 Open Access Article
Open Access ArticleVisible light assisted oxidation of organic compounds by iridium(III)dipyrrinato complexes†
Shekhar
Kumar
 ,
Lalita
Devi
,
Lalita
Devi
 and
Iti
Gupta
and
Iti
Gupta
 *
*
Indian Institute of Technology Gandhinagar, Palaj Campus, Gandhinagar, Gujarat-382355, India. E-mail: iti@iitgn.ac.in
First published on 26th May 2025
Abstract
Photo-assisted oxidation of organic compounds is a greener and more sustainable approach, which utilizes a very small amount of photocatalyst and visible light or sunlight. Ir(III) cyclometalated complexes have been explored extensively for photocatalytic applications in the past. Alternatively, Ir(III)dipyrrinato complexes can be employed for photocatalysis as they have attractive optical properties with high singlet oxygen generation ability. An efficient protocol for the photocatalytic aerobic oxidation of amines and sulfides by utilizing only 0.05 mol% Ir(III)dipyrrinato complexes is reported. The reactions are completed in 2 hours, providing imines and sulfoxides, in excellent yields. Additionally, a methodology for the hydroxylation of aryl boronic acids using 0.05 mol% Ir(III)dipyrrinato catalyst is reported. These methods display excellent substrate tolerance and offer an effective strategy for synthesizing a diverse range of functionalized imines, sulfoxides and phenols in a highly efficient manner, which validates the versatility of Ir(III)dipyrrinato complexes as photocatalysts for various organic transformations. Notably, these photochemical transformations require only a minimal catalyst loading of 0.05 mol% for these reactions, which demonstrates their exceptional cost-effectiveness.
Introduction
The oxidation of organic compounds plays an important role in organic synthesis. Compounds such as imines, phenols and sulfoxides are very important classes of compounds. Imines are key intermediates to synthesize many fine chemicals, dyes and pharmaceutical drugs.1,2 The condensation between primary amines and carbonyl compounds leads to the formation of imines, but the high reactivity of carbonyl compounds leads to the formation of unwanted products. Phenols are frequently occurring scaffolds in biologically active compounds such as natural products and pharmaceuticals.3 They are also key intermediates in organic synthesis.4,5 Phenols are traditionally synthesized through nucleophilic aromatic substitution on aryl halides containing electron-withdrawing groups, as well as by the hydrolysis of arene diazonium salts. However, this method has very poor functional group tolerance, which leads to a limited substrate scope.6 Sulfoxides are crucial intermediates in organic synthesis7–10 and are also present in many pharmaceutical drugs, such as omeprazole, modafinil, sulindac, sulforaphane and fipronil.1,11,12 Traditionally, strong oxidizing agents, such as m-CPBA,13,14 2-iodobenzoic acid (IBX),15 or Oxone,16 are needed for sulfoxidation, and in many cases, high temperatures and high reaction times are also required. These harsh conditions result in the over-oxidation of the sulfide to a sulfone.The above-mentioned issues necessitate the development of efficient, sustainable and selective oxidation strategies. In recent years, visible-light assisted photocatalysis has emerged as an efficient and environmentally benign approach for the synthesis of fine chemicals.17–20 Given its high natural abundance and clean energy profile, visible-light-driven photocatalysis is a promising alternative to traditional oxidation methods.12,17,21–28 Molecular oxygen (O2) is a widely available and environmentally friendly oxidant that can be employed in oxidative photocatalytic transformations, including the oxidative coupling of benzylamines to imines,25,29–31 oxidation of sulfides to sulfoxides,22,24,26–28,32 and oxidative hydroxylation of boronic acids to phenols.23,33–37 However, challenges such as high catalyst loading and long reaction times often limit the practical application of these methodologies.
Metal-dipyrrinato complexes have recently garnered significant attention as efficient photocatalysts due to their high absorption coefficients in the visible region and long-lived triplet excited states, making them excellent photosensitizers.38,39 In this work, we have utilized previously reported three meso-substituted Ir(III)dipyrrinato complexes and investigated their catalytic efficiency for a number of photocatalytic reactions for the first time. These complexes have a high absorption coefficient in visible light and high singlet oxygen quantum yield. We demonstrated the photocatalytic application of Ir(III)dipyrrinato complexes using only 0.05 mole % catalytic loading for the oxidative coupling of benzylamine to an imine, conversion of sulfides to sulfoxides and oxidative hydroxylation of boronic acids to phenols.
Results and discussion
In this work, three Ir(III)dipyrrinato complexes (Ir1–Ir3) (as shown in Fig. 1) containing electron-donating and electron-withdrawing moieties at the C-5 position of the dipyrrin ligand (phenyl, N-butylcarbazole and pentafluorophenyl groups) were prepared as per the reported method and employed for photocatalytic applications.40,41Singlet oxygen generation
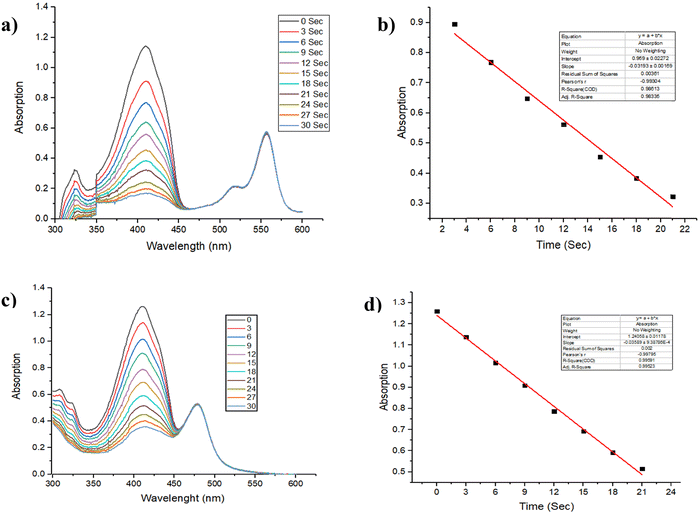
In the case of photocatalytic oxidation, molecular oxygen plays a crucial role as an oxidant. The generation of singlet oxygen became very important because it acts as one of the reagents in the reaction. Therefore, there is a significant demand for catalysts with exceptional singlet oxygen generation capability. A singlet oxygen generation experiment was conducted to determine the singlet oxygen quantum yields of the Ir(III)dipyrrinato complexes (Ir1, Ir2 and Ir3) by following the standard protocol42 using Rose Bengal43 as the reference in methanol solvent.Fig. 2 shows plots of the Ir3 complex's singlet oxygen generation assay, whereas data for the Ir1 and Ir2 complexes can be found in the ESI† (Fig. S1). The figures show that Ir3 produced a singlet oxygen quantum yield of 90%, while the Ir1 and Ir2 complexes produced singlet oxygen quantum yields of 87% and 81%, respectively.
Photo-oxidative benzylamine coupling
The excellent singlet oxygen quantum yield of these Ir(III)dipyrrinato complexes encouraged us to utilize them as photosensitizers for various photo-oxidation reactions. At first, we tested them for the photo-oxidative benzylamine coupling reaction, and the reaction optimization results are summarized in Table 1.| Entry | Light | Solvent | Catalyst | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: benzylamine (1 mmol), solvent (4 mL), catalyst (0.05 mol%), irradiation of white light (24 W, 96 mW cm−2) for 2 h under an O2 atmosphere at rt. b Yield was calculated by 1H-NMR using 1,3,5-trimethylbenzene (1 mmol) as an internal standard. c Time of reaction 12 h. d Time of reaction 12 h. | ||||
| 1 | White | ACN | Ir3 | 97 |
| 2 | White | ACN | Ir2 | 64 |
| 3 | White | ACN | Ir1 | 84 |
| 4 | Sunlight | ACN | Ir3 | 97 |
| 5 | White | MeOH | Ir3 | 23 |
| 6 | White | Hexane | Ir3 | 25 |
| 7 | White | THF | Ir3 | 32 |
| 8 | White | Chloroform | Ir3 | 54 |
| 9c | White | ACN | — | 3 |
| 10d | — | ACN | Ir3 | 2 |
When white light was used as an excitation source in acetonitrile (ACN), Ir3 exhibited the highest catalytic efficiency and afforded 2a in 97% yield (entry 1). Under the same conditions, Ir2 and Ir1 demonstrated moderate activity, yielding the desired product in 64% and 84% yield, respectively (entries 2 and 3). The reaction proceeded efficiently under natural sunlight, with a 97% yield (entry 4). The solvent effect was further explored using Ir3 as a photocatalyst under white light. When methanol (MeOH) and hexane were used as the solvent, the reaction yield dropped significantly to 23% and 25%, respectively (entries 5 and 6). Tetrahydrofuran (THF) and chloroform showed slightly improved performance, with yields of 32% and 54%, respectively (entries 7 and 8). The control experiments further confirmed the necessity of both the photocatalyst and the light source. In the absence of a catalyst and light, the reaction in ACN afforded the product only in 3% and 2% yields, respectively (entries 9 and 10).
These optimization reactions suggest that Ir3 works best for the photo-oxidative benzylamine coupling reaction in the presence of the white LED or sunlight and ACN solvent. A variety of substrate scopes were examined by utilizing the optimized reaction conditions (Scheme 1). In the case of unsubstituted benzylamine, the product (2a) yield was 97% under both the white LED and sunlight. Electron-donating substituents, such as para-methoxy and ortho-methyl groups, were also well tolerated and afforded 2b and 2c products with very good yields in both sunlight and white light. In contrast, fluorine derivatives showed excellent conversion, including para-fluoro (2d, 94%/97%), ortho-fluoro (2e, 96%/95%) and trifluoromethyl (2f, 89%/92%), indicating strong compatibility with the reaction conditions, while para-substituted bromine (2g, 90%/92%) and chlorine (2i, 92%/94%) derivatives of benzylamine also yielded high conversions. However, ortho-substituted bromobenzylamine showed a decrease in reactivity, yielding product 2h in only 59% yield under a white LED and 61% yield under sunlight, likely due to steric hindrance. However, in the case of secondary amines, dibenzylamine afforded 2a with 96% yield and 1,2,3,4-tetrahydroisoquinoline also afforded the desired imine product 2j with 67% and 65% yields under both white LEDs and sunlight, respectively.
Sulfoxidation
In the case of photocatalytic sulfoxidation, the influence of different parameters, such as catalyst, solvent and different light, was investigated to achieve high selectivity and conversion. For the optimization, methylphenyl sulfide (1 mmol, 3a) was selected as the model substrate to assess the efficiency of the oxidation process. In all cases, molecular oxygen (O2) was purged in the reaction mixture for 15 minutes and placed under an O2 atmosphere to ensure a sufficient oxygen supply for the catalytic transformation. The effect of different solvents and catalysts on the photocatalytic sulfoxidation of methylphenyl sulfide was systematically investigated (Table 2).| Entry | Solvent | Catalyst | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: methylphenyl sulfide (1 mmol), catalyst (0.05 mol%), irradiation with white light (24 W, 96 mW cm−2) under an O2 atmosphere at rt. b Yield was calculated by 1H-NMR using 1,3,5-trimethylbenzene (1 mmol) as an internal standard. c No light reaction time 12 h. d No catalyst reaction time 12 h. | |||
| 1 | MeOH 8 mL | Ir1 | 76 |
| 2 | MeOH 8 mL | Ir2 | 67 |
| 3 | MeOH 8 mL | Ir3 | 98 |
| 4 | MeOH 4 mL | Ir3 | 64 |
| 5 | EtOH 8 mL | Ir3 | 94 |
| 6 | THF 8 mL | Ir3 | 12 |
| 7 | DMF 8 mL | Ir3 | 17 |
| 8 | ACN 8 mL | Ir3 | 58 |
| 9c | MeOH 8 mL | Ir3 | 2 |
| 10d | MeOH 8 mL | — | 4 |
Initially, methanol (MeOH) was used as the solvent to assess the catalytic performance of various Ir(III)dipyrrinato photocatalysts. Among them, Ir3 exhibited the highest efficiency, affording the sulfoxide product in 98% yield (entry 2). Ir1 and Ir2 demonstrated moderate activity, yielding the product in 76% and 67% yield, respectively (entries 1 and 3). Subsequently, the effect of solvent variation was explored using Ir3 as a photocatalyst. When the volume of MeOH was reduced to 4 mL, the product yield decreased to 64% (entry 4). Switching to ethanol (EtOH) as the solvent resulted in a slightly lower yield of 94% (entry 5), while the use of tetrahydrofuran (THF) and N,N-dimethylformamide (DMF) led to significantly lower conversions, affording the products in 12% and 17% yields, respectively (entries 6 and 7). Acetonitrile (ACN) demonstrated moderate efficiency, yielding 58% of the desired product (entry 8). In the absence of light and catalyst, only 2% and 4% yields were observed, respectively, confirming the necessity of light and photocatalysts for effective oxidation (entries 9 and 10). Light optimization (Table S1, ESI†) suggested that the white LED and sunlight work best for sulfoxidation reactions. These optimization results indicate that Ir3 is most effective for sulfoxidation reactions, when exposed to white LED light or sunlight in methanol.
The substrate scope of the photocatalytic sulfoxidation reaction was explored using various substituted thioanisole derivatives under the optimized reaction conditions (Scheme 2). The reaction proceeded efficiently for substrates having electron-donating groups, such as methyl (4b, 97%/97%) and methoxy (4c, 83%/87%), yielding high conversion under white light and sunlight. Similarly, substrates containing halogen substituents, including fluorine (4d, 94%/95%), chlorine (4e, 78%/77%) and bromine (4f, 88%/90%), demonstrated good to excellent yields, indicating that halogenated substrates are well tolerated in the reaction, while the yield of meta-substituted (4g, 76%/75% and 4i, 73%/74%) and ortho-substituted (4h, 64%/65%) bromine was lower compared to that of para-substituted (4f, 88%/90%) bromine, indicating a negative effect of steric hindrance on product yield. In contrast, substrates with electron-withdrawing groups, such as nitro (4j, 38%/39%) and cyano (4k, 43%/46%), provided lower yields. Diverse functional groups such as hydroxy-substituted compounds and heterocyclic sulfoxide also provided good yields (4l, 77%/78%) and (4m, 82%/83%), respectively, in both sunlight and white light. The alkyl-substituted sulfides, including tert-butyl (4n, 57%/55%) and n-butyl (4o, 86%/83%), also demonstrated efficient photo-oxidation. Biphenyl thioether produced 4p with a yield of only 18% in white light and 20% in sunlight, also indicating steric hindrance effects. In the case of phenothiazine, the product (4q) yield was only 41% and 44% in white light and sunlight, respectively. Also, diallylsulfane produces 4r and benzyl(methyl)sulfane produces 4s with excellent yields. In all cases, comparable yields were obtained under both white light and sunlight irradiation.
To understand the reaction mechanism, inhibition experiments were conducted (Table 3) in the case of KI, which is an inhibitor of h+ (PC+˙).24 The yield of sulfoxidation and benzylamine coupling reactions decreased to 36% and 6%, respectively (entry 1), which indicates that h+ (PC+˙) is responsible for the product formation in both cases. In the presence of NaN3 and benzoquinone, which act as scavengers of singlet oxygen (1O2)44 and peroxide radicals (O2˙−),45 respectively (entries 1 and 2), a significant decrease in the yield of both products is observed, suggesting that both singlet oxygen (1O2) and peroxide radicals (O2˙−) were involved in product formation.45 The presence of thioether cationic radicals is also confirmed by 1,4-dimethoxy-benzene,44 whereas no significant change in yield was observed in the presence of isopropanol, which suggests no involvement of the hydroxyl radical (˙OH) in the reaction mechanism.46 With the help of these inhibition experiments, the following mechanism was proposed (Fig. 3), which indicates that the reaction follows both energy and electron transfer mechanisms. In the case of energy transfer, when light falls on the catalyst, it gets excited to a singlet excited state Ir3*(S1) and, then through ISC, gets converted to a triplet excited state Ir3*(T1). The triplet excited state Ir3*(T1) reacts with O2 and forms singlet oxygen (1O2); when this 1O2 reacts with benzylamine, intermediate benzaldehyde is generated and then it reacts with another benzylamine to form the desired photocoupled imine product.
| Entry | Scavengersc | Inhibited species | Yieldb (%) (sulfoxidation) | Yieldb (%) (benzylamine coupling) |
|---|---|---|---|---|
| a Reaction conditions: methylphenyl sulfide/benzylamine (1.0 mmol), Ir3 (0.05 mol%), MeOH/ACN 8/4 mL, irradiation with white light (24 W, 96 mW cm−2) under an O2 atmosphere at rt for 2 h. b 1H-NMR yields. c Scavengers (1.0 mmol). d Only used in the sulfoxidation reaction. | ||||
| 1 | KI | PC+˙ (h+) | 36 | 6 |
| 2 | NaN3 | 1O2 | 10 | 11 |
| 3 | Benzoquinone | O2˙− | 9 | 34 |
| 4d | 1,4-Dimethoxy-benzene | Thioether cationic radical | 65 | — |
| 5 | Isopropanol | ˙OH | 94 | 93 |
 | ||
| Fig. 3 Proposed mechanisms for visible light-induced oxidation of sulfides and oxidative benzylamine coupling using Ir3 as a photosensitizer (PS) through both electron transfer and energy transfer. | ||
In the case of electron transfer, the excited state of the photocatalyst (Ir3*) donates an electron to oxygen to generate an oxygen anionic radical (O2−˙) and Ir3* gets oxidised to Ir3+˙ which, upon reaction with benzylamine, generates an intermediate I and returns to its ground state (Ir3). Intermediate I reacts with O2−˙ and generates benzaldehyde. Then, benzaldehyde reacts with another benzylamine to form the desired imine. In the case of sulfoxidation, energy transfer and electron transfer mechanisms follow the same cycle until singlet oxygen (1O2) or the oxygen anionic radical (O2−˙) is generated. After that, singlet oxygen (1O2) and the oxygen anionic radical (O2−˙) react with thioether and intermediate II, respectively, which generates an intermediate III. Then, another molecule of thioether reacts with intermediate III to form the desired sulfoxide.
Aerobic hydroxylation of boronic acids
Apart from benzylamine coupling and sulfoxidation, the aerobic hydroxylation of boronic acid was also catalysed by the Ir(III)dipyrrinato catalyst system. In this reaction, phenylboronic acid is converted into phenol in the presence of light, which is an additive that acts as a sacrificial electron transfer reagent and catalyst in open air only.The influence of various additives and solvents on the photocatalytic aerobic hydroxylation of boronic acid was systematically investigated using different Ir(III)dipyrrinato photocatalysts (Table 4). When diethyl amine was used as an additive in acetonitrile (ACN), Ir3 showed the highest catalytic efficiency, affording product 6a with 98% yield (entry 3). Under the same conditions, Ir1 and Ir2 demonstrated 75% and 48% product yields, respectively (entries 1 and 2). The role of different additives was further explored using Ir3 in ACN. Triethylamine (TEA) facilitated the reaction efficiently, yielding 93% (entry 4), whereas diisopropylamine (DIPA) and triphenylamine led to significantly lower yields of 47% and 32%, respectively (entries 5 and 6). The effect of solvent was also examined using Ir3 with diethyl amine as an additive. While the reaction proceeded efficiently in methanol (MeOH) and toluene, affording the product in 76% and 83% yields, respectively (entries 7 and 9), in the case of dimethyl sulfoxide (DMSO), only 5% product yield was observed (entry 8). Notably, when chloroform (CHCl3) was employed, an excellent yield of 96% was obtained (entry 10). When water is used for the reaction, the yield of the reaction decreases to 17% (entry 11). Notably, in the absence of a photocatalyst, no product formation was observed (entry 12), while without additives or without light, a negligible amount of product was observed (entries 13 and 14), which confirms that a catalyst, additive and white light are much needed for the reaction.
| Entry | Catalyst | Additive | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: (4-methoxyphenyl)boronic acid (0.5 mmol), catalyst (0.05 mol%), additive (1 mmol), solvent (4 mL), irradiation with white light (24 W, 96 mW cm−2) in the open air at rt for 20 h. b Isolated from column chromatography. c No light. | ||||
| 1 | Ir1 | Diethyl amine | ACN | 75 |
| 2 | Ir2 | Diethyl amine | ACN | 48 |
| 3 | Ir3 | Diethyl amine | ACN | 98 |
| 4 | Ir3 | TEA | ACN | 93 |
| 5 | Ir3 | DIPA | ACN | 47 |
| 6 | Ir3 | Triphenyl amine | ACN | 32 |
| 7 | Ir3 | Diethyl amine | MeOH | 76 |
| 8 | Ir3 | Diethyl amine | DMSO | 5 |
| 9 | Ir3 | Diethyl amine | Toluene | 83 |
| 10 | Ir3 | Diethyl amine | CHCl3 | 96 |
| 11 | Ir3 | Diethyl amine | H2O | 17 |
| 12 | No catalyst | Diethyl amine | ACN | — |
| 13 | Ir3 | — | ACN | 3 |
| 14c | Ir3 | Diethyl amine | ACN | 2 |
These findings established that Ir3 as a photocatalyst, diethyl amine as an additive and ACN as a solvent in the presence of a white LED work best for the photocatalytic aerobic hydroxylation of boronic acid reaction.
The substrate scope of the photocatalytic hydroxylation of aryl boronic acids was investigated under the optimized conditions (Scheme 3). Electron-donating substituents, such as methoxy (6a, 98%) and methyl (6b, 96%), exhibited excellent yields, while in the case of meta (6c) and ortho (6d), methyl-substituted groups also showed good conversion with 89% and 83% yields, respectively. In contrast, the dimethoxy-substituted derivative (6e) showed only 57% yield. In the case of phenylboronic acid, phenol (6f) was produced with a 94% yield. Electron-withdrawing substituents such as nitrile (6g, 92%), nitro (6h, 91%) and trifluoromethyl (6i, 64%) groups were well tolerated and gave good to excellent yields, while halogens, including chloro (6j, 91%) and bromo (6k, 80%), maintained high reactivity. Functionalized substrates with ketone (6l, 87%), carboxylic acid (6m, 94%), ethyl ester (6n, 90%) and aldehydes (6o, 78%) demonstrated excellent reactivity, while extended aromatic systems, such as naphthalene-derivatives resulted hydroxylation product (6p, 45%) and (6q, 53%), in moderate yields. Overall, the results demonstrate the broad functional group tolerance of the photocatalytic hydroxylation system, making it a versatile approach for the selective hydroxylation of aryl boronic acids.
In the past, Ir or Ru cyclometalated complexes were employed for similar reactions. In Table 5 the results from this work are compared with those of the previously reported catalysts. It is clear that previous systems used a higher amount of catalyst loading (0.25–2 mol%), whereas the Ir(III)dipyrrinato catalyst works very well at lower catalytic loading (0.05 mol%) with a reduced reaction time.
| Ref. | Reaction | Catalyst | Catalytic loading (mol%) | Time (h) | Maximum yield (%) |
|---|---|---|---|---|---|
| 32 | Benzylamine coupling | Ir(III) based catalyst | 0.25 | 5 | 92 |
| 26 | Sulfoxidation | Rose Bengal | 2 | 6–48 | 99 |
| Ru(bpy)32+ | 2 | 24 | 50 | ||
| Ir(ppy)3 | 2 | 24 | — | ||
| 29 | Sulfoxidation | Ir(III) based catalyst | 0.5 | 4 | 98 |
| 37 | Hydroxylation of aryl boronic acids | Ir(ppy)3 | 2 | 84 | 90 |
| Ru(bpy)32+ | 2 | 26 | 96 | ||
| 36 | Hydroxylation of aryl boronic acids | Ir(III) based catalyst | 2 | 5–12 | 95 |
| This work | Benzylamine coupling | Ir3 | 0.05 mol% | 2 | 97 |
| Sulfoxidation | Ir3 | 0.05 | 2 | 98 | |
| Hydroxylation of aryl boronic acids | Ir3 | 0.05 | 20 | 98 |
Conclusion
In summary, Ir(III)dipyrrinato complexes have proven to be highly efficient photocatalysts for aerobic oxidation reactions, including photo-oxidative benzylamine coupling, oxidation of sulfides to sulfoxides and the hydroxylation of aryl boronic acids. These reactions proceed with excellent yields within a short reaction time, showcasing the high reactivity and efficiency of these complexes. The exceptional singlet oxygen generation quantum yields, long-lived triplet excited states and strong visible-light absorption make these complexes particularly well-suited for photochemical applications. Furthermore, the remarkably low catalyst loading of only 0.05 mol% compared to other catalytic systems highlights their cost-effectiveness and sustainability. The broad substrate tolerance and versatility of these systems provide an effective strategy for synthesizing diverse functionalized organic molecules.Experimental section
General procedure for the photocatalytic benzylamine coupling reaction
Benzylamine (1 mmol) and the Ir(III)dipyrrinato catalyst (0.05 mol%) were dissolved in acetonitrile and added to a 10 mL borosilicate round-bottom flask. The solution was periodically saturated with oxygen by slow purging with a long needle every hour. The reaction mixture was stirred at room temperature and irradiated using a Smartchem Synth Photoreactor (24 W, white LED) for 2 hours. The reaction progress was monitored via1H NMR. Upon completion, the product was purified using basic silica gel column chromatography with hexane/ethyl acetate as an eluent and subsequently characterized by 1H and 13C NMR spectroscopy using a Bruker Avance-500 MHz spectrometer.General procedure for the sulfoxidation reaction
Sulfide (1 mmol) and the Ir(III)dipyrrinato catalyst (0.05 mol%) were dissolved in methanol and added to a 25 mL borosilicate round-bottom flask. To ensure oxygen saturation, the solution was gently purged with oxygen using a long needle every hour for 15–20 minutes. The reaction mixture was stirred at room temperature while being irradiated with a Smartchem Synth Photoreactor (24 W, white LED) for 2 hours. After completion, the product was purified using silica gel column chromatography with a hexane/ethyl acetate/methanol eluent system. The purified compound was then characterized by 1H and 13C NMR spectroscopy using a Bruker Avance-500 MHz spectrometer.General procedure for photocatalytic aerobic hydroxylation of boronic acid
Boronic acid (0.5 mmol), additive (1 mmol) and the Ir(III)dipyrrinato catalyst (0.05 mol%) were dissolved in 4 mL of acetonitrile and added to a 10 mL borosilicate round-bottom flask in open air. The reaction mixture was stirred at room temperature and irradiated using a Smartchem Synth Photoreactor (24 W, white LED) for 20 hours. The reaction progress was monitored via thin-layered chromatography. Upon completion, the product was purified using basic silica gel column chromatography with hexane/ethyl acetate as an eluent and subsequently characterized by 1H and 13C NMR spectroscopy using a Bruker Avance-500 MHz spectrometer.Data availability
All data will be made available on request, and the supporting data have been included as part of the ESI.†Conflicts of interest
No conflict of interest is declared.Acknowledgements
Financial support from IIT Gandhinagar and CSIR, GoI (grant no. 01/3132/23/EMR-II) is greatly acknowledged. S. K. thanks IIT Gandhinagar for the fellowship and infrastructural support. L. D. thanks SERB for the fellowship.References
- S. Murahashi, Angew. Chem., Int. Ed. Engl., 1995, 34, 2443–2465 CrossRef CAS.
- S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626–2704 Search PubMed.
- J. H. P. Tyman, Synthetic and natural phenols, Elsevier, 1996, vol. 52 Search PubMed.
- A. Kotschy and G. Timári, Heterocycles from transition metal catalysis: formation and functionalization, Springer Science & Business Media, 2005, vol. 28 Search PubMed.
- C. A. Fyfe, The Chemistry of the Hydroxyl Group, ed. S. Patai, Wiley-Interscience, New York, 1971, vol. 1 Search PubMed.
- Z. V. I. Rappoport, The chemistry of phenols, John Wiley & Sons, 2004 Search PubMed.
- K. C. Nicolaou, C. J. N. Mathison and T. Montagnon, Angew. Chem., Int. Ed., 2003, 42, 4077–4082 CrossRef CAS.
- H. Pellissier, Tetrahedron, 2006, 62, 5559–5601 CrossRef CAS.
- I. Fernández and N. Khiar, Chem. Rev., 2003, 103, 3651–3706 CrossRef.
- R. A. Volkmann, Comprehensive Organic Synthesis, Oxford, 1st edn, 1991 Search PubMed.
- J. Legros, J. Dehli and C. Bolm, Adv. Synth. Catal., 2005, 347, 19–31 CrossRef CAS.
- E. Skolia, P. L. Gkizis and C. G. Kokotos, ChemPlusChem, 2022, 87, e202200008 CrossRef CAS.
- C. G. Overberger and R. W. Cummins, J. Am. Chem. Soc., 1953, 75, 4250–4254 Search PubMed.
- R. Curci, R. DiPrete, J. O. Edwards and G. Modena, J. Org. Chem., 1970, 35, 740–745 Search PubMed.
- V. G. Shukla, P. D. Salgaonkar and K. G. Akamanchi, J. Org. Chem., 2003, 68, 5422–5425 CrossRef CAS PubMed.
- B. Yu, A.-H. Liu, L.-N. He, B. Li, Z.-F. Diao and Y.-N. Li, Green Chem., 2012, 14, 957 RSC.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed.
- A. Jain, S. Kumar, Sanyam, A. Mondal and I. Gupta, J. Catal., 2024, 438, 115705 Search PubMed.
- K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035–10074 Search PubMed.
- M. Fagnoni, D. Dondi, D. Ravelli and A. Albini, Chem. Rev., 2007, 107, 2725–2756 Search PubMed.
- A. Jain and I. Gupta, SynOpen, 2024, 8, 153–168 CrossRef CAS.
- A. Sridhar, R. Rangasamy and M. Selvaraj, New J. Chem., 2019, 43, 17974–17979 Search PubMed.
- Y. Zou, J. Chen, X. Liu, L. Lu, R. L. Davis, K. A. Jørgensen and W. Xiao, Angew. Chem., 2012, 3, 808–812 CrossRef.
- J. Jiang, R. Luo, X. Zhou, Y. Chen and H. Ji, Adv. Synth. Catal., 2018, 360, 4402–4411 CrossRef CAS.
- H. Nai, J. Hou, J. Li, X. Ma, Y. Yang, K. Qu, X. Huang and L. Li, RSC Adv., 2024, 14, 7924–7931 RSC.
- X. Gu, X. Li, Y. Chai, Q. Yang, P. Li and Y. Yao, Green Chem., 2013, 15, 357–361 Search PubMed.
- A. Casado-Sánchez, R. Gómez-Ballesteros, F. Tato, F. J. Soriano, G. Pascual-Coca, S. Cabrera and J. Alemán, Chem. Commun., 2016, 52, 9137–9140 RSC.
- J. Dad’ová, E. Svobodová, M. Sikorski, B. König and R. Cibulka, ChemCatChem, 2012, 4, 620–623 CrossRef.
- S. Li, L. Li, Y. Li, L. Dai, C. Liu, Y. Liu, J. Li, J. Lv, P. Li and B. Wang, ACS Catal., 2020, 10, 8717–8726 CrossRef CAS.
- E.-T. Wu and M. H. Huang, ACS Catal., 2023, 13, 14746–14752 CrossRef CAS.
- J. Jin, H. W. Shin, J. H. Park, J. H. Park, E. Kim, T. K. Ahn, D. H. Ryu and S. U. Son, Organometallics, 2013, 32, 3954–3959 CrossRef CAS.
- L. P. Li and B. H. Ye, Inorg. Chem., 2019, 58, 7775–7784 CrossRef CAS PubMed.
- L. Hao, G. Ding, D. A. Deming and Q. Zhang, Eur. J. Org. Chem., 2019, 7307–7321 CrossRef CAS.
- Y. Wan, X. Li, J. Ma and G. Zhang, Eur. J. Org. Chem., 2025, e202401152 CrossRef CAS.
- D. A. Nicewicz and T. M. Nguyen, ACS Catal., 2014, 4, 355–360 CrossRef CAS.
- H. Yu, C. Liu, X. Dai, J. Wang and J. Qiu, Tetrahedron, 2017, 73, 3031–3035 CrossRef CAS.
- Y. Q. Zou, J. R. Chen, X. P. Liu, L. Q. Lu, R. L. Davis, K. A. Jørgensen and W. J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 784–788 CrossRef CAS.
- N. Manav, R. Singh, A. Janaagal, A. K. S. Yadav, V. Pandey and I. Gupta, New J. Chem., 2022, 46, 19310–19321 RSC.
- S. Kumar, K. Agarwal, Sanyam, A. Mondal and I. Gupta, Chem. – Asian J., 2024, 19, e202400680 CrossRef CAS PubMed.
- H. Zhang, H. Wang, K. Tanner, A. Schlachter, Z. Chen, P. D. Harvey, S. Chen and W. Y. Wong, Dalton Trans., 2021, 50, 10629–10639 RSC.
- N. Manav, M. Y. Lone, M. K. Raza, J. Chavda, S. Mori and I. Gupta, Dalton Trans., 2022, 51, 3849–3863 RSC.
- V. Pandey, A. Janaagal, A. Jain, S. Mori and I. Gupta, Dyes Pigm., 2023, 209, 110861 CrossRef CAS.
- F. Wilkinson, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1993, 22, 113–262 CrossRef CAS.
- S. Lacombe and T. Pigot, Catal. Sci. Technol., 2016, 6, 1571–1592 RSC.
- S. M. Bonesi, I. Manet, M. Freccero, M. Fagnoni and A. Albini, Chem. – Eur. J., 2006, 12, 4844–4857 CrossRef CAS.
- E. Baciocchi, T. Del Giacco, F. Elisei, M. F. Gerini, M. Guerra, A. Lapi and P. Liberali, J. Am. Chem. Soc., 2003, 125, 16444–16454 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization data such as 1H NMR, 13C NMR and 19F-NMR of selected compounds. Singlet oxygen generation plots for Ir1 and Ir2. See DOI: https://doi.org/10.1039/d5nj01795e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction conditions: benzylamine (1 mmol), Ir3 (0.05 mol%), solvent ACN (4 mL), irradiation with white light (24 W, 96 mW cm−2)/sunlight under an O2 atmosphere at rt for 2 h.
Reaction conditions: benzylamine (1 mmol), Ir3 (0.05 mol%), solvent ACN (4 mL), irradiation with white light (24 W, 96 mW cm−2)/sunlight under an O2 atmosphere at rt for 2 h.


