 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Colloidal synthesis and etching yield monodisperse plasmonic quasi-spherical Mg nanoparticles†
Andrey
Ten
 ab,
Christina
Boukouvala
ab,
Christina
Boukouvala
 ab,
Vladimir
Lomonosov
ab,
Vladimir
Lomonosov
 *ab and
Emilie
Ringe
*ab and
Emilie
Ringe
 *ab
*ab
aDepartment of Materials Science and Metallurgy, University of Cambridge, 27 Charles Babbage Road, Cambridge CB3 0FS, UK. E-mail: vl318@cam.ac.uk; er407@cam.ac.uk
bDepartment of Earth Sciences, University of Cambridge, Downing Street, Cambridge CB2 3EQ, UK
First published on 22nd May 2025
Abstract
Mg is a low-cost, earth-abundant, and biocompatible plasmonic metal. Fine tuning of its optical response, required for successful light-harvesting applications, can be achieved by controlling Mg nanoparticle size and shape. Mg's hexagonal close packed crystal structure leads to the formation of a variety of unique shapes in colloidal synthesis, ranging from single crystalline hexagonal platelets to twinned rods. Yet, shape control in colloidal Mg nanoparticle synthesis is challenging due to complex nucleation and growth kinetics. Here, we present an approach to manipulate Mg nanoparticle shape by one-pot synthesis followed by colloidal etching with polycyclic aromatic hydrocarbons. We demonstrate how tips and edges in faceted Mg nanoparticles can be preferentially etched to produce quasi-spherical nanoparticles with smooth surfaces. The developed approach provides an essential shape control tool in colloidal Mg synthesis potentially applicable to other oxidising metals.
New conceptsThe size and shape of nano-objects determine their properties, including localised surface plasmon resonance energy in plasmonic metals. Such dependence provides a large tuning range but exerts strong pressures on the homogeneity of materials. This paper reports an approach for colloidal etching of metallic nanoparticles that leads to monodisperse quasi-spherical structures. Specifically, the etching is performed by complexation of the surface metal atoms with polycyclic aromatic hydrocarbons, an approach departing from the established oxygen-based etching. This new concept enables the manipulation of shape in oxidation-prone metals, which we exemplify here by transforming faceted nanostructures of Mg into smooth quasi-spheres. We then show that the metallic character and associated plasmonic response of Mg nanoparticles are maintained through the etching process. We anticipate that this concept of oxygen-free etching will unlock the synthesis of new shapes in a monodisperse fashion for other oxidation-sensitive metallic nanoparticles. |
Mg nanostructures sustain localised surface plasmon resonances (LSPRs), coherent oscillations of free electrons driven by an electric field. LSPRs have been traditionally studied and utilised in Au and Ag nanostructures but more recently alternative metals such as Cu,1,2 Al,3,4 and Mg5 have been investigated.6 Mg stands out for its inherent biocompatibility, low cost, earth-abundance, and competitive plasmonic performance spanning ultraviolet, visible, and near-infrared (UV-vis-NIR) frequencies.5 Consequently, a wide range of applications including optical devices,7,8 sensing,9–13 light-enhanced catalysis,14–16 surface-enhanced Raman scattering,16 and photothermal heating17,18 are explored to utilise Mg's plasmonic properties.
