Understanding the catalytic properties for synthesizing NOx derivative NH3 by an alternate gas-switching process†
Ryutaro
Wakabayashi‡
 ,
Yuxiao
Zhang‡
,
Atsuko
Tomita
and
Tatsuo
Kimura
,
Yuxiao
Zhang‡
,
Atsuko
Tomita
and
Tatsuo
Kimura
 *
*
National Institute of Advanced Industrial Science and Technology (AIST), Sakurazaka, Moriyama-ku, Nagoya 463-8560, Japan. E-mail: t-kimura@aist.go.jp
First published on 3rd February 2025
Abstract
Reactive nitrogen compounds like NOx are not environment-friendly but have potential as some of the chemical sources for the low-energy synthesis of NH3 and value-added chemicals. Our recent initiatives have revealed catalytic conversion to NH3via a gas-switching NOx storage and reduction process. This concept was demonstrated absolutely by using nanocomposite catalysts based on mesoporous metal oxides with noble metal catalysts and NOx storage components like BaO/Pt@mAl2O3 and BaO/Pt@mTiO2. Here, we investigate and compare the properties of another BaO/Pt@mCeO2-type nanocomposite catalyst. After stabilization by zirconium doping, BaO/Pt@mCe0.8Zr0.2O2 exhibited acceptable NOx storage properties at temperatures higher than 250 °C with extra NOx storage by the CeO2-based support in addition to the major capacity of BaO. However, Pt nanoparticles were made to be very fine by a strong metal–support interaction to CeO2 and possibly worked very well, which was not preferable for the formation of NH3. From this viewpoint, the hydrogenation property was suppressed by lowering the reaction temperature, leading to an improvement in the NH3 selectivity from less than 10% to more than 70% even in the low NOx storage state.
1. Introduction
Various recycling processes of nitrogen compounds, such as nitrogen oxides (NOx) and nitrate (NO3−), as ammonia (NH3) have been investigated for overcoming issues with the environment, energy and resources.1–3 This approach is quite promising for the design of a nitrogen circular economy, where NH3 is used to obtain value-added organonitrogen compounds like amines.4–7 Accordingly, the reaction converting harmful NOx to valuable NH3 (named as the NTA reaction) is worth investigating through the rational design of heterogeneous catalysts showing high NH3 selectivity. As one of the interesting approaches, the NOx storage reduction, NSR, process has been applied by using nanocomposite catalysts, e.g., BaO/Pt/γ-Al2O3, for the selective synthesis of NH3.8–10 The chemical composition of the catalysts is similar to that developed for the purification to nitrogen (N2) by NSR.11–13 In the NTA reaction by modified NSR, the use of hydrogen (H2) is the standard process for the reduction of NOx to NH3.14,15 From this viewpoint, the chemical composition of the catalysts, such as metal oxide supports and novel metal catalysts, should be investigated further for understanding the catalytic properties. The oxidation of NO, the storage of NO2, the activation of H2 and the hydrogenation of the stored NO3− proceeded sequentially as the major reactions.Fundamental study progressed through the use of well-designed nanocomposite catalysts (e.g., BaO/Pt@mAl2O3 and BaO/Pt@mTiO2) for understanding the properties arising from typical components.16,17 Metal oxide supports such as alumina (Al2O3) and titania (TiO2) were surveyed by combining barium oxide (BaO) and calcium oxide (CaO) as the NOx storage components and platinum (Pt) as the metal catalyst for activating oxygen (O2) and H2. After learning about common NSR catalysts for the purification of NOx to N2 in the lean-burn gasoline engine system of automobiles, we proposed a gas-switching NSR process for the synthesis of NH3 as a game changing technology.14–17 By modifying the NSR process as a completely different process for the recycling of NOx, a selectivity towards NH3 of up to 90% or more was reached in a flow of 5% H2 by using BaO/Pt@mAl2O3 at around 250 °C and CaO/Pt@mAl2O3 at around 300 °C.16 Due to the surface property of the metal oxide support, which supply dissociated hydrogen species, the selectivity of NH3 was almost maintained in a flow of 1% H2 by using BaO/Pt@mTiO2 even at a low temperature (e.g., 175 °C).17 In this study, for further understanding the significance of the metal oxide support for our gas-switching NSR process for the selective synthesis of NH3, an additional nanocomposite catalyst (e.g., BaO/Pt@mCeO2) was prepared in the same way using mesoporous ceria (mCeO2) and compared to BaO/Pt@mAl2O3 and BaO/Pt@mTiO2.
2. Experimental
2.1. Chemicals
Cerium nitrate hexahydrate (Ce(NO3)3·6H2O), zirconyl nitrate dihydrate (ZrO(NO3)2·2H2O) and citric acid (C(OH)(CH2COOH)2COOH) were obtained from FUJIFILM Wako Pure Chemical Co. Hydrogen hexachloroplatinate(IV) hexahydrate (H2PtCl6·6H2O) aqueous solution was purchased from Tanaka Precious Metals. Pluronic P123 (EO20PO70PO20), as one of the commercial poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) (EOnPOmEOn) triblock copolymers, was purchased from Sigma-Aldrich. Barium acetate (Ba(CH3COO)2) was also obtained from FUJIFILM Wako Pure Chemical Co.2.2. Synthesis of BaO/Pt@mCe0.8Zr0.2O2
The Pluronic P123-templated mesoporous structure of pure CeO2 was not stable and was improved by mixing an appropriate amount of zirconia (ZrO2). Ce(NO3)3·6H2O (4 mmol, 17.4 g), ZrO(NO3)2·2H2O (1 mmol, 2.7 g) and citric acid (10 mmol, 19.2 g) were dissolved in an aqueous solution of Pluronic P123 (5 g in 120 mL H2O) and stirred for 90 min. After combining with another aqueous solution of H2PtCl6·6H2O (405.5 μL, 200 g L−1 Pt) and stirring for another 30 min, the resultant precursor solution was solidified by using a spray dryer (Yamato Scientific Co., Ltd., ADL311S-A). The inlet temperature and the blower rate were 170 °C and 1.0 m3 min−1, respectively. After calcination at 400 °C (heating rate; 2 °C min−1) for 3 h in a flow of dry air, Pt@mCe0.8Zr0.2O2 (1.01 g) was dispersed in H2O (20 mL), followed by the dropwise addition of an aqueous solution of Ba(CH3COO)2 (5 mL containing 0.1 g of Ba) under stirring for 1 h and recovered by using a rotary evaporator at 60 °C under vacuum. The weight ratio of Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pt
Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce0.8Zr0.2O2 was 10
Ce0.8Zr0.2O2 was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The resultant powder sample was finally calcined at 500 °C (10 °C min−1) for 3 h in a flow of dry air.
100. The resultant powder sample was finally calcined at 500 °C (10 °C min−1) for 3 h in a flow of dry air.
2.3. Characterization of BaO/Pt@mCe0.8Zr0.2O2
Low- and wide-angle X-ray diffraction (XRD) patterns were recorded by using a Rigaku RINT 2100 with monochromated Fe Kα radiation and a Rigaku RINT 2000 with monochromated Cu Kα radiation, respectively. N2 adsorption–desorption isotherms were measured by using an Anton Paar Autosorb-iQ at 77 K after the sample was degassed at 110 °C for 6 h under vacuum. The specific surface area was calculated by the Brunauer–Emmett–Teller (BET) method. The total pore volume was estimated by using the volume of N2 at P/P0 = 0.99. The pore size distribution curve was drawn by the Barrett–Joyner–Halenda (BJH) method using the desorption branch. Field-emission scanning electron microscopic (FE-SEM) images were taken by using a HITACHI SU9000, operated at 1.0 kV. Transmission electron microscopy (TEM) images were taken by using an FEI Tecnai Osiris, operated at 200 kV, in high-angle annular dark field scanning TEM (HAADF-STEM) mode to enhance the contrast of Pt nanoparticles (NPs) for obtaining clear elemental mapping by energy dispersive X-ray spectroscopy (EDS).2.4. Catalytic test using BaO/Pt@mCe0.8Zr0.2O2
According to our previous study,16,17 the catalytic properties were investigated through our typical gas-switching process between the storage of NOx and the reduction of stored NOx (typically NO3−) species. The inlet gas was alternated between 1000 ppm NO with 10% O2 for 60 min and 1% H2 for 60 min at temperatures ranging from 300 °C down to 175 °C using a fixed-bed flow reactor. All the flow rates were 100 mL min−1 balanced with N2. During the gas-switching operation, the reactor was purged with N2 for 5 min after every process. Initially, BaO/Pt@mCe0.8Zr0.2O2 (approximately 100 mg) was placed on quartz glass wool in a quartz glass tube reactor and pre-treated at 300 °C for 60 min in a flow of 1% H2. The amount of stored NOx was evaluated on the basis of total NOx in the gas phase. The formation of nitrogen compounds (e.g., NO, NO2, N2O and NH3) arising from the hydrogenation of the NO3− species was monitored using a Fourier-transform infrared (FT-IR) spectrometer (Nicolet iS20, Thermo Fisher Scientific) equipped with a deuterated triglycine sulfate (TGS) detector and a 2.4 m gas cell (Pike Technologies).Temperature-programmed reduction (TPR) profiles in a flow of H2 were measured by using a BELCAT II (Microtrac BEL) equipped with a thermal conductivity detector (TCD). A powder sample (approx. 50 mg) was placed inside a triple-walled quartz tube and pre-treated in a flow of O2 at 300 °C to oxidize adequately, and then cooled down to 40 °C in a flow of He. TPR measurement was conducted at temperatures ranging from 40 °C to 900 °C with a heating rate of 10 °C min−1 in a flow of 4% H2 (Ar balanced, 30 mL min−1) where the resultant water was trapped during the measurement by using a molecular sieve before introduction into the detector.
3. Results and discussion
High-surface-area metal oxides are very important for designing high-performance heterogeneous catalysts. A huge number of papers have so far demonstrated the synthesis of mesoporous metal oxides by applying a drying process of clear precursor solutions containing soluble metal oxide species and an amphiphilic organic compound. However, it is still not easy to reproduce ordered mesoporous metal oxides in powder forms with the self-assembly of amphiphilic organic molecules. We have therefore developed several aerosol-assisted synthetic methods for obtaining powder samples of mesoporous metal oxides, such as Al2O3 and TiO2.18–22 However, a wet-impregnation method cannot be applied for preparing Pt-supported catalysts based on EOnPOmEOn (e.g., Pluronic P123)-templated mesoporous metal oxides owing to their lower stability in a highly acidic solution of H2PtCl6.23,24 Accordingly, a series of nanocomposite catalysts have been carefully designed by using such mesoporous metal oxides as the catalyst support, especially those prepared using EOnPOmEOn. Alkali earth metal species (e.g., BaO) were impregnated after the direct introduction of the metal catalysts (e.g., Pt) during the spray-drying process. The growth of Pt nanoparticles (NPs) progressed during calcination to remove organic moieties with the crystallization of the metal oxide frameworks. Here, another BaO/Pt@mCe0.8Zr0.2O2-type nanocomposite catalyst was prepared by the same synthetic process. A Pluronic P123-templated and stabilized mesoporous CeO2 (mCeO2)containing Pt species was obtained in one-pot with the preferable addition of ZrO2 (Pt@mCe0.8Zr0.2O2). Pt NPs were mainly grown during calcination at 400 °C for 3 h in dry air with the crystallization of CeO2-based frameworks.3.1. Structural characterization of Pt@mCe0.8Zr0.2O2 and BaO/Pt@mCe0.8Zr0.2O2
Low- and wide-angle XRD patterns, N2 adsorption–desorption isotherms and SEM images of Pt@mCe0.8Zr0.2O2 and BaO/Pt@mCe0.8Zr0.2O2 are shown in Fig. 1. A single dominant peak was observed at 2θ = 1.4° (d = 7.9 nm) in the low-angle XRD pattern of Pt@mCe0.8Zr0.2O2 (Fig. 1a), indicating the presence of a mesostructural ordering derived from the self-assembly of EO20PO70EO20 molecules. The wide-angle XRD pattern clearly showed several peaks that can be assigned to the cubic structure of CexZr1−xO2 (Fig. 1b). The peaks were slightly shifted to higher diffraction angles compared to those observed for pure CeO2 (Table S1, ESI†). For example, the (111) peak was shifted from 2θ = 28.5° to 28.8° without any peak splitting, suggesting the isomorphous substitution of large Ce4+ (ionic radius; 0.97 Å) with small Zr4+ (0.84 Å).25 The N2 adsorption–desorption isotherm was type IV with a hysteresis loop (Fig. 1c), indicating the presence of mesopores. The BET surface area and the total pore volume were 97 m2 g−1 and 0.10 cm3 g−1, respectively. The average pore diameter was calculated to be 3.6 nm by the BJH method. A representative SEM image showed partially crushed spherical particles with mesopores (Fig. 1d).After the impregnation of Ba(CH3COO)2 followed by calcination at 500 °C for 3 h in a flow of dry air, a modest peak was still observed at almost the same 2θ position in the low-angle XRD pattern (Fig. 1e). This result revealed that the original mesoporous structure of Pt@mCe0.8Zr0.2O2 was maintained and accommodated the resultant barium species like BaO. As in the case of our previous BaO/Pt@mAl2O3 and BaO/Pt@mTiO2 catalysts,16,17,23,24 the formation of crystalline BaO species was not confirmed by XRD, but additional peaks were detected at 2θ = 23.9° and 42.0° due to the presence of witherite-type BaCO3 (Fig. 1f).23 This arises from the thermal decomposition of Ba(CH3COO)2 to BaO and subsequent reaction with atmospheric CO2. The N2 adsorption–desorption isotherm showed a type IV behavior with a hysteresis loop (Fig. 1g). The BET surface area (52 m2 g−1) and the total pore volume (0.07 cm3 g−1) decreased with the impregnation of Ba species, which is related to the presence of Ba species inside the mesopores (3.6 nm). A partially crushed spherical porous morphology was also found through SEM observation even after the impregnation of Ba species (Fig. 1h).
Here, spherical mesopores (pore size; 3.6 nm) were designed through the formation of Ce0.8Zr0.2O2-type frameworks with about 1 mass% Pt using a general-purpose spray dryer where the specific surface area and the total pore volume were 97 m2 g−1 and 0.10 cm3 g−1, respectively. These values are comparable to those reported in several successful literature studies.26,27 2D hexagonal mesoporous Ce0.5Zr0.5O2 (specific surface area; 132 m2 g−1, pore volume; 0.19 cm3 g−1 and pore size; 3.7 nm) was prepared by drying a precursor solution containing Pluronic P123, followed by calcination at 400 °C (heating rate; 1 °C min−1).26 An intense peak was observed at the d-spacing of 9.0 nm in the low diffraction angle region after calcination at 400 °C, which deformed with the increase in calcination temperature and almost disappeared after calcination at 600 °C. In another case of Ce0.8Zr0.2O2, the intensity of the corresponding XRD peaks was weakened after calcination at 400 °C where the specific surface area, pore volume and pore size were 107 m2 g−1, 0.20 cm3 g−1 and 3.7 nm, respectively. Such 2D hexagonal mesoporous Ce0.5Zr0.5O2 (specific surface area; 98 m2 g−1, pore volume; 0.08 cm3 g−1 and pore size; 4.0 nm after calcination at 400 °C) was reproduced using Pluronic P123.27 The mesoporous structure can be confirmed by TEM but seemed to deviate (specific surface area; 56 m2 g−1, pore volume; 0.01 cm3 g−1 and pore size; 7.8 nm).
XRD peaks due to the formation of Pt NPs were not detected in the wide-angle XRD patterns of Pt@mCe0.8Zr0.2O2 after calcination at 400 °C in a flow of dry air (see Fig. 1b) and Ba/Pt@mCe0.8Zr0.2O2 even after calcination at 500 °C (see Fig. 1f). The presence of metallic Pt species is typically confirmed by the presence of the (111) reflection at around 2θ = 40°. The results possibly suggest that the resultant Pt species are extremely fine, being quite different from those observed in our previous case of BaO/Pt@mTiO2 calcined by the same process.17 The presence of Pt NPs was then surveyed by HAADF-STEM combined with EDS analysis. Typical bright-field TEM observations of Pt@mCe0.8Zr0.2O2 and BaO/Pt@mCe0.8Zr0.2O2 were not successful (see Fig. S1, ESI†) because Ce and Zr are both high atomic number atoms. However, HAADF-STEM, detecting scattered electrons, was quite helpful for illustrating the internal structure at an identical acceleration voltage, revealing a partially hollow morphology of the spray-dried spherical particles (Fig. 2a and e). The presence of Pt NPs was not observed directly by HAADF-STEM, but the EDS spectra suggested the presence of reasonable Pt species (see Fig. S2, ∼0.8 mass%, ESI†). The elemental mappings of Ce, Zr, Pt and/or Ba can be illustrated by using the data (Fig. 2b–d and f–i). The resultant size of the Pt NPs is possibly smaller than ∼1 nm, under the detection limit by HAADF-STEM. However, the presence of Pt NPs with an average size of around 1 nm was confirmed after the catalytic test (see Fig. S3, ESI†). In addition, a small amount (0.1–0.3 wt%) of chlorine (Cl) was detected for Pt@Ce0.8Zr0.2O2 and Ba/Pt@Ce0.8Zr0.2O2, which arose from the utilization of H2PtCl6·6H2O as the Pt source.
 | ||
| Fig. 2 (a) and (e) HAADF-STEM images and (b)–(d) and (f)—(i) EDS mappings overlapped with Ce, Zr, Pt and/or Ba of (a)–(d) Pt@mCe0.8Zr0.2O2 and (e)–(i) BaO/Pt@mCe0.8Zr0.2O2. | ||
3.2. Catalytic properties of BaO/Pt@mCe0.8Zr0.2O2 during the gas-switching NSR process
Catalytic reduction of stored NO3− using BaO/Pt@mCe0.8Zr0.2O2 was investigated by an alternate switching of the inlet gas every 60 min between 1000 ppm NO with 10% O2 and 1% H2 at temperatures ranging from 300 °C down to 175 °C. As in the case of our recent study,16,17 Pt NPs were enveloped inside the resultant particles of mCe0.8Zr0.2O2 through the spray-drying of a precursor solution with Pt species followed by calcination at 400 °C. The BaO species were then impregnated using stepwise design to obtain a BaO/Pt@mCeO2 type nanocomposite catalyst for the storage of NOx and the subsequent hydrogenation to NH3 and/or N2. The typical raw data that can be collected under our standard reaction conditions are shown in Fig. 3. The formation of NH3 was favorable at lower temperatures (e.g., 200 °C and lower). Before starting the reaction process, the electric state of CeO2 would be initially changed in part from Ce4+ to reduced Ce3+ in addition to the reduction of Pt NPs to metallic Pt(0) by pretreatment at 300 °C for 60 min in a flow of H2. In the gas-switching process, it is rational to consider that Ce3+ was oxidized to Ce4+ during the storage process in a flow of 1000 ppm NO with 10% O2 and reduced again to Ce3+ during the reduction process in 1% H2.28 This kind of NSR process is mainly divided into 4 catalytic reactions such as (1) the oxidation of NO by Pt NPs, (2) the storage of NO2 with BaO, (3) the activation of H2 over Pt NPs and (4) the hydrogenation of stored NO3−. The reaction steps utilizing BaO/Pt@mCe0.8Zr0.2O2 were assessed one-by-one to understand the role of the CeO2 support and compared to those reported for our BaO/Pt@mAl2O3 and BaO/Pt@mTiO2-type catalysts.The NO oxidation activity over BaO/Pt@mCe0.8Zr0.2O2 in a flow of 1000 ppm NO with 10% O2 is summarized in Fig. 4, showing the gradual increase with an increase in the reaction temperature. In a previous study on a similar NSR process using common Pt/BaO/CeO2 (500 ppm NO with 5% O2 ↔ 5% H2), the steady-state conversion of NO to NO2 was increased up to 300 °C and saturated at the equilibrium (e.g., 400 °C).29 However, the NO oxidation property of BaO/Pt@mCe0.8Zr0.2O2 was not sufficient even at higher temperature (e.g., 250 °C and 300 °C). Additionally, the amount of NOx, mainly stored as NO3− with BaO, is quantified in Fig. 5 at each temperature. This reaction step can be progressed even in a continuous flow of NO2 as the minor component after a little oxidation of NO by O2. The storage of NOx reached up to 0.58 mmol g−1 for 60 min after the 3rd cycle at 300 °C, being superior to those observed for our BaO/Pt@mAl2O3 (∼0.5 mmol g−1) and BaO/Pt@mTiO2 (∼0.2 mmol g−1 at 300 °C and 0.26 mmol g−1 at 250 °C at the maximum).16,17 This is very reasonable because CeO2 can work as an additional NOx storage component in addition to BaO.30,31 The amount of stored NOx was also abundant even at 250 °C (e.g., 0.43 mmol g−1) but drastically decreased at around 200 °C (0.20 mmol g−1) or lower (0.20 mmol g−1 at 175 °C). This tendency for the storage of NOx was not noticeable in our cases using BaO/Pt@mAl2O3 and BaO/Pt@mTiO2-type catalysts.
 | ||
| Fig. 4 Temperature-dependence of the NO oxidation property, calculated as the ratio of NO2/(NO + NO2) at the end of the 3rd NOx storage process. | ||
The amount of resultant NH3 during the reduction process in a flow of 1% H2 is also displayed in Fig. 5. The slight formation of N2O was confirmed at the beginning of the reduction process, being similar to the case using BaO/Pt@mTiO2.17 After the activation of H2 and subsequent hydrogenation of the stored NOx, the recovery of NH3 was very low at temperatures higher than 250 °C (∼0.05 mmol g−1), where the formation rate of NH3 was below 10%. This reaction step was promoted at lower temperatures (0.13 mmol g−1 at 200 °C and 0.12 mmol g−1 at 175 °C) with ∼70% selectivity of NH3. This behavior is mainly caused by the presence of fine Pt NPs smaller than those grown in mAl2O3 (∼21 nm as the average) and mTiO2 (∼11 nm as the average).16,17 As observed by HAADF-STEM of BaO/Pt@mCe0.8Zr0.2O2 (see Fig. 2), the hydrogenation of the stored NOx was accelerated by the presence of extremely fine Pt NPs (smaller than 1 nm). In the case using CeO2, further oxidation of the resultant NH3 to N2 is an extra issue at around 300 °C in the presence of oxidated CeO2 and thus suppressed below 200 °C.32,33
The average size of the resultant Pt NPs is changed with the synthetic process, typically the calcination temperature. Metallic Pt species are dispersed over the surfaces of metal oxides depending on the relationship between the strength of interaction and the amount of interactive sites. For example, Pt interacts strongly with unsaturated penta-coordinated Al3+ species (Alpenta3+) and is then anchored over the γ-Al2O3 surface.34 Electronic defects are designed at the surfaces of reducible metal oxides (e.g., CeO2 and TiO2), leading to strong metal–support interaction (abbreviated as SMSI).35,36 From this viewpoint, the resultant size of the Pt NPs in Pt@mCeO2 (less than 1 nm, calcined at 400 °C) was very reasonable, being smaller than those observed for Pt@mTiO2 (ave. 11 nm, calcined at 400 °C) and much smaller than those observed for Pt@mAl2O3 (ave. 21 nm, calcined at 850 °C). The hydrogenation was completed very quickly in the presence of highly active smaller Pt NPs preferable for the formation of N2. Accordingly, lower temperature is very suitable for the selective synthesis of NH3 (∼70% at 200 °C, smaller than the ∼90% observed for BaO/Pt@mTiO2 at 175 °C).17
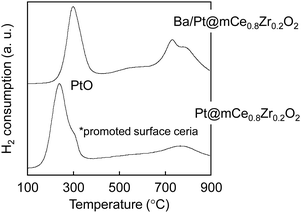
This consideration was supported by TPR measurements in a flow of H2. As shown in Fig. 6, a major peak was observed for Pt@mCe0.8Zr0.2O2 at around 250 °C (starting at around 200 °C) and shifted to higher temperature in the presence of Ba species (around 300 °C, starting at around 250 °C). This peak is assignable to the reduction of PtO to Pt, which is promoted with the surface reduction of CeO2.28 This variation is due to the decrease of accessible Pt surfaces with the coverage through the impregnation of the Ba species. Additionally, the reduction temperature (around 250 °C, starting at around 200 °C) is higher than the case using common Pt/Ce0.25Zr0.75O2 that was prepared by impregnating the same 1.0 wt% Pt and comparable to that observed for Ce0.25Zr0.75O2 having very low Pt (0.001 wt%).37 It would be better to consider that some of the Pt NPs smaller than 1 nm are possibly embedded inside the framework of Ce0.8Zr0.2O2 with a decrease in the NO oxidation property. As a specific feature of the CeO2-based catalyst support, a possible formation route of NH3 by using BaO/Pt@mCe0.8Zr0.2O2 is illustrated in Fig. 7. During the gas-switching NTA process by the storage of NOx through the oxidation of NO and subsequent reduction by H2, lower temperature (e.g., 200 °C and lower) is eventually suitable for the high recovery of NH3 (improved up to ∼70% NH3 selectivity).
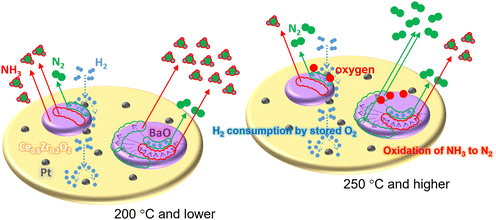 | ||
| Fig. 7 Possible routes for the formation of NH3 and N2 through the reduction of stored NOx by H2 utilizing BaO/Pt@mCe0.8Zr0.2O2. | ||
4. Conclusions
The general properties of a BaO/Pt@mCeO2-type nanocomposite catalyst, specifically those of BaO/Pt@mCe0.8Zr0.2O2, were investigated for the synthesis of NH3 through an alternate switching of the inlet gas between 1000 ppm NO with 10% O2 and 1% H2 at temperatures below 300 °C. Considering the major components for the catalytic conversion of NOx in high-temperature combustion gas, we have designed several nanocomposite catalysts, including BaO/Pt@mCe0.8Zr0.2O2. The resultant size of the Pt NPs was predominantly determined by the synthetic conditions, mainly the calcination temperature. Furthermore, SMSI was very significant for the formation of fine Pt NPs. As a result, the resultant fine Pt NPs (smaller than 1 nm for fresh BaO/Pt@mCe0.8Zr0.2O2), were highly active as a catalyst, but not fully accessible. Accordingly, high temperature is suitable for the oxidation of NO and subsequent storage as NO3− by extra CeO2 in addition to BaO. For the synthesis of NH3 by the hydrogenation of stored NOx species after the activation of H2, rapid hydrogenation is not suitable for the formation of NH3.16,17 This is the main reason that lower reaction temperature is preferable for the high recovery of NH3. In addition, due to the specific features of CeO2, like the oxygen storage properties, the oxidation of NH3 as well as the consumption of H2 should be suppressed by lowering the reaction temperature, which allows the oxygen storage properties of the CeO2-based frameworks to be shown (∼250 °C for BaO/Pt@mCe0.8Zr0.2O2).Data availability
Date will be made available on request. Extra data supporting this article are included as a part of the ESI.†Conflicts of interest
The author declares no competing financial interest.Acknowledgements
This work has been mainly supported as one of the research projects (JPNP18016, commissioned by the New Energy and Industrial Technology Development Organization, NEDO) in the “Moonshot R&D program (Moonshot Goal 4: Realization of sustainable resource circulation to recover the global environment by 2050).”References
- J. G. Chen, R. M. Crooks, L. C. Seefeldt, K. L. Bren, R. M. Bullock, M. Y. Darensbourg, P. L. Holland, B. Hoffman, M. J. Janik, A. K. Jones, M. G. Kanatzidis, P. King, K. M. Lancaster, S. V. Lymar, P. Pfromm, W. F. Schneider and R. R. Schrock, Science, 2018, 360, eaar6611 CrossRef PubMed.
- M. Nazemi and M. A. El-Sayed, Acc. Chem. Res., 2021, 54, 4294–4304 CrossRef PubMed.
- Q. Wu, F. Zhu, G. Wallace, X. Yao and J. Chen, Chem. Soc. Rev., 2024, 53, 557–565 RSC.
- Y. Xiong, Yu Wang, J. Zhou, F. Liu, F. Hao and Z. Fan, Adv. Mater., 2024, 36, 2304021 CrossRef PubMed.
- P. Liao, J. Kang, R. Xiang, S. Wang and G. Li, Angew. Chem., Int. Ed., 2024, 63, e202311752 CrossRef PubMed.
- S. Jia, L. Wu, H. Liu, R. Wang, X. Sun and B. Han, Angew. Chem., Int. Ed., 2024, 63, e202400033 CrossRef PubMed.
- S. R. Udayasurian and T. Li, Nanoscale, 2024, 16, 2805–2819 RSC.
- L. Hollevoet, F. Jardali, Y. Gorbanev, J. Creel, A. Bogaerts and J. A. Martens, Angew. Chem., Int. Ed., 2020, 59, 23825–23829 CrossRef.
- L. Hollevoet, E. Vervloessem, Y. Gorbanev, A. Nikiforov, N. De Geyter, A. Bogaerts and J. A. Martens, ChemSusChem, 2022, 15, e202102526 CrossRef CAS.
- F. V. Steenweghen, L. Hollevoet and J. A. Martens, Green Chem., 2024, 26, 2534–2539 RSC.
- S. Roy and A. Baiker, Chem. Rev., 2009, 109, 4054–4091 CrossRef CAS PubMed.
- P. Granger and V. I. Parvulescu, Chem. Rev., 2011, 111, 3155–3207 CrossRef CAS PubMed.
- J. R. Gonález-Velasco, B. Pereda-Ayo, U. De-La-Torre, M. Urrutxua and R. López-Fonseca, ChemCatChem, 2018, 10, 2928–2940 CrossRef.
- T. Kimura, Chem. Rec., 2024, 24, e202400094 CrossRef CAS PubMed.
- T. Kimura, J. Jpn. Pet. Inst., 2024, 67, 209–216 CrossRef CAS.
- A. Tomita, R. Wakabayashi and T. Kimura, Catal. Sci. Technol., 2023, 13, 2927–2936 RSC.
- Y. Zhang, A. Tomita, R. Wakabayashi and T. Kimura, J. Mater. Chem. A, 2024, 12, 8262–8271 RSC.
- H. Maruoka, A. Tomita, Z. Liu and T. Kimura, Langmuir, 2018, 34, 13781–13787 CrossRef.
- T. Kimura and H. Maruoka, Chem. Commun., 2019, 55, 10003–10006 RSC.
- H. Maruoka and T. Kimura, Bull. Chem. Soc. Jpn., 2019, 92, 1859–1866 CrossRef.
- M. I. Saidul, R. Wakabayashi and T. Kimura, Dalton Trans., 2021, 50, 7191–7197 RSC.
- Y. Zhang, R. Wakabayashi and T. Kimura, Dalton Trans., 2023, 52, 1543–1550 RSC.
- R. Wakabayashi, A. Tomita and T. Kimura, J. Hazardous Mater., 2020, 398, 122791 CrossRef PubMed.
- R. Wakabayashi, A. Tomita and T. Kimura, Chem. – Eur. J., 2021, 27, 6706–6712 CrossRef PubMed.
- G. Colón, M. Pijolat, F. Valdivieso, H. Vidal, J. Kašpar, E. Finocchio, M. Daturi, C. Binet, J. C. Lavalley, R. T. Baker and S. Bernal, J. Chem. Soc., Faraday Trans., 1998, 94, 3717–3726 RSC.
- Q. Yuan, Q. Liu, W.-G. Song, W. Feng, W.-L. Pu, L.-D. Sun, Y.-W. Zhang and C.-H. Yan, J. Am. Chem. Soc., 2007, 129, 6698–6699 CrossRef PubMed.
- L. Xu, H. Song and L. Chou, Int. J. Hydrogen Energy, 2012, 37, 18001–18020 CrossRef CAS.
- Y. Zhang, Y. Yu and H. He, Catal. Sci. Technol., 2016, 6, 3950–3962 RSC.
- Y. Ren and M. P. Harold, ACS Catal., 2011, 1, 969–988 CrossRef.
- S. Philipp, A. Drochner, J. Kunert, H. Vogel, J. Theis and E. S. Lox, Top. Catal., 2004, 30, 235–238 CrossRef.
- A. Filtschew, P. Beato, S. B. Rasmussen and C. Hess, Phys. Chem. Chem. Phys., 2021, 23, 1874–1887 RSC.
- B. Pereda-Ayo, U. D. L. Torre, M. P. González-Macros and J. R. González-Velasco, Catal. Today, 2015, 241, 133–142 CrossRef.
- N. L. Phuc, X. Courtois, F. Can, S. Royer, P. Marecot and D. Duprez, Appl. Catal., B, 2011, 102, 362–371 CrossRef.
- J. H. Kwak, J. Hu, D. Mei, C.-W. Yi, D. H. Kim, C. H. F. Peden, L. F. Allard and J. Szanyi, Science, 2009, 325, 1670–1673 CrossRef PubMed.
- J. Jones, H. Xiong, A. T. DeLaRiva, E. J. Peterson, H. Pham, S. R. Challa, G. Qi, S. Oh, M. H. Wiebenga, X. I. P. Hernández, Y. Wang and A. K. Datye, Science, 2016, 353, 150–154 CrossRef PubMed.
- J. Shan, C. Ye, Y. Jiang, M. Jaroniec, Y. Zheng and S.-Z. Qiao, Sci. Adv., 2022, 8, eabo0762 CrossRef.
- N. Kamiuchi, M. Haneda and M. Ozawa, Catal. Today, 2014, 232, 179–184 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj05459h |
| ‡ These authors equally contributed to this research. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |