Engineering of Lewis acid–base interfaces in Cu2S/ZnIn2S4 hollow hetero-nanocages for enhanced photocatalytic CO2 reduction†
Yuanyuan Zhaoa,
Kangjie Gaob,
Jiaxin Lia,
Huanhuan Liu*b,
Fang Chen*c,
Wentao Wang *d,
Yijun Zhonga and
Yong Hu
*d,
Yijun Zhonga and
Yong Hu *b
*b
aKey Laboratory of the Ministry of Education for Advanced Catalysis Materials, Department of Chemistry, Zhejiang Normal University, Jinhua, 321004, China
bInstitute of Nanocatalysis and Energy Conversion, College of Chemistry and Materials Engineering, Zhejiang A&F University, Hangzhou, 311300, China. E-mail: yonghu@zafu.edu.cn; hhuanliu@zafu.edu.cn; Web: https://www.x-mol.com/groups/yonghu
cHangzhou Institute of Advanced Studies, Zhejiang Normal University, Hangzhou, 311231, China. E-mail: chenfang@zjnu.edu.cn
dGuizhou Provincial Key Laboratory of Computational Nano-Material Science, Guizhou Education University, Guiyang, 550018, China. E-mail: wtwang@gznc.edu.cn
First published on 20th June 2025
Abstract
Selective photocatalytic CO2 reduction (PCR) to CH4 remains challenging due to the sluggish charge transfer kinetics and the involved complicated C1 intermediates. Herein, deliberate engineering of Lewis acid–base interfaces in Cu2S/ZnIn2S4 hollow hetero-nanocages (HHNCs) was carried out, and enhanced PCR activity and selectivity were achieved due to accelerated electron transfer and stabilized intermediates. Both experimental and theoretical results have demonstrated the construction of a Lewis base interface with Cu2S and a Lewis acid interface with ZnIn2S4, which exhibited strong CO2 adsorption and reduction of the Gibbs free energy in the hydrogenation step (*CO to *CHO). As a consequence, a CH4 yield of 23.3 μmol g−1 h−1 under visible light irradiation (λ > 400 nm) was obtained with the Cu2S/ZnIn2S4 HHNCs, approximately 13.7, 10.1 and 6.3 times higher than those of bare Cu2S, ZnIn2S4 and a physically mixed sample (Cu2S/ZnIn2S4-mix), respectively. The product selectivity of CH4 was as high as 93.2%, in sharp contrast with 59.5% for the Cu2S/ZnIn2S4-mix, 53.1% for Cu2S and 35.4% for ZnIn2S4. This work demonstrates a rational strategy to engineer heterogenous Lewis acid–base interfaces for improving PCR activity and selectivity.
New conceptsSelective photocatalytic CO2 reduction (PCR) to CH4 is highly coveted yet remains challenging due to sluggish charge transfer kinetics and the involvement of various C1 intermediates. Constructing frustrated Lewis pair catalysts by assembling surface defect states, such as oxygen vacancies, is regarded as vital for CO2 adsorption and activation. However, the thermodynamically unstable defect states inhibit the sustainability of PCR, which is averse to applications in industry. In this work, we have proposed a new strategy involving the deliberate construction of Lewis acid–base interfaces, in which nucleophilic O atoms and electrophilic C in CO2 can be activated by combinations of electron acceptor (Lewis acid) interfaces and electron donor (Lewis base) interfaces to accelerate multiple proton coupled electron transfer and stabilize intermediates, aiming to enhance PCR activity and selectivity. These significant breakthroughs in this current forefront fundamental research make us believe that both the science and technique of this work are interesting and convincing enough for the broad readership. |
1. Introduction
Photocatalytic CO2 reduction (PCR) toward high value-added chemical products (such as CO, CH4, C2H4, and CH3OH) is considered to be an ingenious strategy for addressing global energy demand and environmental problems.1,2 Among these hydrocarbons, methane (CH4) is very important hydrocarbon feedstock for the storage of hydrogen and generation of multiple-carbon hydrocarbon molecules (such as C2H4 and C2H6) by non-oxidative coupling of methane and other commodity chemicals.3,4 However, due to the dissociation energy of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond in CO2 being as high as 750 kJ mol−1, the PCR performance has remained limited up to now.5 Furthermore, special attention is paid to the conversion of CO2 to CH4, which requires the most electrons (8 electrons) of all the C1 chemicals and is therefore one of the most challenging processes.6,7 Recently, the design of dual-metal sites has been recognized as an excellent concept to intensify CO2 adsorption and stabilize C1 intermediates, enhancing PCR activity and selectivity.8,9 Constructing frustrated Lewis pair catalysts by assembling surface defect states, such as oxygen vacancies, is regarded as vital for CO2 adsorption and activation.10,11 However, the thermodynamically unstable defect states inhibit the sustainability of PCR, which is averse to applications in industry.12,13
O bond in CO2 being as high as 750 kJ mol−1, the PCR performance has remained limited up to now.5 Furthermore, special attention is paid to the conversion of CO2 to CH4, which requires the most electrons (8 electrons) of all the C1 chemicals and is therefore one of the most challenging processes.6,7 Recently, the design of dual-metal sites has been recognized as an excellent concept to intensify CO2 adsorption and stabilize C1 intermediates, enhancing PCR activity and selectivity.8,9 Constructing frustrated Lewis pair catalysts by assembling surface defect states, such as oxygen vacancies, is regarded as vital for CO2 adsorption and activation.10,11 However, the thermodynamically unstable defect states inhibit the sustainability of PCR, which is averse to applications in industry.12,13
Metal sulfide semiconductors with a broad photo-responsive range and advantageous band structures are suitable photocatalysts for PCR.14,15 Among these materials, ZnIn2S4 is a commonly employed photocatalyst due to its chemical stability, non-toxic nature, and a suitable band gap for PCR. Nevertheless, the pristine ZnIn2S4 exhibits low PCR activity because of its low carrier mobility and limited CO2 activation ability.15,16 Conversely, Cu2S features a narrow band gap and rapid carrier mobility.17,18 Owing to the distinct photogenerated carrier migration rates of ZnIn2S4 and Cu2S, in the presence of light, photogenerated electrons in the heterojunction photocatalyst will undergo migration from one catalyst to another, resulting in the formation of an electron-rich (Lewis base) side and an electron-poor (Lewis acid) side at the heterojunction interfaces, where Lewis acid–base interfaces will be established (Scheme 1).19,20 Considering the coexistence of electrophilic C and nucleophilic O atoms in CO2, when CO2 is adsorbed on Lewis acid–base interfaces, the CO2 molecule can be activated by combinations of electron acceptor (Lewis acid) interfaces and electron donor (Lewis base) interfaces to form a stable IA–O–C–IB configuration (where IA and IB represent Lewis acid interfaces and Lewis base interfaces, respectively).21,22 The Lewis acid–base interfaces, formed by the accumulation of photogenerated electrons at a heterogeneous interface under light irradiation, exhibit enhanced stability compared to traditional frustrated Lewis pairs generated through the construction of defect sites.
Inspired by the above considerations, the Lewis acid–base interfaces in Cu2S/ZnIn2S4 hollow hetero-nanocages (HHNCs) were deliberately designed and fabricated to accelerate electron transfer and hydrogenate *CO to *CHO for enhancing the selective photocatalytic reduction of CO2 to CH4. The experimental results show that the electron aggregation region around Cu2S interfaces and the electron depletion region around ZnIn2S4 interfaces upon irradiation lead to the generation of Lewis base sites at the Cu sites and Lewis acid sites at the In sites, which can dramatically enhance CO2 adsorption and activation and accelerate the hydrogenation of *CO rather than desorption. Density functional theory (DFT) calculations further verified that the hydrogenation of *CO to *CHO becomes significantly favorable in contrast to its desorption to gaseous CO, thus contributing to the selectivity of CH4. As a result, the as-prepared Cu2S/ZnIn2S4 HHNCs achieved a CH4 yield of 23.3 μmol g−1 h−1 under visible light irradiation (λ > 400 nm), which is about 6.3, 13.7 and 10.1 times that over a physically mixed sample (Cu2S/ZnIn2S4-mix, 3.7 μmol g−1 h−1), Cu2S (1.7 μmol g−1 h−1) and ZnIn2S4 (2.3 μmol g−1 h−1), respectively. In addition, the selectivity achieved is as high as 93.2% than the Cu2S/ZnIn2S4-mix (59.5%), Cu2S (53.2%) and ZnIn2S4 (35.4%).
2. Results and discussion
2.1 Composition and microstructure of Cu2S/ZnIn2S4 HHNCs
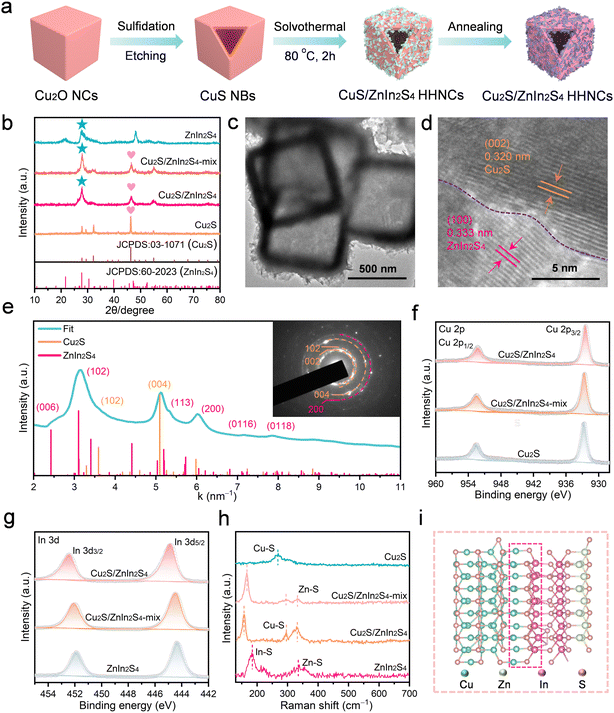
Cu2S/ZnIn2S4 HHNCs were obtained through a facile multistep templating strategy. Initially, the CuS NBs were synthesized by partial sulfidation and core etching of the Cu2O NCs.23,24 Subsequently, the CuS NBs were coated with ZnIn2S4 NSs and then annealed to yield the final Cu2S/ZnIn2S4 HHNCs (Fig. 1a). The cubic phase Cu2S (JCPDS no. 03-1071) and the hexagonal phase (JCPDS no. 60-2023) ZnIn2S4 were verified by powder X-ray diffraction (XRD) patterns (Fig. 1b).24,25 The field-emission scanning electron microscopy (FESEM) image in Fig. S2 (ESI†) and the transmission electron microscopy (TEM) image in Fig. 1c show uniform morphology of Cu2S/ZnIn2S4 HHNCs with abundant intersected ZnIn2S4 NSs on the surface of hollow Cu2S NBs. The uniform distribution of Cu, Zn, In, and S elements within the whole Cu2S/ZnIn2S4 HHNCs (Fig. S3, ESI†) was observed in elemental mapping images. The high-resolution TEM (HRTEM) image in Fig. 1d and that in Fig. S4 (ESI†) show lattice spacings of 0.320 nm and 0.333 nm, belonging to the (002) plane of cubic Cu2S and the (100) plane of hexagonal ZnIn2S4, respectively, in agreement with the XRD results. Moreover, the heterointerface structure that existed on the Cu2S/ZnIn2S4 HHNCs marked with purple imaginal lines is clearly observed. Additionally, geometric phase analysis (GPA) simulation was carried out to determine the interface environment of the Cu2S/ZnIn2S4 HHNCs.26,27 As shown in Fig. S5 (ESI†), the GPA images demonstrated obvious internal strain at Cu2S and ZnIn2S4 of Cu2S/ZnIn2S4 HHNCs. The distinct structural distortion stems from the lattice mismatch at the interface between Cu2S and ZnIn2S4.28,29 This lattice mismatch intensifies the built-in electric field, thus accelerating the migration of carriers and further promoting the formation of a Lewis acid–base interface at the interfaces of Cu2S and ZnIn2S4.26,29,30 The reciprocal spacings of the selected area electron diffraction (SAED) pattern and their relative intensity along with an integrated intensity-spacing profile were obtained, as shown in Fig. 1e. A set of composite diffraction patterns containing Cu2S and ZnIn2S4 were observed with no impurity signal detected, demonstrating the successful fabrication of Cu2S/ZnIn2S4 HHNCs.X-ray photoelectron spectroscopy (XPS) was employed to analyze the surface chemical composition and valence states. The survey spectra reveal that the as-prepared Cu2S/ZnIn2S4 HHNCs consist of Cu, S, Zn, and In elements, confirming the successful synthesis of the heterojunction structure (Fig. S6a, ESI†). The Cu 2p XPS spectra of Cu2S NBs at 932.72 and 952.49 eV correspond to Cu 2p3/2 and Cu 2p1/2 of the Cu+ chemical state (Fig. 1f), consistent with the Auger spectra of Cu+ (Fig. S6b, ESI†).31 The S 2p spectrum indicates that the characteristic peaks centered at 161.11 and 162.27 eV can be assigned to S 2p3/2 and S 2p1/2 orbitals of S2− (Fig. S6c, ESI†). Furthermore, two peaks in the Zn 2p XPS spectra for ZnIn2S4 NSs at 1021.88 and 1044.89 eV could be attributed to Zn 2p3/2 and Zn 2p1/2 of Zn2+, respectively (Fig. S6d, ESI†).32,33 The In 3d XPS spectra of ZnIn2S4 NSs (Fig. 1g) contain two symmetry peaks at 444.35 and 451.87 eV, corresponding to In 3d5/2 and In 3d3/2 of In3+, respectively.34,35 Significantly, the observed lower binding energy shift in the Cu 2p spectra and higher binding energy shift in the Zn 2p and In 3d spectra for Cu2S/ZnIn2S4 HHNCs and the Cu2S/ZnIn2S4-mix compared to their pristine counterparts suggest the formation of a strong interaction at the heterogeneous interfaces.36 Furthermore, the more pronounced chemical shift for Cu2S/ZnIn2S4 HHNCs than the physically mixed counterpart (Cu2S/ZnIn2S4-mix) indicates that the in situ growth strategy can create stronger heterogeneous interfaces, which is advantageous for constructing Lewis acid–base interfaces. The existence of Lewis acid–base interfaces on Cu2S/ZnIn2S4 was further confirmed through NH3-temperature-programmed desorption (NH3-TPD) and CO2-temperature-programmed desorption (CO2-TPD). As shown in Fig. S7a (ESI†), the desorption peaks of Cu2S and ZnIn2S4 at 303.6 and 321.5 °C can be attributed to the chemical adsorption of CO2 on the catalyst, respectively.37,38 Compared to Cu2S and ZnIn2S4, Cu2S/ZnIn2S4 exhibited a higher desorption temperature (324.2 °C) and a larger desorption curve integral area, indicating that Cu2S/ZnIn2S4 has more Lewis base sites.37 In the meantime, two desorption peaks for Cu2S/ZnIn2S4 HHNCs at 176.4 and 410.5 °C in NH3-TPD measurements are observed (Fig. S7b, ESI†). Interestingly, there is no obvious NH3-TPD signal for the single Cu2S or ZnIn2S4 sample, which further indicates that the Lewis acid sites are only constructed at the Cu2S/ZnIn2S4 heterojunction interface.38
The chemical environment of the heterointerface for the Cu2S/ZnIn2S4 HHNCs and control samples was investigated by Fourier-transform infrared spectra (Fig. S8, ESI†). In the case of ZnIn2S4 NSs, the obvious absorption band at 1375 cm−1 was assigned to the vibrational mode of In–S bonds.39,40 The absorption peak at 617 cm−1 for Cu2S NBs corresponded to the Cu–S vibration mode.41–43 Importantly, it is observed that the In–S stretching vibrational peak of Cu2S/ZnIn2S4 HHNCs shifts to a lower wave number when compared with that of ZnIn2S4 NSs. Moreover, the Cu–S bonds of Cu2S/ZnIn2S4 HHNCs shift to a higher wave number compared to those of Cu2S NBs. This suggests potential bonding modes between the In atom of ZnIn2S4 NSs and the Cu atom of Cu2S NBs in Cu2S/ZnIn2S4 HHNCs.44 The results were further confirmed by Raman spectroscopy. As shown in Fig. 1h, the peak at 268 cm−1 for Cu2S NBs corresponded to the vibrational mode of the Cu–S bonds.45 For ZnIn2S4 NSs, the Raman peaks at 187 cm−1 and 332 cm−1 were attributed to the In–S and Zn–S bonds, respectively.46–49 Observingly, the remarkable Raman shift of the In–S and Cu–S bonds, along with the steady Raman vibration modes of the Zn–S bonds, indicates a potential connection through Cu–S–In bonds in the Cu2S/ZnIn2S4-mix and Cu2S/ZnIn2S4 HHNCs (Fig. 1i).50,51 The more significant Raman peak shift observed in Cu2S/ZnIn2S4 HHNCs, compared to the Cu2S/ZnIn2S4-mix, suggests a robust interface interaction between Cu2S NBs and ZnIn2S4 NSs facilitated by an in situ growth strategy.
2.2 Band structures and electron transfer pathways
The band structure of Cu2S/ZnIn2S4 HHNCs and control samples was investigated by UV-vis diffuse reflectance spectroscopy (UV-vis DRS) and ultraviolet photoemission spectroscopy (UPS). Bare ZnIn2S4 exhibits an absorption edge at approximately 500 nm (Fig. 2a), corresponding to a band gap of about 2.62 eV estimated by the Tauc plot, while Cu2S demonstrates a narrow band gap of 1.87 eV (Fig. 2b). UPS analysis was conducted to determine the band structures of Cu2S NBs and ZnIn2S4 NSs from the Ecutoff (secondary electron cutoff) and Eedge (Fermi edge) recorded in Fig. 2c and d, respectively. The Φ values were calculated by subtracting the secondary electron cutoff (Ecutoff) from the excitation energy (21.22 eV), resulting in values of 5.09 and 4.70 eV for Cu2S and ZnIn2S4 (vs. vacuum), respectively. The EVB of Cu2S and ZnIn2S4 was calculated to be −5.70 and −6.57 eV vs. vacuum (0.85 and 1.72 eV vs. NHE, at pH = 7), respectively.52 The ECB was thus estimated to be −3.83 and −3.95 eV vs. vacuum (−1.02 and −0.90 eV vs. NHE, at pH = 7) for Cu2S and ZnIn2S4, respectively.In situ irradiated XPS of Cu2S/ZnIn2S4 HHNCs was employed to further investigate the actual mechanism of electron transfer. The Cu 2p spectra (Fig. 2e) of Cu2S/ZnIn2S4 HHNCs at 932.73 and 932.50 eV demonstrate a significant shift of 0.35 eV toward a higher binding energy under irradiation than those under dark conditions, while the In 3d and Zn 2p spectra moved towards moderately lower values of 0.44 and 0.39 eV, respectively (Fig. 2f and Fig. S9, ESI†), attributed to the injection of electrons from Cu2S to ZnIn2S4 across the heterogeneous interfaces. Typically, the band alignment structure of bare Cu2S and ZnIn2S4 heterojunctions is depicted in Fig. 2g. Under dark conditions, electron transfer occurs from ZnIn2S4 to Cu2S in Cu2S/ZnIn2S4 HHNCs until equilibrium is achieved due to the disparity in Ef, which induces energy band bending in the space charge layer and the establishment of a built-in electric field pointing from ZnIn2S4 to Cu2S. Upon irradiation, the synergistic effect of the built-in electric field and energy band bending leads to the spontaneous transfer of photoexcited electrons from the ECB of Cu2S to that of ZnIn2S4. Simultaneously, the holes at the EVB of ZnIn2S4 are transferred to that of Cu2S, establishing a type-II pathway. These results confirm that the electron transfer occurs from Cu2S to ZnIn2S4 in the Cu2S/ZnIn2S4 heterostructure under irradiation, resulting in the generation of an electron depletion layer at the side of ZnIn2S4 and an electron accumulation layer around Cu2S, as depicted in Fig. 2h, and thereby forming Lewis acid–base interfaces in Cu2S/ZnIn2S4 HHNCs.
2.3 PCR performances
The formation of Lewis acid–base interfaces has a significant impact on the activity and selectivity of PCR to CH4. As illustrated in Fig. 3a and Fig. S10 and Table S1 (ESI†), ZnIn2S4 NSs predominantly produce CO (4.2 μmol g−1 h−1) with low CH4 species (2.3 μmol g−1 h−1) during PCR under visible light irradiation (λ > 400 nm), consistent with previous ZnIn2S4 species.53,54 H2 was not detected due to the gas–solid reactor system with CO2 and water vapor, where sustained CO2 exposure on catalytic surfaces effectively suppressed hydrogen generation through water reduction.55,56 The CH4 selectivity of ZnIn2S4 NSs is approximately 35.4%. Although the selectivity of Cu2S (53.1%) has indeed shown improvement compared to ZnIn2S4, the limited PCR activity (1.7 μmol g−1 h−1) hinders broader applications. Surprisingly, the in situ assembled heterojunction (Cu2S/ZnIn2S4 HHNCs) demonstrates the highest CH4 evolution rate (23.3 μmol g−1 h−1) with trace CO gas (1.3 μmol g−1 h−1), surpassing previously reported Cu-based, Zn-based and In-based photocatalysts (Table S2, ESI†). Liquid products (such as HCOOH) in the photocatalytic CO2 reduction system were also not detected by 1H nuclear magnetic resonance spectroscopy, indicating high specificity of Cu2S/ZnIn2S4 in photocatalytic CO2 reduction (Fig. S11, ESI†).24,57 The CH4 selectivity achieved is as high as 93.2% (Fig. 3b). Additionally, the photogenerated holes participate in H2O oxidation during PCR, while the O2 product rate for the Cu2S/ZnIn2S4 HHNCs is about 50.3 μmol g−1 h−1 (Fig. S12, ESI†). However, the high PCR activity and selectivity of Cu2S/ZnIn2S4 HHNCs are not reprinted by the Cu2S/ZnIn2S4-mix (3.7 μmol g−1 h−1 for CH4 and 2.5 μmol g−1 h−1 for CO), suggesting that the unique and compact interfacial arrangement plays a significant role in shaping the reactivity and specificity towards PCR. The activity and selectivity remained stable even after ten cycles (Fig. 3c). In addition, the consistent phase constitution (Fig. S13a, ESI†), morphology of the hollow cubic structure (Fig. S13b, ESI†) and chemical state of the element (Fig. S14, ESI†) provide inherent evidence that the Cu2S/ZnIn2S4 HHNCs exhibit excellent photostability for PCR to CH4. Control experiments (Fig. S15, ESI†) demonstrated that no products were detected in the absence of photocatalysts, H2O, light irradiation, or under an Ar atmosphere, confirming that the production of CH4 resulted from the reduction of CO2 triggered using the Cu2S/ZnIn2S4 HHNCs under light irradiation. Furthermore, the PCR activity of Cu2S/ZnIn2S4 HHNCs is significantly weakened when a trace amount of pyridine or pyrrole is introduced into the reaction system (Fig. S16, ESI†), which is due to the adsorption of pyridine and pyrrole on Lewis acid and basic sites of the Cu2S/ZnIn2S4 heterojunction, respectively.58 This result further suggests that the Lewis acid–base interfaces of Cu2S/ZnIn2S4 HHNCs play a crucial role in the PCR process. The isotopic labeling experiment was further performed to investigate the origin of CH4 and CO through the replacement of 12CO2 with 13CO2. Ethane (C2H6) was employed as an internal standard substance (ISS). As shown in Fig. S17–S19 (ESI†), two peaks at m/z = 29 and 17 were identified and attributed to 13CO and 13CH4, respectively. The peaks of 13CO and 13CH4 gradually increased with the extension of the light exposure time (Fig. 3d), verifying that the products were exclusively derived from the reduction of CO2.19,592.4 Insights into the PCR mechanism
In situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS) measurements were conducted to investigate the proposed mechanism of high CH4 selectivity for Cu2S/ZnIn2S4 HHNCs by examining the intermediates and reaction pathways during the PCR. As presented in Fig. 4a and Fig. S20a (ESI†), the absorption peak of Cu2S/ZnIn2S4 HHNCs at 1218 cm−1 was attributed to the b-HCO3− species. Monodentate carbonate groups (m-CO32−) at around 1394, 1419, 1518 and 1540 cm−1 and bidentate carbonate groups (b-CO32−) at 1243 and 1364 cm−1 were also detected well.60 All of the b-CO32−, b-HCO3− and m-CO32− are generated from CO2 and H2O groups, which are considered as possible intermediates for the subsequent generation of C1 fuels. The adsorption bands at 1558 and 1653 cm−1 were assigned to the typical *COOH intermediate during CO2 reduction to CO and CH4.61–63 In addition, the observed *CHO and *CH2O groups at 1468 and 1506 cm−1, respectively, are regarded as crucial intermediates for the conversion of CO2 to CH4.4,34,64 The negative in situ DRIFTS signals of Cu2S/ZnIn2S4 HHNCs might be attributed to the fast consumption of reactants under light illumination due to the excellent photocatalytic CO2 reduction capacity.65,66 More importantly, the inconspicuous absorption peaks of *CHO and *CH2O for ZnIn2S4 NSs (Fig. 4b and S20b, ESI†) indicate that the photocatalytic CO2 methanation is insensitive, highlighting the pivotal role of interfacial reaction in this process, which is attributed to the construction of a Lewis acid–base interface.DFT calculations were employed to investigate the effects of the electronic structure and the CO2 reduction mechanism by constructing Lewis acid–base interfaces on the Cu2S/ZnIn2S4 HHNCs. Fig. 4c illustrates that the charge redistribution of Cu2S/ZnIn2S4 HHNCs is primarily confined to the heterostructure interfaces, where there is an accumulation of charge at the Cu sites of Cu2S and a depletion of charge at the In sites of ZnIn2S4. This observation aligns with our hypothesis that Lewis acid–base interfaces are formed on the heterostructure interfaces of Cu2S/ZnIn2S4 HHNCs. The asymmetric charge redistribution helps to perturb the spatial electronic distribution and increase the adsorption capacity of nonpolar CO2 molecules. The adsorption of CO2 represents a crucial aspect of PCR, directly influencing the photocatalytic activity. The N2 adsorption–desorption measurements (Fig. S21, ESI†) reveal that the Cu2S/ZnIn2S4 HHNCs (69.7 m2 g−1) demonstrate an intermediate specific surface area between ZnIn2S4 NSs (188.1 m2 g−1) and Cu2S NBs (4.4 m2 g−1). However, the CO2 uptake of Cu2S/ZnIn2S4 HHNCs was 4.6 cm3 g−1 (Fig. S22, ESI†), which was superior to that of bare Cu2S (3.3 cm3 g−1) and ZnIn2S4 (4.1 cm3 g−1). The enhanced CO2 adsorption capacity of Cu2S/ZnIn2S4 is attributed to the formation of a Lewis acid–base interface. Based on the polarized electronic structure characteristics of the CO2 molecule, the C atom in the CO2 molecule is electrophilic, while the O atoms with lone pairs are nucleophilic. According to Lewis acid–base theory, the C atom and the O atom of the activated CO2 molecule are regarded as Lewis acid and base sites, respectively. Therefore, the C atom tends to anchor at the basic site, while the O atom preferentially binds with the acidic site on the catalyst surface, forming an IA–O–C–IB configuration. The IA–O–C–IB intermediate facilitates the formation of the CHO* intermediate, which is further protonated to generate hydrocarbons,21 resulting in the formation of the Lewis acid–base interface facilitating the adsorption and activation of CO2. The strong CO2 adsorption property was further confirmed through theoretical calculation of CO2 adsorption energy (Fig. 4d). The Cu2S/ZnIn2S4 HHNCs with Cu–In dual sites adsorbing C and O, respectively, exhibit more negative adsorption energy (−1.68 eV) than the control sample compared to isolated Cu (0.16 eV) and In active sites (0.18 eV) of Cu2S/ZnIn2S4 HHNCs demonstrating that interfacial dual sites are favorable for CO2 adsorption capacity.66 Furthermore, the lower adsorption energy of CO2 in the case of Cu2S/ZnIn2S4 compared to Cu2S and ZnIn2S4 demonstrates that the Lewis acid–base interface structure with Cu–In dual sites favors CO2 adsorption and facilitates subsequent activation.67
The electron transfer between the intermediate states and Cu2S/ZnIn2S4, Cu2S, and ZnIn2S4 was visualized and quantified using charge density difference and Bader charge analysis. The Bader charge of the adsorbed *COOH changes from 0.365 |e| on Cu2S (Fig. S23, ESI†) and 0.322 |e| on the ZnIn2S4 surface (Fig. S24, ESI†) to 0.391 |e| on the Cu2S/ZnIn2S4 surface (Fig. S25, ESI†), indicating a more pronounced interaction between the *COOH intermediate and Cu2S/ZnIn2S4 than that of Cu2S or ZnIn2S4.68 Theoretical calculations were further performed to analyze the reaction pathway and energy barriers during PCR. The Gibbs free energy barrier (ΔG) of the rate-determining step (RDS) on Cu2S (Fig. 4e and Fig. S26, ESI†) and ZnIn2S4 (Fig. 4f and Fig. S27, ESI†) for the conversion of *CO2 into *COOH was 1.46 and 2.22 eV, respectively. By contrast, the Gibbs free energy barrier for *COOH formation in Cu2S/ZnIn2S4 (Fig. 4g and Fig. S28, ESI†) is significantly lower (0.37 eV). Furthermore, the RDS shifts from *COOH formation in Cu2S and ZnIn2S4 to *CHO formation in Cu2S/ZnIn2S4. While CO desorption is more favorable relative to subsequent hydrogenation in bare Cu2S (Fig. 4e) and ZnIn2S4 (Fig. 4f), the desorption of *CO presents a challenge for Cu2S/ZnIn2S4 HHNCs (Fig. 4g) due to a high energy requirement (ΔG = 2.59 eV), whereas the process of *CO hydrogenation leading to CHO* demonstrates a low energy barrier (ΔG = 1.48 eV), facilitating the photoreduction of CO2 to CH4 and illustrating remarkable CO-to-CH4 selectivity.69 The spontaneous desorption of *CO with an exothermic nature, rather than hydrogenation to *CHO intermediates for Cu2S/ZnIn2S4 at bare Cu and In sites (Fig. S29, ESI†), suggests that the formation of Lewis acid–base interfaces is advantageous for CO2-to-CH4 conversion. In addition, the temperature-programmed desorption of CO (CO-TPD) was used to further illustrate the preference for CH4 evolution on the Cu2S/ZnIn2S4 surface. Compared to bare Cu2S and ZnIn2S4, Cu2S/ZnIn2S4 has a higher CO desorption temperature and the greatest CO adsorption capacity, which suggests that the surface of Cu2S/ZnIn2S4 is more inclined to the CO hydrogenation process (Fig. S30, ESI†). Based on the aforementioned findings, it can be inferred that the construction of Lewis acid–base interfaces among Cu2S/ZnIn2S4 HHNCs effectively facilitates the activation of CO2, enhances the adsorption of *CO intermediates, and improves the selectivity for CH4 production.
3. Conclusions
In conclusion, Cu2S/ZnIn2S4 HHNCs featuring Lewis acid–base interfaces have been successfully synthesized using a straightforward multistep templating strategy. The experimental results show that the formation of an electron aggregation region around the Cu2S interface and an electron depletion region around the ZnIn2S4 interface upon irradiation leads to the creation of Lewis base sites on the Cu sites and Lewis acid sites on the In sites. DFT calculations further reveal that Cu2S/ZnIn2S4 with Lewis acid–base interfaces exhibits a more negative CO2 adsorption energy and favorable hydrogenation of *CO to form *CHO compared to Cu2S and ZnIn2S4, suggesting that CH4 is the primary product in PCR progress. Under visible light irradiation (λ > 400 nm), the prepared Cu2S/ZnIn2S4 HHNCs achieved a CH4 yield of approximately 23.3 μmol g−1 h−1, which is approximately 6.3, 13.7, and 10.1 times higher than those over the Cu2S/ZnIn2S4-mix, Cu2S, and ZnIn2S4 respectively. The achieved product selectivity of CH4 is 93.2%, surpassing that of the Cu2S/ZnIn2S4-mix (59.5%), Cu2S (53.1%), and ZnIn2S4 (35.4%). This work extends the concept of Lewis pairs from 0D (Lewis acid–base sites) to 2D (Lewis acid–base interfaces).Author contributions
Yuanyuan Zhao: investigation, data curation, writing – original draft, and visualization. Kangjie Gao: investigation and validation. Jiaxin Li: visualization and formal analysis. Huanhuan Liu: conceptualization, methodology, and writing – review & editing. Fang Chen: methodology, validation, and data curation. Wentao Wang: software and validation. Yijun Zhong: methodology and formal analysis. Yong Hu: conceptualization, methodology, writing – review & editing, and supervision.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (22272150 and 22402181), the Zhejiang Provincial Ten Thousand Talent Program (2021R51009), a Project Supported by the Scientific Research Fund of Zhejiang Provincial Education Department (Y202457290), and the Jinhua Science and Technology Plan Project (2024-4-021). The work is carried out at the Shanxi Supercomputing Center of China, and the calculations are performed on TianHe-2. This research is also supported by the advanced computing resources provided by the Supercomputing Center of the USTC.References
- X.-Y. Li, Z.-L. Zhu, F. W. Dagnaw, J.-R. Yu, Z.-X. Wu, Y.-J. Chen, M.-H. Zhou, T. Wang, Q.-X. Tong and J.-X. Jian, Nat. Commun., 2024, 15, 5882 CrossRef CAS PubMed.
- L. Li, X. Dai, M. Lu, C. Guo, S. M. Wabaidur, X.-L. Wu, Z. Lou, Y. Zhong and Y. Hu, Adv. Powder Mater., 2024, 3, 100170 CrossRef.
- H. Su, J.-T. Han, B. Miao, M. Salehi and C.-J. Li, Nat. Commun., 2024, 15, 6435 CrossRef CAS PubMed.
- W. Xie, Y. Liu, X. Zhang, H. Yan, X.-H. Liu, X. Zhang, Q. Zhao and H. Huang, Angew. Chem., Int. Ed., 2024, 63, e202314384 CrossRef CAS PubMed.
- S. Karmakar, S. Barman, F. A. Rahimi, D. Rambabu, S. Nath and T. K. Maji, Nat. Commun., 2023, 14, 4508 CrossRef CAS PubMed.
- S. Si, H. Shou, Y. Mao, X. Bao, G. Zhai, K. Song, Z. Wang, P. Wang, Y. Liu, Z. Zheng, Y. Dai, L. Song, B. Huang and H. Cheng, Angew. Chem., Int. Ed., 2022, 61, e202209446 CrossRef CAS PubMed.
- Z. Wang, J. Zhu, X. Zu, Y. Wu, S. Shang, P. Ling, P. Qiao, C. Liu, J. Hu, Y. Pan, J. Zhu, Y. Sun and Y. Xie, Angew. Chem., Int. Ed., 2022, 61, e202203249 CrossRef CAS PubMed.
- M. Zhang, Y. Mao, X. Bao, P. Wang, Y. Liu, Z. Zheng, H. Cheng, Y. Dai, Z. Wang and B. Huang, ACS Catal., 2024, 14, 5275–5285 CrossRef CAS.
- H. Li, Y. Wen, M. Jiang, Y. Yao, H. Zhou, Z. Huang, J. Li, S. Jiao, Y. Kuang and S. Luo, Adv. Funct. Mater., 2021, 31, 2011289 CrossRef CAS.
- S. Zhang, Z. Xia, Y. Zou, F. Cao, Y. Liu, Y. Ma and Y. Qu, J. Am. Chem. Soc., 2019, 141, 11353–11357 CrossRef CAS PubMed.
- Z.-Q. Huang, L.-P. Liu, S. Qi, S. Zhang, Y. Qu and C.-R. Chang, ACS Catal., 2018, 8, 546–554 CrossRef CAS.
- J. Yang, Y. Guo, R. Jiang, F. Qin, H. Zhang, W. Lu, J. Wang and J. C. Yu, J. Am. Chem. Soc., 2018, 140, 8497–8508 CrossRef CAS PubMed.
- C. Wang, Y. Zhao, C. Cheng, Q. Li, C. Guo and Y. Hu, Coord. Chem. Rev., 2024, 521, 216177 CrossRef CAS.
- J. Wang, S. Lin, N. Tian, T. Ma, Y. Zhang and H. Huang, Adv. Funct. Mater., 2021, 31, 2008008 CrossRef CAS.
- S. Chandrasekaran, L. Yao, L. Deng, C. Bowen, Y. Zhang, S. Chen, Z. Lin, F. Peng and P. Zhang, Chem. Soc. Rev., 2019, 48, 4178–4280 RSC.
- X. Jiao, Z. Chen, X. Li, Y. Sun, S. Gao, W. Yan, C. Wang, Q. Zhang, Y. Lin, Y. Luo and Y. Xie, J. Am. Chem. Soc., 2017, 139, 7586–7594 CrossRef CAS PubMed.
- S. Wang, X. Bai, Q. Li, Y. Ouyang, L. Shi and J. Wang, Nanoscale Horiz., 2021, 6, 661–668 RSC.
- A. Manzi, T. Simon, C. Sonnleitner, M. Döblinger, R. Wyrwich, O. Stern, J. K. Stolarczyk and J. Feldmann, J. Am. Chem. Soc., 2015, 137, 14007–14010 CrossRef CAS PubMed.
- M. Zhou, H. Wang, R. Liu, Z. Liu, X. Xiao, W. Li, C. Gao, Z. Lu, Z. Jiang, W. Shi and Y. Xiong, Angew. Chem., Int. Ed., 2024, 63, e202407468 CrossRef CAS PubMed.
- D. W. Stephan, Science, 2016, 354, aaf7229 CrossRef PubMed.
- S. Deng, R. Wang, X. Feng, R. Zheng, S. Gong, X. Chen, Y. Shangguan, L. Deng, H. Tang, H. Dai, L. Duan, C. Liu, Y. Pan and H. Chen, Angew. Chem., Int. Ed., 2023, 62, e202309625 CrossRef CAS PubMed.
- D. Voicu, M. Abolhasani, R. Choueiri, G. Lestari, C. Seiler, G. Menard, J. Greener, A. Guenther, D. W. Stephan and E. Kumacheva, J. Am. Chem. Soc., 2014, 136, 3875–3880 CrossRef CAS PubMed.
- Y. Fang, D. Luan, Y. Chen, S. Gao and X. W. Lou, Angew. Chem., Int. Ed., 2020, 59, 7178–7183 CrossRef CAS PubMed.
- L. Li, X. Dai, D. L. Chen, Y. Zeng, Y. Hu and X. W. D. Lou, Angew. Chem., Int. Ed., 2022, 61, e202205839 CrossRef CAS PubMed.
- Y. He, H. Rao, K. Song, J. Li, Y. Yu, Y. Lou, C. Li, Y. Han, Z. Shi and S. Feng, Adv. Funct. Mater., 2019, 29, 1905153 CrossRef CAS.
- J. Hu, B. Li, X. Li, T. Yang, X. Yang, J. Qu, Y. Cai, H. Yang and Z. Lin, Adv. Mater., 2024, 36, 2412070 CrossRef CAS PubMed.
- S. Wan, W. Wang, B. Cheng, G. Luo, Q. Shen, J. Yu, J. Zhang, S. Cao and L. Zhang, Nat. Commun., 2024, 15, 9612 CrossRef CAS PubMed.
- X. Yue, L. Cheng, F. Li, J. Fan and Q. Xiang, Angew. Chem., Int. Ed., 2022, 61, e202208414 CrossRef CAS PubMed.
- C. Guan, X. Yue and Q. Xiang, Adv. Mater., 2025 DOI:10.1002/adma.202501209.
- A. Sabbah, I. Shown, M. Qorbani, F.-Y. Fu, T.-Y. Lin, H.-L. Wu, P.-W. Chung, C.-I. Wu, S. R. M. Santiago, J.-L. Shen, K.-H. Chen and L.-C. Chen, Nano Energy, 2022, 93, 106809 CrossRef CAS.
- Y. Zhang, L. Hu, Y. Zhang, X. Wang and H. Wang, Appl. Catal., B, 2022, 315, 121540 CrossRef CAS.
- C. Cheng, H. Xu, M. Ni, C. Guo, Y. Zhao and Y. Hu, Appl. Catal., B, 2024, 345, 123705 CrossRef CAS.
- X. Hao, Y. Wang, J. Zhou, Z. Cui, Y. Wang and Z. Zou, Appl. Catal., B, 2018, 221, 302–311 CrossRef CAS.
- J. Y. Li, C. L. Tan, M. Y. Qi, Z. R. Tang and Y. J. Xu, Angew. Chem., Int. Ed., 2023, 62, e202303054 CrossRef CAS PubMed.
- H. Xu, Y. Wang, X. Dong, N. Zheng, H. Ma and X. Zhang, Appl. Catal., B, 2019, 257, 117932 CrossRef CAS.
- L. Li, X. Dai, K. Gao, H. Yu, F. Chen, W. Wang, J. Ning and Y. Hu, Chem. Eng. J., 2025, 514, 163193 CrossRef CAS.
- W. Lin, H. Chen, G. Lin, S. Yao, Z. Zhang, J. Qi, M. Jing, W. Song, J. Li, X. Liu, J. Fu and S. Dai, Angew. Chem., Int. Ed., 2022, 61, e202207807 CrossRef CAS PubMed.
- L. Ma, Y. Wang, Y. Chen, R. Han, D. Xu, D. Jiao, D. Wang and X. Yang, Adv. Funct. Mater., 2025 DOI:10.1002/adfm.202503358.
- K. Saravanakumar, K. Yun, V. Maheskumar, Y. Yea, G. Jagan and C. M. Park, Chem. Eng. J., 2023, 451, 138933 CrossRef CAS.
- C. Huang, Y. Hong, X. Yan, L. Xiao, K. Huang, W. Gu, K. Liu and W. Shi, RSC Adv., 2016, 6, 40137–40146 RSC.
- Y. Wang, L. Zhang, H. Jiu, N. Li and Y. Sun, Appl. Surf. Sci., 2014, 303, 54–60 CrossRef CAS.
- K. G. Schmitt, R. Schmidt, H. F. von-Horsten, G. Vazhenin and A. A. Gewirth, J. Phys. Chem. C, 2015, 119, 23453–23462 CrossRef CAS.
- M. Saranya, C. Santhosh, R. Ramachandran, P. Kollu, P. Saravanan, M. Vinoba, S. K. Jeong and A. N. Grace, Powder Technol., 2014, 252, 25–32 CrossRef CAS.
- C. Wang, B. Zhang, Z. Zhao, Z. Zhang, S. Zhang, H. Bala and Z. Zhang, Appl. Surf. Sci., 2023, 641, 158458 CrossRef CAS.
- O. Siiman and P. R. Carey, J. Inorg. Biochem., 1980, 12, 353–362 CrossRef CAS.
- D. Ma, Z. Wang, J.-W. Shi, M. Zhu, H. Yu, Y. Zou, Y. Lv, G. Sun, S. Mao and Y. Cheng, Chem. Eng. J., 2020, 399, 125785 CrossRef CAS.
- W. Vallejo, C. Díaz-Uribe and K. Rios, Adv. Phys. Chem., 2017, 2017, 6358601 Search PubMed.
- L. Babedi, B. P. von der Heyden, P. H. Neethling and M. Tadie, Vib. Spectrosc., 2019, 105, 102968 CrossRef CAS.
- Z. Ye, L. Kong, F. Chen, Z. Chen, Y. Lin and C. Liu, Optik, 2018, 164, 345–354 CrossRef CAS.
- X. Wang, X. Wang, J. Huang, S. Li, A. Meng and Z. Li, Nat. Commun., 2021, 12, 4112 CrossRef CAS PubMed.
- F. Li, B. Xu, W. Yang, Z. Qi, C. Ma, Y. Wang, X. Zhang, Z. Luo, D. Liang, D. Li, Z. Li and A. Pan, Nano Res., 2020, 13, 1053–1059 CrossRef CAS.
- F. Liu, R. Shi, Z. Wang, Y. Weng, C.-M. Che and Y. Chen, Angew. Chem., Int. Ed., 2019, 58, 11791–11795 CrossRef CAS PubMed.
- Y. Zhang, J. Li, W. Zhou, X. Liu, X. Song, S. Chen, H. Wang and P. Huo, Appl. Catal., B, 2024, 342, 123449 CrossRef CAS.
- Y. Zhang, Y. Wu, L. Wan, H. Ding, H. Li, X. Wang and W. Zhang, Appl. Catal., B, 2022, 311, 121255 CrossRef CAS.
- J. Fu, K. Jiang, X. Qiu, J. Yu and M. Liu, Mater. Today, 2020, 32, 222–243 CrossRef CAS.
- S. Xie, Y. Wang, Q. Zhang, W. Deng and Y. Wang, ACS Catal., 2014, 4, 3644–3653 CrossRef CAS.
- H. Liu, F. Zhang, H. Wang, J. Xue, Y. Guo, Q. Qian and G. Zhang, Energy Environ. Sci., 2021, 14, 5339–5346 RSC.
- X. Liang, X. Wang, X. Zhang, S. Lin, M. Ji and M. Wang, ACS Catal., 2023, 13, 6214–6221 CrossRef CAS.
- M. Melchionna and P. Fornasiero, ACS Catal., 2020, 10, 5493–5501 CrossRef CAS.
- B. Kim, D. Kwon, J.-O. Baeg, M. Austeria P, G. H. Gu, J.-H. Lee, J. Jeong, W. Kim and W. Choi, Adv. Funct. Mater., 2023, 33, 2212453 CrossRef CAS.
- L. Sun, Z. Zhang, J. Bian, F. Bai, H. Su, Z. Li, J. Xie, R. Xu, J. Sun, L. Bai, C. Chen, Y. Han, J. Tang and L. Jing, Adv. Mater., 2023, 35, 2300064 CrossRef CAS PubMed.
- S. Yang, X. Guo, X. Li, T. Wu, L. Zou, Z. He, Q. Xu, J. Zheng, L. Chen, Q. Wang and Z. J. Xu, Angew. Chem., Int. Ed., 2024, 63, e202317957 CrossRef CAS PubMed.
- Y.-N. Gong, J.-H. Mei, W.-J. Shi, J.-W. Liu, D.-C. Zhong and T.-B. Lu, Angew. Chem., Int. Ed., 2024, 63, e202318735 CrossRef CAS PubMed.
- M. Ni, Y. Zhu, C. Guo, D.-L. Chen, J. Ning, Y. Zhong and Y. Hu, ACS Catal., 2023, 13, 2502–2512 CrossRef CAS.
- L. Li, H. Liu, C. Cheng, X. Dai, F. Chen, J. Ning, W. Wang and Y. Hu, ACS Catal., 2024, 14, 10204–10213 CrossRef CAS.
- X. Wu, W. Zhang, J. Li, Q. Xiang, Z. Liu and B. Liu, Angew. Chem., Int. Ed., 2023, 62, e202213124 CrossRef CAS PubMed.
- J. Yin, X. Song, C. Sun, Y. Jiang, Y. He and H. Fei, Angew. Chem., Int. Ed., 2024, 63, e202316080 CrossRef CAS PubMed.
- X. Zhang, H. Su, P. Cui, Y. Cao, Z. Teng, Q. Zhang, Y. Wang, Y. Feng, R. Feng, J. Hou, X. Zhou, P. Ma, H. Hu, K. Wang, C. Wang, L. Gan, Y. Zhao, Q. Liu, T. Zhang and K. Zheng, Nat. Commun., 2023, 14, 7115 CrossRef CAS PubMed.
- K. Wang, Q. Cheng, W. Hou, H. Guo, X. Wu, J. Wang, J. Li, Z. Liu and L. Wang, Adv. Funct. Mater., 2024, 34, 2309603 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and characterization section; DFT calculation details and structure models; SEM, TEM, EDS, FTIR, XPS, TPD, and BET results of Cu2S/ZnIn2S4 HHNCs; and photocatalytic performance and comparison tables of Cu2S/ZnIn2S4 HHNCs. See DOI: https://doi.org/10.1039/d5nh00355e |
| This journal is © The Royal Society of Chemistry 2025 |