High-performance optoelectronics enabled by solution-based sintering of perovskite nanocrystals†
Karthika
Vijayan
 ab,
Yu-Xiang
Chen
cde,
Pradyumna Kumar
Chand
ab,
Yu-Xiang
Chen
cde,
Pradyumna Kumar
Chand
 abf,
Ting-Chun
Huang
abf,
Ting-Chun
Huang
 c,
Ya-Ping
Hsieh
c,
Ya-Ping
Hsieh
 c and
Mario
Hofmann
c and
Mario
Hofmann
 *a
*a
aDepartment of Physics, National Taiwan University, Taipei, 10617, Taiwan. E-mail: mario@phys.ntu.edu.tw
bNanoscience and Technology Program, Taiwan International Graduate Program, Academia Sinica, Taipei, 105210, Taiwan
cInstitute of Atomic and Molecular Sciences, Academia Sinica, Taipei, 10617, Taiwan
dMolecular Science and Technology Program, Taiwan International Graduate Program, Academia Sinica, Taipei, 105210, Taiwan
eInternational Graduate Program of Molecular Science and Technology, National Taiwan University, Taipei 10617, Taiwan
fDepartment of Chemistry, National Taiwan University, Taipei, 10617, Taiwan
First published on 4th July 2025
Abstract
Perovskite nanocrystals have emerged as promising constituents for optoelectronic applications due to their exceptional and tunable properties and their scalable synthesis. However, their integration into devices faces challenges such as defects, poor carrier transport, and ligand interference. We present a liquid-in-liquid impingement process that achieves the mechanical coalescence of lead–bromide perovskite nanocrystals into large, free-standing flakes under ambient conditions. This approach leverages localized shear forces generated during impingement to achieve nanocrystal sintering, ligand removal, and solvent exchange. Microscopic analysis reveals the formation of large surface-sintered domains that overcome previous issues of defectiveness and environmental stability. This process results in significant improvements of the sintered nanocrystal properties compared to random perovskite assemblies. We demonstrate a significant decrease in trap density leading to enhanced chemical stability, charge transport and radiative charge recombination. Enhancements in carrier mobility enable the fabrication of photodetectors with exceptional response speed and sensitivity, surpassing conventional methods. These findings highlight the potential of liquid impingement processing for advancing perovskite-based optoelectronics through scalable and efficient nanocrystal assembly.
New conceptsIn this work, we demonstrate a novel liquid-in-liquid impingement process where individual lead bromide perovskite nanoparticles are mechano-coalesced, forming larger free-standing sintered flakes at ambient conditions. Conventional methods often face scalability issues with high defect density affecting the crystallinity, carrier mobility, and even optoelectronic properties of the material. Our method enables fusion of dispersed nanocubes due to ligand abstraction and thereby supports sintering at the liquid–liquid interface, resulting in superior quality assemblies with prolonged stability and fewer defects, evidenced by spectroscopic and electrochemical analyses. Unlike traditional assembly methods, where nanoparticles are assembled adjacent to a polymer or grid as a structural scaffold, our approach coalesces the particles without physical support. This fusion facilitates improved connectivity, augmenting carrier transport and environmental stability, subsequently offering remarkable promise for photovoltaics and electrochemical storage devices. |
Introduction
Perovskite materials have garnered significant attention due to their exceptional optoelectronic properties, making them promising for applications such as solar cells,1,2 light-emitting diodes,3 and photodetectors.4,5 Among the different perovskite morphologies, nanocrystals have captured the attention of many researchers due to their combination of several attractive properties:6,7 The low-temperature synthesis from earth-abundant constituents makes them commercially appealing. Their composition can be widely adjusted through co-synthesis and doping. Compared to bulk crystals, they exhibit higher environmental stability.8 High precision in controlling their shape and the formation of core–shell structures has been reported.9,10 Finally, complex three-dimensional nanocrystal assemblies can be produced that enhance the active area of heterojunctions.11,12Unfortunately, the transition from nanocrystal synthesis to device integration presents critical challenges. The assembly of nanocrystals into macroscopic structures is plagued by non-uniformity issues and defects.13 Moreover, the presence of different surface types and textures introduces large tunneling barriers between neighboring particles that hinder carrier transport through nanocrystal films.14 Consequently, the performance of nanocrystal-based electronic devices remains below bulk perovskites.15
Substantial efforts have been invested in realizing assembly methods that retain the scalability and cost advantages of perovskites. Blade and spray coating,16,17 self-assembly methods,18 and precipitation approaches19,20 have shown varying success in exerting control over the assembly process. However, the presence of ligands that stabilize the nanocrystals during synthesis hinder carrier transport after assembly. Moreover, the misalignment of crystal surfaces produces charge scatterers and traps that decrease carrier mobility.21 To overcome these issues, researchers have investigated the formation of composites where perovskites are encapsulated in a glass matrix to limit interaction with the environment, assist the removal of surface defects, enhance stability, and decrease toxicity.22 Subsequent sintering of the matrix could produce extended grain structures with enhanced optoelectronic properties. While this approach can increase the quality and stability of perovskite nanocrystals, the presence of the host introduces large barriers to carrier transport and limits its applicability in the envisioned optoelectronic devices.23 Moreover, elevated temperatures are required for these approaches, which affect the stability of the perovskite components.
Recently, fundamental studies have highlighted the impact of pressure in directly sintering perovskite assemblies without the use of a host. By using a diamond-anvil cell, Hills-Kimball et al. merged nanocrystals into larger platelet structures that exhibited increased crystallinity.24 This approach achieves carrier delocalization across dimensions much larger than a nanocrystal, making it promising for solar cells and LEDs.25 However, these experiments only achieved pressure-induced coalescence between a few crystals, and the anvil-cell approach cannot be easily translated toward perovskite production due to the complex equipment and slow nature of the process.
We here demonstrate a novel approach to produce large-scale assemblies of sintered perovskite nanocrystals using liquid processing. A liquid-in-liquid impingement process was devised that achieves mechanical coalescence of lead–bromide perovskite nanocrystals into large-scale, free-standing membranes at ambient conditions. Microscopic characterization reveals the merging of nanocrystals into large domains and the abstraction of ligands. Optical spectroscopy demonstrates the increased crystalline quality and suppression of defects. This enhancement leads to improvements in radiative recombination and enhanced stability compared to conventional approaches. Finally, a significantly enhanced carrier transport of the mechano-coalesced perovskite assemblies is observed, which opens up exciting potential in future optoelectronics. We illustrate this ability by realizing photodetectors with superior speed and sensitivity.
Results
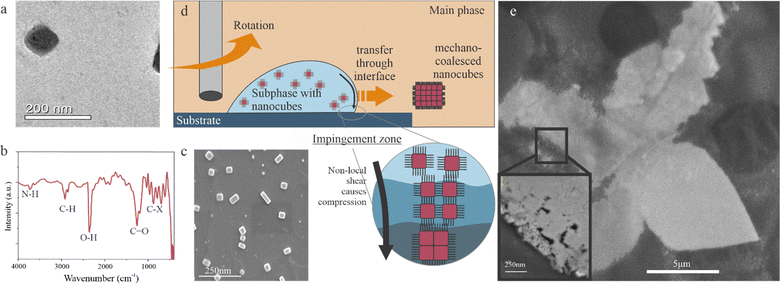
Impingement processes are prevalent in solution processing, such as spray coating, ink-jet printing, spinning disk reactors, and curtain coating, where a fluid deforms while interacting with a solid substrate.26 During impingement, viscous forces produce localized changes in shear within the fluid region27–30 that exert significant forces on particles within the fluid.31,32To demonstrate the suitability of such impingement processing on perovskite coalescence, we devise a simple and scalable approach that overcomes the complex requirements of coating setups and liquid feeding. Our approach utilizes a liquid-in-liquid impingement process. Water is chosen as an insoluble and stationary main phase. A droplet of toluene containing premade perovskite nanocrystals is introduced as a secondary phase. The toluene droplet is positioned at the center of a spinning disk that acts as a moving solid surface (Fig. S6, ESI†). During operation, the droplet moves radially across the disk within the main phase. The simultaneous existence of the stationary main phase, the spinning solid surface, and the rotating secondary phase induces large shear forces in the impingement zone around the triple contact line.30
We investigate the liquid–liquid impingement (LLI) process using lead iodine perovskite nanocrystals due to their established promise in optoelectronics.9 Nanosized CsPbBr3 nanocubes were premade through a reprecipitation method following previous reports.33–35 Transmission electron microscopy confirms the formation of cubes with a side length of 50 nm (Fig. 1(a)). These nanocubes are stabilized by a ligand cap, as demonstrated by the occurrence of amine, carbonyl, and alkane groups in the FTIR spectrum (Fig. 1(b) and Fig. S1, ESI†). Due to the protective ligand cap, the nanocubes are well-dispersed in the aqueous subphase, as confirmed by SEM after drop-casting deposition of a water-CsPbBr3 on a motionless substrate (Fig. 1(c). (Deviations from the ideal cubic shapes are thought to originate from uncontrolled crystal growth dynamics36 and assembly due to non-uniform solvent evaporation rates37 during deposition).
The result changes drastically when the main phase is introduced. Different from the drop casting condition, no deposition is observed on the substrate, and the subphase remains separate from the main phase. When the substrate is rotated at 100 rpm, micrometer-sized flakes occur at the surface of the main phase. This observation suggests that impingement induces the transfer of perovskite particles from the good solvent of the subphase to the bad solvent of the main phase through the liquid–liquid interface (Fig. 1(d)). (More details on the optimization of parameters and an overview of different solvent combinations are included in the ESI†).
The promise of the LLI to sinter perovskite nanocrystals is demonstrated when evaluating the resulting material's morphology. Large flakes are found in the main phase that are mechanically strong enough to retain their integrity even after harvesting from the liquid. Scanning electron microscopy demonstrates their freestanding structure (Fig. 1(e)). The flakes exhibit a continuous morphology with discontinuities only occurring around the edges. Closer inspection of these defective areas confirms the assembly from individual nanocubes (Inset image of Fig. 1(e)). These results indicate that LLI can produce a new assembly type that we term “mechano-coalesced” nanostructures.
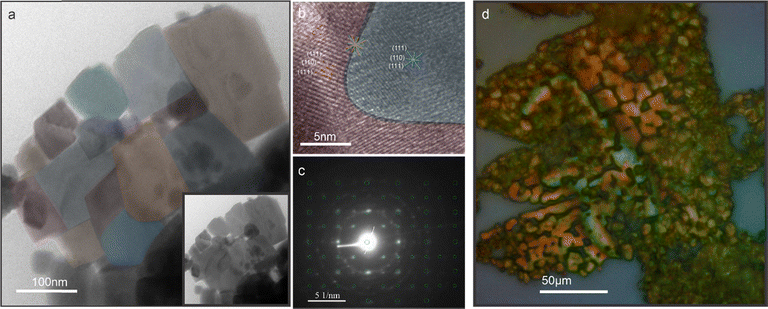
To further elucidate the mechano-coalescing process, we conduct transmission electron microscopy of perovskites after LLI. At the flake edges, the original nanocube structure can still be discerned (Fig. 2(a)). The retention of the microstructure is expected for sintering processes since they proceed below the melting point and coalescence proceeds by interfacial interdiffusion.33,38 Close inspection of the interface between two particles reveals the merging of (110) and (111) crystal planes (identified by their lattice spacing) without a capping layer between them (Fig. 2(b)). This observation further confirms that the pressure experienced during liquid processing is sufficient to strip the ligand cap, as previously observed for high-pressure transitions,39 causing rearrangement at the interface into larger domains. Selected area electron diffraction (SAED) indicates that the orthorhombic structure of the perovskite constituents is retained and that mechano-coalescence produces polycrystalline assemblies (Fig. 2(c)).
The dimension and morphology of the mechano-coalesced material can be adjusted by the processing conditions. The thickness of the flakes depends on the extent of the impingement zone within the subphase droplet, and most of the observed flakes are hundreds of nanometers in thickness. Lateral sintering seems to occur continuously throughout the motion of the droplet and we observe some flakes that exhibit hierarchical microstructures which are composed of well-sintered regions interspersed with less ordered areas (Fig. 2(d)).
Finally, the overall size of the flake is controlled by the expulsion of the sintered flake from the subphase. This transition occurs when the centrifugal force on the growing flake exceeds the surface tension of the liquid–liquid interface. Consequently, a low rotation speed (of 100 rpm) will favor efficient expulsion of small flakes, whereas thicker flakes of up to 100 μm (Fig. S2, ESI†) are produced at high rotation speeds. The radial travel distance of the droplet provides a lower boundary for the rotation speed, and in our current setup, we are able to produce flake with over 100 μm lateral dimension.
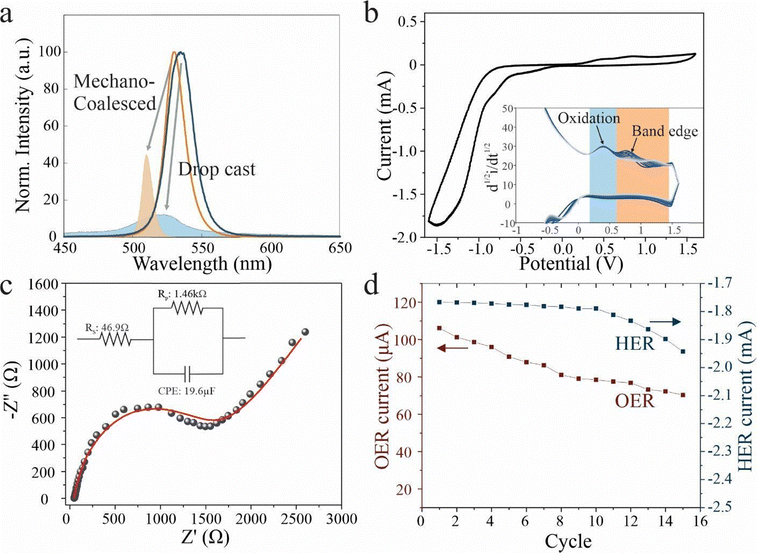
With the LLI process validated, we turn to investigate the impact of mechano-coalescence on the perovskite nanocrystal properties. Optical characterization was conducted on mechano-coalesced perovskites and compared to nanocrystals in solution and drop cast samples. Photoluminescence (PL) spectroscopy demonstrates that deposition produces a red-shift compared to the dispersed nanocrystals, which is indicative of agglomeration:40 Due to the increase of crystallite size, electronic confinement effects are relaxed and the exciton binding energy decreases.41 Moreover, the PL intensity is found to decrease for drop-casting assemblies compared to the pristine material, whereas mechano-coalescence increases it (Fig. 3(a)). This trend agrees with the hypothesis that mechanocoalescence decreases the amount of ligands that control the non-radiative recombination and limit the PL yield through surface states.42
To further test this hypothesis, we carry out temperature-dependent PL measurements. Drop-casted samples show a decrease in peak energy with temperature that indicates the contribution of shallow trap states in the band gap.43 Instead, mechano-coalesced perovskites show fundamentally different behavior with a positive temperature coefficient of emission energy that is similar to previous reports on the intrinsic response of perovskites (Fig. 3(b)).44
To further corroborate the decrease in trap states brought about by mechano-coalescence, we conduct time-resolved photoluminescence (TRPL) (Fig. 3(c)). The TRPL curves for assembled and sintered perovskites were fitted with a conventionally employed bi-exponential decay function.45
I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) + y0 exp(−t/τ2) + y0 |
This model considers the trap-assisted non-radiative recombination at defects associated with a decay lifetime τ1 and radiative recombination with a lifetime τ2. We observe values of τ1 = 1 ns and τ2 = 10 ns. Previous work assigned these decay times to radiative recombination and recombination vs shallow trap states, respectively.46 Based on this characterization,47,48 we observe an increase in radiative contribution from 9% to 74% between drop-cast and mechano-coalesced material. This increase indicates the importance of trap-assisted recombination in limiting our conventional perovskite assemblies and the impact of our enhanced crystallinity on their emission properties.
The decreased defect density also imparts the mechano-coalesced perovskites with superior stability. We find that the PL intensity of conventional assemblies decreases significantly over time (Fig. 4(a)). Instead, the mechano-coalesced sample retains a significantly higher emission intensity and a sharper peak shape after 4 months in ambient conditions. This behavior makes our assembly process promising for overcoming the issues of degradation that are plaguing current perovskite devices.
The demonstrated environmental stability furthermore allows us to explore novel applications of perovskites beyond optoelectronics. Perovskites are considered promising for third-generation photocatalysts that combine easy manufacturing with powerful tunability of exciton generation and conduction pathways.49 However, their limited stability has precluded the use of perovskites in water splitting and CO2 reduction applications.50 To explore the impact of the LLI-enhanced stability, we conduct electrochemical characterization. Cyclic voltammetry demonstrates the occurrence of two oxidation peaks at 0.45 V and 0.8 V, respectively (Fig. 4(b)). Previous work suggested that the first peak originated from the oxidation of Cs.51,52 Upon repeated cycling of the electrochemical oxidation and reduction process, we observe similar reaction currents, suggesting the reversibility of the Cs oxidation process. This observation indicates that Cs oxidation only occurs at the surface and can be reversed by deposition without changes to the overall structure. The second peak represents the electron injection from the valence band into the electrolyte.52 A continuous shift toward higher oxidation energies can be observed (inset Fig. 4(b)), which indicates the gradual p-doping of the material and provides a promising route toward adjusting the electronic properties of electrochemically stable perovskites.
We carry out electrochemical impedance spectroscopy on mechano-coalesced devices that were laterally contacted and then immersed into an electrolyte (Fig. 4(c)). This situation can be modeled with a simple equivalent circuit that contains the electrolyte resistance in series with an interfacial RC circuit. Despite the disadvantageous electrode geometry, we extract an RC constant τ of 28.3 ms which is significantly lower than previous reports53 indicating the enhanced heterogeneous charge transfer rate for electrochemical reactions.
The demonstrated stability of our new perovskite assemblies and good electrocatalytic performance opens up exciting routes for applications in photocatalytic water splitting. We demonstrate electrochemical oxygen and hydrogen evolution reactions on perovskite-based electrodes and find that both electrochemical processes exhibit only small degradation even after extended cycling (Fig. 4(d)), indicating the stability of the electrodes and highlighting the potential of mechano-coalesced perovskites for future applications.
Finally, we investigate the impact of mechano-coalescence on carrier transport due to its known sensitivity to the assembly morphology54 and its practical relevance. For this purpose, we deposit two Au electrodes of 50 μm width with a separation of 100 μm. The current–voltage characteristics exhibit a pronounced non-linearity that is indicative of conduction within a semiconductor. The high-field resistivity was estimated to be 2 × 10−2 Ω m−1 (Fig. 5(a)). This value is orders of magnitude lower than previously reported nanocrystal perovskite assemblies,55 which emphasizes the potential of our mechano-coalescing assembly.
 | ||
| Fig. 5 (a) Output characteristics of mechano-coalesced perovskite device, (b) current–time graph under pulsed illumination, (c) comparison of extracted detectivity to previously reported values on different materials systems (reference details in the ESI†). | ||
The combination of enhanced carrier transport and improved optoelectronic performance opens up new routes to apply perovskite assemblies in future electronics. To illustrate the advance of our work, we apply mechano-coalesced perovskites to photosensing. We toggle a 405 nm laser excitation of 10 mW power and observe a large difference in dark and bright currents. This high photosensitivity and the high transition speeds confirm the optoelectronic performance of our structure (Fig. 5(b)). We quantify this observation by calculating the specific detectivity according to
Conclusion
In conclusion, our work introduces a mechano-coalescence process to assemble perovskite nanocrystals into freestanding flake morphologies with macroscopic dimensions and enhanced optoelectronic properties. Through a liquid–liquid impingement process, localized pressure gradients were realized that abstracted the ligands and merged the crystal planes of neighboring CsPbBr3 nanocubes. This process was shown to decrease the defect density of perovskite assemblies, leading to an 8-fold enhancement in radiative quantum yield and increased environmental stability. This new processing route enables the integration of nanocrystal perovskites into electronic devices with high performance, as demonstrated in electrochemical storage devices and record-breaking photodetectors. The liquid-based sintering approach is not limited to CsPbBr3 perovskites but will be applicable to nanostructured materials that exhibit comparable melting temperature, particle size, and surface energy.58Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as a part of the ESI,† including materials and methods, synthesis of nanocubes and mechano-coalesced flakes, details of equipment used in characterizations, picture of the experimental setup, table of references of previously measured detectivity values, and different solvent systems.References
- D. Zhou, T. Zhou, Y. Tian, X. Zhu and Y. Tu, J. Nanomater., 2018, 2018, 8148072 CrossRef.
- A. Djurišić, F. Z. Liu, H. W. Tam, M. Wong, A. Ng, C. Surya, W. Chen and Z. B. He, Prog. Quantum Electron., 2017, 53, 1–37 CrossRef.
- S. A. Veldhuis, P. P. Boix, N. Yantara, M. Li, T. C. Sum, N. Mathews and S. G. Mhaisalkar, Adv. Mater., 2016, 28, 6804–6834 CrossRef CAS PubMed.
- H. Wang and D. H. Kim, Chem. Soc. Rev., 2017, 46, 5204–5236 RSC.
- Y. Zhao, C. Li and L. Shen, Chin. Phys. B, 2018, 27, 127806 CrossRef CAS.
- M. Curri, R. Comparelli, M. Striccoli and A. Agostiano, Phys. Chem. Chem. Phys., 2010, 12, 11197–11207 RSC.
- H. Huang, L. Polavarapu, J. A. Sichert, A. S. Susha, A. S. Urban and A. L. Rogach, NPG Asia Mater., 2016, 8, e328 CrossRef CAS.
- S. K. Avugadda, A. Castelli, B. Dhanabalan, T. Fernandez, N. Silvestri, C. Collantes, D. Baranov, M. Imran, L. Manna and T. Pellegrino, ACS Nano, 2022, 16, 13657–13666 CrossRef CAS PubMed.
- Z. Liang, S. Zhao, Z. Xu, B. Qiao, P. Song, D. Gao and X. Xu, ACS Appl. Mater. Interfaces, 2016, 8, 28824–28830 CrossRef CAS PubMed.
- S. D. Adhikari, A. F. G. Reyes, S. Paul, J. Torres, B. Escuder, I. Mora-Seró and S. Masi, Chem. Sci., 2023, 14, 8984–8999 RSC.
- C. McDonald, C. Ni, P. Maguire, P. Connor, J. T. Irvine, D. Mariotti and V. Svrcek, Nanomaterials, 2019, 9, 1481 CrossRef CAS PubMed.
- Y. Mu, Z. He, K. Wang, X. Pi and S. Zhou, iScience, 2022, 25(11), 105371 CrossRef CAS PubMed.
- X. Zheng, Y. Hou, H.-T. Sun, O. F. Mohammed, E. H. Sargent and O. M. Bakr, J. Phys. Chem. Lett., 2019, 10, 2629–2640 CrossRef CAS PubMed.
- M. Liu, N. Yazdani, M. Yarema, M. Jansen, V. Wood and E. H. Sargent, Nat. Electron., 2021, 4, 548–558 CrossRef.
- L. Piveteau, M. Aebli, N. Yazdani, M. Millen, L. Korosec, F. Krieg, B. M. Benin, V. Morad, C. Piveteau and T. Shiroka, ACS Cent. Sci., 2020, 6, 1138–1149 CrossRef CAS PubMed.
- A. E. Shalan, Mater. Adv., 2020, 1, 292–309 RSC.
- F. L. d Araújo, A. F. Nogueira and J. N. d Freitas, J. Braz. Chem. Soc., 2023, 34, 794–808 Search PubMed.
- A. Jana, A. Meena, S. A. Patil, Y. Jo, S. Cho, Y. Park, V. G. Sree, H. Kim, H. Im and R. A. Taylor, Prog. Mater. Sci., 2022, 129, 100975 CrossRef CAS.
- A. Jancik Prochazkova, M. C. Scharber, C. Yumusak, J. Jančík, J. Másilko, O. Brüggemann, M. Weiter, N. S. Sariciftci, J. Krajcovic and Y. Salinas, Sci. Rep., 2020, 10, 15720 CrossRef CAS PubMed.
- H. Wang, C. Luo, P. Tian, D. Li, C. Jiang, C. Zhong, S. Chen, R. Huang, H. Lin and H. Peng, J. Colloid Interface Sci., 2018, 529, 575–581 CrossRef CAS PubMed.
- Y. Xing, N. Yazdani, W. M. Lin, M. Yarema, R. Zahn and V. Wood, ACS Appl. Electron. Mater., 2022, 4, 631–642 CrossRef CAS.
- I. Konidakis, A. Karagiannaki and E. Stratakis, Nanoscale, 2022, 14, 2966–2989 RSC.
- J. Hou, P. Chen, A. Shukla, A. Krajnc, T. Wang, X. Li, R. Doasa, L. H. Tizei, B. Chan and D. N. Johnstone, Science, 2021, 374, 621–625 CrossRef CAS PubMed.
- Y. Nagaoka, K. Hills-Kimball, R. Tan, R. Li, Z. Wang and O. Chen, Adv. Mater., 2017, 29, 1606666 CrossRef PubMed.
- K. Hills-Kimball, H. Yang, T. Cai, J. Wang and O. Chen, Adv. Sci., 2021, 8, 2100214 CrossRef CAS PubMed.
- K. Miyamoto and Y. Katagiri, Liquid film coating: scientific principles and their technological implications, Springer, 1997, pp. 463–494 Search PubMed.
- X. Li, F. Bodziony, M. Yin, H. Marschall, R. Berger and H.-J. Butt, Nat. Commun., 2023, 14, 4571 CrossRef CAS PubMed.
- D. Jacqmin, J. Fluid Mech., 2000, 402, 57–88 CrossRef CAS.
- H. Li, Y. Zhang, X. S. Gu, H. Qi, J. Yu and J. Zhuang, Colloids Surf., A, 2022, 653, 130046 CrossRef CAS.
- T. D. Blake, J. Colloid Interface Sci., 2006, 299, 1–13 CrossRef CAS PubMed.
- D. Bolleddula, A. Berchielli and A. Aliseda, Adv. Colloid Interface Sci., 2010, 159, 144–159 CrossRef CAS PubMed.
- M. Nicolas, J. Fluid Mech., 2005, 545, 271–280 CrossRef.
- S. Ullah, J. Wang, P. Yang, L. Liu, S.-E. Yang, T. Xia, H. Guo and Y. Chen, Mater. Adv., 2021, 2, 646–683 RSC.
- S. Akhil, V. V. Dutt and N. Mishra, Nanoscale Adv., 2021, 3, 2547–2553 RSC.
- S. Sun, D. Yuan, Y. Xu, A. Wang and Z. Deng, ACS Nano, 2016, 10, 3648–3657 CrossRef CAS PubMed.
- C. Zuo, A. D. Scully, W. L. Tan, F. Zheng, K. P. Ghiggino, D. Vak, H. Weerasinghe, C. R. McNeill, D. Angmo and A. S. Chesman, Commun. Mater., 2020, 1, 33 CrossRef.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed.
- F. Palazon, S. Dogan, S. Marras, F. Locardi, I. Nelli, P. Rastogi, M. Ferretti, M. Prato, R. Krahne and L. Manna, J. Phys. Chem. C, 2017, 121, 11956–11961 CrossRef CAS PubMed.
- L. Lu, S. Zou and B. Fang, ACS Catal., 2021, 11, 6020–6058 CrossRef CAS.
- L. Xu, J. Li, T. Fang, Y. Zhao, S. Yuan, Y. Dong and J. Song, Nanoscale Adv., 2019, 1, 980–988 RSC.
- J. Zhang, J. He, L. Yang and Z. Gan, Molecules, 2020, 25, 1151 CrossRef CAS PubMed.
- S. Xiong, F. Tian, F. Wang, A. Cao, Z. Chen, S. Jiang, D. Li, B. Xu, H. Wu and Y. Zhang, Nat. Commun., 2024, 15, 5607 CrossRef CAS PubMed.
- S. Kahmann, E. K. Tekelenburg, H. Duim, M. E. Kamminga and M. A. Loi, Nat. Commun., 2020, 11, 2344 CrossRef CAS PubMed.
- H. Zheng and J. Dai, Mater. Lett., 2017, 188, 232–234 CrossRef CAS.
- E. V. Péan, S. Dimitrov, C. S. De Castro and M. L. Davies, Phys. Chem. Chem. Phys., 2020, 22, 28345–28358 RSC.
- Y.-H. Chen, K.-A. Tsai, T.-W. Liu, Y.-J. Chang, Y.-C. Wei, M.-W. Zheng, S.-H. Liu, M.-Y. Liao, P.-Y. Sie and J.-H. Lin, J. Phys. Chem. Lett., 2022, 14, 122–131 CrossRef PubMed.
- J. M. Cleveland, T. A. Welsch, E. Y. Chen, D. B. Chase, M. F. Doty and H. Y. Ramírez-Gómez, J. Phys. Chem. C, 2024, 5893–5904 Search PubMed.
- M. R. Subramaniam, A. K. Pramod, S. A. Hevia and S. K. Batabyal, J. Phys. Chem. C, 2022, 126, 1462–1470 CrossRef CAS.
- J. George, A. P. Joseph and M. Balachandran, Int. J. Energy Res., 2022, 46, 21856–21883 CrossRef.
- K. Bienkowski, R. Solarska, L. Trinh, J. Widera-Kalinowska, B. Al-Anesi, M. Liu, G. K. Grandhi, P. Vivo, B. Oral and B. Yılmaz, ACS Catal., 2024, 14, 6603–6622 CrossRef CAS PubMed.
- V. Kumar, S. K. Patel, V. Vyas, D. Kumar, E. S. S. Iyer and A. Indra, Chem. Sci., 2024, 15, 13218–13226 RSC.
- G. F. Samu, R. A. Scheidt, P. V. Kamat and C. Janáky, Chem. Mater., 2018, 30, 561–569 CrossRef CAS PubMed.
- B. Roose, K. Dey, M. R. Fitzsimmons, Y.-H. Chiang, P. J. Cameron and S. D. Stranks, ACS Energy Lett., 2024, 9, 442–453 CrossRef CAS PubMed.
- R. Ollearo, J. Wang, M. J. Dyson, C. H. Weijtens, M. Fattori, B. T. Van Gorkom, A. J. Van Breemen, S. C. Meskers, R. A. Janssen and G. H. Gelinck, Nat. Commun., 2021, 12, 7277 CrossRef CAS PubMed.
- R. Hawrami, L. Matei, E. Ariesanti, V. Buliga, H. Parkhe, A. Burger, J. Stewart, A. Piro, F. De Figueiredo and A. Kargar, Materials, 2024, 17, 5360 CrossRef CAS PubMed.
- M. T. Hossain, M. Das, J. Ghosh, S. Ghosh and P. Giri, Nanoscale, 2021, 13, 14945–14959 Search PubMed.
- J. Wang, J. Zhang, Y. Zhou, H. Liu, Q. Xue, X. Li, C.-C. Chueh, H.-L. Yip, Z. Zhu and A. K. Jen, Nat. Commun., 2020, 11, 177 CrossRef CAS PubMed.
- J. Guo, R. Floyd, S. Lowum, J.-P. Maria, T. Herisson de Beauvoir, J.-H. Seo and C. A. Randall, Annu. Rev. Mater. Res., 2019, 49, 275–295 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: (i) material, methods and characterization, (ii) FTIR, SEM and TEM, (iii) temperature-dependent photoluminescence (TDPL), (iv) I–V measurements (v) detectivity comparison table of different material composites, (vi) experimental setup and (vii) table of solvent system trials. See DOI: https://doi.org/10.1039/d5nh00272a |
| This journal is © The Royal Society of Chemistry 2025 |