Fe3O4-loaded carbon nanotubes to enhance electromagnetic shielding of PAM/L hydrogels†
Hongwei
Wang
a,
Yuhuan
Xu
 a,
Teng
Zhou
a,
Kunlan
Diao
a,
Teng
Zhou
a,
Kunlan
Diao
 a,
Xuebin
Long
b,
Daohai
Zhang
a,
Xuebin
Long
b,
Daohai
Zhang
 *a and
Shuhao
Qin
*b
*a and
Shuhao
Qin
*b
aSchool of Chemical Engineering of Guizhou Minzu University, Guizhou, Guiyang 550025, China. E-mail: zhangdaohai6235@163.com
bNational Engineering Research Center for Compounding and Modification of Polymer Materials, Guizhou, Guiyang 550014, China. E-mail: qinshuhao@126.com
First published on 13th May 2025
Abstract
Carbon nanotubes (CNTs) are common fillers, but their nanoscale radial size still offers great potential for processability. In this study, the mechanical strength and electrical conductivity of hydrogels were significantly improved by modulating the introduction strategies of CNTs and Fe3O4@CNTs, followed by synergistic interaction with Linum fiber (L). The results showed that the maximum compressive strength of the prepared hydrogel PAM/L/4-CNTs reached 17.82 kPa, and the electrical conductivity of PAM/L/6-CNTs reached 2.91 S m−1. In addition, the interfacial polarisation effect enhanced the electromagnetic shielding (EMI) performance, with an average shielding effectiveness (SET) of 43.29 dB for PAM/L/6-CNTs, while the SET of Fe3O4@CNTs reached 41.52 dB at 4 wt%. All samples exhibited an absorption mechanism for electromagnetic shielding.
1. Introduction
Hydrogels can be microscopically described as solids with a tendency to flow,1 as the name suggests. They can contain a large amount of water or other liquids, allowing the molecular chains within their internal structure to move freely and easily deform.2 Similarly, the solid form materializes various functional particles, which serve as effective carriers for them.3 In addition, hydrogels have significant internal space, which can be easily filled with various types of fillers to impart specific functionalities,4,5 such as in conductive hydrogels. They not only retain the high water content and good biocompatibility of hydrogels but also exhibit good electrical conductivity.6,7 Conductive hydrogels combine the advantages of intrinsically conductive materials with the physicochemical properties of hydrogels and the ease of regulation, offering great potential for applications in tissue engineering, drug delivery, biosensing, flexible electronic devices, and other fields.8–12 Structurally, conductive hydrogel consist of a polymer network, water, and conductive materials.13,14 The polymer mesh structure serves as a carrier to retain a large amount of water, while conductive materials are uniformly distributed within the mesh, utilizing physicochemical effects for electron and ion transport, thereby rendering the hydrogel electrically conductive.15,16 The hydrogel functions both as a water reservoir and a medium for the uniform distribution of conductive materials within the mesh structure.Conductive media used in the hydrogels can be broadly classified into metal ions, liquid metals and nano-scale conductive materials.17,18 Most metallic elements are naturally positively charged substances, and when mixed with water or other solvents, they form metal salt solutions.19 In these solutions, metal cations and anions from the solvent ionize and move in specific patterns, resulting in ionic conductivity.20 Notably, a hydrogel containing large amounts of water or other solution provides an ideal medium for ionisation. For example, Jiang et al.21 synthesized polyvinyl alcohol (PVA)-based hydrogels with high electrical conductivity and anticorrosive properties by using sodium chloride (NaCl)-induced self-assembly of sodium alginate (SA). The results showed that an immersion time of 150 minutes in saturated NaCl solution yielded optimal properties. The resulting PVA/SA-150 hydrogel exhibited excellent toughness (1.32 MPa, 400%) and electron conductivity (3.62 S m−1). Similarly, Yu et al.22 developed stretchable, transparent and conductive NFC/SA/CaCl2 hydrogels using low-temperature (−190 °C) freezing, hydrogen and ester bonding, and ionic cross-linking. The raw materials included nanofibrillated cellulose (NFC), SA, and CaCl2. These NFC/SA/CaCl2 hydrogels demonstrated excellent tensile properties (∼900%) and electrical conductivity (∼2.34 S cm−1).
