Open Access Article
Open Access ArticleFunctionalization at the C3 position of the cephalosporin pharmacophore by palladium-catalyzed cross-coupling reactions†
Fanny
Faure
 a,
Margot
Zambon
a,
Yen
Vo-Hoang
b,
Patricia
Licznar-Fajardo
b,
Jean-Denis
Docquier
a,
Margot
Zambon
a,
Yen
Vo-Hoang
b,
Patricia
Licznar-Fajardo
b,
Jean-Denis
Docquier
 c,
Suzanne
Peyrottes
c,
Suzanne
Peyrottes
 a and
Laurent
Gavara
a and
Laurent
Gavara
 *a
*a
aInstitut des Biomolécules Max Mousseron, Univ Montpellier, CNRS, ENSCM, Montpellier, France. E-mail: laurent.gavara@umontpellier.fr
bHSM, Univ Montpellier, CNRS, IRD, CHU, Montpellier, France
cDipartimento di Biotecnologie Mediche, Università di Siena, I-53100 Siena, Italy
First published on 24th March 2025
Abstract
Herein, we describe a new methodology for selective cephalosporin functionalization at the C3 position. First, an optimization study of the reaction conditions was performed and allowed favourable parameters for the Suzuki–Miyaura coupling reaction to be defined. Then, the scope of the reaction was evaluated using various boronic acids, and the reaction demonstrated a good functional-group tolerance profile. Finally, the formation of some side-products was also investigated, and the main limitations of the reaction were defined.
1. Introduction
Since the discovery of penicillin G in 1928, structurally characterized by a β-lactam moiety, mankind has moved into the modern era of antibiotics. Subsequently, several other antibiotic families were developed around this crucial 4-membered ring, such as monobactam, carbapenem or cephalosporin.1 Today, β-lactam agents account for more than 60% of prescribed antibiotics worldwide, profoundly impacting global human health.2 However, this major milestone in modern medicine is being challenged by the emergence and spread of bacterial resistance, threatening the efficacy of the current antibiotic arsenal.3,4 To secure our future, it is necessary to explore new β-lactam analogues and consequently new methodologies are required to synthesize them. Among the various β-lactam families, cephalosporin represents the most extensively developed scaffold, with numerous marketed drugs, acting as antibacterial or adjuvant agents.5 The corresponding structure–activity relationships (SARs) are well-established and all last-generation cephalosporins share common features, such as the oxime moiety to reduce β-lactamase recognition, and the amino-thiazole heterocycle for optimal penicillin-binding protein (PBP) inhibition profile (Fig. 1).6 The C3 position of the cephalosporin pharmacophore remains the last tunable site, with a wide variety of substituents already described. It is a crucial position to modulate several parameters, such as enzyme inhibition potency, aqueous solubility or even periplasm accumulation. A sp2–sp2 bond on the C3 position is of great interest, as demonstrated by the marketed cefditoren and cefdinir (Fig. 1), ranked in the US Top300 prescription drugs.7Nevertheless, the industrial synthetic pathways involved require harmful and environmentally costly reagents, offering limited opportunities for divergent synthesis approaches. The introduction of palladium-catalyzed coupling reactions has been a game-changer in the medicinal chemistry field.8 For the cephalosporin core, only Stille coupling reactions are described to decorate the C3 position. This methodology was successfully implemented in the synthesis of PBP and β-lactamase inhibitors.9–13 While it represents a viable and versatile approach, Stille coupling has some notable drawbacks, especially the acute toxicity of mandatory tin-based reagents. To overcome this limitation, we decided to explore the safer Suzuki–Miyaura coupling for C3 functionalization on the cephalosporin core. We recently published a similar approach for the synthesis of monobactam conjugates, based on Buchwald–Hartwig amination.14