Controllable and cost-effective synthetic approaches are essential to leverage Mg's advantages over other plasmonic metals. Colloidal synthesis is a scalable bottom-up approach that produces nanoparticles (NPs) that can be easily stored, modified, and transported.19 In colloidal synthesis, the size and shape of NPs are affected by several factors including the type of precursor, reducing agent, and capping ligand, as well as reaction conditions.20,21 In addition, synthesis can be performed in multiple steps, for instance via seed-mediated growth to further enable tuning of size and shape.22–24
The reduction of an Mg halide with an alkali metal has first been demonstrated in the 1970s by Rieke et al.,25,26 and inspired recent explorations for the colloidal synthesis of Mg NPs by reducing di-n-butyl magnesium (MgBu2) with lithium in the presence of a polycyclic aromatic hydrocarbon electron carrier.27–31 The synthetic details are now established for Mg single crystalline hexagonal platelets and twinned rods of 80 to 1300 nm.28 A seed-mediated growth method for Mg NPs was also reported recently, yielding faceted Mg NPs with narrow size distribution.29 Colloidally synthesised Mg NPs remain stable and plasmonic in air up to 400 °C owing to the spontaneous formation of a protective, self-limiting surface oxide layer.32
The plasmonic properties of NPs are directly affected by their shape and size. NPs adopt a shape that either minimises their thermodynamic surface energy (thermodynamic regime) or is controlled by facet-specific growth rate (kinetic regime) as captured by various Wulff constructions.33,34 Colloidally synthesised Mg NPs naturally form faceted shapes, as do many metallic NPs.35,36 Of the various shapes, spheres provide the most homogeneous optical response, which is particularly important for applications involving NP self-assemblies or aggregates.37,38 Oxidative etching is a common approach to produce metallic nanospheres.39,40 Particularly, halide ions in the presence of oxygen can selectively etch low-coordinated surface atoms of Au,41–47 Ag,48,49 Pd,50 Pt,51 and Rh52 NPs, leading to corner rounding and aspect ratio reduction. Roundness can be further improved by iterative growth and etching steps.45–47 Under certain conditions, oxidative etching can even selectively remove twinned NPs53–55 and slow down growth kinetics,56 making it an effective method to control colloidal synthesis.40 Balancing oxidative processes with reductive steps allows for the formation of NPs with unique shapes and sizes.41–59 However, oxidative etching cannot be used for readily oxidising metals due the formation of a surface oxide layer that prevents further morphological modifications.
Here, we report a one-pot colloidal etching approach akin to oxidative etching using polycyclic aromatic hydrocarbons in the absence of oxygen. When applied to Mg colloidal synthesis, this methodology transforms faceted Mg NPs into monodisperse quasi-spherical Mg NPs. We first investigate the reaction dynamics of seed-mediated growth of Mg to establish when the reduction slows down sufficiently to enable a successive etching step. We then find that the addition of excess naphthalene to colloidal Mg NP synthesis selectively etches edges and corners of faceted Mg NPs and produces plasmonic quasi-spherical Mg NPs. The etching mechanism reported here is a promising method for shape-controlled synthesis of oxidising metals such as Mg, and provides an approach for reproducible and monodisperse NPs.
The seed-mediated growth of faceted NPs and subsequent etching (leading to rounding) into quasi-spheres were conducted under an inert atmosphere in one pot. The reaction scheme (Fig. 1) consists of two reduction stages leading to monodisperse faceted Mg NPs, followed by the etching stage where the NPs are transformed into smooth quasi-spheres. Poly(vinyl pyrrolidone) (PVP) was added from the beginning of the synthesis to minimise NP aggregation. The effects of PVP in Mg NP synthesis have been studied by Wayman et al.30 The reaction was quenched with acetone at different times to analyse the reaction progress. The resulting NPs were imaged with scanning electron microscopy (SEM) and their average size was measured by fitting over a hundred NPs per sample to an ellipse using an automated procedure and obtaining their major axis lengths (details in ESI†). The aspect ratios in all cases were close to 1 and in the following text we will describe this length as diameter.
The first stage in the growth of faceted Mg NPs involves reducing MgBu2 with lithium naphthalene dianion (Li2Napht). The highly negative reduction potential of Li2Napht, comparable to that of Li0 (reduction potential of Li is E0 = −3.04 V),60 generated monodisperse Mg NPs. The nucleation and growth dynamics in this step were tracked by collecting aliquots from the same flask over the course of the reaction (Fig. 2). After 3 min of reaction, NPs were grown to 57 ± 9 nm (diameter ± standard deviation) and continued growing to 84 ± 6 nm after 11 min (Fig. 2A, D, and Fig. S1, ESI†). At shorter reaction times, SEM and transmission electron microscopy (TEM) images suggest the presence of smaller NPs. However, their size could not be measured reliably because Mg NPs were aggregated and covered by surrounding contaminants produced from reaction quenching when significant MgBu2 is present. The low atomic number (Z) of Mg produces limited contrast between the NPs and the contaminants. Additionally, surface oxidation in NPs <50 nm leads to a majority oxide volume and could be associated with shape and size changes, leading to unrepresentative images. Between 3 and 11 min, both the reaction yield (conversion of the organometallic Mg2+ precursor into solid Mg) obtained from inductively coupled plasma optical emission spectroscopy (ICP-OES) and the average volume (ESI†) of a single NP increased linearly (Fig. 2E and Table S1, ESI†). Together, these trends confirm that Mg ions reduced to metal during this period are mainly involved in the growth of NPs, i.e., that nucleation wanes by 3 min. Longer reactions in the first reduction stage yielded a bimodal colloid, as large hexagonal platelets begin forming in the reaction mixture beyond 11 min (Fig. S2, ESI†), an outcome previously reported when Li2Napht depletes to ∼30% of its initial concentration.29
 | ||
| Fig. 2 Reaction evolution of the first reduction stage of the growth of faceted Mg NPs, sampled between 3 and 11 min of reaction time. (A) SEM images of representative NPs, labelled with the reaction time as well as average diameter and its standard deviation. (B) Colloidal Mg NPs and (C) their UV-vis-NIR extinction spectra. (D) Size distribution histogram showing the diameter of Mg NPs obtained from SEM images after 5 and 9 min of reaction, N = 166 and 100, respectively. Equivalent data for other reaction times are reported in Fig. S1 (ESI†). (E) Reaction yield from ICP-OES (circles, black solid line) and average NP volume from SEM images (triangles, grey dashed line) as a function of reaction time showing NP growth; lines represent the linear best fit. | ||
The Mg NPs obtained after one reduction step were plasmonic. The presence of Mg0 and long-term air stability were confirmed by powder X-ray diffraction (XRD) of dried Mg NPs in ambient air, revealing peaks corresponding to hexagonal close packed (HCP) Mg (Fig. S3, ESI†). The UV-vis-NIR extinction spectrum displayed an LSPR peak that redshifted from 400 to 473 nm and became more intense as NPs grew from 57 to 84 nm in diameter (Fig. 2C). The narrowness of this peak indicates homogeneity in size and shape as well as high dispersity of the NPs (i.e., low aggregation). With resonances in the visible region, the colloids displayed strong colours ranging from yellow to brownish red with increasing NP size (Fig. 2B); however, this distinct colour change is in large parts due to an increase in extinction caused by larger and more concentrated NPs (Fig. S4, ESI†), with a small contribution from the 73 nm LSPR redshift.
Converting Li2Napht to lithium naphthalene radical anion (LiNapht, E0 = −2.73 V, from Wawzonek and Laitinen61 and Meerholz and Heinze62) leads to uniform growth of the Mg NPs formed at the first reduction stage and suppresses the formation of a bimodal colloid.29 The addition of stoichiometric quantities of naphthalene instantly converted Li2Napht into LiNapht, as confirmed by the change in colour from purple to dark green.29,63 The timing of naphthalene injection, i.e., the start of the second reduction stage, influences the outcome obtained after 1 h of growth. Injection of naphthalene within the first 5 min of the first stage produced a mixture of faceted NPs with single crystalline and twinned Mg platelets (Fig. S5, ESI†). The presence of the latter indicates the formation of new nuclei in LiNapht environment. Indeed, starting the reaction directly from the second stage (only LiNapht, no Li2Napht) also produces such Mg platelets.28 Therefore, the precursor concentration must be sufficiently lowered before the Li2Napht to LiNapht conversion to avoid nucleation at the second stage. Meanwhile, conversion between 5 and 9 min of the first stage produced monodisperse Mg NPs (Fig. S6, ESI†). Lastly, conversion beyond 9 min resulted in bimodal colloids (Fig. S5, ESI†), grown from the mixture of faceted NPs and hexagonal platelets produced from longer reaction times (over 9 min) in the first stage (Fig. S2, ESI†).
The growth dynamics over the course of the second reduction stage, following a 5 min reaction in the first stage, revealed that Mg NPs grow steadily for 15 min, then the reduction considerably slows down. The diameter of NPs doubled from 82 ± 8 (1 min) to 170 ± 17 nm within the first 15 min and remained constant thereafter as measured from SEM images and confirmed by DLS (Fig. 3, Fig. S7, S8, and Table S2, ESI†). Similar trends were observed in reaction yield and average single NP volume: both increased rapidly in the first 10 min and plateaued by 20 min (Fig. 3E). The overlap of these trends confirms previous observations that reduced Mg atoms contribute to the growth of existing Mg NPs rather than nucleating new NPs. By 10 min of the second stage, the yield reached 28%, much higher than the 1% yield obtained in a single-step reduction of MgBu2 with LiNapht, reported previously for the same reaction time.30 The rate of reduction in the seed-mediated synthesis is thus much higher compared to that of the single-step approach.
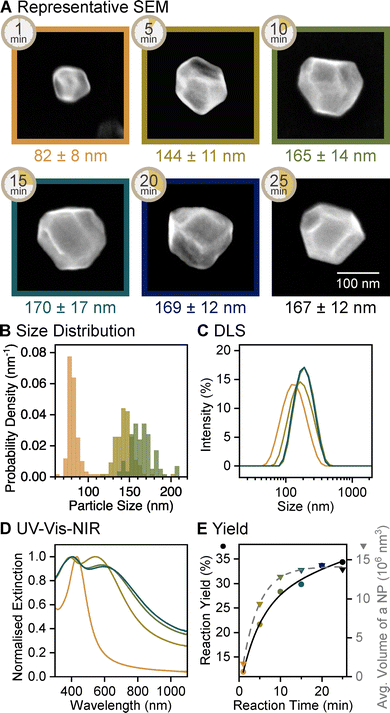 | ||
| Fig. 3 Reaction evolution of the second reduction stage of the growth of faceted Mg NPs, sampled between 1 and 25 min of reaction time within this stage, following a 5 min reaction in the first reduction stage. (A) SEM images of representative NPs, labelled with the reaction time as well as average diameter and its standard deviation. (B) Size distribution histogram showing the diameter of Mg NPs obtained from SEM images after 1, 5, and 10 min of reaction, N = 119, 109, and 117, respectively. Equivalent data for other reaction times are reported in Fig. S7 (ESI†). (C) Size distribution of Mg NPs after 1, 5, 10, and 15 min of reaction obtained from DLS and (D) their UV-vis-NIR extinction spectra. Equivalent data for other reactions are reported in Fig. S8 and S9 (ESI†). (E) Reaction yield from ICP-OES (circles, black solid line) and average NP volume measured from SEM images (triangles, grey dashed line) as a function of reaction time; lines represent the best exponential (association) fit. | ||
Mg NPs remain Mg0 throughout their growth. The dipolar LSPR mode in their UV-vis-NIR extinction spectra (Fig. 3D and Fig. S9, ESI†) redshifted to 600 nm as NPs grew to their final size. A quadrupolar mode, characterised by an extinction maximum around 400 nm, appeared as NPs reached 144 ± 11 nm in diameter, after 10 min of growth. Such changes to the extinction profiles indicate the growth of Mg metal (Fig. S10, ESI†); an increase of the oxide layer thickness resulting in the same size change was ruled out as we calculated it would cause a <100 nm redshift and no dominant quadrupolar mode (Fig. S11, ESI†). The presence of Mg0 and stability of the final Mg NPs were further confirmed by obtaining clear HCP Mg XRD pattern from a freshly dried sample and the same sample left in ambient air for 15 days (Fig. S3, ESI†).
Naphthalene and anthracene can etch Mg NPs. Either of the two polycyclic aromatic hydrocarbons was added to the reaction mixture in equal amounts as the reducing agent after 60 min of second stage reaction, when no Mg yield change was detected. Naphthalene addition caused etching of the sharp edges and corners in faceted NPs. Under stirring with naphthalene, faceted NPs of 166 ± 12 nm were etched into quasi-spheres of 154 ± 14 nm in diameter over the course of 60 min at room temperature, as measured by SEM (Fig. 4A, B and Fig. S12, ESI†). The resulting Mg NPs had no discernible extended flat facets, edges, or corners (Fig. 4C and Fig. S13, ESI†). Equivalent experiments with anthracene as the etchant resulted in significant dissolution of Mg NPs. After 60 min, the amount of Mg considerably decreased and NP surface was rough (Fig. S14, ESI†), indicating a rapid and non-uniform etching that is more aggressive than with naphthalene. The extent of etching with anthracene further increased at higher reaction temperature (Fig. S14, ESI†). By contrast, for naphthalene, increasing the temperature to 40 °C led to further growth of Mg NPs instead of etching, indicating that the rate of etching with naphthalene is outpaced by the promoted growth rate.
 | ||
| Fig. 4 Reaction evolution and outcome of the etching stage in quasi-spherical Mg NP synthesis, sampled between 1 and 60 min of reaction time within this stage, following 5 and 60 min reaction times in first and second reduction stages, respectively. (A) SEM images of representative NPs, labelled with the reaction time as well as average diameter and its standard deviation. (B) Size distribution showing the diameter of Mg NPs obtained from SEM images after 1 and 60 min of reaction, N = 101 and 119, respectively. Equivalent data after 30 min of reaction are reported in Fig. S12 (ESI†). (C) High-angle annular dark-field scanning TEM (HAADF-STEM) image of quasi-spherical Mg NPs. (D) UV-vis-NIR extinction spectra after 1, 30, and 60 min of reaction. (E) XRD pattern of quasi-spherical Mg NPs after 60 min of reaction, displaying peaks corresponding to HCP Mg (PDF 00-035-0821). | ||
The etching process likely occurs by redissolution of Mg via complex formation with naphthalene or anthracene. Complexing of Mg with polycyclic aromatic hydrocarbons has been reported with anthracene in THF,64 and is expected to occur for naphthalene based on their chemical similarity. During etching with naphthalene, the volume of Mg NPs decreased by 15% (Table 1). This volume loss was consistent with the 14% decrease, from 0.81 to 0.70 mg mL−1, in amount of Mg in the colloid measured by ICP-OES. Therefore, etched Mg is understood to have been redissolved in the solution and subsequently removed during centrifugation and resuspension steps.
| Reaction time in rounding stage (min) | Average NP size (diameter, nm) | Average NP volume (nm3) | Concentration of NPs (NPs per mL) |
|---|---|---|---|
| 1 | 166 ± 12 | 1.36 × 107 | 3.44 × 1010 |
| 30 | 158 ± 13 | 1.20 × 107 | 3.49 × 1010 |
| 60 | 154 ± 14 | 1.15 × 107 | 3.53 × 1010 |
Colloidal etching with naphthalene and anthracene occurs only for Mg0, as would be expected from the formation of a complex. Indeed, post-synthetic addition of naphthalene and anthracene after oxide formation (through quenching and cleaning) did not result in shape change (Fig. S15, ESI†).
Mg NPs consisted mainly of Mg0 after etching with naphthalene, as confirmed by XRD (Fig. 4E), and therefore remained plasmonic (Fig. 4D). The decrease in the NP size upon etching is reflected by the blueshift of the dipolar LSPR peak in the UV-vis-NIR extinction spectra (Fig. 4D), as shape change alone does not lead to such shift (Fig. S16, ESI†).
In this work, we described a one-pot synthetic approach for colloidal etching of Mg NPs by redissolution of Mg0 in the presence of polycyclic aromatic hydrocarbons in the absence of oxygen. Naphthalene was incorporated in the third stage of the reaction to preferentially etch corners and edges of faceted NPs to yield smooth quasi-spherical Mg NPs. The approach presented here provides an important shape control tool in colloidal NP synthesis. The resulting protocol produces quasi-spherical Mg NPs, a notable addition to the Mg NP library, as the most isotropic colloidal Mg NP available to date.
Author contributions
Andrey Ten: data curation, formal analysis, investigation, methodology, software, validation, writing – original draft, writing – review & editing. Christina Boukouvala: formal analysis, methodology, software, writing – original draft. Vladimir Lomonosov: conceptualization, investigation, methodology, project administration, supervision, validation, writing – original draft, writing – review & editing. Emilie Ringe: funding acquisition, investigation, project administration, resources, supervision, writing – original draft, writing – review & editing.Data availability
Additional experimental and analytical details, calculated average volumes and numbers, SEM images, size distributions from SEM and DLS, photographs, UV-vis-NIR spectra, XRD patterns, shape used for numerical calculations, and simulated extinction cross sections of Mg NPs supporting this article have been included as part of the ESI.† All SEM images used for size measurements are available in Cambridge University's Apollo repository at https://doi.org/10.17863/CAM.117186. The code for Stratify65 can be found at https://gitlab.com/iliarasskazov/stratify. The version of the code employed for this study is version 1.1. The code for DDSCAT66 can be found at https://ddscat.wikidot.com. The version of the code employed for this study is version 7.3.2.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Authors thank Dr Nigel Howard and Viyaleta Tratsiakova for ICP-OES acquisition. Support for this project was provided by the EU Framework Programme for Research and Innovation Horizon 2020 (ERC Starting Grant SPECs 804523) and the Engineering and Physical Sciences Research Council (EPSCR) grant EP/W015986/1 (MagNanoThermo).References
- S. Jeong, Y. Liu, Y. Zhong, X. Zhan, Y. Li, Y. Wang, P. M. Cha, J. Chen and X. Ye, Nano Lett., 2020, 20, 7263–7271 CrossRef CAS PubMed.
- V. Mkhitaryan, K. March, E. N. Tseng, X. Li, L. Scarabelli, L. M. Liz-Marzán, S.-Y. Chen, L. H. G. Tizei, O. Stéphan, J.-M. Song, M. Kociak, F. J. García de Abajo and A. Gloter, Nano Lett., 2021, 21, 2444–2452 CrossRef CAS PubMed.
- D. F. Swearer, H. Zhao, L. Zhou, C. Zhang, H. Robatjazi, J. M. P. Martirez, C. M. Krauter, S. Yazdi, M. J. McClain, E. Ringe, E. A. Carter, P. Nordlander and N. J. Halas, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 8916–8920 CrossRef CAS PubMed.
- M. W. Knight, N. S. King, L. Liu, H. O. Everitt, P. Nordlander and N. J. Halas, ACS Nano, 2014, 8, 834–840 CrossRef CAS PubMed.
- E. Ringe, J. Phys. Chem. C, 2020, 124, 15665–15679 CrossRef CAS PubMed.
- E. R. Hopper, C. Boukouvala, J. Asselin, J. S. Biggins and E. Ringe, J. Phys. Chem. C, 2022, 126, 10630–10643 Search PubMed.
- T. Gong, P. Lyu and M. S. Leite, ACS Appl. Opt. Mater., 2023, 1, 825–831 CrossRef CAS PubMed.
- X. Duan, S. Kamin and N. Liu, Nat. Commun., 2017, 8, 14606 CrossRef CAS PubMed.
- X. Duan, S. Kamin, F. Sterl, H. Giessen and N. Liu, Nano Lett., 2016, 16, 1462–1466 CrossRef CAS PubMed.
- H. H. Jeong, A. G. Mark and P. Fischer, Chem. Commun., 2016, 52, 12179–12182 RSC.
- J. M. Sanz, D. Ortiz, R. Alcaraz de la Osa, J. M. Saiz, F. González, A. S. Brown, M. Losurdo, H. O. Everitt and F. Moreno, J. Phys. Chem. C, 2013, 117, 19606–19615 CrossRef CAS.
- F. Sterl, N. Strohfeldt, R. Walter, R. Griessen, A. Tittl and H. Giessen, Nano Lett., 2015, 15, 7949–7955 CrossRef CAS PubMed.
- F. Sterl, H. Linnenbank, T. Steinle, F. Mörz, N. Strohfeldt and H. Giessen, Nano Lett., 2018, 18, 4293–4302 Search PubMed.
- V. Lomonosov, T. M. R. Wayman, E. R. Hopper, Y. P. Ivanov, G. Divitini and E. Ringe, Nanoscale, 2023, 15, 7420–7429 Search PubMed.
- S. J. Patil, V. Lomonosov, E. Ringe and D. Kurouski, J. Phys. Chem. C, 2023, 127, 7702–7706 CrossRef CAS PubMed.
- A. Ten, V. Lomonosov, C. Boukouvala and E. Ringe, ACS Nano, 2024, 18, 18785–18799 CrossRef CAS PubMed.
- C. A. West, V. Lomonosov, Z. S. Pehlivan and E. Ringe, Nano Lett., 2023, 23, 10964–10970 Search PubMed.
- Q. Wang, L. Xie, Z. He, D. Di and J. Liu, Int. J. Nanomed., 2012, 7, 4715–4725 CrossRef CAS PubMed.
- P. Yang, J. Zheng, Y. Xu, Q. Zhang and L. Jiang, Adv. Mater., 2016, 28, 10508–10517 CrossRef CAS PubMed.
- A. R. Tao, S. Habas and P. Yang, Small, 2008, 4, 310–325 Search PubMed.
- M. Wuithschick, B. Paul, R. Bienert, A. Sarfraz, U. Vainio, M. Sztucki, R. Kraehnert, P. Strasser, K. Rademann, F. Emmerling and J. Polte, Chem. Mater., 2013, 25, 4679–4689 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Langmuir, 2001, 17, 6782–6786 Search PubMed.
- Y. Xia, K. D. Gilroy, H.-C. Peng and X. Xia, Angew. Chem., 2017, 56, 60–95 CrossRef CAS PubMed.
- J. S. Googasian, G. R. Lewis, Z. J. Woessner, E. Ringe and S. E. Skrabalak, Chem. Commun., 2022, 58, 11575–11578 RSC.
- R. D. Rieke and P. M. Hudnall, J. Am. Chem. Soc., 1972, 94, 7178–7179 CrossRef CAS.
- R. D. Rieke and S. E. Bales, J. Am. Chem. Soc., 1974, 96, 1775–1781 CrossRef CAS.
- J. S. Biggins, S. Yazdi and E. Ringe, Nano Lett., 2018, 18, 3752–3758 CrossRef CAS PubMed.
- E. R. Hopper, T. M. R. Wayman, J. Asselin, B. Pinho, C. Boukouvala, L. Torrente-Murciano and E. Ringe, J. Phys. Chem. C, 2022, 126, 563–577 CrossRef CAS PubMed.
- V. Lomonosov, E. R. Hopper and E. Ringe, Chem. Commun., 2023, 59, 5603–5606 RSC.
- T. M. R. Wayman, V. Lomonosov and E. Ringe, J. Phys. Chem. C, 2024, 128, 4666–4676 CrossRef CAS PubMed.
- W. Liu and K.-F. Aguey-Zinsou, J. Mater. Chem. A, 2014, 2, 9718–9726 RSC.
- V. Lomonosov, J. Yang, Y. Fan, S. Hofmann and E. Ringe, Nano Lett., 2024, 24, 7084–7090 CrossRef CAS PubMed.
- E. Ringe, R. P. Van Duyne and L. D. Marks, J. Phys. Chem. C, 2013, 117, 15859–15870 CrossRef CAS.
- C. Boukouvala, J. Daniel and E. Ringe, Nano Convergence, 2021, 8, 26 CrossRef CAS PubMed.
- M. R. Langille, J. Zhang, M. L. Personick, S. Li and C. A. Mirkin, Science, 2012, 337, 954–957 CrossRef CAS PubMed.
- E. Ringe, IUCrJ, 2014, 1, 530–539 CrossRef CAS PubMed.
- J. Lee, J.-H. Huh, K. Kim and S. Lee, Adv. Funct. Mater., 2018, 28, 1707309 CrossRef.
- K. J. Park, J.-H. Huh, D.-W. Jung, J.-S. Park, G. H. Choi, G. Lee, P. J. Yoo, H.-G. Park, G.-R. Yi and S. Lee, Sci. Rep., 2017, 7, 6045 CrossRef PubMed.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., 2009, 48, 60–103 CrossRef CAS PubMed.
- Y. Zheng, J. Zeng, A. Ruditskiy, M. Liu and Y. Xia, Chem. Mater., 2014, 26, 22–33 CrossRef CAS.
- J. Rodríguez-Fernández, J. Pérez-Juste, P. Mulvaney and L. M. Liz-Marzán, J. Phys. Chem. B, 2005, 109, 14257–14261 CrossRef PubMed.
- C. M. Aguirre, T. R. Kaspar, C. Radloff and N. J. Halas, Nano Lett., 2003, 3, 1707–1711 CrossRef CAS.
- C.-K. Tsung, X. Kou, Q. Shi, J. Zhang, M. H. Yeung, J. Wang and G. D. Stucky, J. Am. Chem. Soc., 2006, 128, 5352–5353 CrossRef CAS PubMed.
- S. Hong, K. L. Shuford and S. Park, Chem. Mater., 2011, 23, 2011–2013 CrossRef CAS.
- M. N. O’Brien, M. R. Jones, K. A. Brown and C. A. Mirkin, J. Am. Chem. Soc., 2014, 136, 7603–7606 CrossRef PubMed.
- Y.-J. Lee, N. B. Schade, L. Sun, J. A. Fan, D. R. Bae, M. M. Mariscal, G. Lee, F. Capasso, S. Sacanna, V. N. Manoharan and G.-R. Yi, ACS Nano, 2013, 7, 11064–11070 CrossRef CAS PubMed.
- D.-K. Kim, Y. J. Hwang, C. Yoon, H.-O. Yoon, K. S. Chang, G. Lee, S. Lee and G.-R. Yi, Phys. Chem. Chem. Phys., 2015, 17, 20786–20794 RSC.
- Y. Jung, Y. Kim, Y. Lee, J. Son, M. Lim and J.-M. Nam, J. Am. Chem. Soc., 2024, 146, 10591–10598 CrossRef CAS PubMed.
- B. J. Wiley, Y. Chen, J. M. McLellan, Y. Xiong, Z.-Y. Li, D. Ginger and Y. Xia, Nano Lett., 2007, 7, 1032–1036 CrossRef CAS PubMed.
- Z. Liu, Z. Mu, K. Liu, X. Wang, Z. Qiao, Z. Zhang and C. Gao, Mater. Chem. Front., 2022, 6, 2905–2912 RSC.
- J. Wu, W. Gao, H. Yang and J.-M. Zuo, ACS Nano, 2017, 11, 1696–1703 CrossRef CAS PubMed.
- N. Zettsu, J. M. McLellan, B. Wiley, Y. Yin, Z.-Y. Li and Y. Xia, Angew. Chem., 2006, 45, 1288–1292 CrossRef CAS PubMed.
- B. Wiley, T. Herricks, Y. Sun and Y. Xia, Nano Lett., 2004, 4, 1733–1739 CrossRef CAS.
- Y. Xiong, J. Chen, B. Wiley, Y. Xia, S. Aloni and Y. Yin, J. Am. Chem. Soc., 2005, 127, 7332–7333 CrossRef CAS PubMed.
- B. J. Wiley, Y. Xiong, Z.-Y. Li, Y. Yin and Y. Xia, Nano Lett., 2006, 6, 765–768 CrossRef CAS PubMed.
- B. Wiley, Y. Sun and Y. Xia, Langmuir, 2005, 21, 8077–8080 CrossRef CAS PubMed.
- Y. Xiong, B. Wiley, J. Chen, Z.-Y. Li, Y. Yin and Y. Xia, Angew. Chem., 2005, 44, 7913–7917 CrossRef CAS PubMed.
- Y. Xiong, J. M. McLellan, Y. Yin and Y. Xia, Angew. Chem., 2007, 46, 790–794 CrossRef CAS PubMed.
- M. Tsuji, N. Miyamae, M. Hashimoto, M. Nishio, S. Hikino, N. Ishigami and I. Tanaka, Colloids Surf., A, 2007, 302, 587–598 CrossRef CAS.
- I. Blasco, H. Pérez and A. Guijarro, J. Phys. Org. Chem., 2015, 28, 388–395 CrossRef CAS.
- S. Wawzonek and H. A. Laitinen, J. Am. Chem. Soc., 1942, 64, 2365–2368 Search PubMed.
- K. Meerholz and J. Heinze, J. Am. Chem. Soc., 1989, 111, 2325–2326 Search PubMed.
- J. Smid, J. Am. Chem. Soc., 1965, 87, 655–656 Search PubMed.
- B. Bogdanović, S.-T. Liao, R. Mynott, K. Schlichte and U. Westeppe, Chem. Ber., 1984, 117, 1378–1392 Search PubMed.
- I. L. Rasskazov, P. S. Carney and A. Moroz, OSA Continuum, 2020, 3, 2290–2306 CrossRef CAS.
- B. T. Draine and P. J. Flatau, J. Opt. Soc. Am. A, 1994, 11, 1491–1499 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5nh00205b |
| This journal is © The Royal Society of Chemistry 2025 |