Carbonaceous nanomaterials, including carbon powder (C), graphene oxide (GO), and carbon nanotubes (CNTs),23–25 not only possess good intrinsic electrical conductivity,26 but also, when used as fillers, can form physical electrostatic entanglements with polymer chains. This increases intermolecular forces27 and promotes the formation of a more robust cross-linked network.28 Nan et al.29 used negatively charged SA and positively charged metallic Ni material, crosslinked via Coulomb forces, and then added Fe3O4-loaded CNTs to construct a three-dimensional composite in a synergistic manner. Although this material exhibited a high electromagnetic interference shielding effectiveness EMI SE of 32 dB under very thin and lightweight conditions, its lack of degradability remains a significant limitation.
In this study, Fe3O4@CNTs were synthesized using the hydrothermal method. An alkaline urea solution was then employed to dissolve the Linum fiber, after which acrylamide (AM) was added to the cellulose solution as a monomer, MBA as a cross-linking agent, and APS as an initiator. Different amounts of CNTs/Fe3O4@CNTs were incorporated, and various types of PAM/L/CNTs and PAM/L/Fe3O4@CNTs hydrogels were prepared. Their properties, including electrical conductivity and electromagnetic shielding, were subsequently studied and investigated.
2. Results and discussion
2.1. Morphology of PAM/L/CNTs and PAM/L/Fe3O4@CNTs hydrogels
We reported a detailed description of the technical route (Fig. 1A and B) and preparation process (in ESI†) for PAM/L/CNTs and PAM/L/Fe3O4@CNTs hydrogels. PAM and L formed the hydrogel's 3D network structure, and the addition of CNTs enhanced the hydrogel layers (Fig. 1C). Furthermore, CNTs attached to the surface of Linum fibers and filled the gaps between the Linum fibers and PAM molecular chains, thereby densifying the hydrogel structure.9 The entry of Fe3O4@CNTs, on the other hand, interacted with the PAM molecular chains, which not only further filled the pores12 but also provided better support and connectivity for the PAM molecular chains (Fig. 1D). | ||
| Fig. 1 (A) and (B) Schematic of the technical route. (C) SEM image of PAM/L/6-CNTs hydrogel. (D) SEM image of PAM/L/4-Fe3O4@CNTs hydrogel. | ||
As shown in Fig. 2A and B, the prepared hydrogel exhibited a stretching band at 1080 cm−1, corresponding to the rocking vibration of the amino group (–NH2) in the composite.30 The characteristic peaks at 1620 cm−1 and 3330 cm−1 corresponded to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and the stretching vibration of –OH,1,9 respectively. The transmittance and shape of some peaks changed with an increase in the CNTs content, indicating that CNTs interacted with the PAM/L matrix, affecting the arrangement of the polymer molecular chains and the functional group environment. Similarly, with the incorporation of Fe3O4@CNTs into PAM/L, it was observed that the incorporation of Fe3O4@CNTs did not result in the formation of new substances.22 Therefore, it can be surmised that physical cross-linking structures were formed in the composite hydrogel.
O and the stretching vibration of –OH,1,9 respectively. The transmittance and shape of some peaks changed with an increase in the CNTs content, indicating that CNTs interacted with the PAM/L matrix, affecting the arrangement of the polymer molecular chains and the functional group environment. Similarly, with the incorporation of Fe3O4@CNTs into PAM/L, it was observed that the incorporation of Fe3O4@CNTs did not result in the formation of new substances.22 Therefore, it can be surmised that physical cross-linking structures were formed in the composite hydrogel.
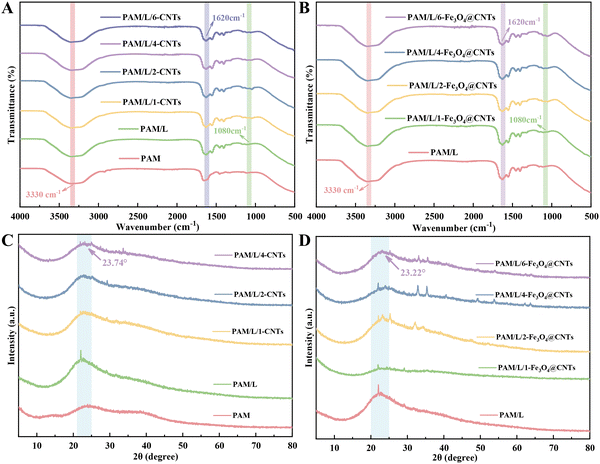 | ||
| Fig. 2 (A) FTIR spectra of PAM/L/CNTs hydrogels. (B) FTIR spectra of PAM/L/Fe3O4@CNTs hydrogels. (C) XRD spectra of PAM/L/CNTs hydrogels. (D) XRD spectra of PAM/L/Fe3O4@CNTs hydrogels. | ||
In Fig. 2C, the PAM diffraction intensity showed no obvious sharp crystalline phase peaks. It showed only broadened diffuse peaks in the low-angle region.5,19 The addition of flax diffraction peaks did not change significantly, having a minimal effect on the overall crystalline structure, particularly in the region around 23.74°.
After the addition of Fe3O4@CNTs, several new diffraction peaks appeared in the region around 23.22° for each curve (Fig. 2D). This indicated that the addition of Fe3O4@CNTs introduced a new crystalline structure with enhanced peak intensities and increased crystalline content,23 suggesting that the incorporation of Fe3O4@CNTs had a major impact on the crystalline architecture of the system.
From Fig. 3A, it can be observed that the crystallisation temperature of pure PAM is −17.9 °C, which decreased with the addition of flax and varying CNT contents. This was attributed to the interaction among flax, CNTs and PAM molecular chains, which disrupted the otherwise regular arrangement of PAM molecular chains.7 Notably, the interaction forces among these three components (CNTs, PAM and Linum fibers) redistributed the molecular chain forces. This redistribution enabled the chains to acquire sufficient energy to overcome intermolecular interactions, facilitating molecular mobility at lower temperatures and resulting in a reduced melting temperature of the composite hydrogel.14 Previously, the loading of Fe3O4 onto CNTs was found to promote the formation of chemical cross-links and physical entanglements with other molecular chains, encouraging directional crystallization of the hydrogel's chain segments (Fig. 3B). Surprisingly, at higher Fe3O4@CNTs content, agglomerations occurred. These agglomerates disrupted the regularity of polymer chain segments and occupied substantial space, leading to a site-barrier effect.20 This spatial hindrance restricted chain mobility and order, ultimately reducing crystallinity.
 | ||
| Fig. 3 (A) Melting curves of PAM/L/CNTs hydrogels. (B) Melting curves of PAM/L/Fe3O4@CNTs hydrogels. (C) Water content of PAM/L/CNTs hydrogels. (D) Water content of PAM/L/Fe3O4@CNTs hydrogels. | ||
The water content of pure PAM hydrogel reached 82.64% (Fig. 3C). With the addition of flax, its rigidity and irregular shape caused the network structure of the hydrogel to become looser,8 and the water content of the PAM/L hydrogel decreased to 58.47%. However, it is worth mentioning that the high aspect ratio of CNTs allows them to be interspersed between the PAM and Linum fibers in the composite hydrogel. This arrangement transforms the original loose network structure into a denser and more orderly one,4 which effectively restricts the evaporation of water molecules, consequently leading to an increase in water content. For the PAM/L/Fe3O4@CNTs hydrogel, Fe3O4@CNTs, with its larger specific surface area, interacted with PAM/L molecular chains, making the network structure of the hydrogel more regular and less loose,6 thus providing more adsorption sites and accommodation space for water molecules. This allowed water molecules to more easily enter and remain in the hydrogel network,11 resulting in increased water content. However, as the content of Fe3O4@CNTs increased further, agglomeration occurred, disrupting the original regular hydrogel network structure. This made it difficult to distribute water molecules uniformly, leading to a decrease in the binding capacity of water molecules.
2.2. Characterization of PAM/L/CNTs and PAM/L/Fe3O4@CNTs hydrogels
PAM hydrogels face several limitations in practical applications due to their low mechanical strength. However, the addition of flax and CNTs forms a stable three-dimensional mesh structure, and hydrogen bonds can form between the two,16 thereby improving their mechanical properties to some extent (Fig. 4A and B). The maximum compressive strength of the PAM hydrogel was 16.14 kPa, while those of PAM/L and PAM/L/4-CNTs were 16.65 kPa and 17.82 kPa, respectively. This corresponded to increases of 3.16% and 10.41% compared to pure PAM. On this basis, when the hydrogel was subjected to external forces, the interaction between Fe3O4@CNTs, Linum fibers and PAM molecular chains enhanced toughness through an energy dissipation mechanism.27 The interaction between the three led to the formation of a stable polymer network structure in the hydrogel, resulting in excellent mechanical strength. Eventually, PAM/L/6-Fe3O4@CNTs achieved a maximum compressive strength of 17.12 kPa, which was 2.76% higher than that of PAM/L.As shown in Fig. 4C, due to the conductivity of CNTs themselves, and their interaction with the PAM/L molecular chains, a localized conductive loop was formed inside the hydrogel,3,29 which improved the conductivity of the PAM hydrogel. As the content of CNTs increased, the distance between the lamellar structures decreased, increasing the contact area and improving the electron conduction rate.30 When the content of CNTs was 6 wt%, the electrical conductivity of the PAM/L/6-CNTs hydrogel was the highest at 2.91 S m−1, which was 6.7 times higher than that of pure PAM hydrogel.
In the case of Fe3O4@CNTs, their addition promoted numerous physical contacts, both among Fe3O4@CNTs particles and with PAM/L molecular chains. These served as electron hopping and transport sites, thereby reducing the activation energy for electron movement and accelerating charge migration.31,32 As a result, the electrical conductivity was significantly enhanced. However, excessive loading of Fe3O4@CNTs led to agglomeration, precluding uniform dispersion and reducing effective contact area. This created a physical barrier to charge transfer, ultimately decreasing electrical conductivity,33,34 The highest electrical conductivity reached was 2.94 S m−1 for PAM/L/4-Fe3O4@CNTs, representing an 87.26% improvement compared to the PAM/L composite hydrogel.
The average EMI SET of pure PAM hydrogel was 30.83 dB, whereas that of PAM/L hydrogels was 33.22 dB (Fig. 5), demonstrating that the incorporation of flax enhanced interfacial polarization.35 As expected, the EMI SET of PAM/L/CNTs hydrogels gradually increased with the increase of CNTs content. Linum fibre also helped in dispersing CNTs uniformly throughout the hydrogel, contributing to a well-distributed conductive network that enhanced both conduction and polarization losses.36,37 Among these, PAM/L/6-CNTs achieved the highest SET of 43.29 dB. Moreover, the SEA values of PAM/L/CNTS hydrogels were significantly higher than their SER values (Fig. 5B and C), indicating that absorption was the primary shielding mechanism. This suggested that the hydrogels exhibited excellent wave-absorbing properties and could minimize secondary electromagnetic pollution caused by wave reflection.
From Fig. 5D–F, it can be seen that there is an interface between the Fe3O4@CNTs and the hydrogel matrix. Since the conductivity of Fe3O4@CNTs and PAM/L are different, interfacial polarization is enhanced when the incident electromagnetic wave interacts with the surface of the hydrogel matrix, which consumes the energy of the electromagnetic wave and improves the electromagnetic shielding performance. However, with the increase of Fe3O4@CNTs content, the electromagnetic shielding performance of the hydrogel decreased, which could be attributed to the addition of a conductive filler beyond its diffusion threshold. When the content of Fe3O4@CNTs exceeded this threshold, further increasing its dosage led to an increase in the viscosity of the system, and the distance between the particles became too small, resulting in the formation of a blocking effect that hindered the migration of electrons and the propagation of electromagnetic waves,38 thus reducing the material's shielding ability for electromagnetic waves. Therefore, when the content of Fe3O4@CNTs was 4 wt%, the maximum SET was 41.52 dB and SEA was 37 dB, compared to the SET of 33.22 dB and SEA of 28.92 dB for PAM/L, which were 24.98% and 27.94% higher, respectively.
3. Conclusions
In this study, multifunctional hydrogels were prepared by introducing Linum fiber (L), carbon nanotubes (CNTs) and Fe3O4@CNTs into a polyacrylamide (PAM) matrix. CNTs and Fe3O4@CNTs modulated the arrangement of polymer molecular chains through physical cross-linking to form dense network structure, which significantly enhanced the mechanical strength and electrical conductivity of the hydrogel. FTIR analysis showed that the vibrational peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1620 cm−1) and –OH (3330 cm−1) confirmed the enhanced intermolecular interactions. The maximum compressive strength of PAM/L/4-CNTs reached 17.82 kPa. The conductivity increased with CNTs content, reaching 2.91 S m−1 at 6 wt%. In addition, CNTs and Fe3O4@CNTs optimized the electromagnetic shielding performance through the interfacial polarization effect, and SET of PAM/L/6-CNTs was 43.29 Db. The average shielding effectiveness (SET) of Fe3O4@CNTs at 4 wt% was also measured. This study provides a new strategy for the development of smart hydrogels with high mechanical strength, electrical conductivity and electromagnetic shielding properties, which could be extended to flexible electronics and biomedical applications in the future.
O (1620 cm−1) and –OH (3330 cm−1) confirmed the enhanced intermolecular interactions. The maximum compressive strength of PAM/L/4-CNTs reached 17.82 kPa. The conductivity increased with CNTs content, reaching 2.91 S m−1 at 6 wt%. In addition, CNTs and Fe3O4@CNTs optimized the electromagnetic shielding performance through the interfacial polarization effect, and SET of PAM/L/6-CNTs was 43.29 Db. The average shielding effectiveness (SET) of Fe3O4@CNTs at 4 wt% was also measured. This study provides a new strategy for the development of smart hydrogels with high mechanical strength, electrical conductivity and electromagnetic shielding properties, which could be extended to flexible electronics and biomedical applications in the future.
Data availability
Additional data are available in the ESI† of this manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Project No. 52163001), the Guizhou Provincial Science and Technology Program (Project Grants: Qiankehe Platform Talents-CXTD [2021]005, Qiankehe Platform Talents-GCC [2022]010-1, Qiankehe Platform Talents-GCC[2023]035, Qiankehe Platform Talents-CXTD[2023]003, Qiankehe Platform Talents-KXJZ [2024]022), the Guizhou Minzu University Research Platform (Grant No. GZMUGCZX [2021]01), the Central Guided Local Science and Technology Development Funds (Qiankehe Zhong Yindi [2023]035), the Doctor Startup Fund of Guizhou Minzu University (Grant No. GZMUZK [2024] QD77) and the Guizhou Province Special Fund for Innovative Capacity Building of Scientific Research Institutions (Qiankehe Fuqi [2023]001, Qiankehe Fuqi [2024]002-1).References
- Y. Xu, M. Pei, J. Du, R. Yang, Y. Pan, D. Zhang and S. Qin, New J. Chem., 2023, 47, 13721–13728 RSC.
- Z. Deng, H. Wang, P. X. Ma and B. Guo, Nanoscale, 2020, 12, 1224–1246 RSC.
- D. Li, Q. Luo, H. Xin, C. Wang, Y. Zhao, H. Bai and F. Ma, ACS Sustainable Chem. Eng., 2023, 11, 5462–5472 CrossRef CAS.
- R. Hu, G. Ji, Y. Wang, J. Zhao and J. Zheng, Extreme Mech. Lett., 2021, 42, 101136 CrossRef.
- C. Igwe Idumah, Cleaner Mater., 2022, 5, 100103 CrossRef.
- C. Wu, L. Zeng, G. Chang, Y. Zhou, K. Yan, L. Xie, B. Xue and Q. Zheng, Adv. Compos. Hybrid Mater., 2023, 6, 31 CrossRef CAS.
- J. Xu, Z. Wang, J. You, X. Li, M. Li, X. Wu and C. Li, Chem. Eng. J., 2020, 392, 123788 CrossRef CAS.
- H. Xu, D. Liu, Y. Song, Y. Xie, Z. Shi, C. Xiong and Q. Yang, Compos. Sci. Technol., 2022, 228, 109679 CrossRef CAS.
- Y. Xu, M. Pei, X. Zhan, H. Wang, D. Zhang and S. Qin, New J. Chem., 2023, 47, 21475–21484 RSC.
- S. Zhao, Y. Yan, A. Gao, S. Zhao, J. Cui and G. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 26723–26732 CrossRef CAS.
- W. Yang, B. Shao, T. Liu, Y. Zhang, R. Huang, F. Chen and Q. Fu, ACS Appl. Mater. Interfaces, 2018, 10, 8245–8257 CrossRef.
- Z. Zeng, H. Jin, M. Chen, W. Li, L. Zhou and Z. Zhang, Adv. Funct. Mater., 2016, 26, 303–310 CrossRef.
- Q. Duan, B. Lan and Y. Lv, ACS Appl. Mater. Interfaces, 2022, 14, 1973–1982 CrossRef PubMed.
- Y. Zhang and J. Gu, Nano-Micro Lett., 2022, 14, 89 CrossRef.
- Y. Yang, C.-L. Luo, X.-D. Chen and M. Wang, Compos. Sci. Technol., 2023, 233, 109913 CrossRef.
- R. Yang, X. Chen, Y. Zheng, K. Chen, W. Zeng and X. Wu, J. Mater. Chem. C, 2022, 10, 5380–5399 RSC.
- J. Yang, J. Cheng, G. Qi and B. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 17163–17174 CrossRef PubMed.
- Z. Yan, Y. Ding, M. Huang, J. Li, Q. Han, M. Yang and W. Li, ACS Appl. Nano Mater., 2023, 6, 6141–6150 CrossRef.
- J.-R. Tao, X.-H. Tang, Q.-M. He and M. Wang, Compos. Sci. Technol., 2022, 229, 109715 CrossRef.
- L. Tang, J. Han, Z. Jiang, S. Chen and H. Wang, Carbohydr. Polym., 2015, 117, 230–235 CrossRef PubMed.
- X. Jiang, N. Xiang, H. Zhang, Y. Sun, Z. Lin and L. Hou, Carbohydr. Polym., 2018, 186, 377–383 CrossRef PubMed.
- K. Yu, L. Yang, S. Zhang, N. Zhang, S. Wang, Y. He and H. Liu, Food Hydrocolloids, 2024, 152, 109938 CrossRef.
- H. Wei, D. Kong, T. Li, Q. Xue, S. Wang, D. Cui, Y. Huang, L. Wang, S. Hu, T. Wan and G. Yang, ACS Sens., 2021, 6, 2938–2951 CrossRef CAS PubMed.
- Y. Chao, S. Yu, H. Zhang, D. Gong, J. Li, F. Wang, J. Chen, J. Zhu and J. Chen, ACS Appl. Bio Mater., 2023, 6, 1525–1535 CrossRef CAS.
- W. Liu, R. Xie, J. Zhu, J. Wu, J. Hui, X. Zheng, F. Huo and D. Fan, npj Flex Electron., 2022, 6, 68 CrossRef CAS.
- T. Wang, J. Wang, Z. Li, M. Yue, X. Qing, P. Zhang, X. Liao, Z. Fan and S. Yang, J. Appl. Polym. Sci., 2022, 139, 51627 CrossRef CAS.
- H. Yue, L. Yuan, W. Zhang, S. Zhang, W. Wei and G. Ma, J. Mater. Chem. B, 2018, 6, 393–400 RSC.
- Y. Zhao, Y. Ohm, J. Liao, Y. Luo, H.-Y. Cheng, P. Won, P. Roberts, M. R. Carneiro, M. F. Islam, J. H. Ahn, L. M. Walker and C. Majidi, Nat. Electron., 2023, 6, 206–215 CrossRef CAS.
- N. Zhou, Q. An, Z. Xiao, S. Zhai and Z. Shi, ACS Sustainable Chem. Eng., 2017, 5, 5394–5407 CrossRef CAS.
- W. Hanif, A. Hardiansyah, A. Randy and L. A. T. W. Asri, RSC Adv., 2021, 11, 29029–29041 RSC.
- X. Liang, G. Chen, I. M. Lei, P. Zhang, Z. Wang, X. Chen, M. Lu, J. Zhang, Z. Wang, T. Sun, Y. Lan and J. Liu, Adv. Mater., 2023, 35, 2207587 CrossRef CAS.
- C. Liu, R. Zhang, Y. Wang, J. Qu, J. Huang, M. Mo, N. Qing and L. Tang, J. Mater. Chem. A, 2023, 11, 2002–2013 RSC.
- M. B. Lawrence, J. A. E. Desa and V. K. Aswal, Mater. Res. Express, 2018, 5, 015315 CrossRef.
- Y. Xu, M. Pei, X. Zhan, J. Du and D. Zhang, Prog. Org. Coat., 2024, 194, 108592 CrossRef CAS.
- T. Kuang, J. Zhang, G.-M. Huang, T. Liu and Z.-X. Huang, Nano Energy, 2024, 128, 109877 CrossRef CAS.
- B. Quan, Y. Chen, Y. Wang, X. Lu, T. Guo, M. Zhang and X. Huang, Carbon, 2023, 206, 392–401 CrossRef CAS.
- B. Quan, Y. Wang, Y. Chen, X. Lu, S. Teng, X. Zhou, M. Qiao, H. Lai and X. Huang, Carbon, 2023, 210, 118045 CrossRef CAS.
- Y. Wang, X. Lu, Y. Chen, H. Lai, S. Teng and B. Quan, J. Mater. Chem. A, 2021, 9, 24503–24509 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5nj01366f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |


