Open Access Article
Open Access ArticleMarine natural products†
Anthony R.
Carroll
 *ab,
Brent R.
Copp
*ab,
Brent R.
Copp
 c,
Tanja
Grkovic
c,
Tanja
Grkovic
 d,
Robert A.
Keyzers
d,
Robert A.
Keyzers
 e and
Michèle R.
Prinsep
e and
Michèle R.
Prinsep
 f
f
aSchool of Environment and Science, Griffith University, Gold Coast, Australia. E-mail: A.Carroll@griffith.edu.au
bGriffith Institute for Drug Discovery, Griffith University, Brisbane, Australia
cSchool of Chemical Sciences, University of Auckland, Auckland, New Zealand
dNatural Products Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, Molecular Targets Program, Center for Cancer Research, National Cancer Institute, Frederick, MD, USA
eCentre for Biodiscovery, School of Chemical and Physical Sciences, Victoria University of Wellington, Wellington, New Zealand
fSchool of Science, University of Waikato, Hamilton, New Zealand
First published on 6th February 2025
Abstract
Covering: January to the end of December 2023
This review covers the literature published in 2023 for marine natural products (MNPs), with 582 citations (541 for the period January to December 2023) referring to compounds isolated from marine microorganisms and phytoplankton, green, brown and red algae, sponges, cnidarians, bryozoans, molluscs, tunicates, echinoderms, the submerged parts of mangroves and other intertidal plants. The emphasis is on new compounds (1220 in 340 papers for 2023), together with the relevant biological activities, source organisms and country of origin. Pertinent reviews, biosynthetic studies, first syntheses, and syntheses that led to the revision of structures or stereochemistries, have been included. An analysis of the progress in the study of prokaryote involvement in macro-invertebrate MNP production is discussed.
1 Introduction
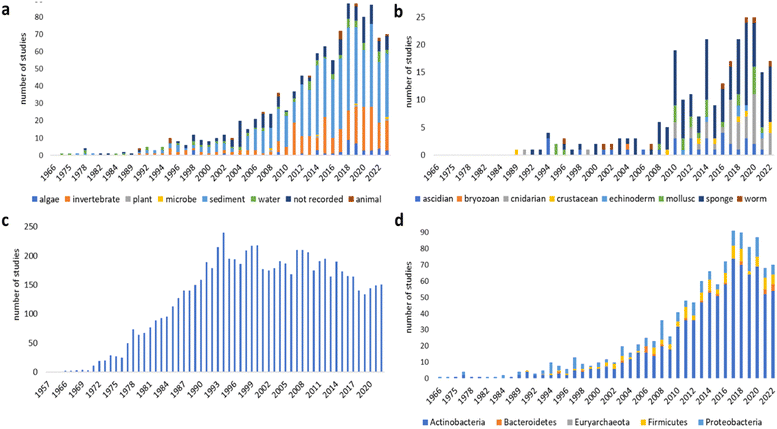
This review is of the literature for 2023 and describes 1220 new compounds from 340 papers, compared to 1417 new compounds in 384 papers reported for 2022.1 In addition, 25 known NPs were reported from a marine source for the first time, 9 artefacts were identified and 45 known MNPs had their structures revised. Only new MNP structures or previously reported compounds where there has been a structural revision, or a newly established stereochemistry are shown in this review. The review also covers previously reported MNPs with significant new bioactivities or ones that have been synthesised for the first time, but their structures are generally not shown. A † symbol on the identifying diagram number is used to distinguish structures where the absolute configuration has been determined for all stereogenic centres, axes and/or planes in a compound. Reports of new MNPs that were identified based solely on a combination of gene cluster information, MS/MS data and/or Global Natural Products Social (GNPS)-based molecular networking, with compounds not isolated and no NMR data recorded, are excluded from the review. Only a selection of highlighted structures (58) is shown in the review. Compound numbers for structures not highlighted in the review are italicised, and all structures are available for viewing, along with their names, taxonomic origins, collection locations, and biological activities, in an associated ESI document.† Access to the curated MNP data held in the Marinlit database2 provides all the structural and literature data used to prepare this review. The section reporting MNPs from mangrove-associated fungi that appeared in previous installments of this review has now been amalgamated into a general marine-sourced fungi section.Trends in the number of new MNPs reported annually over the semi-decade show a substantial drop in new MNPs reported from bacteria in 2023. A decreasing trend in reporting of cyanobacterial MNPs continues. New sponge metabolites are at a decadal low. The number of new compounds reported from the brown algae rebounded after an anomalous low in the previous year (Fig. 1).
 | ||
| Fig. 1 Trends in new MNPs. The bars represent the total number of new MNPs reported each year over the last five years. | ||
2 Marine microorganisms and phytoplankton
2.1 Marine-sourced bacteria
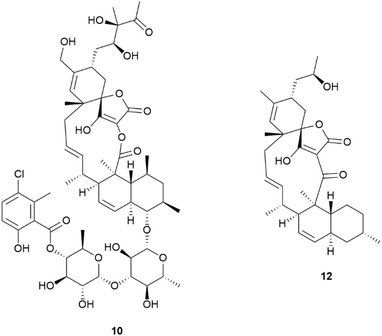
Actinobacteria were the most common source of bacterial MNPs with 94 new structures reported. A sponge-derived Actinoalloteichus cyanogriseus yielded three new cyclolipopeptides, cyanogripeptides A–C 1–3.3 Based on the annotations from the genome mining tool antiSMASH, the candidate biosynthetic gene cluster (BGC) cgpV was proposed to be responsible for the assembly of the compounds. A cyclic tetrapeptide, arthropeptide B 4 was isolated from Arthrobacter humicola sourced from composted material of marine origin,4 and a new diketopiperazine janibatide A 5 was reported from a deep-sea sediment-derived Janibacter sp.5 A new pyrroline, nocarpyrroline A 6 was reported from a krill-derived Nocardiopsis sp.,6 and two new furan derivatives, nicardifurans D 7 and E 8 were isolated from a sediment-sourced Nocardiopsis sp.7A coral-derived Micromonospora sp. yielded a new phenolic acid, 1-(6-methylsalicyloyl)glycerol 9, the absolute configuration of which was confirmed via total synthesis.8 Three new spirotetronate polyketides, phocoenamicins D 10 and E 11 and maklamicin B 12 were reported from a sediment-derived Micromonospora endophytica.9 When tested against a panel of human pathogens, only the aglycone 12 showed weak activity against methicillin-resistant S. aureus (MRSA), and M. tuberculosis, and moderate activity against E. faecium.
Pyrrolizine alkaloids, phenopyrrolizins A 13 and B 14 were isolated as racemates from a sediment-derived Micromonospora sp., and their structures confirmed by X-ray diffraction analysis (XRD).10 A series of new benzoxazole alkaloids, microechmycins A–F 15–20 were reported to be the products of a BGC mich originally found in Micromonospora sp. but here, heterologously expressed in the host Streptomyces albus.11 A large-scale, 70 L culture of Salinispora arenicola yielded three new rifamycin analogues, salinisporamycins C 21 and D 22, and salinifuran A 23.12
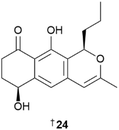
The genus Streptomyces continues to be the largest source of chemical novelty with 71 new MNPs reported in 2023. Prealnumycin B 24 was isolated from a polychaete-derived Streptomyces sundarbansensis.13 The putative BGC als was identified to be responsible for the biosynthesis of the compound and, when expressed in a heterologous host Streptomyces coelicolor, an additional new MNP phaeochromycin L 25 was detected and isolated.
Five new aromatic polyketides, RM18 C–G 26–32, were reported from a mangrove soil-derived Streptomyces sp.14 Compounds RM-18 E and F were isolated as racemates and separation by chiral HPLC allowed the absolute configurations of the enantiomers 28/29 and 30/31 to be assigned. Strategies involving one strain many conditions (OSMAC) and addition of epigenetic modifiers yielded five new aromatic polyketides 33–37 from a cnidarian-derived Streptomyces griseorubiginosus,15 and wailupemycins Q 38 and R 39 from a green alga-derived Streptomyces sp.16 A marine sediment-derived Streptomyces massiliensis yielded a new indanone derivative streptinone 40,17 and two new lactones 41, 42 were reported from a sediment-derived Streptomyces sp.18 Three new angucyclines, angumycinones E 43 and F 44, and kanglemycin E 45 were reported from a Streptomyces sp. engineered to overexpress the native global regulator cyclic AMP-receptor protein (Crp).19 A coral-derived Streptomyces sp. yielded naphthohydroquinones, iseoic acids A 46 and B 47 and a new naphthoquinone propanoic acid dimer bis-iseoate 48.20 Two new, ether-bridged C-glycosyl benz[a]anthracenes tandocyclinones A 49 and B 50 were isolated from a sediment-sourced Streptomyces sp.,21 and a cold-seep-derived Streptomyces olivaceus yielded four new linear ansamycin analogues olimycins E–H 51–54.22
Four new enediyne-derived, cycloaromatised polyketides, jejucarbosides B–E 55–58 were isolated from a sediment-sourced Streptomyces sp., with only 58 showing sub-micromolar cytotoxic activity against a panel of five human tumour cell lines (HTCLs), demonstrating that carbonate and methoxy functional groups are crucial for the activity of the series.23 Two new Streptomyces-sourced antimycin analogues have been reported, antimycin Q 59,24 and antimycin A2c 60.25 A hydrothermal vent sediment-derived Streptomyces sp. yielded two new linear polyketides kueishanamides A 61 and B 62 that exhibited weak antifungal activity against the pathogenic fungus Cryptococcus neoformans with no cytotoxicity against two HTCLs.26
Two new chlorinated, pyrrole-containing alkaloids, streptopyrroles B 63 and C 64 were reported from a sediment-derived Streptomyces zhaozhouensis.27 Both compounds showed moderate activities against Gram-positive bacteria but no activity against Gram-negative strains and only weak activity against a panel of six HTCLs. A new naphthyridine MNP, actinoquinazolinone 65 was isolated from a sediment-derived Streptomyces sp.,28 and a new 3-hydroxybutanoic acid-containing quinazolinone streptonaphthyridine A 66 was reported from a Streptomyces sp. strain derived from a sediment sample collected from a submarine canyon.29 Two new diphenazines, baraphenazine H 67 and izumiphenazine E 68 were reported from a sediment-derived Streptomyces sp.30 Structural and stereochemical elucidation of these proton-deficient compounds was aided by computationally-predicted NMR and ECD spectra and the established methodology was used to revise absolute configurations of three co-occurring known dimeric phenazines, namely phenazinolin D 69,31 izumiphenazine A 70,32 and baraphenazine G 71,33 which were also reported as MNPs for the first time.
Structures and absolute configurations of four new polyketide-peptide hybrid macrolide lactams, somalactams A–D 72, 73–75 isolated from an Arctic sponge-derived Streptomyces somaliensis, were unambiguously assigned by XRD.34 Somalactams A 72 and B 73 possess a novel hexahydro-2H-cyclopenta[b]furo[2,3-d]furan tricyclic ring system. This structural motif has also been reported in argenteolide A 76 isolated together with a simpler analogue argenteolide B 77 from a deep-sea sediment-derived Streptomyces argenteolus.35 Notably, while the planar structure of the macrocyclic ring is almost identical in 72 and 76, the assigned absolute configurations at multiple stereogenic centres are different. An additional new glycosylated macrolide lactam, haneummycin 78 was isolated from a sediment-derived Streptomyces sp.36 Two new 24- and 26-membered macrolactones, marinolides A 79 and B 80 were reported from a sponge-derived marine bacterium that was identified by partial 16S rDNA analysis to likely be a new genus within the Streptomycetaceae family.37 Recognising the challenges of assigning the structures and absolute configurations of complex macrolactones, the authors identified a 97 kb BGC mld to be responsible for the assembly of the compounds and used bioinformatic analyses of ketoreductase and enoylreductase domains within the BGC to predict the structures and configuration of 13 out of 16 stereogenic centres in 79 and 80. Full structural and stereochemical assignment of the two compounds was achieved through complete NMR analysis and XRD data and this matched the bioinformatic predictions.
Four new ribosomally synthesised and post-translationally modified peptides (RiPPs), cihunamides A–D 81, 82–84, were isolated from a volcanic island sediment-derived Streptomyces sp.38 The compounds possess a rare C–N crosslink between the two tryptophan units formed through oxidative coupling catalysed by cytochrome P450. The authors proposed a new naming classification for this RiPP family, the “bitryptides”, defined by a single biaryl linkage between two tryptophan units and canonical atropisomerism. A cyclic undecapeptide, streptnatamide A 85 was reported from a sponge-derived Streptomyces sp.39 Peptides such as 85 that contain non-canonical amino acids that can adopt different conformers in solution represent significant structural elucidation challenges by NMR due to insolubility or line broadening. To facilitate fast structural elucidation, the authors developed a MS-based structure confirmation tool using isotopic fine structure (IFS) analysis and an in-house MS2 analysis workflow. Other new peptide MNPs sourced from sediment-derived Streptomyces sp. included octadepsipeptides, quinomycins K 86 and L 87,40 and piperazic acid-containing decapaptides, lenziamides B1 89 and D1 88.41
Four new sesquiterpenoids, pentalenomycins A–C 90–92, and bolinane A 93 were reported from a sediment collection of Streptomyces qinglanensis,42 and four new geosmin- and germacrane-type sesquiterpenoids, odoripenoids A–D 94–97 were isolated from a sponge-derived Streptomyces sp.43
Five new MNPs were identified from the phylum Firmicutes. An intertidal mudflat sediment-derived Bacillus sp. yielded two new glycosylated macrolactin analogues succinyl glyco-oxydifficidin 98 and succinyl macrolactin O 99,44 while a cold-seep sediment-derived Bacillus sp. collection yielded a further new macrolactin analogue 6′-O-succinyl methyl ester macrolactin O 100, as well as two new hydroxy unsaturated fatty acids 101 and 102.45
Eight new MNPs were reported from the phylum Pseudomonadota. A coral-derived Microbulbifer sp. yielded a new ureidohexapeptide bulbiferamide 103, with a rare N-aminoacylated indole linkage between tryptophan and leucine residues.46 The same structure, referred to as bulbiferamide A, was also reported from a sponge-derived Microbulbifer sp., together with a related bulbiferamide analogue 104 where the terminal threonine unit was dehydrated to a dehydrobutyrine residue.47 The latter study identified a putative BGC bulb to be responsible for the assembly of the bulbiferamides. Another Microbulbifer sp. strain derived from the sponge Smenospongia aurea yielded four related linear analogues, the pseudobulbiferamides A–C 105, 106, 107 and a truncated shunt metabolite 108, and their BGC was identified and named mbp.48 Interestingly, the BGCs for both pseudobulbiferamides and the bulbiferamides were present in this strain, with the pseudobulbiferamide BGC mbp found to be plasmid encoded, while the bulbiferamide BGC bulb was chromosomally encoded. The strain was shown to be capable of producing both families of ureidopeptides, and mass spectrometry imaging showed they occupy different physical spaces within the colony when grown on solid media. A genome-mining strategy targeting BGCs with siderophore related genes identified a marine-derived Tistrella mobilis as a possible producer of siderophores, from which two new C-diazeniumdiolate-containing MNPs, tistrellabactins A 109 and B 110 were identified.49 Both compounds were found to coordinate Fe(III), but were also photoreactive upon exposure to UV light, releasing an equivalent of NO and H+ from the bacterial cells in the process.
As with previous years, a small number of MNPs published in the literature from marine bacteria did not have adequate spectrometric and spectroscopic data to support the proposed structures.50–54 Some MNPs reported in 2023 had structures proposed from mass spectrometric data, but without full NMR structural characterisation and were omitted from this review.47,48,55 Total synthesis of the reported structure of cahuitamycin A revealed significant inconsistencies in the 1H NMR spectroscopic data, putting the proposed structure in doubt.56 Other total syntheses of bacterial NPs included rac-abyssomicin 2 and rac-neoabyssomicin B,57 chejuenolides A–C,58rac-cyanogramide D,59 dixiamycins A and B,60 enhypyrazinone A,61 levesquamide,62 lysiformine,63 marinoququinoline A,64 mindapyrroles A and B,65 neaumycin B,66 (–)-nenestatin A,67 and sorangiolide A.68
Reviews focused on marine bacterial NPs published during 2023 included structures and biological activities of NPs reported from sponge-derived microorganisms,69,70 deep-sea sourced actinobacteria,71 phylum Bacillota,72 and the genus Pseudoalteromonas.73 Specific classes of bacteria-sourced MNPs reviewed included peptides with antimicrobial activity,74 biosynthesis and biological activities of Streptomyces-sourced lipopopeptides,75 and structures and biological activities of pederin-type polyketides.76 MNPs from marine-derived-bacteria with antibiotic and antibiofilm activities were reviewed,77,78 as were various strategies for the co-culture of marine-sourced bacteria.79
2.2 Cyanobacteria
The lower number of MNPs reported from cyanobacteria in 2023 also coincided with a decrease in the chemical diversity of new structures. Another general observation is an increase in the isolation of new NPs from mixed assemblages of cyanobacteria. A mixed cyanobacterial collection of predominantly Lyngbya and Dichothrix spp. yielded a new peptide–polyketide hybrid NP, iezoside B 111,80 while a South China Sea collection of Lyngbya sp. yielded two new aplysiatoxin analogues, neo-debromoaplysiatoxin I 112, and neo-debromoaplysiatoxin J 113.81 A new cyclic depsipeptide, alotamide B 114 was reported from a mixed assemblage comprised mostly of Moorena sp. (annotated as Moorena sp. in the manuscript)82 and a new cyclopropane-containing fatty acid derivative, benderadiene 115 was reported from a bloom forming assemblage of Lyngbya sp.83 Notably, there is still significant inconsistency in reporting of the correct naming of this genus with Moorena being the accepted genus name.Four new peptide glycosides, suomilides B–D 116–119 were isolated from a laboratory cultivation of Nostoc sp.84 Nostocyclophanes E–J 120–123, 124, 125, were minor cyclophane metabolites reported from a 240 L culture of Nostoc linckia with the compounds showing weak to moderate cytotoxicity against the breast epithelial adenocarcinoma MDA-MB-231 cell line with GI50 ranges from 0.72 to 8.2 μM.85
Akunolides A–D 126, 127–129, four new 16-membered macrolide glycosides bearing alkylated substitution at C-15, were isolated from the cyanobacterium Okeania sp.86 Akunolide A 126 possesses a rare terminal alkyne structure in the alkylated sidechain. The structure of 30-methyloscillatoxin reported in 2019,87 was revised to 7-epi-30-methyloscillatoxin D 130 following a comparison of the NMR data with that of synthesised analogues.88 Moreover, the taxonomic classification of the producing organism, initially assigned via morphological observations under light microscopy, was changed from Moorea producens to Okeania hirsuta based on 16S rRNA phylogenetic analysis. This taxonomic revision has implications for over 20 other new cyanobacterial MNPs in seven other articles reported from this collection (and obtained from a single extraction) over the last four years.87,89–94
Two new lipopeptides, okeaniamides A 131 and B 132 were isolated from a coastline collection of Okeania sp.95 The compounds showed no cytotoxicity at 10 μM but demonstrated an increase in adipocyte differentiation of 3T3-L1 pre-adypocyte cells in the presence of insulin when tested at concentrations of 5 and 10 μM. Finally, a black band disease-forming, filamentous cyanobacterium Roseofilum reptotaenium collected from the massive starlet coral Sidarastrea siderea yielded a new mixed polyketide/peptide 20-membered macrocycle, looekeyolide D 133.96
Reviews focused on marine cyanobacteria included summaries of various biological activities annotated for cyanobacterial MNPs including antifungal,97 antiviral,98 and cytotoxic activity against human cancer cell lines.99 Notable work on the biosynthesis of cyanobacterial MNPs has included identification and characterisation of the putative BGC lynB for the assembly of lyngbyapeptin B,100 a comprehensive review on the incorporation of fatty acids in the biosynthesis of cyanobacterial NPs,101 and a review on NPs from symbiotic cyanobacteria and their biosynthesis.102 Total syntheses of cyanobacterial MNPs included the polyketides (10E/Z)-trichotoxin A and dechlorotrichotoxin A103 as well as caldorazole104 and the peptides ikoamide,105 odookeanynes A and B,106 and a high yielding synthesis of gallinamide A which was achieved in nine steps of longest linear sequence and an overall yield of 32%.107 The large-scale synthesis of complex polyketides has been hampered by access to complex chiral building blocks. Access to the apratoxin A fragment, (2R,3R,5R,7R)-3,7-dihydroxy-2,5,8,8-tetramethylnonanoic acid, has been achieved through heterologous expression in the cyanobacterium Anabaena sp. PCC78120.108
2.3 Marine-sourced fungi
The sesquiterpenoid marinobazzanan 134 was isolated from an Acremonium species and shown to decrease cancer cell migration and invasion at nontoxic concentrations by downregulating transcription factors and modulating the expression level of other enzymes involved in cell motility and β-catenin expression. Additionally, 134 reduced the number of metastatic nodules in an intraperitoneal xenograft mouse model.109 Myrochromanol analogues 135–142 were obtained from a culture of Alfimbria verrucaria110 and an Alternaria species yielded territrem F 143, a boronic ester of the co-isolated drimane meroterpenoid territrem B, both of which were weak synchronous Ca2+ oscillation inhibitors.111 Culture of Amphicorda felina resulted in isolation of meroterpenoids 144–152.112,113Three Arthrinium strains isolated from mangrove sediments contained an oxime 153 and pyridyl derivative 154,114 four sesterterpenoids; arthproliferins A–D 155–158,115 and two tetrahydroisobenzofurans arthrinones A 159 and B 160, respectively.116 A further Arthrinium strain was the source of the pyridine alkaloids arthpyrones M–O 161–163,117 of which 161, inhibited growth and metastasis of gastric cancer in vivo via targeting a signalling pathway.118
The Aspergillus genus was once again a source of many new metabolites including the dimeric tetrahydroxanthones, aculeaxanthones A–E 164–168,119 and benzoic-acid containing alkaloids, 169–176.120 Production of a further alkaloid 177, by Aspergillus aculeatus, was induced via chemical epigenetic regulation with suberohydroxamic acid121 and culture of A. austwickii yielded polyketides 178 and 179, 2,3-dihydrobenzofuran derivative 180 and kojic acid derivative 181.122 Isocoumarin 182,123 quinazoline alkaloids felicarnezolines A–E 183–187,124 oxygenated chromene derivative 188,124 anthraquinone derivative 189 and 2-aminoprop-2-enoic acid derivative 190,125 were all obtained from various Aspergillus cultures. A. chevalieri was the source of indole diketopiperazine alkaloids 191–195,126 and nonadride 196,127 cyclohexanone derivative 197 and drimane sesquiterpenoids 198 and 199 were obtained from a seagrass-derived strain.128 Sediment-derived Aspergillus strains yielded indole alkaloid 200,129 thiodiketopiperazines 201–203,130 oxygen bridged phenolics 204 and 205 and dimeric isobenzofuran 206,131 whilst sponge-derived strains were the source of numerous indoloquinazoline alkaloids 207–227 and depsidone 228.132,133 Alkaloids were also obtained from several Aspergillus strains derived from various sources; sediment-derived A. noonimiae yielded indole diterpenoid glycosides noonindoles G–L 229–234,134 diketopiperazines 235–238 and 239–241 were obtained from coral-derived A. puniceus135 and sponge-derived A. sclerotiorum136 respectively. Culture of deep-sea-derived Aspergillus strains led to the isolation of cyclopentapeptides 242–248,137 bisabolane sesquiterpenoids 249 and 250–253 (the last four as racemates separated by chiral chromatography)138,139 and N-acyl adenosine derivative 254.140A. terreus strains were the source of chlorinated biphenyls 255–258,141 maleimides 259, 260 and butenolides 261 and 262,142 sesquiterpenoid 263 and nitrobenzene derivatives 264 and 265 (the last two known synthetic compounds but new NPs).143A. terreus strains also produced terrein derivatives 266 and 267, octahydrocoumarin derivative 268 and eurylene 269, (the last a known terrestrial NP but new MNP).144 Deep-sea-derived A. versicolor strains yielded pyrazinopyrimidine 270–273 and quinolinone 274, 275 alkaloids (the last two known synthetics but new NPs),145 diketopiperazine alkaloids 276–279, (276 and 278 not new but the absolute configuration as determined),146 macrolactone 280, quinazoline alkaloid 281 (ref. 147) and phenolic bisabolene sesquiterpenoids 282–292,148 while other strains of A. versicolor were the source of pyrroloindoline-containing cycloheptapeptide 293, (also synthesised from the co-isolated asperversiamide A),149 indole diketopiperazine alkaloids 294 and 295,150 dimeric citrinin derivatives 296–299, isochromene derivative 300 and acetamide 301.151 Indole alkaloids, including dimeric diketopiperazines (302–307)152,153 and indole diterpenoids 308–315,154 austalide derivative 316,155 ophiobolin sesterterpenoid 317 and drimane sesquiterpenoids 318–322,156 phenolic bisabolane 323,157 benzofuran derivative 324,158 glyoxylate-containing benzene derivative 325,159 unsaturated fatty acid 326,160 azaspirenes 327–331,161 in addition to the p-terphenyls, asperterphenyls A–N 332–352, were also obtained from various Aspergillus strains.162
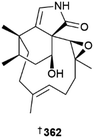
Asteromyces cruciatus was the source of anthraquinone derivatives 353–355,163 an integrated genomics and metabolomics approach was utilised to isolate cyclopeptides 356–361 from Beauveria felina,164 and a combination of metabolomics, chemometrics and traditional NP techniques resulted in isolation of phomactinine 362, the first nitrogen containing phomactin, from a Biatriospora strain.165 Chlorinated azaphilones 363–365 were isolated from strains of Chaetomium globosum166,167 and diterpenoids 366 and 367, sesquiterpenoids 368 and 369 and ecdysteroid 370 were all obtained from Cladosporium oxysporum.168 Synthesis of all possible enantiomers of the indole alkaloids colletotrichindoles A–E, 371–377, was utilised in their structure determination. These, along with further indole alkaloids 378–382, were obtained from a culture of Colletotrichum gloeosporioides,169 while α-pyrone derivatives 383 and 384 were isolated from a Curvularia strain170 and 4a-O-methoxyarugosin H 385 was isolated from Emericella nidulans but may be an artefact resulting from the use of MeOH in the isolation procedure.171
Various sediment-derived fungal strains have yielded a range of compounds. Emericellopsis maritima was the source of eremophilane sesquiterpenoids 386–389,172 a chlorogentisyl alcohol derivative 390 was isolated from Epicoccum sorghinum,173Eutypella strains yielded prenylated dihydroisocoumarin 391 and chromene amide derivative 392,174 pimarane diterpenoids 393–395 and cytosporin derivatives 396–402,175,176 epipolthiodioxopiperazines, graphiumins K–N 403–406 were obtained from Exophiala mesophila,177 two anthraquinones 407 and 408 were obtained from a mangrove sediment-derived Fusarium sp.178 and culture of F. solani led to isolation of the polyketides, fusarisolins F–K 409–414 and another polyketide 415.179 This last compound was named fusarin I but this name has already been used for another compound isolated from F. solani previously.180 Culture of Hamigera avellanea strains yielded pentaketides 416–419 and p-hydroxyphenyl-2-pyridone derivative 420,181 and enantiomeric alkaloids 421 and 422 (resolved by chiral chromatography).182 Steroidal lactone 423 was obtained from a deep-sea mussel-derived Hypocrea strain,183 thioketopiperazines, lecanicilliums A–G 424–429 were reported from Lecanicillium kalimantanense isolated from mangrove sediment184 and supplementation of growth media with amino acids led to isolation of alkaloids 430–432 and sterol 433, (the last two are new MNPs but are a known synthetic product and plant metabolite, respectively).185 A seawater-derived Meira strain was the source of thiolactones 434 and 435 and steroids 436 and 437. Thiolactone 435 is a known terrestrial fungal metabolite but isolated here as a new MNP and the absolute configuration was revised from 3R,4S to 3R,4R.186 A Metarhizium strain, also derived from seawater, yielded α-pyrone glycosides 438–440 and phenolic glycoside 441.187 The chlorinated benzopyrone 442, and two known terrestrial (but new to marine) NPs, a dichloropyrol-2,5-dione 443 and meroterpenoid 444 were reported from mangrove sediment-derived Mollisia sp.188 Fungal cultures derived from sediment were the source of a number of metabolites; cultures of Paraconiothyrium sporulosum yielded eremophilane 445–451 and santalane sesquiterpenoids 452–454,189,190 and isobenzofuranones 455 and 456,190 whilst Paraphoma radicina was the source of isobenzofuranone 457 and polyketide amino acid hybrid 458.191
The Penicillium genus has always been extensively studied as a source of new metabolites but this year, there were even more studies reported on this genus than on Aspergillus. Cultures of Penicillium antarcticum yielded β-resorcylic acid derivatives 459–463,192 cyclopiane diterpenoids 464 and 465 and pentaketide derivative 466,193 seawater-derived cultures of P. chrysogenum were the source of cerebroside A aglycone 467 and tyrosine derivative 468,194,195 sediment-derived P. citrinum strains yielded indole diterpenoid 469,196 polyketides 470–472 and three pairs of C-9 verrucosidin epimers 473–478,197,198 (the first two pairs revising C-6 configuration from 6S to 6R), while P. citrinum strains sourced from crustaceans were the source of alkaloid 479 and unusual hirsutellone analogues 480 and 481–486.199,200 Of these, perpyrrospirone A 480 consists of an unprecedented 6/5/6/8/5/13/6 oxahexacyclic scaffold with a peroxide-bridged 8,9-dioxa-2-azaspiro[4,7]dodecane core. Indole diketopiperazine alkaloid 487, obtained from fermentation of P. dimorphosporum, resensitised drug-resistant prostate cancer cells to the antiandrogen drug enzalutamide through specific downregulation of the androgen receptor without associated toxicity.201
Polycyclic alkaloid communesin M 488, was isolated from a culture of P. expansum and synthesised from co-isolated communesin A.202 Co-culture of P. janthinellum with Paecilomyces formosus (both collected from the same source) led to isolation of nine indole diterpenoids, janthinellumines A–I 489–497,203 meroterpenoid 498 was obtained from axenic culture of Penicillium ochrochloron and on co-cultivation with the bacterium Bacillus subtilis, the known synthetic compound but new NP, ochrocholonic acid 499 was produced.204 Sesterterpenoid 500 was obtained from P. oxalicum,205 as was phenalenone derivative 501.206 Verrucosidin derivatives, poloncosidins G–K 502–506 were isolated from cold-seep sediment-derived P. polonicum,207 β-carboline 507–509 and 2-quinolinone alkaloids 510 and 511 were obtained from P. raistrichii;208 deep-sea coral-derived P. rubens was the source of polyketide 512, sesquiterpenoid 513 and steroid 514, the last of which exhibited potent activity against E. coli and Vibrio parahaemolyticus.209,210 Co-culture of a natural complex/association of sea urchin-derived P. sajarovii and Aspergillus protuberus led to production of polyketides 515 and 516;211 seven meroterpenoids 517–523 and a range of azaphilones were also obtained from P. sclerotiorum.212,213 Of these, 524–527 were obtained from a red alga-derived strain by Taiwanese researchers, with 524 and 525 designated penicilazaphilones H and I, however, the name penicilazaphilone H had already been very recently designated to a brominated analogue by Chinese researchers and this same group isolated further azaphilones 528–534 from a sponge-derived strain, including 524 and 525 (here named penicilazaphilones L and K respectively).214 Very close timing/overlap in the publication process of these reports has led to this confusion; 524 and 525 were reported first but should be renamed. Green algal-derived P. stecki cultures yielded tanzawaic acid 535–541 and benzene 542 derivatives,215 in addition to fusarin derivatives 543–547.216 Deep-sea sediment-derived Penicillium strains yielded sulfated isonitrile, sulfoxanthicillin 548 and xanthones 549 and 550,217,218 whilst other sediment-derived strains were the source of citrinin derivatives 551–559,219 polyketides 560 and 561,220 indole alkaloid 562,221 eremophilane sesquiterpenoid 563 and meroterpenoids 564 and 565.222,223 A soft coral-derived strain yielded the linear peptides, penicamides A 566 and B 567 and alkaloids 568 and 569 and butenolide 570 were obtained from a sponge-derived Penicillium strain.224,225
Two studies on a Penicillium species isolated from the roots of the Chinese mangrove Lumnitzera litorea furnished 15 rearranged merosesquiterpenoids, littoreanoids A–O 571–585, one (573) possessing a rare oxetan-2-one ring226 and nine related merosesquiterpenoids, peniciacetals A–I 586–594.227
Other MNPs isolated from mangrove-derived Penicillium spp. were meromonoterpenoids cyclohexenoneterpenes A–J 595–604,228 sesquiterpenoid 605,229 diterpenoids 606 and 607, merosesquiterpenoid 608, oxime 609, carboxylic acid 610, stilbene 611 (known, but absolute configuration now determined), phenol 612 and indoloditerpenoids 613–616.230,231
Polyketides 617–623, were reported from culture of a Peroneutypa strain, the latter being a known terrestrial NP but new MNP and with absolute configuration determined for the first time.232 Another compound was claimed as new but the structure subsequently corrected to that of known NP daidzein.233 Farnesyl hydroquinones 624 and 625 were obtained from culture of Pestalotiopsis diploclisia,234 phaeosphaerins A–E 626–630 are isocoumarins isolated from a Phaeosphaeriopsis strain,235 a Phoma strain was the source of polyketides and a sesquiterpenoid, 631–633,236 and a mangrove sediment-derived Phomopsis sp. contained isocoumarins 634–636, and an α-pyrone 637.237 Tetracyclic steroids 638–643 were obtained from a Rhizopus strain,238 culture of Samsoniella hepiali led to isolation of aminated fusaric acid derivatives 644–646 and PKS-NRPS derived polyketide 647,239 a Spiromastix strain yielded chlorinated diphenyl ethers 648–650 and cyclopentanone 651,240 phenyl spirodrimanes 652–656 (with 655 being a known semi-synthetic product but new NP) were isolated from culture of Stachybotrys strains241,242 and cyclopropane derivatives 657, 658 and α-pyrones 659–665 were obtained from a Stagonospora strain.243 In the course of the structural determination of the latter α-pyrones, computational chemistry and NMR analyses suggested that the structures of the terrestrial plant metabolites, chenopodalans A–F should be revised from furopyrans to α-pyrones.243Talaromyces strains were the source of a number of polyketides, including azaphilone derivatives 666 and 667, (C-8 epimers) 668–670,244 nonadride derivatives talarodrides G 671 and H 672 and depsidone derivative botryorhodine K, 673,245 and the spirocyclic talaromyacins A–C 674–676.246 Dibenzodioxepinones 677–680, diphenyl ether 681, benzopyran 682, benzophenones 683 and 684,247 maleic anhydrides 685 and 686, and three simple isoprenyl phenyl ethers 687–689,248 were obtained from mangrove sediment-derived Talaromyces species. The glucosidic polyketides, talaminiosides A–C 690–692, enantiomers 693 and 694, (resolved by chiral chromatography) and azaphilones 695 and 696 were isolated from a culture of T. minioluteus.249T. pinophilus yielded a range of metabolites including known terrestrial fungal metabolites bacillisporins A 697 and B 698 as new MNPs, hybrid phenalenone dimer talaropinophilone 699, azaphilone 700, phthalide dimer 701, steroid 702 and 1-deoxyrubralactone 703, the configuration of which was revised to 11S.250 The structure of talarolide A was revised to 704 after its isolation from a Talaromyces strain along with three analogues, talarolides B–D 705–707. Talarolide B 705 was prepared via solid phase peptide synthesis.251 Heterologous expression of a silent BGC from a Talaromyces strain in Aspergillus nidulans led to isolation of labdane diterpenoid derivatives, talarobicins A–E 708–712, with three P450 enzymes determined to catalyse multi-step reactions in the biosynthetic pathway.252 Culture of a Tolypocladium strain resulted in the isolation of lipopeptaibols, tolypocaibols A 713 and B 714,253 whilst phomalone derivatives, tricholichenones A–D 715–718, were obtained from deep-sea sediment-derived Trichobotrys effusa.254Trichoderma strains derived from red or brown algae were the source of numerous metabolites including bisabolene sesquiterpenoids 719–722, cyclopentene 723 and cyclopentenone 724, derivatives,255 γ-lactone trichonafurin A 725,256 sorbicillinoid derivative 726,257 carotane sesquiterpenoid, trichocarotin N 727,258 harziane diterpenoid, harziaketal A 728 and sterol, trichosterol A 729,259 hydroxylated lipids, trichoderols B–G 730–735,260 and cyclopentenone 736 and wickerol 737 derivatives.261 Further Trichoderma strains yielded sesquiterpenoid (738–743) and diterpenoid (744 and 745) aminoglycosides,262 and the peptaibols, trichorzins A–G 746–752.263
Computational methods were used to show that the structure assigned to janthinolide A obtained from coral-derived Penicillium janthinellum is incorrect and that the compound isolated was actually the known terrestrial NP, janthinolide C 753.264 The isolation was the first report of janthinolide C from the marine environment however.264 The absolute configuration of vismione E, previously obtained from a sponge-derived Aspergillus strain, was established as 754.265
Total synthesis of aflaquinone I was achieved by two parallel strategies in nine and four steps respectively,266 and a divergent synthetic strategy was employed to synthesise conidiogenones E, F and 12β-hydroxyconidiogenone C.267 Chemoenyzymatic synthesis of 13-oxoveeruculogen was achieved in ten steps from commercially available materials,268 gram scale preparation of a key intermediate facilitated the successful synthesis of tanzawaic acid B269 and syntheses of marilines B and C were accomplished utilising a multicomponent reaction method.270 Syntheses of colletopeptide A and colletotrichamide A were achieved via a cyclic tridepsipeptide derivative as a key intermediate271 and varioxiranols B and C were prepared by convergent strategies.272
Penicopeptide A was shown to promote osteoblast-related bone formation, indicating its potential in osteoporosis prevention,273 pimarane diterpenoid scoparasin B was shown to inhibit angiogenesis, vascular mimicry and tumour growth,274 naphtho-γ-pyrone aurasperone F inhibited amyloid-β (Aβ) aggregation and exhibited a protective effect against Aβ toxicity so could be useful in treatment of Alzheimer's disease275 and pretrichodermamide B inhibited the transcription activator STAT3 in vivo, also promoting cell cycle arrest and apoptosis.276
Gene deletion, heterologous expression, and biochemical characterisation were utilised to demonstrate that a unique fungal P450 enzyme, CtdY catalyses amide bond cleavage in the 2,5-diazabicyclo[2.2.2]octane system and subsequent decarboxylation to form the 6/5/5/6/6 pentacyclic ring system in (21R)-citrinadin A. Seven enzymes were implicated in subsequent post-translational modification to produce the metabolite.277 The biosynthetic pathway to the dipeptide (+)-azonazine was reconstituted using four enzymes and the study revealed that the route to the benzofuranoindoline core occurs via an oxidative coupling reaction catalysed by the P450 enzyme AznC.278 The BGC responsible for biosynthesis of (−)-protubonine B was identified via heterologous expression, gene deletion experiments and isolation of subsequently accumulated products.279 Techniques utilised for activation of silent BGCs in fungi such as epigenetic regulation, co-culture, precursor feeding, heterologous expression and altering fermentation conditions were reviewed.280
2.4 Dinoflagellates
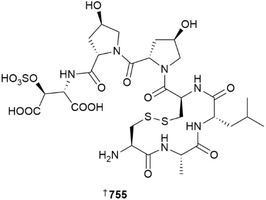
The number of reported dinoflagellate and diatom derived MNPs continues to decline.1 A novel peptide 755 from Seminavis robusta, obtained from a culture collection, is the first sex-inducing pheromone of diatoms known. Compound 755 induces production of a diproline diketopiperazine dimer as an attraction pheromone at doses estimated to be in the low fM range. Assuming the peptide is chemically stable, each S. robusta cell produces ∼150–400 amol of 755 within five days, leading to nanomolar concentrations at a cell density of 20 k cm−2. Stable isotope incorporation (13C/15N) during culturing was crucial to providing material suitable for NMR-based structural studies.281A new ergosterol derivative 756 has been obtained from a Vietnamese Thraustochytrium pachydermum, although it was inactive against seven bacterial strains.282 As is commonplace, new azaspiracid and gambierone congeners were proposed based solely on mass spectrometric studies and so are not definitively assigned, therefore, these structures are not shown here.283,284 The first total synthesis of amphidinolide S has been achieved using allyl alcohol as a key acrolein equivalent. The same study also completed the syntheses of amphidinolides J and R in half the number of steps previously needed.285 A “two-phase” synthetic approach has resulted in the total synthesis of portimine B 757, resulting in its structural revision, and has also provided portimine A in amounts suitable to probe its molecular target using photoaffinity labelling studies; portimine A targets the 60S ribosomal exporter NMD3.286 The reported structure of prorocentin, and its subsequent revised form 758, have been synthesised for the first time.287 The relative configuration of the C-61 to C-83 segment of the super carbon chain compound symbiodinolide 759 has been established by synthesis.288
A review of the low molecular weight carbohydrate-derived MNPs of microalgae has been published,289 as has a summary of the synthesis and mechanisms of action of the amphidinolides.290 The genus Amphidinium is one of the most prolific producers of toxic metabolites known. Rather than focusing on their biotoxins, the antimicrobial, anticancer and antifungal properties of Amphidinium-derived metabolites have been examined.291 The role microalgal compounds may play as biopesticides has also been reviewed.292
The mechanism of action of okadaic acid (OA) continues to intrigue researchers. OA downregulates the metabolism of xenobiotics in the liver by activating NF-κB signalling that stimulates the release of various interleukins, with downstream activation of JAK (Janus Kinase)-signalling.293 Domoic acid (DA) is a potent neurotoxin produced by diatoms of the genus Pseudonitzschia. A recent study has shown that even trace amounts of DA can alter the make-up and biodiversity of marine protists, altering the ecosystems they inhabit at a functional level, largely driven by alteration of phototroph composition and subsequent downstream effects.294 In a different study, Desulfovibrio and Clostridiales bacteria have been shown to metabolise DA via a novel reductive biotransformation pathway.295
A new method to perform metabolite fingerprinting using magic angle spinning solid-state NMR spectroscopy has been applied to study biofuels and nutritional components from three microalgal species.296 Biotoxins produced by harmful algal blooms (HABs) continue to plague communities around the world through contamination of shellfish food stocks. SoundToxins is a new research and monitoring partnership in Puget Sound, Washington State, established to help monitor HABs in the area. Over 30 partner organisations including indigenous peoples, local citizens and aquaculture fisheries, use real-time monitoring of their local areas to help provide critical information in a timely fashion to regulators and testing agencies.297
3 Green algae
A species of Bryopsis (Mie Province, Japan) was the source of new kahalalide congeners Z3760 and Z4761, both of which are weakly cytotoxic against murine fibroblasts. To support their assignment of absolute configuration using standard Marfey's approaches, the authors also analysed the metagenomic DNA from the Bryopsis specimen to determine a genetic reasoning for the differences in amino acid configuration between the new compounds and earlier members of the class. The presence of a key E-domain was found to control the configuration of the kahalalide products, based upon A-domain specificities. In addition, the authors identified a suitable BGC from symbiotic bacteria belonging to a new taxon Candidatus Endrobryopsis kahalalidefaciens. They related kahalalide biosynthesis to the presence of different strains. Notably, the lack of production of both kahalalide F and Z within a single strain implies that different strains have evolved independently without genetic crossing.298 Other peptide-based MNPs were reported from a green alga but the structures were identified only using mass spectrometry and therefore are not shown here.299The synthesis of a weakly cytotoxic, 22-amino acid linear peptide from B. plumosa has been achieved,300 as has a revised total synthesis of indolocarbazole racemosin B in two steps with over 50% yield.301In vivo testing of acetylated carotenoid siphonein, isolated from the edible green algae Caulerpa lentillifera and Codium fragile, has shown that it is absorbed in the gut (mouse model) with minimal degradation whilst still exhibiting anti-inflammatory effects by inhibiting production of pro-inflammatory cytokines.302 Whole genome sequencing of Chrysophaeum taylorii, which has been linked to coastal algal blooms, has revealed that the alga has an extensive suite of BGCs alongside a small microbiome with limited biosynthetic potential.
4 Brown algae
It is surprising that all of the new compounds reported from brown algae were exclusively terpenoid in origin. There were two reports of macrocarquinoid meroditerpenoids from Sargassum macrocarpum, one from a Japanese specimen (762 and 763) and the other sourced from Korea (764–767), respectively.303,304 A new nor-meroterpenoid sargasilol A 768 and eight related compounds, sargassilols B–I 769–776 were reported from S. siliquastrum.305 Five new xenicane meroditerpenoids, including rare lactams 777, 778, and 779, a butanone 780 and a more standard structure 781, were isolated from a Chinese Dictyota coriacea; all exhibited weak anti-oxidant activity with three as likely artefacts from extraction with EtOH.306 A Chinese (Hainan Province) collection of Sargassum polycystum yielded an unusual spiro-cyclic sesquiterpenoid 782 of the spheciospongone series.307 A series of new diterpenoids from different biosynthetic classes 783–790 with varying levels of ability to inhibit NO production were obtained from the invasive alga Rugulopteryx okamurae sourced from Punta Carnero, Spain.308Further structures were claimed in another publication, but one of the authors of this review (RAK) has independently raised concerns about the spectroscopic data to the publishing journal.309,310
A review of the diterpenoid metabolites of Dictyota and Canistrocarpus algae sourced from Brazil has been published,311 as has a summary of Ochrophyta compounds with potential to treat neurodegenerative diseases.312 Two reviews focusing on Sargassum have been published; one is a review of antioxidant metabolites sourced from the genus,313 while the other focuses specifically on the bioactivities and active principles of the edible alga S. fusiforme.314 As noted above, R. okamurae is a member of an invasive genus of algae that has spread from native waters in Asia through to Europe. A review of R. okamurae and potential applications of it as an economic resource during efforts to control its spread has been published.315 Bioinformatic analysis has suggested that a series of non-canonical PKS genes from the host alga are responsible for the biosynthesis of the antibiotic, macrocyclic halogenated ether, chrysopaentin A.316
5 Red algae
New C15-acetogenins were reported from Laurencia species collected in Japan (791) and Egypt (792–800), respectively.317,318 Chlorobenzoate solieriate 801 was isolated from a Solieria species from Zhangjiang City, Guangdong Province, China,319 while a Laurencia species from the same geographical area yielded halogenated laurenhalogens A 802 and B 803, respectively.320 A tribrominated diphenyl methane 804 was obtained from Symphyocladia latiuscula, although the metabolite was inactive as an antioxidant.321 A series of new mycosporine-like amino acids (MAAs) were isolated from two intertidal species, Bostrychia scorpioides (805–810) and Catenella caespitosa (811, 812), respectively, both collected from France (Brittany). Such MAAs help protect the producing alga from UV damage.322The Rhodophyta are well known for their repertoire of polyhalogenated monoterpenoids. Portieria hornemannii, collected at the Penghu (Pescadore) Is., was the source of three halogenated linear monoterpenoids and while 813 was a weak inhibitor of TNF-α expression, congeners 814 and 815 were inactive, hinting at a potential SAR.323 Two brominated aplysin derivatives 816 and 817 showed activity against settling of the mussel Mytilus galloprovinciallis at 0.16 μmol cm−2 so may be antifouling leads.324
Two separate collections of Fijian Peysonnelia spp. have resulted in the isolation of sulfated triterpenoid glycosides peyssobaricanosides A–C 818, 819–820. Genetically and otherwise chemically similar Peysonnelia samples from the Solomon Is., collected from similar geological locations, did not produce these metabolites, indicating population-level differences in metabolite profiles. Cryo-electron microscopy-based micro-crystal electron diffraction (microED) was used to establish the absolute configuration of 819.325
Red algal metabolites that have been synthesised include a butyl dibromobenzoate,326 dibromoindole glossobalol,327 and histidine-derived alkaloid colensolide A.328 A survey of the chemistry and bioactivity of metabolites from genus Gelidium has been published.329 Both enantiomers of elatol have been assessed for activity against the primary amoebic meningoencephalitis-causing Naegleria fowleri; (+)-elatol showed weak to moderate activity against two strains of the amoeba while the (−)-enantiomer was inactive.330 Laurequinone (Laurencia johnstonii) shows weak to moderate anti-leishmanicidal activity against the promastigote form of Leishmania amazonensis and seems to cause apoptosis of the parasite.331 Analysis of seasonal variation of metabolites of Jania rubens (Israeli Mediterranean coast) coupled with bioactivity against non-small cell lung cancer has highlighted essential fatty acid eicosapentaenoic acid as the main driver of activity.332 Polyhalogenated carbazoles are increasingly detected in the environment and are products of both human and natural sources. A study of the bromoperoxidase reaction has determined the regiospecificity of halogenation of carbazole and has subsequently detected the products in algal samples from the South China Sea.333
6 Sponges
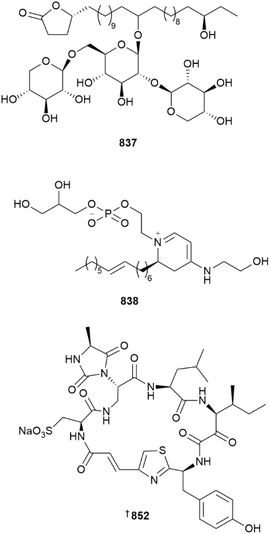
A series of sixteen new phytoceramides 821–836 were obtained from Monanchora clathrata collected in Western Australia.334Assimiloside A 837 is an unusual, branched glycolipid lactone isolated from a dredged (160 m) sample of Hymeniacidon assimilis collected from the Urup Is. The structure of 837 was determined using a combination of spectroscopic, computational and degradative studies, although the configuration at C-16 remains unresolved. Assimiloside A stimulated both ROS production and lysosomal activity in RAW 264.7 cells at non-toxic concentrations between 0.01 to 10 μM, making it a new immunomodulatory lead.335
A specimen of Clathria faviformis yielded a dihydropyridinium-containing lipid, favilipid A 838. This lipid contains a chromophore and hence is UV-active, which is unusual for the class. The dihydropyridinium core exhibited slow protium–deuterium exchange of carbon-bound hydrogen nuclei when stored in CD3OD NMR solvent. In addition, 838 is a weak inhibitor of five of 24 kinases, three of which are involved in immune system regulation.336
Two pairs of enantiomeric butenolide lipids 839/840 and 841/842 were obtained from a Chinese Suberites sponge.337 Both the first isolation and total synthesis of adamantane-like arsenicin D 843 were achieved,338 while a new valine-containing formamide 844, baeriamide, was obtained from a Haliclona baeri.339 SAR analysis of two new onnamide congeners 845, and 846, which are weak to moderately cytotoxic to mammalian cell lines, revealed the importance of the sidechain alkene geometry for bioactivity.340 The genus Phakellia remains a rich source of new peptides, with phakellisin A–E 847–851 being isolated from a Chinese sample; note the names given to these compounds are very similar to other Phakellia-derived metabolites (for example the pyrrole imidazole alkaloid phakellin) from different biosynthetic classes and readers should exercise care to not confuse them.341
An Australian (Coral Sea, Far North Queensland) Theonella species gave six new cyclotheonellazoles 852, 853–857. All incorporate non-proteogenic amino acids including the key protease transition state mimic, 3-amino-4-methyl-2-oxohexanoic acid. It is therefore logical that all six isolates are potent (IC50 16–61 nM) inhibitors of mammalian elastase but at non-toxic concentrations (IC50 > 100 μM vs. 3 HTCLs).342
Somewhat surprisingly, there was only one report of new sponge-derived macrolides in 2023, that of four new enigmazole congeners 858–861 from a Papua New Guinean Cinachyrella enigmatica.343 Two new brominated diphenyl ethers 862 and 863 came from a Dysidea fragilis from Mozambique.344
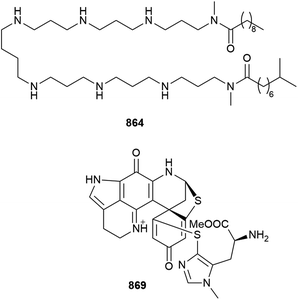
Aaptolobamine A 864 is a polyamine from Aaptos lobata. Analysis of mass spectrometric fragmentation data was key to assigning the structure of this metabolite, and also highlighted the presence of other homologues in the sponge extract. The purified compound showed a broad range of activities against cancer cell lines, bacterial strains, and the mixture of homologues also inhibited α-synuclein aggregation.345
The isolation and total synthesis of 2-piperidone alkaloid dysidone A 865 has been reported,346 while two new naphthyridine isomers 866 and 867 came from an Aaptos sponge collected at the Xisha Is., China.347 Geobarrettin D 868 is a bromoindole alkaloid containing the rare herbipoline motif.348
A semisynthetic approach has been used to assess SAR within the discorhabdin class of aromatic alkaloids. Three new metabolites of the class (869, 870, 871) have been isolated although 869 is likely an artefact of methanolic extraction. The effects of structural modification upon the bioactivity within the discorhabdin B, C and L series against Merkel cell carcinoma have been assessed. The presence of sulfur on the E-ring, as well as a protonated imine B-ring, are important for potent activity.349
A partnership between the United States of America National Institute of Allergy and Infectious Disease and the National Cancer Institute (NCI) to screen the NCI's collection of over 326![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 extract fractions for antimicrobial activity has led to the isolation of two new amphimedine alkaloids from a Malaysian Petrosia sponge. Both 2-bromo- and 3-bromo-deoxyamphimedine 872, 873 are moderate to potent antimicrobial agents with activity against five of nine ESKAPE pathogens. The screening campaign resulted in ∼3000 leads from the prefractionated library, representing ∼1% hit-rate.350
000 extract fractions for antimicrobial activity has led to the isolation of two new amphimedine alkaloids from a Malaysian Petrosia sponge. Both 2-bromo- and 3-bromo-deoxyamphimedine 872, 873 are moderate to potent antimicrobial agents with activity against five of nine ESKAPE pathogens. The screening campaign resulted in ∼3000 leads from the prefractionated library, representing ∼1% hit-rate.350
A dredged (97 m) Isabela sponge from Zuytdorp, Western Australia, was the source of three new porphyrin metabolites isabellins A 874 and B 875, and Fe3+-containing isabellihemin A 876. Heavily reduced porphyrin 874 is potently active against two HTCLs while the other isolates were inactive. Other related Fe-complexes were also detected by LCMS but were not isolated and fully characterised due to paramagnetic effects of the bound metal. NMR characterisation of the compounds required extensive analysis of NOESY correlations.351
Terpenoid MNPs continue to dominate the new metabolites reported from Porifera. A seco-meroterpenoid, dysambiol 877, is moderately potent as an anti-inflammatory agent but is non-toxic against RAW 264.7 cells at concentrations up to 20 μM.352 Ten new merosesquiterpenoids, pseudoceranoids A–J 878–887 were isolated from a Xisha Is. (China) sample of Pseudoceratina purpurea,353 while merosesquiterpenoid dimer thorectidiol A 888, isolated as a racemate from a Papua New Guinean Dactylospongia elegans, is a moderate inhibitor of the SARS-CoV-2 spike protein receptor-binding domain interaction with the host ACE2 receptor.354 Meroditerpenoid alkaloids 889–891, along with the first time isolation of core adenine derivative 892, were reported from a Taiwanese Agelas nakamurai.355 Five strongylophorine-class metabolites 893–897 came from a Solomon Is. collection of Petrosia sp. It is likely 893 and 894 are artefacts from 895 and 896, respectively.356 Linear (898–904) and cyclic (905) sesquiterpenoids have been reported from the Aaptos and Cliona genera, respectively.357,358
The conscinoderines are a series of unusual pyridinium-containing terpenoid-based alkaloids. Conscioderines A–J 906, 907–915 were obtained from Coscinoderma bakusi collected at Fannuk Is., Chuuk (Federated States of Micronesia). The conscioderines possess a rare 1,2,5-trisubstituted pyridinium motif. Alkaloids are very uncommon metabolites from this genus.359
Diterpenoids from sponges reported include kalihiacyloxyamides A–H 916–923 from Acanthella cavernosa,360 and spongian-class metabolites 924 and 925 from Spongia officinalis and 926 and 927 from Dendrilla sp. respectively.361,362 A series of di- and sesterterpenoids 928–935 were isolated from a Chinese Sarcotragus specimen collected from the South China Sea.363 The suberitienones 936–944, obtained from an Antarctic collection of Suberites, are sesterterpenoids with a new carbon skeleton.364 A large number of scalarane-class metabolites were reported from Lendenfeldia (945–948), Hyrtios (949–954), and Phyllospongia (955–974) specimens, all of which were collected in the waters surrounding China and Taiwan.365–370 Rhabdastrellosides A 975 and B 976 are new isomalabaricane triterpenoids from Rhabdastrella globostellata,371 while a Papua New Guinean Melophus sarasinorum yielded seven new triterpenoids (977–983) although none were found to be bioactive.372 Surprisingly, no steroids were reported from sponges in 2023.
The published structures of peptide solomonamide B and alkaloid 1-(1-H-indol-3-yloxy)propan-2-ol have been synthesised but are spectroscopically different from the NP suggesting that their structures should be revised.373,374 Sponge NPs that have been synthesised for the first time are enigmazole B,375 lissodendoric acid A,376 which was also the subject of a review,377 and njaoamine C which also established the absolute configuration 984 as shown.378 Pyrroloiminoquinone NPs discorhabdin H, K and V and related compound aleutianamine have been synthesised by two independent groups.379–381 Alkaloids (−)-chelonin A,382 naamidine J,383 nagelamide W,384 longamide F, agelasines A and B, and nakamurine B,385 respectively, have also been synthesised for the first time. Although the total synthesis of (−)-agelastatin A has already been achieved, a recent development of a flow-based photorearrangement to generate the central core has resulted in a scalable route to gram-level production of the compound with the use of only a single protecting group.386 The total syntheses of merosesquiterpenoids dysiherbol B, D and E,387 diterpenoids dysidealactams E and F, dysidealactone B,388 mycaperoxide B, C, D, and G methyl ester,389 and hamigerans C, I and debromo-I,390 were also reported.
Notable sponge-related reviews include a summary of the development of the anti-proliferative polyketide plocabulin,391 macrolide neopeltolide,392 and the synthesis and bioactivity of fascaplysin and the aplysinopsins respectively.393,394 Summaries of the cytotoxicity and anti-inflammatory activities of the nortopsentins,395 and of the general chemistry of dimeric pyrrole–imidazole alkaloids,396 and of sponge sterol and triterpenoid glycosides,397 have also been published. Taxa specific reviews include a focus on the sterols obtained from Theonella spp.,398 the biosynthesis of compounds from the Theonellidae,399 and of the MNPs obtained from Acanthella spp.400 Case studies of the application of GNPS in studying Australian sponge chemistry have also been reviewed.401
Several reports of new biological activity for known sponge metabolites have been reported. Bis-indole dragmacidin D has been found to selectively induce apoptosis in aggressive triple negative breast cancer spheroids, and can work synergistically with paclitaxel.402 Curcuphenol, an aromatic marine sesquiterpenoid that is also commonly found in food spices, rescues immune recognition of metastatic cancers by restoring expression of antigen presentation machinery. This is achieved by eliciting histone deacetylase-enhancing activity, which causes changes resembling those caused by interferon-γ, a cytokine that has an important role in regulating the innate and adaptive immune systems.403 Fusion of a GFP-label to defensive steroid formoside has shown it distributes to the lips, tastebuds and olfactory epithelium in zebrafish as a model of fish predator–prey interactions, which helps to explain chemoreception in an ecological setting.404
A comparison of the metabolic and bioactivity profiles of two farmed and wild Mediterranean sponge species, Agelas oroides and Sarcotragus foetidus, has shown that both populations shared similar chemical profiles. The antibiotic activities of the sponge extracts were also generally similar, albeit slightly lower in the farmed sponges, while only S. foetidus extracts from both treatment groups were weakly cytotoxic.405 A chemoecological study of Indonesian Aaptos suberitoides has explored the effects of the sponge microbiome upon the composition of aaptamine and other alkaloids. A wide variability of alkaloid concentrations across the sponges sampled showed no direct correlation with the presence of microbial symbionts, and no direct link for microbial biosynthesis of aaptamine could be found.406
An assessment of the combination of DFT-calculated NMR chemical shifts, as determined with several variants of the DP4+ algorithm combined with artificial neural network pattern recognition, along with a comparison of calculated and experimental chiroptical analyses, has confirmed the relative and absolute configurations of a marine endoperoxide as proposed using biogenic reasoning.407 Several new poly-arsenic compounds, like arsenicins A–D, have been reported from sponges in the preceding years. However, DFT-methods, including study of the use of different functionals and basis sets for calculating their NMR chemical shifts for comparison with experimental data, has been lacking. A recent report describes a systematic examination of the use of two DFT methods, four functionals and five basis sets to establish the best approach for calculating 1H and 13C NMR chemical shifts and coupling constants within this growing class of metabolite, and validated by comparison with experimental data.408 An evaluation of currently accessible, state of the art computational tools for assisting with structure elucidation has been carried out, in particular, focusing on their application by non-specialist users. The tools assessed included HOSE, CASCADE, DP4, DP4+ and ML-J-DP4. The study was exemplified by a computational examination of the compound dysiherbol A, the structure of which was recently reassigned following total synthesis, and where the erroneous structure proposed could have been flagged using the assistance of digital technologies prior to the synthetic campaign beginning. A pathway for structural confirmation prior to publication and embarking on a total synthesis was proposed.409
7 Cnidarians
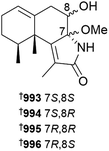
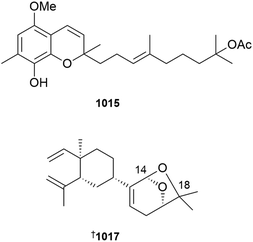
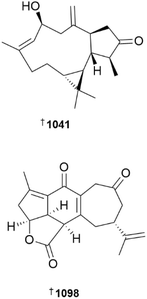
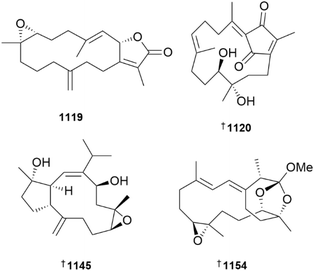
In addition to an anti-inflammatory cembranoid (discussed later), investigation of a Red Sea collection of Sarcophyton glaucum afforded δ-lactone sarcoglaucanoate 985.410 As well as three known alkyl glycerol ethers, a new example 3-(n-henicosyloxy)propane-1,2-diol 986 was isolated from the soft coral Nephthea mollis, also collected in the Red Sea.411 A large number of sesquiterpenoid and merosesquiterpenoid NPs were reported from soft corals. Two farnesane-type sesquiterpenoids, sinulalides A 987 and B 988 were isolated from extracts of the South China Sea soft coral Sinularia scabra.412 The capnellene skeleton sesquiterpenoid 989 was isolated from an Orchid Is. Taiwan collection of Capnella imbricata and is somewhat unusual in possessing hydroxylation at C-15.413 A guaiane sesquiterpenoid, litoarbolide A 990 was purified from a Red Sea collection of Litophyton arboretum414 while a eudesmane sesquiterpenoid, cespilamide F 991 was isolated from a Taiwanese collection of Cespitularia sp.415 – both MNPs were inactive when evaluated for anti-P. falciparum (the former), and cytotoxicity and anti-inflammatory (the latter) properties. Thirteen examples of nitrogen-containing nardosinane sesquiterpenoids, clavukoelloids A–M 992–1004 were isolated from a South China Sea collection of Clavularia koellikeri.416 The stereochemical relationships between clavukoelloids B–E (993–996) were secured by CP3 analysis of calculated NMR chemical shifts, comparison of calculated and experimental ECD spectra and, in the cases of clavukoelloid B and E, by XRD analysis. The absolute configuration of clavukoelloid H was also secured by XRD analysis. In addition to several dolabellane diterpenoids (discussed later), a Taiwanese collection of Clavularia sp. afforded five eudensamane-type sesquiterpenoids, clasamanes A–E 1005–1009, with clasamane E having a peroxide bridge.417New congeners were added to the tuaimenal family of merosesquiterpenoids, with tuaimenals B–H 1010–1016 being isolated from deep-sea collections of the Irish soft coral Duva florida.418 Tuaimenal G 1015 displayed selectively potent in vitro cytotoxicity towards a HPV-negative cervical human cancer cell line. Soft corals continue to be an excellent source of diterpenoids with over one hundred and sixty examples reported in 2023. Lobocatalens A–G 1017, 1018–1023 are lobane skeleton diterpenoids isolated from a Xisha Is. collection of Lobophytum catalai.419 The structure of lobocatalen A contains an unusual ether linkage between C-14 and C-18. Fifteen diterpenoids, including lobane examples related to the known MNP fuscol, were identified in extracts of Klyxum molle and named xishaklyanes A–O 1024–1038.420 Xishaklyanes D and K exhibited moderate activity towards the fish pathogenic bacteria Lactococcus garvieae and Streptococcus parauberis, respectively. Lactone diterpenoids sinulatones A 1039 and B 1040 were isolated from extracts of the South China Sea soft coral Sinularia scabra.412 Absolute configurations were assigned by TDDFT calculations of ECD spectra. Sinulatone B was a weak inhibitor of osteoclastogenesis. Sinularia nanolobata specimens collected in the South China Sea were the source of diterpenoids nanolobatones A–E 1041, 1042–1045 and nanolobaperoxides A–D 1046–1049.421 The structures of nanolobatones A and B and nanolobaperoxide A were secured by XRD analysis.
Seven examples of diterpenoids containing the rare flexibilane scaffold, paraflexinols A–G 1050–1056, were reported from a Green Is., Taiwan, collection of Paralemnalia thyrsoides.422 The authors speculated that acetoxy analogues 1053 and 1054 may be artefacts. Fourteen verticillane diterpenoids, heterolactone 1057 and heterolactams A–M 1058–1070 were isolated from the soft coral Heteroxenia ghardaqensis.423 A further example of a verticillane, cespitulactam M 1071 was identified in extracts of Cespitularia sp. collected at Green Is., Taiwan.415 Targeted isolation using molecular networking analysis led to the identification of twelve dolabellane diterpenoids clavirolides J–U 1072–1083 from extracts of Clavularia viridis collected at Yongxing Is., South China Sea.424 Clavirolide L exhibited weak inhibition of HIV-1 but it lacked the ability to inhibit reverse transcriptase. The authors considered clavirolide O to be an artefact derived from EtOH solvent used in the extraction process. Xisha Is. collections of C. viridis afforded clavusins A–E 1084–1088, with structures and absolute configuration of clavusins A and E being secured by XRD analysis,425 while a Green Is. collection of Clavularia sp. yielded dolabellanes clabellanes A–C 1089–1091.417 New examples of capnosane diterpenoids, sarcocrassolins A–F 1092–1097, were isolated from a Nansha Is. collection of Sarcophyton crassocaule, with the structure of sarcocrassolin B being secured by XRD.426 Further investigation of the biological activities of the casbane-type diterpenoid sinueracabanone D have revealed it to be capable of inducing apoptosis in HepG2 cells via a mechanism that in-part involves enhanced generation of reactive oxygen species.427 With over fifty new examples, cembranoids remain the dominant sub-structural class of diterpenoids reported from soft corals. The structures and biological activities of cembranoids isolated from marine, and terrestrial, organisms between 2011 and 2022 have been reviewed.428 The structures of previously reported norcembrane diterpenoids yonarolide and scabrolide B were revised and absolute configuration assigned to 1098 and 1099, respectively, by XRD analysis.429 It should be noted that the structure now assigned to scabrolide B is identical to that previously reported for sinuscalide D, isolated from Sinularia scabra.430 A new synthetic route to (−)-scabrolide A was reported, with a subsequent dehydration step using Burgess reagent to afford (−)-yonarolide, providing further confirmation of structure and absolute configuration of the latter.431
Sinulaflexiolide Q 1100, isolated from Sinularia flexibilis, was inactive in a Bugula neritina larvae settlement assay.432 The structures and absolute configurations of Sinularia-sourced cembranoids sinupendunculide A 1101,433 sinulariaone A 1102 and previously reported chlorofurancembranoid B 1103 were established or confirmed by XRD analyses.434 Of two related α-methylene-ε-lactonic cembranoids, querciformolides G 1104 and H 1105, isolated from Sinularia querciformis, only the former exhibited anti-inflammatory activity, weakly inhibiting the release of elastase from activated neutrophils.435 The α-methylene-δ-lactone flexibanone 1106, isolated from a Taiwanese collection of Sinularia flexibilis, was inactive towards a panel of three HTCLs.436 Additional examples of cembranoids isolated from soft corals of the genus Sinularia included 1107–1109, along with casbane 1110, from a South China Sea collection of Sinularia nanolobata,437 the absolute configurations of which were determined using TDDFT calculations of ECD spectra, and situmulins A 1111 and B 1112 isolated from specimens of Sinularia tumulosa, also collected in the South China Sea.438 Two α-methylene-γ-lactone-containing cembranoids, ximaolobophytolides A 1113 and B 1114 were reported from Lobophytum sp.439 Despite the presence of electrophilic functionality, ximaolobophytolide A was inactive against the HEL tumour cell line in vitro while structurally-related known co-metabolites were cytotoxic. Soft corals of the genus Sarcophyton were a prolific source of additional examples of cembranoids including variants with unusual substitution patterns and some new examples of dimers. Cembranoids 1115–1118, derived from an extract of Sarcophyton trocheliophorum, were found to be inactive against two bacterial strains and influenza A virus H1N1.440 The structurally-related sarcophine derivative 1119, isolated from a Red Sea collection of S. glaucum, exhibited an interesting range of anti-oxidant and anti-inflammatory activities against indomethacin-induced gastric injury in rats.410 A structurally-diverse set of cembranoid diterpenoids, sarcoelegans A–H 1120, 1121–1130 were reported from a South China Sea collection of Sarcophyton elegans.441 Sarcoelegans A–C, E and G were isolated as optically active MNPs while the remaining examples were isolated as racemates, subsequently being separated by chiral HPLC and absolute configuration was assigned to each of the enantiomers. The structures of sarcoelegans A, D, F and G were also secured by XRD analysis. Three cembranoids isolated from a Hainan collection of Sarcophyton sp., sarcophynoids A–C 1131–1133, are variants on the simple cembran-tetraene (first) and sarcophytonolide (last two) scaffolds.442 All three were inactive against a panel of three bacterial strains. Four new examples of isosarcophytoxides 1134–1137, notable for containing a 2,5-dihydrofuran moiety, were reported from a Xiao Liuqiu Is. collection of S. cinereum.443 Despite MeOH being used in the isolation of the metabolites, the authors did not discuss the possibility that the methoxy-containing examples were artefactual. New examples of unsaturated-lactone containing cembranoids, isoehrenbergol D 1138 and sarcoehrenolides F–L 1139–1144, were isolated from a Weizhou Is. collection of S. ehrenbergi.444 None of the MNPs were able to inhibit the production of TNF-α in a cell-based anti-inflammatory assay. While sartrocheliol A 1145 is a capnosane-type diterpenoid, the remaining diterpenoids isolated from a Ximao Is. collection of S. trocheliophorum, sartrocheliols B–F 1146–1150, were cembranoids.445 The structure and absolute configuration of sartrocheliol A were secured by XRD analysis. All of the MNPs were deemed inactive when evaluated against a panel of HTCLs and microorganisms. Amongst the cembranoids, sarcomililatol H 1151 isolated from a Xigu Is., South China Sea collection of S. mililatensis446 and two further examples, sarcoboettgerols D 1152 and E 1153, isolated from a Weizou Is. collection of S. boettgeri.447 Sarcomililatol H contains a rarely encountered C-2 to C-12 ether linkage, while sarcoboettgerol E has an ether linkage between C-17 and C-12. A diverse set of terpenoids and bis-terpenoids were isolated from S. tortuosum, collected from Ximao Is., South China Sea.448 Of the first two, sarcotortin A 1154 is a cembranoid that contains an unusual orthoester moiety, while sarcotorolide A 1155 contains a ring closure between C-2 and C-11 of the cembranoid framework, making it a eunicellane-type scaffold. The structures and absolute configurations of both metabolites were secured by XRD analysis. The structure of co-metabolite sarcostolide G was revised to the C-2 epimer 1156 also by the analysis of XRD data. In addition, biscembranoids, ximaolides M 1157 and N 1158 were isolated from the soft coral – the structure and absolute configuration of the former was again secured by XRD analysis. Of all the isolated compounds, only ximaolide M exhibited bioactivity, being found to be a weak inhibitor of PTP1B. Two additional examples of biscembranoids, sarcotroxides A 1159 and B 1160 were isolated from aquaculture-derived S. trocheliophorum – the latter MNP was found to be a weak inhibitor of superoxide generation by stimulated neurophils.449
New biological activities were reported for cnidarian-derived cembranoids including the finding that 11-epi-sinulariolide acetate can induce apoptosis in oral cancer cells via the PI3K/AKT/FOXO pathway,450 that sinularin induces, amongst other things, ferroptosis, which is apoptosis induced in an iron-dependent mechanism,451 and that crassolide can stimulate anticancer immune responses in part by blocking mitogen-activated protein kinase 14 activation.452 In two publications, new congeners of the briavioid family of briarane diterpenoids, briavioids D–G 1161–1164 were reported from specimens of Briareum violaceum grown in acquaculture.453,454 The latter study also reported an XRD structure of briavioid A 1165, defining its absolute configuration for the first time. Briarlide S 1166 was isolated from an Okinawa Prefecture collection of Pachyclavularia (Briareum) violacea.455Briareum stechei was the source of three new briaranes, 12-epi-briacavatolide B 1167, and briastecholides M 1168 and N 1169, with the structure and absolute configuration of the last being secured by XRD analysis.456–458 Of 45 briarane diterpenoids isolated from a South China Sea collection of Junceella juncea, 16 new analogues, juncelactones A–P 1170–1185 were characterised.459 Juncelactone K exhibited weak ability to inhibit RANKL-stimulated osteoclastogenesis activity. Comprehensive biological evaluation was undertaken on the major (known) MNP in the extract, praelolide, identifying the briarane to be a potential new lead in the treatment of osteoclastogenic bone disease. Investigation of an extract of a Southern Taiwan collection of J. fragilis afforded a series of briaranes of which one, fragilide Y 1186, was new.460 The structure of fragilide Y contains the 3(E),5(16)-diene system in a s-cis form, the determination of which was aided by XRD analysis of two previously reported co-metabolites (−)-frajunolide H 1187 and fragilide P 1188. These structures correct those originally reported for the diterpenoids, both of which were depicted with a s-trans diene. Similar changes from s-trans to s-cis were proposed for the structures of junceellolides B 1189 and C 1190. In a major achievement, the first syntheses of the xenicin class diterpenoids (+)-waixenicin A 1191 and (+)-9-deacetoxy-14,15-deepoxyxeniculin 1192 have been reported.461 This is particularly noteworthy as the former MNP is a potent irreversible inhibitor of transient receptor potential melastatin 7 (TRPM7) channels, with potential applications in neurodegenerative disorders, cardiovascular disease and cancer chemotherapies. The authors also noted that reaction of 1192 with mild base (K2CO3 in MeOH) induced a rearrangement to another known xenicin NP, xeniafaraunol A 1193. In an extension to their previous work identifying and characterising the BGCs associated with cnidarian terpenoid biosynthesis, the Schmidt lab have reported that expressing the relevant terpene cyclase in the yeast Saccharomyces cerevisiae can yield fermentation titres of close to 100 mg L−1 for the production of (−)-klysimplexin R.462 Subsequent semi-synthetic transformations allowed for rapid preparation of analogues belonging to several different structural classes. The biosynthesis and enzymology associated with eunicellane biosynthesis has been reviewed.463 Specimens of the soft coral Sinularia depressa collected at Xisha Is. in the South China Sea were the source of three ergosterol and one cholesterol analogue, sinulasterols D–G 1194–1197,464 while 7-hydroperoxides of gorgosterol 1198, 1199 and campesterol 1200 were isolated from a Taiwanese collection of Cespitularia sp.415 None of the MNPs exhibited bioactivity in a range of HTCL cytotoxicity, antibacterial and anti-inflammatory assays. The absolute configuration of the ring-A-aromatised bile acid analogue 1201, isolated from an Irish deep-sea collection of Duva florida, was established by XRD analysis.418 Two spiroketal steroids, 24-dehydrohippuristanol 1202 and hippuristanol 11-one 1203, the latter being first reported as a NP, were isolated from an Orchid Is., Taiwan collection of Isis hippuris.465 The former MNP exhibited weak cytotoxicity towards 2 HTCLs. Three steroids, one being a 7-one 1204 and two being epimeric 7-hydroperoxides 1205 and 1206 were isolated from a Xisha Is., South China Sea collection of Lobophytum sarcophytoides.466 The steroidal 3,23-diketone dendronestadione 1207, derived from an extract of a Red Sea Dendronephthya sp., exhibited weak cytotoxicity towards the prostate PC-3 cell line.467 Enone and dienone steroids lobosteroids A–E 1208–1212 and 5α,8α-epidioxy-containing lobosteroid F 1213 exhibited weak to moderate activities towards three species of pathogenic fish bacteria.468 A single isomer of a previously reported cnidarian-derived sterol, 25(R)-26-acetoxy-3β,5α-dihydroxycholest-6-one, has been synthesised starting from diosgenin.469 The semi-synthetic compound was inactive against respiratory syncytial virus, but did show weak cytotoxicity towards two HTCLs. The therapeutic potential of sea anemone neurotoxins and pore forming peptides has been reviewed.470 Investigation of ethanolic extracts of the invasive anemone Condylactis sp. led to the characterisation of a diverse range of small molecules including bispyridinyl-1,2,4-thiadiazole 1214, a previously reported synthetic compound, thionicotinamide 1215 and (−)-betonicine 1216, a known higher plant NP.471
A number of reviews were published that are relevant to the chemistry and biology of metabolites derived from cnidarians. Genus or species specific reviews summarised the chemistry of the Cladiella genus, covering the period 2006 to August 2022,472 the genus Litophyton covering from the 1970s until July 2023,473 and the structural diversity of terpenoids reported from Sarcophyton trocheliophorum, covering the period 1976 to October 2022.474 In addition to these, two reviews focused on biological activities, with one summarising neutrophilic anti-inflammatory NPs isolated from cnidarians covering the period 1995 to April 2023,475 and the other covering NPs with enzyme inhibiting properties published between 1974 and 2022.476
While no new MNPs isolated from zoanthids and hard corals were reported, further study of zoanthid-derived NPs identified that extremely low doses (low pM) of palytoxin exhibit anti-leukaemic activity in cell-based and zebrafish xenograft assays,477 and that epi-oxyzoanthamine, sourced from Zoanthus vietnamensis, reduced skin cell damage and hyperplasia, suggesting it has potential in the treatment of atopic dermatitis.478 Molecular networking has been used to analyse the chemical relationships between four species of hard corals, Pocillopora meandrina, Seriatopora hystrix, Acropora formosa and Fungia fungites, collected in the South China Sea.479 The study revealed notable differences in amino acid, peptide and lipid profiles that were discernible using principal component analysis. Variation of tissue lipid composition of seven species of Porites hard corals with changes in temperature and pCO2 concentrations identified, amongst other changes, increases in free fatty acids and campesterol with increasing temperature while seawater pCO2 concentrations generally had no significant effect.480 Two reviews summarised the extensive lipid chemistry of reef-building corals and their dinoflagellate symbionts.481,482 The hydrozoan-derived indole-oxazole alkaloids, breitfussin G and H, have been synthesised for the first time, using a convergent, one-pot Friedel–Crafts/Robinson–Gabriel method.483
8 Bryozoans
No new metabolites were reported from bryozoans over the past year but two syntheses of bryozoan metabolites were accomplished. Total synthesis of the tetrabrominated alkaloid aspidostomide G was achieved in five steps and an overall yield of 16%,484 and trichlorinated alkaloid caulamidine A was prepared in 11 steps.485 The potential of bryostatin-1 for treatment of neurological disorders was reviewed.4869 Molluscs
A comprehensive review of the chemical structures, chemical ecology and pharmaceutical potential of MNPs derived from marine molluscs, covering the period 2011–2021, has been published.487 MS-directed investigation of bioactive extracts of the sea hare Aplysia kurodai collected from the Shima Peninsula, Japan, afforded new members 1217, 1218–1222 of the aplaminone family of dopamine-terpenoid hybrids.488 Cytotoxicity towards the HCT 116 HTCL ranged from inactive to moderate in potency. Amongst the set, isoaplaminone 1217 is structurally unusual in that it contains a reverse prenyl substituent. LCMS analysis of alga species collected in the vicinity of the sea hare collection site identified the presence of known aplaminones A and B in the red alga Palisada intermedia, and that the levels of the molecules varied with location, leading the authors to speculate that the MNPs were produced by microbes.Investigation of Vietnamese collections of Aplysia dactylomela afforded three nor-chamigrane and bisabolane sesquiterpenoids, dactylomelanins C–E 1223–1225.489 The absolute configuration of a co-metabolite, 2-chloro-3,7-epoxychamigran-9-one 1226, previously reported from the red alga Laurencia obtusa, was secured by TDDFT ECD calculations.
A new example of an irieane diterpenoid, 12-hydroxypinnaterpene C 1227, was isolated from specimens of Aplysia argus collected at Ikei Is. in the Okinawa Prefecture.490 Syntheses of the sea hare metabolites aplysiasecosterols A and B, the latter for the first time, have been reported, using a late-stage convergent strategy.491 In two separate accounts, new metabolites have been reported from South China Sea collections of the nudibranch Hexabranchus sanguineus. In the first of these, nine diterpenoids, sanyanolides A–I 1228–1236 were purified from an extract of the nudibranch's internal organs.492 The majority of these metabolites were also identified in extracts of the sponge Chelonaplysilla sp. collected in the immediate area. Antimicrobial testing against a panel of eight human pathogens identified sanyanolide E as being weakly active against two species of Streptococcus, while none were found to inhibit NO production by LPS stimulated macrophages. Further investigation of extracts of the same organism from the same location afforded thirteen sesquiterpenoids sanyagunins A–H 1237–1244, sanyalides A–C 1245–1247 and sanyalactams A 1248 and B 1249.493 The absolute configuration of known MNP herbabysidolide 1250 was secured by XRD. Sodium borohydride reduction of sanyagunin C gave two diastereomeric allylic alcohols which were identical to two previously reported MNPs. Mosher's derivatisation led to determination of the secondary alcohol configuration in 1251 and 1252, requiring reversal of the original assignments. A number of γ-pyrone polypropionates were reported from the photosynthetic mollusc Placobranchus ocellatus.494 Ocellatuspyrones A 1253 and B 1254 were both isolated as racemic mixtures; the former was resolved using chiral HPLC and the relative and absolute configuration assigned using DP4+ analysis and Mosher's derivatisation.
Ocellatuspyrone C 1255 shares close structural similarity to the known polypropionate co-metabolite tridachiapyrone J 1256. The absolute configuration of tridachiapyrone J was established using XRD analysis, which allowed determination of that of ocellatuspyrone C. Reduction of the hydroperoxide moiety in tridachiapyrone J afforded co-metabolite tridachiapyrone G 1257, securing its absolute configuration as well as that of the diastereomer tridachiapyrone H 1258. Four new structurally simpler polypropionates were also characterised from the extract, ocellatuspyrones D–G 1259–1262, with relative and absolute configurations assigned by combinations of DP4+ and ECD analysis. Three ceramides, bathymodiolamides C–E 1263–1265 were isolated from extracts of the mussel Bathymodiolus azoricus collected near deep-sea hydrothermal vents.495 An unusual aspect of their structures is the presence of a methoxy ethylene ether substituent. The authors revealed that the structures they previously reported for congeners bathymodiolamides A and B were incorrectly drawn in their original publication496 and should be represented as 1266 and 1267, respectively. It is disappointing that such a simple error in structure drawing, compounded by a factual mistake regarding a stereochemical model, were not recognised by the authors, nor was it caught during the peer review process. Unfortunately, those errors led to a subsequent total synthesis study to target an incorrect diastereomer of bathymodiolamides A and B which, unsurprisingly, exhibited NMR data that did not match those reported for the isolated NPs.497
The presence of the polyunsaturated fatty acid docosahexaenoic acid in marine bivalves appears to play a role in increasing the levels of esterification of diarrhetic shellfish toxins and activating the Nrf2 signalling pathway, leading to a reduction in damage to the digestive glands by the toxins.498 A seven-year study of spirolide toxins in bivalve molluscs of the Galicia Coast (NW Spain) has identified the mussel Mytilus galloprovincialis to be the major accumulator of 13-desmethyl spirolide C (13-desmSPXC), while the presence of an isomer, likely an epimer based upon high similarity of MS/MS fragmentation patterns, was restricted to cockles and two clam species.499 The highest levels of 13-desmSPXC occurred during the autumn–winter months while the isomer had peak levels in spring–summer. A second, 21 months study, also undertaken on the Galicia Coast, identified the presence of multiple lipophilic toxins in non-traditional vectors, including 13-desmSPXC and pinnatoxin G in cephalopods and ascidians, and 13-desmSPXC, OA and dinophysistoxin 2 in polychaete worms.500 Overall it was concluded that the mussel Mytilus galloprovincialis could be used as a biological indicator for toxicity. Mice treated with sublethal concentrations of extracts of the cockle Acanthocardia tuberculatum, known to harbour saxitoxins, found that the toxins induced oxidative stress and affected energy metabolism, but with limited to no impairment of renal function.501
Racemic synthetic N-(2-ozoazepan-3-yl)-pyrrolidine-2-carboxamide, previously reported from Octopus vulgaris ink, was evaluated for a range of antiproliferative and anti-inflammatory activities but the responses did not reach a threshold for inclusion in this review.502 Two steroidal glycosides 1268 and 1269 were reported as weakly anti-inflammatory constituents of the octopus Cistopus indicus.503 The biological activities of 1-O-alkylglycerol ethers, reported from a number of different marine organisms including cartilaginous fish, cnidarians and molluscs, which can include reduction of oxidative stress, and stimulation of hematopoiesis and immune responses have been reviewed.504 Dietary supplements containing crude preparations of 1-O-alkylglycerol ethers derived from the squid Berryteuthis magister, when given to obese patients with asthma, afforded improved pulmonary function and reduction in plasma levels of several inflammatory cytokines and oxylipins.505 Several reviews pertaining to cone snail toxins have been published, including a bibliometric summary of toxins published from 2000 to 2022,506 a perspective on those toxins that target voltage-gated sodium channels,507 and an update of the Conus-derived toxins reported from snails collected off the coast of Brazil.508 Ten cysteine-free conopeptides, based upon transcriptomes previously determined for the vermivorous snail Conus betulinus, were synthesised and evaluated for insecticidal activity against mealworm (Tenebrio molitor) larvae leading to the identification of several analogues with nanomolar potency.509 Two studies reported investigation of structure–activity relationships of conotoxins. In the first, a series of alanine insertion and truncated loop 2 mutants of α4/6-conotoxin TXID were prepared and demonstrated similar or weaker inhibition of α3β4 nACh receptors than the parent toxin.510 In the second study, it was found that swapping the position 11 L-arginine for D-arginine in α-conotoxin RgIA led to reduced ability to block α9α10 nAChR activity, but instead led to introduction of the ability to block α7 nAChR activity.511
The solution structure of mono-disufide-containing Conus monile-derived peptide Mo1853 has been investigated using 2D NMR spectroscopy, identifying the presence cis and trans Lys–Pro conformers.512
10 Tunicates (ascidians)
In addition to summaries of the biological activities reported for ascidian MNPs, the clinical status of ascidian-derived therapeutics has been reviewed.513 Halorotetin A 1270 is a terpenoid isolated from the tunic of the edible ascidian Halocynthia rotetzi.514 Although the authors reported many biological assay results for the compound, including antiproliferative and gene expression profiles, the potency of activity did not reach the threshold used by this review. Bioassay-directed fractionation of extracts of Australian collections of the ascidian Polycarpa procera led to isolation of the butenolide procerolide E 1271, methylprocerolate A 1272 and 3-bromo-4-methoxyphenylacetamide 1273, the latter being reported as a NP for the first time.515 In addition to a set of known related MNPs also isolated from the ascidian, all three new metabolites exhibited the ability to bind to α-synuclein in an affinity mass spectrometry assay. Follow up testing in a biochemical amyloid aggregation assay showed the former two compounds to be inhibitors. Five examples of hexacyclic alkaloids, isocaulamidines B–D 1274–1276 and caulamidines C 1277 and D 1278 were reported from a Palau collection of Polyandrocarpa sp.516 All five MNPs shared similar ECD spectra to the previously reported co-metabolite caulamidine B, and so were assigned absolute configurations corresponding to that of caulamidine B, which had been determined using TDDFT calculations. Within months, enantioselective syntheses of (−)-caulamidine D 1279 and (−)-isocaulamidine D 1280 were reported, leading to reassignment of absolute configuration of the isolated NPs to their respective enantiomers.517 Two studies reported new biological activities for lepadin alkaloids, including the finding that lepadin A activates the intrinsic apoptosis pathway in human melanoma cells leading to induction of immunogenic cell death,518 and that lepadins E and H can induce an iron-dependent accumulation of phospholipid peroxides that lead to mitochondrial shrinkage, cell membrane perforation and cell death, a process known as ferroptosis.519 Lepadin H exhibited in vivo activity against B16F10 melanoma cells with limited to no toxicity towards internal organs.Two analogues of siladenoserinol A, that explored variation in substituent configuration about the dioxabicyclooctane central skeleton, were less active in their abilities to inhibit p53-Hdm2 interaction than the NP.520 In addition to the first reported synthesis of meridianin B, a library of analogues were synthesised and evaluated for antibiofilm activity against Acinetobacter baumannii.521 None were found to be active, but in additional testing, several analogues demonstrated the ability to synergistically improve the antibiotic activity of gentamicin and ceftriaxone. Analogues of the diarylpyrrole ascidian alkaloid lukianol A were prepared and screened for human aldose reductase inhibition properties, identifying two analogues with comparable activity to the NP.522 The study noted the importance of at least two of the phenolic hydroxyl groups for bioactivity. A series of pyrazole-containing, lamellarin O-type analogues were prepared and found to exhibit variable levels of cytotoxicity, ranging from inactive to weak, against a panel of three HTCLs.523 Closer analysis using HCT116 cells showed two of the compounds to promote G2/M-phase cell cycle arrest. N-9-Alkylated analogues of the β-carboline eudistomin Y are at best weakly cytotoxic towards a panel of five HTCLs.524 The presence of electron-rich groups in the N-9 substituent restored fluorescence, enabling subcellular localisation studies that identified lysosomes as the location of accumulation.
11 Echinoderms
Pentaoside protonodososide 1281 was isolated from a Vietnamese collection of the starfish Protoreaster nodosus.525 It was found to be inactive towards a panel of five HTCLs. A study exploring the saponin and fatty acid profiles of body wall extracts of the sea cucumber Holothuria atra led to the characterisation of the C-12 epimer of desholothurin B (desulfated holothurin B) 1282.526 Far Eastern specimens of the sea cucumber Paracaudina chilensis afforded chilensosides E–G 1283–1285, all of which were inactive towards a panel of HTCLs and were also non-haemolytic.527 In two studies, new triterpenoid glycosides djakonoviosides A, A1 and A21286–1288, B1–B41289–1292, C1, D1, E1 and F11293–1296 were reported from extracts of the Far Eastern sea cucumber Cucumaria djakonovi.528,529 Three of the glycosides, djakonoviosides A2 (1288), B2 and B4 contained an unusual 23,16-hemiketal linkage.Palmitic acid, isolated from Holothuria leucospilota, is active in Caenorhabditis elegans models of Parkinson's disease, improving locomotion, extending the lifespan and decreasing α-synuclein aggregation.530
As with previous years, a number of studies have reported additional biological activities for echinochrome A including prevention of heart failure after myocardial infarction in mice,531 preventing diabetic nephropathy,532 induced inhibition of Ca2+-permeable cation channels,533 and ability to prevent asthma in a mouse model via inhibition of inflammation and oxidative stress.534 Phase II metabolic profiling of echinochrome A using rat and human hepatic preparation identified mono-methylated and mono-glucuronide conjugates as the principle phase II metabolites.535 Further biological activities reported for known echinoderm MNPs include the finding that asterosaponin P1 causes significant toxicity and embryonic effects towards zebrafish embryos,536 and that holothurin A inhibits the epithelial–mesenchymal transition that drives apoptosis in prostate cancer cells.537 Two studies were reported using the saponin frondoside A whereby a detailed study of HTCL cytotoxicity and differential gene expression analysis provided further evidence that frondoside A regulates multiple pathways in tumour cells,538 and that an immunomodulator can synergistically potentiate the antiproliferative and anti-migration properties of frondoside A against human bladder cancer cells and that the combination was significantly active in an in vivo xenograft model.539
Several reviews have been published covering specific aspects of the chemistry of echinoderms, including a commentary on saponins derived from North Sea holothurians,540 an in silico analysis of cytotoxic sea cucumber compounds,541 fatty acids from sea cucumbers,542 and a discussion of the synthesis and structure elucidation of dimeric hydroxynaphthazarins.543
12 Miscellaneous
In an excellent exposition of NP research, the structure elucidation of an ellagic acid derivative, lumnitzeralactone 1297 isolated from root extracts of the mangrove Lumnitzera racemosa, was achieved using combinations of NMR, including 1,1-ADEQUATE and 1,n-ADEQUATE data, computer-assisted structure elucidation, and DFT calculated NMR chemical shifts with DP4+ analysis.544 Synthesis was achieved by photo-oxidation of ellagic acid to an intermediate that was then subjected to thermal decarboxylation to afford the NP. Lumnitzeralactone was only detected in two of thirty-one Lumnitzera sp. samples, leading the authors to speculate that the NP is biosynthesised by associated microorganisms.Previous studies have identified that Chinese medicinal use of Hai-Long, the fish Syngnathus acus, is associated with antitumour activity. Investigation of extracts afforded two glycerolipids, syngaculipids A 1298 and B 1299 as well as triallyl isocyanurate 1300, with the last being reported as a NP for the first time.545 The use of marine annelids to investigate the mechanism of action of toxic microalgae on marine invertebrates has been reviewed.546 A racemic synthesis of a thienothiochromene-based luciferin of the polychaete worm Odontosyllis undecimdonta has been reported.547 Tetrodotoxin and congeners are present in larvae of the toxic ribbon worm Cephalothrix cf. simula, with developmental time course analysis suggesting the larvae were not capable of independent production and that the toxins were obtained from the maternal organism.548 A study involving the comparative amino acid sequence, 3D structure analysis and biological evaluation of BRICHOS-domain antimicrobial peptides isolated from three polychaete worm species, including known peptides alvinellacin from Alvinella pompejana (deep-sea, hydrothermal) and arenicin from Arenicola marina (temperate, coastal) and a new peptide polaricin from Amphitritides sp. (polar, coastal), concluded that production of the specific peptides was the result of environmental pressures to afford antimicrobial agents that would function under specific conditions and against specific microbial targets.549 Temporal analysis of the fish Lagocephalus sceleratus collected in Antalya Bay, Mediterranean Sea, has identified the gonads to contain the highest levels of tetrodotoxin with levels peaking in the late autumn to winter (November–December) months, identifying this invasive pufferfish as being a public health risk.550 A similar study of the same fish species, covering a two-month period using specimens collected from Rhodes Is., Greece, identified a wider array of tetrodotoxin analogues, with liver and gonad tissues being the most toxic and with the highest concentrations being observed in ovaries of female fish.551 One of the lethal symptoms of tetrodotoxin poisoning is severe hypotension, which has now been attributed to the ability of the toxin to block voltage-gated sodium channels in resistance arteries leading to a decrease in vascular tone.552 The ability of fish intestinal gut derived gangliosides to bind bacteria of the genus Vibrio has been reviewed.553 The linear antimicrobial peptide epinecidin-1, originally isolated from the orange-spotted grouper Epinephelus coioides, has been found to have good characteristics as a preservative for raw milk, preventing spoiling and reducing the incidence of milk-borne pathogens.554 Phlorizin, a known dihydrochalcone glycoside isolated from the seagrass Syringodium isoetifolium, induces apoptosis in HepG2 cells, and exhibits in vivo activity against diethylnitrosamine + CCl4 induced hepatocellular carcinoma.555 Using Mediterranean collections of Nanozostera noltei, phenolic metabolites, including rosmarinic acid and zosteranoic acid appear to be useful markers of sea grass health.556
13 Conclusion
The decline in new prokaryote MNP discoveries in recent years may reflect the challenges involved in finding uniquely marine microbes since easily accessible prokaryotes obtained from marine environments produce chemical diversity typical of terrestrial sourced strains. This section summarises the current knowledge.In the mid 1980s MNP researchers began to speculate that microorganisms were likely to play a key role in the production of some NPs isolated from marine invertebrates. The similarity in structures of some marine invertebrate NPs to those reported from terrestrial bacteria and marine and freshwater cyanobacteria was the basis for this hypothesis.557 The isolation of diketopiperazines from both the sponge Tedania ignis and cultures of the actinomycete bacteria Micrococcus sp. isolated from the sponge in 1988 provided the first example of MNPs ascribed to the sponge being produced by an associated bacterium.558
The seminal work of John Faulkner and others in the early 1990s highlighted that, through cell separation techniques, some of the complex polyketide and peptidic NPs reported from the sponges Theonella swinhoei and Dysidea herbacea were co-located in bacterial cell fractions and this provided circumstantial evidence for their bacterial origins.559 These findings intersected with the growing realisation that sustainable production of some biomedically important and complex invertebrate-derived MNPs such as ET-743, bryostatins and didemnin B would require a different approach to sourcing the compounds by wild invertebrate harvest. The promise of fermentation of microorganisms, speculated to be the true source of these compounds, resulted in growing interest in marine microorganisms for MNP discovery. Cultivation of marine bacteria led in part by the groundbreaking discoveries and methodological advancements of Fenical and Jensen at Scripps Institution of Oceanography in the early 1990s, and the impactful “Natural Products as Sources of New Drugs over the Last 25 Years” by Newman and Cragg in 2007 that urged a significant expansion in NP research focused on marine microbes, resulted in a rapid increase in interest in marine bacteria as a new source of MNP chemical diversity (Fig. 2).559–561
Over the last 20 years an exponential increase in studies aimed at discovering new MNPs from marine microbes rather than from marine macro-organisms has occurred. In contrast, marine invertebrate NP studies reached a zenith in 1994 and have been in slow decline since (Fig. 2).
The study of NPs from marine prokaryotes (excluding cyanobacteria) includes isolates obtained from the surface or within macro-organism tissues as well as from water and sediment. Over the last 67 years there have been 1151 papers that have reported new MNPs from marine bacteria. Of these, 648 have been from water or sediment samples, with corals, sponges, echinoderms, algae, ascidians, fish and bryozoans contributing to most of the remainder. In contrast, MNPs reported from cyanobacteria are almost exclusively derived from wild harvested material (Fig. 2).
The Actinobacteria are the most studied phylum of marine bacteria, and the organisms are also a prolific source of terrestrial bacterial NPs. However, the marine environment is different from terrestrial biomes and genomic data obtained from cultured, metagenomic and single amplified genome sources from marine environments (sediment and sea water) suggest that Actinobacteria only represent a small proportion of marine bacterial biodiversity with Proteobacteria being a dominant group (Fig. 3).562–564
 | ||
| Fig. 3 Marine bacterial diversity (a) proportion of bacteria phyla yielding new MNPs (b) proportion of bacteria phyla found in marine environments based on metagenomic analysis. | ||
In 2022 we highlighted the dissimilarity of marine microorganism NP chemistry with that of marine macro-organisms and further highlighted the high similarity between terrestrial and marine microbial NP chemical diversity.565
Research efforts to discover new MNPs from marine prokaryotes are striking in that only a few of the studies on cultures obtained from marine habitats and reporting new chemistry have produced known MNPs isolated previously from marine invertebrates. The serendipitous discoveries of prokaryotes isolated from marine habitats that have yielded didemnin B and the lobatamides that were originally isolated from ascidians are two examples but these microbes were not found in the ascidian hosts that yielded these MNPs.566,567 Chemical diversity analysis using a self-organising map (Fig. 4) shows that there is little overlap between NPs reported from either sponges, cnidarians or ascidians and the microbes that have been isolated and cultured from their tissues.
 | ||
Fig. 4 Self-organising map depicting chemical diversity of sponge derived MNPs (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 267) (blue) and MNPs (272) obtained from bacteria isolated from sponge (red). 267) (blue) and MNPs (272) obtained from bacteria isolated from sponge (red). | ||
The one report that stands out is that by Berlinck et al. of the isolation of a Pseudovibrio strain from a Brazilian Demospongiae sponge (Arenosclera brasiliensis, order: Haplosclerida) that produces bromotyrosine alkaloids that were only previously reported from sponges of the Demospongiae order Verongiida.568,569 This begs the question, do Verongiida sponges also harbour Pseudovibrio species with the capacity of making bromotyrosine alkaloids? Previous cell localisation experiments suggested that Veroingiid sponge cells accumulated the alkaloids but definitive proof of their biosynthesis within sponge cells is lacking.567 Somewhat perplexing is the fact that the sponge Arenosclera brasiliensis harbouring Pseudovibrio denitrificans has not been reported to produce bromotyrosine alkaloids.
Although there is specific and circumstantial evidence to support the bacterial origin of some macro-organism NPs, the last 20 years of research has not provided many examples where bacteria isolated from marine macro-organisms have generated cultures that can be used for sustainable production of MNPs in the lab. In fact, there are only three examples (manzamine A Acanthostrongylophora ingens/Micromonospora M42; patellamides Lissoclinum patella/Prochloron didemni; and diketopiperazines Tedania ignis/Minococcus sp.) where a viable bacteria/cyanobacterial strain that produces the NP has been isolated from the macro-organism host that contains the MNP.558,570–572 However, the manzamine A result must be considered provisional since more recent studies have shown that the original strain no longer produces the compound.573
Notably, an increasing number of studies have definitively identified microbial sources for NPs initially reported from marine invertebrates. The seminal studies by Piel, Schmidt and Jaspars are highlights here.571,572,574 Current evidence has shown that many of the symbiotic microorganisms found in marine macro-organisms that produce the NP are obligate symbionts that are unlikely to be culturable. These obligate symbionts are mainly in bacterial phyla that, to date, remain unculturable and therefore have not been shown to produce isolable NPs.574 This highlights the need to find alternative methods to exploit their biosynthetic genes through heterologous gene expression. There have been examples where bacteria isolated from sediment or seawater have yielded NPs that are the same or closely related to macro-organism NPs, but these are the exceptions rather than the rule and, in most cases, a prokaryote sourced from a terrestrial habitat has yielded NPs closely linked to a marine invertebrate-derived NP.
Analysis of data reported in reviews574–577 (including previous installments of this review), and compound matching within chemical databases (MarinLit vs. NP Atlas)2,578 suggests that marine invertebrate NPs from 46 compound classes have been definitively identified to be biosynthesised by prokaryotes (see Tables S1 and Fig S1 in the ESI†) (Fig. 5). These associations have been established through (i) BGC analysis which has identified 28 compound classes (one identified from two different bacteria phyla), (ii) analysis of NPs from separated prokaryote cells obtained from macro-organism hosts has identified four compound classes or (iii) culturing of randomly sourced bacteria (seven compound classes) and wild harvest of cyanobacteria (two compound classes) with a further six compound classes initially isolated from prokaryote cultures then later found in invertebrates. A further 17 invertebrate MNP structure classes have been associated with prokaryote sources by inference because similar molecules have been reported from microbes and marine invertebrates.
Within these 64 MNP structure classes, 39 have been identified as having a marine prokaryote origin, 20 are associated with terrestrial prokaryotes and two are associated with both marine and terrestrial prokaryotes. Invertebrate MNP compound classes that are also found in marine prokaryote cultures are from Cyanobacteria (patellamides, palyotoxin, swinholide), Actinomycetes (manzamine, diketopiperazine) and Proteobacteria (fistularin, dideminin). Terrestrial microbial cultures Proteobacteria (2), Actinomycete (5), Myxococcota (1), and Amoebozoa (1) are the source of eight invertebrate MNP compound classes. There are four marine invertebrate compound classes that have similar compounds in marine microbes, two are from Cyanobacteria and two are from Proteobacteria. The 13 compound classes from invertebrates that are similar to terrestrial microbial compounds are found in Proteobacteria (4), Myxococcota (6), Cyanobacteria (4), and Actinomycetes (2). Three structure classes are found in two different prokaryote sources. Microbial BGCs that encode enzymes that biosynthesise MNPs originally found in marine invertebrates are associated with microbes from Tectomicrobia (11) Proteobacteria (5), Cyanobacteria (5), Verrucomicrobia (3), Kiritimatiellaeota (1), Acidobacteriota (1), Bacteroidata (1), and one unknown phylum.
Microorganism-associated MNPs isolated from marine invertebrates have primarily been investigated within sponges and ascidians. There are 41 sponge derived structures (18 polyketides, 15 peptides, 6 alkaloids, 1 terpenoid, 1 phenol) that have been ascribed to a prokaryotic source (either through genomics, microbial culture, cell separation/imaging or structural similarity). Congeners of these structures represent 604 sponge-derived MNPs (5.8% of all sponge MNPs). Of the structures (and their congeners) definitively ascribed to prokaryotes by BGC analysis or cultivation, 8.5% of peptides, 8% of polyketides and 10.6% of alkaloids. This suggests that to date only 4.2% of all sponge NPs can been ascribed a microbial origin. A similar analysis of ascidian NPs shows that 14 structures have been ascribed to prokaryotes. When congeners of these structures are included, 101 ascidian derived NPs (7.8% of ascidian NPs) are likely to be biosynthesised by prokaryotes and these represent 43% of peptides, 27.5% of polyketides and 3.2% of the alkaloids reported from ascidians.
The average time between the discovery of a marine invertebrate NP and ascribing its biosynthesis to a symbiotic microbe is >15 years (Fig. 6). BGC analysis has proven to be the most successful technique to identify a bacterial source within an invertebrate that produces the NP. However, this method has only been successful for PKS, NRPS and RiPP NP structure classes. This is most likely because the biosynthesis of PKS, NRPS and RiPP NPs is associated with clustered genes that produce enzymes that are co-located in the cell. Other classes of NPs appear to have more complex biosynthetic pathways, and the enzymes used to make them may not be clustered. This is particularly the case with some alkaloids. The full biosynthetic pathway to ET-743, for example remains to be discovered.579
Since the structural uniqueness of MNPs continues to be linked to wild harvested macro-organisms and cyanobacteria, but annually over half of all new MNPs are reported from marine microbes, the lessons learnt from identifying the prokaryote producers of marine macro-organism derived MNPs should be applied to aid in the selection of uniquely marine microbes for the discovery and exploitation of unique microbial MNP chemical diversity.
Furthermore, the realisation that many of the marine microorganisms responsible for macro-organism derived MNPs have eluded culturing has meant that other methods to access their rich biosynthetic diversity are required. Heterologous expression of BGCs identified from metagenomic analysis of environmental samples (accessed from sediment, seawater or macro-organism hosts) in culturable hosts is one way to access this chemical diversity.580–583
Recent genomic studies have also started to reveal the capabilities of marine invertebrates to manufacture NPs de novo.584–586 Both cnidarians and sponges have capabilities to biosynthesise terpenoids, a structure class that represents 89% and 65% of NPs reported from these phyla, respectively. Alkaloids possess some of the most diverse structures and play a predominant role to drug development, yet their biosynthesis and true origins remain mostly unresolved.
Finally, the number of invertebrate species that have been shown to contain NPs of prokaryote origin is also very limited. Five genera (four sponge and one ascidian) account for half of the compound classes ascribed to prokaryotes Theonella (structure classes: swinholide, onnamide, theopederin, konbamide, cyclotheronamide, keramamide, pseudotheonamide, nazumamide, polytheonamide, motuporin, theopalauamide, theonellamide) Discoderma (structure classes: calyculin, discodermide, kasumigamide), Mycale (peloruside, mycalamides, pateamine), Dysidea (structure classes: bromodiphenyl ethers, trichloropeptides, arenastatin, dysonosin, dysidazirine), Lissoclinum (structure classes: patellamides, lissoclinamide, patellins trunkamides, ascidiacyclamide, patellazoles, mandelalide, lissoclinolide, haterumalide). It is difficult to know if these limited examples reflect the difficulty in studying symbiosis or if these super-producer sponge and ascidian species have a unique ability to host diverse microbiomes that manufacture a range of NPs compared to most invertebrates.
Although there is growing evidence for the contribution of marine prokaryotes to MNP production in marine invertebrates, this knowledge has not translated into widespread sustainable production of marine invertebrate-derived NPs in culturable microbes. Furthermore, the promise of targeting the culture of marine prokaryotes sampled from marine habitats as an alternative way to discover the unique chemical diversity of marine invertebrates has so far not eventuated. Metagenomics has certainly highlighted the huge potential of marine bacteria to yield new chemical diversity based on the predicted uniqueness of BGCs that they host, and heterologous gene expression appears to be the most promising way forward to harness this diversity. A complimentary approach would be to expand studies of marine invertebrates using cheminformatics tools to identify unique chemicals. Flipping the focus of outputs generated by GNPS to target the unique unknown singletons rather than the clusters of unknown analogues within well studied structure classes may be a good starting point.
14 Conflicts of interest
There are no conflicts to declare.15 Acknowledgements
We thank Royal Society of Chemistry for the provision of data used in this review, adapted from the MarinLit database with permission from the Royal Society of Chemistry.2 We thank Jeroen Gillard from the Pohnert Group at the Max Planck Institute for Chemical Ecology, Jena, Germany for the image of the diatom that was used to create the image in the TOC graphic.16 References
- A. R. Carroll, B. R. Copp, T. Grkovic, R. A. Keyzers and M. R. Prinsep, Nat. Prod. Rep., 2024, 41, 162–207, 10.1039/D3NP00061C.
- http://pubs.rsc.org/marinlit, accessed July 2024.
- S.-S. Zhang, L. Li, Y. Wu, Z.-M. Wu, C. Kong, L.-L. Hong, S. Zhang, X.-L. Lin, H.-W. Lin and S.-P. Wang, J. Nat. Prod., 2023, 86, 1708–1714, DOI:10.1021/acs.jnatprod.3c00127.
- J. S. Gomez, M. Shaikhet, A. K. Loganathan, M. G. Darnowski, C. N. Boddy, D. R. McMullin and T. J. Avis, J. Chem. Ecol., 2023, 49, 528–536, DOI:10.1007/s10886-023-01438-x.
- W. Ding, Y. Li, X. Tian, Z. Xiao, R. Li, S. Zhang and H. Yin, Molecules, 2023, 28, 2133, DOI:10.3390/molecules28052133.
- T. Shi, Y.-J. Li, Z.-M. Wang, Y.-F. Wang, B. Wang and D.-Y. Shi, Mar. Drugs, 2023, 21, 127, DOI:10.3390/md21020127.
- Y. Tian, Y. Jiang, Z. Wen, L. Guan, X. Ouyang, W. Ding and Z. Ma, Int. J. Mol. Sci., 2023, 24, 2822, DOI:10.3390/ijms24032822.
- E. Harunari, H. Doyo, W. Phongsopitanun, S. Tanasupawat, M. Sutthacheep, T. Yeemin and Y. Igarashi, J. Antibiot., 2023, 76, 83–87, DOI:10.1038/s41429-022-00578-8.
- M. Kokkini, D. Oves-Costales, P. Sánchez, Á. Melguizo, T. A. Mackenzie, M. Pérez-Bonilla, J. Martín, A. Giusti, P. de Witte, F. Vicente, O. Genilloud and F. Reyes, Mar. Drugs, 2023, 21, 443, DOI:10.3390/md21080443.
- H. Zhang, X. Ren, H. Xu, H. Qi, S. Du, J. Huang, J. Zhang and J. Wang, Molecules, 2023, 28, 7672, DOI:10.3390/molecules28227672.
- Z. Cheng, Q. Zhang, J. Peng, X. Zhao, L. Ma, C. Zhang and Y. Zhu, Molecules, 2023, 28, 821, DOI:10.3390/molecules28020821.
- C. V. Anh, J. S. Kang, J.-W. Yang, J.-H. Kwon, C.-S. Heo, H.-S. Lee and H. J. Shin, Mar. Drugs, 2023, 21, 494, DOI:10.3390/md21090494.
- R. Xu, H. Zhu, H. Zhang, J. Ju, Q. Li and S. Fu, J. Nat. Prod., 2023, 86, 1512–1519, DOI:10.1021/acs.jnatprod.3c00202.
- L. Sun, H. Zhu, L. Zhang, Y. Zhu, D. Ratnasekera, C. Zhang and Q. Zhang, J. Nat. Prod., 2023, 86, 979–985, DOI:10.1021/acs.jnatprod.2c01169.
- V. R. Martín-Aragón, F. R. Millán, C. Cuadrado, A. H. Daranas, A. F. Medarde and J. M. S. López, Mar. Drugs, 2023, 21, 526, DOI:10.3390/md21100526.
- K. Xia, J. Shang, J. Sun, W. Zhu and P. Fu, ACS Omega, 2023, 8, 28886–28897, DOI:10.1021/acsomega.3c04199.
- H.-S. Lee, D. P. Nagahawatta, Y.-J. Jeon, M. A. Lee, C.-S. Heo, S. J. Park and H. J. Shin, Mar. Drugs, 2023, 21, 640, DOI:10.3390/md21120640.
- B. Zhang, Y.-Y. Zhao, Z. P. Huang, K. T. Zhang, X. R. Xu, Q. Zhao and X.-M. Zhang, Nat. Prod. Res., 2023, 37, 2144–2150, DOI:10.1080/14786419.2022.2030734.
- X. Xu, F. Zhang, L. Zhou, Y. Chang, Q. Che, T. Zhu, D. Li and G. Zhang, Mar. Drugs, 2023, 21, 240, DOI:10.3390/md21040240.
- Z. Zhang, E. Harunari and Y. Igarashi, J. Antibiot., 2023, 76, 618–622, DOI:10.1038/s41429-023-00644-9.
- T.-H. Huynh, E. S. Bae, B. E. Heo, J. Lee, J. S. An, Y. Kwon, S.-J. Nam, K.-B. Oh, J. Jang, S. K. Lee and D.-C. Oh, Mar. Drugs, 2023, 21, 500, DOI:10.3390/md21090500.
- Y. Dong, E. Jin, R. Wang, Y. Bao and H. Li, Chem. Biodiversity, 2023, 20, e202300689, DOI:10.1002/cbdv.202300689.
- J. H. Im, Y.-H. Shin, E. S. Bae, S. K. Lee and D.-C. Oh, Mar. Drugs, 2023, 21, 405, DOI:10.3390/md21070405.
- Y. Bai, D. Ren, F. Li, J. Hu and H. Pan, Chem. Biodiversity, 2023, 20, e202300715, DOI:10.1002/cbdv.202300715.
- W. Zhang, Q. Che, H. Tan, X. Qi, D. Li, T. Zhu and M. Liu, Life Sci., 2023, 330, 121998, DOI:10.1016/j.lfs.2023.121998.
- Y. Ge, Y. Ma, C. Zhou, Z. Zhang, Q. Yin, X. Zhang, Z. Zhang and B. Wu, Chem. Biodiversity, 2023, 20, e202301345, DOI:10.1002/cbdv.202301345.
- C.-S. Heo, J. S. Kang, J.-H. Kwon, C. V. Anh and H. J. Shin, Mar. Drugs, 2023, 21, 167, DOI:10.3390/md21030167.
- S. Pulat, D.-A. Kim, P. F. Hillman, D.-C. Oh, H. Kim, S.-J. Nam and W. Fenical, Mar. Drugs, 2023, 21, 489, DOI:10.3390/md21090489.
- L. Qin, K. Yong, X.-Y. Lian and Z. Zhang, Nat. Prod. Res., 2023, 37, 478–483, DOI:10.1080/14786419.2021.1981315.
- Y. Zhuang, F. Yang, A. Menon, J. M. Song, R. V. Espinoza, P. J. Schultz, A. L. Garner and A. Tripathi, J. Nat. Prod., 2023, 86, 1801–1814, DOI:10.1021/acs.jnatprod.3c00306.
- Z.-G. Ding, M.-G. Li, J. Ren, J.-Y. Zhao, R. Huang, Q.-Z. Wang, X.-L. Cui, H.-J. Zhu and M.-L. Wen, Org. Biomol. Chem., 2011, 9, 2771–2776, 10.1039/c1ob05044c.
- M. S. Abdelfattah, T. Kazufumi and M. Ishibashi, J. Nat. Prod., 2010, 73, 1999–2002, DOI:10.1021/np100400t.
- X. Wang, M. Abbas, Y. Zhang, S. I. Elshahawi, L. V. Ponomareva, Z. Cui, S. G. V. Lanen, I. Sajid, S. R. Voss, K. A. Shaaban and J. S. Thorson, J. Nat. Prod., 2019, 82, 1686–1693, DOI:10.1021/acs.jnatprod.9b00289.
- F. Yang, M. Sang, J.-R. Lu, H.-M. Zhao, Y. Zou, W. Wu, Y. Yu, Y.-W. Liu, W. Ma, Y. Zhang, W. Zhang and H.-W. Lin, Angew. Chem., Int. Ed., 2023, 62, e202218085, DOI:10.1002/anie.202218085.
- Z. Liu, Y. Yashiroda, P. Sun, H. Ma, Y. Wang, L. Li, F. Yan and Y. Sun, Org. Lett., 2023, 25, 571–575, DOI:10.1021/acs.orglett.2c03290.
- M. Uemura, K. Kobayashi, N. Sato, K. Nagai, R. Seki, M. Kamio, T. Fukuda, T. Tsubouchi, H. Tomoda, T. Ohshiro, T. Kobayashi and T. Terahara, J. Antibiot., 2023, 76, 650, DOI:10.1038/s41429-023-00648-5.
- M. C. Kim, J. M. Winter, R. Cullum, A. J. Smith and W. Fenical, Mar. Drugs, 2023, 21, 367, DOI:10.3390/md21060367.
- J. S. An, H. Lee, H. Kim, S. Woo, H. Nam, J. Lee, J. Y. Lee, S.-J. Nam, S. K. Lee, K. -B. Oh, S. Kim and D. -C. Oh, Angew. Chem., Int. Ed., 2023, 62, e202300998, DOI:10.1002/anie.202300998.
- J. -X. Yan, Q. Wu, M. Maity, D. R. Braun, I. Alas, X. Wang, X. Yin, Y. Zhu, B. A. Bell, S. R. Rajski, Y. Ge, D. D. Richardson, W. Zhong and T. S. Bugni, Chem.–Eur. J., 2023, 29, e202301813, DOI:10.1002/chem.202301813.
- Q. Lu, G. Wu, X. Hao, X. Hu, H. Cai, X. Liu, X. You, H. Guo and C. Sun, Mar. Drugs, 2023, 21, 143, DOI:10.3390/md21030143.
- H. Huang, L. Yue, F. Deng, X. Wang, N. Wang, H. Chen and H. Li, J. Nat. Prod., 2023, 86, 2122–2130, DOI:10.1021/acs.jnatprod.3c00310.
- C. V. Anh, J. S. Kang, J.-W. Yang, J.-H. Kwon, C.-S. Heo, H.-S. Lee, C. H. Park and H. J. Shin, Mar. Drugs, 2023, 21, 361, DOI:10.3390/md21060361.
- H. Wen, D. Zhang, H. Zhao, Y. Zhang, X. Yan, W. Lin, S. He and L. Ding, Phytochemistry, 2023, 213, 113779, DOI:10.1016/j.phytochem.2023.113779.
- J. Cui, S. Ye, D. Shin, I. Cho, H. Y. Kim, Y. Kwon, K. Park, S.-J. Nam, Y. Kim and D.-C. Oh, Mar. Drugs, 2023, 21, 67, DOI:10.3390/md21020067.
- X. Huang, Y. Wang, L. Zhou, W. Wang, K. Anjum, J. Zhang, G. Zhang, T. Zhu, D. Li and Q. Che, Tetrahedron Lett., 2023, 121, 154477, DOI:10.1016/j.tetlet.2023.154477.
- S. Lu, Z. Zhang, A. R. Sharma, J. Nakajima-Shimada, E. Harunari, N. Oku, A. Trianto and Y. Igarashi, J. Nat. Prod., 2023, 86, 1081–1086, DOI:10.1021/acs.jnatprod.2c01083.
- W. Zhong, J. M. Deutsch, D. Yi, N. H. Abrahamse, I. Mohanty, S. G. Moore, A. C. McShan, N. Garg and V. Agarwal, ChemBioChem, 2023, 24, e202300190, DOI:10.1002/cbic.202300190.
- W. Zhong, N. Aiosa, J. M. Deutsch, N. Garg and V. Agarwal, J. Nat. Prod., 2023, 86, 2414–2420, DOI:10.1021/acs.jnatprod.3c00595.
- C. Makris, J. K. Leckrone and A. Butler, J. Nat. Prod., 2023, 86, 1770–1778, DOI:10.1021/acs.jnatprod.3c00230.
- J. Chen, Y. Guo, Q. Wu, W. Wang, J. Pan, M. Chen, H. Jiang, Q. Yin, G. Zhang, B. Wei, H. Zhang and H. Wang, J. Agric. Food Chem., 2023, 71, 6584–6593, DOI:10.1021/acs.jafc.3c00234.
- J. Jia, X. Wang, J. Sang, Z. Li, S. Lin, Z. Deng and T. Huang, Nat. Prod. Res., 2023, 37, 3647–3653, DOI:10.1080/14786419.2022.2098496.
- A. N. Al-Rawahi, R. M. M. Abed, N. Ur Rehman, K. Rafiq, A. Khan, A. L. Khan, M. Khan, S. A. Halim, F. Al-Senafi, H. Mahmoud and A. Al-Harrasi, Chem. Nat. Compd., 2023, 59, 346–350, DOI:10.1007/s10600-023-03990-0.
- B. S. Kalyani, P. S. Krishna, E. Laxminarayana and K. Sreenivasulu, Pharm. Chem. J., 2023, 57, 834–841, DOI:10.1007/s11094-023-02957-3.
- L. Ding, W. Li, X. Zhong, F. Feng, Y. Xin, X. Yan, J. E. H. Lazaro, B. Zhang, Y. Shi, G.-J. Yang and S. He, J. Mol. Struct., 2023, 1290, 135809, DOI:10.1016/j.molstruc.2023.135809.
- R. Giugliano, G. D. Sala, C. Buonocore, C. Zannella, P. Tedesco, F. P. Esposito, C. Ragozzino, A. Chianese, M. V. Morone, V. Mazzella, L. Nunez-Pons, V. Folliero, G. Franci, A. D. Filipps, M. Galdiero and D. de Pascale, Pharmaceutics, 2023, 15, 2139–2165, DOI:10.3390/pharmaceutics15082139.
- J. A. Shapiro, S. J. Post, G. C. Smith and W. M. Wuest, Org. Lett., 2023, 25, 9243–9248, DOI:10.1021/acs.orglett.3c03993.
- A. Canko, G. D. Athanassopoulou, V. Psycharis, C. P. Raptopoulou, J. M. Herniman, V. Mouchtouris, A. S. Foscolos, E. A. Couladouros and V. P. Vidali, Org. Biomol. Chem., 2023, 21, 3761–3765, 10.1039/D3OB00476G.
- B. Zhang, K. Zheng and R. Hong, ACS Cent. Sci., 2023, 9, 84–92, DOI:10.1021/acscentsci.2c01096.
- D. M. Sarnes, V. Struck, P. G. Jones and T. Lindel, Synthesis, 2023, 56, 408–417, DOI:10.1055/a-2202-7145.
- R. Nandi, S. Niyogi, S. Kundu, V. R. Gavit, M. Munda, R. Murmu and A. Bisai, Chem. Sci., 2023, 14, 8047, 10.1039/D2SC07119C.
- T. Kim, H. S. Kim and Y. T. Han, Tetrahedron Lett., 2023, 123, 154549, DOI:10.1016/j.tetlet.2023.154549.
- Y. Jiang, X. Liu, W. Li, X. Jiao and P. Xie, J. Org. Chem., 2023, 88, 3981–3986, DOI:10.1021/acs.joc.2c03066.
- S. X. Lin and J. Sperry, Tetrahedron Lett., 2023, 127, 154671, DOI:10.1016/j.tetlet.2023.154671.
- M. Damai, N. Guzzardi, V. Lewis, Z. X. Rao, D. Sykes and B. Patel, RSC Adv., 2023, 13, 29561–29567, 10.1039/D3RA05952A.
- R. A. Allen and W. M. Wuest, ChemMedChem, 2023, 18, e202300235, DOI:10.1002/cmdc.202300235.
- J. Ding and A. B. Smith, J. Am. Chem. Soc., 2023, 145, 18240–18246, DOI:10.1021/jacs.3c06573.
- M. C. Frischling and S. B. Herzon, Org. Lett., 2023, 25, 3723–3727, DOI:10.1021/acs.orglett.3c01175.
- M. H. Sahana, D. Paul, H. Sharma and R. K. Goswami, Org. Lett., 2023, 25, 7827–7831, DOI:10.1021/acs.orglett.3c03066.
- J. Liang, J. She, J. Fu, J. Wang, Y. Ye, B. Yang, Y. Liu, X. Zhou and H. Tao, Mar. Drugs, 2023, 21, 236, DOI:10.3390/md21040236.
- H. U. Devkar, N. L. Thakur and P. Kaur, Can. J. Microbiol., 2023, 69, 1–16, DOI:10.1139/cjm-2022-0147.
- G. Siro, L. Donald and A. Pipite, Diversity, 2023, 15, 30, DOI:10.3390/d15010030.
- U. M. Chukwudulue, N. Barger, M. Dubovis and T. L. Knaan, Mar. Drugs, 2023, 21, 569, DOI:10.3390/md21110569.
- O. C. Eze, D. P. Berebon, S. C. Emencheta, S. A. Evurani, C. N. Okorie, V. M. Balcão and M. M. D. C. Vila, Pharmaceuticals, 2023, 16, 1091, DOI:10.3390/ph16081091.
- W. Wang, L. Gu, J. Wang, X. Hu, B. Wei, H. Zhang, H. Wang and J. Chen, Mar. Drugs, 2023, 21, 547, DOI:10.3390/md21100547.
- S. Zhang, Y. Chen, J. Zhu, Q. Lu, M. J. Cryle, Y. Zhang and F. Yan, Nat. Prod. Rep., 2023, 40, 557–594, 10.1039/D2NP00044J.
- M. A. Tammam and A. El-Demerdash, Curr. Res. Biotechnol., 2023, 6, 100145, DOI:10.1016/j.crbiot.2023.100145.
- J. T. Wibowo, A. Bayu, W. D. Aryati, C. Fernandes, A. Yanuar, A. Kijjoa and M. Y. Putra, Mar. Drugs, 2023, 21, 50, DOI:10.3390/md21010050.
- R. N. Morgan, A. A. Ali, M. Y. Alshahrani and K. M. Aboshanab, Microorganisms, 2023, 11, 2444, DOI:10.3390/microorganisms11102444.
- X. Li, H. Xu, Y. Li, S. Liao and Y. Liu, Molecules, 2023, 28, 6371, DOI:10.3390/molecules28176371.
- S. Kokkaliari, D. Luo, V. J. Paul and H. Luesch, Mar. Drugs, 2023, 21, 378, DOI:10.3390/md21070378.
- Z. Chen, N. Chen, P. Fu, W. Wang, S. Bian, H. Zhang, S. Shen and B. Han, Molecules, 2023, 28, 2786, DOI:10.3390/molecules28062786.
- K. Aihara, F. Nakamura and Y. Nakao, Chem. Lett., 2023, 52, 270–272, DOI:10.1246/cl.230035.
- N. F. Salleh, J. Wang, B. Kundukad, E. T. Oluwabusola, D. X. Y. Goh, M. Y. Phyo, J. J. L. Tong, S. Kjelleberg and L. T. Tan, Molecules, 2023, 28, 3965, DOI:10.3390/molecules28093965.
- Y. K.-H. Schneider, A. Liaimer, J. Isaksson, O. S. B. Wilhelmsen, J. H. Andersen, K. Ø. Hansen and E. H. Hansen, Front. Microbiol., 2023, 14, 1130018, DOI:10.3389/fmicb.2023.1130018.
- J. Dai, C. S. Philbin, C. Wakano, W. Y. Yoshida and P. G. Williams, Mar. Drugs, 2023, 21, 101, DOI:10.3390/md21020101.
- K. Umeda, A. Iwasaki, R. Taguchi, N. Kurisawa, G. Jeelani, T. Nozaki and K. Suenaga, J. Nat. Prod., 2023, 86, 2529–2538, DOI:10.1021/acs.jnatprod.3c00742.
- H. Nagai, M. Watanabe, S. Sato, M. Kawaguchi, Y.-Y. Xiao, K. Hayashi, R. Watanabe, H. Uchida and M. Satake, Tetrahedron, 2019, 75, 2486, DOI:10.1016/j.tet.2019.03.020.
- N. Kanda, B.-T. Zhang, A. Shinjo, M. Kamiya, H. Nagai, H. Uchida, Y. Araki, T. Nishikawa and M. Satake, Nat. Prod. Commun., 2023, 18, 1, DOI:10.1177/1934578X231173799.
- W. Jiang, Y. Bu, M. Kawaguchi, H. Osada, M. Fukuoka, H. Uchida, R. Watanabe, T. Suzuki and H. Nagai, Phytochem. Lett., 2017, 22, 163–166, DOI:10.1016/j.phytol.2017.09.025.
- H. Nagai, S. Shingo, I. Kaori, H. Kazutaka, K. Mioko, U. Hajime and S. Masayuki, Toxins, 2018, 11, 366–372, DOI:10.3390/toxins11060366.
- M. Kawaguchi, S. Masayuki, B.-T. Zhang, Y.-Y. Xiao, M. Fukuoka, H. Uchida and H. Nagai, Molecules, 2020, 25, 457–463, DOI:10.3390/molecules25030457.
- A. Murakami, J.-I. Hayashi, K. Igawa, M. Tsutsumi, K. Tomooka, H. Nagai and T. Nehira, Chirality, 2020, 32, 1152–1159, DOI:10.1002/chir.23264.
- K. Iguchi, M. Satake, Y. Nishio, B.-T. Zhang, K. Kawashima, H. Uchida and H. Nagai, Heterocycles, 2021, 102, 1287–1293, DOI:10.3987/COM-21-14447.
- M. Satake, K. Iguchi, R. Watanabe, H. Uchida and H. Nagai, Results Chem., 2021, 3, 1000206–1000208, DOI:10.1016/j.rechem.2021.100206.
- K. Ozaki, Y. Asato, N. Natsume, S. Tojo, S. Sumimoto, A. Iwasaki, K. Suenaga and T. Teruya, J. Nat. Prod., 2023, 86, 1564–1570, DOI:10.1021/acs.jnatprod.3c00256.
- J. L. Meyer, S. P. Gunasekra, A. L. Brown, Y. Ding, S. Miller, M. Teplinski and V. J. Paul, Mar. Drugs, 2023, 21, 76, DOI:10.3390/md2120076.
- S. C. do Amaral, L. P. Xavier, V. Vasconcelos and A. V. Santos, Mar. Drugs, 2023, 21, 359, DOI:10.3390/md21060359.
- U. Singh, H. A. Gandhi, Nikita, J. Bhattacharya, R. Tandon, G. L. Tiwari and R. Tandon, Arch. Microbiol., 2023, 205, 164, DOI:10.1007/s00203-023-03514-y.
- A. Gupta, P. R. Singh, A. P. Singh, N. Kumari, J. Jaiswal, N. Sahu, S. Mishra, J. Pathak and R. P. Sinha, Curr. Protein Pept. Sci., 2023, 24, 805–819, DOI:10.2174/1389203724666230411091726.
- F. Kudo, T. Chikuma, M. Nambu, T. Chisuga, S. Sumimoto, A. Iwasaki, K. Suenaga, A. Miyanaga and T. Eguchi, ACS Chem. Biol., 2023, 18, 875–883, DOI:10.1021/acschembio.3c00011.
- P. N. Leão, T. P. Martins, K. Abt, J. P. A. Reis, S. Figueiredo, R. Castelo-Branco and S. Freitas, Chem. Commun., 2023, 59, 4436–4446, 10.1039/D3CC00136A.
- P. M. D'Agostino, Nat. Prod. Rep., 2023, 40, 1701–1717, 10.1039/D3NP00011G.
- D. Bae, J.-W. Nam, H. Park, P. Chaudhary, J.-A. Kim, H. Lee and B.-S. Jeong, J. Nat. Prod., 2023, 86, 2585–2591, DOI:10.1021/acs.jnatprod.3c00629.
- Y. Miyamoto, A. Iwasaki, H. Fujimura, C. Kudo, N. Kurisawa, O. Ohno and K. Suenaga, J. Org. Chem., 2023, 88, 3208–3216, DOI:10.1021/acs.joc.2c03007.
- Y.-H. Lo, A. Iwasaki and K. Suenaga, J. Org. Chem., 2023, 88, 10565–10573, DOI:10.1021/acs.joc.3c00595.
- Y.-H. Lo, K. Umeda, N. Kurisawa, G. Jeelani, T. Nozaki and K. Suenaga, Tetrahedron Lett., 2023, 130, 154763, DOI:10.1016/j.tetlet.2023.154763.
- A. H. Tang and R. J. Payne, Tetrahedron, 2023, 139, 133445, DOI:10.1016/j.tet.2023.133445.
- D. Dhakal, D. Kallifidas, M. Chen, S. Kokkaliari, Q.-Y. Chen, V. J. Paul, Y. Ding and H. Luesch, Org. Lett., 2023, 25, 2238, DOI:10.1021/acs.orglett.3c00462.
- S. Pulat, P. F. Hillman, S. Kim, R. N. Asolkar, H. Kim, R. Zhou, İ. Taş, C. D. B. Gamage, M. Varlı, S.-Y. Park, S. C. Park, I. Yang, J. Shin, D.-C. Oh, H. Kim, S.-J. Nam and W. Fenical, Mar. Drugs, 2023, 21, 153, DOI:10.3390/md21030153.
- S.-T. Fang, Y.-P. Song, F.-P. Miao, X.-L. Yin and N.-Y. Ji, Phytochemistry, 2023, 209, 113645, DOI:10.1016/j.phytochem.2023.113645.
- H.-L. Wang, R. Li, M. Zhao, Z.-Y. Wang, H. Tang, Z.-Y. Cao, G.-L. Zheng and W. Zhang, J. Nat. Prod., 2023, 86, 429–433, DOI:10.1021/acs.jnatprod.2c01028.
- M. Jiang, H. Guo, Q. Wu, X. Lu, Y. Zou, Q. Fu, S. Chen, L. Liu, B. Peng and S. Chen, Bioorg. Chem., 2023, 139, 106715, DOI:10.1016/j.bioorg.2023.106715.
- M. Jiang, Q. Wu, H. Guo, X. Lu, S. Chen, L. Liu and S. Chen, J. Nat. Prod., 2023, 86, 2651–2660, DOI:10.1021/acs.jnatprod.3c00664.
- Y. Hu, K. Zhao, J. Zhu, X. Qi, W. Chen, Y. Song, X. Pang, Y. Liu and J. Wang, J. Ocean Univ. China, 2023, 22, 1446–1451, DOI:10.1007/s11802-023-5439-2.
- B. Yang, C. Li, Y. Chen, Y. He, J. She, X. Zhou, H. Tao and B. Peng, Molecules, 2023, 28, 7246, DOI:10.3390/molecules28217246.
- Y. Hu, X. Qi, W. Chen, S. Liao, D. Yang, M. Wang, Y. Liu, Y. Tan and J. Wang, Chem. Biodiversity, 2023, 20, e202300551, DOI:10.1002/cbdv.202300551.
- Y. Hu, S. Ma, X. Pang, M. Cong, Q. Liu, F. Han, J. Wang, W. Feng, Y. Liu and J. Wang, Phytochemistry, 2023, 213, 113765, DOI:10.1016/j.phytochem.2023.113765.
- S. Ma, Y. Hu, J. Chen, X. Wang, C. Zhang, Q. Liu, G. Cai, H. Wang, J. Zheng, Q. Wang, L. Zhong, B. Yang, S. Zhou, Y. Liu, F. Han, J. Wang and J. Wang, Chem.-Biol. Interact., 2023, 382, 110618, DOI:10.1016/j.cbi.2023.110618.
- J. Wu, H. Shui, M. Zhang, Y. Zeng, M. Zheng, K.-K. Zhu, S.-B. Wang, H. Bi, K. Hong and Y.-S. Cai, Front. Microbiol., 2023, 14, 1138830, DOI:10.3389/fmicb.2023.1138830.
- Z. Hu, Y. Zhu, J. Chen, J. Chen, C. Li, Z. Gao, J. Li and L. Liu, J. Agric. Food Chem., 2023, 71, 4298–4305, DOI:10.1021/acs.jafc.2c09141.
- X. Shi, Y. Sun, J. Liu, W. Liu, Y. Xing, Z. Xiu and Y. Dong, Molecules, 2023, 28, 218, DOI:10.3390/molecules28010218.
- Y. Chen, Y. He, X. Pang, X. Zhou, Y. Liu and B. Yang, Mar. Drugs, 2023, 21, 567, DOI:10.3390/md21110567.
- M. S. Ramadhan, R. Riga and E. H. Hakim, J. Microbiol. Biotechnol. Food Sci., 2023, 13, e9467, DOI:10.55251/jmbfs.9467.
- E. B. Belousova, O. I. Zhuravleva, E. A. Yurchenko, G. K. Oleynikova, A. S. Antonov, N. N. Kirichuk, V. E. Chausova, Y. V. Khudyakova, A. S. Menshov, R. S. Popov, E. S. Menchinskaya, E. A. Pislyagin, V. V. Mikhailov and A. N. Yurchenko, Biomolecules, 2023, 13, 741, DOI:10.3390/biom13050741.
- X. Xu, C.-J. Lu, Z.-Z. Tang, Y.-H. Liu, C.-H. Gao, H.-Y. Li, G.-S. Zhang and Z.-W. Su, Rec. Nat. Prod., 2023, 17, 343–351, DOI:10.25135/rnp.355.2207.2518.
- L.-H. Yan, F.-Y. Du, X.-M. Li, S.-Q. Yang, B.-G. Wang and X. Li, Mar. Drugs, 2023, 21, 195, DOI:10.3390/md21030195.
- B. N. S. Ningsih, V. Rukachaisirikul, S. Phongpaichit, S. Preedanon, J. Sakayaroj and C. Muanprasat, Nat. Prod. Res., 2023, 37, 2311–2318, DOI:10.1080/14786419.2022.2039651.
- Z. Hu, J. Chen, Q. Liu, Q. Wu, S. Chen, J. Wang, J. Li, L. Liu and Z. Gao, Chem. Biodiversity, 2023, 20, e202300424, DOI:10.1002/cbdv.202300424.
- Y. Guo, Y. Ying, Q. Wu, B. Wei, J. Chen, H. Wang and J. Ocean, J. Limnol., 2023, 41, 1159–1167, DOI:10.1007/s00343-022-2007-3.
- F.-Y. Lv, A. Mándi, X.-M. Li, L.-P. Chi, X. Li, B.-G. Wang, T. Kurtán and L.-H. Meng, Deep-Sea Res. Part I, 2023, 195, 104004, DOI:10.1016/j.dsr.2023.104004.
- W. Zhao, Y. Zeng, W. Chang, H. Chen, H. Wang, H. Dai and F. Lv, Mar. Drugs, 2023, 21, 157, DOI:10.3390/md21030157.
- X. Guo, A. Fan, X. Qi, D. Liu, J. Huang and W. Lin, Bioorg. Chem., 2023, 141, 106873, DOI:10.1016/j.bioorg.2023.106873.
- N. T. H. Anh, N. M. Anh, V. T. T. Huyen, P. T. Dao, D. T. M. Huong, P. V. Cuong, D. T. Xuan, B. H. Tai, L. T. H. Minh and P. V. Kiem, Chem. Biodiversity, 2023, 20, e202301660, DOI:10.1002/cbdv.202301660.
- S. Kankanamge, Z. G. Khalil, T. Sritharan and R. J. Capon, J. Nat. Prod., 2023, 86, 508–516, DOI:10.1021/acs.jnatprod.
- Q. Hong, M.-M. Guo, J. Yang, X. Wei, L. Liao, X.-J. Xin, D. Zhang and F.-L. An, Phytochemistry, 2023, 214, 113816, DOI:10.1016/j.phytochem.2023.113816.
- J.-Q. Mao, Y.-Y. Zheng, C.-Y. Wang, Y. Liu and G.-S. Yao, Mar. Drugs, 2023, 21, 219, DOI:10.3390/md21040219.
- W. Ding, D. Tian, M. Chen, Z. Xia, X. Tang, S. Zhang, J. Wei, X. Li, X. Yao, B. Wu and J. Tang, J. Nat. Prod., 2023, 86, 1919–1930, DOI:10.1021/acs.jnatprod.3c00287.
- X. Yang, H. Yu, J. Ren, L. Cai, L. Xu and L. Liu, J. Fungi, 2023, 9, 347, DOI:10.3390/jof9030347.
- Y. Jiang, C. Cui, C. Chen, N. Wang, H. Liao, Q. Li, L. Mao, N. Ding, J. Kang, J. Zhou, H. Zhu, Y. Lai, Z. Wang, Q. Zhou and Y. Zhang, Chem. Biodiversity, 2023, 20, e202301047, DOI:10.1002/cbdv.202301047.
- M. Qader, L. L. Mweetwa, T. Rämä, B. Thissera, B. F. Milne, U. R. Abdelmohsen, R. Orfali, A. Tawfike, M. Esheli, E. T. Oluwabusola, L. Jaysainghe, M. Jaspars and M. E. Rateb, J. Appl. Microbiol., 2023, 134, 1–12, DOI:10.1093/jambio/lxad158.
- J.-X. Li, Q.-H. Xu, R.-Y. Shang, Q. Liu, X.-C. Luo, H.-W. Lin and W.-H. Jiao, Chem. Biodiversity, 2023, 20, e202300010, DOI:10.1002/cbdv.202300010.
- H. Fan, L. Wang, Z.-K. Zhang, P.-P. Wu, Y.-P. He, L.-Y. Chen, Q. Wang and C.-X. Zhang, Bioengineering, 2023, 10, 805, DOI:10.3390/bioengineering10070805.
- J. Wu, L. Qiu, Y. Zhou, S. Yao and D. Zhu, Rec. Nat. Prod., 2023, 516–521, DOI:10.25135/rnp.375.2211.2623.
- Y. Ying, S. Tu, J. Ni, X. Lu, X. Hu, P. Lei, X. Li, Y. Wang, G. Jin and H. Wang, Fitoterapia, 2023, 170, 105662, DOI:10.1016/j.fitote.2023.105662.
- Y.-L. Dong, X.-M. Li, Y.-R. Wang, X.-S. Shi, B.-G. Wang and L.-H. Meng, Fitoterapia, 2023, 168, 105559, DOI:10.1016/j.fitote.2023.105559.
- Y.-L. Dong, X.-M. Li, X.-S. Shi, Y.-R. Wang, B.-G. Wang and L.-H. Meng, Mar. Drugs, 2023, 21, 293, DOI:10.3390/md21050293.
- F. Magot, G. Van Soen, L. Buedenbender, F. Li, T. Soltwedel, L. Grauso, A. Mangoni, M. Blümel and D. Tasdemir, Mar. Drugs, 2023, 21, 95, DOI:10.3390/md21020095.
- Y. Li, J. Shi, R. Liu, Y. Liu, R. Liu, Z. Wu, W. Xu, H. Ma, H.-B. Luo and Z. Cheng, J. Nat. Prod., 2023, 86, 830–841, DOI:10.1021/acs.jnatprod.2c01022.
- Y.-Q. Han, Q. Zhang, W.-F. Xu, Y. Hai, R. Chao, C.-F. Wang, X.-M. Hou, M.-Y. Wei, Y.-C. Gu, C.-Y. Wang and C.-L. Shao, Mar. Life Sci. & Technol., 2023, 5, 85–93, DOI:10.1007/s42995-022-00157-8.
- J.-S. Hu, Y.-P. He, F.-G. Zhou, P.-P. Wu, L.-Y. Chen, C. Ni, Z.-K. Zhang, X.-J. Xiao, L.-K. An, X.-X. He and C.-X. Zhang, Chem. Biodiversity, 2023, 20, e202300301, DOI:10.1002/cbdv.202300301.
- S.-H. Lin, Q.-X. Yan, Y. Zhang, T.-Z. Wu, Z.-B. Zou, Q.-M. Liu, J.-Y. Jiang, M.-M. Xie, L. Xu, Y.-J. Hao, Z. Liu, G.-M. Liu and X.-W. Yang, Mar. Drugs, 2023, 21, 504, DOI:10.3390/md21100504.
- Y.-J. Zhao, L. Li, Y.-H. Zhang, Y.-Y. Yang, L.-F. Li, K. Yang, Y.-F. Liu and F. Cao, Appl. Microbiol. Biotechnol., 2023, 107, 6459–6467, DOI:10.1007/s00253-023-12739-2.
- X. Li, J. Xu, P. Wang and W. Ding, Mycology, 2023, 14, 1–10, DOI:10.1080/21501203.2022.2069173.
- F. Zhang, L. Yang, Q.-Y. Xie, J.-C. Guo, Q.-Y. Ma, L.-T. Dai, L.-M. Zhou, H.-F. Dai, F.-D. Kong, D.-Q. Luo and Y.-X. Zhao, Phytochemistry, 2023, 216, 113888, DOI:10.1016/j.phytochem.2023.113888.
- M. S. Elnaggar, A. M. Elissawy, F. S. Youssef, M. Kicsák, T. Kurtán, A. N. B. Singab and R. Kalscheuer, RSC Adv., 2023, 13, 16480–16487, 10.1039/D3RA02632A.
- S.-T. Fang, Z.-Z. Shi, Y.-P. Song, X.-L. Yin and N.-Y. Ji, Fitoterapia, 2023, 170, 105659, DOI:10.1016/j.fitote.2023.105659.
- W. Xu, X. Liu, W. Lin, M. Ling, C. Guo, H. Meng, Y. Guo, X. Du and R. Liu, Rec. Nat. Prod., 2023, 17, 561–565, DOI:10.25135/rnp.377.2202.2454.
- W. Li, Q. Gao, Y. Hu, Y. Shi, X. Yan, L. Ding and S. He, J. Mol. Struct., 2023, 1271, 134082, DOI:10.1016/j.molstruc.2022.134082.
- X. Qi, W.-H. Chen, X.-P. Lin, S.-R. Liao, B. Yang, X.-F. Zhou, Y.-H. Liu, J.-F. Wang and Y. Li, Nat. Prod. Res., 2023, 37, 441–448, DOI:10.1080/14786419.2021.1978994.
- Z. Tan, Y.-Y. Liu, H.-Z. Zhang, G.-Y. Chen and X.-H. Nong, Chem. Nat. Compd., 2023, 59, 219, DOI:10.1007/s10600-023-03960-6.
- F. W. Jiao, Y. N. Xing, T. Y. Liu, F. Wu, W. Li and R. H. Jiao, Tetrahedron Lett., 2023, 123, 154566, DOI:10.1016/j.tetlet.2023.154566.
- X. Qi, W. Chen, L. Chen, Y. Hu, X. Wang, W. Han, J. Xiao, X. Pang, X. Yao, S. Liu, Y. Li, J. Yang, J. Wang and Y. Liu, Bioorg. Chem., 2023, 132, 106357, DOI:10.1016/j.bioorg.2023.106357.
- O. I. Zhuravleva, E. A. Chingizova, G. K. Oleinikova, S. S. Starnovskaya, A. S. Antonov, N. N. Kirichuk, A. S. Menshov, R. S. Popov, N. Y. Kim, D. V. Berdyshev, A. R. Chingizov, A. S. Kuzmich, I. V. Guzhova, A. N. Yurchenko and E. A. Yurchenko, Mar. Drugs, 2023, 21, 431, DOI:10.3390/md21080431.
- M. Jiang, S. Chen, X. Lu, H. Guo, S. Chen, X. Yin, H. Li, G. Dai and L. Liu, J. Agric. Food Chem., 2023, 71, 9782–9795, DOI:10.1021/acs.jafc.3c02415.
- L. da Silva Oliveira, C. M. Crnkovic, M. R. de Amorim, A. Navarro-Vázquez, T. A. Paz, V. F. Freire, M. Takaki, T. Venâncio, A. G. Ferreira, R. de Freitas Saito, R. Chammas and R. G. S. Berlinck, J. Nat. Prod., 2023, 86, 2065–2072, DOI:10.1021/acs.jnatprod.3c00383.
- Y. Fan, C. Jiang, P. Li, C. Wang, H. Chen and J. Ocean, J. Limnol., 2023, 41, 1145–1151, DOI:10.1007/s00343-022-2017-1.
- Z.-J. Gao, L.-L. Cao, H.-P. Ren, H. Yu and Y. Wang, Front. Microbiol., 2023, 14, 1252563, DOI:10.3389/fmicb.2023.1252563.
- D.-D. Li, X. Luo, W. Ying, E. L. Kim, J. Hong, J.-H. Lee and J. H. Jung, Chem. Biodiversity, 2023, 20, e202300851, DOI:10.1002/cbdv.202300851.
- Q. Huang, M.-J. Hao, L.-Y. Wang, F. Wu, H.-J. Li, J. Yuan, J. Xu, T. Mahmud and W.-J. Lan, Phytochemistry, 2023, 209, 113612, DOI:10.1016/j.phytochem.2023.113612.
- Q.-H. Yan, L. Yang, Q.-Y. Ma, Q.-Y. Xie, H.-F. Dai, Y. Fu and Y.-X. Zhao, Phytochem. Lett., 2023, 55, 6–11, DOI:10.1016/j.phytol.2023.03.005.
- M. Lin, Z. Tang, J. Wang, H. Lu, C. Wang, Y. Zhang, X. Liu, C. Gao, Y. Liu and X. Luo, J. Zhejiang Univ., Sci., B, 2023, 24, 275–280, DOI:10.1631/jzus.B2200622.
- J. R. Virués-Segovia, C. Millán, C. Pinedo, V. E. González-Rodríguez, S. Papaspyrou, D. Zorrilla, T. A. Mackenzie, M. C. Ramos, M. de la Cruz, J. Aleu and R. Durán-Patrón, Mar. Drugs, 2023, 21, 634, DOI:10.3390/md21120634.
- N. Xing, Z. Luo, Y. Cheng, C. Gao, Y. Liu and X.-Q. Chen, Chem. Nat. Compd., 2023, 59, 666–669, DOI:10.1007/s10600-023-04082-9.
- J. Yi, K. Shi, B. Wu, W. Li and G. Chen, Chin. J. Org. Chem., 2023, 43, 295, DOI:10.6023/cjoc202206046.
- Y. Ning, S. Zhang, T. Zheng, Y. Xu, S. Li, J. Zhang, B. Jiao, Y. Zhang, Z. Ma and X. Lu, Mar. Drugs, 2023, 21, 541, DOI:10.3390/md21100541.
- H.-B. Yu, Z. Ning, B. Hu, Y.-P. Zhu, X.-L. Lu, Y. He, B.-H. Jiao and X.-Y. Liu, Mar. Drugs, 2023, 21, 382, DOI:10.3390/md21070382.
- M. Cheng, X. Tang, Z. Shao, G. Li and Q. Yao, J. Nat. Prod., 2023, 86, 2342–2347, DOI:10.1021/acs.jnatprod.3c00534.
- M. Chen, J.-T. Wu, B.-C. Hao, C.-L. Jin, L. Shen, C.-J. Zheng, L.-K. Zhang and X.-J. Zhou, Chem. Nat. Compd., 2023, 59, 237, DOI:10.1007/s10600-023-03965-1.
- C. Lin, R. Huang, J. Liu, H. Li, L. Zhu, X. Huang, B. Ding, L. Liu, H. Huang and Y. Tao, J. Fungi, 2023, 9, 875, DOI:10.3390/jof9090875.
- G. Luo, L. Zheng, Q. Wu, S. Chen, J. Li and L. Liu, Mar. Drugs, 2021, 19, 305, DOI:10.3390/md19060305.
- R. Klaram, T. Dethoup, F. P. Machado, L. Gales, D. Kumla, S. H. Ghoran, E. Sousa, S. Mistry, A. M. S. Silva and A. Kijjoa, Mar. Drugs, 2023, 21, 344, DOI:10.3390/md21060344.
- L. T. H. Minh, N. M. Anh, V. T. T. Huyen, V. T. Quyen, P. T. Dao, D. T. M. Huong, P. V. Cuong, C. D. Tuan, P. V. Kiem and B. H. Tai, Chem. Biodiversity, 2023, 20, e202301425, DOI:10.1002/cbdv.202301425.
- L. Xu, H.-B. Ma, T. Wu, L.-F. Liu, M.-M. Xie, M.-Y. Hu, Y.-B. Gai, T.-H. Zhong and X.-W. Yang, Chem. Biodiversity, 2023, 20, e202300753, DOI:10.1002/cbdv.202300753.
- L.-F. Zhong, J. Ling, L.-X. Luo, C.-N. Yang, X. Liang and S.-H. Qi, Mar. Drugs, 2023, 21, 575, DOI:10.3390/md21110575.
- H.-X. Liang, M.-J. Hao, G.-Y. Zhang, H.-J. Li, W.-Z. Ma, J. Xu and W.-J. Lan, Fitoterapia, 2023, 166, 105433, DOI:10.1016/j.fitote.2023.105433.
- H. J. Shin, M. A. Lee, H.-S. Lee and C.-S. Heo, Mar. Drugs, 2023, 21, 246, DOI:10.3390/md21040246.
- Z.-L. Ma, Z.-P. Yu, Y.-Y. Zheng, N. Han, Y.-H. Zhang, S.-Y. Song, J.-Q. Mao, J.-J. Li, G.-S. Yao and C.-Y. Wang, J. Fungi, 2023, 9, 28, DOI:10.3390/jof9010028.
- J. Cai, X. Wang, X. Gan, Q. Zhou, X. Luo, B. Yang, Y. Liu, D. Ratnasekera and X. Zhou, Mar. Drugs, 2023, 21, 32, DOI:10.3390/md21010032.
- B. Sun, D. Wang, J. Ren, C. Wang, P. Yan, K. R. Gustafson and W. Jiang, J. Nat. Prod., 2023, 86, 1360–1369, DOI:10.1021/acs.jnatprod.3c00221.
- L.-H. Zhang, W.-K. Gao, S.-W. Li, X.-Y. Song, H.-H. Wu, H.-F. Wang, G. Chen, S.-X. Wang and Y.-H. Pei, Phytochemistry, 2023, 211, 113691, DOI:10.1016/j.phytochem.2023.113691.
- Y. Jin, K.-T. Lee, T. Kim, J. Kim, J. W. Lee and S. H. Shim, J. Antibiot., 2023, 76, 474–480, DOI:10.1038/s41429-023-00626-x.
- E. V. Leshchenko, A. S. Antonov, G. V. Borkunov, J. Hauschild, O. I. Zhuravleva, Y. V. Khudyakova, A. S. Menshov, R. S. Popov, N. Y. Kim, M. Graefen, C. Bokemeyer, G. von Amsberg, A. N. Yurchenko and S. A. Dyshlovoy, Mar. Drugs, 2023, 21, 178, DOI:10.3390/md21030178.
- A. N. Yurchenko, O. I. Zhuravleva, O. O. Khmel, G. K. Oleynikova, A. S. Antonov, N. N. Kirichuk, V. E. Chausova, A. I. Kalinovsky, D. V. Berdyshev, N. Y. Kim, R. S. Popov, E. A. Chingizova, A. R. Chingizov, M. P. Isaeva and E. A. Yurchenko, Mar. Drugs, 2023, 21, 584, DOI:10.3390/md21110584.
- Y.-Z. Qiu, Y.-Q. Zhu, H. Lu, X.-B. Li, K.-C. Liu, P.-H. Li, L.-Z. Wang, X.-M. Zhang, H. Chen, H.-W. Lin and S.-S. Zhang, Z. Naturforsch. C, 2023, 78, 345–352, DOI:10.1515/znc-2022-0198.
- P. Li, D. Xie, H. Chen, Y. Qiu, X. Zhang, S. Zhang, L. Wang, H. Lin, X. Li and K. Liu, Biochem. Syst. Ecol., 2023, 107, 104625, DOI:10.1016/j.bse.2023.104625.
- S. Pang, Z.-G. Guo, L. Wang, Q.-F. Guo and F. Cao, Nat. Prod. Res., 2023, 37, 586–591, DOI:10.1080/14786419.2022.2078820.
- Y. Zhang, C.-L. Xie, Y. Wang, X.-W. He, M.-M. Xie, Y. Li, K. Zhang, Z.-B. Zou, L.-H. Yang, R. Xu and X.-W. Yang, Mar. Drugs, 2023, 21, 538, DOI:10.3390/md21100538.
- Y.-H. Li, A. Mándi, H.-L. Li, X.-M. Li, X. Li, L.-H. Meng, S.-Q. Yang, X.-S. Shi, T. Kurtán and B.-G. Wang, Mar. Life Sci. & Technol., 2023, 5, 223–231, DOI:10.1007/s42995-023-00173-2.
- H.-Y. Yu, Y. Li, M. Zhang, Z.-B. Zou, Y.-J. Hao, M.-M. Xie, L.-S. Li, D.-L. Meng and X.-W. Yang, Chem. Biodiversity, 2023, 20, e202301507, DOI:10.1002/cbdv.202301507.
- Y. Ding, Y. Jiang, S. Xu, X. Xin and F. An, Chin. Chem. Lett., 2023, 34, 107562, DOI:10.1016/j.cclet.2022.05.076.
- S. A. Dyshlovoy, O. I. Zhuravleva, J. Hauschild, T. Busenbender, D. N. Pelageev, A. N. Yurchenko, Y. V. Khudyakova, A. S. Antonov, M. Graefen, C. Bokemeyer and G. von Amsberg, Mar. Drugs, 2023, 21, 54, DOI:10.3390/md21010054.
- T. P. T. Hoang, C. Roullier, L. Evanno, I. Kerzaon, E. Gentil, T. R. du Pont, E. -H. Nazih, Y. F. Pouchus, S. Bertrand, E. Poupon and O. Grovel, Chem.–Eur. J., 2023, 29, e202300103, DOI:10.1002/chem.202300103.
- F. Cao, X.-M. Liu, X. Wang, Y.-H. Zhang, J. Yang, W. Li, D.-Q. Luo and Y.-F. Liu, Bioorg. Chem., 2023, 141, 106863, DOI:10.1016/j.bioorg.2023.106863.
- P. M. Eze, Y. Liu, V. E. Simons, S. S. Ebada, T. Kurtán, S. B. Király, C. O. Esimone, F. B. C. Okoye, P. Proksch and R. Kalscheuer, Phytochem. Lett., 2023, 55, 101–104, DOI:10.1016/j.phytol.2023.04.004.
- T. H. A. Nguyen, T. Q. Do, T. L. Nguyen, H. M. L. Thi, M. A. Nguyen, B. T. Murphy, T. Xuan Dam, D. T. M. Huong and P. V. Cuong, Nat. Prod. Commun., 2023, 18, 1–5, DOI:10.1177/1934578X231191636.
- X. Qi, B. Liu and Z. Jiang, Nat. Prod. Res., 2023, 37, 1397–1400, DOI:10.1080/14786419.2021.2008388.
- Y.-H. Li, S.-Q. Yang, X.-M. Li, X. Li, B.-G. Wang and H. Li, Fitoterapia, 2023, 165, 105387, DOI:10.1016/j.fitote.2022.105387.
- M. Zhong, H. Kang, W. Liu, L. Ma and D. Liu, Front. Microbiol., 2023, 14, 1279140, DOI:10.3389/fmicb.2023.1279140.
- Z. Ying, X.-M. Li, S.-Q. Yang, B.-G. Wang, H.-L. Li and L.-H. Meng, Chem. Biodiversity, 2023, 20, e202300229, DOI:10.1002/cbdv.202300229.
- Z. Ying, X.-M. Li, B.-G. Wang, H.-L. Li and L.-H. Meng, J. Antibiot., 2023, 76, 563–566, DOI:10.1038/s41429-023-00634-x.
- E. V. Leshchenko, D. V. Berdyshev, E. A. Yurchenko, A. S. Antonov, G. V. Borkunov, N. N. Kirichuk, V. E. Chausova, A. I. Kalinovskiy, R. S. Popov, Y. V. Khudyakova, E. A. Chingizova, A. R. Chingizov, M. P. Isaeva and A. N. Yurchenko, Int. J. Mol. Sci., 2023, 24, 16568, DOI:10.3390/ijms242316568.
- X. Liu, M. Zhao, J. Chen, W.-C. Pan, S.-L. Tan, H. Cui and Z.-X. Zhao, Fitoterapia, 2023, 165, 105428, DOI:10.1016/j.fitote.2023.105428.
- S.-R. Chen, S.-W. Wang, C.-Y. Chen, T.-Y. Ke, J.-J. Lin, T.-L. Hwang, Y.-T. Huang, Y.-C. Huang and Y.-B. Cheng, Bull. Chem. Soc. Jpn., 2023, 96, 1–7, DOI:10.1246/bcsj.20220235.
- Y. Zeng, Z. Wang, W. Chang, W. Zhao, H. Wang, H. Chen, H. Dai and F. Lv, Mar. Drugs, 2023, 21, 75, DOI:10.3390/md21020075.
- Y. Song, Y. Tan, J. She, C. Chen, J. Wang, Y. Hu, X. Pang, J. Wang and Y. Liu, J. Nat. Prod., 2023, 86, 1171–1178, DOI:10.1021/acs.jnatprod.2c00865.
- Y. Song, J. She, W. Chen, J. Wang, Y. Tan, X. Pang, X. Zhou, J. Wang and Y. Liu, Mar. Drugs, 2023, 21, 532, DOI:10.3390/md21100532.
- J. Yang, Y. Song, Z. Zhou, Y. Huang, S. Wang, J. Yuan, N.-K. Wong, Y. Yan and J. Ju, J. Antibiot., 2023, 76, 113–120, DOI:10.1038/s41429-022-00593-9.
- Y.-J. Hao, Z.-B. Zou, M.-M. Xie, Y. Zhang, L. Xu, H.-Y. Yu, H.-B. Ma and X.-W. Yang, Mar. Drugs, 2023, 21, 234, DOI:10.3390/md21040234.
- C. Lai, D. Tian, M. Zheng, B. Li, J. Jia, J. Wei, B. Wu, H. Bi and J. Tang, Appl. Microbiol. Biotechnol., 2023, 107, 6607–6619, DOI:10.1007/s00253-023-12738-3.
- Q. Chen, Y. Chen, Y. Lu and J. Zhao, Rec. Nat. Prod., 2023, 17, 367–371, DOI:10.25135/rnp.363.2210.2593.
- J.-X. Zhang, B.-D. Zhang, Y. Shi, Y.-N. Zhai, J.-W. Ren, L. Cai, L.-Y. Sun and L. Liu, Magn. Reson. Chem., 2023, 61, 554–559, DOI:10.1002/mrc.5389.
- L. Qiu, J. Wu and Y. Zhou, Rec. Nat. Prod., 2023, 17, 671, DOI:10.25135/rnp.390.2303.2747.
- H. Ku, Y. Lee, S. Lee, J. W. Lee, H.-S. Kang, H. Joo and S. H. Shim, J. Antibiot., 2023, 76, 57–64, DOI:10.1038/s41429-022-00587-7.
- F.-H. Yao, X. Liang and S.-H. Qi, J. Asian Nat. Prod. Res., 2023, 25, 941–948, DOI:10.1080/10286020.2023.2189107.
- Y. Ye, J. Liang, J. She, X. Lin, J. Wang, Y. Liu, D. Yang, Y. Tan, X. Luo and X. Zhou, Mar. Drugs, 2023, 21, 27, DOI:10.3390/md21010027.
- Y.-Y. Qin, W.-S. Li, X. Zhang, Z.-X. Yu, X.-B. Li, H.-Y. Chen, Y.-R. Lv, C.-R. Han and G.-Y. Chen, Chin. J. Chem., 2023, 41, 2507–2517, DOI:10.1002/cjoc.202300231.
- Y. Qin, L. Zou, X. Lei, J. Su, R. Yang, W. Xie, W. Li and G. Chen, Bioorg. Chem., 2023, 130, 106271, DOI:10.1016/j.bioorg.2022.106271.
- T. Chen, Y. Liu, Y. Huang, W. Yang, B. Sun, Q. Tan, T. Wei, B. Wang, J. Yuan and Z. She, Phytochemistry, 2023, 215, 113868, DOI:10.1016/j.phytochem.2023.113868.
- R.-N. Ji, J.-T. Wu, B.-C. Hao, X.-H. Zhu, J.-C. Xue, C.-J. Zheng and M. Chen, Chem. Nat. Compd., 2023, 59, 285, DOI:10.1007/s10600-023-03978-w.
- Y. Wang, W. Chen, Z. Xu, Q. Bai, X. Zhou, C. Zheng, M. Bai and G. Chen, Mar. Drugs, 2023, 21, 22, DOI:10.3390/md21010022.
- X.-X. Wang, Z.-L. Chen, J.-S. Zhang, H.-S. Liu, R.-P. Ma, X.-P. Liu, M.-Y. Li, D. Ge, J. Bao and H. Zhang, Mar. Drugs, 2023, 21, 593, DOI:10.3390/md21110593.
- M. R. de Amorim, C. D. S. Barbosa, T. A. Paz, L. P. Ióca, K. J. Nicácio, L. F. P. de Oliveira, M. O. Goulart, J. M. Paulino, M. O. da Cruz, A. G. Ferreira, M. Furlan, S. P. de Lira, R. A. dos Santos, A. Rodrigues, R. V. C. Guido and R. G. S. Berlinck, J. Nat. Prod., 2023, 86, 1476–1486, DOI:10.1021/acs.jnatprod.3c00175.
- M. R. de Amorim, C. D. S. Barbosa, T. A. Paz, L. P. Ióca, K. J. Nicácio, L. F. P. de Oliveira, M. O. Goulart, J. M. Paulino, M. O. da Cruz, A. G. Ferreira, M. Furlan, S. P. de Lira, R. A. dos Santos, A. Rodrigues, R. V. C. Guido and R. G. S. Berlinck, J. Nat. Prod., 2023, 86, 1884, DOI:10.1021/acs.jnatprod.3c00532.
- T. Bunyapaiboonsri, S. Yoiprommarat, S. Nithithanasilp, W. Choowong, S. Preedanon and S. Suetrong, Nat. Prod. Res., 2023, 37, 24–30, DOI:10.1080/14786419.2021.1946536.
- P. Wang, H. Wang, J. Yang, L. Yang, C. Cai, J. Yuan, F. Wu, C. Gai, W. Mei and H. Dai, Mar. Drugs, 2023, 21, 150, DOI:10.3390/md21030150.
- D. Pan, S. Lin, X. Huang, D. Lv, Q. Lai, J. Xia, Z. Shao and W. Wang, Fitoterapia, 2023, 168, 105546, DOI:10.1016/j.fitote.2023.105546.
- Z. Guo, B. Chen, D. Chen, X. Deng, J. Yuan, S. Zhang, Z. Xiong and J. Xu, Molecules, 2023, 28, 3756, DOI:10.3390/molecules28093756.
- Z.-H. He, C.-L. Xie, T. Wu, Y.-T. Yue, C.-F. Wang, L. Xu, M.-M. Xie, Y. Zhang, Y.-J. Hao, R. Xu and X.-W. Yang, J. Nat. Prod., 2023, 86, 157–165, DOI:10.1021/acs.jnatprod.2c00866.
- Z.-B. Zou, T.-Z. Wu, L.-H. Yang, X.-W. He, W.-Y. Liu, K. Zhang, C.-L. Xie, M.-M. Xie, Y. Zhang, X.-W. Yang and J.-S. Wang, Mar. Drugs, 2023, 21, 596, DOI:10.3390/md21110596.
- C. Cai, Y. Chen, L. Zhou, N. Gong, H. Zhang, C. Sun, J. Ma and J. Ju, J. Nat. Prod., 2023, 86, 589–595, DOI:10.1021/acs.jnatprod.2c00900.
- L. Xiang, W. He, Q. Y. Ling, W. Li, Z. Y. Yan, B. Zhang and R. X. Tan, Tetrahedron Lett., 2023, 119, 154426, DOI:10.1016/j.tetlet.2023.154426.
- M. Dayras, E. Sfecci, E. Bovio, O. Rastoin, M. Dufies, F. Fontaine-Vive, E. Taffin-de-Givenchy, T. Lacour, G. Pages, G. C. Varese and M. Mehiri, Mar. Drugs, 2023, 21, 135, DOI:10.3390/md21030135.
- Z. Wu, H. Guo, Q. Wu, M. Jiang, J. Chen, B. Chen, H. Li, L. Liu and S. Chen, Bioorg. Chem., 2023, 136, 106542, DOI:10.1016/j.bioorg.2023.106542.
- Z. Liu, J. Kou, M. Li, Y. Chen, S. Li, H. Li and W. Zhang, Tetrahedron, 2023, 138, 133410, DOI:10.1016/j.tet.2023.133410.
- Y. Wang, Z. Ying, X.-M. Li, S.-Q. Yang, H.-L. Li, B.-G. Wang and L.-H. Meng, J. Antibiot., 2023, 76, 699–705, DOI:10.1038/s41429-023-00664-5.
- X. Cao, Y. Ge, D. Lan, X. Wu and B. Wu, Fitoterapia, 2023, 164, 105359, DOI:10.1016/j.fitote.2022.105359.
- H. Lv, H. Su, Y. Xue, J. Jia, H. Bi, S. Wang, J. Zhang, M. Zhu, M. Emam, H. Wang, K. Hong and X.-N. Li, Mar. Life Sci. & Technol., 2023, 5, 232–241, DOI:10.1007/s42995-023-00170-5.
- B. Peng, J. Cai, Z. Xiao, M. Liu, X. Li, B. Yang, W. Fang, Y.-Y. Huang, C. Chen, X. Zhou and H. Tao, Mar. Drugs, 2023, 21, 327, DOI:10.3390/md21060327.
- S.-Q. Yang, Q. Song, X.-M. Li, X. Li, H.-L. Li, L.-H. Meng and B.-G. Wang, Org. Biomol. Chem., 2023, 21, 2575–2585, 10.1039/D3OB00058C.
- F. P. Machado, I. C. Rodrigues, A. Georgopolou, L. Gales, J. A. Pereira, P. M. Costa, S. Mistry, S. H. Ghoran, A. M. S. Silva, T. Dethoup, E. Sousa and A. Kijjoa, Mar. Drugs, 2023, 21, 194, DOI:10.3390/md21030194.
- A. A. Salim, W. M. Hussein, P. Dewapriya, H. N. Hoang, Y. Zhou, K. Samarasekera, Z. G. Khalil, D. P. Fairlie and R. J. Capon, Mar. Drugs, 2023, 21, 487, DOI:10.3390/md21090487.
- F. Zhang, C. Ma, Q. Che, T. Zhu, G. Zhang and D. Li, Mar. Drugs, 2023, 21, 628, DOI:10.3390/md21120628.
- N. J. Morehouse, A. J. Flewelling, D. Y. Liu, H. Cavanagh, R. G. Linington, J. A. Johnson and C. A. Gray, J. Nat. Prod., 2023, 86, 1529–1535, DOI:10.1021/acs.jnatprod.3c00233.
- H.-B. Huang, Y.-C. Chen, T.-Y. Wen, S.-N. Li, Z.-M. Liu, W.-M. Zhang and X.-X. Gao, Chem. Biodiversity, 2023, 20, e202301512, DOI:10.1002/cbdv.202301512.
- H.-L. Li, X.-M. Li, Z. Ying, Y.-H. Li and B.-G. Wang, Phytochemistry, 2023, 210, 113644, DOI:10.1016/j.phytochem.2023.113644.
- L.-D. Zhai, W. Gao, L.-T. Liu, K. Zhang and D.-L. Zhang, Rec. Nat. Prod., 2023, 17, 721, DOI:10.25135/rnp.388.2302.2714.
- X.-Y. Ma, Z.-Z. Shi and N.-Y. Ji, Nat. Prod. Res., 2023, 37, 369–374, DOI:10.1080/14786419.2021.1980793.
- W.-T. Yang, W. Gao, J.-A. Liu, X.-X. Liu and L.-C. Jia, Rec. Nat. Prod., 2023, 17, 363–366, DOI:10.25135/rnp.349.2206.2492.
- Z.-Z. Shi, X.-L. Yin, Y.-P. Song and N.-Y. Ji, Chem. Biodiversity, 2023, 20, e202301099, DOI:10.1002/cbdv.202301099.
- Z.-Z. Shi, X.-L. Yin and N.-Y. Ji, Mar. Drugs, 2023, 21, 453, DOI:10.3390/md21080453.
- X.-L. Yin, Y.-P. Song, X.-H. Liu and N.-Y. Ji, Nat. Prod. Res., 2023, 37, 277–282, DOI:10.1080/14786419.2021.1969568.
- H. Li, X. Liu, Z. Hu and L. Wang, Mar. Drugs, 2023, 21, 7, DOI:10.3390/md21010007.
- X. Lin, Z. Tang, Y. Gan, Z. Li, X. Luo, C. Gao, L. Zhao, L. Chai and Y. Liu, J. Nat. Prod., 2023, 86, 994–1002, DOI:10.1021/acs.jnatprod.3c00014.
- R. W. Bates, M. Elyashberg, A. G. Kutateladze and C. M. Williams, Eur. J. Org Chem., 2023, 26, e202300910, DOI:10.1002/ejoc.202300910.
- E. V. Girich, P. T. H. Trinh, L. E. Nesterenko, R. S. Popov, N. Y. Kim, A. B. Rasin, E. S. Menchinskaya, A. S. Kuzmich, E. A. Chingizova, A. S. Minin, N. T. D. Ngoc, T. T. T. Van, E. A. Yurchenko, A. N. Yurchenko and D. V. Berdyshev, Int. J. Mol. Sci., 2023, 24, 8150, DOI:10.3390/ijms24098150.
- F. W. Guo, Y. Gao, Y.-C. Gu and C.-L. Shao, Tetrahedron, 2023, 134, 133299, DOI:10.1016/j.tet.2023.133299.
- J. Kim, S. Lee, S. Han and H.-Y. Lee, Chem, 2023, 9, 1270–1280, DOI:10.1016/j.chempr.2023.01.018.
- J. Yang, B. Singh, G. Cohen and C. P. Ting, J. Am. Chem. Soc., 2023, 145, 19189–19194, DOI:10.1021/jacs.3c07078.
- T. Murata, H. Tsutsui, T. Yoshida, H. Kubota, S. Hiraishi, H. Natsukawa, Y. Suzuki, D. Hiraga, T. Mori, Y. Maekawa, S. Tateyama, K. Toyoyama, K. Ito, K. Suzuki, K. Yonekura, N. Shibata, T. Sato, Y. Tasaki, T. Inohana, A. Takano, N. Egashira, M. Honda, Y. Umezaki and I. Shiina, ACS Omega, 2023, 8, 27703–27709, DOI:10.1021/acsomega.3c03634.
- Y. F. Wong and T. R. R. Pettus, Synthesis, 2023, 55, 3297, DOI:10.1055/s-0041-1738443.
- J. Chen, Y. Jiang, J. Yan, C. Xu and T. Ye, Molecules, 2023, 28, 7194, DOI:10.3390/molecules28207194.
- Y. Li, Y. Cui, Y. Jing, J. Liu and Y. Du, Tetrahedron, 2023, 145, 133621, DOI:10.1016/j.tet.2023.133621.
- C.-L. Xie, Y.-T. Yue, J.-P. Xu, N. Li, T. Lin, G.-R. Ji, X.-W. Yang and R. Xu, Pharmacol. Res., 2023, 197, 106968, DOI:10.1016/j.phrs.2023.106968.
- K. Lin, L. Huang, Y. Zhang, M. Chen, Z. Li, K. K. L. Yung, S. Lv, Q. Pan, W. Zhang, J. Fu, W. Li and Q. Deng, J. Nat. Prod., 2023, 86, 368–379, DOI:10.1021/acs.jnatprod.2c00979.
- K. Fujihara, T. Hashimoto, H. Sasaki, K. Koyama and K. Kinoshita, J. Nat. Med., 2023, 77, 516–522, DOI:10.1007/s11418-023-01696-9.
- R. Li, Y. Zhou, X. Zhang, L. Yang, J. Liu, S. M. Wightman, L. Lv, Z. Liu, C.-Y. Wang and C. Zhao, Mar. Life Sci. & Technol., 2023, 5, 94–101, DOI:10.1007/s42995-022-00162-x.
- S. Liu, Q. Nie, Z. Liu, S. Patil and X. Gao, J. Am. Chem. Soc., 2023, 145, 14251–14259, DOI:10.1021/jacs.3c02109.
- M. Liu, M. Ohashi, Q. Zhou, J. N. Sanders, E. P. McCauley, P. Crews, K. N. Houk and Y. Tang, Angew. Chem., Int. Ed., 2023, 62, e202311266, DOI:10.1002/anie.202311266.
- D. J. Janzen, H. Wang and S.-M. Li, J. Nat. Prod., 2023, 86, 1779–1785, DOI:10.1021/acs.jnatprod.3c00274.
- R. Zou, B. Chen, J. Sun, Y.-W. Guo and B. Xu, Fitoterapia, 2023, 167, 105503, DOI:10.1016/j.fitote.2023.105503.
- F. A. Klapper, C. Kiel, P. Bellstedt, W. Vyverman and G. Pohnert, Angew. Chem., Int. Ed., 2023, 62, e202307165, DOI:10.1002/anie.202307165.
- N. T. T. Thuy, H. T. M. Hien, N. C. Ha, L. T. Thom, D. D. Hong, N. van Thinh, N. T. Dan, N. D. Hoi, V. T. Loan, H. D. Quang, D. T. T. Hang, P. van Kiem, N. T. Dat and N. X. Nhiem, Nat. Prod. Commun., 2023, 18, 1–5, DOI:10.1177/1934578X231157145.
- J. Tebben, C. Zurhelle, A. Tubaro, I. A. Samdal, B. Krock, J. Kilcoyne, S. Sosa, V. L. Trainer, J. R. Deeds and U. Tillmann, Harmful Algae, 2023, 124, 102388, DOI:10.1016/j.hal.2023.102388.
- X. Liu, Y. Ma, J. Wu, Q. Yin, P. Wang, J. Zhu, L. L. Chan and B. Wu, Mar. Drugs, 2023, 21, 3, DOI:10.3390/md21010003.
- C. C. Meyer, K. L. Verboom, M. M. Evarts, W.-O. Jung and M. J. Krische, J. Am. Chem. Soc., 2023, 145, 8242–8247, DOI:10.1021/jacs.3c01809.
- J. Tang, W. Li, T. Y. Chiu, F. Martínez-Peña, Z. Luo, C. T. Chong, Q. Wei, N. Gazaniga, T. J. West, Y. Y. See, L. L. Lairson, C. G. Parker and P. S. Baran, Nature, 2023, 622, 507–513, DOI:10.1038/s41586-023-06535-1.
- R. J. Zachmann, K. Yahata, M. Holzheimer, M. Jarret, C. Wirtz and A. Fürstner, J. Am. Chem. Soc., 2023, 145, 2584–2595, DOI:10.1021/jacs.2c12529.
- H. Takamura, K. Hattori, T. Ohashi, T. Otsu and I. Kadota, Org. Biomol. Chem., 2023, 21, 8837–8848, 10.1039/D3OB01420G.
- V. A. Stonik and I. V. Stonik, Mar. Drugs, 2023, 21, 427, DOI:10.3390/md21080427.
- A. M. Costa, Molecules, 2023, 28, 5249, DOI:10.3390/molecules28135249.
- I. Orefice, S. Balzano, G. Romano and A. Sardo, Life, 2023, 13, 2164, DOI:10.3390/life13112164.
- L. M. Casanova, A. Macrae, J. E. de Souza, A. Neves Junior and A. B. Vermelho, Plants, 2023, 12, 1896, DOI:10.3390/plants12091896.
- L. T. D. Wuerger, F. Kudiabor, J. Alarcan, M. Templin, O. Poetz, H. Sieg and A. Braeuning, Cells, 2023, 12, 770, DOI:10.3390/cells12050770.
- Z. Li, J. Wang, J. Fan, H. Yue and X. Zhang, Mar. Environ. Res., 2023, 191, 106131, DOI:10.1016/j.marenvres.2023.106131.
- M. Du, Y. Jin, J. Fan, S. Zan, C. Gu and J. Wang, Environ. Sci. Pollut. Res., 2023, 30, 5150, DOI:10.1007/s11356-022-22368-3.
- C. da Silva Canielles Caprara, T. K. Mathias, M. D. F. C. Santos, M. G. M. D'Oca, C. D. R. M. D'Oca, F. Roselet, P. C. Abreu and D. F. Ramos, Metabolites, 2023, 13, 202, DOI:10.3390/metabo13020202.
- V. L. Trainer and T. L. King, Toxins, 2023, 15, 189, DOI:10.3390/toxins15030189.
- Y. Yamazaki, S. Yamabe, S. Komori, K. Yoshitake, M. Fukuoka, S. Sato and K. Takada, J. Nat. Prod., 2023, 86, 2539–2545, DOI:10.1021/acs.jnatprod.3c00760.
- W. He, J. Xie, Z. Xia, X. Chen, J. Xiao, Y. Cao and X. Liu, Food Funct., 2023, 14, 5576–5588, 10.1039/D3FO00383C.
- H. Kim, H.-T. Kim, S.-H. Jung, J. W. Han, S. Jo, I.-G. Kim, R.-K. Kim, Y.-J. Kahm, T.-I. Choi, C.-H. Kim and J. H. Lee, Mar. Drugs, 2023, 21, 607, DOI:10.3390/md21120607.
- Y. Li, J. Jin, W. Fan and D. Huang, Org. Lett., 2023, 25, 8284–8289, DOI:10.1021/acs.orglett.3c03346.
- Y. Manabe, S. Takagi-Hayashi, S. Mohri and T. Sugawara, J. Nutr. Sci. Vitaminol., 2023, 69, 62–70, DOI:10.3177/jnsv.69.62.
- S. Shinoda, Y. Tozawa, S.-I. Kurimoto, H. Shigemori and M. Sekiguchi, J. Nat. Med., 2023, 77, 508–515, DOI:10.1007/s11418-023-01693-y.
- J.-Y. Kim, D.-C. Choi, J. M. Lee, H.-S. Kim, D.-W. Ki, S.-C. Ko, M.-J. Yim, G.-W. Oh, K. Woo Kim, C. H. Kim, K. Lee, K. Baek and D.-S. Lee, Nat. Prod. Commun., 2023, 18, 1–6, DOI:10.1177/1934578X231191857.
- Y. Qi, Z. Wang, J. Zhang, S. Tang, H. Zhu, B. Jiang, X. Li, J. Wang, Z. Sun, M. Zhao, H. Zhu and P. Yan, J. Nat. Prod., 2023, 86, 1284–1293, DOI:10.1021/acs.jnatprod.3c00087.
- Y. Qi, G. Liu, C. Fang, C. Jing, S. Tang, G. Li, C. Wang, H. Zhu, M. Zhao, Z. Sun, J. Wu and P. Yan, ACS Omega, 2023, 8, 8034–8044, DOI:10.1021/acsomega.2c07891.
- M. Yu, Y.-B. Gong, Y. Chen, Y.-J. Zhang, X.-L. He, G.-L. Huang and C.-J. Zheng, Chem. Nat. Compd., 2023, 59, 505–507, DOI:10.1007/s10600-023-04036-1.
- B. Cuevas, A. I. Arroba, C. de los Reyes and E. Zubía, Mar. Drugs, 2023, 21, 252, DOI:10.3390/md21040252.
- A. Thambi and K. Chakraborty, Nat. Prod. Res., 2023, 37, 713–724, DOI:10.1080/14786419.2022.2087182.
- R. A. Keyzers, Nat. Prod. Res., 2024, 38, 1–2, DOI:10.1080/14786419.2024.2372837.
- A. D. N. P. Georgii and V. L. Teixeira, Mar. Drugs, 2023, 21, 484, DOI:10.3390/md21090484.
- A. Gonçalves, M. Fernandes, M. Lima, J. P. Gomes, F. Silva, S. Castro, F. Sampaio and A. C. Gomes, Appl. Sci., 2023, 13, 1883, DOI:10.3390/app13031883.
- M. D. Catarino, R. Silva-Reis, A. Chouh, S. Silva, S. S. Braga, A. M. S. Silva and S. M. Cardoso, Mar. Drugs, 2023, 21, 172, DOI:10.3390/md21030172.
- X. Wang, C. Huang, X. Fu, Y.-J. Jeon, X. Mao and L. Wang, J. Agric. Food Chem., 2023, 71, 16452–16468, DOI:10.1021/acs.jafc.3c05135.
- L. Barcellos, C. K. Pham, G. Menezes, R. Bettencourt, N. Rocha, M. Carvalho and H. P. Felgueiras, Mar. Drugs, 2023, 21, 40, DOI:10.3390/md21010040.
- J. R. Davison, R. Rajwani, G. Zhao and C. A. Bewley, Sci. Rep., 2023, 13, 11944, DOI:10.1038/s41598-023-38042-8.
- T. Ishii, A. Tahara, K. Taba, M. Iwatsuki, M. Honsho, Y. Asami, K. Nonaka, H. Hanaki and T. Teruya, J. Asian Nat. Prod. Res., 2023, 25, 704–710, DOI:10.1080/10286020.2022.2130266.
- M. A. Tammam, M. G. Daskalaki, N. Tsoureas, O. Kolliniati, A. Mahdy, S. C. Kampranis, C. Tsatsanis, V. Roussis and E. Ioannou, Mar. Drugs, 2023, 21, 79, DOI:10.3390/md21020079.
- X.-Y. Lian, T.-T. Liu, X.-J. Liao, S.-H. Xu and B.-X. Zhao, J. Asian Nat. Prod. Res., 2023, 25, 899–904, DOI:10.1080/10286020.2022.2162886.
- Z.-C. Wang, Y. Wang, L.-Y. Huang, X.-J. Liao, Z.-H. Jiang, S.-H. Xu and B.-X. Zhao, J. Asian Nat. Prod. Res., 2023, 25, 61–67, DOI:10.1080/10286020.2022.2056029.
- G. Zhao and C.-L. Jiao, Rec. Nat. Prod., 2023, 17, 726, DOI:10.25135/rnp.394.2304.2764.
- M. Orfanoudaki, M. Alilou, A. Hartmann, J. Mayr, U. Karsten, H. Nguyen-Ngoc and M. Ganzera, Mar. Drugs, 2023, 21, 543, DOI:10.3390/md21100543.
- Y.-J. Wu, T.-Y. Huang, C.-Y. Huang, C.-C. Lin, W.-L. Wang, H.-C. Huang, S.-Y. V. Liu, C.-H. Chao and J.-H. Sheu, Mar. Drugs, 2023, 21, 493, DOI:10.3390/md21090493.
- R. Fukada, Y. Yamagishi, M. Nagasaka, D. Osada, K. Nimura, I. Oshima, K. Tsujimoto, M. Kirihara, S. Takizawa, N. Kikuchi, T. Ishii and T. Kamada, Chem. Biodiversity, 2023, 20, e202300888, DOI:10.1002/cbdv.202300888.
- B. K. Chhetri, N. Mojib, S. G. Moore, D. A. Delgadillo, J. E. Burch, N. H. Barrett, D. A. Gaul, L. Marquez, K. Soapi, H. M. Nelson, C. L. Quave and J. Kubanek, ACS Omega, 2023, 8, 13899–13910, DOI:10.1021/acsomega.3c00301.
- C. Bayrak, P. Taslimi, N. Kilinc, I. Gulcin and A. Menzek, Chem. Biodiversity, 2023, 20, e202300469, DOI:10.1002/cbdv.202300469.
- Y. Yu and J. Sperry, Tetrahedron Lett., 2023, 129, 154724, DOI:10.1016/j.tetlet.2023.154724.
- E. Savelson and J. J. Tepe, Org. Lett., 2023, 25, 3698–3701, DOI:10.1021/acs.orglett.3c01139.
- M. D. N. Meinita, D. Harwanto, A. Md. Abdul Hannan, G.-T. Jeong, I. S. Moon and J.-S. Choi, J. Appl. Phycol., 2023, 35, 1499–1523, DOI:10.1007/s10811-023-02956-7.
- I. Arberas-Jiménez, N. Nocchi, J. Chao-Pellicer, I. Sifaoui, A. Ribeiro Soares, A. R. Díaz-Marrero, J. J. Fernández, J. E. Piñero and J. Lorenzo-Morales, Mar. Drugs, 2023, 21, 224, DOI:10.3390/md21040224.
- S. García-Davis, A. López-Arencibia, C. J. Bethencourt-Estrella, D. San Nicolás-Hernández, E. Viveros-Valdez, A. R. Díaz-Marrero, J. J. Fernández, J. Lorenzo-Morales and J. E. Piñero, Mar. Drugs, 2023, 21, 333, DOI:10.3390/md21060333.
- N. Krupnik, A. Israel and D. Meiri, Sci. Rep., 2023, 13, 15559, DOI:10.1038/s41598-023-42497-0.
- M. Zhang, P. Li, Q. Wang, L. Huang and K. Lin, Environ. Sci. Technol., 2023, 57, 6673–6681, DOI:10.1021/acs.est.3c00311.
- E. A. Santalova, A. S. Kuzmich, E. A. Chingizova, E. S. Menchinskaya, E. A. Pislyagin and P. S. Dmitrenok, Biomolecules, 2023, 13, 677, DOI:10.3390/biom13040677.
- E. K. Kudryashova, T. N. Makarieva, L. K. Shubina, A. G. Guzii, R. S. Popov, A. S. Menshov, D. V. Berdyshev, E. A. Pislyagin, E. S. Menchinskaya, B. B. Grebnev and V. A. Stonik, J. Nat. Prod., 2023, 86, 2073–2078, DOI:10.1021/acs.jnatprod.3c00456.
- S. Scarpato, R. Teta, P. De Cicco, F. Borrelli, J. R. Pawlik, V. Costantino and A. Mangoni, Mar. Drugs, 2023, 21, 58, DOI:10.3390/md21020058.
- D.-C. Li, H.-X. Liang, X.-J. Liao, X.-W. Xing, S.-H. Xu and B.-X. Zhao, Chem. Biodiversity, 2023, 20, e202300950, DOI:10.1002/cbdv.202300950.
- J. Vigna, D. Sighel, E. F. Rosatti, A. Defant, M. Pancher, V. Sidarovich, A. Quattrone and I. Mancini, Mar. Drugs, 2023, 21, 186, DOI:10.3390/md21030186.
- Y.-F. Xiao, J.-Y. Xu, L.-Z. Cui, C.-B. Wang, Y. Lei, X.-J. Liao, S.-H. Xu and B.-X. Zhao, Nat. Prod. Res., 2023, 37, 1–7, DOI:10.1080/14786419.2021.1941950.
- F. Nakamura, H. Kimura, N. Fusetani and Y. Nakao, Molecules, 2023, 28, 2524, DOI:10.3390/molecules28062524.
- C. Kong, Y. Wu, H.-M. Zhao, S.-S. Zhang, Z.-M. Wu, X.-B. Li, K.-C. Liu, H.-W. Lin and S.-P. Wang, Bioorg. Chem., 2023, 139, 106699, DOI:10.1016/j.bioorg.2023.106699.
- D. C. Holland, W. A. Schroder, M. J. Calcott, E. Kaemmerer, V. M. Avery, M. G. Ekins and A. R. Carroll, J. Nat. Prod., 2023, 86, 2216–2227, DOI:10.1021/acs.jnatprod.3c00633.
- K. Takada, N. Oku, M. L. Peach, T. T. Ransom, C. J. Henrich and K. R. Gustafson, J. Org. Chem., 2023, 88, 10996–11002, DOI:10.1021/acs.joc.3c00963.
- N. K. Utkina, V. V. Isakov and E. A. Chingizova, Chem. Nat. Compd., 2023, 59, 1179–1181, DOI:10.1007/s10600-023-04223-0.
- T. M. Voser, J. B. Hayton, D. W. Prebble, J. Jin, G. Grant, M. G. Ekins and A. R. Carroll, J. Nat. Prod., 2023, 86, 475–481, DOI:10.1021/acs.jnatprod.2c01125.
- Y. Lei, B. Li, X. Liao, X. Xing, P. Feng, B. Zhao and S. Xu, RSC Adv., 2023, 13, 29316–29319, 10.1039/D3RA06115A.
- M. Liu, Z. Zhang, Q. He, S. Qi, K. Sun, S. Miao, Y. Wu and K. Gong, Chem. Biodiversity, 2023, 20, e202300410, DOI:10.1002/cbdv.202300410.
- X. Di, I. Hardardottir, J. Freysdottir, D. Wang, K. R. Gustafson, S. Omarsdottir and T. F. Molinski, Molecules, 2023, 28, 2937, DOI:10.3390/molecules28072937.
- M. Orfanoudaki, E. A. Smith, N. T. Hill, K. A. Garman, I. Brownell, B. R. Copp, T. Grkovic and C. J. Henrich, Mar. Drugs, 2023, 21, 474, DOI:10.3390/md21090474.
- L. Martínez-Fructuoso, S. J. R. Arends, V. F. Freire, J. R. Evans, S. DeVries, B. D. Peyser, R. K. Akee, C. C. Thornburg, R. Kumar, S. Ensel, G. M. Morgan, G. D. McConachie, N. Veeder, L. R. Duncan, T. Grkovic and B. R. O'Keefe, ACS Infect. Dis., 2023, 9, 1245, DOI:10.1021/acsinfecdis.3c00067.
- S. Sala, S. Moggach, G. Nealon, J. Fromont, O. Gomez, D. Vuong, E. Lacey and G. Flematti, Mar. Drugs, 2023, 21, 41, DOI:10.3390/md21010041.
- W.-H. Jiao, J.-X. Li, H.-Y. Liu, X.-C. Luo, T.-Y. Hu, G.-H. Shi, D.-D. Xie, H.-F. Chen, B.-H. Cheng and H.-W. Lin, Org. Lett., 2023, 25, 6391–6395, DOI:10.1021/acs.orglett.3c02409.
- X. Yu, X. Han, Y. Cui, A. Fu, K. Liu, W. Zhang, X. Tang and G. Li, J. Nat. Prod., 2023, 86, 2710–2717, DOI:10.1021/acs.jnatprod.3c00877.
- D. E. Williams, J. Cassel, J.-L. Zhu, J.-X. Yang, N. J. de Voogd, T. Matainaho, J. M. Salvino, Y. A. Wang, L. J. Montaner, I. Tietjen and R. J. Andersen, J. Nat. Prod., 2023, 86, 582–588, DOI:10.1021/acs.jnatprod.2c01030.
- C.-W. Fu, L. Chiang, C.-H. Chao, Y.-L. Huang, S.-F. Chiou, L.-C. Wang, H.-W. Chang, S.-L. Chen, H.-C. Wang, M.-C. Yu, H.-C. Huang and J.-H. Sheu, Tetrahedron, 2023, 149, 133745, DOI:10.1016/j.tet.2023.133745.
- B. K. Chhetri, R. Bhanushali, Y. Liang, M. R. Cepeda, A. K. Niradininoco, K. Soapi, B. Wan, M. Qader, S. G. Franzblau and J. Kubanek, J. Nat. Prod., 2023, 86, 574–581, DOI:10.1021/acs.jnatprod.2c01003.
- A. H. El-Desoky, K. Eguchi, I. Kagiyama, Y. Hitora, H. Kato, Y. Ise, F. Losung, R. E. P. Mangindaan and S. Tsukamoto, Phytochemistry, 2023, 216, 113872, DOI:10.1016/j.phytochem.2023.113872.
- Y. Zhou, H. Liang, Z. Wang, X. Liao, S. Xu and B. Zhao, J. Mar. Sci. Eng., 2023, 11, 131, DOI:10.3390/jmse11010131.
- H. N. K. Tran, M. J. Kim, A.-Y. Shin, L. V. H. Tran, J. Lee and Y.-J. Lee, J. Nat. Prod., 2023, 86, 2145–2150, DOI:10.1021/acs.jnatprod.3c00358.
- Z. Wang, Y. Li, X. Han, D. Zhang, H. Hou, L. Xiao and G. Li, Phytochemistry, 2023, 206, 113512, DOI:10.1016/j.phytochem.2022.113512.
- T. Jin, P. Li, C. Wang, X. Tang, X. Yv, K. Li, L. Luo, H. Ou and G. Li, Nat. Prod. Res., 2023, 37, 216–226, DOI:10.1080/14786419.2021.1961137.
- S. Sala, P. J. C. James, G. L. Nealon, J. Fromont, O. Gomez, D. Vuong, E. Lacey and G. R. Flematti, J. Nat. Prod., 2023, 86, 482–489, DOI:10.1021/acs.jnatprod.2c01087.
- J. Xu, M. Wang, Z. Liu, W. Zhang, J. Ma, G. Li and P. Li, J. Nat. Prod., 2023, 86, 330–339, DOI:10.1021/acs.jnatprod.2c00937.
- J. Bracegirdle, S. S. H. Olsen, M. N. Teng, K. C. Tran, C. D. Amsler, J. B. McClintock and B. J. Baker, Mar. Drugs, 2023, 21, 107, DOI:10.3390/md21020107.
- B.-R. Peng, L.-G. Zheng, L.-Y. Chen, M. El-Shazly, T.-L. Hwang, J.-H. Su, M.-H. Lee, K.-H. Lai and P.-J. Sung, Pharmaceuticals, 2023, 16, 1258, DOI:10.3390/ph16091258.
- M.-Z. Su, Q. Zhang, L.-G. Yao, B. Wu and Y.-W. Guo, J. Asian Nat. Prod. Res., 2023, 25, 741–747, DOI:10.1080/10286020.2022.2150614.
- L.-L. Sun, Y.-R. Shen, J. Li, J.-R. Wang, X.-W. Li and Y.-W. Guo, Tetrahedron, 2023, 137, 133388, DOI:10.1016/j.tet.2023.133388.
- H.-B. Yu, B. Hu, G.-F. Wu, Z. Ning, Y. He, B.-H. Jiao, X.-Y. Liu and H.-W. Lin, J. Nat. Prod., 2023, 86, 1754–1760, DOI:10.1021/acs.jnatprod.3c00218.
- H.-B. Yu, B. Hu, Z. Ning, Y. He, X.-L. Men, Z.-F. Yin, B.-H. Jiao, X.-Y. Liu and H.-W. Lin, Mar. Drugs, 2023, 21, 507, DOI:10.3390/md21100507.
- D. Lu, X.-C. Luo, J. Liu, G.-L. Wu, Y. Yu, Y.-N. Xu, H.-W. Lin and F. Yang, Tetrahedron, 2023, 137, 133382, DOI:10.1016/j.tet.2023.133382.
- A. B. Kozhushnaya, S. A. Kolesnikova, E. A. Yurchenko, E. G. Lyakhova, A. S. Menshov, A. I. Kalinovsky, R. S. Popov, P. S. Dmitrenok and N. V. Ivanchina, Mar. Drugs, 2023, 21, 554, DOI:10.3390/md21110554.
- S. O'Brien, R. Lacret, M. M. Reddy, L. K. Jennings, P. Sánchez, F. Reyes, A. Mungkaje, K. Calabro and O. P. Thomas, J. Nat. Prod., 2023, 86, 2730–2738, DOI:10.1021/acs.jnatprod.3c01045.
- G. R. Jachak, K. Kashinath, N. Vasudevan, P. R. Athawale, R. Choudhury, S. S. Dange, H. Agarwal, M. K. Barthwal and D. S. Reddy, J. Org. Chem., 2023, 88, 17088–17133, DOI:10.1021/acs.joc.3c01987.
- M. Kimata and T. Abe, Chemistry, 2023, 5, 2772–2784, DOI:10.3390/chemistry5040177.
- Y. Goda and H. Fuwa, Org. Lett., 2023, 25, 8402–8407, DOI:10.1021/acs.orglett.3c03002.
- F. M. Ippoliti, N. J. Adamson, L. G. Wonilowicz, D. J. Nasrallah, E. R. Darzi, J. S. Donaldson and N. K. Garg, Science, 2023, 379, 261–265, DOI:10.1126/science.ade0032.
- H. Tian and L. Zhang, Chin. J. Nat. Med., 2023, 21, 161–162, DOI:10.1016/S1875-5364(23)60417-0.
- T. Varlet, S. Portmann and A. Fürstner, J. Am. Chem. Soc., 2023, 145, 21197–21202, DOI:10.1021/jacs.3c08410.
- B. C. Derstine, A. J. Cook, J. D. Collings, J. Gair, J. Saurí, E. E. Kwan and N. Z. Burns, Angew. Chem., Int. Ed., 2023, 63, e202315284, DOI:10.1002/anie.202315284.
- M. Shimomura, K. Ide, J. Sakata and H. Tokuyama, J. Am. Chem. Soc., 2023, 145, 18233–18239, DOI:10.1021/jacs.3c06578.
- H. Yu, Z. P. Sercel, S. P. Rezgui, J. Farhi, S. C. Virgil and B. M. Stoltz, J. Am. Chem. Soc., 2023, 145, 25533–25537, DOI:10.1021/jacs.3c10212.
- N. Gunawan, M. J. Nutt, A. C. Bissember, J. A. Smith and S. G. Stewart, Synlett, 2023, 34, 1524–1528, DOI:10.1055/a-1982-5433.
- P.-P. Fu, Q. Wang, Q. Zhang, Y. Jin, J. Liu, K.-X. Chen, Y.-W. Guo, S.-H. Liu and X.-W. Li, J. Med. Chem., 2023, 66, 5427–5438, DOI:10.1021/acs.jmedchem.2c01702.
- D. E. George and J. J. Tepe, J. Org. Chem., 2023, 88, 9306–9312, DOI:10.1021/acs.joc.3c00867.
- C. Chen, T. Yuan, P. Lan, L. V. White, J. Chen and M. G. Banwell, Eur. J. Org Chem., 2023, 26, e202300003, DOI:10.1002/ejoc.202300003.
- J. R. Vale, M. A. G. Fortunato, K. H. S. Andrade, Â. M. R. Rocha, C. A. M. Afonso and F. Siopa, Adv. Synth. Catal., 2023, 365, 2240–2247, DOI:10.1002/adsc.202300560.
- C. Chong, L. Chang, I. Grimm, Q. Zhang, Y. Kuang, B. Wang, J. Kang, W. Liu, J. Baars, Y. Guo, H.-G. Schmalz and Z. Lu, Chem. Sci., 2023, 14, 3302–3310, 10.1039/D3SC00173C.
- Y. Chen, P. Lan, L. V. White, W. Yang and M. G. Banwell, Synlett, 2023, 34, 1529, DOI:10.1055/a-2002-8680.
- M. D. Kerim, L. Evanno and L. Ferrié, Chem.–Eur. J., 2023, 29, e202203004, DOI:10.1002/chem.202203004.
- B. Jiang and M. Dai, J. Am. Chem. Soc., 2023, 145, 18731–18736, DOI:10.1021/jacs.3c06031.
- E. Turrini, F. Maffei and C. Fimognari, Mar. Drugs, 2023, 21, 38, DOI:10.3390/md21010038.
- S. I. Peña-Corona, H. Hernández-Parra, S. A. Bernal-Chávez, N. Mendoza-Muñoz, A. Romero-Montero, M. L. Del Prado-Audelo, H. Cortés, D. A. Ateşşahin, S. Habtemariam, Z. M. Almarhoon, A. F. A. Razis, B. Modu, J. Sharifi-Rad and G. Leyva-Gómez, Front. Pharmacol, 2023, 14, 1206334, DOI:10.3389/fphar.2023.1206334.
- C. Wang, S. Wang, H. Li, Y. Hou, H. Cao, H. Hua and D. Li, Mar. Drugs, 2023, 21, 226, DOI:10.3390/md21040226.
- E. R. El-Sawy and G. Kirsch, Mar. Drugs, 2023, 21, 268, DOI:10.3390/md21050268.
- C. Pecoraro, F. Terrana, G. Panzeca, B. Parrino, S. Cascioferro, P. Diana, E. Giovannetti and D. Carbone, Molecules, 2023, 28, 6450, DOI:10.3390/molecules28186450.
- M.-J. Chu, M. Li and Y. Zhao, Bioorg. Chem., 2023, 133, 106332, DOI:10.1016/j.bioorg.2022.106332.
- N. V. Ivanchina and V. I. Kalinin, Molecules, 2023, 28, 2503, DOI:10.3390/molecules28062503.
- C. Festa, S. De Marino, A. Zampella and S. Fiorucci, Mar. Drugs, 2023, 21, 291, DOI:10.3390/md21050291.
- T. Wakimoto, Chem. Pharm. Bull., 2023, 71, 1–8, DOI:10.1248/cpb.c22-00715.
- S. R. M. Ibrahim, K. F. Ghazawi, S. F. Miski, D. F Alsiyud, S. G. A. Mohamed and G. A. Mohamed, Mar. Drugs, 2023, 21, 257, DOI:10.3390/md21040257.
- S. Khushi, A. A. Salim and R. J. Capon, Mar. Drugs, 2023, 21, 413, DOI:10.3390/md21070413.
- E. A. Guzmán, T. A. Peterson and A. E. Wright, Mar. Drugs, 2023, 21, 642, DOI:10.3390/md21120642.
- S. L. S. Ellis, S. Dada, L. L. Nohara, I. Saranchova, L. Munro, C. G. Pfeifer, B. A. Eyford, T. Morova, D. E. Williams, P. Cheng, N. A. Lack, R. J. Andersen and W. A. Jefferies, Front. Pharmacol, 2023, 14, 1119620, DOI:10.3389/fphar.2023.1119620.
- S. J. Mascuch, B. K. Chhetri, N. Mojib and J. Kubanek, J. Exp. Biol., 2023, 226, jeb246246, DOI:10.1242/jeb.246246.
- D. Varamogianni-Mamatsi, M. J. Nunes, V. Marques, T. I. Anastasiou, E. Kagiampaki, E. Vernadou, T. Dailianis, N. Kalogerakis, L. C. Branco, C. M. P. Rodrigues, R. G. Sobral, S. P. Gaudêncio and M. Mandalakis, Mar. Drugs, 2023, 21, 612, DOI:10.3390/md21120612.
- N. K. D. Cahyani, N. Kasanah, D. S. Kurnia and M. T. Hamann, Mar. Biotechnol., 2023, 25, 1158–1175, DOI:10.1007/s10126-023-10268-7.
- A. N. L. Batista, L. H. Martorano, A. L. Valverde and F. M. dos Santos, New J. Chem., 2023, 47, 10834–10841, 10.1039/D3NJ01762A.
- A. Defant and I. Mancini, Mar. Drugs, 2023, 21, 511, DOI:10.3390/md21100511.
- I. Cortés and A. M. Sarotti, J. Org. Chem., 2023, 88, 14156–14164, DOI:10.1021/acs.joc.3c01738.
- N. O. Bawakid, H. S. Alorfi, N. M. Alqarni, A. B. Abdel-Naim and W. M. Alarif, Naunyn-Schmiedeberg's Arch. Pharmacol., 2023, 396, 289–300, DOI:10.1007/s00210-022-02313-4.
- A. H. Eissa, A. M. Abdel-Tawab, E. S. A. E. Hamed, F. Z. El-Ablack and S. -E. N. Ayyad, Chem. Biodiversity, 2023, 20, e202301208, DOI:10.1002/cbdv.202301208.
- K. Wang, J. Liu, J. Huang, X. Leng, T. Li, H. Ouyang, W. Lin, X. Yan and S. He, Phytochemistry, 2023, 209, 113616, DOI:10.1016/j.phytochem.2023.113616.
- I.-T. Wu, Y.-C. Fan, G.-Z. Lin, Y.-L. Wang, T.-L. Hwang, K.-H. Lai and H.-M. Chung, J. Mol. Struct., 2023, 1271, 133995, DOI:10.1016/j.molstruc.2022.133995.
- M. M. A. Ahmed, E. A. Ragab, A. Zayed, E. M. El-Ghaly, S. K. Ismail, S. I. Khan, Z. Ali, A. G. Chittiboyina and I. A. Khan, Nat. Prod. Res., 2023, 37, 542–550, DOI:10.1080/14786419.2022.2071268.
- C.-W. Fu, Y.-C. Lin, S.-F. Chiou, S.-L. Chen, C.-C. Lin, H.-C. Wang, C.-F. Dai and J.-H. Sheu, Molecules, 2023, 28, 1521, DOI:10.3390/molecules28041521.
- X. Han, Z. Wang, A. Fu, K. Liu, Z. Ma, X. Tang, D. Zhang and G. Li, Chin. J. Chem., 2023, 41, 1491–1496, DOI:10.1002/cjoc.202200827.
- M.-Y. Cheng, Y.-T. Chuang, H.-W. Chang, Z.-Y. Lin, C.-Y. Chen and Y.-B. Cheng, Mar. Drugs, 2023, 21, 529, DOI:10.3390/md21100529.
- J. T. Welsch, T. B. Smalley, J. K. Matlack, N. E. Avalon, J. M. Binning, M. P. Johnson, A. L. Allcock and B. J. Baker, J. Nat. Prod., 2023, 86, 182–190, DOI:10.1021/acs.jnatprod.2c00898.
- J. Zhang, H. Ma, S. Jin, X. Liu, L. Li, Z. Liu, G. Li and P. Li, Mar. Drugs, 2023, 21, 223, DOI:10.3390/md21040223.
- J.-D. Yu, D.-D. Yu, M.-Z. Su, Y.-C. Gu, H. Wang and Y.-W. Guo, Mar. Drugs, 2023, 21, 362, DOI:10.3390/md21060362.
- J. Liu, S.-W. Li, Q.-M. Zhao, Z.-Y. Zhang, L.-G. Yao, Y.-C. Gu, L.-F. Lan and Y.-W. Guo, Chem.–Eur. J., 2023, 29, e202300055, DOI:10.1002/chem.202300055.
- H.-C. Huang, A. F. Ahmed, C.-F. Dai and J.-H. Sheu, Tetrahedron, 2023, 143, 133561, DOI:10.1016/j.tet.2023.133561.
- X. Han, K. Liu, A. Fu, Z. Ma, Z. Wang, X. Li, X. Tang, D. Zhang and G. Li, J. Nat. Prod., 2023, 86, 2131–2138, DOI:10.1021/acs.jnatprod.3c00330.
- X. Dong, J. Wu, H. Jia, S. Cen, W. Cheng and W. Lin, ACS Omega, 2023, 8, 21254–21264, DOI:10.1021/acsomega.3c02429.
- Y.-Q. Du, Y. Gao, Y. Zang, J. Li, X.-W. Li and Y.-W. Guo, Phytochemistry, 2023, 210, 113671, DOI:10.1016/j.phytochem.2023.113671.
- H. Peng, Y. Zeng, H. Wang, W. Chang, H. Chen, F. Zhou, H. Dai and X. Wang, Mar. Drugs, 2023, 21, 645, DOI:10.3390/md21120645.
- W. M. Alarif, D. F. Baamer, M. A. Ghandourah, H. S. Alorfi, N. A. Alburae, F. Budiyanto and A. B. Abdel-Naim, Environ. Sci. Pollut. Res., 2023, 30, 56920–56929, DOI:10.1007/s11356-023-26466-8.
- N. Zhang, W. Xu, Y. Yan, M. Chen, H. Li and L. Chen, Phytochemistry, 2023, 212, 113703, DOI:10.1016/j.phytochem.2023.113703.
- Y. Du, L. Yao, X. Li and Y. Guo, Chin. Chem. Lett., 2023, 34, 107512, DOI:10.1016/j.cclet.2022.05.026.
- J. Liu, Q. Tang, J. Huang, T. Li, H. Ouyang, W.-H. Lin, X.-J. Yan, X. Yan and S. He, J. Org. Chem., 2022, 87, 9806–9814, DOI:10.1021/acs.joc.2c00784.
- R. Serrano, Y. D. Boyko, L. W. Hernandez, A. Lotuzas and D. Sarlah, J. Am. Chem. Soc., 2023, 145, 8805–8809, DOI:10.1021/jacs.3c02317.
- Z.-W. Wu, Z.-X. Wang, Y.-Q. Guo, S.-A. Tang and D.-Q. Feng, J. Asian Nat. Prod. Res., 2023, 25, 85, DOI:10.1080/10286020.2022.2046562.
- H. -R. Wang, Y. Jin, R. -N. Sun, L. -G. Yao, J. -H. Xia, Y. Huang, S. -H. Liu, W. -D. Zhang and X. -W. Li, Chem. Biodiversity, 2023, 20, e202300214, DOI:10.1002/cbdv.202300214.
- H.-J. Tseng, L.-M. Kuo, Y.-C. Tsai, H.-C. Hu, P.-J. Chen, S.-Y. Chien, J.-H. Sheu and P.-J. Sung, RSC Adv., 2023, 13, 10408–10413, 10.1039/D3RA01589K.
- T.-A. Kung, L.-Y. Chen, C.-Y. Li, B.-R. Peng, T.-L. Hwang, P.-J. Sung, K.-H. Lai and H.-M. Chung, Phytochem. Lett., 2023, 55, 12–16, DOI:10.1016/j.phytol.2023.03.004.
- C.-J. Tai, H.-W. Zhang, H.-C. Wang, Y.-T. Chuang, H.-W. Chang, F.-R. Chang, P.-J. Sung and J.-H. Sheu, Tetrahedron Lett., 2023, 129, 154753, DOI:10.1016/j.tetlet.2023.154753.
- D.-D. Yu, L.-M. Ke, J. Liu, S.-W. Li, M.-Z. Su, L.-G. Yao, H. Luo and Y.-W. Guo, Molecules, 2023, 28, 6892, DOI:10.3390/molecules28196892.
- S. -H. Zhu, D. -D. Yu, M. -Z. Su, H. Luo, L. -G. Yao, F. Gu, L. -F. Liang, H. Wang and Y. -W. Guo, Chem. Biodiversity, 2023, 20, e202300589, DOI:10.1002/cbdv.202300589.
- Y. -T. Song, D. -D. Yu, M. Z. Su, H. Luo, J. -G. Cao, L. -G. Yao, L. -F. Liang, Y. -W. Guo and F. Yang, Chem. Biodiversity, 2023, 20, e202300217, DOI:10.1002/cbdv.202300217.
- L. Ren, X. Leng, T. Li, J. Liu, W. Wang, X. Yan, X. Yan and S. He, Nat. Prod. Res., 2023, 37, 39–46, DOI:10.1080/14786419.2021.1948041.
- C. Wang, J. Zhang, Y. Gan, M. Wang, X. Li, X. Liu, X. Shi, Y. Mi, K. Liu, Y. Zhang, G. Li and P. Li, Phytochemistry, 2023, 207, 113578, DOI:10.1016/j.phytochem.2022.113578.
- X. Leng, J. Liu, X. Han, T. Li, H. Ouyang, X. Yan and S. He, Chem. Biodiversity, 2023, 20, e202200966, DOI:10.1002/cbdv.202200966.
- C.-H. Chao, Y.-J. Wu, T.-Y. Huang, C.-J. Tai, Y.-J. Chen, C.-Y. Huang, C.-C. Lin, C.-F. Dai, H.-C. Huang and J.-H. Sheu, Molecules, 2023, 28, 641, DOI:10.3390/molecules28020641.
- M.-J. Wu, D.-D. Yu, Y.-Q. Du, J. Zhang, M.-Z. Su, C.-S. Jiang and Y.-W. Guo, Phytochemistry, 2023, 206, 113549, DOI:10.1016/j.phytochem.2022.113549.
- Y.-T. Song, D.-D. Yu, M.-Z. Su, H. Luo, J.-G. Cao, L.-F. Liang, F. Yang and Y.-W. Guo, Mar. Drugs, 2023, 21, 69, DOI:10.3390/md21020069.
- M. Yang, Z. -Y. Ge, Z. -H. Chen, L. -G. Yao, L. -F. Liang and Y. -W. Guo, Chem. Biodiversity, 2023, 20, e202300267, DOI:10.1002/cbdv.202300267.
- J.-D. Yu, M.-Z. Su, Y.-C. Gu, D.-D. Yu, L.-G. Yao, S.-M. Shen, Y.-W. Guo and H. Wang, Chem. Biodiversity, 2023, 20, e202300662, DOI:10.1002/cbdv.202300662.
- Z. -H. Chen, D. -D. Yu, C. Li, M. -Z. Su, Q. Wu, Z. -Y. Zhang, J. -R. Wang, J. Li and Y. -W. Guo, Chem.–Eur. J., 2023, 29, e202203487, DOI:10.1002/chem.202203487.
- B.-R. Peng, N. B. A. Nguyen, L.-Y. Chen, M. El-Shazly, T.-L. Hwang, J.-H. Su and K.-H. Lai, Aquat. Res., 2023, 33, 101835, DOI:10.1016/j.aqrep.2023.101835.
- T.-S. Chang, J.-J. Lin, K.-C. Cheng, J.-H. Su, Y.-Y. She and Y.-J. Wu, Anticancer Res., 2023, 43, 2625–2634, DOI:10.21873/anticanres.16429.
- Z. Wu, M. Su, H. Chen, X. Chen, C.-Y. Chen, L. An, Z. Shao, X. Liu, Y. Lin, A.-J. OuYang and C.-M. Liu, Anti-Cancer Agents Med. Chem., 2023, 23, 1457–1468, DOI:10.2174/1871520623666230331083744.
- K.-C. Tsai, C.-S. Chen, J.-H. Su, Y.-C. Lee, Y.-H. Tseng and W.-C. Wei, Mar. Drugs, 2023, 21, 225, DOI:10.3390/md21040225.
- T. H. Huynh, C.-Y. Ko, H.-T. Liu, Y.-P. Chen, M. M. Zhang, L. K. Tsou, Z.-H. Wen, S.-Y. Chien and P.-J. Sung, Tetrahedron Lett., 2023, 118, 154407, DOI:10.1016/j.tetlet.2023.154407.
- T. H. Huynh, C.-J. Liu, Y.-H. Liu, S.-Y. Chien, Z.-H. Wen, L.-S. Fang, J.-J. Chen, Y.-C. Wu, J.-H. Su and P.-J. Sung, Mar. Drugs, 2023, 21, 124, DOI:10.3390/md21020124.
- M. Nagasaka, K. Tani, M. Wada, M. Wakatsuki, S.-Y. Ng and T. Ishii, Chem. Nat. Compd., 2023, 59, 697–700, DOI:10.1007/s10600-023-04090-9.
- P.-J. Sung, C.-J. Liu, H.-T. Liu, T. H. Huynh, Z.-H. Wen, C.-C. Liaw, J.-J. Chen and J.-H. Su, Heterocycles, 2023, 106, 358–365, DOI:10.3987/COM-22-14792.
- T. H. Huynh, Z.-H. Wen, J.-H. Su, Z.-K. Yao, L.-G. Zheng, Y.-C. Tsai, J. Tanaka, C.-C. Liaw and P.-J. Sung, Chem. Pharm. Bull., 2023, 71, 183–187, DOI:10.1248/cpb.c22-00605.
- P.-J. Sung, H.-T. Lee, Y.-Y. Chen, Y.-W. Liu, S.-Y. Chien, Y.-C. Chen, F.-C. Chiu, P.-W. Huang, I.-L. Su and Z.-H. Wen, Heterocycles, 2023, 106, 2116, DOI:10.3987/COM-23-14911.
- X. Qi, X. Zhang, J. Meng, J. Wu, W. Cheng, J. Huang and W. Lin, Eur. J. Med. Chem., 2023, 246, 114948, DOI:10.1016/j.ejmech.2022.114948.
- Y.-H. Hsieh, Y.-W. Liu, S.-Y. Chien, Y.-C. Lin, Y.-J. Wu, J.-H. Su, C.-C. Liaw, Y.-C. Tsai, Y.-C. Chang and P.-J. Sung, Tetrahedron, 2023, 145, 133626, DOI:10.1016/j.tet.2023.133626.
- C. Steinborn, T. Huber, J. Lichtenegger, I. Plangger, K. Wurst and T. Magauer, J. Am. Chem. Soc., 2023, 145, 11811–11817, DOI:10.1021/jacs.3c03366.
- P. Scesa and E. Schmidt, Angew. Chem. Int. Ed., 2023, 62, e202311406, DOI:10.1002/anie.202311406.
- Z. Li and J. D Rudolf, J. Ind. Microbiol. Biotechnol., 2023, 50, kuad027, DOI:10.1093/jimb/kuad027.
- T. Xu, Q.-M. Zhao, L.-G. Yao, L.-F. Lan, S.-W. Li and Y.-W. Guo, Steroids, 2023, 192, 109182, DOI:10.1016/j.steroids.2023.109182.
- Y.-Q. Huang, A. M. Illias, P.-J. Chen, S.-Y. Chien, Y.-H. Kuo, C.-Y. Ko, C.-F. Chang, Z.-H. Wen, T.-L. Hwang and P.-J. Sung, Tetrahedron Lett., 2023, 123, 154540, DOI:10.1016/j.tetlet.2023.154540.
- S. -W. Li, J. Liu, Y. Fu, L. -G. Yao, H. -Y. Zhang, H. Wang and Y. -W. Guo, Chem. Biodiversity, 2023, 20, e202300821, DOI:10.1002/cbdv.202300821.
- M. A. Ghandourah, Open Chem., 2023, 21, 20220327, DOI:10.1515/chem-2022-0327.
- Z.-Y. Xia, M.-M. Sun, Y. Jin, L.-G. Yao, M.-Z. Su, L.-F. Liang, H. Wang and Y.-W. Guo, Mar. Drugs, 2023, 21, 457, DOI:10.3390/md21080457.
- M. Maimaitiming, L. Lv, X. Zhang, S. Xia, X. Li, P. Wang, Z. Liu and C.-Y. Wang, Mar. Drugs, 2023, 21, 191, DOI:10.3390/md21030191.
- Z.-L. Wang, S.-Y. Zhang, S.-L. Hao and W.-X. Yang, Histol. Histopathol., 2023, 38, 9–28, DOI:10.14670/HH-18-500.
- A. F. Ahmed, C.-F. Dai, Y.-H. Kuo and J.-H. Sheu, Metabolites, 2023, 13, 392, DOI:10.3390/metabo13030392.
- Y. -Q. Du, L. -F. Liang and Y. -W. Guo, Chem. Biodiversity, 2023, 20, e202201065, DOI:10.1002/cbdv.202201065.
- X.-Y. Yan, L. Zhang, Q.-B. Yang, Z.-Y. Ge, L.-F. Liang and Y.-W. Guo, Mar. Drugs, 2023, 21, 523, DOI:10.3390/md21100523.
- Q.-B. Yang, Q. Wu, J.-K. Chen and L.-F. Liang, Mar. Drugs, 2023, 21, 30, DOI:10.3390/md21010030.
- N. B. A. Nguyen, M. El-Shazly, P.-J. Chen, B.-R. Peng, L.-Y. Chen, T.-L. Hwang and K.-H. Lai, Mar. Drugs, 2023, 21, 456, DOI:10.3390/md21080456.
- A. Córdova-Isaza, S. Jiménez-Mármol, Y. Guerra and E. Salas-Sarduy, Mar. Drugs, 2023, 21, 104, DOI:10.3390/md21020104.
- J. Kim, S. Ji, J.-Y. Lee, J. Lorquin, B. Orlikova-Boyer, C. Cerella, A. Mazumder, F. Muller, M. Dicato, O. Detournay and M. Diederich, Mar. Drugs, 2023, 21, 233, DOI:10.3390/md21040233.
- C.-C. Huang, Y.-H. Lo, Y.-J. Hsu, Y.-B. Cheng, C.-C. Kung, C.-W. Liang, D.-C. Chang, K.-L. Wang and C.-F. Hung, Mar. Drugs, 2023, 21, 447, DOI:10.3390/md21080447.
- J. Pei, Y. Zhou, S. Chen, K. Yu, Z. Qin, R. Zhang and Y. Wang, Acta Oceanol. Sin., 2024, 42, 127–135, DOI:10.1007/s13131-023-2173-y.
- N. S. H. von Xylander, S. A. Young, C. Cole, T. K. Smith and N. Allison, Coral Reefs, 2023, 42, 551–566, DOI:10.1007/s00338-023-02356-w.
- T. V. Sikorskaya, Mar. Drugs, 2023, 21, 335, DOI:10.3390/md21060335.
- A. B. Imbs and V. M. Dembitsky, Mar. Drugs, 2023, 21, 539, DOI:10.3390/md21100539.
- E. Savelson and J. J. Tepe, J. Org. Chem., 2023, 88, 755–761, DOI:10.1021/acs.joc.2c00033.
- B.-Y. Wu, F.-Y. Shih, Y.-T. Tsai, C.-Y. Chu and C.-K. Lin, Org. Biomol. Chem., 2023, 21, 4601, 10.1039/D3OB00672G.
- Z. Zhu and T. J. Maimone, J. Am. Chem. Soc., 2023, 145, 14215–14220, DOI:10.1021/jacs.3c04493.
- Z. Tian, X.-T. Lu, X. Jiang and J. Tian, Front. Pharmacol, 2023, 14, 1187411, DOI:10.3389/fphar.2023.1187411.
- Z.-H. Chen, Y.-W. Guo and X.-W. Li, Nat. Prod. Rep., 2023, 40, 509–556, 10.1039/D2NP00021K.
- Y. Hioki, T. Sato, T. Iwao, J. Ikuma, A. Kawamura, T. Ohyoshi, Y. Tsunematsu and M. Kita, Eur. J. Org Chem., 2023, 26, e202300084, DOI:10.1002/ejoc.202300084.
- P. T. Binh, D. T. Trang, V. T. Trung, K. T. P. Linh, N. V. Phong, N. P. Thao, N. X. Cuong, D. C. Thung, N. H. Nam and N. V. Thanh, Phytochem. Lett., 2023, 53, 92–97, DOI:10.1016/j.phytol.2022.11.016.
- M. Nagasaka, H. Isa, A. Tahara, R. Fukada, T. Kamada and T. Ishii, Chem. Biodiversity, 2023, 20, e202300791, DOI:10.1002/cbdv.202300791.
- T. Ohyoshi, H. Iizumi, S. Hosono, H. Tano and H. Kigoshi, Org. Lett., 2023, 25, 4725–4729, DOI:10.1021/acs.orglett.3c01692.
- S. -M. Shen, S. -W. Li, M. -Z. Su, L. -G. Yao, G. Appendino and Y. -W. Guo, Chem.–Eur. J., 2023, 29, e202203858, DOI:10.1002/chem.202203858.
- S. -M. Shen, M. -Z. Su, D. -D. Yu, H. Luo, X. -W. Li and Y. -W. Guo, Chem.–Eur. J., 2023, 29, e202300457, DOI:10.1002/chem.202300457.
- S.-W. Li, D.-D. Yu, M.-Z. Su, L.-G. Yao, H. Wang, X. Liu and Y.-W. Guo, Mar. Life Sci. & Technol., 2023, 5, 373–386, DOI:10.1007/s42995-023-00179-w.
- E. H. Andrianasolo, L. Haramaty, K. L. McPhail and R. A. Lutz, J. Shellfish Res., 2023, 42, 177–197, DOI:10.2983/035.042.0201.
- E. H. Andrianasolo, L. Haramaty, K. L. McPhail, E. White, C. Vetriani, P. Falkowski and R. Lutz, J. Nat. Prod., 2011, 74, 842–846, DOI:10.1021/np100601w.
- A. Bär, S. I. Bär, M. Röder and R. Schobert, J. Org. Chem., 2021, 86, 1868–1873, DOI:10.1021/acs.joc.0c02726.
- K.-K. Yuan, Z.-M. Chen, Y.-X. Liu, H.-Y. Li and W.-D. Yang, Mar. Drugs, 2023, 21, 155, DOI:10.3390/md21030155.
- J. Blanco, F. Arévalo, Á. Moroño, J. Correa, A. E. Rossignoli and J. P. Lamas, Toxins, 2023, 15, 13, DOI:10.3390/toxins15010013.
- A. E. Rossignoli, B. Ben-Gigirey, M. Cid, C. Mariño, H. Martín, S. Garrido, F. Rodríguez and J. Blanco, Toxins, 2023, 15, 631, DOI:10.3390/toxins15110631.
- N. Takati, H. Azeddoug, M. N. Benchekroun, M. Blaghen and M. M. Ennaji, Cell. Mol. Biol., 2023, 69, 95–100, DOI:10.14715/cmb/2023.69.6.15.
- M. S. Hernández-Zazueta, J. S. García-Romo, I. Luzardo-Ocampo, Á. A. Carbonell-Barrachina, P. Taboada-Antelo, E. C. Rosas-Burgos, J. M. Ezquerra-Brauer, J. M. Martínez-Soto, M. del Carmen Candia-Plata, H. del Carmen Santacruz-Ortega and A. Burgos-Hernández, Food Chem. Toxicol., 2023, 177, 113829, DOI:10.1016/j.fct.2023.113829.
- S. K. Paulose and K. Chakraborty, Nat. Prod. Res., 2023, 37, 891–902, DOI:10.1080/14786419.2022.2095634.
- R. Sultanov, E. Ermolenko, T. Poleshchuk and S. Kasyanov, Mar. Drugs, 2023, 21, 4, DOI:10.3390/md21010004.
- Y. Denisenko, T. Novgorodtseva, M. Antonyuk, A. Yurenko, T. Gvozdenko, S. Kasyanov, E. Ermolenko and R. Sultanov, Mar. Drugs, 2023, 21, 351, DOI:10.3390/md21060351.
- L. T. T. Nguyen, D. J. Craik and Q. Kaas, Mar. Drugs, 2023, 21, 154, DOI:10.3390/md21030154.
- J. R. Groome, Mar. Drugs, 2023, 21, 209, DOI:10.3390/md21040209.
- H. B. Fiorotti, S. G. Figueiredo, F. V. Campos and D. C. Pimenta, J. Venom. Anim. Toxins Incl. Trop. Dis., 2023, 29, e20220052, DOI:10.1590/1678-9199-jvatitd-2022-0052.
- J. Chen, X. Zhang, C. Lin and B. Gao, Toxicon, 2023, 233, 107253, DOI:10.1016/j.toxicon.2023.107253.
- J. Dong, P. Zhang, J. Xie, T. Xie, X. Zhu, D. Zhangsun, J. Yu and S. Luo, Mar. Drugs, 2023, 21, 286, DOI:10.3390/md21050286.
- Y. Wu, J. Zhang, J. Ren, X. Zhu, R. Li, D. Zhangsun and S. Luo, Mar. Drugs, 2023, 21, 326, DOI:10.3390/md21060326.
- A. Kancherla and S. Sarma, Indian J. Biochem. Biophys., 2023, 60, 710, DOI:10.56042/ijbb.v60i9.4061.
- K. Cooreman, B. De Spiegeleer, C. Van Poucke, D. Vanavermaete, D. Delbare, E. Wynendaele and B. De Witte, Environ. Toxicol. Pharmacol., 2023, 102, 104254, DOI:10.1016/j.etap.2023.104254.
- J. Li, S. Han, Y. Zhu and B. Dong, Mar. Drugs, 2023, 21, 51, DOI:10.3390/md21010051.
- D. W. Prebble, D. C. Holland, J. B. Hayton, F. Ferretti, L. K. Jennings, J. Everson, M. Xu, M. J. Kiefel, G. D. Mellick and A. R. Carroll, J. Nat. Prod., 2023, 86, 533–540, DOI:10.1021/acs.jnatprod.2c01140.
- X. Tian, D. Wang, W. Jiang, H. R. Bokesch, B. A. P. Wilson, B. R. O'Keefe and K. R. Gustafson, J. Nat. Prod., 2023, 86, 1855–1861, DOI:10.1021/acs.jnatprod.3c00393.
- H. Yu, J. Zhang, D. Ma, X. Li and T. Xu, J. Am. Chem. Soc., 2023, 145, 22335–22340, DOI:10.1021/jacs.3c08714.
- D. Carbone, C. Gallo, G. Nuzzo, G. Barra, M. Dell'Isola, M. Affuso, O. Follero, F. Albiani, C. Sansone, E. Manzo, G. d'Ippolito and A. Fontana, Nat. Prod. Bioprospect., 2023, 13, 34, DOI:10.1007/s13659-023-00401-3.
- W. Wang, F. Ma, Y. T. Cheung, G. Zeng, Y. Zhou, Z. Chen, L. Liang, T. Luo and R. Tong, J. Med. Chem., 2023, 66, 11201–11215, DOI:10.1021/acs.jmedchem.3c00659.
- M. Yoshida, K. Sasaoka, K. Hano, Y. Sugiyama, Y. Hitora, T. Doi, S. Tsukamoto and H. Kigoshi, Bull. Chem. Soc. Jpn., 2023, 96, 1283–1297, DOI:10.1246/bcsj.20230197.
- H. Geng, F. Chen, Y. Zhao, B. Guo, L. Tang and Y.-Y. Yang, J. Org. Chem., 2023, 88, 3927–3934, DOI:10.1021/acs.joc.2c02837.
- F. Ishibashi, S. Zha, T. Kondo, M. Sakamoto, M. Ueno and T. Fukuda, Biosci., Biotechnol., Biochem., 2023, 87, 148, DOI:10.1093/bbb/zbac193.
- K. Dzedulionytė, N. Fuxreiter, E. Schreiber-Brynzak, A. Žukauskaitė, A. Šačkus, V. Pichler and E. Arbačiauskienė, RSC Adv., 2023, 13, 7897–7912, 10.1039/D3RA00972F.
- G. Yang, H. Xie, C. Wang, C. Zhang, L. Yu, L. Zhang, X. Liu, R. Xu, Z. Song, R. Liu and M. Ueda, Eur. J. Med. Chem., 2023, 250, 115193, DOI:10.1016/j.ejmech.2023.115193.
- L. T. Vien, T. T. H. Hanh, T. H. Quang, N. V. Thanh, N. X. Cuong, N. H. Nam, T. D. Cong and P. V. Kiem, J. Asian Nat. Prod. Res., 2023, 25, 1229–1235, DOI:10.1080/10286020.2023.2219616.
- Y. E. Puspitasari, E. Tuenter, K. Foubert, H. Herawati, A. M. Hariati, A. Aulanni’am, L. Pieters, T. De Bruyne and N. Hermans, Nutrients, 2023, 15, 1033, DOI:10.3390/nu15041033.
- A. S. Silchenko, S. A. Avilov, R. S. Popov, P. S. Dmitrenok, E. A. Chingizova, B. B. Grebnev, A. B. Rasin and V. I. Kalinin, Mar. Drugs, 2023, 21, 114, DOI:10.3390/md21020114.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, R. S. Popov, P. S. Dmitrenok, E. A. Chingizova, E. S. Menchinskaya, E. G. Panina, V. G. Stepanov, V. I. Kalinin and V. A. Stonik, Int. J. Mol. Sci., 2023, 24, 11128, DOI:10.3390/ijms241311128.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, R. S. Popov, E. A. Chingizova, E. S. Menchinskaya, E. A. Zelepuga, E. G. Panina, V. G. Stepanov, V. I. Kalinin and P. S. Dmitrenok, Mar. Drugs, 2023, 21, 602, DOI:10.3390/md21120602.
- T. Sanguanphun, S. Promtang, N. Sornkaew, N. Niamnont, P. Sobhon and K. Meemon, Mar. Drugs, 2023, 21, 141, DOI:10.3390/md21030141.
- X. Tang, A. Nishimura, K. Ariyoshi, K. Nishiyama, Y. Kato, E. A. Vasileva, N. P. Mishchenko, S. A. Fedoreyev, V. A. Stonik, H.-K. Kim, J. Han, Y. Kanda, K. Umezawa, Y. Urano, T. Akaike and M. Nishida, Mar. Drugs, 2023, 21, 52, DOI:10.3390/md21010052.
- T. K. Pham, T. H. T. Nguyen, H. R. Yun, E. A. Vasileva, N. P. Mishchenko, S. A. Fedoreyev, V. A. Stonik, T. T. Vu, H. Q. Nguyen, S. W. Cho, H. K. Kim and J. Han, Mar. Drugs, 2023, 21, 222, DOI:10.3390/md21040222.
- S. E. Kim, E. D. S. Chung, E. A. Vasileva, N. P. Mishchenko, S. A. Fedoreyev, V. A. Stonik, H. K. Kim, J. H. Nam and S. J. Kim, Mar. Drugs, 2023, 21, 78, DOI:10.3390/md21020078.
- I. A. Abdelmawgood, N. A. Mahana, A. M. Badr, A. S. Mohamed, A. M. A. Shawoush, T. Atia, A. E. Abdelrazak and H. I. Sakr, Mar. Drugs, 2023, 21, 455, DOI:10.3390/md21080455.
- D.-G. Han, J. Kwak, E. Choi, S.-W. Seo, E. A. Vasileva, N. P. Mishchenko, S. A. Fedoreyev, V. A. Stonik, H. K. Kim, J. Han, J. H. Byun, I. H. Jung, H. Yun and I.-S. Yoon, Biomed. Pharmacother., 2023, 162, 114589, DOI:10.1016/j.biopha.2023.114589.
- Q. Xu, X. Yang, R. Zhang, Y. Li, Z. Yan, X. Li, B. Ma, Y. Liu, A. Lin, S. Han, K. Li and L. Chen, Toxics, 2023, 11, 137, DOI:10.3390/toxics11020137.
- S. Janta, K. Pranweerapaiboon, P. Vivithanaporn, A. Plubrukarn, A. Chairoungdua, P. Prasertsuksri, S. Apisawetakan and K. Chaithirayanon, Mar. Drugs, 2023, 21, 345, DOI:10.3390/md21060345.
- G. Liu, S. Zhang, R. Lin, X. Cao and L. Yuan, Front. Oncol., 2023, 13, 1307838, DOI:10.3389/fonc.2023.1307838.
- R. Ru, G. Chen, X. Liang, X. Cao, L. Yuan and M. Meng, Nutrients, 2023, 15, 378, DOI:10.3390/nu15020378.
- O. Fagbohun, J. Joseph, O. Oriyomi and H. Rupasinghe, Mar. Drugs, 2023, 21, 262, DOI:10.3390/md21050262.
- A. Zare, S. Izanloo, S. Khaledi, M. N. Maratovich, A. A. Kaliyev, N. A. Abenova, F. Rahmanifar, M. Mahdipour, S. Bakhshalizadeh, R. Shirazi, N. Tanideh and A. Tamadon, Mar. Drugs, 2023, 21, 283, DOI:10.3390/md21050283.
- N. V. Zhukova, Mar. Drugs, 2023, 21, 21, DOI:10.3390/md21010021.
- D. N. Pelageev, K. L. Borisova and V. P. Anufriev, Mar. Drugs, 2023, 21, 407, DOI:10.3390/md21070407.
- J. Kappen, J. Manurung, T. Fuchs, S. P. B. Vemulapalli, L. M. Schmitz, A. Frolov, A. Agusta, A. N. Muellner-Riehl, C. Griesinger, K. Franke and L. A. Wessjohann, Mar. Drugs, 2023, 21, 242, DOI:10.3390/md21040242.
- Z. Gao, M. -X. Wang, C. -L. Gao, S. Chen and X. -W. Li, Chem. Biodiversity, 2023, 20, e202300616, DOI:10.1002/cbdv.202300616.
- E. Pires, P. da Cunha Lana and L. L. Mafra Jr, Harmful Algae, 2023, 122, 102373, DOI:10.1016/j.hal.2022.102373.
- Y. V. Bolt, M. A. Dubinnyi, V. V. Litvinenko, A. A. Kotlobay, O. A. Belozerova, R. I. Zagitova, V. I. Shmygarev, O. N. Yatskin, E. B. Guglya, V. S. Kublitski, M. S. Baranov, I. V. Yampolsky, Z. M. Kaskova and A. S. Tsarkova, Org. Lett., 2023, 25, 4892–4897, DOI:10.1021/acs.orglett.3c01696.
- G. V. Malykin, P. V. Velansky, D. I. Melnikova and T. Y. Magarlamov, Mar. Biotechnol., 2023, 25, 918–934, DOI:10.1007/s10126-023-10249-w.
- R. Bruno, C. Boidin-Wichlacz, O. Melnyk, D. Zeppilli, C. Landon, F. Thomas, M.-A. Cambon, M. Lafond, K. Mabrouk, F. Massol, S. Hourdez, M. Maresca, D. Jollivet and A. Tasiemski, Sci. Total Environ., 2023, 879, 162875, DOI:10.1016/j.scitotenv.2023.162875.
- A. R. Kosker, M. Karakus, P. Katikou, I. Dal, M. Durmus, Y. Ucar, D. Ayas and F. Özogul, Mar. Drugs, 2023, 21, 527, DOI:10.3390/md21100527.
- T. I. Anastasiou, E. Kagiampaki, G. Kondylatos, A. Tselepides, P. Peristeraki and M. Mandalakis, Mar. Drugs, 2023, 21, 520, DOI:10.3390/md21100520.
- J. Park, C. Proux, W. Ehanno, L. Réthoré, E. Vessières, J. Bourreau, J. Favre, G. Kauffenstein, C. Mattei, H. Tricoire-Leignel, D. Henrion, C. Legendre and C. Legros, Mar. Drugs, 2023, 21, 196, DOI:10.3390/md21030196.
- M. Ito, S. Chisada, N. Matsunaga and N. Okino, Glycoconj. J., 2023, 40, 315–322, DOI:10.1007/s10719-023-10110-1.
- Z. Salimnejhad, H. Hassanzadazar and M. Aminzare, Food Nutr. Sci., 2023, 11, 5573, DOI:10.1002/fsn3.3514.
- P. Perumal, U. Arthanari and E. Sanniyasi, J. S. Afr. Bot., 2023, 155, 1–15, DOI:10.1016/j.sajb.2023.02.002.
- M. Grignon-Dubois and B. Rezzonico, Diversity, 2023, 15, 1210, DOI:10.3390/d15121210.
- S. Matsunaga, N. Fusetani and S. Konosu, Tetrahedron Lett., 1984, 25, 5165–5168, DOI:10.1016/S0040-4039(01)81553-7.
- A. C. Stierle, J. H. Cardellina II and F. L. Singleton, Experientia, 1988, 44, 1021, DOI:10.1007/BF01939910.
- D. John Faulkner, M. D. Unson and C. A. Bewley, Pure Appl. Chem., 1994, 66, 1983–1990, DOI:10.1351/pac199466101983AC4.
- W. Fenical, J. Antibiot., 2020, 73, 481–487, DOI:10.1038/s41429-020-0331-4.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2007, 70, 461–477, DOI:10.1021/np068054v.
- E. J. Biers, S. Sun and E. C. Howard, Appl. Environ. Microbiol., 2009, 75, 2221–2229, DOI:10.1128/AEM.02118-08A.
- T. Hoshino, H. Doi, G.-I. Uramoto, L. Wörmer, R. R. Adhikari, N. Xiao, Y. Morono, S. D'Hondt, K.-U. Hinrichs and F. Inagaki, Proc. Natl. Acad. Sci. U.S.A., 2020, 117, 27587–27597, DOI:10.1073/pnas.1919139117.
- B. Wei, G.-A. Hu, Z.-Y. Zhou, W.-C. Yu, A.-Q. Du, C.-L. Yang, Y.-L. Yu, J.-W. Chen, H.-W. Zhang, Q. Wu, Q. Xuan, X.-W. Xu and H. Wang, Microbiome, 2023, 11, 144, DOI:10.1186/s40168-023-01573-3AC9.
- T. M. Voser, M. D. Campbell and A. R. Carroll, Nat. Prod. Rep., 2022, 39, 7–19, 10.1039/D1NP00051A.
- R. Ueoka, R. A. Meoded, A. Gran-Scheuch, A. Bhushan, M. W. Fraaije and J. Piel, Angew. Chem., Int. Ed., 2020, 59, 7761–7765, DOI:10.1002/anie.201916005.
- M. Tsukimoto, M. Nagaoka, Y. Shishido, J. Fujimoto, F. Nishisaka, S. Matsumoto, E. Harunari, C. Imada and T. Matsuzaki, J. Nat. Prod., 2011, 74, 2329–2331, DOI:10.1021/np200543.
- K. J. Nicacio, L. P. Ióca, A. M. Fróes, L. Leomil, L. R. Appolinario, C. C. Thompson, F. L. Thompson, A. G. Ferreira, D. E. Williams, R. J. Andersen, A. S. Eustaquio and R. G. S. Berlinck, J. Nat. Prod., 2017, 80, 235–240, DOI:10.1021/acs.jnatprod.6b00838.
- I. Mohanty, S. Tapadar, S. G. Moore, J. S. Biggs, C. J. Freeman, D. A. Gaul and N. Garg, mSystems, 2021, 6, e0138720, DOI:10.1128/msystems.01387-20.
- A. L. Waters, O. Peraud, N. Kasanah, J. W. Sims, N. Kothalawala, M. A. Anderson, S. H. Abbas, K. V. Rao, V. R. Jupally, M. Kelly, A. Dass, R. T. Hill and M. T. Hamann, Front. Mar. Sci., 2014, 54 DOI:10.3389/fmars.2014.00054.
- M. S. Donia, J. Ravel and E. W. Schmidt, Nat. Chem. Biol., 2008, 4, 341–343, DOI:10.1038/nchembio.8.
- P. Long, W. Dunlap, C. Battershill and M. Jaspars, ChemBioChem, 2005, 6, 1760, DOI:10.1002/cbic.200500210.
- A. T. Piwko, B. G. Miller and J. M. Smith, Nat. Prod. Rep., 2023, 40, 964, 10.1039/d2np00082b.
- M. F. Freeman, A. L. Vagstad and J. Piel, Curr. Opin. Chem. Biol., 2016, 31, 8–14, DOI:10.1016/j.cbpa.2015.11.002.
- E. P. McCauley, I. C. Piña, A. D. Thompson, K. Bashir, M. Weinberg, S. L. Kurz and P. Crews, J. Antibiot., 2020, 73, 504–525, DOI:10.1038/s41429-020-0330-5.
- O. K. Radjasa, Y. M. Vaske, G. Navarro, H. C. Vervoort, K. Tenney, R. G. Linington and P. Crews, Bioorg. Med. Chem. Lett., 2011, 19, 6658–6674, DOI:10.1016/j.bmc.2011.07.017.
- T. Wakimotoa, Chem. Pharm. Bull., 2023, 71, 1–8, DOI:10.1248/cpb.c22-00715.
- J. A. van Santen, G. Jacob, A. L. Singh, V. Aniebok, M. J. Balunas, D. Bunsko, F. C. Neto, L. Castaño-Espriu, C. Chang, T. N. Clark, J. L. C. Little, D. A. Delgadillo, P. C. Dorrestein, K. R. Duncan, J. M. Egan, M. M. Galey, F. P. J. Haeckl, A. Hua, A. H. Hughes, D. Iskakova, A. Khadilkar, J.-H. Lee, S. Lee, N. LeGrow, D. Y. Liu, J. M. Macho, C. S. McCaughey, M. H. Medema, R. P. Neupane, T. J. O'Donnell, J. S. Paula, L. M. Sanchez, A. F. Shaikh, S. Soldatou, B. R. Terlouw, T. A. Tran, M. Valentine, J. J. J. van der Hooft, D. A. Vo, M. Wang, D. Wilson, K. E. Zink and R. G. Linington, ACS Cent. Sci., 2019, 5, 1824–1833, DOI:10.1021/acscentsci.9b00806.
- M. M. Schofield, S. Jain, D. Porat, G. J. Dick and D. H. Sherman, Environ. Microbiol., 2015, 17, 3964–3975, DOI:10.1111/1462-2920.12908.
- J. K. B. Cahn and J. Piel, Angew. Chem., Int. Ed., 2021, 60, 18412–18428, DOI:10.1002/anie.201900532.
- R. D. Kersten, N. Ziemert, D. J. Gonzalez, B. M. Duggan, V. Nizet, P. C. Dorrestein and B. S. Moore, Proc. Natl. Acad. Sci. U.S.A., 2013, 110, E4407–E4116, DOI:10.1073/pnas.1315492110.
- C. Hermans, M. L. De Mol, M. Mispelaere, A.-S. De Rop, J. Rombaut, T. Nusayr, R. Creamer, S. L. De Maeseneire, W. K. Soetaert and P. Hulpiau, Mar. Drugs, 2023, 21, 449, DOI:10.3390/md21080449.
- A. E. Kadjo and A. S. Eustáquio, J. Ind. Microbiol. Biotechnol., 2023, 50, 1–12, DOI:10.1093/jimb/kuad044.
- P. D. Scesa, Z. Lin and E. W. Schmidt, Nat. Chem. Biol., 2022, 18, 659–663, DOI:10.1038/s41589-022-01027-1.
- I. Burkhardt, T. de Rond, P. Y.-T. Chen and B. S. Moore, Nat. Chem. Biol., 2022, 18, 664–669, DOI:10.1038/s41589-022-01026-2.
- K. Wilson, T. de Rond, I. Burkhardt, T. S. Steele, R. J. B. Schäfer, S. Podell, E. E. Allen and B. S. Moore, Proc. Natl. Acad. Sci. U.S.A., 2023, 120, e2220934120, DOI:10.1073/pnas.2220934120.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4np00067f |
| This journal is © The Royal Society of Chemistry 2025 |