A reflection on ‘A new strategy for developing superior electrode materials for advanced batteries: using a positive cycling trend to compensate the negative one to achieve ultralong cycling stability’
Hong Han
Choo
 ,
Dan-Yang
Wang
,
Dan-Yang
Wang
 and
Qingyu
Yan
and
Qingyu
Yan
 *
*
School of Material Science and Engineering, Nanyang Technological University, 639798 Singapore, Singapore. E-mail: alexyan@ntu.edu.sg
First published on 25th June 2025
Abstract
The development of durable, high-capacity electrode materials remains a critical challenge in battery research. At the time of our study (2015–2016), conventional anodes often exhibited a trade-off between high initial capacity and poor cycling stability. Our Nanoscale Horizons article in 2016 (D.-H. Liu, H.-Y. Lü, X.-L. Wu, J. Wang, X. Yan, J.-P. Zhang, H. Geng, Y. Zhang and Q. Yan, Nanoscale Horiz., 2016, 1(6), 496–501, https://doi.org/10.1039/C6NH00150E) discovered that manganese oxide (MnO), when embedded in a conductive matrix, unexpectedly demonstrated capacity enhancement with repeated cycling. By integrating this with volume-expanding silicon into a hierarchical core–shell Si@MnO structure, encased in reduced graphene oxide, we achieved long-term cycling stability with good capacities and high current densities. This breakthrough introduced a new paradigm in composite electrode design. Our work not only led to extensive citations across sodium- and zinc-ion battery systems but also influenced further studies on materials like FeS–ZnS, VOx, and SnPS3. Reflecting on this journey, we recognize that modern in situ/operando techniques and AI-guided material screening could have further accelerated optimization. Today, as multivalent and beyond-Li battery technologies advance, the foundational ideas of dynamic capacity pairing and structural synergy continue to inform next-generation electrode innovation.
1. Introduction
The continuing growth of rechargeable battery technology is essentially tied to the development of electrode materials capable of providing high energy density, robust cycle stability, and favourable rate capability. Over the last two decades, lithium-ion batteries (LIBs) have emerged as the dominant energy storage technology, powering a diverse range of consumer gadgets, electric vehicles, and grid-scale energy systems. This success is largely due to their great energy density and efficiency.1 However, as the global demand for longer-lasting and higher-performance batteries grows, substantial constraints in the stability and longevity of LIB electrode materials have come to light.2 The problem of creating high-capacity anode materials that can preserve structural integrity and electrochemical performance over long periods of cycling is particularly difficult. Silicon (Si), an archetypal high-capacity anode material, has received significant interest due to its remarkable theoretical specific capacity (∼4200 mA h g−1), which much exceeds that of common graphite (∼372 mA h g−1).3 However, Si experiences substantial volume increases (up to 300%) during lithiation and delithiation, resulting in severe particle fracture, electrode pulverisation, and unstable solid–electrolyte interphase (SEI) production.2,4 These concerns emerge as low cycling stability and quick capacity fading, making pure Si unsuitable for commercial use without considerable structural modifications (https://doi.org/10.1039/C6NH00150E).5By 2015–2016, the most common solutions for addressing these challenges were nano-structuring, composite design, and surface coating, all of which attempted to reduce volume expansion while increasing electronic and ionic conductivity. Despite modest gains, many of these techniques failed to overcome the inherent trade-off between initial capacity and long-term cycling stability. Most high-capacity materials showed rapid capacity decline, whereas more stable materials usually offered insufficient capacity. This duality spurred a fundamental rethinking of how to design composite electrodes with more dynamic, synergistic performance characteristics. During this period, our group became particularly interested in the underexplored potential of materials that exhibit capacity enhancement over cycling, a phenomenon observed sporadically in certain transition metal oxides and amorphous materials, often attributed to electrochemical activation, structural rearrangement, or improved wettability. Specifically, manganese oxide (MnO), though commonly dismissed due to poor intrinsic conductivity and structural instability, was found to exhibit a positive capacity evolution when embedded in a conductive matrix.6 This counterintuitive behaviour led us to propose a new conceptual framework for electrode design: the positive cycling trend strategy. This method entails integrating two functionally complementary materials, the first one that normally exhibits a decreasing capacity trend (e.g., Si) and another that exhibits a rising or stabilising capacity trend (e.g., MnO) in a single electrode layout. We hypothesised that the dynamic compensation between these two materials would stabilise electrode performance, resulting in increased longevity without losing energy density. To test this theory, we created a hierarchical core–shell structure in which Si nanoparticles were placed within a MnO shell and then enclosed in a conductive carbon/reduced graphene oxide (rGO) matrix to improve electrical connectivity and buffer mechanical stress.7,8 The Si@MnO@C-rGO composite outperformed individual components and conventional composites with a reversible capacity of 800–820 mA h g−1 over 1500 cycles at high current densities. Beyond its empirical success, the positive cycling trend method signified a conceptual breakthrough in how cycling behaviour may be used constructively rather than just mitigated. It established a new paradigm in the logical design of composite electrodes, motivating further research on alternative high-capacity materials and hybrid designs. Our work has been mentioned in studies on FeS–ZnS, VOx, and SnPS3 materials, as well as in alternative batteries with sodium-ion and zinc-ion battery chemistries, highlighting its broad applicability and influence (Fig. 1).5
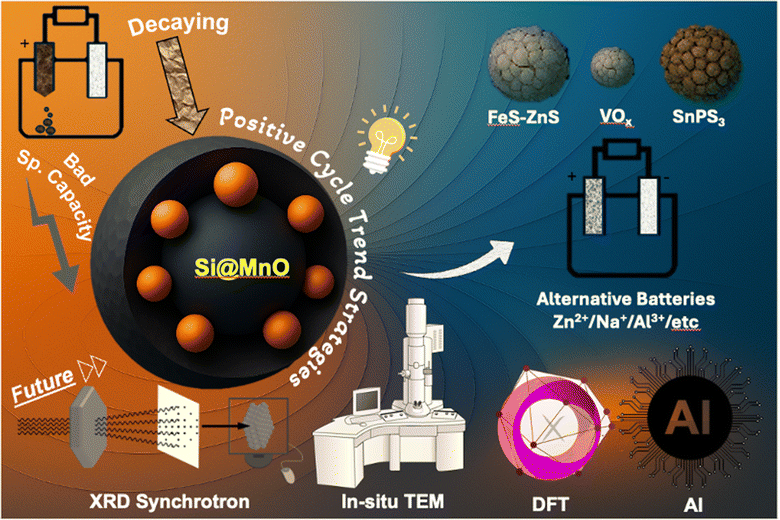 | ||
| Fig. 1 Illustration of the positive–negative cycling trend strategy and its scientific trajectory—from instability and poor performance to robust cycling and broader impact. | ||
2. Positive cycling trend
The “positive cycling trend” technique is a paradigm shift in the design of electrode materials for lithium-ion batteries (LIBs), specifically addressing the challenges associated with high-capacity anode materials such as silicon (Si). This approach combines materials with complementing cycling behaviours to improve overall performance and lifetime.5Silicon has a theoretical capacity of around 4200 mA h g−1 in LIBs.1,3,9 However, during lithiation and delithiation processes, the volume expands by approximately 300%. This expansion causes particle fracture, electrode pulverisation, and unstable solid–electrolyte interphase (SEI) development, resulting in rapid capacity loss. Materials like manganese oxide (MnO), which are commonly disregarded due to weak intrinsic conductivity and structural instability, have shown capacity gains over cycling when implanted in conductive matrices.10 Therefore the research on the positive cycling trend method makes use of this behaviour by combining a material with a declining capacity trend (e.g., Si) with one with a rising or stabilising capacity trend (e.g., MnO). The dynamic compensation between these materials seeks to stabilise electrode performance, increasing longevity while maintaining energy density. To validate this method, our team created a hierarchical core–shell structure in which Si nanoparticles were contained within a MnO shell and then enclosed in a conductive carbon/reduced graphene oxide (rGO) matrix. This design sought to increase electrical connectivity and protect against mechanical stress. The Si@MnO@C-rGO compound demonstrated excellent electrochemical performance, with a reversible capacity of 800–820 mA h g−1 over 1500 cycles at high current densities.5
The positive cycling trend concept has sparked further research into various high-capacity materials and hybrid designs. Research on FeS–ZnS composite nanosheets has shown improved lithium storage characteristics, while studies on hydrogenated vanadium oxides and SnPS3 materials have proven the usefulness of this method in other alternative battery chemistries.11–13 This method has also inspired developments in sodium-ion and zinc-ion batteries,11,14 as indicated by research on inverted opal manganese dioxide cathodes and hydrated sodium vanadate publications.
Reflecting on this methodology, the integration of current advanced characterization techniques, such as in situ and operando analyses, alongside AI-driven material discovery, could accelerate the optimization process. These tools offer real-time insights into dynamic electrode behaviours, facilitating more informed design decisions. As the field progresses, the integration of such technologies will be pivotal in developing next-generation electrode materials with superior performance and durability.15
3. Integration of current technologies
Reflecting on our original methods, it is clear that using modern in situ and operando characterisation techniques may have significantly improved our understanding of the dynamic behaviours of our Si@MnO@C-rGO composite electrodes. Our dependence on ex situ investigations, while instructive, hampered our capacity to capture real-time changes and transitory occurrences during battery operation.Modern in situ and operando techniques, including synchrotron-based X-ray diffraction (XRD) and X-ray absorption spectroscopy (XAS), enable real-time monitoring of structural and chemical changes in electrode materials during electrochemical cycling.16 These approaches provide information about phase transitions, oxidation state variations, and the evolution of the solid–electrolyte interphase (SEI), which is important for understanding degradation causes and performance limitations. When combined with an operando setup, high-resolution transmission electron microscopy (HRTEM) allows for the visualisation of nanoscale morphological changes such as particle breakage, phase transitions, and SEI creation.17,18 This level of detail is quite useful for comparing structural dynamics to electrochemical performance.
Using these advanced techniques on our Si@MnO@C-rGO composites would have allowed us to monitor the lithiation and delithiation processes in real time, giving us a better understanding of how the MnO shell and carbon matrix interact with the silicon core during cycling. We could have identified the onset of structural degradation and nano-structuring, monitored the stability of the SEI layer, and evaluated the carbon matrix’s ability to accommodate volume variations.19 Furthermore, operando investigations may have revealed the mechanisms underlying the observed positive cycling trend in MnO, perhaps suggesting techniques to reduce electrolyte dissolution and improve long-term stability. Understanding these mechanisms on a fundamental level is critical for optimising composite electrode designs.
The combination of in situ and operando data with computer modelling, such as density functional theory (DFT) and machine-learning techniques, provides a strong tool for predicting material behaviour and guiding the design of next-generation electrode materials. By simulating different structural configurations and cycling circumstances, we may have sped up the creation of composites with specialised qualities for specific applications.20 Furthermore, the current era of artificial intelligence (AI) and machine learning (ML) in materials research has created new opportunities for expediting the discovery and optimisation of battery materials. By analysing vast datasets from experimental and computational investigations, AI/ML models can forecast material attributes, identify performance trends, and recommend interesting material combinations.21 Integrating AI-driven methodologies into our study could have accelerated the screening of prospective electrode materials and composite architectures, allowing for more efficient exploration of the design space and faster creation of high-performance battery systems.22
4. Conclusion and future perspective
Reflecting on our 2016 work, it was a watershed event in our research career, providing a fresh way to address long-standing issues in battery technology. The concept of integrating materials with complementary cycle behaviours, as demonstrated by our Si@MnO@C-rGO composite, demonstrated the power of interdisciplinary collaboration and inventive thinking in creating energy storage solutions. Since then, the landscape of battery research has shifted dramatically. The incorporation of modern characterisation techniques, such as in situ TEM and operando synchrotron-based XRD, has allowed more insight into the dynamic processes that occur within batteries during operation. These methods have helped to untangle complex systems, enable more accurate material optimisation, and accelerate the development of high-performance batteries. Furthermore, the introduction of DFT modelling, AI and ML has transformed material discovery and design. AI/ML systems can anticipate material qualities, optimise electrode layouts, and estimate battery performance by analysing large datasets, shortening the invention cycle. This computational ability supplements experimental efforts, creating a synergistic environment for rapid advances in battery technology. Looking ahead, the future of battery research depends on embracing these current technologies and approaches. The confluence of sophisticated characterisation, computational modelling, and interdisciplinary collaboration will be critical in overcoming current restrictions and opening new possibilities in energy storage. As we continue to investigate novel materials and architectures, our dedication to innovation and collaboration remains unwavering.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the funding support from the ASTAR MTC programmatic project under grant no. M23L9b0052, Indonesia-NTU Singapore Institute of Research for Sustainability and Innovation INSPIRASI under contract no. 6635/E3/KL.02.02/2023, and Singapore NRF Singapore-China flagship program under grant no. 023740-00001.References
- N. Nasajpour-Esfahani, H. Garmestani, M. Bagheritabar, D. J. Jasim, D. Toghraie, S. Dadkhah and H. Firoozeh, Comprehensive Review of Lithium-Ion Battery Materials and Development Challenges, Renewable Sustainable Energy Rev., 2024, 203, 114783, DOI:10.1016/j.rser.2024.114783.
- X. Shen, X.-Q. Zhang, F. Ding, J.-Q. Huang, R. Xu, X. Chen, C. Yan, F.-Y. Su, C.-M. Chen, X. Liu and Q. Zhang, Advanced Electrode Materials in Lithium Batteries: Retrospect and Prospect, Energy Mater. Adv., 2021, 2021, 1205324, DOI:10.34133/2021/1205324.
- C. Heubner, U. Langklotz and A. Michaelis, Theoretical Optimization of Electrode Design Parameters of Si Based Anodes for Lithium-Ion Batteries, J. Energy Storage, 2018, 15, 181–190, DOI:10.1016/j.est.2017.11.009.
- Y. Cheng, Z. Guo, C. Zheng, L. Zhang, S. Wang and H. Du, Revisiting the Core Problem Impeding the Commercialization of Silicon-Based Lithium-Ion Batteries, Energy Mater. Devices, 2025, 3(1), 9370055, DOI:10.26599/EMD.2025.9370055.
- D.-H. Liu, H.-Y. Lü, X.-L. Wu, J. Wang, X. Yan, J.-P. Zhang, H. Geng, Y. Zhang and Q. Yan, A New Strategy for Developing Superior Electrode Materials for Advanced Batteries: Using a Positive Cycling Trend to Compensate the Negative One to Achieve Ultralong Cycling Stability, Nanoscale Horiz., 2016, 1(6), 496–501, 10.1039/C6NH00150E.
- D.-H. Liu, H.-Y. Lü, X.-L. Wu, B.-H. Hou, F. Wan, S.-D. Bao, Q. Yan, H.-M. Xie and R.-S. Wang, Constructing the Optimal Conductive Network in MnO-Based Nanohybrids as High-Rate and Long-Life Anode Materials for Lithium-Ion Batteries, J. Mater. Chem. A, 2015, 3(39), 19738–19746, 10.1039/C5TA03556B.
- A. R. Park, J. S. Kim, K. S. Kim, K. Zhang, J. Park, J. H. Park, J. K. Lee and P. J. Yoo, Si–Mn/Reduced Graphene Oxide Nanocomposite Anodes with Enhanced Capacity and Stability for Lithium-Ion Batteries, ACS Appl. Mater. Interfaces, 2014, 6(3), 1702–1708, DOI:10.1021/am404608d.
- U. V. Kawade, S. R. Kadam, M. V. Kulkarni and B. B. Kale, Synergic Effects of the Decoration of Nickel Oxide Nanoparticles on Silicon for Enhanced Electrochemical Performance in LIBs, Nanoscale Adv., 2020, 2(2), 823–832, 10.1039/C9NA00727J.
- M. Gu, Y. He, J. Zheng and C. Wang, Nanoscale Silicon as Anode for Li-Ion Batteries: The Fundamentals, Promises, and Challenges, Nano Energy, 2015, 17, 366–383, DOI:10.1016/j.nanoen.2015.08.025.
- J. Ma, H. Song, Z. He, Y. Chen and M. Luo, Selection of High Rate Capability and Cycling Stability MnO Anode Material for Lithium-Ion Capacitors: Effect of the Carbon Source, J. Electroanal. Chem., 2024, 974, 118717, DOI:10.1016/j.jelechem.2024.118717.
- Q. Liang, Y. Zheng, C. Du, Y. Luo, J. Zhao, H. Ren, J. Xu and Q. Yan, Asymmetric-Layered Tin Thiophosphate: An Emerging 2D Ternary Anode for High-Performance Sodium Ion Full Cell, ACS Nano, 2018, 12(12), 12902–12911, DOI:10.1021/acsnano.8b08229.
- C. Xu, H. T. Tan, M. Ulaganathan and Q. Yan, FeS–ZnS Composite Nanosheets for Enhanced Lithium Storage Properties, ChemNanoMat, 2017, 3(6), 420–427, DOI:10.1002/cnma.201700059.
- Y. Zhang, H. Wang, J. Yang, H. Fan, Y. Zhang, Z. Dai, Y. Zheng, W. Huang, X. Dong and Q. Yan, Hydrogenated Vanadium Oxides as an Advanced Anode Material in Lithium Ion Batteries, Nano Res., 2017, 10(12), 4266–4273, DOI:10.1007/s12274-017-1582-7.
- H. Ren, J. Zhao, L. Yang, Q. Liang, S. Madhavi and Q. Yan, Inverse Opal Manganese Dioxide Constructed by Few-Layered Ultrathin Nanosheets as High-Performance Cathodes for Aqueous Zinc-Ion Batteries, Nano Res., 2019, 12(6), 1347–1353, DOI:10.1007/s12274-019-2303-1.
- C. Hu, L. Lv, J. Xue, M. Ye, L. Wang and L. Qu, Branched Graphene Nanocapsules for Anode Material of Lithium-Ion Batteries, Chem. Mater., 2015, 27(15), 5253–5260, DOI:10.1021/acs.chemmater.5b01398.
- J. Tan, D. Liu, X. Xu and L. Mai, In Situ/Operando Characterization Techniques for Rechargeable Lithium–Sulfur Batteries: A Review, Nanoscale, 2017, 9(48), 19001–19016, 10.1039/C7NR06819K.
- E. Peled and S. Menkin, Review—SEI: Past, Present and Future, J. Electrochem. Soc., 2017, 164(7), A1703–A1719, DOI:10.1149/2.1441707jes.
- X. H. Liu, Y. Liu, A. Kushima, S. Zhang, T. Zhu, J. Li and J. Y. Huang, In Situ TEM Experiments of Electrochemical Lithiation and Delithiation of Individual Nanostructures, Adv. Energy Mater., 2012, 2(7), 722–741, DOI:10.1002/aenm.201200024.
- H. Wu and Y. Cui, Designing Nanostructured Si Anodes for High Energy Lithium Ion Batteries, Nano Today, 2012, 7(5), 414–429, DOI:10.1016/j.nantod.2012.08.004.
- H. Tan, D. Chen, W. Liu, C. Liu, B. Lu, X. Rui and Q. Yan, Free-Standing Hydrated Sodium Vanadate Papers for High-Stability Zinc-Ion Batteries, Batteries Supercaps, 2020, 3(3), 254–260, DOI:10.1002/batt.201900145.
- K. T. Butler, D. W. Davies, H. Cartwright, O. Isayev and A. Walsh, Machine Learning for Molecular and Materials Science, Nature, 2018, 559(7715), 547–555, DOI:10.1038/s41586-018-0337-2.
- D. Jha, K. Choudhary, F. Tavazza, W. Liao, A. Choudhary, C. Campbell and A. Agrawal, Enhancing Materials Property Prediction by Leveraging Computational and Experimental Data Using Deep Transfer Learning, Nat. Commun., 2019, 10(1), 5316, DOI:10.1038/s41467-019-13297-w.
| This journal is © The Royal Society of Chemistry 2025 |