2. Results and discussion
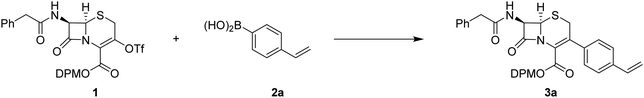
The cephalosporin triflate 1 was prepared from the corresponding commercially available enol, after optimization of the literature protocol, in a good yield (see ESI†). As a model substrate, we selected the 4-vinylphenylboronic acid 2a. Firstly, we intended to develop a room temperature reaction to avoid any side reactions, especially the well-known double bond Δ3–Δ2 isomerization of the cephalosporin, and the protodeboronation phenomenon.15,16 The starting catalytic conditions were based on the standard palladium acetate–triphenylphosphine mixture using potassium carbonate as a base. All reactions were monitored by liquid chromatography–mass spectrometry (LC-MS) and reaction times were adjusted according to the observed conversion rate and are detailed in Table 1.| Entry | Precatalyst | Ligand | Base | Time (h) | Temperature | Solvent | Yield (conversion rate)b |
|---|---|---|---|---|---|---|---|
| a DPM = diphenylmethyl, Ph2CH. Reaction conditions: 1, 0.16 mmol, 1 eq.; 2, 1.2 eq.; precatalyst, 10%; ligand, 20%; base, 3 eq. solvent, 3.2 mL. b Isolated yield after column chromatography and conversion rate determined by LC-MS in brackets. c Not isolated. d Product only detected by LC-MS. e Precatalyst, 5%; ligand, 10%. f No product detected. g Palladium-ligand precatalyst at 10%. | |||||||
| 1 | Pd(OAc)2 | PPh3 | K2CO3 | 1 | rt | Toluene | (4%)c |
| 2 | Pd(OAc)2 | PPh3 | K2CO3 | 5 | rt | Dioxane | (30%)c |
| 3 | Pd(OAc)2 | PPh3 | K2CO3 | 1 | rt | CH3CN | —f |
| 4 | Pd(OAc)2 | PPh3 | K2CO3 | 1 | rt | DMF | —f |
| 5 | Pd(OAc)2 | PPh3 | K2CO3 | 24 | rt | THF | 64% (100%) |
| 6 | Pd(OAc) 2 | PPh 3 | K 2 CO 3 | 1 | 65 °C | THF | 70% (100%) |
| 7 | Pd(OAc)2 | PPh3 | K2CO3 | 1 | rt | THF-H2O | Traced |
| 8 | Pd(OAc)2 | PPh3 | K2CO3 | 4 | 65 °C | 2-Me-THF | 58% (100%) |
| 9 | Pd(OAc)2 | PPh3 | K2CO3 | 1 | 65 °C | THF | 55% (100%)e |
| 10 | Pd2(dba)3 | PPh3 | K2CO3 | 5 | rt | THF | Traced |
| 11 | Pd(OAc)2 | PPh3 | Cs2CO3 | 1 | rt | THF | (23%)d |
| 12 | Pd(OAc)2 | JohnPhos | K2CO3 | 1 | rt | THF | —f |
| 13 | Pd(OAc)2 | XPhos | K2CO3 | 1 | rt | THF | (8%)d |
| 14 | PEPPSI-iPrg | K2CO3 | 24 | rt | THF | —f | |
| 15 | XPhos-Pd G4g | K2CO3 | 2 | rt | THF | —f | |
| 16 | XPhos-Pd G4g | K2CO3 | 5 | 65 °C | THF | 55% (100%) | |
The influence of the solvent was preliminary investigated, and we firstly selected the two most common solvents, i.e. toluene and dioxane.17 In both cases, the starting cephalosporin 1 was degraded and only a small amount of the desired coupled cephalosporin 3a was detected (Table 1, entries 1 and 2). The use of polar aprotic solvents, like acetonitrile or DMF, led only to rapid degradation of the starting material, without the formation of the desired compound (Table 1, entries 3 and 4). However, THF as the solvent demonstrated a better ability to perform the reaction, and after 24 hours at rt, a full conversion was reached. The reaction time can be efficiently reduced to 1 h under reflux conditions, without any side-product formation. In both cases, the isolated cephalosporin 3a was obtained in a good yield (Table 1, entries 5 and 6). Sometimes, the addition of a small amount of water in THF can improve the coupling kinetic reaction.18 In our case, the use of this mixture (Table 1, entry 7) led only to the degradation of the starting triflate 1, with only traces of the desired compound 3a. To provide a greener approach, 2-Me-THF was also investigated and led to clean conversion (Table 1, entry 8).19,20 Concomitantly, we also demonstrated that it was possible to reduce the catalytic charge (10 mol% of ligand, 5 mol% catalyst) leading to 55% yield (Table 1, entry 9). The palladium source can be divided between two parts, according to the oxidative state of the metal: Pd(II) or Pd(0). The Pd2(dba)3 complex was used as a stable Pd(0) source (Table 1, entry 10), instead of the regular Pd(II) acetate, but only a poor conversion rate was observed. This may demonstrate that the reduction step, to form in situ the Pd(0) entity, is not a limiting factor, or that the Pd2(dba)3 complex is too stable to proceed to the next oxidative addition step. Even though potassium carbonate is economically attractive, cesium carbonate has provided better reaction rates in numerous examples in the literature. In our case, this modification had a deleterious impact, with only a weak conversion rate (Table 1, entry 11). As the nature of the ligand used is often a key parameter in Pd-catalyzed coupling reactions, we also investigated this parameter. Among the abundance of available ligands, we selected a panel of representative phosphines with different behaviors. The development of biaryl phosphanes provided numerous new insights into metal-catalyzed reactions and we choose JohnPhos and XPhos ligands, based on the distinct substituent size on the phenyl part.21 Sadly, neither biaryl phosphanes exhibited any significant catalytic activity (Table 1, entries 12 and 13). Finally, we explored “all-in-one” precatalysts with the electron-rich carbene PEPPSI-iPr and the N-substituted XPhos-Pd G4.22,23 No significant reaction occurred in these conditions at room temperature and a moderate yield was reached with XPhos-Pd G4 under reflux of THF for 5 h (Table 1, entries 14–16).
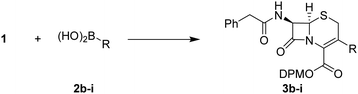
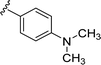
Once the best conditions were identified (Table 1, entry 6), the scope of the reaction was studied, with a focused library of eight boronic acids 2b–i, variously substituted. Initially, we intended to introduce a phenyl group by using two boron-based reagents: the boronic acid 2b and the corresponding pinacol ester (data not shown). Only the boronic acid reagent led to the desired 3-phenyl-cephalosporin 3b in good yield (Table 2, entry 1) and no product was detected with the less reactive pinacol ester.24 To further establish the functional-group tolerance of the coupling reaction, a boronic acid containing a diol (2c) was synthesized (see ESI,† for detailed protocols). Using this, no reaction was detected (Table 2, entry 2). As the corresponding protected acetal 2d and the 6-membered acetal ring 2e were also obtained as intermediates, they were also tested in this reaction. In both cases, full conversion into the corresponding cephalosporins was observed. Nevertheless, partial removal of the acetal protecting group occurred during the reaction, especially in the case of the 1,3-dioxane ring, leading to a complex reaction mixture. Consequently, HCl treatment was performed before any purification attempt on both intermediates, to give rise to the corresponding diol compounds 3d–e in a good yield (Table 2, entries 3 and 4). The presence of an amino group protected by a Boc group and an ester function did not deteriorate the coupling reaction. Nevertheless, it was necessary to perform a TFA deprotecting step (see ESI† for details) to obtain the corresponding cephalosporin 3f with a good yield and purity (Table 2, entry 5). The electronic effect was also studied with the introduction of electron-withdrawing and electron-donating groups on the arylboronic acid reagent 2. In the case of an ester function, a rapid conversion into the expected product 3g was observed, with a good isolated yield (Table 2, entry 6). In the case of the N,N-dimethylaniline boronic acid 2h, the formation of the corresponding compound 3h was observed, but it underwent an immediate degradation, preventing any purification (Table 2, entry 7). This chemical instability may result from hyperconjugation between the nitrogen lone pair of the aniline and the double bond of the cephalosporin, weakening the condensed ring nuclei.
| Entry | Cpd | R | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1, 0.16 mmol, 1 eq.; 2b–g, 1.2 eq.; Pd(OAc)2, 10%; PPh3, 20%; K2CO3, 3 eq.; THF, 3.2 mL; reflux. b Isolated yield. c No product detected by LC-MS. d Isolated yield after acetal deprotection (see ESI). e Isolated after TFA deprotection of BOC and DPM groups, see details in the ESI. f 100% conversion by LC-MS, but chemically unstable. g Gram-scale synthesis (1 g–1.58 mmol). | ||||
| 1 | b |
|
1 | 73 |
| 2 | c |

|
1 | —c |
| 3 | d |

|
1 | 59d |
| 4 | e |

|
1 | 86d |
| 5 | f |

|
1 | 45e |
| 6 | g |

|
1 | 77 |
| 7 | h |
|
1 | —f |
| 8 | i |
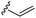
|
2 | 74 |
| 9 | 2 | 65g | ||
Finally, the introduction of double bonds was investigated with the vinyl boronic acid 2i. The coupling reaction led to the valuable cephalosporin-vinyl synthon 3i (Table 2, entry 8), this compound being an industrial intermediate for the synthesis of the marketed cefdinir (Fig. 1). To demonstrate the industrial suitability of our methodology, a gram-scale synthesis was performed and the key intermediate 3i was obtained with a good yield (Table 2, entry 9). Obviously, a specific development will be necessary to optimize this step. Nevertheless, our approach provides a straightforward synthetic alternative, by-passing numerous costly steps, in the preparation of the 7-AVCA intermediate.25
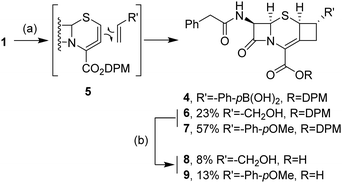
During the optimization process with the vinyl boronic acid 2a, two recurrent side products were identified, sharing the same molecular weight and close HPLC retention times, but never detected with other boronic acid substrates 2b–i. The Heck reaction on the vinyl group was first considered, but it was not consistent with NMR spectrum data. Finally, the structures were elucidated and consisted of cyclobutane derivative 4 as a mixture of isomers. This side-reaction has already been reported in the literature and involves a [2+2] cycloaddition between the allene intermediate 5, generated by the elimination of the triflate, and the vinyl bond.26,27 The presence of the boronic acid function on cephalosporin 4 prevented any successful purification.
Two other analogues, with allyl alcohol and 4-vinylanisole, were investigated and led to the corresponding cyclobutene compounds 6 and 7 (Scheme 1) as pure isomers. The stereogenic center configurations were determined based on the specific NMR chemical shift, splitting signals and coupling constants, as previously established (see ESI,† for detailed NMR spectra).26,27 Even if this side-reaction was already known, the microbiological evaluation of the resulting fused cephalosporins has never been reported. Therefore, the DPM group was removed by acidic treatment to release the carboxylic acid, which is critical for affinity toward PBPs. Both deprotected cephalosporins 8 and 9 were submitted to MIC determination by the broth micro-dilution method toward Gram-positive and Gram-negative strains, S. aureus and E. coli, respectively (see ESI† for detailed protocols). None of them exhibited any significant antibacterial activity, probably due to the steric hindrance induced by the condensed cyclobutane ring.
3. Conclusion
To fight bacterial resistance and to always keep one step ahead of pathogens, the development of new antibiotics is crucial. In this context, the β-lactam agents, especially cephalosporin drugs, have received considerable attention in recent years, and represent the first line of defence in the current therapeutic arsenal against resistant bacteria. We developed a straightforward protocol to selectively decorate the key C3 position of the cephalosporin core, based on a Suzuki–Miyaura coupling reaction. This methodology allows synthetic access to critical described intermediates and to original motifs. The reaction can be performed at room temperature or under reflux conditions, without any detectable degradation. The scope of the reaction shows great functional-group tolerance and opens the way to a wide variety of original cephalosporin scaffolds.Author contributions
Fanny Faure: methodology; investigation and writing – original draft; Margot Zambon: methodology and investigation; Yen Vo-Hoang: investigation; Patricia Licznar-Fajardo: investigation; Jean-Denis Docquier: conceptualization; Suzanne Peyrottes: conceptualization and supervision; Laurent Gavara: conceptualization, writing – original draft and project administration; all authors contributed to writing – reviewing and editing.Data availability
No data was used for the research described in the article.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was funded by the Agence Nationale de la Recherche (ANR-19-CE18-0003-01) and the LabUM chimie of the University of Montpellier through the “Investissements d’avenir” program of the ANR with reference number ANR-16-IDEX-0006 (F. F. PhD grant). We would like to thank Elodie Carretero, Luc Brunel and Dr Pascal Verdié, and the SynBio3 platform (IBMM, UMR 5247 Montpellier) which was supported by GIS IBISA and whose equipment was publicly funded through the I-SITE Excellence Program of the University of Montpellier, under the Investissements France 2030. We also want to thank analytical platforms, in particular Aurélien Lebrun for NMR experiments and Pierre Sanchez for mass spectrometry analyses. J. D. D. was supported in part by the Italian MUR (Ministero dell’Università e della Ricerca) in the frame of the PNRR PE-13 (“Piano Nazionale di Ripresa e Resilienza, Partenariato Esteso 13, Malattie infettive emergenti”) INF-ACT project (“One Health Basic and Translational Research Actions addressing Unmet Needs on Emerging Infectious Diseases”, funded by the European Union - Next generation EU, MUR PNRR M4 C2 Inv. 1.5 CUP B63C22001400007).References
- K. Bush, Past and Present Perspectives on β-Lactamases, Antimicrob. Agents Chemother., 2018, 62, e01076–18 CAS.
- B. Hamad, The antibiotics market, Nat. Rev. Drug Discovery, 2010, 9, 675–676, DOI:10.1038/nrd3267.
- E. M. Darby, E. Trampari, P. Siasat, M. S. Gaya, I. Alav, M. A. Webber and J. M. A. Blair, Molecular mechanisms of antibiotic resistance revisited, Nat. Rev. Microbiol., 2023, 21, 280–295 CrossRef CAS.
- WHO bacterial priority pathogens list, 2024, https://www.who.int/publications/i/item/9789240093461, (accessed June 17, 2024).
- X. Lin and U. Kück, Cephalosporins as key lead generation beta-lactam antibiotics, Appl. Microbiol. Biotechnol., 2022, 106, 8007–8020 CrossRef CAS PubMed.
- E. Kanis, J. Parks and D. L. Austin, Structural Analysis and Protein Binding of Cephalosporins, ACS Pharmacol. Transl. Sci., 2023, 6, 88–91 CrossRef CAS.
- Y. Inamoto, T. Chiba, T. Kamimura and T. Takaya, FK 482, a new orally active cephalosporin synthesis and biological properties, J. Antibiot., 1988, 41, 828–830 CrossRef CAS.
- A. Biffis, P. Centomo, A. Del Zotto and M. Zecca, Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review, Chem. Rev., 2018, 118, 2249–2295 CrossRef CAS.
- S. J. Hecker, I.-S. Cho, T. W. Glinka, Z. J. Zhang, M. E. Price, V. J. Lee, B. G. Christensen, A. Boggs, S. Chamberland, F. Malouin, T. R. Parr, T. Annamalai, J. Blais, E. L. Bond, L. Case, C. Chan, J. Crase, R. Frith, D. Griffith, L. Harford, N. Liu, M. Ludwikow, K. Mathias, D. Rea and R. Williams, Discovery of MC-02,331, a new cephalosporin exhibiting potent activity against methicillin-resistant Staphylococcus aureus, J. Antibiot., 1998, 51, 722–734 CAS.
- G. P. Roth and C. Sapino, Palladium in cephalosporin chemistry: an inexpensive triflate replacement for palladium acetate mediated coupling reactions, Tetrahedron Lett., 1991, 32, 4073–4076 CAS.
- J. D. Buynak, L. Vogeti, V. R. Doppalapudi, G. M. Solomon and H. Chen, Cephalosporin-derived inhibitors of β-Lactamase. Part 4: The C3 substituent, Bioorg. Med. Chem. Lett., 2002, 12, 1663–1666 CrossRef CAS PubMed.
- J. D. Buynak, L. Vogeti and H. Chen, Coupling Reactions of Cephalosporin Sulfones:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Stable 3-Stannylated Cephem, Org. Lett., 2001, 3, 2953–2956 CrossRef CAS.
A Stable 3-Stannylated Cephem, Org. Lett., 2001, 3, 2953–2956 CrossRef CAS. - V. Farina, S. R. Baker, D. A. Benigni, S. I. Hauck and C. Sapino, Palladium Catalysis in Cephalosporin Chemistry: General Methodology for the Synthesis of Cephem Side Chains, J. Org. Chem., 1990, 55, 5833–5847 CrossRef CAS.
- M. Rosales-Hurtado, F. Faure, F. Sannio, F. Verdirosa, G. Feller, E. Carretero, Y. Vo-Hoang, P. Licznar-Fajardo, S. Peyrottes, J.-D. Docquier and L. Gavara, Synthesis of β-lactam-zidovudine pronucleosides as potential selective narrow-spectrum antibacterial agents, Org. Biomol. Chem., 2025, 23, 389–399 CAS.
- M. Botta, M. C. De Rosa, R. Di Fabio, C. Mozzetti, A. Santini and F. Corelli, Experimental and theoretical investigations on the cephalosporin Δ3-Δ2 isomerization, Electron. J. Theor. Chem., 1996, 1, 52–59 CAS.
- H. L. D. Hayes, R. Wei, M. Assante, K. J. Geogheghan, N. Jin, S. Tomasi, G. Noonan, A. G. Leach and G. C. Lloyd-Jones, Protodeboronation of (Hetero)Arylboronic Esters: Direct versus Prehydrolytic Pathways and Self-/Auto-Catalysis, J. Am. Chem. Soc., 2021, 143, 14814–14826 CAS.
- D. S. Surry and S. L. Buchwald, Dialkylbiaryl phosphines in Pd-catalyzed amination: a user's guide, Chem. Sci., 2010, 2, 27–50 Search PubMed.
- S. D. Kuduk, R. K. Chang, C. Ng, K. L. Murphy, R. W. Ransom, C. Tang, T. Prueksaritanont, R. M. Freidinger, D. J. Pettibone and M. G. Bock, Bradykinin B1 antagonists: SAR studies in the 2,3-diaminopyridine series, Bioorg. Med. Chem. Lett., 2005, 15, 3925–3929 CAS.
- D. F. Aycock, Solvent Applications of 2-Methyltetrahydrofuran in Organometallic and Biphasic Reactions, Org. Process Res. Dev., 2007, 11, 156–159 CAS.
- M. Mondal and U. Bora, Eco-friendly Suzuki–Miyaura coupling of arylboronic acids to aromatic ketones catalyzed by the oxime-palladacycle in biosolvent 2-MeTHF, New J. Chem., 2016, 40, 3119–3123, 10.1039/C5NJ02734A.
- D. S. Surry and S. Buchwald, Biaryl Phosphane Ligands in Palladium-Catalyzed Amination, Angew. Chem., Int. Ed., 2008, 47, 6338–6361 CrossRef CAS PubMed.
- N. Hadei, E. A. B. Kantchev, C. J. O’Brie and M. G. Organ, The First Negishi Cross-Coupling Reaction of Two Alkyl Centers Utilizing a Pd−N-Heterocyclic Carbene (NHC) Catalyst, Org. Lett., 2005, 7, 3805–3807 CrossRef CAS.
- N. C. Bruno, N. Niljianskul and S. L. Buchwald, N-Substituted 2-Aminobiphenylpalladium Methanesulfonate Precatalysts and Their Use in C–C and C–N Cross-Couplings, J. Org. Chem., 2014, 79, 4161–4166 CAS.
- A. J. J. Lennox and G. C. Lloyd-Jones, Selection of boron reagents for Suzuki–Miyaura coupling, Chem. Soc. Rev., 2014, 43, 412–443 CAS.
- M. Chao and A. Hao, An Improved and Scaleable Preparation of 7-Amino-3-vinylcephem-4-carboxylic acid, Org. Process Res. Dev., 2009, 13, 924–927 CAS.
- R. L. Elliott, N. H. Nicholson, F. E. Peaker, A. K. Takle, C. M. Richardson, J. W. Tyler, J. White, M. J. Pearson, D. S. Eggleston and R. C. Haltiwanger, Cycloadditions of Cephalosporins. A Comprehensive Study of the Reaction of Cephalosporin Triflates with Olefins, Acetylenes, and Dienes to Form [2+2] and [4+2] Adducts, J. Org. Chem., 1997, 62, 4998–5016 CAS.
- R. L. Elliott, A. K. Takle, J. W. Tyler and J. White, Cycloadditions of cephalosporins. A general synthesis of novel 2,3-fused cyclobutane and cyclobutene cephems, J. Org. Chem., 1993, 58, 6954–6955 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5nj00243e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |