An efficient visible light-driven double-well-based Pt/CdS/3D-graphene heterostructure for electrocatalytic and photo-electrocatalytic methanol oxidation†
Habib
Ullah‡
a,
Muqaddas Fatima
Mumtaz‡
a,
Asad
Mumtaz
 *a,
Hina
Sajid
*a,
Hina
Sajid
 b,
Sani
Zahra
a,
Sabahat
Sardar
c,
Uzma
Naz
b,
Qamir Ullah
Niazi
b,
Shahid
Iqbal
b,
Sani
Zahra
a,
Sabahat
Sardar
c,
Uzma
Naz
b,
Qamir Ullah
Niazi
b,
Shahid
Iqbal
 d,
Syed Farooq
Adil
d,
Syed Farooq
Adil
 e,
Mohammad Rafe
Hatshan
e,
Mujeeb
Khan
e,
Mohammad Rafe
Hatshan
e,
Mujeeb
Khan
 e,
Jaweria
Ambreen
e,
Jaweria
Ambreen
 b,
Muhammad Imran
Irshad
b,
Muhammad Imran
Irshad
 f and
Muhammad
Ahmad
g
f and
Muhammad
Ahmad
g
aDepartment of Chemistry, School of Natural Sciences, National University of Sciences and Technology, 44000, Islamabad, Pakistan. E-mail: asad_032@yahoo.com
bDepartment of Chemistry, COMSATS University Islamabad, Park Road, 45550, Islamabad, Pakistan
cResearch & Development Division, Al Qasim Traders, H-13, 44000, Islamabad, Pakistan
dNottingham Ningbo China Beacons of Excellence Research and Innovation Institute, University of Nottingham Ningbo China, Ningbo 315100, China
eDepartment of Chemistry, College of Science, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia
fDepartment of Physics, Khawaja Fareed University of Engineering and Information Technology (KFUEIT), Rahim Yar Khan, Pakistan
gDepartment of Chemistry, Division of Science and Technology, University of Education, 54770, Lahore, Pakistan
First published on 2nd December 2024
Abstract
In this article, the impact of the loading of CdS between Pt and 3D graphene was investigated determining enhanced visible light absorption and efficient charge separation at the interfaces that have improved methanol oxidation reaction activities. The structural, morphological, optical and electrochemical properties of photoanodes were investigated. The highest current density reached 264 mA cm−2 at 0.28 V vs. Ag/AgCl under one sun illumination for Pt/10-CdS/3D-Gr@Ni-Foam as compared to 116 mA cm−2 at 0.26 V for Ag/AgCl in the dark at a scan rate of 10 mV s−1. The diffusion coefficient of electron transfer is also enhanced to 3.99 × 10−3 cm2 s−1 under illumination as compared to 2.971 × 10−3 cm2 s−1 under dark conditions for the Pt/10-CdS/3D-Gr@Ni-Foam heterostructure. The decrease in the charge transfer resistance (Rct) from 34.31 Ω to 2.38 Ω indicated that the introduction of CdS enhanced the separation and transportation of photoexcited charges and also improved the kinetics of the electron transfer reaction. The Pt/10-CdS/3D-Gr@Ni-Foam exhibited a significantly enhanced net donor density (ND) of 2.5 × 1020, surpassing the donor density of 1.5 × 1020 of the Pt/3D-Gr@Ni-Foam. Both Pt and 3D-graphene are being utilized as a well, for efficient charge separation and transportation at either side of the CdS sandwich, resulting in effective transfer of charges at the corresponding Pt/CdS/3D-Gr@Ni-Foam heterostructure interfaces and increased electron density on Pt showed its unprecedented potential to be utilized for electrocatalytic and photo-electrocatalytic applications.
Introduction
The primary factor behind the escalation of CO2 levels in our atmosphere is the excessive utilization of fossil fuels, spurred by the tremendous growth in energy consumption across various sectors of human society. As a potential solution, researchers are exploring using fuel cells, which are gentler for the environment.1,2 Direct methanol fuel cells (DMFCs) have been a significant research focus due to their ability to convert chemical energy into electrical energy. Their clean and eco-friendly properties, high energy conversion efficiency, and uncomplicated battery structure make them a compelling choice.3,4 In recent years, efforts have been made to improve methanol electrocatalytic oxidation in DMFCs, with platinum (Pt) and Pt-based materials emerging as the leading catalysts.Extensive theoretical and experimental research has focused on integrating graphene with noble metal nanoparticles,5 nanocrystals,6 and metal oxides,7–9 among others, to use as electrocatalysts/photocatalysts or supports. The emergence of three-dimensional graphene (3D-Gr) has sparked widespread curiosity among researchers and industry leaders.10 One of the most promising developments with 3D-graphene lies in its continuous and interconnected porous design, which facilitates rapid and efficient transport of both mass and electrons. This unique feature makes 3D-graphene an ideal substrate for electrocatalysts, enabling its potential for use in synthesizing innovative hierarchical-structured hybrid nanomaterials that can significantly enhance the electrochemical performance of catalytic materials. Chen et al. (2016) successfully developed NiCo2O4@MnO2 core–shell nanowire arrays on 3D graphene (3D-Gr) for a high-performance supercapacitor.11 Similarly, Yu et al. (2017) synthesized NiCo2O4 nanoneedles on 3D-Gr as electrodes for a supercapacitor and for the methanol oxidation reaction (MOR).12 In a separate study, Sun et al. (2014) produced Co3O4 sheets on 3D-Gr, which showcased excellent performance as hybrid materials for lithium-ion batteries.13 One of the critical benefits of 3D-Gr-based hybrid nanomaterials was their ability to facilitate effective connection between the electrolyte and the electrode materials in electrochemical energy storage devices. In addition, these nanomaterials also significantly improve the electron and ion transport rate at the electrode/electrolyte interface, enhancing the efficiency of energy storage/conversion devices. According to the study conducted by Pattanayak et al., a combination of cuprous oxide, polypyrrole, and graphene oxide is a highly effective catalyst for the MOR, which was found to have a power density of 31 mW cm2 and a maximum current density of 155 mA cm2 at a much higher potential of +0.2 V.3
Other semiconductor classes like metal chalcogenides,14,15 metal oxides16, metal carbides,17,18 metal phosphides19, metal nitrides20 and metal organic frameworks (MOFs)21 are used as supports or as sensitizers22 for enhancing the electrocatalytic and photoelectrocatalytic oxidation of alcohols for power generation. Other options for improving semiconductor performance include doping, modification, loading cocatalysts, and constructing heterojunctions with other materials. However, creating a heterojunction appeared as the most effective technique as it permits the full utilization of each semiconductor's advantages in the hybrid system. The cobalt sulfide nanoneedles (CS-NNs) on 3D graphene (3DG) decorated nickel foam and without binders offered a dual advantage: preventing aggregation to maximize the efficiency of catalytically active sites and acting as a “pathway” for electron collection in the axial direction.23 A novel electrode design for efficient methanol oxidation, consisting of 2D-cobalt sulfide nanosheets on a porous microstructure of 3D-graphene/nickel foam, was strategically engineered to provide numerous benefits such as rapid ionic transportation facilitated by porous three-dimensional graphene, and a large surface area optimized for loading an impressive amount of 2D-cobalt sulfide NSs.24 High-efficiency electrocatalysts made of cobalt and iron layered double hydroxide nanorods and graphene oxide on nickel foam have shown excellent performance in methanol electro-oxidation. This activity is attributed to its many exposed active sites, abundant oxygen vacancies, and efficient electron transfer abilities between the catalyst and the electrode surface.25 Poor current density in the lower potential window from −0.5 to 0 V is a major issue to be resolved to have high current densities over the said voltage windows. Also, transition metal based electrocatalysts and photoelectrocatalysts have shown their redox reaction chemistry that creates issues in identifying the main reaction chemistry of the methanol oxidation reaction. Therefore, utilizing a noble metal as a basic reaction site with its heterostructure formation using a single well is reported in the literature,16–20,26 but a double well based strategy is yet to be explored further for different electrocatalytic and photoelectrocatalytic heterojunctions.
Herein, being a visible light driven photocatalyst, CdS is sandwiched in between Pt and graphene (Scheme 1) and photoexcited charge extraction capability is investigated in the Pt/CdS/3D-Gr heterojunction. The obtained Pt/CdS/3D-Gr@Ni-Foam electrocatalysts with a hierarchical architecture exhibit improved electrocatalytic and photoelectrocatalytic methanol oxidation reaction activity in an alkaline medium with a specific tailoring effect of the CdS loading on the sandwich. The structural, morphological, optical and electrochemical properties are explored to investigate the structure–activity relationships. Electrocatalytic methanol oxidation activity is observed in the Pt/CdS/3D-Gr@Ni-Foam heterostructure, and is boosted to more than 100% under illumination conditions. Tuning the CdS loading at the Pt/3D–graphene interfaces effectively increased the photoexcited charge separation efficiency as well as MOR activity. Noticeably, 10 SILAR cycles of CdS in the Pt/CdS/3D-Gr@Ni-Foam heterostructure showed the highest current density of 264 mA cm−2 at 0.28 V vs. Ag/AgCl under illumination in comparison to 116 mA cm−2 at 0.26 V vs. Ag/AgCl in the dark at a scan rate of 10 mV s−1. The diffusion coefficient of electron transfer is enhanced to 3.99 × 10−3 cm2 s−1 under light compared to 2.971 × 10−3 cm2 s−1 under dark conditions for the Pt/10-CdS/3D-Gr@Ni-Foam heterostructure. Furthermore, in-depth analysis is carried out using electrochemical impedance spectroscopy. It detailed that 10 SILAR cycles of CdS in the Pt/10-CdS/3D-Gr@Ni-Foam heterostructure optimized and improved the charge transportation at the electrode–electrolyte interface. The sensitizer loading (CdS) at the interfaces of both Pt-CdS and CdS-graphene with higher work function materials was optimized and the double-well strategy played a key role in promoting the activity of the methanol oxidation reaction. Unprecedented photocurrent density and photostability are observed in comparison to those of the previously reported work that makes this strategy a potential one. This strategy paved its ways to further explore different high work function materials and/or sensitizers to improve and explore the electrocatalytic and photoelectrocatalytic activities of hetero-structures for different applications.
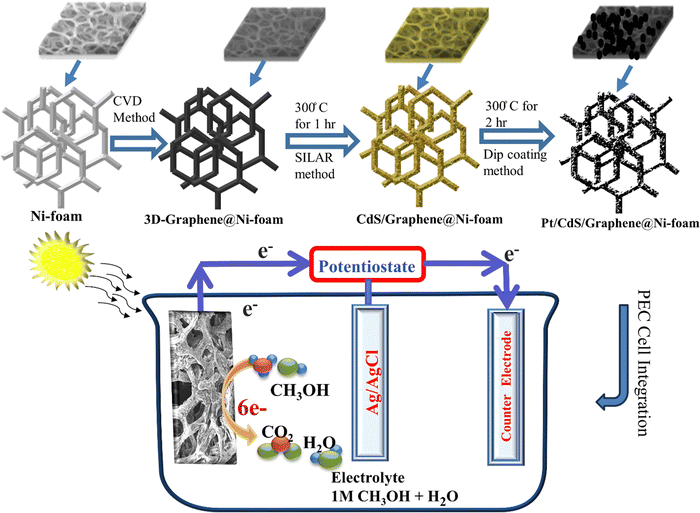 | ||
| Scheme 1 Schematic representation of Pt/CdS/3D-Gr@Ni-Foam preparation and its integration into PEC assembly for methanol oxidation. | ||
Materials and methods
Fabrication of 3D graphene
3D graphene is developed on Ni-foam via the chemical vapor deposition method. A 1-inch Ni-foam (Sigma Scientific) was placed in an air tight CVD tube furnace. A gaseous mixture of Ar (80 Sccm, 99.99%) and H2 (29 Sccm, 99.99%) is purged until atmospheric pressure is reached after the tube has been evacuated. The furnace is heated to a temperature of 850 °C with a ramping rate of 2 °C min−1 and kept there for ten minutes under H2 (10 Sccm) and Ar (40 Sccm) flow. Then, Ni foam is exposed to 40 sccm Ar, 5 sccm H2, and 5 Sccm acetylene (99.99%) for ten minutes at 850 °C. Then suddenly cooled down to 450 °C followed by further cooling to room temperature at 25 °C. During this process, a few graphene layers formed on the Ni-foam surface. The graphene–Ni composite was submerged in an aqueous solution containing 0.5 M Fe(NO3)3 at 80 °C for 20 hours in order to dissolve the Ni-substrate. Graphene foam is cleaned with deionized water. After that, isopropanol was used to rinse it, obtaining an ambient environment for characterization.27Synthesis of CdS on 3D-Gr@Ni-Foam
Deposition of cadmium sulfide on the 3D-Gr@Ni-Foam is carried out by the SILAR method as reported in our previous paper.28 Briefly, the 3D-Gr@Ni-Foam sample was placed in 0.05 M Cd(NO3)2 in a 70 mL aqueous solution for about 5 minutes. After this, the sample was rinsed with DI water and slowly dried at 50 °C on a hotplate. After this, the sample was placed in 0.05M Na2S in a 70 mL aqueous solution for 5 minutes. After this, it was rinsed with DI water and slowly dried on a hotplate at 80 °C temperature that completes one SILAR cycle, as shown in Scheme 1. Similarly, 5, 10, and 15 SILAR cycles were repeated to deposit respective CdS layers on the 3D-Gr@Ni-Foam. After the SILAR process is completed, samples were annealed in order to improve crystallization and to increase the electrical conductivity at 300 °C for 1 hour in an argon environment in a tube furnace. Samples were designated as 5-CdS/3D-Gr@Ni-Foam, 10-CdS/3D-Gr@Ni-Foam and 15-CdS/3D-Gr@Ni-Foam.Synthesis of platinum on CdS/3D-Gr@Ni-Foam
A dip coating technique was used for coating Pt over the CdS/3D-Gr@Ni-Foam. The CdS/3D-Gr@Ni-Foam was dipped in PtCl4 (0.178M in 5 mL of acetone) solution in a beaker for 5 min to adsorb Pt ions. After that, the sample was removed from the Pt solution, and the sample was dried over the hotplate at 70 °C. Furthermore, the sample is reduced in a hydrogen environment at 300 °C for 120 min with 100 Sccm constant flow of H2 gas. This step was repeated for all the samples with different SILAR layers of CdS in the CdS/3D-Gr@Ni-Foam and the samples are designated as Pt/5-CdS/3D-Gr@Ni-Foam, Pt/10-CdS/3D-Gr@Ni-Foam and Pt/15-CdS/3D-Gr@Ni-Foam.Material characterization
The X-ray diffraction (XRD) patterns of the catalysts are obtained using the Bruker D8 PXRD instrument. The hetero-structure morphology is observed using a SEM JSM6490 operated at acceleration voltages between 0.5 and 30 kV. A A Renishaw 2000 Raman spectrometer is used to obtain the Raman spectra. The electrochemical and photo-electrochemical studies were performed using potentiostat/galvanostat/ZRA (model; INTERFACE1000E-12095) in a three-electrode system with platinum (Pt) as the counter electrode, Ag/AgCl electrode as the reference electrode and the aforementioned photo-anodes as the working electrode (apparent area = 1 cm2) under a Halogen lamp as the light source with 100 mW cm−2, calibrated with a standard silicon solar cell. All electrochemical studies are done using 1 M KOH as a supporting electrolyte and 1 M CH3OH as a fuel. Cyclic voltammetry is performed at a potential range of −0.1 V to 0.5 V at different scan rates, including 1 mV s−1, 2 mV s−1, 5 mV s−1, 10 mV s−1, 20 mV s−1, 40 mV s−1, 60 mV s−1, 80 mV s−1 and 100 mV s−1, respectively.Results and discussion
Structural and morphological properties
The crystallinity of the pure Ni foam, Gr@Ni-Foam, CdS/3D-Gr@Ni-Foam and Pt/CdS/3D-Gr@Ni-Foam is investigated using XRD. The diffraction peaks at 2θ = 44.7°, 52.2°, and 76.6°, as displayed in Fig. 1A(a), are associated with the (111), (200), and (311) planes of the Ni foam (JCPDS no. 04-0850).29 The red curve, as seen in Fig. 1((A) and (B)(b)), has a peak from graphene at 26.3°, which is the graphite (002) diffraction peak. Diffraction peaks at 2θ = 24.8°, 26.1°, 28.1°, 43.6°, and 47.8°, for (100), (111), (101), (110), (103), and (112), respectively (JCPDS card # 892944)30 are observed in the XRD pattern of the synthesized CdS on Gr@Ni-Foam displayed in Fig. 1A(c). The XRD pattern fully represents the characteristics of composite materials, with the diffraction peaks around 26.1°and 26.4° attributed to C (002) and CdS (111), respectively, and at 44.7° and 52.2 attributed to Ni (111) (200) and CdS (110) (112), respectively. JCPDS 04-0802 indicates that the extra diffraction peaks are located at 40.1°, 46.85°, and 67.9° as shown in Fig. 1A(d), and are indexed to the metallic platinum crystal planes (111), (200), and (220), respectively.31 | ||
| Fig. 1 XRD patterns of (A) (a) Ni foam, (b) 3D-Gr@Ni-Foam, (c) CdS/3D-Gr@Ni-Foam, and (d) Pt/CdS/3D-Gr@Ni-Foam; (B) amplified from 23°–30° showing graphene peaks. | ||
The morphology of the electrodes is examined using SEM, revealing details as illustrated in Fig. 2. Specifically, Fig. 2(A) highlights a fascinating SEM image of graphene over the 3D Ni foam (NF) showcasing a smooth and porous 3-D framework. Furthermore, the SEM images in Fig. 2(B) and (C) at varying magnifications provide a glimpse of the 10-CdS/3D-graphene@Ni-Foam, showing a densely packed arrangement on the NF substrate. In Fig. 2(D) and (E), it is evident that Pt was uniformly deposited over CdS/3D-graphene@Ni-Foam as evidenced by charged surfaces throughout Fig. 2(D). This unique arrangement resembles a flowerbed, composed of numerous individual nanorods, thus significantly increasing the surface area and reaction sites.
 | ||
| Fig. 2 SEM micrographs of (A) 3D-Gr@Ni-Foam, (B) and (C) 10-CdS/3D-Gr@Ni-Foam and (D) and (E) Pt/10-CdS/3D-Gr@Ni-Foam. | ||
When used to characterize graphene, Raman spectroscopy can yield extensive insights into the nature of the material. The two distinct peaks at 1578 cm−1 (G-band) and 2702 cm−1 (2D-band) in the typical graphene Raman spectra, as illustrated in Fig. 3(A), indicate that few-layer graphene films make up the 3D graphene network. In the meantime, there is a substantial suppression of the D-band at 1344 cm−1, which is linked to the disordered defects of graphene. This suggests that the graphene network is of good quality and can offer charge carriers quick transport pathways.32 The highest value of ID/IG is calculated to be 0.18 for 3D-Gr@Ni-Foam, indicating the graphitic sp2 hybridized bonds with reduced defects in 3D-graphene.33 The existence of a well-developed 2D band at 2702 cm−1 indicates the 3D structure, while the value of I2D/IG is calculated to be 0.12, illustrating few layered graphene sheets over Ni foam. Fig. 3(A), also indicates that after deposition of CdS over 3D-Gr@Ni-Foam, characteristic bands of individual components of the heterostructure are evident, illustrating the successful development of the heterojunction of CdS with 3D-Gr@Ni-Foam. The interaction between CdS and the graphene substrate may slightly shift the peak position due to strain, size effects, or the bonding environment between CdS and the graphene surface. This peak indicates successful deposition of CdS on the graphene and the resultant changes in vibrational modes due to this interaction. The Raman spectrum of Pt/CdS/3D-Gr@Ni-Foam, shown in Fig. S1a (ESI†), does not display characteristic bands but instead exhibits enhanced background noise.34 Moreover, the UV-visible spectra of 3D-graphene@Ni-Foam and Pt/10-CdS/3D-Gr@Ni-Foam are obtained using a spectrophotometer of Agilent as shown in Fig. S1b (ESI†). It is observed that 3D-graphene@Ni-Foam shows poor absorbance, in other words, it shows maximum reflectance in the visible region of the solar spectrum. However, Pt/10-CdS/3D-Gr@Ni-Foam shows its absorbance edge nearly in the 560–600 nm range of the visible spectrum. The band gap is determined using Tauc plot and found to be around 2.4 eV for the CdS edge of Pt/10-CdS/3D-Gr@Ni-Foam, which is in good agreement with the literature.35
Electrochemical studies
A cyclic voltammetry study is performed to record the current voltage response for the methanol oxidation reaction (MOR). The photocurrent density is evaluated both under dark and light conditions. In Fig. 4A, the photocurrent densities of Pt/3D-Gr@Ni-Foam, Pt/5-CdS/3D-Gr@Ni-Foam, Pt/10-CdS/3D-Gr@Ni-Foam and Pt/15-CdS/3D-Gr@Ni-Foam are calculated to be 63, 71, 116, and 90 mA cm−2, respectively, under dark conditions at a voltage range of −1 to 0.2 V. Pt/10-CdS/3D-Gr@Ni-Foam showed the maximum current density for MOR activity under dark conditions which is 84% higher than the MOR activity of Pt/3D-Gr@Ni-Foam. The forward peaks, which are located between 0.07 and 0.13 V, are caused by the oxidation of methanol molecules. In contrast, the backward peaks, which are located between −0.29 and 0.26 V, are caused by the further electrooxidation of some intermediates generated in earlier scans.However, the photocurrent densities of Pt/3D-Gr@Ni-Foam, Pt/5-CdS/3D-Gr@Ni-Foam, Pt/10-CdS/3D-Gr@Ni-Foam and Pt/15-CdS/3D-Gr@Ni-Foam are calculated to be 112, 98, 265 and 211 mA cm−2, respectively, under light conditions at a voltage range of −1 to 0.2 V as shown in Fig. 4(B). Under dark and light illumination conditions, current density is the highest for Pt/10-CdS/3D-Gr@Ni-Foam. As shown in Fig. 4, maximum photocurrent density under light at voltage 0.28 V is observed to be 265 mA cm−2 and a high If/Ib ratio is due to the efficient charge separation, fast transportation and lower recombination rate. Pt/10-CdS/3D-Gr@Ni-Foam showed the maximum current density for MOR activity under illumination conditions which is 136% higher than the MOR activity of Pt/3D-Gr@Ni-Foam without CdS. Noticeably, Pt/15-CdS/3D-Gr@Ni-Foam showed lower photocurrent density for MOR activity under illumination conditions which is 25% lower than MOR activity of Pt/10-CdS/3D-Gr@Ni-Foam. This decrease is due to the diffusion restrictions of the photoexcited charges caused due to overloading of CdS at the interface of Pt/3D-Gr@Ni-Foam. Furthermore, it is observed that CdS is the major source of photoexcited charges that transferred on both the interfaces; Pt-CdS36 and CdS-graphene,37 whereby graphene and Pt acted as a double well on either side of CdS with higher work function than the work function of CdS. Also, the plasmonic effect of Pt also played an important role in enhancing the MOR activity.26 CdS being a visible light driven material with a band gap of 2.4 eV38 acts as a photocatalyst and plays a key role in enhancing the MOR activity as demonstrated in Fig. 4(B) in comparison to Fig. 4(A). A high If/Ib factor has been considered a crucial parameter for an effective MOR electrocatalyst. For the MOR reaction, it is important to know the ratio of the forward anodic peak current density (If) to the reverse anodic peak current density (Ib), or If/Ib. This ratio determines if the catalyst can withstand CO poisoning. A high If/Ib number is ideal because it shows that methanol is being converted to CO2 rapidly, which reduces the amount of leftover carbon species that build up on the catalyst and improves CO tolerance.39Table 1 lists the measured cyclic voltammetry parameters, including the If/Ib ratio under both light and dark conditions.
| Photoanodes | Dark | Light | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V f (V) | I f (mA) | V b (V) | I b (mA) | I f/Ib | V f (V) | I f (mA) | V b (V) | I b (mA) | I f/Ib | |
| Pt/3D-Gr@Ni-Foam | 0.12 | 62.79 | −0.25 | 20.16 | 3.11 | 0.26 | 122.3 | −0.19 | 58.44 | 2.09 |
| Pt/5-CdS/3D-Gr@Ni-Foam | 0.05 | 70.91 | −0.26 | 24.17 | 2.93 | 0.15 | 98.06 | −0.21 | 37.74 | 2.59 |
| Pt/10-CdS/3D-Gr@Ni-Foam | 0.26 | 116.5 | −0.25 | 25.06 | 4.64 | 0.28 | 264.4 | −0.13 | 62.4 | 4.23 |
| Pt/15-CdS/3D-Gr@Ni-Foam | 0.13 | 89.29 | −0.28 | 23.01 | 3.88 | 0.18 | 210.66 | −0.32 | 45.27 | 4.65 |
Fig. 4 shows the comparison of activity of Pt/10-CdS/3D-Gr@Ni-Foam and Pt/15-CdS/3D-Gr@Ni-Foam at a scan rate of 10 mV s−1 under dark (Fig. 4A) and light conditions (Fig. 4B). It could be seen that the oxidation of methanol is enhanced under light illumination than in the dark. At 10 mV s−1, the difference of current density between light and dark conditions of Pt/CdS/3D-Gr@Ni-Foam is relatively small, as seen in Fig. 4(C). This difference is increased further by the cycles of CdS because it enhances charge carrier separation at the interface of 3D-graphene and CdS and plasmon-induced charge carrier separation at the interface of CdS & Pt under light illumination. The most significant difference between light and dark peak current is shown by Pt/10-CdS/3D-Gr@Ni-Foam than Pt/15-CdS/3D-Gr@Ni-Foam. This could be due to the overloading of CdS, which blocks the active sites and reduces the active surface area.
Electrochemical studies are also carried out by using working electrodes Pt/3D-Gr@Ni-Foam and Pt/CdS/3D-Gr@Ni-Foam in 1 M KOH and then adding 1 M CH3OH at different scan rates i.e., 1 mV s−1, 2 mV s−1, 5 mV s−1, 10 mV s−1, 20 mV s−1, 40 mV s−1, 60 mV s−1, 80 mV s−1 and 100 mV s−1, respectively. The cyclic voltammogram of Pt/10-CdS/3D-Gr@Ni-Foam with different scan rates in 1 M methanol is shown in Fig. 5. When the scan rate increases from 1 mV s−1 to 100 mV s−1, the current density increases according to the reaction kinetics. Under dark conditions, the current density is low, i.e., 156.6 mA cm−2 at 0.24V (Fig. 5(A)) as compared to under light illumination where the current density is 367.7 mA cm−2 at 0.28 V which is higher (Fig. 5(B)). Under light illumination, the voltage shifts towards a greater value, which indicates it will show enhanced electrocatalytic activity.
The cyclic voltammograms of Pt/3D-Gr@Ni-Foam and Pt/CdS/3D-Gr@Ni-Foam (5 and 15 cycles) with different scan rates in 1 M methanol are shown in Fig. S2 in the ESI.† When the scan rate is increased, it refers to the speed at which the working electrode's potential is changing during a cyclic voltammetry experiment. This change in potential affects both the kinetics and mass transport processes, leading to an increase in current. The higher the rate of change of potential, the higher the charges that accumulate in a short time, so the current increases with the scan rate but mass transport causes the oxidation potential peaks to be observed at higher potentials than usual.
To determine whether the process is diffusion-controlled, capacitive controlled or a combination of both, the logarithmic value of current is plotted against the logarithmic value of the scan rate for all the photo-anodes using the following equations.40
| i = avb | (1) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i = b i = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + log v + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i shows a linear relationship with log v having a slope value (b value) of less than 0.5 which indicates that the process is diffusion-controlled41 for all the photo-anodes as shown in Fig. S3(A) (ESI†). The value of “b” in between 1 and 0.5 indicates diffusion and capacitive-controlled processes.41,42
i shows a linear relationship with log v having a slope value (b value) of less than 0.5 which indicates that the process is diffusion-controlled41 for all the photo-anodes as shown in Fig. S3(A) (ESI†). The value of “b” in between 1 and 0.5 indicates diffusion and capacitive-controlled processes.41,42
Gradual enhancement in current density with the increase in the scan rate indicates the obeying of the Randles–Sevcik circuit. A linear relationship between the square root of the scan rate and peak current densities of Pt/CdS/3D-Gr@Ni-Foam for 10 cycles is shown in Fig. 6(C), and for 5 and 15 cycles, it is is given in Fig. S3(B) of the ESI.† The plot indicates that it is a diffusion-controlled process. Furthermore, the diffusion-coefficient (D) has also been calculated by measuring the linearity and rate constant of the charge transfer on the electrode and surface deposited layer. The diffusion coefficient (D) is calculated using the Randles Sevcik equation,43
 | (3) |
| S. no | Photoanodes | Diffusion coefficient/ (cm2 s−1) | Tafel slope/ (mV dec−1) | |
|---|---|---|---|---|
| Dark | Light | |||
| 1 | Pt/3D-Gr@Ni-Foam (dark) | 2.969 × 10−3 | 833 | 670 |
| 2 | Pt/5-CdS/3D-Gr@Ni-Foam (dark) | 2.722 × 10−3 | 1101 | 633 |
| 3 | Pt/15-CdS/3D-Gr@Ni-Foam (dark) | 2.891 × 10−3 | 817 | 641 |
| 4 | Pt/10-CdS/3D-Gr@Ni-Foam (dark) | 2.271 × 10−3 | 728 | 570 |
Also, the Tafel slope of the Pt/10-CdS/3D-Gr@Ni-Foam under light is estimated to be 570 mV dec−1, which is lower than that of the Pt/10-CdS/3D-Gr@Ni-Foam under dark conditions (728 mV dec−1), as demonstrated in Fig. 5(D). The Tafel slope tells us about the kinetics of the electron transfer process.43 This trend suggests the enhanced MOR kinetics of the Pt/CdS/3D-Gr@Ni-Foam for 10 cycles under light conditions. The Tafel slope values of the Pt/CdS/3D-Gr@Ni-Foam for 5, 10, and 15 cycles are given in Table 2. Fig. S4 of the ESI† indicates the enhanced MOR kinetics of the Pt/CdS/3D-Gr@Ni-Foam for 5 and 15 cycles under light conditions as compared to dark.
The Nyquist plot is shown in Fig. 6(A) and (B) as a change of the imaginary component of impedance (Z′′) with respect to the real analogy (Z′). Electrochemical impedance spectroscopy (EIS) is a well-established technique routinely employed to support cyclic voltammetry to investigate the charge transfer mechanism and kinetics of catalysts in electrode–electrolyte interfaces. By capturing electrochemical reactions at varying frequencies, EIS offers valuable insights into these vital properties.44 Impedance spectroscopy analysis reveals that the a semicircle with a smaller diameter indicates a significantly lower charge transfer resistance, indicating a higher catalytic activity.45,46 Conversely, a semicircle with a larger diameter indicates the presence of CO adsorbates on the Pt surface, which hinder the continuous oxidation of methanol. There is a noticeable decrease in steady current and a larger semicircle is observed.47 The EIS Nyquist plot shows a large arc resistance semicircle for Pt/3D-Gr@Ni-Foam under dark and light conditions, as shown in Fig. 6 A(a) and B(a). The Nyquist plot shows a curve in the low-frequency region for Pt/x-CdS/3D-Gr@Ni-Foam (x = 5, 10, and 15 cycles) compared to the Pt/3D-Gr@Ni-Foam. By comparing the results, it is concluded that Pt/10-CdS/3D-Gr@Ni-Foam showed the least resistance to the transfer of charges across the circuit. The Pt/3D-Gr@Ni-Foam exhibits a significantly larger arc radius resistance compared to the Pt/x-CdS/3D-Gr@Ni-Foam, resulting in a more efficient charge transfer resistance (Rct) at the electrode–electrolyte interface (Fig. 6A and B). As a result, this reduction in the arc diameter signifies a noteworthy improvement in the photoelectrochemical activity of the coated materials.
The Pt/CdS/3D-Gr@Ni-Foam exhibited a significantly larger arc radius resistance in the absence of light (Fig. 6A) compared to its smaller resistance in the presence of light (Fig. 6B). This can be attributed to the decreased charge transfer resistance (Rct) at the electrode–electrolyte interface under illumination, enhancing photoelectrochemical performance. Consequently, the samples showed superior activity under light conditions, highlighting the importance of light influence in this system. Data for the charge transfer resistance from the photoelectrode (Rct) are derived from the Nyquist plots. Fig. 6(A) and (B) show that the arc decreases in the following order: Pt\Gr@Ni-Foam > Pt/5-CdS/3D-Gr@Ni-Foam > Pt/10-CdS/3D-Gr@Ni-Foam > Pt/15-CdS/3D-Gr@Ni-Foam and the estimated Rct values are given in Table 2. The decreased Rct value suggests that heterojunctions facilitate charge transfer and separation and change the kinetics of the electron transfer reaction with loading cycles. Fig. 6(C) and (D) show the Bode phase plot of the prepared samples under dark and light conditions. τe (lifetime) for recombination can be calculated according to the equation,48
| τe = 1/(2πfmax) | (4) |
| Photoanodes | R ct (Ω) | Frequency (Hz) | τ e (ms) | ||
|---|---|---|---|---|---|
| EIS | EIS at OCP | ||||
| Dark | Pt/3D-Gr@Ni-Foam | 255.9 | 240.3 | 0.5 | 318.5 |
| Pt/5-CdS/3D-Gr@Ni-Foam | 22.42 | 230.54 | 0.622 | 256 | |
| Pt/10-CdS/3D-Gr@Ni-Foam | 2.602 | 17.71 | 12.4 | 12.8 | |
| Pt/15-CdS/3D-Gr@Ni-Foam | 2.77 | 46.23 | 5.0 | 31.8 | |
| Light | Pt/3D-Gr@Ni-Foam | 34.31 | 273.2 | 1.27 | 125.4 |
| Pt/5-CdS/3D-Gr@Ni-Foam | 3.261 | 47.5 | 3.15 | 50.5 | |
| Pt/10-CdS/3D-Gr@Ni-Foam | 2.385 | 7.525 | 19.86 | 8.0 | |
| Pt/15-CdS/3D-Gr@Ni-Foam | 3.163 | 22.3 | 13.92 | 11.4 | |
EIS open current potential also supports the results of cyclic voltammetry and EIS. Fig. S5(A) and (B) (ESI†) demonstrates the EIS OCP. The Nyquist plot with the real analogy (Z′) is represented as a variation of the imaginary part of impedance (Z′′). The EIS OCP Nyquist plot exhibits a large arc resistance semicircle for Pt/3D-Gr@Ni-Foam under dark and light conditions as shown in Fig. S5(A) and (B). The Nyquist plot shows a curve in the low-frequency area for Pt/x-CdS/3D-Gr@Ni-Foam (x = 5, 10, and 15 cycles) compared to the Pt/3D-Gr@Ni-Foam. By comparing the results, it can be concluded that Pt/10-CdS/3D-Gr@Ni-Foam showed the least resistance to the transfer of charges.
To find the photoelectrochemical stability of Pt/3D-Gr@Ni-Foam and Pt/CdS/3D-Gr@Ni-Foam, the chronoamperometric V–t curve is plotted. The test is conducted for a total of 3600 seconds. Every material experiences an initial decrease in current density, which is a well-established phenomenon. However, the current material can maintain the current density for an extended length of time. The reduction in current density is largely attributed to the rapid decline of catalytic activity, caused by the discharge of the double-layer and the accumulation of trapped and chemically inert intermediate compounds on the reactive sites. This laboratory finding further supports the assumption that platinum poisoning contributes to the decrease in current density.49 Initially, the pores and active sites are open, but as the reaction proceeds, the photoelectrode takes up the space and limits the reaction kinetics. In Fig. S6(A) (ESI†), an increase in the photostability is observed for the Pt/CdS/3D-Gr@Ni-Foam electrode compared to the Pt/3D-Gr@Ni-Foam. Pt/10-CdS/3D-Gr@Ni-Foam shows maximum photostability, however, Pt/15-CdS/3D-Gr@Ni-Foam exhibits a decreased photostability as compared to Pt/10-CdS/3D-Gr@Ni-Foam implying an efficient separation and migration for the photogenerated charge carrier under light irradiation.50,51 The non-sandwich Pt/3D-graphene showed prominent CO poisoning, while 5CdS loading cycles in the sandwich reduced the CO poisoning as the photoexcited electrons of CdS started transferring to Pt surface sites and boosted the methanol oxidation reaction (Fig. S6-A, ESI†). Furthermore, CdS loading cycles for achieving 10 layers enhanced the photoexcited charge separation and facilitated the oxidation of methanol by releasing CO present on the Pt sites by providing photogenerated electrons to Pt. Hence, CdS acts as a photocatalyst to accelerate the methanol oxidation reaction occurring on the surface of Pt, and therefore the sandwich of Pt/10-CdS/3D-Gr@Ni-Foam showed excellent photostabilty over the investigated time interval due to regeneration of Pt active sites. On the other hand, 15 layers of CdS in the Pt/3D-graphene sandwich may create imbalance of the photogenerated charges during their separation and transportation on either side of the interface formed by the double well based strategy due to two reasons; firstly, CdS overloading causes sufficient electron–hole pair recombination,52 secondly poor transportation of photoexcited charges to Pt active sites due to poor diffusion of electrons, hence, Pt/15-CdS/3D-Gr@Ni-Foam showed reduced photostability as well as the current density in comparison to the Pt/10-CdS/3D-Gr@Ni-Foam double well based photocatalyst. The efficient transfer of CdS charges on either side of its interface provides a potential map to explore other semiconductors for the sandwich based double well strategy.
Mott–Schottky plots of Pt/3D-Gr@Ni-Foam and Pt/x-CdS/3D-Gr@Ni-Foam (x = 5, 10, and 15) are obtained to determine the position of the conduction band potentials in order to understand the underlying reaction mechanism of the Pt/CdS/3D-Gr@Ni-Foam photoanodes. The flat-band potential (Vfb) and carrier concentration (ND) of the Pt/3D-Gr@Ni-Foam and Pt/x-CdS/3D-Gr@Ni-Foam are obtained (Table 4 and Fig. S6(B), ESI†) from the X-axis intercept and the gradient of the linear fit of the Mott–Schottky equation used.26
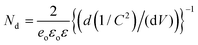 | (5) |
| Photoanodes | Intercept | Slope | Donor density | V fb |
|---|---|---|---|---|
| Pt/Gr@Ni-Foam | 5.123 × 1011 | 4.069 × 1011 | 1.5 × 1020 | −0.47 |
| Pt/5-CdS/Gr@Ni-Foam | 1.251 × 1012 | 1.642 × 1011 | 1.6 × 1020 | −0.85 |
| Pt/10-CdS/Gr@Ni-Foam | 6.199 × 1010 | 9.599 × 1010 | 2.5 × 1020 | −1.41 |
| Pt/15-CdS/Gr@Ni-Foam | 9.616 × 1011 | 3.845 × 1011 | 6.3 × 1019 | 3.78 |
Pt/3D-Gr@Ni-Foam possessed the lowest carrier concentration and flat-band potential, according to the computed results. Given in Table 4, increased net donor density (ND) in the Pt/CdS/3D-Gr@Ni-Foam can be attributed to the current density's improvement. Among all the coating cycles of CdS, Pt/10-CdS/3D-Gr@Ni-Foam showed the highest negative flat-band voltage (Vfb). The development of donor levels, which effectively shift the relative energy band locations and promote band bending, may cause the increase in Vfb resulting from adding CdS.54 The various Vfb values observed provide evidence for the occurrence of different relative conduction band levels in the grown Pt/10-CdS/3D-Gr@Ni-Foam, hence indicating the need for additional research into their energy band structures.
Pt-based anodes are widely utilized because of their vital activity in the electrocatalytic methanol oxidation process. However, the reaction intermediates also negatively impact the high cost and availability of Pt sites.55 Increased band bending is caused by the creation of donor levels, which effectively alter the relative energy band locations when CdS is coated on graphene and have a well-known band gap of 2.4 eV.56 A proposed mechanism of charge transfer across the interface of graphene, CdS, and Pt is illustrated in Fig. 7. The electron energy level on the conduction band of CdS is substantially greater than that of graphene, indicating that the process of moving electrons from the conduction band of CdS to graphene is energetically advantageous.35 When exposed to light, CdS can efficiently absorb the incident light and create pairs of photogenerated charges, or electrons (e−) and holes (h+). A photo-current is produced when the photo-generated electrons are quickly caught and transferred from excited CdS to the (i) counter electrode Pt because of graphene's improved electrical conduction and (ii) surface plasmonic metal platinum that effectively undergoes methanol oxidation. Hydrogen gases are produced at the Pt electrode when the hydrogen ion is reduced by photogenerated electrons. Current increases as a result of photogenerated electrons undergoing methanol oxidation as well as photogenerated holes. More precisely, strong oxidized holes can react directly with OH−/H2O adsorbed on the surface to produce strong oxidative hydroxyl radicals (˙OH). ˙OH radicals have the ability to oxidize the adsorbed methanol molecules on the surface of Pt/CdS/3D-Gr@Ni-Foam, resulting in the photooxidation processes. Additionally, extremely active ˙OH radicals oxidize the carbon-containing intermediates, which effectively suppresses poisoning and results in a backward anodic current peak in cyclic voltammetry. CdS loading provides increased absorption of visible light, increases charge separation at the interfaces, and increases the electron density on Pt NPs, which enhances the methanol oxidation due to the surface plasmon effect.57 Photoexcited charge separation and transportation from CdS to graphene and CdS to Pt provide maximum absorption, excellent separation and fastest transportation at the respective interfaces of the Pt/10-CdS/3D-Gr heterojunction and hence facilitate the double well strategy.
 | ||
| Fig. 7 The proposed mechanism of the charge transfer and enhanced methanol oxidation activity over Pt/10-CdS/3D-Gr@Ni-Foam. | ||
Conclusion
This study focuses on improving the efficiency of methanol oxidation by sandwiching CdS successfully in Pt/3D-Gr@Ni-Foam and monitoring its loading effect. When utilized as an anode in an electrochemical cell, the catalyst formed after 10 cycles of CdS loading in Pt/3D-Gr@Ni-Foam exhibited exceptional performance with both the highest current density and maximum negative onset potential. Specifically, the current density reached 116 mA cm−2 at 0.26 V vs. Ag/AgCl in the absence of light and further increased to 264 mA cm−2 at 0.28 V vs. Ag/AgCl under illumination for Pt/10-CdS/3D-Gr@Ni-Foam. The sandwich and non-sandwich-based catalysts showed prominent CO-poisoning, as evidenced by decreased current densities in the chronoamperometric results, and optimizing the loading cycles of CdS in Pt/3D-Gr@Ni-Foam could enhance catalytic activity by strengthening charge transfer and separation, thus reducing CO accumulation on the active sites. The diffusion coefficient of electron transfer is found to be enhanced to 3.99 × 10−3 cm2 s−1 under light in comparison to 2.971 × 10−3 cm2 s−1 under dark conditions for Pt/10-CdS/3D-Gr@Ni-Foam photoanodes compared to all other electrocatalysts and photoelectrocatalysts under investigation. Also, the high Tafel slope value of 833 mV dec−1 for Pt/3D-Gr@Ni-Foam dropped dramatically to 570 mV dec−1 for Pt/10-CdS/3D-Gr@Ni-Foam. The Pt/10-CdS/3D-Gr@Ni-Foam exhibited a significantly improved net donor density (ND) of 2.5 × 1020, surpassing the 1.5 × 1020 donor density of Pt/3D-Gr@Ni-Foam that increases density on Pt. The Pt/10-CdS/3D-Gr@Ni-Foam exhibited a significantly smaller charge carrier lifetime of 8.0 ms, surpassing the 318.5 ms lifetime of the Pt/3D-Gr@Ni-Foam. This lifetime resulted in improved photoexcited charge separation and collection efficiency, ultimately leading to enhanced photoelectrocatalytic performance in the methanol oxidation reaction (MOR). Additionally, the decrease in the charge transfer resistance (Rct) indicated that the introduction of CdS enhanced the charge transfer and separation processes, as well as improved the kinetics of the electron transfer reaction. This effect is further magnified with an increase in the loading cycles of CdS from 5 to 15 in the Pt/3D-Gr@Ni-Foam, demonstrating the significant role of CdS in promoting efficient charge transfer and separation, and Pt/3D-Gr@Ni-Foam can be used as an electrocatalyst. Furthermore, under illumination conditions, this groundbreaking study presents a novel approach for constructing double-well based heterostructures, offering a robust mechanism for efficient methanol oxidation.Author contributions
Habib Ullah: curation of data and writing first draft, Muqaddas Fatima Mumtaz: curation of data and writing first draft, Asad Mumtaz: conceptualization, methodology, validation, supervision, software, editing and review, funding resources and administration, Hina Sajid: curation of data and writing first draft, validation and analysis, Sani Zahra: curation of data and writing first draft, validation and analysis, Sabahat Sardar: methodology, validation, editing and review and analysis, Uzma Naz: methodology, validation, editing and review, Qamir Ullah Niazi: methodology, validation, editing and review, Shahid Iqbal: methodology, validation, editing and review, software, and administration, Syed Farooq Adil: methodology, validation, editing and review, Mohammad Rafe Hatshan: methodology, validation, funding resources, editing and review, Mujeeb Khan: methodology, validation, editing and review, Jaweria Ambreen: validation, editing and review, Muhammad Imran Irshad: methodology, validation, editing and review, and Muhammad Ahmad: methodology, editing and review.Data availability
The experimental data including tables and figures are available with the authors and would be provided if required.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the internal funding provided by the Department of Chemistry, School of Natural Sciences, National University of Sciences and Technology, Islamabad, Pakistan. The authors also acknowledge the funding from the Researchers Supporting Project number (RSP2024R222), King Saud University, Riyadh, Saudi Arabia.References
- K. A. D. P. Baruah, Electrochemically active site-rich nanocomposites of two-dimensional materials as anode catalysts for direct oxidation fuel cells: new age beyond graphene, Nanoscale Adv., 2021, 3(13), 3681–3707 RSC
.
- A. Shabbir, S. Sardar and A. Mumtaz, Mechanistic Investigations of Emerging Type-II, Z-scheme and S-scheme Heterojunctions for Photocatalytic Applications-A Review, J. Alloys Compd., 2024, 175683 CrossRef CAS
.
- P. N. Pattanayak P, P. Kumar and P. P. Kundu, Fabrication of cost-effective non noble metal supported on conducting polymer composite such as copper/poly pyrrole graphene oxide (Cu2O/PPy GO) as an anode catalyst for methanol oxida tion in DMFC, Int. J. Hydrogen Energy, 2018, 43, 11505–11519 Search PubMed
.
- L. X. Lei H, C. Sun, J. Zeng, S. S. Siwal and Q. Zhang, Galvanic replacement-mediated syn thesis of Ni-supported Pd nanoparticles with strong metal-support interaction for methanol electro-oxidation, Small, 2019, 15, 1804722 CrossRef
.
- A. Ali and P. K. Shen, Recent advances in graphene-based platinum and palladium electrocatalysts for the methanol oxidation reaction, J. Mater. Chem. A, 2019, 7(39), 22189–22217 Search PubMed
.
- S. Cui, S. Mao, G. Lu and J. Chen, Graphene coupled with nanocrystals: opportunities and challenges for energy and sensing applications, J. Phys. Chem. Lett., 2013, 4(15), 2441–2454 Search PubMed
.
- K. Tarantseva, K. Tarantsev and E. Polyanskova, Catalytic activity and stability of ethanol oxidation catalysts based on transition metals and transition metal spinels in alkaline ethanol media, Chem. Petrol. Eng., 2024, 1–7 Search PubMed
.
- K. Baruah,
et al., A versatile non-precious metal based electrode material endowed by layer-on-layer structure for methanol oxidation and supercapacitor applications, J. Energy Storage, 2024, 84, 110867 Search PubMed
.
- A. K. Pradhan, S. Halder and C. Chakraborty, Metal–organic framework derived synergistic carbon nanoarchitectures boost bifunctional electrocatalytic performances toward methanol oxidation and oxygen reduction in Pt-nanoparticles, Surf. Interfaces, 2024, 44, 103816 Search PubMed
.
- Y. Wu, J. Zhu and L. Huang, A review of three -dimensional graphene -based materials: Synthesis and applications to energy conversion/storage and environment, Carbon, 2019, 143, 610–640 Search PubMed
.
- H. Chen, C. K. Hsieh, Y. Yang, X. Y. Liu, C. H. Lin, C. H. Tsai, Z. Q. Wen, F. Dong and Y. X. Zhang, Hierarchical Nickel Cobaltate/Manganese Dioxide Core -Shell Nanowire Arrays on Graphene -Decorated Nickel Foam for High -Performance Supercapacitors, ChemElectroChem, 2017, 4, 2414–2422 CrossRef CAS
.
- M. Yu, J. Chen, J. Liu, S. Li, Y. Ma, J. Zhang and J. An, Mesoporous NiCo2O4 nanoneedles grown on 3D graphene -nickel foam for supercapacitor and methanol electro -oxidation, Electrochim. Acta, 2015, 151, 99–108 CrossRef CAS
.
- H. Sun, Y. Liu, Y. Yu, M. Ahmad, D. Nan and J. Zhu, Mesoporous Co3O4 nanosheets -3D graphene networks hybrid materials for high-performance lithium ion batteries, Electrochim. Acta, 2014, 118, 1–9 CrossRef CAS
.
- L. Chang,
et al., A review of methanol photoreforming: elucidating the mechanisms, photocatalysts and recent advancement strategies, Mater. Today Chem., 2023, 27, 101334 CrossRef CAS
.
- N. Alam,
et al., Efficient charge separation and transportation using 1D iron-sulfide@ titania heterojunctions as photoanodes for improved interface stability and photoelectrochemical activity to produce hydrogen, New J. Chem., 2024, 48(9), 3998–4008 RSC
.
- F. Wang,
et al., Tunable Pt-NiO interaction-induced efficient electrocatalytic water oxidation and methanol oxidation, Chem. Sci., 2024, 15(26), 10172–10181 RSC
.
- F. Fathirad, Ni and Fe mixed oxide anchored on CNTs, GONRs, and Ti3C2 Mxene as efficient nanocatalysts for direct power generation from methanol electro-oxidation, Mater. Chem. Phys., 2023, 305, 127976 CrossRef CAS
.
- N. Narayanan,
et al., Spinel Nickel–Cobalt Oxide Nanorods Supported on Ti3C2T x-MXene Nanosheets as Electrocatalysts for the Methanol Oxidation Reaction via a CO-Free Pathway, ACS Appl. Nano Mater., 2024, 7(4), 3907–3917 Search PubMed
.
- M. M. Mohamed, M. Khairy and S. Eid, Phosphorous, boron and sulphur-doped silver tungstate-based nanomaterials toward electrochemical methanol oxidation and water splitting energy applications, Int. J. Hydrogen Energy, 2024, 50, 1232–1245 Search PubMed
.
- I. Ahmed and S. H. Jhung, Catalytic oxidation reactions for environmental remediation with transition metal nitride nanoparticles, J. Environ. Chem. Eng., 2024, 112907 CrossRef CAS
.
- S. Sheikhi and F. Jalali, Copper selenide-Porous carbon derived from metal-organic frameworks as an efficient electrocatalyst for methanol oxidation, Int. J. Hydrogen Energy, 2024, 55, 864–874 CrossRef CAS
.
- S. Saipanya,
et al., Photo-enhanced electrocatalytic methanol oxidation of carbon black-modified Pt and Mo-based oxide for acidic and basic solutions, Materialia, 2024, 35, 102120 CrossRef CAS
.
- C.-K. Cheng,
et al., The hybrid nanostructure of vertically aligned cobalt sulfide nanoneedles on three-dimensional graphene decorated nickel foam for high performance methanol oxidation, Surf. Coat. Technol., 2017, 320, 536–541 CrossRef CAS
.
- K.-H. Luo,
et al., Highly-porous hierarchically microstructure of graphene-decorated nickel foam supported two-dimensional quadrilateral shapes of cobalt sulfide nanosheets as efficient electrode for methanol oxidation, Surf. Coat. Technol., 2020, 393, 125850 CrossRef CAS
.
- H. Yang,
et al., A high-efficiency noble metal-free electrocatalyst of cobalt-iron layer double hydroxides nanorods coupled with graphene oxides grown on a nickel foam towards methanol electrooxidation, J. Taiwan Inst. Chem. Eng., 2020, 112, 212–221 CrossRef CAS
.
- H. Sajid,
et al., Efficient CO release from plasmonic Pt using 1D BiVO 4@ TiO 2 heterojunctions for improved electrocatalytic and photoelectrocatalytic methanol oxidation, New J. Chem., 2024, 48(8), 3551–3562 RSC
.
- A. C. Sotolongo, M. M. Messina, F. J. Ibañez and R. G. Wuilloud, Hybrid ionic liquid-3D graphene–Ni foam for on-line preconcentration and separation of Hg species in water with atomic fluorescence spectrometry detection, Talanta, 2020, 210, 120614 CrossRef CAS PubMed
.
- A. Mumtaz,
et al., Mutual effect of extrinsic defects and electronic carbon traps of M-TiO2 (M = V, Co, Ni) nanorod arrays on photoexcited charge extraction of CdS for superior photoelectrochemical activity of hydrogen production, Int. J. Hydrogen Energy, 2018, 43(31), 14388–14405 CrossRef CAS
.
- X. Hu, X. Tian, Y. W. Lin and Z. Wang, Nickel foam and stainless steel mesh as electrocatalysts for hydrogen evolution reaction, oxygen evolution reaction and overall water splitting in alkaline media, RSC Adv., 2019, 9(54), 31563–31571 RSC
.
- P. R. Nikam, P. K. Baviskar, J. V. Sali, K. V. Gurav, J. H. Kim and B. R. Sankapal, CdS surface encapsulated ZnO nanorods: synthesis to solar cell application, J. Alloys Compd., 2016, 689, 394–400 CrossRef CAS
.
- Y. Li, L. Tang and J. Li, Preparation and electrochemical performance for methanol oxidation of Pt/graphene nanocomposites, Electrochem. Commun., 2009, 11(4), 846–849 CrossRef CAS
.
- J. Nong, D. Liu, W. Wei, C. Li, J. Yang, T. Sun, L. Yu, H. Shi, C. Du and D. Wei, CdS nanowire-modified 3D graphene foam for high-performance photo-electrochemical anode, J. Alloys Compd., 2016, 688, 37–43 CrossRef CAS
.
- X. Guo, H. F. Xiang, T. P. Zhou, X. K. Ju and Y. C. Wu, Morphologies and structures of carbon coated on Li4Ti5O12 and their effects on lithium storage performance, Electrochim. Acta, 2014, 130, 470–476 CrossRef CAS
.
- M.-H. Park,
et al., Mechanism of Pt loading on multi-walled carbon nanotubes, J. Nanosci. Nanotechnol., 2011, 11(7), 6293–6297 CrossRef CAS PubMed
.
-
G. W. Kumarage, et al., Enhancing the Photovoltaic Performance of Cd (1− x) ZnxS Thin Films Using Seed Assistance and EDTA Treatment. in Micro. 2023. MDPI Search PubMed
.
- Z. Meng,
et al., Dynamics of electron transfer in CdS photocatalysts decorated with various noble metals, Small, 2024, 20(21), 2308952 CrossRef CAS
.
- P. Wu,
et al., Precise Engineering of the electrocatalytic activity of FeN4-embedded graphene on oxygen electrode reactions by attaching electrides, J. Phys. Chem. Lett., 2024, 15(4), 1121–1129 CrossRef CAS
.
- P. Tan,
et al., CdS QDs decorated on 3D flower-like Sn 3 O 4: a hierarchical photocatalyst with boosted charge separation for hydrogen production, New J. Chem., 2024, 48(1), 300–308 Search PubMed
.
- Z. Zhang,
et al., Methanol electrooxidation with platinum decorated hematene Nanosheet, J. Electrochem. Soc., 2019, 166(4), H135 Search PubMed
.
- J. Liu, J. Wang, C. Xu, H. Jiang, C. Li, L. Zhang, J. Lin and Z. X. Shen, Advanced energy storage devices: basic principles, analytical methods, and rational materials design, Adv. Sci., 2018, 5(1), 1700322 Search PubMed
.
- T. Kim,
et al., Applications of Voltammetry in Lithium Ion Battery Research, J. Electrochem. Sci. Technol., 2020, 11(1), 14–25 Search PubMed
.
- K. Mielech-Łukasiewicz, H. Puzanowska-Tarasiewicz and A. Panuszko, Electrochemical Oxidation of Phenothiazine Derivatives at Glassy Carbon Electrodes and Their Differential Pulse and Square-wave Voltammetric Determination in Pharmaceuticals, Anal. Lett., 2008, 41(5), 789–805 Search PubMed
.
- K. S. Bhavani, T. Anusha and P. K. Brahman, Fabrication and characterization of gold nanoparticles and fullerene-C60 nanocomposite film at glassy carbon electrode as potential electro-catalyst towards the methanol oxidation, Int. J. Hydrogen Energy, 2019, 44(47), 25863–25873 Search PubMed
.
- C. Qin,
et al., The in situ etching assisted synthesis of Pt–Fe–Mn ternary alloys with high-index facets as efficient catalysts for electro-oxidation reactions, Nanoscale, 2019, 11(18), 9061–9075 Search PubMed
.
- D. Y. Chung, K.-J. Lee and Y.-E. Sung, Methanol electro-oxidation on the Pt surface: revisiting the cyclic voltammetry interpretation. The, J. Phys. Chem. C, 2016, 120(17), 9028–9035 Search PubMed
.
- M. Adachi,
et al., Determination of parameters of electron transport in dye-sensitized solar cells using electrochemical impedance spectroscopy, J. Phys. Chem. B, 2006, 110(28), 13872–13880 Search PubMed
.
- C. Zhai,
et al., Enhanced photoelectrocatalytic performance of titanium dioxide/carbon cloth based photoelectrodes by graphene modification under visible-light irradiation, J. Hazard. Mater., 2013, 263, 291–298 CrossRef CAS PubMed
.
- H. A. Gasteiger,
et al., Temperature-Dependent Methanol Electro-Oxidation on Well-Characterized Pt–Ru Alloys, J. Electrochem. Soc., 1994, 141(7), 1795 CrossRef CAS
.
- Y. Zhao,
et al., Super-long aligned TiO2/carbon nanotube arrays, Nanotechnology, 2010, 21(50), 505702 Search PubMed
.
- H. A. Altaleb, A. Salah and B. M. Thamer, Hydrogel Nanocomposite-Derived Nickel Nanoparticles/Porous Carbon Frameworks as Non-Precious and Effective Electrocatalysts for Methanol Oxidation, Gels, 2022, 8(9), 542 Search PubMed
.
- L. Mao,
et al., Supercritical CH3OH-Triggered Isotype Heterojunction and Groups in g-C3N4 for Enhanced Photocatalytic H2 Evolution, ACS nano, 2024, 18(21), 13939–13949 Search PubMed
.
- L. Mao,
et al., Simultaneous bulk and surface modifications of g-C3N4 via supercritical CO2-assisted post-treatment towards enhanced photocatalytic activity, Appl. Catal., B, 2024, 124712 Search PubMed
.
- S. Kang,
et al., 2D semiconducting materials for electronic and optoelectronic applications: potential and challenge, 2D Mater., 2020, 7(2), 022003 Search PubMed
.
- R. K. Kumarasinghe,
et al., A comparative study on CdS film formation under variable and steady bath-temperature conditions, Semiconductors, 2020, 54, 838–843 Search PubMed
.
- N. Zhang,
et al., Assembly of CdS nanoparticles on the two-dimensional graphene scaffold as visible-light-driven photocatalyst for selective organic transformation under ambient conditions, J. Phys. Chem. C, 2011, 115(47), 23501–23511 CrossRef CAS
.
- M. H. de Sá,
et al., Recent Advances in the Development of Nanocatalysts for Direct Methanol Fuel Cells, Energies, 2022, 15(17), 6335 CrossRef
.
- J. Nong,
et al., CdS nanowire-modified 3D graphene foam for high-performance photo-electrochemical anode, J. Alloys Compd., 2016, 688, 37–43 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nj04462b |
| ‡ First authorship. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |




