N. Rinkenberger, I. R. Dunay and L. D. Sibley, Nat. Rev. Microbiol., 2021, 19, 467–480 CrossRef CAS PubMed.
- F. Derouin, Curr. Opin. Invest. Drugs, 2001, 2, 1368 CAS.
- G. C. Olivera, E. C. Ross, C. Peuckert and A. Barragan, Elife, 2021, 10, e69182 CrossRef CAS PubMed.
- S. K. Matta, N. Rinkenberger, I. R. Dunay and L. D. Sibley, Nat. Rev. Microbiol., 2021, 19, 467–480 CrossRef CAS PubMed.
- M. P. Barrett, D. E. Kyle, L. D. Sibley, J. B. Radke and R. L. Tarleton, Nat. Rev. Microbiol., 2019, 17, 607–620 CrossRef CAS PubMed.
- G. Lapage, Nature, 1960, 187, 815–816 CrossRef.
- H. D. Alan Lindquist and J. H. Cross, Infectious Diseases, 4th edn, 2017, vol. 2, pp. 1763–1779 Search PubMed.
- P. Chakraborty, V. Aravindhan and S. Mukherjee, Int. J. Biol. Macromol., 2023, 241, 124649 CrossRef CAS PubMed.
- W. Harnett and M. M. Harnett, Parasite Immunol., 2006, 28, 535–543 CrossRef CAS PubMed.
- D. W. T. Crompton and M. C. Nesheim, Annu. Rev. Nutr., 2002, 22, 35–59 CrossRef CAS PubMed.
- R. Kaminsky, P. Ducray, M. Jung, R. Clover, L. Rufener, J. Bouvier, S. S. Weber, A. Wenger, S. Wieland-Berghausen, T. Goebel, N. Gauvry, F. Pautrat, T. Skripsky, O. Froelich, C. Komoin-Oka, B. Westlund, A. Sluder and P. Maeser, Nature, 2008, 452, 176–180 CrossRef CAS PubMed.
- M. A. Verjee, Res. Rep. Trop. Med., 2019, 10, 153–163 Search PubMed.
- T. J. C. Anderson and M. T. Duraisingh, Science, 2020, 369, 1562–1564 CrossRef CAS PubMed.
- The Lymphatic Filariases, Lancet, 1985, 325, 1135–1136 CrossRef.
- A. Hadermann, L.-J. Amaral, G. Van Cutsem, J. N. S. Fodjo and R. Colebunders, Trends Parasitol., 2023, 39, 126–138 CrossRef PubMed.
- M. J. Taylor, A. Hoerauf and M. Bockarie, Lancet, 2010, 376, 1175–1185 CrossRef PubMed.
- S. L. Croft and S. Ward, Sci. Transl. Med., 2015, 7, 316ed14 Search PubMed.
- E. Hurlimann, D. Hofmann and J. Keiser, Trends Parasitol., 2023, 39, 272–284 CrossRef CAS PubMed.
- WHO, New drug being tested in Africa for river blindness, 2010, https://www.who.int/news/item/11-12-2010-new-drug-being-tested-in-africa-for-river-blindness Search PubMed.
- D. Grace, F. Mutua, P. Ochungo, R. Kruska and F. Ogutu, Mapping of Poverty and Likely Zoonoses Hotspots, ILRI, Nairobi, Kenya, 2012 Search PubMed.
- M. M. Islam, E. Farag, K. Eltom, M. M. Hassan, D. Bansal, F. Schaffner, J. M. Medlock, H. Al-Romaihi and Z. Mkhize-Kwitshana, Pathogens, 2021, 10, 139 CrossRef PubMed.
- R. G. Maggi, Animal Health: Ectoparasites, Encyclopedia of Agriculture and Food Systems, 2014, vol. 1, pp. 315–326 Search PubMed.
- P. M. Selzer and C. Epe, Trends Parasitol., 2021, 37, 77–89 CrossRef CAS PubMed.
- D. Engelman, P. T. Cantey, M. Marks, A. W. Solomon, A. Y. Chang, O. Chosidow, W. Enbiale, D. Engels, R. J. Hay, D. Hendrickx, P. J. Hotez, J. M. Kaldor, M. Kama, C. D. Mackenzie, J. S. McCarthy, D. L. Martin, B. Mengistu, T. Maurer, N. Negussu, L. Romani, O. Sokana, M. J. Whitfeld, L. C. Fuller and A. C. Steer, Lancet, 2019, 394, 81–92 CrossRef PubMed.
- Y. Stienstra, D. T. Beeres, R. Phillips, M. Vonk and S. J. Ravensbergen, Lancet, 2019, 394, 2068 CrossRef PubMed.
- C. Karimkhani, D. V. Colombara, A. M. Drucker, S. A. Norton, R. Hay, D. Engelman, A. Steer, M. Whitfeld, M. Naghavi and R. P. Dellavalle, Lancet Infect. Dis., 2017, 17, 1247–1254 CrossRef PubMed.
- R. Balestri, M. Magnano, S. D. Infusino, L. Rizzoli, C. R. Girardelli and G. Rech, J. Eur. Acad. Dermatol. Venereol., 2021, 35, e889–e891 CrossRef CAS PubMed.
- K. E. Mounsey, D. C. Holt, J. McCarthy, B. J. Currie and S. F. Walton, Future Microbiol., 2008, 3, 57–66 CrossRef CAS PubMed.
- L. Romani, M. J. Whitfeld, J. Koroivueta, M. Kama, H. Wand, L. Tikoduadua, M. Tuicakau, A. Koroi, R. Andrews, J. M. Kaldor and A. C. Steer, N. Engl. J. Med., 2015, 373, 2305–2313 CrossRef CAS PubMed.
- W. L. Shoop, Parasitol. Today, 1993, 9, 154 CrossRef CAS PubMed.
- Australian Prescriber, New drugs-Ivermectin, 1997, http://www.australianprescriber.com/magazine/20/3/77/9/new-drugs/149/ivermectin, last accessed 19 June 2015 Search PubMed.
- S. Khalil, O. Abbas, A. G. Kibbi and M. Kurban, PLoS Neglected Trop. Dis., 2017, 11, e0005920 CrossRef PubMed.
- L. Mancini, I. Lacchetti, F. Chiudioni, W. Cristiano, K. Di Domenico, S. Marcheggiani, M. Carere, L. Bindi and S. Borrello, Ann. Ist. Super. Sanita, 2020, 56, 492–496 CAS.
- R. L. Coop, M. A. Taylor, D. E. Jacobs and F. Jackson, Trends Parasitol., 2002, 18, 55–56 CrossRef PubMed.
- X. F. Shang, L. X. Dai, H. Pan and J. Y. Zhang, J. Tradit. Chin. Vet. Med., 2020, 39, 92–96 Search PubMed.
- L. A. Durden and G. R. Mullen, Medical and Veterinary Entomology Third Edition Introduction, 2019 Search PubMed.
- G. P. Kaaya and S. Hassan, Exp. Appl. Acarol., 2000, 24, 913–926 CrossRef PubMed.
- O. T. Adenubi, F. O. Fasina, L. J. McGaw, J. N. Eloff and V. Naidoo, S. Afr. J. Bot., 2016, 105, 178–193 CrossRef.
- L. Grisi, R. C. Leite, J. R. de Souza Martins, A. T. Medeiros de Barros, R. Andreotti, P. H. Duarte Cancado, A. A. Perez de Leon, J. B. Pereira and H. S. Villela, Rev. Bras. Parasitol. Vet., 2014, 23, 150–156 CrossRef PubMed.
- A. A. Marchiondo, P. A. Holdsworth, L. J. Fourie, D. Rugg, K. Hellmann, D. E. Snyder and M. W. Dryden, Vet. Parasitol., 2013, 194, 84–97 CrossRef CAS PubMed.
- R. Pavela, A. Canale, H. Mehlhorn and G. Benelli, Res. Vet. Sci., 2016, 109, 1–9 CrossRef PubMed.
- M. K. Obaid, N. Islam, A. Alouffi, A. Z. Khan, I. d. S. Vaz Jr, T. Tanaka and A. Ali, Front. Cell. Infect. Microbiol., 2022, 12, 941831 CrossRef CAS PubMed.
- S. M. A. Selles, M. Kouidri, M. G. Gonzalez, J. Gonzalez, M. Sanchez, A. Gonzalez-Coloma, J. Sanchis, L. Elhachimi, A. S. Olmeda, J. M. Tercero and F. Valcarcel, Pathogens, 2021, 10, 1379 CrossRef CAS PubMed.
- Z. Gan, Z. Liang, X. Chen, X. Wen, Y. Wang, M. Li and Y. Ni, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1011, 99–107 CrossRef CAS PubMed.
- R. Hofstetter, G. M. Fassauer and A. Link, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2018, 1076, 77–83 CrossRef CAS PubMed.
- A. Mannu, M. Blangetti, S. Baldino and C. Prandi, Materials, 2021, 14, 2494 CrossRef CAS PubMed.
- G. He, Y. Yin, X. Yan and Y. Wang, J. Food Process Eng., 2017, 40, e12392 CrossRef.
- P. Wangchuk and A. Loukas, Nat. Prod. Drug Discovery, Elsevier, Amsterdam, Netherlands, 2018, pp. 435–465 Search PubMed.
- C. Phakeovilay, S. Bourgeade-Delmas, P. Perio, A. Valentin, F. Chassagne, E. Deharo, K. Reybier and G. Marti, Molecules, 2019, 24, 4536 CrossRef CAS PubMed.
- U. R. Abdelmohsen, C. Cheng, C. Viegelmann, T. Zhang, T. Grkovic, S. Ahmed, R. J. Quinn, U. Hentschel and R. Edrada-Ebel, Mar. Drugs, 2014, 12, 1220–1244 CrossRef CAS PubMed.
- N. D. Yuliana, A. Khatib, Y. H. Choi and R. Verpoorte, Phytother. Res., 2011, 25, 157–169 CrossRef CAS PubMed.
- S. Pidot, K. Ishida, M. Cyrulies and C. Hertweck, Angew. Chem., Int. Ed., 2014, 53, 7856–7859 CrossRef CAS PubMed.
- S. Chowdhury, T. Mukherjee, R. Mukhopadhyay, B. Mukherjee, S. Sengupta, S. Chattopadhyay, P. Jaisankar, S. Roy and H. K. Majumder, EMBO Mol. Med., 2012, 4, 1126–1143 CrossRef CAS PubMed.
- P. Saudagar and V. K. Dubey, Biol. Chem., 2011, 392, 1113–1122 Search PubMed.
- B. O. Bachmann, S. G. Van Lanen and R. H. Baltz, J. Ind. Microbiol. Biotechnol., 2014, 41, 175–184 CrossRef CAS PubMed.
- G. L. Challis, J. Med. Chem., 2008, 51, 2618–2628 CrossRef CAS PubMed.
- X.-W. Zhang, N. Feng, Y.-C. Liu, Q. Guo, J.-K. Wang, Y.-Z. Bai, X.-M. Ye, Z. Yang, H. Yang, Y. Liu, M.-M. Yang, Y.-H. Wang, X.-M. Shi, D. Liu, P.-F. Tu and K.-W. Zeng, Sci. Adv., 2022, 8, eabo0789 CrossRef CAS PubMed.
- J. R. Yates III, Nat. Methods, 2011, 8, 633–637 CrossRef.
- S.-Z. Zheng, X.-W. Zhang, X.-M. Song, Z. Yang, L. Yao, P.-F. Tu and K.-W. Zeng, Pharmacol. Res., 2022, 177, 106093 CrossRef CAS PubMed.
- N. O. Opoko, D. K. Bakajika, E. M. Kanza, H. Howard, G. L. Mambandu, A. Nyathirombo, M. M. Nigo, K. Kasonia, S. L. Masembe, M. Mumbere, K. Kataliko, J. P. Larbelee, M. Kpawor, K. M. Bolay, F. Bolay, S. Asare, S. K. Attah, G. Olipoh, M. Vaillant, C. M. Halleux and A. C. Kuesel, Lancet, 2018, 392, 1207–1216 CrossRef PubMed.
- Y. Bu, Q. Hu, T. Bao, X. Xie and S. Wang, TrAC, Trends Anal. Chem., 2022, 151, 116601 CrossRef CAS.
- L. Zhang, J. Song, L. Kong, T. Yuan, W. Li, W. Zhang, B. Hou, Y. Lu and G. Du, Pharmacol. Ther., 2020, 216, 107686 CrossRef CAS PubMed.
- C. M. Bjerum, B. G. Koudou, A. F. Ouattara, D. Lew, C. W. Goss, P. T. Gabo, C. L. King, P. U. Fischer, G. J. Weil and P. J. Budge, PLoS Neglected Trop. Dis., 2023, 17, e0011633 CrossRef CAS PubMed.
- L. J. Thean, L. Romani, D. Engelman, H. Wand, A. Jenney, J. Mani, J. Paka, T. Cua, S. Taole, M. Silai, K. Ashwini, A. Sahukhan, M. Kama, M. Tuicakau, J. Kado, M. Parnaby, N. Carvalho, M. Whitfeld, J. Kaldor and A. C. Steer, Lancet Reg. Health West. Pac., 2022, 22, 100433 Search PubMed.
- R. M. Andrade, J. D. Chaparro, E. Capparelli and S. L. Reed, PLoS Neglected Trop. Dis., 2014, 8, e2973 CrossRef PubMed.
- B. Gopu, P. Kour, R. Pandian and K. Singh, Int. Immunopharmacol., 2023, 114, 109591 CrossRef CAS PubMed.
- F. E. Koehn and G. T. Carter, Nat. Rev. Drug Discovery, 2005, 4, 206–220 CrossRef CAS PubMed.
- SuperNatural 3.0, 2023, https://bioinf-applied.charite.de/supernatural_3/index.php Search PubMed.
- J. Gu, Y. Gui, L. Chen, G. Yuan, H.-Z. Lu and X. Xu, PLoS One, 2013, 8, e62839 CrossRef CAS PubMed.
- R. K. Petersen, K. B. Christensen, A. N. Assimopoulou, X. Frette, V. P. Papageorgiou, K. Kristiansen and I. Kouskoumvekaki, J. Comput.-Aided Mol. Des., 2011, 25, 107–116 CrossRef CAS PubMed.
- R. L. Clark, B. F. Johnston, S. P. Mackay, C. J. Breslin, M. N. Robertson and A. L. Harvey, Drug Discovery Today, 2010, 15, 679–683 CrossRef PubMed.
- K.-W. Chang, T.-Y. Tsai, K.-C. Chen, S.-C. Yang, H.-J. Huang, T.-T. Chang, M.-F. Sun, H.-Y. Chen, F.-J. Tsai and C. Y.-C. Chen, J. Biomol. Struct. Dyn., 2011, 29, 243–250 CrossRef CAS PubMed.
- D. Schuster, L. Kern, D. P. Hristozov, L. Terfloth, B. Bienfait, C. Laggner, J. Kirchmair, U. Grienke, G. Wolber, T. Langer, H. Stuppner and J. M. Rollinger, Comb. Chem. High Throughput Screening, 2010, 13, 54–66 CrossRef CAS PubMed.
- F. Ntie-Kang, D. Zofou, S. B. Babiaka, R. Meudom, M. Scharfe, L. L. Lifongo, J. A. Mbah, L. M. Mbaze, W. Sippl and S. M. N. Efange, PLoS One, 2013, 8, e78085 CrossRef CAS PubMed.
- M. Valli, R. N. dos Santos, L. D. Figueira, C. H. Nakajima, I. Castro-Gamboa, A. D. Andricopulo and V. S. Bolzani, J. Nat. Prod., 2013, 76, 439–444 Search PubMed.
- MarinLit, Dedicated to marine natural products research, 2018, http://pubs.rsc.org/marinlit Search PubMed.
- https://www.neotrident.com/index.php/product/proinfo/33 .
- J. A. van Santen, E. F. Poynton, D. Iskakova, E. McMann, T. A. Alsup, T. N. Clark, C. H. Fergusson, D. P. Fewer, A. H. Hughes, C. A. McCadden, J. Parra, S. Soldatou, J. D. Rudolf, E. M. L. Janssen, K. R. Duncan and R. G. Linington, Nucleic Acids Res., 2022, 50, D1317–D1323 CrossRef CAS PubMed.
- P. Wangchuk, J. Biol. Act. Prod. Nat., 2018, 8, 1–20 Search PubMed.
- https://www.routledge.com/go/the_dictionary_of_natural_products .
- G. Rinaldi, A. Loukas, P. J. Brindley, J. T. Irelan and M. J. Smout, Int. J. Parasitol.: Drugs Drug Resist., 2015, 5, 141–148 Search PubMed.
- M. K. Sundaraneedi, B. A. Tedla, R. M. Eichenberger, L. Becker, D. Pickering, M. J. Smout, S. Rajan, P. Wangchuk, F. R. Keene, A. Loukas, J. G. Collins and M. S. Pearson, PLoS Neglected Trop. Dis., 2017, 11, e0006134 CrossRef PubMed.
- S. Stone, D. J. Newman, S. L. Colletti and D. S. Tan, Nat. Prod. Rep., 2022, 39, 20–32 RSC.
- S. Nwaka and A. Hudson, Nat. Rev. Drug Discovery, 2006, 5, 941–955 CrossRef CAS PubMed.
- G. W. Ashdown, M. Dimon, M. Fan, F. S.-R. Teran, K. Witmer, D. C. A. Gaboriau, Z. Armstrong, D. M. Ando and J. Baum, Sci. Adv., 2020, 6, eaba9338 CrossRef CAS PubMed.
- Z. B. Mackey, A. M. Baca, J. P. Mallari, B. Apsel, A. Shelat, E. J. Hansell, P. K. Chiang, B. Wolff, K. R. Guy, J. Williams and J. H. McKerrow, Chem. Biol. Drug Des., 2006, 67, 355–363 CrossRef CAS PubMed.
- C. R. Chong, X. Chen, L. Shi, J. O Liu and D. J. Sullivan, Jr., Nat. Chem. Biol., 2006, 2, 415–416 Search PubMed.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2020, 83, 770–803 Search PubMed.
- R. da Rosa, B. P. Dambros, M. H. de Moraes, L. Grand, M. Jacolot, F. Popowycz, M. Steindel, E. P. Schenkel and L. S. Campos Bernardes, Bioorg. Chem., 2022, 119, 105492 CrossRef CAS PubMed.
- F. M. Kuhlmann and J. M. Fleckenstein, Infect. Dis., 2017, 2, 1345–1372 Search PubMed.
- M. Pascolutti and R. J. Quinn, Drug Discovery Today, 2014, 19, 215–221 CrossRef CAS PubMed.
- Y. Du, A. R. Jamasb, J. Guo, T. Fu, C. Harris, Y. Wang, C. Duan, P. Lio, P. Schwaller and T. L. Blundell, Nat. Mach. Intell., 2024, 6, 589–604 CrossRef.
- C. Sheng and W. Zhang, Med. Res. Rev., 2013, 33, 554–598 CrossRef CAS PubMed.
- S. Nwaka and R. G. Ridley, Nat. Rev. Drug Discovery, 2003, 2, 919–928 CrossRef CAS PubMed.
- M. Said and E. Zerhouni, Nat. Rev. Drug Discovery, 2014, 13, 789–790 CrossRef CAS PubMed.
- V. A. Nagaraj, D. Mukhi, V. Sathishkumar, P. A. Subramani, S. K. Ghosh, R. R. Pandey, M. C. Shetty and G. Padmanaban, Nat. Commun., 2015, 6, 8775 CrossRef PubMed.
- J.-M. Lu, G.-N. Jin, Y.-N. Lu, X.-D. Zhao, H.-W. Lan, S.-R. Mu, X.-Y. Shen, G.-H. Xu, C.-H. Jin, J. Ma, X. Jin, X. Xu and L.-X. Piao, Eur. J. Pharmacol., 2021, 910, 174497 CrossRef CAS PubMed.
- Y. N. Lu, X. Y. Shen, J. M. Lu, G. N. Jin, H. W. Lan, X. Xu and L. X. Piao, Phytomedicine, 2023, 108, 154522 CrossRef CAS PubMed.
- X.-Y. Shen, J.-M. Lu, Y.-N. Lu, G.-N. Jin, J.-W. Ma, J.-H. Wang, Y. Wang, X. Xu and L.-X. Piao, Int. Immunopharmacol., 2023, 118, 110031 CrossRef CAS PubMed.
- G. N. Jin, J. M. Lu, H. W. Lan, Y. N. Lu, X. Y. Shen, X. Xu and L. X. Piao, Int. Immunopharmacol., 2022, 112, 109176 CrossRef CAS PubMed.
- L. J. Roberts, S. E. Huffam, S. F. Walton and B. J. Currie, J. Infect., 2005, 50, 375–381 CrossRef CAS PubMed.
- A. Puri, R. P. Saxena, Sumati, P. Y. Guru, D. K. Kulshreshtha, K. C. Saxena and B. N. Dhawan, Planta Med., 1992, 58, 528–532 CrossRef CAS PubMed.
- W. Liu, Y. Wang, L. Xia and J. Li, Nutrients, 2024, 16, 797 CrossRef CAS PubMed.
- A. Simoni, L. Schwartz, G. Y. Junquera, C. B. Ching and J. D. Spencer, Nat. Rev. Urol., 2024, 21, 707–722 CrossRef PubMed.
- B. Bajgai, M. Suri, H. Singh, M. Hanifa, J. S. Bhatti, P. K. Randhawa and A. Bali, Phytomedicine, 2024, 130, 155707 CrossRef CAS PubMed.
- A. C. B. B. Candido, M. C. Pagotti, D. A. dos Santos, L. A. d. L. Paula, R. C. S. Veneziani, J. K. Bastos, S. R. Ambrosio and L. G. Magalhaes, Pharmaceuticals, 2024, 17, 1243 CrossRef CAS PubMed.
- P. M. Loiseau, S. Pomel and S. L. Croft, Molecules, 2020, 25, 4123 CrossRef CAS PubMed.
- N. Singh, B. B. Mishra, S. Bajpai, R. K. Singh and V. K. Tiwari, Bioorg. Med. Chem., 2014, 22, 18–45 CrossRef CAS PubMed.
- E. Caballero, J. I. Manzano, P. Puebla, S. Castanys, F. Gamarro and A. San Feliciano, Bioorg. Med. Chem. Lett., 2012, 22, 6272–6275 CrossRef CAS PubMed.
- P. M. Cheuka, G. Mayoka, P. Mutai and K. Chibale, Molecules, 2017, 22, 58 CrossRef PubMed.
- S. Malvolti, M. Malhame, C. F. Mantel, E. A. Le Rutte and P. M. Kaye, PLoS Neglected Trop. Dis., 2021, 15, e0009742 CrossRef PubMed.
- R. R. Tumer and R. R. Woodward, The chemistry of the Cinchona alkaloids, in The Alkaloids, ed. R. H. F. Manske, Academic Press, New York, 1953, p. 16 Search PubMed.
- S. Kapishnikov, T. Staalso, Y. Yang, J. Lee, A. J. Perez-Berna, E. Pereiro, Y. Yang, S. Werner, P. Guttmann, L. Leiserowitz and J. Als-Nielsen, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 22946–22952 CrossRef CAS PubMed.
- N. Tajuddeen and F. R. Van Heerden, Malar. J., 2019, 18, 404 CrossRef PubMed.
- C. W. Wright, J. D. Phillipson, S. O. Awe, G. C. Kirby, D. C. Warhurst and J. Quetin-Leclerq, Phytother. Res., 1996, 10, 361–363 CrossRef CAS.
- G. L. Tona, Clinical trial of Pr259ct1 versus artesunate-amodiaquine (ASAQ) in patients with uncomplicated malaria in the Democratic Republic of Congo (DRC), 2009, http://www.edctpforum2009.org/fileadmin/documents/forum09/Forum2009_programme_book.pdf Search PubMed.
- M. L. Willcox, B. Graz, J. Falquet, O. Sidibe, M. Forster and D. Diallo, Trans. R. Soc. Trop. Med. Hyg., 2007, 101, 1190–1198 CrossRef PubMed.
- B. Graz, M. L. Willcox, C. Diakite, J. Falquet, F. Dackuo, O. Sidibe, S. Giani and D. Diallo, Trans. R. Soc. Trop. Med. Hyg., 2010, 104, 33–41 CrossRef CAS PubMed.
- C. S. Jang, F. Y. Fu, C. Y. Wang, K. C. Huang, G. Lu and T. C. Chou, Science, 1946, 103, 59 CrossRef CAS PubMed.
- S. Zhu, Q. Zhang, C. Gudise, L. Wei, E. Smith and Y. Zeng, Bioorg. Med. Chem., 2009, 17, 4496–4502 CrossRef CAS PubMed.
- T. N. C. Wells, Malar. J., 2011, 10, S3 CrossRef CAS PubMed.
- G. Bringmann, G. Zhang, T. Oelschlaeger, A. Stich, J. Wu, M. Chatterjee and R. Brun, Phytochemistry, 2013, 91, 220–228 CrossRef CAS PubMed.
- J. Li, R. Seupel, D. Feineis, V. Mudogo, M. Kaiser, R. Brun, D. Bruennert, M. Chatterjee, E.-J. Seo, T. Efferth and G. Bringmann, J. Nat. Prod., 2017, 80, 443–458 CrossRef CAS PubMed.
- M. Kubo, W. Yatsuzuka, S. Matsushima, K. Harada, Y. Inoue, H. Miyamoto, M. Matsumoto and Y. Fukuyama, Chem. Pharm. Bull., 2016, 64, 957–960 CrossRef CAS PubMed.
- L. S. Fernandez, M. L. Sykes, K. T. Andrews and V. M. Avery, Int. J. Antimicrob. Agents, 2010, 36, 275–279 CrossRef CAS PubMed.
- R. A. Davis, M. S. Buchanan, S. Duffy, V. M. Avery, S. A. Charman, W. N. Charman, K. L. White, D. M. Shackleford, M. D. Edstein, K. T. Andrews, D. Camp and R. J. Quinn, J. Med. Chem., 2012, 55, 5851–5858 CrossRef CAS PubMed.
- R. A. Davis, S. Duffy, S. Fletcher, V. M. Avery and R. J. Quinn, J. Org. Chem., 2013, 78, 9608–9613 CrossRef CAS PubMed.
- D. Staerk, E. Lemmich, J. Christensen, A. Kharazmi, C. E. Olsen and J. W. Jaroszewski, Planta Med., 2000, 66, 531–536 CrossRef CAS PubMed.
- T. S. Kam, K. M. Sim, T. Koyano and K. Komiyama, Phytochemistry, 1999, 50, 75–79 CrossRef CAS.
- D. B. da Silva, E. C. O. Tulli, G. C. G. Militao, L. V. Costa-Lotufo, C. Pessoa, M. O. de Moraes, S. Albuquerque and J. M. de Siqueira, Phytomedicine, 2009, 16, 1059–1063 CrossRef CAS PubMed.
- H. Istanbullu, G. Bayraktar, G. Karakaya, H. Akbaba, N. E. Perk, I. Cavus, C. Podlipnik, K. Yereli, A. Ozbilgin, B. D. Butuner and V. Alptuzun, Eur. J. Med. Chem., 2023, 247, 115049 CrossRef CAS PubMed.
- G. W. Seifu, Y. S. Birhan, B. Y. Beshay, A. Hymete and A. A. Bekhit, BMC Chem., 2022, 16, 107 CrossRef CAS PubMed.
- S. Hazra, S. Ghosh, S. Debnath, S. Seville, V. K. Prajapati, C. W. Wright, S. Sundar and B. Hazra, Parasitol. Res., 2012, 111, 195–203 CrossRef PubMed.
- A. J. Oluwafemi, E. O. Okanla, P. Camps, D. Munoz-Torrero, Z. B. Mackey, P. K. Chiang, S. Seville and C. W. Wright, Nat. Prod. Commun., 2009, 4, 193–198 CrossRef CAS PubMed.
- B. R. Copp, O. Kayser, R. Brun and A. F. Kiderlen, Planta Med., 2003, 69, 527–531 CrossRef CAS PubMed.
- A. Secrieru, I. C. C. Costa, P. M. O'Neill and M. L. S. Cristiano, Molecules, 2020, 25, 1574 CrossRef CAS PubMed.
- H. Abd Elgawad, S. M. Alhusseiny, A. Taman, M. Y. Youssef, B. Mansour, M. Massoud and A. Handousa, Exp. Parasitol., 2019, 206, 107756 CrossRef PubMed.
- M. J. McPhillie, Y. Zhou, M. R. Hickman, J. A. Gordon, C. R. Weber, Q. Li, P. J. Lee, K. Amporndanai, R. M. Johnson, H. Darby, S. Woods, Z.-h. Li, R. S. Priestley, K. D. Ristroph, S. B. Biering, K. El Bissati, S. Hwang, F. E. Hakim, S. M. Dovgin, J. D. Lykins, L. Roberts, K. Hargrave, H. Cong, A. P. Sinai, S. P. Muench, J. P. Dubey, R. K. Prud'homme, H. A. Lorenzi, G. A. Biagini, S. N. Moreno, C. W. Roberts, S. V. Antonyuk, C. W. G. Fishwick and R. McLeod, Front. Cell. Infect. Microbiol., 2020, 10, 203 CrossRef CAS PubMed.
- M. Zhang, Q. Zhang, Q. Zhang, X. Cui and L. Zhu, Mar. Drugs, 2023, 21, 84 CrossRef CAS PubMed.
- C. Ji-xiang and S. Bao-an, J. Integr. Agric., 2021, 20, 2015–2031 CrossRef.
- L. M. P. Vermeulen, S. C. De Smedt, K. Remaut and K. Braeckmans, Eur. J. Pharm. Biopharm., 2018, 129, 184–190 CrossRef CAS PubMed.
- L.-X. Dai, J.-C. Li, X.-L. Miao, X. Guo, X.-F. Shang, W.-W. Wang, B. Li, Y. Wang, H. Pan and J.-Y. Zhang, Ind. Crops Prod., 2021, 162, 113288 CrossRef CAS.
- X.-F. Shang, Z.-M. Zhao, J.-C. Li, G.-Z. Yang, Y.-Q. Liu, L.-X. Dai, Z.-J. Zhang, Z.-G. Yang, X.-L. Miao, C.-J. Yang and J.-Y. Zhang, Ind. Crops Prod., 2019, 137, 508–520 CrossRef CAS.
- B. B. Mishra, J. K. Gour, N. Kishore, R. K. Singh, V. Tripathi and V. K. Tiwari, Nat. Prod. Res., 2013, 27, 480–485 Search PubMed.
- L. Zhang, G. Zhang, S. Xu and Y. Song, Eur. J. Med. Chem., 2021, 223, 113632 CrossRef CAS PubMed.
- A. T. Hudson, Parasitol. Today, 1993, 9, 66 CrossRef CAS PubMed.
- O. P. S. Patel, R. M. Beteck and L. J. Legoabe, Eur. J. Med. Chem., 2021, 210, 113084 Search PubMed.
- J. A. Dimmer, F. V. Cabral, S. C. N. Montoya and M. S. Ribeiro, Photodiagn. Photodyn. Ther., 2023, 42, 103525 CrossRef CAS PubMed.
- R. W. Winter, K. A. Cornell, L. L. Johnson, L. M. Isabelle, D. J. Hinrichs and M. K. Riscoe, Bioorg. Med. Chem. Lett., 1995, 5, 1927–1932 CrossRef CAS.
- A. Fournet, A. A. Barrios, V. Munoz, R. Hocquemiller and A. Cave, Trop. Med. Parasitol., 1992, 43, 219–222 Search PubMed.
- K. Mori-Yasumoto, R. Izumoto, H. Fuchino, T. Ooi, Y. Agatsuma, T. Kusumi, M. Satake and S. Sekita, Bioorg. Med. Chem., 2012, 20, 5215–5219 CrossRef CAS PubMed.
- A. A. Sittie, E. Lemmich, C. E. Olsen, L. Hviid, A. Kharazmi, F. K. Nkrumah and S. B. Christensen, Planta Med., 1999, 65, 259–261 CrossRef CAS PubMed.
- M. P. M. Portela and A. O. M. Stoppani, Biochem. Pharmacol., 1996, 51, 275–283 CrossRef PubMed.
- J. N. Lopes, F. S. Cruz, R. Docampo, M. E. Vasconcellos, M. C. Sampaio, A. V. Pinto and B. Gilbert, Ann. Trop. Med. Parasitol., 1978, 72, 523–531 CrossRef CAS PubMed.
- M. R. Dhananjeyan, Y. P. Milev, M. A. Kron and M. G. Nair, J. Med. Chem., 2005, 48, 2822–2830 CrossRef CAS PubMed.
- S. de Ridder, F. van der Kooy and R. Verpoorte, J. Ethnopharmacol., 2008, 120, 302–314 CrossRef CAS PubMed.
- F. S. Li and J. K. Weng, Nat. Plants, 2017, 3, 17109 CrossRef PubMed.
- Y. Tu, Nat. Med., 2011, 17, 1217–1220 CrossRef CAS PubMed.
- N. J. White, S. Pukrittayakamee, H. Tran Tinh, M. A. Faiz, O. A. Mokuolu and A. M. Dondorp, Lancet, 2014, 383, 723–735 CrossRef PubMed.
- J. L. Vennerstrom, S. Arbe-Barnes, R. Brun, S. A. Charman, F. C. K. Chiu, J. Chollet, Y. X. Dong, A. Dorn, D. Hunziker, H. Matile, K. McIntosh, M. Padmanilayam, J. S. Tomas, C. Scheurer, B. Scorneaux, Y. Q. Tang, H. Urwyler, S. Wittlin and W. N. Charman, Nature, 2004, 430, 900–904 CrossRef CAS PubMed.
- B. Zhou, Y. Wu, S. Dalal, E. F. Merino, Q. F. Liu, C. H. Xu, T. Yuan, J. Ding, D. G. I. Kingston, M. B. Cassera and J. M. Yue, J. Nat. Prod., 2017, 80, 96–107 CrossRef CAS PubMed.
- R. Somo-Moyou, M. L. Mittelholzer, F. Sorenson, L. Haller and D. Sturchler, Trop. Med. Parasitol., 1994, 45, 288–291 CAS.
- S. Challand and M. Willcox, J. Altern. Complementary Med., 2009, 15, 1231–1237 CrossRef PubMed.
- N. Valecha, C. U. Devi, H. Joshi, V. K. Shahi, V. P. Sharma and S. Lal, Curr. Sci., 2000, 78, 1120–1122 Search PubMed.
- N. Germonprez, L. Maes, L. Van Puyvelde, M. Van Tri, D. A. Tuan and N. De Kimpe, J. Med. Chem., 2005, 48, 32–37 CrossRef CAS PubMed.
- K. Nagamune, L. M. Hicks, B. Fux, F. Brossier, E. N. Chini and L. D. Sibley, Nature, 2008, 451, 207–210 Search PubMed.
- T. F. Frezza, C. N. Fernandes de Oliveira, T. M. Banin, V. L. Garcia Rehder, S. Boaventura Jr and S. M. Allegretti, Rev. Biol. Trop., 2013, 42, 309–321 Search PubMed.
- J. Utzinger, S.-H. Xiao, M. Tanner and J. Keiser, Curr. Opin. Invest. Drugs, 2007, 8, 105–116 Search PubMed.
- M. A. Chama, H. A. Onyame, C. Fleischer, D. Osei-Safo, R. Waibel, J. Otchere, I. Addae-Mensah and M. Wilson, Heliyon, 2020, 6, e04460 Search PubMed.
- J. Mikus, M. Harkenthal, D. Steverding and J. Reichling, Planta Med., 2000, 66, 366–368 CrossRef CAS PubMed.
- D. Tasdemir, M. Kaiser, R. Brun, V. Yardley, T. J. Schmidt, F. Tosun and P. Rüedi, Antimicrob. Agents Chemother., 2006, 50, 1352–1364 CrossRef CAS PubMed.
- B. Räz, Isolation and evaluation of antiparasitic lead compounds from African medicinal plants, Ph.D. thesis, Universität Basel, 1998, p. 216.
- I. Ramírez, A. Carabot, P. Meléndez, J. Carmona, M. Jimenez, A. V. Patel, T. A. Crabb, G. Blunden, P. D. Cary, S. L. Croft and M. Costa, Phytochemistry, 2003, 64, 645–647 CrossRef PubMed.
- N. Tiwari, A. Kumar, A. K. Singh, S. Bajpai, A. K. Agrahari, D. Kishore, V. K. Tiwari and R. K. Singh, Discovery and Development of Therapeutics from Natural Products against Neglected Tropical Diseases, Elsevier, 2019, pp. 293–350 Search PubMed.
- M. S. Osman, T. A. Awad, S. W. Shantier, E. A. Garelnabi, W. Osman, R. A. Mothana, F. A. Nasr and R. I. Elhag, Arabian J. Chem., 2022, 15, 103717 CrossRef CAS.
- V. Gouri, S. Upreti and M. Samant, Parasitol. Int., 2022, 91, 102622 CrossRef CAS PubMed.
- B. Mittra, A. Saha, A. R. Chowdhury, C. Pal, S. Mandal, S. Mukhopadhyay, S. Bandyopadhyay and H. K. Majumder, Mol. Med., 2000, 6, 527–541 CAS.
- L. Zhai, M. Chen, J. Blom, T. G. Theander, S. B. Christensen and A. Kharazmi, J. Antimicrob. Chemother., 1999, 43, 793–803 Search PubMed.
- L. Jiang, B. Liu, S. Hou, T. Su, Q. Fan, E. Alyafeai, Y. Tang, M. Wu, X. Liu, J. Li, Y. Hu, W. Li, Z. Zheng, Y. Liu and J. Wu, Eur. J. Med. Chem., 2022, 234, 114244 CrossRef CAS PubMed.
- D. A. Abugri, W. H. Witola, A. E. Russell and R. M. Troy, Chem. Biol. Drug Des., 2018, 91, 194–201 CrossRef CAS PubMed.
- M. T. Islam, M. Martorell, B. Salehi, W. N. Setzer and J. Sharifi-Rad, Phytother. Res., 2020, 34, 1761–1769 CrossRef CAS PubMed.
- T. A. S. Oliveira, M. C. Pagotti, L. G. Magalhaes and A. E. M. Crotti, Chem. Biodiversity, 2022, 19, e202100909 CrossRef CAS PubMed.
- N. A. Parreira, L. C. Magalhaes, D. R. Morais, S. C. Caixeta, J. P. B. de Sousa, J. K. Bastos, W. R. Cunha, M. L. A. Silva, N. P. D. Nanayakkara, V. Rodrigues and A. A. da Silva Filho, Chem. Biodiversity, 2010, 7, 993–1001 CrossRef CAS PubMed.
- M. H. Soares, H. J. Dias, T. M. Vieira, M. G. M. de Souza, A. F. F. Cruz, F. R. Badoco, H. D. Nicolella, W. R. Cunha, M. Groppo, C. H. G. Martins, D. C. Tavares, L. G. Magalhaes and A. E. M. Crotti, Chem. Biodiversity, 2017, 14, e1700149 CrossRef PubMed.
- A. M. Barakat, H. A. M. El Fadaly, A. Gareh, K. A. Abd El-Razik, F. A. Z. Ali, A. A. Saleh, S. A. S. Sadek, N. Dahran, A. E.-N. G. El-Gendy, M. F. El-Khadragy and E. K. Elmahallawy, Animals, 2022, 12, 3069 CrossRef PubMed.
- G. Elango and A. A. Rahuman, Parasitol. Res., 2011, 108, 513–519 CrossRef PubMed.
- B. S. Tanwer and R. V. Rekha Vijayvergia, Herba Pol., 2010, 56, 71–77 CAS.
- N. H. M. Nisha and N. Packialakshmi, Int. J. Pharm. Res., 2014, 4, 22–24 Search PubMed.
- C. Steinbeck, V. Spitzer, M. Starosta and G. vonPoser, J. Nat. Prod., 1997, 60, 627–628 CrossRef CAS.
- A. d. S. Lima, J. G. do Nascimento Sousa Filho, S. G. Pereira, G. M. Skelding Pinheiro Guillon, L. d. S. Santos and L. M. Costa Junior, Parasitol. Res., 2014, 113, 107–112 CrossRef PubMed.
- C. F. C. Lam, A. N. Pearce, S. H. Tan, M. Kaiser and B. R. Copp, Mar. Drugs, 2013, 11, 3472–3499 CrossRef CAS PubMed.
- H. R. El-Seedi, M. Azeem, N. S. Khalil, H. H. Sakr, S. A. M. Khalifa, K. Awang, A. Saeed, M. A. Farag, M. F. AlAjmi, K. Palsson and A. K. Borg-Karlson, Exp. Appl. Acarol., 2017, 73, 139–157 CrossRef CAS PubMed.
- J. F. Carroll, N. Tabanca, M. Kramer, N. M. Elejalde, D. E. Wedge, U. R. Bernier, M. Coy, J. J. Becnel, B. Demirci, K. H. C. Baser, J. Zhang and S. Zhang, J. Vector Ecol., 2011, 36, 258–268 CrossRef PubMed.
- H. R. El-Seedi, N. S. Khalil, M. Azeem, E. A. Taher, U. Goransson, K. Palsson and A. K. Borg-Karlson, J. Med. Entomol., 2012, 49, 1067–1075 CrossRef CAS PubMed.
- W. Wanzala, A. Hassanali, W. R. Mukabana and W. Takken, J. Parasitol. Res., 2014, 2014, 434506 Search PubMed.
- W. Lwande, A. J. Ndakala, A. Hassanali, L. Moreka, E. Nyandat, M. Ndungu, H. Amiani, P. M. Gitu, M. M. Malonza and D. K. Punyua, Phytochemistry, 1999, 50, 401–405 CrossRef CAS.
- G. D. da Silva, H. G. de Lima, H. F. de Freitas, S. S. da Rocha Pita, Y. d. S. Luz, M. P. de Figueiredo, R. S. Uzeda, A. Branco, S. L. Costa, M. J. Moreira Batatinha and M. B. Botura, Ticks Tick-Borne Dis., 2021, 12, 101643 CrossRef PubMed.
- M. Salman, R. Z. Abbas, M. Israr, A. Abbas, K. Mehmood, M. K. Khan, Z. U. D. Sindhu, R. Hussain, M. K. Saleemi and S. Shah, Vet. Parasitol., 2020, 283, 109178 CrossRef CAS PubMed.
- S. S. Weber, K. P. Kaminski, J. L. Perret, P. Leroy, A. Mazurov, M. C. Peitsch, N. V. Ivanov and J. Hoeng, Food Chem. Toxicol., 2019, 132, 110660 CrossRef PubMed.
- F. Fang, K. Candy, E. Melloul, C. Bernigaud, L. Chai, C. Darmon, R. Durand, F. Botterel, O. Chosidow, A. Izri, W. Huang and J. Guillot, Parasites Vectors, 2016, 9, 594 CrossRef PubMed.
- S. F. Walton, M. McKinnon, S. Pizzutto, A. Dougall, E. Williams and B. J. Currie, Arch. Dermatol., 2004, 140, 563–566 CAS.
- M. Bahmani, K. Saki, B. Ezatpour, S. Shahsavari, Z. Eftekhari, M. Jelodari, M. Rafieian-Kopaei and R. Sepahvand, Asian Pac. J. Trop. Biomed., 2015, 5, 673–679 CrossRef CAS.
- K. M. Choi, J. Gang and J. Yun, Int. J. Antimicrob. Agents, 2008, 32, 360–362 CrossRef CAS PubMed.
- T. Efferth, F. Herrmann, A. Tahrani and M. Wink, Phytomedicine, 2011, 18, 959–969 CrossRef CAS PubMed.
- H. N. Sharma, J. Catrett, O. D. Nwokeocha, M. Boersma, M. E. Miller, A. Napier, B. K. Robertson and D. A. Abugri, Sci. Rep., 2023, 13, 8667 CrossRef CAS PubMed.
- N. Kavitha, R. Noordin, K. L. Chan and S. Sasidharan, BMC Complementary Altern. Med., 2012, 12, 91 CrossRef PubMed.
- C. C. Barbosa de Castro, M. M. Dias, T. P. de Rezende, L. G. Magalhaes and A. A. Da Silva Filho, Fighting Multidrug Resist. Herb. Extr., Essent. Oils Their Compon., 2013, 109–134 CAS.
- F. Yousif, G. Wassel, L. Boulos, T. Labib, K. Mahmoud, S. El-Hallouty, S. El Bardicy, S. Mahmoud, F. Ramzy, L. Gohar, M. El-Manawaty, M. A. M. El Gendy, W. Fayad and B. El-Menshawi, Pharm. Biol., 2012, 50, 732–739 CrossRef PubMed.
- H. F. Abdel-Hamid, J. Egypt. Soc. Parasitol., 2003, 33, 947 Search PubMed.
- A. M. Elmalawany, G. Y. Osman, M.-A. S. H. Elashwal and A. H. Mohamed, Exp. Parasitol., 2022, 239, 108290 CrossRef CAS PubMed.
- J. G. Mendes Rodrigues, P. S. Veras Albuquerque, J. R. Nascimento, J. A. Viana Campos, A. S. S. Godinho, S. J. Araujo, J. M. Brito, C. M. Jesus, G. S. Miranda, M. C. Rezende, D. A. Negrao-Correa, C. Q. Rocha, L. A. Silva, R. N. M. Guerra and F. R. F. Nascimento, J. Ethnopharmacol., 2021, 264, 113287 CrossRef PubMed.
- R. N. de Oliveira, V. L. Garcia Rehder, A. S. Santos Oliveira, V. d. L. Sierpe Jeraldo, A. X. Linhares and S. M. Allegretti, Exp. Parasitol., 2014, 139, 63–72 CrossRef PubMed.
- A. H. Mohamed, G. Y. Osman, T. A. Salem and A. M. Elmalawany, Exp. Parasitol., 2014, 145, 7–13 CrossRef CAS PubMed.
- J. Zhang, J. Chen, K. Lv, B. Li, B. Yan, L. Gai, C. Shi, X. Wang, H. Si and J. Zhang, Front. Cell. Infect. Microbiol., 2021, 11, 730222 CrossRef CAS PubMed.
- D. d. S. Costa, C. M. Leal, R. A. Cajas, M. C. Gazolla, L. M. Silva, L. S. A. de Carvalho, B. L. Lemes, R. O. de Moura, J. de Almeida, J. de Moraes and A. A. da Silva Filho, J. Ethnopharmacol., 2023, 313, 116607 CrossRef CAS PubMed.
- Y.-H. Du, J.-L. Li, R.-Y. Jia, Z.-Q. Yin, X.-T. Li, C. Lv, G. Ye, L. Zhang and Y.-Q. Zhang, Vet. Parasitol., 2009, 163, 175–178 CrossRef CAS PubMed.
- L. Li, Y. Zhang, T. Liu, R. Xing, S. Peng, X. Song, Y. Zou, X. Zhao, R. Jia, H. Wan, L. Yin, G. Ye, F. Shi, Y. Zhang, G. Yue and Z. Yin, Front. Pharmacol., 2022, 13, 953284 CrossRef CAS PubMed.
- C. Pasay, K. Mounsey, G. Stevenson, R. Davis, L. Arlian, M. Morgan, D. Vyszenski-Moher, K. Andrews and J. McCarthy, PLoS One, 2010, 5, e12079 CrossRef PubMed.
- X.-F. Shang, L.-X. Dai, C.-J. Yang, X. Guo, Y.-Q. Liu, X.-L. Miao and J.-Y. Zhang, J. Adv. Res., 2021, 34, 149–158 CrossRef CAS PubMed.
- X.-F. Shang, Y.-Q. Liu, X. Guo, X.-L. Miao, C. Chen, J.-X. Zhang, X.-S. Xu, G.-Z. Yang, C.-J. Yang, J.-C. Li and X.-S. Zhang, Sci. Rep., 2018, 8, 1609 CrossRef PubMed.
- A. L. Castillo, M. O. Osi, J. D. A. Ramos, J. L. De Francia, M. U. Dujunco and P. F. Quilala, J. Pharmacol. Pharmacother., 2013, 4, 39–46 CrossRef PubMed.
- J. L. Dahlin, D. S. Auld, I. Rothenaigner, S. Haney, J. Z. Sexton, J. W. M. Nissink, J. Walsh, J. A. Lee, J. M. Strelow, F. S. Willard, L. Ferrins, J. B. Baell, M. A. Walters, B. K. Hua, K. Hadian and B. K. Wagner, Cell Chem. Biol., 2021, 28, 356–370 CrossRef CAS PubMed.
- K. M. Nelson, J. L. Dahlin, J. Bisson, J. Graham, G. F. Pauli and M. A. Walters, J. Med. Chem., 2017, 60, 1620–1637 CrossRef CAS PubMed.
- M. Z. Y. Choo and C. L. L. Chai, ChemMedChem, 2022, 17, e202100710 CrossRef CAS PubMed.
- J. Sun, H. Zhong, K. Wang, N. Li and L. Chen, Acta Pharm. Sin. B, 2021, 11, 3417–3432 CrossRef CAS PubMed.
- F. M. Bashore, J. Annor-Gyamfi, Y. Du, V. Katis, F. Nwogbo, R. G. Flax, S. V. Frye, K. H. Pearce, H. Fu, T. M. Willson, D. H. Drewry and A. D. Axtman, J. Med. Chem., 2023, 66, 14434–14446 CrossRef CAS PubMed.
- J. Glaser and U. Holzgrabe, MedChemComm, 2016, 7, 214–223 RSC.
- A. L. Heinzke, B. Zdrazil, P. D. Leeson, R. J. Young, A. Pahl, H. Waldmann and A. R. Leach, Sci. Data, 2024, 11, 1160 CrossRef PubMed.
- S. Omura and A. Crump, Nat. Rev. Microbiol., 2004, 2, 984–989 CrossRef CAS PubMed.
- The Nobel Prize, 2015, https://www.nobelprize.org/prizes/medicine/2015/press-release/ Search PubMed.
- R. T. Jacobs, C. S. Lunde, Y. R. Freund, V. Hernandez, X. Li, Y. Xia, D. S. Carter, P. W. Berry, J. Halladay, F. Rock, R. Stefanakis, E. Easom, J. J. Plattner, L. Ford, K. L. Johnston, D. A. N. Cook, R. Clare, A. Cassidy, L. Myhill, H. Tyrer, J. Gamble, A. F. Guimaraes, A. Steven, F. Lenz, A. Ehrens, S. J. Frohberger, M. Koschel, A. Hoerauf, M. P. Huebner, C. W. McNamara, M. A. Bakowski, J. D. Turner, M. J. Taylor and S. A. Ward, J. Med. Chem., 2019, 62, 2521–2540 CrossRef CAS PubMed.
- M. J. Taylor, W. H. Makunde, H. F. McGarry, J. D. Turner, S. Mand and A. Hoerauf, Lancet, 2005, 365, 2116–2121 CrossRef CAS PubMed.
- M. J. Taylor, T. W. von Geldern, L. Ford, M. P. Huebner, K. Marsh, K. L. Johnston, H. T. Sjoberg, S. Specht, N. Pionnier, H. E. Tyrer, R. H. Clare, D. A. N. Cook, E. Murphy, A. Steven, J. Archer, D. Bloemker, F. Lenz, M. Koschel, A. Ehrens, H. M. Metuge, V. C. Chunda, P. W. N. Chounna, A. J. Njouendou, F. F. Fombad, R. Carr, H. E. Morton, G. Aljayyoussi, A. Hoerauf, S. Wanji, D. J. Kempf, J. D. Turner and S. A. Ward, Sci. Transl. Med., 2019, 11, eaau2086 CrossRef PubMed.
- M. Iwatsuki, S. Takada, M. Mori, A. Ishiyama, M. Namatame, A. Nishihara-Tsukashima, K. Nonaka, R. Masuma, K. Otoguro, K. Shiomi and S. Omura, J. Antibiot., 2011, 64, 183–188 CrossRef CAS PubMed.
- H. A. M. F. Silva, A. L. Aires, C. L. R. Soares, W. N. Siqueira, M. V. Lima, M. C. B. Martins, M. C. P. A. Albuquerque, T. G. Silva, F. A. Brayner, L. C. Alves, A. M. M. A. Melo and N. H. Silva, Acta Trop., 2021, 222, 106044 CrossRef CAS PubMed.
- T. Tanaka, H. Maeda, T. Matsuo, D. Boldbattar, R. Umemiya-Shirafuji, A. Kume, H. Suzuki, X. Xuan, N. Tsuji and K. Fujisaki, Peptides, 2012, 34, 242–250 CrossRef CAS PubMed.
- S. Hou, Y. Liu, Y. Tang, M. Wu, J. Guan, X. Li, Z. Wang, J. Jiang, M. Deng, Z. Duan, X. Tang, X. Han and L. Jiang, Toxicon, 2019, 166, 9–14 CrossRef CAS PubMed.
- L. M. Bastos, R. J. Oliveira Junior, D. A. Oliveira Silva, J. R. Mineo, C. U. Vieira, D. N. Silva Teixeira, M. I. Homsi-Brandeburgo, V. M. Rodrigues and A. Hamaguchi, Exp. Parasitol., 2008, 120, 391–396 CrossRef CAS PubMed.
- D. Yang, X. Liu, J. Li, J. Xie and L. Jiang, Front. Pharmacol., 2023, 14, 1178070 CrossRef CAS PubMed.
- R. S. Compagnone, I. C. Piña, H. R. Rangel, F. Dagger, A. I. Suárez, M. V. R. Reddy and D. J. Faulkner, Tetrahedron, 1998, 54, 3057–3068 CrossRef CAS.
- L. Cartuche, I. Sifaoui, A. Lopez-Arencibia, C. J. Bethencourt-Estrella, D. San Nicolas-Hernandez, J. Lorenzo-Morales, J. E. Pinero, A. R. Diaz-Marrero and J. J. Fernandez, Biomolecules, 2020, 10, 657 CrossRef CAS PubMed.
- C. Imperatore, R. Gimmelli, M. Persico, M. Casertano, A. Guidi, F. Saccoccia, G. Ruberti, P. Luciano, A. Aiello, S. Parapini, S. Avunduk, N. Basilico, C. Fattorusso and M. Menna, Mar. Drugs, 2020, 18, 112 CrossRef CAS PubMed.
- Y. Nakao, T. Shiroiwa, S. Murayama, S. Matsunaga, Y. Goto, Y. Matsumoto and N. Fusetani, Mar. Drugs, 2004, 2, 55–62 CrossRef CAS.
- R. F. S. Menna-Barreto, R. L. S. Goncalves, E. M. Costa, R. S. F. Silva, A. V. Pinto, M. F. Oliveira and S. L. de Castro, Free Radical Biol. Med., 2009, 47, 644–653 CrossRef CAS PubMed.
- G. Chianese, E. Fattorusso, F. Scala, R. Teta, B. Calcinai, G. Bavestrello, H. A. Dien, M. Kaiser, D. Tasdemir and O. Taglialatela-Scafati, Org. Biomol. Chem., 2012, 10, 7197–7207 RSC.
- G. Chianese, F. Scala, B. Calcinai, C. Cerrano, H. A. Dien, M. Kaiser, D. Tasdemir and O. Taglialatela-Scafati, Mar. Drugs, 2013, 11, 3297–3308 CrossRef PubMed.
- Y. Feng, R. A. Davis, M. Sykes, V. M. Avery, D. Camp and R. J. Quinn, J. Nat. Prod., 2010, 73, 716–719 CrossRef CAS PubMed.
- S. M. Pimentel-Elardo, S. Kozytska, T. S. Bugni, C. M. Ireland, H. Moll and U. Hentschel, Mar. Drugs, 2010, 8, 373–380 CrossRef CAS PubMed.
- C. Osterhage, R. Kaminsky, G. M. König and A. D. Wright, J. Org. Chem., 2000, 65, 6412–6417 CrossRef CAS PubMed.
- N. B. Pham, S. Deydier, M. Labaied, S. Monnerat, K. Stuart and R. J. Quinn, Eur. J. Med. Chem., 2014, 74, 541–551 CrossRef CAS PubMed.
- R. Sakai, T. Higa, C. W. Jefford and G. Bernardinelli, J. Am. Chem. Soc., 1986, 108, 6404 CrossRef CAS.
- K. A. El Sayed, M. Kelly, U. A. K. Kara, K. K. H. Ang, I. Katsuyama, D. C. Dunbar, A. A. Khan and M. T. Hamann, J. Am. Chem. Soc., 2001, 123, 1804–1808 CrossRef CAS PubMed.
- K. A. El Sayed, M. T. Hamann, N. E. Hashish, W. T. Shier, M. Kelly and A. A. Khan, J. Nat. Prod., 2001, 64, 522–524 CrossRef CAS PubMed.
- T. L. Perry, A. Dickerson, A. A. Khan, R. K. Kondru, D. N. Beratan, P. Wipf, M. Kelly and M. T. Hamann, Tetrahedron, 2001, 57, 1483–1487 CrossRef CAS.
- N. Chaachouay and L. Zidane, Drugs Drug Candidates, 2024, 3, 184–207 CrossRef.
- M. K. Obaid, N. Islam, A. Alouffi, A. Z. Khan, I. d. S. Vaz Jr, T. Tanaka and A. Ali, Front. Cell. Infect. Microbiol., 2022, 12, 941831 CrossRef CAS PubMed.
- S. B. Mishra, A. Mukerjee and S. Singh, in Evidence Based Validation of Traditional Medicines: A Comprehensive Approach, ed. S. C. Mandal, R. Chakraborty and S. Sen, Springer Singapore, Singapore, 2021, pp. 3–27, DOI:10.1007/978-981-15-8127-4_1.
- X. Xing, M. Sun, Z. Guo, Y. Zhao, Y. Cai, P. Zhou, H. Wang, W. Gao, P. Li and H. Yang, Acta Pharm. Sin. B, 2023, 13, 3802–3816 CrossRef PubMed.
- A. Javid, A. Fatima, M. Hamad and M. Ahmed, S. Afr. J. Bot., 2024, 173, 159–174 CrossRef CAS.
- M. Grigalunas, S. Brakmann and H. Waldmann, J. Am. Chem. Soc., 2022, 144, 3314–3329 CrossRef CAS PubMed.
- Y. Wang, Y.-N. Shi, H. Xiang and Y.-M. Shi, Nat. Prod. Rep., 2024, 41, 1630–1651 RSC.
- C. Herrmann-Pillath, J. Hiedanpää and K. Soini, Nature-Based Solutions, 2022, 2, 100011 CrossRef.
- A. R. Awan, W. M. Shaw and T. Ellis, Adv. Drug Delivery Rev., 2016, 105, 96–106 CrossRef CAS PubMed.
- D. J. Kountz and E. P. Balskus, Acc. Chem. Res., 2021, 54, 2788–2797 CrossRef CAS PubMed.
- L. Yao, X. Wu, X. Jiang, M. Shan, Z. Zhang, Y. Li, A. Yang, Y. Li and C. Yang, Biotechnol. Adv., 2023, 69, 108258 CrossRef CAS PubMed.
- J. Pollier, T. Moses and A. Goossens, Nat. Prod. Rep., 2011, 28, 1897–1916 RSC.
- M. G. Chevrette, K. Gutierrez-Garcia, N. Selem-Mojica, C. Aguilar-Martinez, A. Yanez-Olvera, H. E. Ramos-Aboites, P. A. Hoskisson and F. Barona-Gomez, Nat. Prod. Rep., 2020, 37, 566–599 RSC.
- I. Ri, S. Pak, U. Pak, C. Yun and Z. Tang, Ind. Crops Prod., 2024, 208, 117832 CrossRef CAS.
- E. Skellam, S. Rajendran and L. Li, Commun. Chem., 2024, 7, 89 CrossRef CAS PubMed.
- W. Yeh, T. Hanekamp, S. Tsoka, P. D. Karp and R. B. Altman, Genome Res., 2004, 14, 917–924 CrossRef PubMed.
- L. B. Tulloch, S. K. Menzies, A. L. Fraser, E. R. Gould, E. F. King, M. K. Zacharova, G. J. Florence and T. K. Smith, PLoS Neglected Trop. Dis., 2017, 11, e0005886 CrossRef PubMed.
- D. A. DeGoey and P. B. Cox, in Burger's Medicinal Chemistry and Drug Discovery, 2021, pp. 1–35, DOI:10.1002/0471266949.bmc258.
- A. G. Atanasov, S. B. Zotchev, V. M. Dirsch, C. T. Supuran and The International Natural Product Sciences Taskforce, Nat. Rev. Drug Discovery, 2021, 20, 200–216 CrossRef CAS PubMed.
- L. Zhang, J. Song, L. Kong, T. Yuan, W. Li, W. Zhang, B. Hou, Y. Lu and G. Du, Pharmacol. Ther., 2020, 216, 107686 CrossRef CAS PubMed.
- G. B. Santos, A. Ganesan and F. S. Emery, ChemMedChem, 2016, 11, 2245–2251 CrossRef CAS PubMed.
- F. Chemat, M. Abert-Vian, A. S. Fabiano-Tixier, J. Strube, L. Uhlenbrock, V. Gunjevic and G. Cravotto, TrAC, Trends Anal. Chem., 2019, 118, 248–263 CrossRef CAS.
- C.-Y. Xiao, J.-M. He, J. Huang, X.-M. Guo, P. Yang and Q. Mu, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2024, 1232, 123965 CrossRef CAS PubMed.
- S. P. Gaudencio and F. Pereira, Nat. Prod. Rep., 2015, 32, 779–810 RSC.
- A. Mohamed, N. Canh Hao and H. Mamitsuka, Briefings Bioinf., 2016, 17, 309–321 CrossRef CAS PubMed.
- S. Hoet, F. Opperdoes, R. Brun and J. Quetin-Leclereq, Nat. Prod. Rep., 2004, 21, 353–364 RSC.
- C. Harrison, Nat. Rev. Drug Discovery, 2014, 13, 250 CrossRef PubMed.
- B. E. Kirsop, J. Ind. Microbiol. Biotechnol., 1996, 17, 505–511 CAS.
- R. L. do Monte Neto, P. O. Lourenco Moreira, A. M. de Sousa, M. A. do Nascimento Garcia, S. R. Maran and N. S. Moretti, Mem. Inst. Oswaldo Cruz, 2022, 117, e210403 CrossRef PubMed.
- A. I. Olias-Molero, C. de la Fuente, M. Cuquerella, J. J. Torrado and J. M. Alunda, Microorganisms, 2021, 9, 2500 CrossRef CAS PubMed.
- J. L. Vennerstrom, S. Arbe-Barnes, R. Brun, S. A. Charman, F. C. K. Chiu, J. Chollet, Y. X. Dong, A. Dorn, D. Hunziker, H. Matile, K. McIntosh, M. Padmanilayam, J. S. Tomas, C. Scheurer, B. Scorneaux, Y. Q. Tang, H. Urwyler, S. Wittlin and W. N. Charman, Nature, 2004, 430, 900–904 CrossRef CAS PubMed.
- CHEMnetBASE, Dictionary of Natural Products, Taylor & Francis Group, 2013 Search PubMed.
- V. Sharma and I. N. Sarkar, J. Am. Med. Inform. Assoc., 2013, 20, 668–679 CrossRef PubMed.
- R. A. Dixon, Nature, 2001, 411, 843–847 CrossRef CAS PubMed.
- C. Kamaraj, C. Ragavendran, R. C. S. Kumar, A. Ali, S. U. Khan, Z. u.-R. Mashwani, J. P. Luna-Arias and J. P. R. Pedroza, Phytomed. Plus, 2022, 2, 100377 CrossRef.
- S. P. S. Rao, U. H. Manjunatha, S. Mikolajczak, P. G. Ashigbie and T. T. Diagana, Trends Parasitol., 2023, 39, 260–271 CrossRef CAS PubMed.
- A. M. S. Mayer, A. D. Rodriguez, O. Taglialatela-Scafati and N. Fusetani, Mar. Drugs, 2017, 15, 273 CrossRef PubMed.
- A. M. S. Mayer, Marine Pharmaceuticals: The Clinical Pipeline, 2018, http://marinepharmacology.midwestern.edu/clinPipeline.htm Search PubMed.
- R. Pedrosa, S. P. Gaudencio and V. Vasconcelos, Mar. Drugs, 2020, 18, 40 CrossRef PubMed.
- S. Giraud, Front. Drug Discovery, 2024, 4, 1342866 CrossRef.
- P. Ruenchit, Curr. Res. Parasitol. Vector-Borne Dis., 2025, 7, 100256 CrossRef CAS PubMed.
- H. T. Xue, M. Stanley-Baker, A. W. K. Kong, H. L. Li and W. W. B. Goh, Drug Discovery Today, 2022, 27, 2235–2243 CrossRef CAS PubMed.
- B. B. Basnet, Z.-Y. Zhou, B. Wei and H. Wang, Crit. Rev. Biotechnol., 2025 DOI:10.1080/07388551.2025.2478094.
- D. Meijer, M. A. Beniddir, C. W. Coley, Y. M. Mejri, M. Ozturk, J. J. J. van der Hooft, M. H. Medema and A. Skiredj, Nat. Prod. Rep., 2025, 42, 654–662 RSC.
- J. Wang, C. Paz, G. Padalino, A. Coghlan, Z. Lu, I. Gradinaru, J. N. R. Collins, M. Berriman, K. F. Hoffmann and J. J. Collins, Science, 2020, 369, 1649 CrossRef CAS PubMed.
- J. S. Marchant, W. W. Harding and J. D. Chan, Int. J. Parasitol.: Drugs Drug Resist., 2018, 8, 550–558 Search PubMed.
- S. Arora, S. Chettri, V. Percha, D. Kumar and M. Latwal, J. Biomol. Struct. Dyn., 2024, 42, 3826–3835 CrossRef CAS PubMed.
- A. C. Pushkaran and A. A. Arabi, Artif. Intell. Rev., 2024, 57, 86 CrossRef.
- P. V. Coveney, E. R. Dougherty and R. R. Highfield, Philos. Trans. R. Soc., A, 2016, 374, 20160153 CrossRef PubMed.
- A. A. Khan, M. C. Taylor, A. F. Francisco, S. Jayawardhana, R. L. Atherton, F. Olmo, M. D. Lewis and J. M. Kelly, Clin. Microbiol. Rev., 2024, 37, e00155–00123 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |