Toxicity of nanoplastics: machine learning combined with meta-analysis†
Zhaoxiang
Li‡
 a,
Yuanyi
Zhang‡
bc and
Yueyue
Chen
a,
Yuanyi
Zhang‡
bc and
Yueyue
Chen
 *a
*a
aDepartment of Toxicology and Sanitary Chemistry, School of Public Health, Capital Medical University, Beijing, 100069, PR China. E-mail: ccccc1yy@ccmu.edu.cn
bSino-Danish College, University of Chinese Academy of Sciences, Beijing, 101408, China
cSino-Danish Center for Education and Research, Beijing, 101408, China
First published on 11th June 2025
Abstract
Nanoplastics (NPs) are widespread in ecosystems, and their biohazards are of increasing concern. The hazards posed by NPs to aquatic and terrestrial plants as well as to aquatic animals have been extensively studied; however, their impact on mammals remains underexplored. Herein, we performed a meta-analysis to quantify the extent of the effects of NPs on mice and developed two machine learning methods to predict the correlations of the detrimental effects of NPs. We found that NPs have a wide range of toxic effects on various systems, and their adverse effects are mainly related to toxicity metrics, followed by the size, type, and mass concentration of NPs, as well as exposure routes, exposure time, and gender. These results suggest that the toxicity of NPs to mammals depends on diverse responses ranging from the molecular to the systemic scale and is influenced by the properties of NPs and environmental conditions, which complicate their toxicity and lead to a wide range of effects.
New conceptsThis study pioneers the integration of meta-analysis and machine learning to systematically evaluate and predict nanoplastic biotoxicity. By acquiring data from published research on the toxicity of nanoplastics, we quantified the effects of their key physical and chemical properties (e.g., particle size and surface charge) and exposure factors (e.g., duration and concentration) on different biotoxicity metrics. Unlike previous studies that relied on isolated experiments, our approach ensures a comprehensive and statistically robust assessment. Using machine learning approaches, we constructed RF and XGBoost models to predict nanoplastic toxicity levels, revealing hidden patterns in the biological interactions of nanoplastics. This novel combination of data-driven analysis and predictive modeling provides new insights into nanoplastic risks, enhances toxicity assessment methodologies, and supports regulatory decision-making in nanoscience and nanotechnology. |
1. Introduction
As one of the most widely used materials, plastic offers tremendous convenience to people but causes a lot of environmental problems.1–6 Small-diameter plastic particles pose significantly higher challenges in their recycling and cleaning than plastic fragments that are visible to the naked eye, and their infinitesimally small size allows these plastic particles to penetrate biological barriers and enter biological tissues.7–9 Therefore, the harm caused by small-diameter plastic particles demands more attention. Research on the biotoxicity of nanoplastics (NPs) and microplastics (MPs) has been particularly conspicuous in recent years. The definition of MPs was introduced by Thompson in 2004,10 initially focusing on plastic debris pollution in the ocean. In 2008, Arthur11 identified plastic fragments smaller than 5 mm in diameter as MPs. However, the definition of NPs remains a bit controversial, with some researchers considering plastic particles smaller than 1 μm as NPs,12–16 while others setting the upper limit at 100 nm.17–22 In this work, we considered NPs of sizes 1–100 nm. There are two reasons for this decision: first, the National Nanotechnology Initiative defines nanomaterials as particles sized between 1 and 100 nm, with properties that are not found in larger particles of the same material.23 Alternatively, the European Commission has revised the definition of nanomaterials as small particles of different shapes, with sizes no larger than 100 nm.24 Moreover, the European Chemicals Agency defines MPs as plastic particles of sizes (in all dimensions) between 100 nm–1 μm.25 Second, when the size of plastic particles reaches the nanometer scale, water molecules and ions in a solution will prevent the settling of these plastic particles owing to random collision, and this interaction will cause the particles to move randomly throughout the solution, called Brownian motion. When particles are sized a few microns, Brownian motion becomes apparent, dominating at sizes approaching 100 nm.26NPs are mainly derived from two main sources, namely, primary plastics and secondary plastics.27,28 Primary plastics are plastic particles that are directly produced at the nanoscale at the time of production and disposed into the environment in the nanoform, such as nanomedicine, nanoimaging, nanosensors and personal care products.29–32 Secondary plastics are derived from the breakdown of plastics driven by physical, chemical or biological forces,28,33 such as agricultural plastic mulch, car tire friction, washing machine effluent, etc.34–40 Under natural conditions, the degradation process of plastics is extremely slow,4 which causes plastic waste to exist longer in the environment. In the case of NPs, this property provides more opportunities for primary plastics to invade living organisms, while also giving more time for larger pieces of plastic to break down in the environment and become secondary plastics.
To comprehensively assess the potential effects of NPs on biological systems, researchers have employed a variety of advanced analytical methods, among which meta-analysis and machine learning techniques are particularly prominent. By systematically integrating and analyzing data from multiple independent studies, a meta-analysis reveals the general toxicity trends of nanoplastics in biological systems at different concentrations and forms, thus increasing the statistical efficacy and credibility of the data. In addition, meta-analyses can help identify effects that may have been overlooked by individual studies, such as sub-toxicity effects or low-dose effects, providing a more comprehensive perspective on toxicity assessment. Using meta-analytical methods, a considerable number of studies have been conducted on the biotoxicity of NPs to fish,41 aquatic invertebrates,42–44 plants,45–50 soil invertebrates51 and microorganisms.52 However, the toxicity of NPs to mammals, which is more informative for us humans, has rarely been investigated and summarized.53
Meanwhile, machine learning methods have rarely been applied to the prediction of NP toxicity.46,54 In current NP studies, machine learning is often coupled with scanning electron microscopy,55 Raman spectroscopy,56,57 hyperspectral imaging,58 mass spectrometry,59 and other technological methods60 for the identification and characterization of NPs. Because of their ability to process large-scale and complex data, machine learning techniques can analyze the toxicity mechanisms of NPs from multi-dimensional and multi-scale perspectives and predict their biological responses under different environmental conditions. By training computational models to learn the patterns and trends of experimental data, machine learning can provide personalized toxicity predictions, which form a scientific basis for risk assessment and environmental management decisions.
The combined use of meta-analysis and machine learning can not only provide insights into the toxicity mechanisms and the extent of the effects of NPs but also support the development of scientifically sound environment management policies and biosafety assessment standards. These results not only serve as a guide for the sustainable development and safe application of nanotechnology but also provide a scientific basis and technical path for the design and application of nanomaterials in the future. Fig. 1 summarizes the pathways of NP generation, different routes of mammalian exposure to NPs in the natural environment (mice are used in most experiments), and their toxic effects and toxicity mechanisms on different organs and systems, which are discussed in detail in the following sections. Currently, the key challenge lies in combining quantitative and classified data from intricate and varied published data to consolidate dependable evidence for both risk assessment and evidence-based policy actions.61 Here, we have used meta-analysis to conclude and summarize the current studies on NP toxicity in mice. We analyzed the different NP features and their toxic effects on different systems in mice. Furthermore, two machine learning models (RF and XGboost) are proposed to analyze the toxicity of NPs and their influencing factors and eventually predict the toxicity of NPs with different features.
2. Review methodology
2.1 Literature search and data extraction
Experimental studies published between January 2021 and September 2023 were compiled through a literature search. For systematic analysis, we focused on adverse effects reported in mice using the ISI Web of Science and PubMed databases, and the literature search was conducted according to the workflow presented in Fig. S1 (ESI†). To ensure quality control, we developed the following literature screening criteria. Articles were included if: (1) NPs with a particle size of 1–100 nm were used as experimental materials; (2) control treatments were performed under laboratory conditions with no NP exposure; (3) at least one organ or system response to NPs exposure was documented; and (4) each response was recorded in three or more publications; and (5) mean, standard deviation, and sample size (n ≥ 3) were reported. The initial search yielded a total of 236 studies, of which 45 met all screening criteria. We extracted the mean, standard deviation, and sample size of mice responses from each of the selected articles. Raw data were either obtained from tables or extracted from graphs using the Get-data Graph Digitizer 2.26 software. Data extracted from all included studies are provided as ESI.†2.2 Meta-analysis
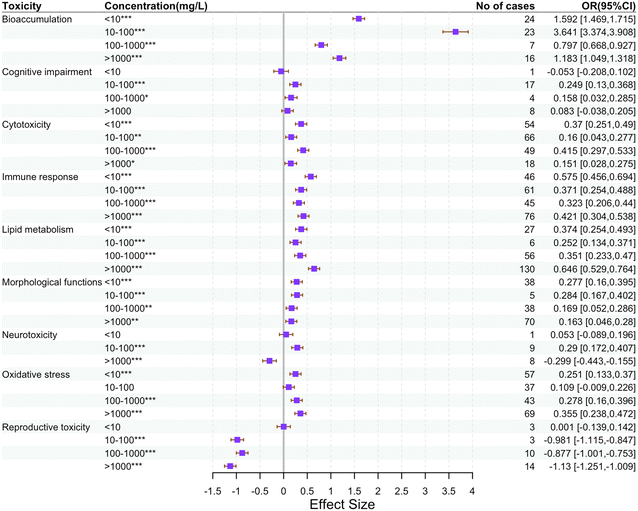
In each study, all mice responses were categorized according to their biological significance. A total of 32 metrics of toxicity were extracted and categorized into 9 major categories, with a database of 1139 observations. The distribution of plastic-type, system, exposure type and time, NP size and concentration, and mice gender the major categories of toxicity metrics are shown in Fig. 2. These major categories included (1) bioaccumulation: fluorescence intensity; (2) morphological function: body weight, placental weight, fetal weight, liver weight, liver coefficient, and weights/coefficients of other organs; (3) cytotoxicity: cell viability, apoptosis percentage, expression levels of genes promoting apoptosis and necrosis, and expression levels of genes inhibiting apoptosis and necrosis; (4) immune response: interleukin-6 (IL-6) content, interleukin-10 (IL-10) content, interleukin-1β (IL-1β) content and tumor necrosis factor-α (TNF-α) content; (5) oxidative stress: malondialdehyde (MDA) content, catalase (CAT) activity, superoxide dismutase (SOD) activity, glutathione (GSH) content, and reactive oxygen species (ROS) content; (6) lipid metabolism: TC (total cholesterol) content, TG (triglyceride) content, aspartate aminotransferase (AST) content, alanine aminotransferase (ALT) content, and expression levels of genes associated with lipid metabolism; (7) reproductive toxicity: sperm number, sperm viability and testosterone levels; (8) cognitive impairment: distance moved in the central area and total distance moved in the open field test (OFT) experiment; (9) neurotoxicity: acetylcholinesterase (AChE) activity and gamma-aminobutyric acid (GABA) content. In addition to these mouse responses, the features of the NPs (size, type, and shape), exposure conditions (mass concentration, exposure route, and exposure time), and mice characteristics (in vivo or in vitro experiments, systemic origin of the cells or tissues and mice age) were considered. Specifically, NPs were classified by size as ≤50 nm and 50–100 nm, and the NP exposure routes in mice included oral (mostly intragastric administration and drinking water), parenteral (intravenous and intraperitoneal injection), culture medium (in vitro experiments), and inhalant and nasal (respiratory methods such as oropharyngeal aspiration). The exposure time was graded as <24 h, 24 h–15 days, 15–30 days, 30 × 45 days, and >45 days. The mass concentration of NPs was converted to concentrations used in the experiments according to the different exposure methods, and the conversion process is described in Text S1 (ESI†). The specific mass concentrations were graded as: <10 mg L−1, 10–100 mg L−1, 100–1000 mg L−1, and >1000 mg L−1. The mice organ systems considered were categorized into: the whole body and nervous, respiratory, circulatory, digestive, excretory, immune, endocrine, and reproductive systems. Information on literature search, effect size calculation, publication bias, and sensitivity analysis are provided as ESI† (Texts S1, S2; Fig. S1 and Tables S1, S2).2.3 Machine learning
The machine learning models developed in this paper used two integrated learning algorithms, Random Forest (RF) and eXtreme Gradient Boosting (XGBoost). RF predicts classification or regression results by bagging (Bootstrap Aggregating) multiple decision trees and reduces overfitting problems through bagging and random feature selection. XGBoost uses a gradient-boosting strategy to build the model sequentially and progressively attempts to reduce residuals from the previous round of predictions to optimize the loss function. At the same time, a regularization term was introduced to avoid overfitting.Among the 1139 data points from the meta-analysis, we retained 1101 data points that contained valid information across the dimensions of cell_type, system, exposure_type, polymer_type, size, concentration, exposure_time, gender, and endpoints1. The remaining 38 data points, in which the endpoint type was “biological aggregation” and the control group data was zero, resulting in an invalid risk ratio (RR), were excluded from further analysis. The key metrics for each sample were extracted from the original dataset to build the dataset for machine learning, which was randomly divided into a training set (80%) and a test set (20%). While training the model, in order to select the best parameters, we applied the grid search algorithm to multiple optional parameters of the model to achieve optimal model performance. The performance of the model was evaluated by the correlation coefficient (R2) and root mean square error (RMSE) from the 5-fold cross-validation (R2_5CV and RMSE_5CV) for the training set and the validation (R2_test and RMSE_test) for the test set. The optimal parameters used for the final models are shown in Table S4 (ESI†). All machine learning models were implemented by scikit-learn 0.24.2 for Python 3.9.7.
To identify which variables were the most influential in predicting the magnitude of toxicity, we used SHapley Additive exPlanations (SHAP) to assign an importance value to each feature, indicating how positively or negatively each feature contributed to the target variable. SHAP 0.43.0 was used for SHAP analysis in this paper.
3. Results and discussion
3.1 Biotoxicity of nanoplastics: a meta-analysis
NPs that originate from the breakdown of larger plastic materials or are intentionally manufactured at the nanoscale, possess unique properties, which raise questions about their potential biological impact. The intricate interplay between NPs and living organisms necessitates a thorough examination of their biological toxicity, with a particular focus on in vivo and in vitro experiments using murine models.62–64This meta-analysis and systematic review aim to provide a comprehensive understanding of existing studies on the biological toxicity of NPs, specifically those involving murine subjects. Through aggregation and critical analysis of research findings from diverse works, this study seeks to uncover patterns, consensus, and gaps in our understanding of how NPs interact with murine organisms at both systemic and cellular levels. The choice of murine models offers a valuable opportunity to bridge the gap between controlled laboratory experiments and the complexities of biological systems, providing insights into their potential effects on mammalian physiology. This meta-analysis encompasses a wide array of studies, including in vitro experiments that elucidate cellular responses to NP exposure and in vivo assessments that explore the systemic impact on murine health. This inclusive approach aims to offer a comprehensive perspective on the diverse facets of NP biotoxicity, ranging from organ-level effects to cellular and molecular interactions. Additionally, the meta-analysis scrutinizes variations in experimental designs, methodologies, and reported outcomes to provide insights into the reliability and comparability of existing studies.
As global concerns regarding plastic pollution intensify, comprehending the biological repercussions of NPs on murine organisms becomes crucial for informed decision-making and the formulation of effective mitigation strategies.65–67 This meta-analysis not only consolidates the current knowledge in this regard but also sheds light on avenues for future research, emphasizing the need for standardized methodologies and a holistic understanding of the intricate nexus between NPs and murine biology. Ultimately, this meta-analysis aspires to contribute to the ongoing discussions surrounding NP bio-toxicity, fostering advancements in research and policies to gear towards a sustainable and plastic-aware future.
Comprehensive and rigorous literature mining (Fig. S1 and Text S1, ESI†) led to a total of 45 studies and 1139 observations containing 32 toxicity metrics (classified into nine main categories) and 8 feature variables (physicochemical properties of NPs, including NPs size, mass concentration and type, sample traits, including organs and systems, and experimental conditions, including exposure time, exposure routine and in vivo or in vitro setting) published between January 2021 and September 2023 (Fig. 3). The references of the included studies are provided in Table S1 (ESI†).
In addition to fluorescent labelling, a number of studies have used metal markers for NP localization and recovery in recent years. Compared with fluorescent labelling, metal labels have lower size detection limits and lower background interference.108 Commonly used metal markers include Pd,109–117 In,111,112,114,118,119 Au,120,121 Eu,122,123 Ag,121 Fe124 and Pt.125 Intriguingly, the possible alteration of NP toxicity via metal labelling was tested. Villacorta et al.126 extracted NPs doped with titanium metal from PET bottles, which did not show significant toxic effects, providing strong evidence that metal labelling methods do not increase the toxicity of NPs. Vicentini et al.127 labelled PSNPs with aluminum metal to quantify the amount of PSNP ingested by Daphnia magna and compared the effects of fluorescent labelling and metal labelling on the toxicity of PSNPs; their results showed that PSNPs synthesized with an Al2O3 nucleus did not show significant toxicity, which was enhanced by the surface modification of PSNPs with palmitic acid in this study. These studies demonstrate that metal labelling of NPs does not increase the biotoxicity of NPs. However, the number of relevant studies is relatively limited, and most of these studies have focused on the implementation of the NP detection method rather than the toxic effects while implementing the metal-labelling method. The biotoxicity of metal-labelled NPs may be a focus area for future research.
Further, NPs show slight cytotoxicity (4.81%) and risk of affecting morphological functions (−5.04%) in mice. The internalization of NPs by living organisms is crucial for their biotoxicity, and macromolecules, such as proteins, lipids, phospholipids and carbohydrates, can form bioprotein coronas around NPs upon intake by humans or animals.128 In the presence of immunoglobulins (IgG),129 the NPs form a corona, leading to an elevated level of cellular uptake.130 When NPs encounter cell membranes, approximately 50% are internalized, and the uptake process can be triggered by112 endocytosis.131,132 As shown in Fig. 1, the mechanisms underlying NP endocytosis are diverse. Initially, NPs enter the cytoplasm via early endosomes and then mature into late endosomes, which subsequently combine with lysosomes.133 NPs then accumulate mainly in lysosomes,134 producing reactive oxygen species (ROS) that can lead to lysosomal membrane damage (LMD).135 In response to LMD, the release of lysosomal cathepsin into the cytoplasm136 results in DNA mutations in the mitochondria, which leads to mitochondrial dysfunction and subsequent ROS release.137–139 Elevated ROS levels induce oxidative stress,140 activating specific signaling pathways that lead to apoptosis, necrosis, and pyroptosis.141,142 Cytotoxicity,143,144 genotoxicity,145,146 inflammation and other effects may occur through this mechanism. As ROS promotes cancer cell growth and proliferation,147,148 the cellular uptake of NPs is also known to pose a potential cancer risk. ROS can be produced by inorganic nanoparticles, such as silver nanoparticles, which may pose a risk to human health.149 However, nanoparticles are generally considered fairly safe as they can be easily removed from the human body based on their properties associated with optimal removal rates.150 Conversely, the accumulation of NPs within the human body may have serious hidden dangers as they cannot be removed readily and may persist in the lysosomal endosomes.151 In addition, nanoparticles can cause vascular endothelial leakage on a microscale,152,153 which may lead to adverse effects, particularly in cancer patients with, accelerating cancer invasion and metastasis. Thus, unremoved NPs may continue to circulate in the body and pose ongoing health risks.154
Based on the mechanism of action described above, the toxicity of NPs is reflected mainly in the induction of oxidative stress (21%), immune response (36%), lipid metabolism (35%), reproductive toxicity (−26%), cognitive impairment (−11%) and neurotoxicity (−22%). Since metrics were available for opposite descriptors of the same toxic effects under the 9 major categories in Fig. 3 (e.g., when NPs cause apoptosis, the expression of genes promoting apoptosis is increased, whereas the expression of genes inhibiting apoptosis is decreased), we conducted subgroup analyses to obtain a more precise interpretation of toxicity.
In terms of morphological functions, the impact of NPs was relatively minor. The organ coefficient is the ratio of the mass of an organ to the body weight of an experimental subject, also known as the organ-to-body weight ratio. This ratio is relatively constant in normal conditions. When the animal subject is poisoned, the heavily damaged organs often undergo pathophysiological or morphological changes, such as swelling, edema, congestion, and hemorrhage, which change their mass and hence the organ coefficients.155 A slight decrease in body weight (−5%), placenta weight (−1%), fetus weight (−9%), and liver weight (−4%) was reported. Conversely, there was a modest increase in liver coefficients (2%) and other organ weights/coefficients (3%). The presence of NPs may interfere with normal nutrient uptake or metabolic pathways, affecting energy production and utilization and thus leading to weight loss.156 The relatively increased burden on the liver, which is a major metabolic organ, (e.g., elevated liver coefficient) may reflect the metabolic stress induced by the NP metabolization and the subsequent detoxification process.157 The decrease in fetal weight may point to direct or indirect adverse effects of NPs on fetal growth, which may involve impaired placental transport or direct toxicity to the fetus.158 The slight increase in other organ weights/factors may indicate specific responses of different organs to NP exposure.159 This may include the relative stabilization of organ dimensions or compensatory growth in response to injury to maintain physiological function.
As for cytotoxicity, NPs exhibited significant pro-apoptotic and pro-necrotic effects. There was a significant decrease in cell viability (−24%) and a decrease in the expression of genes inhibiting apoptosis and necrosis (−42%). Meanwhile, the apoptosis rate (106%) and expression of genes promoting apoptosis and necrosis increased (41%). These data suggest that NP exposure significantly promotes cell death. This pro-apoptotic effect may be due to NP-induced oxidative stress and DNA damage, resulting in the activation of apoptotic signaling pathways in cells.160,161 In addition, NPs may trigger cell death mechanisms by interacting with cell membranes and altering membrane integrity. These changes have a significant impact on the health of organisms and may lead to tissue damage and organ dysfunction.162
NPs also significantly induced an immune response, as evidenced by elevated pro-inflammatory and anti-inflammatory factors. Specifically, pro-inflammatory factors IL-6 (40%), IL-1β (19%) and TNF-α (42%), which are usually associated with acute inflammatory responses, were elevated.163–165 This suggests that exposure to NPs triggers an acute inflammatory response in the organism, whereas elevated IL-10 (17%) may be indicative of a protective response aimed at preventing tissue damage caused by excessive inflammatory response.166–168 Elevated pro-inflammatory factors can lead to localized inflammation, attracting more immune cells to the affected area and further exacerbating the inflammatory response. Anti-inflammatory factors, on the other hand, may serve to balance this response and prevent uncontrolled inflammation. This dynamic change in the immune response demonstrates the complex effects of NPs on the immune system, and the long-term inflammatory response may lead to chronic disease and tissue damage.169–173
In terms of oxidative stress, NPs remarkably affected the intracellular redox balance, as evidenced by a significant increase in MDA (38%) and ROS (57%) levels. In contrast, antioxidant enzymes, such as CAT (−21%) and GSH (−10%), were significantly decreased, although SOD (7%) did not change significantly. These data suggest that exposure to NPs leads to a significant increase in intracellular oxidative stress, mainly through increased ROS production. The increase in ROS may be due to the reaction of NPs with intracellular metal ions, which promotes the generation of free radicals.174,175 Oxidative stress leads to lipid peroxidation, protein damage, and DNA damage, which can trigger a series of cellular damage pathways and lead to cell death. This sustained oxidative stress may lead to cellular dysfunction, further exacerbating the toxic effects of NPs, especially in cells that lack effective antioxidant defense mechanisms.176
These three categories collectively suggest that NPs negatively affect cells and tissues through a variety of mechanisms, including cytotoxicity, immune response and oxidative stress.177 Cytotoxicity is achieved primarily through the induction of apoptosis and necrosis, which may lead to the destruction of tissue structure and function. The induction of immune responses suggests that NP exposure triggers acute and possibly chronic inflammation, further exacerbating tissue damage.178 Increased oxidative stress, on the other hand, is an important mechanism by which NPs cause cellular damage, leading to the disruption of intracellular redox homeostasis through increased ROS production and reduced antioxidant capacity.179,180 Together, these mechanisms project exposure to NPs as a significant health threat, potentially leading to a range of acute and chronic health problems, including inflammation-related diseases, organ dysfunction and potential cancer risk.
As for lipid metabolism, TG (25%), TC (42%), AST (16%), ALT (22%) and the expression of genes related to lipid metabolism (e.g., CD36, DGAT2, GPAT4, etc.) (28%) were increased. The increased TG and TC levels reflect a disturbance in lipid metabolism, suggesting that NPs may interfere with normal lipid absorption, synthesis, catabolism, and transport processes.181 This disturbance may involve a variety of mechanisms by which NPs affect intestinal absorption, hepatic metabolism, or directly affect adipocyte function.182 Increased levels of AST and ALT suggest that NPs may cause hepatic stress or injury, which in turn affects overall lipid homeostasis.183–185 The increased expression of genes related to lipid metabolism further confirms changes in lipid metabolic processes.88,186–188 The increased activity of these genes may reflect that cells attempt to adapt to NP-induced stress by increasing lipid synthesis to reserve energy or repair damaged cell membranes.189
The data on reproductive toxicity showed a 34% decrease in sperm count, a 36.04% decrease in sperm viability and a 32% decrease in testosterone levels. These results suggest that nanoplastic exposure may have significant negative effects on the reproductive system. Firstly, the significant decrease in sperm count and viability may reflect the negative interference of nanoplastics on the spermatogenesis process. This may include direct toxic effects on sperm cells or indirect interference of spermatogenesis by affecting testicular function.190 In addition, the reduced sperm viability may indicate that the energy metabolism and motility of sperm cells are affected, possibly due to nanoplastic-induced oxidative stress and inflammatory responses.191 Oxidative stress generates large amounts of ROS, which are capable of damaging sperm DNA and cell membranes, thereby reducing sperm quality and function.192 At the same time, nanoplastics may trigger an inflammatory response in the testes, causing damage to spermatogenic and supporting cells (Sertoli cells), which in turn affects spermatogenesis and maturation.193 Secondly, the negative effects of nanoplastics on the male reproductive system are further supported by the significant decrease in testosterone levels. The decrease in testosterone levels may be due to the direct toxic effects of nanoplastics on Leydig cells,73 which are responsible for the synthesis and secretion of testosterone, and nanoplastics exposure may reduce testosterone synthesis by inducing apoptosis or necrosis of these cells.194 In addition, nanoplastics may interfere with the regulation of the hypothalamic-pituitary-gonadal axis (HPG axis), leading to abnormal secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which indirectly affects testosterone levels.195 Overall, these results suggest that nanoplastic exposure has significant toxic effects on the male reproductive system, including a reduction in sperm count and viability and lower testosterone levels.
In terms of neurotoxicity and cognitive deficit, except for AChE activity (14%), which showed an increase, there was a decrease in GABA (−45%), as well as the distance in the central area (−2%) and the total distance (−9%) covered by mice in the OFT test. Elevated AChE activity may lead to a rapid breakdown of acetylcholine and lower levels of acetylcholine, which may affect excitability and signal transmission efficiency in the nervous system.196 Decreased levels of GABA may reduce hyperexcitability and/or inhibitory signaling in the nervous system, which may lead to anxiety, overreaction, or other behavioral and emotional problems.197,198 A decrease in the distance moved by mice in the central area and total distance moved in the open field test (OFT) usually means higher levels of anxiety, because of which the animals tend to avoid open areas and show less exploratory behavior.199 This behavioral change may be related to changes in neurotransmitter levels, as described above. These changes may also indicate that NP exposure significantly affects the nervous system of the mice, resulting in a higher stress response in new or open environments. This may be the direct or indirect effect of NPs on neurotransmitter metabolism and neuronal function. For example, reduced levels of acetylcholine may affect the function of the hippocampus, which plays an important role in spatial memory and exploratory behavior.200–202 Decreased levels of GABA, on the other hand, may affect multiple regions of the brain, including the cortex and limbic system, thereby affecting emotion regulation and behavioral responses.203,204 Taken together, NP exposure not only leads to significant changes in neurotransmitter levels but can also trigger a range of behavioral and emotional problems through these changes. These findings suggest the need for further research into the long-term neurological effects of NPs and the potential health risks they may pose.
Lipid metabolism and oxidative stress showed a relatively clear trend of enhanced toxicity with increasing mass concentration, and the rest of the toxicity metrics did not show any obvious trend, and most of them exhibited similar levels of toxicity (Fig. 7). While neurotoxicity showed an opposite effect at >1000 mg mL−1, given that NP size also showed an opposite trend for this toxicity index, we hypothesized that this result might be due to the combination of the NP size and mass concentration used by the researchers.
With regard to experimental design, we analyzed the effects of different exposure times on the toxicity metrics. Immune response, lipid metabolism, and cytotoxicity showed a tendency to increase with exposure time, while reproductive toxicity showed a tendency to decrease with exposure time (Fig. 6), which may be due to the combined effects of other factors, such as the size and mass concentration of the NPs.
In terms of bioaccumulation, NPs of both particle sizes showed a high degree of accumulation. However, the trend of the effect of different exposure times and mass concentrations of NPs on bioaccumulation showed different degrees of facilitation, and notably, bioaccumulation did not increase with an increase in exposure time and mass concentration. This is possibly related to the way cells take up NPs, and higher concentrations or longer exposure times do not result in increased bioaccumulation once the uptake efficiency is saturated.
As for cognitive impairment, NPs of both particle sizes showed similar level of effect size, while shorter (<24 h) and longer (30–45 d and >45 d) exposure times showed higher effect size, and differences in mass concentration had very little effect on cognitive impairment, which may be due to the different exposure protocols used in the experimental design as well as the different conditions in the cellular and mouse experiments. Generally, the cellular experiments used shorter exposure times and lower doses because the direct addition of NPs to the culture medium allowed more access to the cells; this result suggests that cognitive impairments can be brought about by long-term exposure to NPs as these traits are closely related to the nervous system. Regarding neurotoxicity, longer exposure time (30–45 d) led to higher toxicity, which is consistent with the results of cognitive damage, suggesting that they are interrelated.
Concerning cytotoxicity, particles of both sizes showed a similar trend, and the prolongation of exposure time also enhanced cytotoxicity to some extent; the different mass concentrations also showed a consistent trend, which clearly indicates the effect of NPs on cytotoxicity and that their presence would significantly reduce cell viability and bring about damage. The mechanism of cytotoxicity of NPs involves oxidative stress and immune response. Firstly, NPs of 50–100 nm particle size can lead to severe oxidative stress, and 30–45 days exposure time also showed the highest oxidative stress effect. Both particle sizes had higher effects on immune responses; NPs sized 50–100 nm had stronger pro-inflammatory effects and higher levels of inflammatory responses with longer exposure times, while different mass concentrations showed similar trends in terms of oxidative stress and immune responses, indicating lesser promotive effects on both categories. This correlates with the mechanisms of cytotoxicity elicited by NPs, as revealed by the obvious pro-ROS generation and pro-inflammatory effects mentioned earlier.
NPs also had a significant effect on lipid metabolism; NPs of size 50–100 nm showed a stronger trend than the smaller NPs. Moreover, longer exposure time and higher mass concentration of NPs corresponded with higher toxicity, indicating that lipid metabolism was also one of the important targets of NP toxicity, and its correlation with exposure conditions (longer duration and high concentrations) was high. In particular, 30–45 days of exposure produced stronger toxicity in terms of morphological function, similar to previous results, and the toxic effects of long-term exposure to NPs were more obvious.
In terms of reproductive toxicity, the particle size and concentration of NPs had less influence, but the exposure time showed a trend of decreasing toxicity with prolonged exposure, which is very unusual. After confirming the data, we found that all previous studies83,94,96–99 on reproductive toxicity used oral dyeing, but the experimental conditions used for data on prolonged exposure (>45 d) were NPs of 100 size nm and a concentration of only 0.1–10 mg L−1, whereas data up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 was available for other exposure times. Therefore, the lower toxicity produced by prolonged exposure may be due to lower NP concentration rather than the exposure time.
000 mg L−1 was available for other exposure times. Therefore, the lower toxicity produced by prolonged exposure may be due to lower NP concentration rather than the exposure time.
A dose–response relationship analysis was performed for different factors with regard to the mass concentration of NPs (Fig. 8). As mentioned before, some endpoints showed opposite trends for the same effect. To facilitate the analysis, we set the risk ratio such that it positively correlated with the toxicity of NPs, while taking the opposite number of the risk ratio of some endpoints for the dose–response relationship analysis, as follows: (1) cell viability and gene expression (inhibiting apoptosis and necrocytosis): the decrease in cell viability and expression level of genes inhibiting apoptosis and necrocytosis indicates that NPs enhance apoptosis and necrocytosis, which means that NPs exhibit higher toxicity.205 (2) CAT: the decrease in CAT indicates that the body's ability to resist hydrogen peroxide is reduced, and antioxidant defense is weakened, which may render the cells more sensitive to oxidative damage, so lower levels of CAT mean that NPs will have higher toxicity.206 (3) GSH: as an important antioxidant present in almost all cells, GSH plays a key role in protecting cells from oxidative damage, especially in mitigating the harmful effects of free radicals and peroxides. It helps neutralize toxic substances by reacting directly with ROS, reducing them to less active forms and acting as a cofactor in enzymatic reactions.207 Similar to CAT, a decrease in GSH means a decrease in antioxidant capacity in the cell. (4) Sperm viability, sperm number and testosterone: sperm viability and number are usually directly related to fertility,208 and their decrease implies direct damage to the reproductive system; testosterone is a key hormone for sperm production and sexual function, and a decrease in testosterone level may affect the function of the entire reproductive system.209 Moreover, the change in testosterone levels implies that NPs may have endocrine-disrupting properties that affect the production of androgens. (5) The distance moved in the center area (OFT) and the total distance moved (OFT): OFT is a behavioral test that assesses anxiety and exploratory behavior in rodents; the animal is free to move about in an open place, usually with a central area and a peripheral area. In general, bolder or more exploratory animals tend to spend more time exploring the center area in the OFT because it requires them to move away from the periphery, their sanctuary. More anxious or fearful animals tend to stay in the peripheral region and spend less time in the central region because the peripheral region is closer to where they might escape to hide.199 Therefore, if the mice in the experiment were less active in the central area and less active in total, this could be indicative of their anxiety about open spaces and tendency to reduce risk and potential exposure, implying that the NPs may have contributed to cognitive impairment in mice. (6) GABA: as an inhibitory neurotransmitter, GABA plays an important role in regulating nervous system homeostasis and reducing its over-excitation. Decreased levels of GABA may have led to over-excitation of the nervous system, which may be reflected in behavioral anxiety, excitement, or other related neurobehavioral changes, suggesting that NPs may affect the central nervous system of the mice.210
According to the extracted data, in vivo experiments showed a relatively weak mass concentration dependence, while in vitro experiments showed a more obvious trend of increasing risk ratio with increasing concentrations (Fig. 8A). This may be attributed to the fact that NPs produce a variety of complex biochemical reactions in mice, making it difficult for them to enter cells directly to exhibit toxicity, whereas, the cellular experiments give NPs direct access to the cells, leading to a more defined toxic response. In both female and male mice, there was a clear increasing tendency of NP toxicity with elevated mass concentration (Fig. 8B). The toxicity manifestations of NPs were not consistent across systems (Fig. 8C). The absence of significant changes in body indices (e.g., body weight, organ weights, and behavioral indices) is consistent with the widely reported micro toxicity of NPs in mice. Except for the nervous system, the circulatory, digestive, endocrine, excretory, immune, reproductive, and respiratory systems all showed a significant increase in toxicity with increasing mass concentrations of NPs. Nerve toxicity showed a slight decrease with increasing mass concentration, which can possibly result from the combined effect of other factors in the experiment, such as the higher exposure time in the lower dose experiment, which may have resulted in stronger toxicity, or the absence of more toxic NPs (e.g., PS–COOH, PS–NH2) in the higher concentration experiment, resulting in such a scenario.
To minimize potential bias, as stipulated in the Methods section, we included only the studies in which each factor was examined at least three times. Due to limited research on the influence of NP shape on toxicity and the challenges associated with maintaining complete uniformity in NP shape, all studies included in this work employed spherical NPs for toxicity evaluation. However, the impact of NP shape on biological toxicity remains a critical area of investigation, as different NP shapes exhibit significant differences in their ability to penetrate biological barriers (e.g., the intestinal barrier and the blood–brain barrier).
Fibrous nanoplastics, owing to their linear structure, can more efficiently traverse the intestinal barrier and enter the circulatory system, thereby increasing systemic toxicity.211 In contrast, spherical nanoplastics demonstrate lower efficiency in penetrating biological barriers but may exhibit greater accumulation in specific organs, such as the liver and kidneys.212 The shape of nanoplastics also affects their cellular uptake efficiency. Spherical nanoplastics, with a lower surface area-to-volume ratio, are more readily phagocytosed by cells, whereas fibrous nanoplastics, with a larger surface area, are more likely to adhere to cell surfaces, potentially causing membrane damage. Additionally, sheet-like nanoplastics, with a planar structure, may interfere with cellular signal transduction, leading to cellular dysfunction.213,214
Environmental factors (e.g., pH, salinity, and organic content) further influence the relationship between nanoplastic shape and toxicity. In acidic environments, fibrous nanoplastics may be more prone to degradation into smaller fragments, which increases their bioavailability and toxicity.215 Conversely, in high-salinity environments, spherical nanoplastics are more likely to aggregate, which reduces their bioavailability.216
The exposure route of nanoparticles (NPs) also influences their toxic effects on mouse systems (Fig. 8D). In in vitro experiments, cells are directly exposed to NPs, resulting in the highest observed toxicity, with the strongest dose dependence. Inhalation exposure typically involves much higher NP concentrations than oral administration, leading to more pronounced dose-dependent toxicity. Notably, while the overall toxicity caused by inhalation exposure was lower than that of oral exposure, inhalation provides a direct route for NPs to enter the bloodstream.217,218 This difference alters the target organs affected by NP toxicity; inhaled NPs primarily impact the lungs and heart, whereas, orally ingested NPs predominantly affect the intestine and liver.217–219 For biological organisms, the manifestation of similar toxic effects in different target organs implies distinct toxicological consequences. Therefore, further elucidation of mechanisms underlying the toxic effects associated with different exposure routes is of critical importance.
The effects of different exposure times and sizes of NPs were not significantly different (Fig. 8E and F), but a decrease in effect was observed with increasing mass concentration of NPs when the exposure time was over 45 days, which might be because longer exposure times alter the physiological activities of the animals, and they adapt to the high exposure levels and adjust accordingly on their own. However, different surface modifications of PSNPs largely altered their toxicity (Fig. 8G), and it was clearly observed that normal PSNPs were slightly toxic; PS–COOH NPs had a clear concentration-dependent toxicity effect, and PS–NH2 NPs were more toxic in a very wide range of mass concentrations, but their toxicity decreased with increasing mass concentrations. This is probably because the large aggregates formed at high concentrations reduce the efficiency of their cellular uptake or biological activity in the organism.220 At higher NP concentrations, the cellular mechanism for processing the particles may be saturated, leading to stabilization or a decrease in toxicity. This may explain why toxicity does not increase with increasing concentrations. However, due to the lack of relevant studies, more research is needed to verify the actual cause of these trends in their toxicity.
3.2 Biotoxicity of nanoplastics: machine learning model prediction
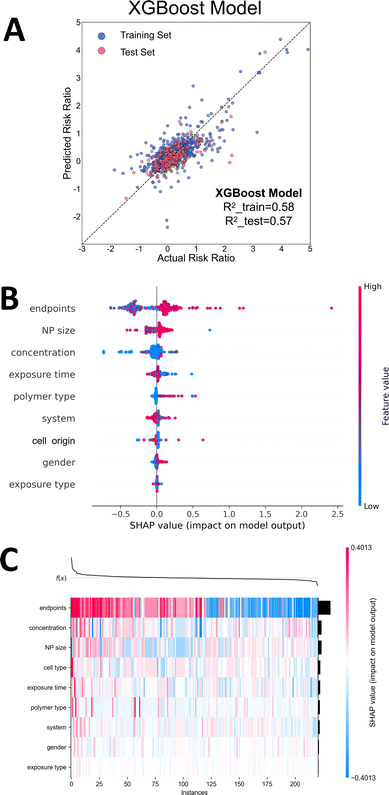
Nine feature values were extracted and constructed from the general dataset of the meta-analysis, including cell type and organ system, exposure route, concentration and duration, NP type and size, gender, and toxicity metrics, with Risk ratio as the prediction target, to train the machine learning models. Before training, the text data were numerically coded in the dataset. A multiple linear regression model was first employed to assess the importance of various variables; however, the performance was notably poor (Fig. S3 and S4, ESI†). This result indicated a highly non-linear relationship between the parameters and the dependent variable. Consequently, we constructed Random Forest (RF) and XGBoost models to further explore this relationship and evaluate variable importance (Table 1). As shown in Fig. 9A and Fig. S2A (ESI†), both RF and XGBoost models achieved good prediction accuracy. In the training set, the R2 of both RF and XGBoost models were over 0.58, while in the test set, the R2 of RF and XGBoost models were 0.53 and 0.57, respectively. Meanwhile, most of the data points were around the reference line, indicating that the two models could result in the robust prediction of NP toxicity. However, several data in the figure show relatively large deviations from the predictions of the models, which is possible because the feature variables in the data that significantly affect NP toxicity might have been ignored in this prediction model. Overall, the two machine learning models constructed in this work can predict the magnitude of NP toxicity to a certain extent.| Model | R 2 | RMSE | MAE | MSE | |
|---|---|---|---|---|---|
| RF | Training set | 0.58 | 0.41 | 0.26 | 0.17 |
| Test set | 0.53 | 0.38 | 0.24 | 0.15 | |
| XGBoost | Training set | 0.58 | 0.41 | 0.27 | 0.17 |
| Test set | 0.57 | 0.37 | 0.24 | 0.13 |
To further explain the output of the machine learning model, we used SHAP to evaluate the contribution of various features to toxicity prediction. Fig. 9B and Fig. S2B (ESI†) show that toxicity metrics, NP size, NP concentration, NP type, cell origin, and exposure time are the main feature variables that affect the significance of NP toxicity. Among them, different toxicity metrics had the most significant contributions to the predicted results of NP toxicity, indicating that NPs have different degrees of influence on different toxicity metrics. On the other hand, the size and concentration of NPs also contributed significantly to the prediction of toxicity, and compared with the previous dose–response relationship analysis, the NP size did not significantly affect the risk ratio (Fig. 8F). This is probably because NPs of a single size were used in most dose–response relationship analyses, which explains its inability to obtain the effect of different sizes on toxicity during the analysis, hence resulting in a similar risk ratio (∼0.25). In machine learning, on the other hand, the combined effects of factors except for the effect of a single variable on the dose–response relationship are accounted for, resulting in different results. Consistent with the factors considered in the traditional experimental design and the results of the dose–response relationship analysis, the concentration, exposure time, and type of NPs clearly influenced the final toxic effect (Fig. 8E and G). In addition to this, the toxic effects of NP exposure were also significantly different in different systems of mice, which is also consistent with the results of the dose–response relationship analysis (Fig. 8C).
The model prediction process is illustrated in Fig. 9C and Fig. S2C (ESI†); the individual samples of the test set are arranged along the horizontal axis and the feature variables are arranged along the vertical axis. Colors indicate the magnitude of SHAP values: red indicates positive SHAP values, blue indicates negative SHAP values, and the shades correspond with the absolute magnitude of the SHAP values. Notably, the SHAP values serve as a local explanation method, reflecting the contribution of features for individual samples. Although we aim to provide a global perspective by aggregating these values, it is important to acknowledge that in nonlinear models, the actual impact of features may vary across different samples.
3.3 Nanoplastic biotoxicity analysis between meta-analysis and machine learning
Unlike traditional animal experiment studies, this study presents a comprehensive meta-analysis and machine-learning-based prediction of the biotoxic effects of different NP features on different health metrics using data from the nanotoxicology literature to improve the understanding and knowledge on the toxicity mechanisms and risk profiles of NPs. The dataset compiled in this paper includes 1101 data points, which is more than many machine learning datasets used in nanotoxicology. In previous studies, some researchers have analyzed NP toxicity and factors influencing NPs, as well as constructed machine learning models, using plants as research subjects.46 However, in vivo experiments on animals have a higher reference value for human health. Compared with previous studies, dose–response relationship analysis under different conditions is presented additionally in this work to analyze the magnitude of NP toxicity effects and the trend of changes in different metrics, providing a more detailed perspective. By combining machine learning methods and meta-analysis, we were able to gain a deeper understanding of the biotoxicity of NPs and their influencing factors. Existing studies indicate that the toxicity of NPs is generally greater than that of MPs, but only a few studies have been published on the toxicity of NPs with different particle sizes. The SHAP results from the machine learning model show that the particle size of NPs contributes significantly to toxicity, and it is only second to the toxicity metrics. However, since most of the experiments reported in the literature have used only NPs of a single particle size, we did not have enough experimental results for different sizes of NPs to observe their toxic effects; and thus, it was difficult to derive the toxicity trend of the toxic effects of NPs in the size range of 0–100 nm on mice. This suggests that there may be an unknown effect of particle size on NP toxicity, and it is worthy of exploration in the future.While our machine learning models, including meta-regression, random forest, and XGBoost, provide valuable insights into the toxicity patterns of nanoplastics in murine models, their limitations must be acknowledged. The predictive accuracy of all these models is inherently constrained by the aggregated experimental uncertainties of the original studies, such as biological heterogeneity in murine responses and inconsistencies in dosage measurement, particularly in in vivo exposure scenarios. Furthermore, the parameter space exhibits significant sampling imbalances, including the overrepresentation of certain polymer types (e.g., PS) relative to others (e.g., PS–COOH), concentration ranges that are primarily clustered around 1–100 μg L−1 with sparse data at environmentally relevant low doses, and a disproportionate focus on acute (≤7 days) versus chronic exposure studies. Additional challenges arise from the non-normal distribution of key endpoints (e.g., inflammatory markers), which violates the assumptions of linear regression. Tree-based models may overfit to outliers from high-dose studies or be disproportionately influenced by categorical variables, while XGBoost is robust to non-normal data distributions; the non-normality of our dataset may limit the applicability of certain parametric statistical inferences. However, this does not affect the predictive performance of our model. Moreover, despite robust cross-validation, the model predictions remain limited to murine physiology, controlled laboratory conditions rather than real-world exposure mixtures, and short-term endpoints (e.g., oxidative stress, inflammation) rather than chronic outcomes. The interpretation of feature importance, including SHAP values, is inherently dependent on training data distribution. Although SHAP provides more consistent explanations than traditional importance measures, the interpretations should be cautiously generalized to groups with substantially different characteristics. These limitations underscore the need for standardized nanoplastic characterization protocols in toxicology studies, targeted experiments to fill existing data gaps, particularly chronic low-dose exposure scenarios, and ensemble modeling approaches to better account for uncertainty propagation.
Despite these limitations, this work provides an informative analysis that combines the use of traditional statistical meta-analysis methods to evaluate the toxicity of NPs with machine learning methods to predict the toxicity of unknown NPs. This study also improves the understanding of NP toxicity and the underlying mechanisms and provides new information for the risk assessment of NPs.
4. Conclusion
We have integrated and analyzed the nanotoxicology research literature by combining meta-analysis and machine-learning methods, focusing on the effects of nanoplastics on the biotoxicity of mice. The results show that nanoplastics have significant effects on mice, and the severity of such effects is influenced by a variety of factors, including their toxicity metrics, specific features of the nanoplastics, traits of the mice, and specific conditions of exposure. Therefore, future studies should document and report these key factors in detail. More importantly, these vital findings indicate a direction for global nanoplastic alleviation solutions based on the optimization of these key features.Conflicts of interest
The authors declare no competing financial interest.Data availability
The dataset used in this paper was produced by the authors from a collection of references. The specific datasets, code and models used in Rstudio and Python are provided in the ESI.†Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (no. 21805197) and the Scientific Research and Innovation Project of Capital Medical University (XSKY2024122). The graphical abstract of this study was drawn using Figdraw.References
- S. B. Borrelle, J. Ringma, K. L. Law, C. C. Monnahan, L. Lebreton, A. McGivern, E. Murphy, J. Jambeck, G. H. Leonard, M. A. Hilleary, M. Eriksen, H. P. Possingham, H. De Frond, L. R. Gerber, B. Polidoro, A. Tahir, M. Bernard, N. Mallos, M. Barnes and C. M. Rochman, Science, 2020, 369, 1515–1518 CrossRef CAS.
- M. MacLeod, H. P. H. Arp, M. B. Tekman and A. Jahnke, Science, 2021, 373, 61–65 CrossRef CAS PubMed.
- V. Nava, S. Chandra, J. Aherne, M. B. Alfonso, A. M. Antão-Geraldes, K. Attermeyer, R. Bao, M. Bartrons, S. A. Berger, M. Biernaczyk, R. Bissen, J. D. Brookes, D. Brown, M. Cañedo-Argüelles, M. Canle, C. Capelli, R. Carballeira, J. L. Cereijo, S. Chawchai, S. T. Christensen, K. S. Christoffersen, E. de Eyto, J. Delgado, T. N. Dornan, J. P. Doubek, J. Dusaucy, O. Erina, Z. Ersoy, H. Feuchtmayr, M. L. Frezzotti, S. Galafassi, D. Gateuille, V. Gonçalves, H.-P. Grossart, D. P. Hamilton, T. D. Harris, K. Kangur, G. B. Kankılıç, R. Kessler, C. Kiel, E. M. Krynak, À. Leiva-Presa, F. Lepori, M. G. Matias, S.-I. S. Matsuzaki, Y. McElarney, B. Messyasz, M. Mitchell, M. C. Mlambo, S. N. Motitsoe, S. Nandini, V. Orlandi, C. Owens, D. Özkundakci, S. Pinnow, A. Pociecha, P. M. Raposeiro, E.-I. Rõõm, F. Rotta, N. Salmaso, S. S. S. Sarma, D. Sartirana, F. Scordo, C. Sibomana, D. Siewert, K. Stepanowska, Ü. N. Tavşanoğlu, M. Tereshina, J. Thompson, M. Tolotti, A. Valois, P. Verburg, B. Welsh, B. Wesolek, G. A. Weyhenmeyer, N. Wu, E. Zawisza, L. Zink and B. Leoni, Nature, 2023, 619, 317–322 CrossRef CAS.
- H. T. Pinheiro, C. MacDonald, R. G. Santos, R. Ali, A. Bobat, B. J. Cresswell, R. Francini-Filho, R. Freitas, G. F. Galbraith, P. Musembi, T. A. Phelps, J. P. Quimbayo, T. E. A. L. Quiros, B. Shepherd, P. V. Stefanoudis, S. Talma, J. B. Teixeira, L. C. Woodall and L. A. Rocha, Nature, 2023, 619, 311–316 CrossRef CAS.
- R. G. Santos, G. E. Machovsky-Capuska and R. Andrades, Science, 2021, 373, 56–60 CrossRef CAS PubMed.
- Y. Zhou, H. Wang, Y. Chen and Q. Jiang, Lancet, 2011, 378, e4 CrossRef.
- K. Mattsson, L. A. Hansson and T. Cedervall, Environ. Sci.: Processes Impacts, 2015, 17, 1712–1721 RSC.
- M. Teng, X. Zhao, C. Wang, C. Wang, J. C. White, W. Zhao, L. Zhou, M. Duan and F. Wu, ACS Nano, 2022, 16, 8190–8204 CrossRef CAS PubMed.
- Y. Li, Z. Liu, M. Li, Q. Jiang, D. Wu, Y. Huang, Y. Jiao, M. Zhang and Y. Zhao, J. Hazard. Mater., 2020, 398, 122990 CrossRef CAS.
- R. C. Thompson, Y. Olsen, R. P. Mitchell, A. Davis, S. J. Rowland, A. W. G. John, D. McGonigle and A. E. Russell, Science, 2004, 304, 838 CrossRef CAS.
- C. Arthur, J. E. Baker and H. A. Bamford, Proceedings of the international research workshop on the occurrence, effects, and fate of microplastic marine debris, https://repository.library.noaa.gov/view/noaa/2509.
- A. L. Andrady, Mar. Pollut. Bull., 2011, 62, 1596–1605 CrossRef CAS PubMed.
- A. Cózar, F. Echevarría, J. I. González-Gordillo, X. Irigoien, B. Úbeda, S. Hernández-León, Á. T. Palma, S. Navarro, J. García-de-Lomas, A. Ruiz, M. L. Fernández-de-Puelles and C. M. Duarte, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 10239–10244 CrossRef PubMed.
- N. P. Ivleva, Chem. Rev., 2021, 121, 11886–11936 CrossRef CAS.
- A. ter Halle, L. Ladirat, X. Gendre, D. Goudouneche, C. Pusineri, C. Routaboul, C. Tenailleau, B. Duployer and E. Perez, Environ. Sci. Technol., 2016, 50, 5668–5675 CrossRef CAS PubMed.
- S. Lambert and M. Wagner, Chemosphere, 2016, 145, 265–268 CrossRef CAS.
- M. Cole and T. S. Galloway, Environ. Sci. Technol., 2015, 49, 14625–14632 CrossRef CAS PubMed.
- M. Cole, P. Lindeque, E. Fileman, C. Halsband and T. S. Galloway, Environ. Sci. Technol., 2015, 49, 1130–1137 CrossRef CAS.
- C. B. Crawford and B. Quinn, in Microplastic Pollutants, ed. C. B. Crawford and B. Quinn, Elsevier, 2017, pp. 57–100 DOI:10.1016/B978-0-12-809406-8.00004-9.
- J. P. da Costa, P. S. M. Santos, A. C. Duarte and T. Rocha-Santos, Sci. Total Environ., 2016, 566–567, 15–26 CrossRef PubMed.
- A. A. Koelmans, E. Besseling and W. J. Shim, in Marine Anthropogenic Litter, ed. M. Bergmann, L. Gutow and M. Klages, Springer International Publishing, Cham, 2015, pp. 325–340 DOI:10.1007/978-3-319-16510-3_12.
- M. Auffan, J. Rose, J.-Y. Bottero, G. V. Lowry, J.-P. Jolivet and M. R. Wiesner, Nat. Nanotechnol., 2009, 4, 634–641 CrossRef CAS.
- NNI, About Nanotechnology, https://www.nano.gov/about-nanotechnology, (accessed 8/26, 2023).
- EU, Chemicals: Commission revises the definition of nanomaterials, https://environment.ec.europa.eu/news/chemicals-commission-revises-definition-nanomaterials-2022-06-10_en, (accessed 10/2, 2023).
- ECHA/RAC/RES-, O-0000006790-71-01/F. In (SEAC), 2020.
- P. A. Hassan, S. Rana and G. Verma, Langmuir, 2015, 31, 3–12 CrossRef CAS PubMed.
- K. Zhang, J. Su, X. Xiong, X. Wu, C. Wu and J. Liu, Environ. Pollut., 2016, 219, 450–455 CrossRef CAS PubMed.
- L. Peng, D. Fu, H. Qi, C. Q. Lan, H. Yu and C. Ge, Sci. Total Environ., 2020, 698, 134254 CrossRef CAS.
- M. Dalela, T. G. Shrivastav, S. Kharbanda and H. Singh, ACS Appl. Mater. Interfaces, 2015, 7, 26530–26548 CrossRef CAS PubMed.
- L. M. Hernandez, N. Yousefi and N. Tufenkji, Environ. Sci. Technol. Lett., 2017, 4, 280–285 CrossRef CAS.
- F. Wang, A. Salvati and P. Boya, Open Biol., 2018, 8, 170271 CrossRef.
- C. M. Rochman, S. M. Kross, J. B. Armstrong, M. T. Bogan, E. S. Darling, S. J. Green, A. R. Smyth and D. Veríssimo, Environ. Sci. Technol., 2015, 49, 10759–10761 CrossRef CAS PubMed.
- Z. Zhang, S.-H. Gao, G. Luo, Y. Kang, L. Zhang, Y. Pan, X. Zhou, L. Fan, B. Liang and A. Wang, Environ. Pollut., 2022, 296, 118737 CrossRef CAS.
- J. Li, Y. Song and Y. Cai, Environ. Pollut., 2020, 257, 113570 CrossRef CAS.
- A. Kolomijeca, J. Parrott, H. Khan, K. Shires, S. Clarence, C. Sullivan, L. Chibwe, D. Sinton and C. M. Rochman, Environ. Sci. Technol., 2020, 54, 1750–1759 CrossRef CAS.
- Y. Huang, X. Qing, W. Wang, G. Han and J. Wang, TrAC, Trends Anal. Chem., 2020, 125, 115821 CrossRef CAS.
- Y. Huang, Q. Liu, W. Jia, C. Yan and J. Wang, Environ. Pollut., 2020, 260, 114096 CrossRef CAS.
- M. Capolupo, L. Sørensen, K. D. R. Jayasena, A. M. Booth and E. Fabbri, Water Res., 2020, 169, 115270 CrossRef CAS PubMed.
- M. Mathissen, V. Scheer, R. Vogt and T. Benter, Atmos. Environ., 2011, 45, 6172–6179 CrossRef CAS.
- M. Sillanpää and P. Sainio, Environ. Sci. Pollut. Res., 2017, 24, 19313–19321 CrossRef PubMed.
- H. Jacob, M. Besson, P. W. Swarzenski, D. Lecchini and M. Metian, Environ. Sci. Technol., 2020, 54, 4733–4745 CrossRef CAS.
- D. Doyle, H. Sundh and B. C. Almroth, Environ. Pollut., 2022, 315, 120434 CrossRef CAS.
- Y. Han, F. Lian, Z. Xiao, S. Gu, X. Cao, Z. Wang and B. Xing, J. Hazard. Mater., 2022, 427, 127870 CrossRef CAS.
- J. Brehm, S. Ritschar, C. Laforsch and M. M. Mair, J. Hazard. Mater., 2023, 458, 131839 CrossRef CAS PubMed.
- J. Ge, P. Jin, S. Xie, J. Beardall, Y. Feng, C. Guo, Z. Ma and G. Gao, Environ. Pollut., 2024, 342, 123127 CrossRef CAS PubMed.
- F. Dang, Q. Wang, X. Yan, Y. Zhang, J. Yan, H. Zhong, D. Zhou, Y. Luo, Y.-G. Zhu, B. Xing and Y. Wang, ACS Nano, 2022, 16, 17157–17167 CrossRef CAS PubMed.
- C. Wang, Q. Luo, J. Zhang, X. Zhang, N. Yang and L. Feng, Environ. Pollut., 2023, 337, 122593 CrossRef CAS PubMed.
- D. Ghosh, A. Sarkar, A. G. Basu and S. Roy, Water Res., 2022, 225, 119114 CrossRef CAS PubMed.
- Z. Guo, J. Li and Z. Zhang, Water Res., 2024, 258, 121706 CrossRef CAS.
- S. Casabianca, A. Bellingeri, S. Capellacci, A. Sbrana, T. Russo, I. Corsi and A. Penna, Environ. Pollut., 2021, 290, 118101 CrossRef CAS PubMed.
- Z. Ji, Y. Huang, Y. Feng, A. Johansen, J. Xue, L. A. Tremblay and Z. Li, Sci. Total Environ., 2021, 788, 147784 Search PubMed.
- Y. Huo, F. A. Dijkstra, M. Possell and B. Singh, Environ. Pollut., 2022, 310, 119892 CrossRef CAS PubMed.
- Y. Hu, M. Shen, C. Wang, Q. Huang, R. Li, G. Dorj, E. Gombojav, J. Du and L. Ren, J. Hazard. Mater., 2024, 465, 133375 CrossRef CAS PubMed.
- H. Xie, C. Wei, W. Wang, R. Chen, L. Cui, L. Wang, D. Chen, Y.-L. Yu, B. Li and Y.-F. Li, J. Hazard. Mater., 2024, 463, 132886 Search PubMed.
- J. S. Stine, N. Aziere, B. J. Harper and S. L. Harper, Micromachines, 2023, 14, 1903 Search PubMed.
- L. Xie, S. Luo, Y. Liu, X. Ruan, K. Gong, Q. Ge, K. Li, V. K. Valev, G. Liu and L. Zhang, Environ. Sci. Technol., 2023, 57, 18203–18214 CrossRef CAS PubMed.
- Y. Luo, X. Zhang, Z. Zhang, R. Naidu and C. Fang, Anal. Chem., 2022, 94, 3150–3157 Search PubMed.
- I. Ishmukhametov, S. Batasheva and R. Fakhrullin, Analyst, 2022, 147, 4616–4628 RSC.
- T. P. Forbes, J. M. Pettibone, E. Windsor, J. M. Conny and R. A. Fletcher, Anal. Chem., 2023, 95, 12373–12382 CrossRef CAS PubMed.
- Y. Tang, T. J. Hardy and J.-Y. Yoon, Biosens. Bioelectron., 2023, 234, 115361 CrossRef CAS PubMed.
- H. I. Labouta, N. Asgarian, K. Rinker and D. T. Cramb, ACS Nano, 2019, 13, 1583–1594 CAS.
- B. Liang, Y. Zhong, Y. Huang, X. Lin, J. Liu, L. Lin, M. Hu, J. Jiang, M. Dai, B. Wang, B. Zhang, H. Meng, J. J. J. Lelaka, H. Sui, X. Yang and Z. Huang, Part. Fibre Toxicol., 2021, 18, 20 CrossRef CAS PubMed.
- D. M. Mitrano, P. Wick and B. Nowack, Nat. Nanotechnol., 2021, 16, 491–500 CrossRef CAS PubMed.
- A. Banerjee and W. L. Shelver, Sci. Total Environ., 2021, 755, 142518 CrossRef CAS PubMed.
- G. M. Zarus, C. Muianga, C. M. Hunter and R. S. Pappas, Sci. Total Environ., 2021, 756, 144010 CrossRef CAS PubMed.
- Y. Su, X. Hu, H. Tang, K. Lu, H. Li, S. Liu, B. Xing and R. Ji, Nat. Nanotechnol., 2022, 17, 76–85 CrossRef CAS PubMed.
- A. C. Morales, J. M. Tomlin, C. P. West, F. A. Rivera-Adorno, B. N. Peterson, S. A. L. Sharpe, Y. Noh, S. M. T. Sendesi, B. E. Boor, J. A. Howarter, R. C. Moffet, S. China, B. T. O’Callahan, P. Z. El-Khoury, A. J. Whelton and A. Laskin, Nat. Nanotechnol., 2022, 17, 1171–1177 CrossRef CAS PubMed.
- X. Y. Liu, Y. C. Zhao, J. B. Dou, Q. H. Hou, J. X. Cheng and X. Y. Jiang, Nano Lett., 2022, 22, 1091–1099 CrossRef CAS PubMed.
- S. Shan, Y. F. Zhang, H. W. Zhao, T. Zeng and X. L. Zhao, Chemosphere, 2022, 298, 134261 CrossRef CAS PubMed.
- R. Jia, J. Han, X. Liu, K. Li, W. Lai, L. Bian, J. Yan and Z. Xi, Toxics, 2023, 11, 127 CrossRef CAS PubMed.
- D. Q. Yang, J. D. Zhu, X. S. Zhou, D. Pan, S. Nan, R. L. Yin, Q. H. Lei, N. Ma, H. M. Zhu, J. G. Chen, L. Han, M. X. Ding and Y. Ding, Environ. Int., 2022, 166, 107362 CrossRef CAS PubMed.
- J. Chen, Z. Xu, Y. Liu, A. Mei, X. Wang and Q. Shi, Ecotoxicol. Environ. Saf., 2023, 252, 114574 CrossRef CAS PubMed.
- Z. B. Sun, Y. Q. Wen, F. Zhang, Z. D. Fu, Y. Y. Yuan, H. B. Kuang, X. D. Kuang, J. Huang, L. P. Zheng and D. L. Zhang, Ecotoxicol. Environ. Saf., 2023, 255, 114796 CrossRef CAS PubMed.
- A. T. B. Guimarães, Í. N. Freitas, N. M. Mubarak, M. M. Rahman, F. P. Rodrigues, A. S. D. L. Rodrigues, D. Barceló, A. R. M. T. Islam and G. Malafaia, J. Hazard. Mater., 2023, 442, 130004 CrossRef PubMed.
- B. Jeong, J. Y. Baek, J. Koo, S. Park, Y. K. Ryu, K. S. Kim, S. Zhang, C. Chung, R. Dogan, H. S. Choi, D. Um, T. K. Kim, W. S. Lee, J. Jeong, W. H. Shin, J. R. Lee, N. S. Kim and D. Y. Lee, J. Hazard. Mater., 2022, 426, 127815 CrossRef CAS PubMed.
- B. Liang, Y. Huang, Y. Zhong, Z. Li, R. Ye, B. Wang, B. Zhang, H. Meng, X. Lin, J. Du, M. Hu, Q. Wu, H. Sui, X. Yang and Z. Huang, J. Hazard. Mater., 2022, 430, 128459 CrossRef CAS PubMed.
- X. B. Chen, L. Y. Xu, Q. L. Chen, S. Y. Su, J. S. Zhuang and D. F. Qiao, Ecotoxicol. Environ. Saf., 2023, 259, 115000 CrossRef CAS PubMed.
- H. Kang, W. Zhang, J. Jing, D. Huang, L. Zhang, J. Wang, L. Han, Z. Liu, Z. Wang and A. Gao, J. Hazard. Mater., 2023, 458, 131949 CrossRef CAS PubMed.
- J. T. Xiao, X. J. Jiang, Y. J. Zhou, G. Sumayyah, L. X. Zhou, B. J. Tu, Q. Z. Qin, J. F. Qiu, X. Qin, Z. Zou and C. Z. Chen, Environ. Pollut., 2022, 292, 118184 CrossRef CAS PubMed.
- J. Tang, W. X. Bu, W. X. Hu, Z. X. Zhao, L. Liu, C. Luo, R. Wang, S. S. Fan, S. L. Yu, Q. Y. Wu, X. K. Wang and X. Y. Zhao, ACS Nano, 2023, 17, 2440–2449 CrossRef CAS PubMed.
- Y. Wang, Z. Wei, K. Xu, X. Wang, X. Gao, Q. Han, S. Wang and M. Chen, Food Chem. Toxicol., 2023, 173, 113642 CrossRef CAS PubMed.
- Y. He, Z. Li, T. Xu, D. Luo, Q. Chi, Y. Zhang and S. Li, Chemosphere, 2022, 307, 135662 CrossRef CAS PubMed.
- T. Huang, W. Zhang, T. Lin, S. Liu, Z. Sun, F. Liu, Y. Yuan, X. Xiang, H. Kuang, B. Yang and D. Zhang, Food Chem. Toxicol., 2022, 160, 112803 CrossRef CAS PubMed.
- M. H. Jin, J. N. Hu, M. Zhang, Z. Meng, G. P. Shi, Z. Wang and W. Li, Environ. Pollut., 2023, 322, 121202 CrossRef CAS PubMed.
- D. Xu, Y. Ma, C. Peng, Y. Gan, Y. Wang, Z. Chen, X. Han and Y. Chen, Environ. Int., 2023, 176, 107968 CrossRef CAS PubMed.
- X. P. Fan, X. J. Wei, H. L. Hu, B. Y. Zhang, D. Q. Yang, H. N. Du, R. J. Zhu, X. T. Sun, Y. R. Oh and N. Gu, Chemosphere, 2022, 288, 132607 CrossRef CAS PubMed.
- L. Li, M. J. Xu, C. He, H. Wang and Q. L. Hu, Environ. Toxicol., 2022, 37, 362–372 CrossRef CAS PubMed.
- X. Wang, Z. Zhao, X. Wang, W. Hu, L. Chao, X. Chu, M. Qian, R. Wang, S. Yu, Q. Wu, J. Tang and X. Zhao, Chemosphere, 2023, 324, 138255 CrossRef CAS.
- Z. Li, T. Xu, L. Peng, X. Tang, Q. Chi, M. Li and S. Li, J. Cell. Physiol., 2023, 238, 151–164 CrossRef CAS PubMed.
- Y. P. Tang, R. Zhao, Q. Y. Pu, C. Yang, L. Yin, Y. Guo, T. Han, Y. Wang, G. Liu, F. Maqbool, L. Xu and J. Zhao, Sci. Total Environ., 2023, 892, 164518 CrossRef PubMed.
- X. M. Meng, J. W. Zhang, W. J. Wang, G. Gonzalez-Gil, J. S. Vrouwenvelder and Z. Y. Li, Chemosphere, 2022, 288, 132631 CrossRef CAS PubMed.
- J. Lian, L. Cheng, X. Zhai, R. Wu, W. Liu, J. Pan, M. J. I. Shohag, X. Xin, Z. He and X. Yang, J. Hazard. Mater., 2022, 440, 129857 CrossRef CAS.
- R. Hu, C. Yao, Y. Li, J. Qu, S. Yu, Y. Han, G. Chen, J. Tang and H. Wei, Ecotoxicol. Environ. Saf., 2022, 248, 114332 CrossRef CAS PubMed.
- P. R. Cai, Y. L. Wang, N. N. Feng, H. Zou, J. H. Gu, Y. Yuan, X. Z. Liu, Z. P. Liu and J. C. Bian, Environ. Toxicol., 2023, 38, 2881–2893 CrossRef CAS PubMed.
- J. Zhang, Y. Zou, L. Hu, Y. Zhao, Y. Fen and H. Xu, J. Sci. Food Agric., 2023, 103, 6452–6462 Search PubMed.
- W. Xu, Y. Yuan, Y. Tian, C. Cheng, Y. Chen, L. Zeng, Y. Yuan, D. Li, L. Zheng and T. Luo, J. Hazard. Mater., 2023, 454, 131470 CrossRef CAS PubMed.
- S. Nikolic, M. Gazdic-Jankovic, G. Rosic, M. Miletic-Kovacevic, N. Jovicic, N. Nestorovic, P. Stojkovic, N. Filipovic, O. Milosevic-Djordjevic, D. Selakovic, M. Zivanovic, D. Seklic, N. Milivojevic, A. Markovic, R. Seist, S. Vasilijic, K. M. Stankovic, M. Stojkovic and B. Ljujic, Environ. Pollut., 2022, 305, 119206 CrossRef CAS PubMed.
- L. X. Zhou, Z. Y. Yu, Y. Y. Xia, S. Q. Cheng, J. Y. Gao, W. Sun, X. J. Jiang, J. Zhang, L. J. Mao, X. Qin, Z. Zou, J. F. Qiu and C. Z. Chen, Environ. Int., 2022, 163, 107220 CrossRef CAS PubMed.
- X. F. Fu, L. Liu, H. Han, Y. Y. Li, S. B. Si, B. Xu, W. J. Dai, H. Yang, T. T. He, X. Du and X. Y. Pei, Environ. Toxicol., 2023, 38, 1277–1291 CrossRef CAS PubMed.
- X. Wang, X. M. Ren, H. He, F. Li, K. Liu, F. Zhao, H. Hu, P. Zhang, B. Huang and X. Pan, Toxicology, 2023, 484, 153391 CrossRef CAS PubMed.
- X. Y. Tang, X. Fan, T. Xu, Y. J. He, Q. R. Chi, Z. Li and S. Li, Environ. Toxicol., 2022, 37, 2552–2565 CrossRef CAS PubMed.
- X. Y. Zhu, L. Peng, E. Q. Song and Y. Song, Chem. Res. Toxicol., 2022, 35, 378–382 Search PubMed.
- X. Wang, Z. Jia, X. Zhou, L. Su, M. Wang, T. Wang and H. Zhang, Sci. Total Environ., 2023, 865, 161271 CrossRef CAS.
- P. Lin, X. Tong, F. Xue, C. Qianru, T. Xinyu, L. Zhe, B. Zhikun and L. Shu, Toxicology, 2022, 480, 153338 CrossRef CAS PubMed.
- X. L. Guo, C. Cheng, L. Wang, D. B. Li, R. H. Fan and X. D. Wei, Environ. Toxicol., 2023, 38, 2487–2498 CrossRef CAS PubMed.
- S. He, J. Wang, L. Zhou, T. Jia, Z. Mao, X. Zhang, L. Zhang, J. Wang, M. Yang and P. Huang, Ecotoxicol. Environ. Saf., 2023, 256, 114906 CrossRef CAS PubMed.
- Y. Wu, Y. Yao, H. Bai, K. Shimizu, R. Li and C. Zhang, Sci. Total Environ., 2023, 855, 158851 CrossRef CAS PubMed.
- Y. Liu, J. Li, B. V. Parakhonskiy, R. Hoogenboom, A. Skirtach and S. De Neve, J. Hazard. Mater., 2024, 462, 132785 CrossRef CAS PubMed.
- D. M. Mitrano, A. Beltzung, S. Frehland, M. Schmiedgruber, A. Cingolani and F. Schmidt, Nat. Nanotechnol., 2019, 14, 362–368 CrossRef CAS PubMed.
- P. E. Redondo-Hasselerharm, G. Vink, D. M. Mitrano and A. A. Koelmans, Environ. Sci.: Nano, 2021, 8, 1761–1770 RSC.
- S. Frehland, R. Kaegi, R. Hufenus and D. M. Mitrano, Water Res., 2020, 182, 115860 CrossRef CAS PubMed.
- A. S. Keller, J. Jimenez-Martinez and D. M. Mitrano, Environ. Sci. Technol., 2020, 54, 911–920 CrossRef CAS PubMed.
- Y. Feng, J.-L. Duan, X.-D. Sun, J.-Y. Ma, Q. Wang, X.-Y. Li, W.-X. Tian, S.-G. Wang and X.-Z. Yuan, Environ. Pollut., 2021, 275, 115755 CrossRef CAS PubMed.
- E. Lahive, R. Cross, A. I. Saarloos, A. A. Horton, C. Svendsen, R. Hufenus and D. M. Mitrano, Sci. Total Environ., 2022, 807, 151022 CrossRef CAS PubMed.
- N. J. Clark, F. R. Khan, C. Crowther, D. M. Mitrano and R. C. Thompson, Sci. Total Environ., 2023, 854, 158765 CrossRef CAS PubMed.
- M. Tamayo-Belda, A. V. Pérez-Olivares, G. Pulido-Reyes, K. Martin-Betancor, M. González-Pleiter, F. Leganés, D. M. Mitrano, R. Rosal and F. Fernández-Piñas, J. Hazard. Mater., 2023, 445, 130625 CrossRef CAS PubMed.
- F. Ribeiro, D. M. Mitrano, C. Hacker, P. Cherek, K. Brigden, S. L. Kaserzon, K. V. Thomas and T. S. Galloway, Environ. Sci. Technol., 2022, 56, 16716–16725 CrossRef CAS PubMed.
- A. H. Tophinke, A. Joshi, U. Baier, R. Hufenus and D. M. Mitrano, Environ. Pollut., 2022, 311, 119933 CrossRef CAS PubMed.
- M. Schmiedgruber, R. Hufenus and D. M. Mitrano, Water Res., 2019, 155, 423–430 CrossRef CAS PubMed.
- R. J. Rauschendorfer, K. M. Whitham, S. Summer, S. A. Patrick, A. E. Pierce, H. Sefi-Cyr, S. Tadjiki, M. D. Kraft, S. R. Emory, D. A. Rider and M. D. Montaño, Front. Toxicol., 2021, 3, 752296 CrossRef PubMed.
- T. Curtis, A. K. Taylor, S. E. Alden, C. Swanson, J. Lo, L. Knight, A. Silva, B. D. Gates, S. R. Emory and D. A. Rider, ACS Omega, 2018, 3, 10572–10588 CrossRef CAS PubMed.
- Y. Luo, L. Li, Y. Feng, R. Li, J. Yang, W. J. G. M. Peijnenburg and C. Tu, Nat. Nanotechnol., 2022, 17, 424–431 CrossRef CAS PubMed.
- F. Abdolahpur Monikh, N. Doornhein, S. Romeijn, M. G. Vijver and W. J. G. M. Peijnenburg, Anal. Methods, 2021, 13, 1576–1583 RSC.
- F. Zhang, Z. Wang, L. Song, H. Fang and D.-G. Wang, Environ. Pollut., 2020, 257, 113451 CrossRef CAS PubMed.
- S. V. Facchetti, R. La Spina, F. Fumagalli, N. Riccardi, D. Gilliland and J. Ponti, Nanomaterials, 2020, 10, 2363 CrossRef CAS PubMed.
- A. Villacorta, L. Vela, M. Morataya-Reyes, R. Llorens-Chiralt, L. Rubio, M. Alaraby, R. Marcos and A. Hernández, Sci. Total Environ., 2023, 880, 163151 CrossRef CAS PubMed.
- D. S. Vicentini, D. J. Nogueira, S. P. Melegari, M. Arl, J. S. Köerich, L. Cruz, N. M. Justino, B. V. Oscar, R. C. Puerari, M. L. N. da Silva, C. Simioni, L. C. Ouriques, M. S. Matias, A. B. de Castilhos Junior and W. G. Matias, Environ. Toxicol. Chem., 2019, 38, 2101–2110 CrossRef CAS PubMed.
- M. Mahmoudi, I. Lynch, M. R. Ejtehadi, M. P. Monopoli, F. B. Bombelli and S. Laurent, Chem. Rev., 2011, 111, 5610–5637 CrossRef CAS PubMed.
- M. W. Smith, N. W. Thomas, P. G. Jenkins, N. G. Miller, D. Cremaschi and C. Porta, Exp. Physiol., 1995, 80, 735–743 CrossRef CAS PubMed.
- F. Nasser and I. Lynch, J. Proteomics, 2016, 137, 45–51 CrossRef CAS PubMed.
- A. Lesniak, A. Salvati, M. J. Santos-Martinez, M. W. Radomski, K. A. Dawson and C. Åberg, J. Am. Chem. Soc., 2013, 135, 1438–1444 CrossRef CAS PubMed.
- P. Kolandhasamy, L. Su, J. Li, X. Qu, K. Jabeen and H. Shi, Sci. Total Environ., 2018, 610–611, 635–640 CrossRef CAS PubMed.
- J. Mosquera, I. García and L. M. Liz-Marzán, Acc. Chem. Res., 2018, 51, 2305–2313 CrossRef CAS PubMed.
- L. Liu, K. Xu, B. Zhang, Y. Ye, Q. Zhang and W. Jiang, Sci. Total Environ., 2021, 779, 146523 CrossRef CAS PubMed.
- C. G. Avio, S. Gorbi, M. Milan, M. Benedetti, D. Fattorini, G. d'Errico, M. Pauletto, L. Bargelloni and F. Regoli, Environ. Pollut., 2015, 198, 211–222 CrossRef CAS PubMed.
- M. E. Guicciardi, M. Leist and G. J. Gores, Oncogene, 2004, 23, 2881–2890 CrossRef CAS PubMed.
- X. Yuan, X. Zhang, L. Sun, Y. Wei and X. Wei, Part. Fibre Toxicol., 2019, 16, 18 CrossRef PubMed.
- R. L. Auten and J. M. Davis, Pediatr. Res., 2009, 66, 121–127 CrossRef CAS PubMed.
- S. E. Lee, Y. Yi, S. Moon, H. Yoon and Y. S. Park, Metabolites, 2022, 12, 897 CrossRef CAS PubMed.
- X. Xie, T. Deng, J. Duan, J. Xie, J. Yuan and M. Chen, Ecotoxicol. Environ. Saf., 2020, 190, 110133 CrossRef CAS PubMed.
- J. Hou, Z. Lei, L. Cui, Y. Hou, L. Yang, R. An, Q. Wang, S. Li, H. Zhang and L. Zhang, Ecotoxicol. Environ. Saf., 2021, 212, 112012 CrossRef CAS PubMed.
- C. Guo, L. Sun, X. Chen and D. Zhang, Neural Regener. Res., 2013, 8, 2003–2014 CAS.
- J. Hwang, D. Choi, S. Han, J. Choi and J. Hong, Sci. Total Environ., 2019, 684, 657–669 CrossRef CAS PubMed.
- J. H. Hwang, T. T. T. Trang, O. Lee, G. Park, A. Zargaran and N. J. Kim, Acta Mater., 2020, 191, 1–12 CrossRef CAS.
- A. Poma, G. Vecchiotti, S. Colafarina, O. Zarivi, M. Aloisi, L. Arrizza, G. Chichiriccò and P. Di Carlo, Nanomaterials, 2019, 9, 1299 CrossRef CAS PubMed.
- M. Cole, C. Liddle, G. Consolandi, C. Drago, C. Hird, P. K. Lindeque and T. S. Galloway, Mar. Pollut. Bull., 2020, 160, 111552 Search PubMed.
- L. Poillet-Perez, G. Despouy, R. Delage-Mourroux and M. Boyer-Guittaut, Redox Biol., 2015, 4, 184–192 CrossRef CAS PubMed.
- Ž. Roje, K. Ilić, E. Galić, I. Pavičić, P. Turčić, Z. Stanec and I. V. Vrček, Arh. Hig. Rada Toksikol., 2019, 70, 310–314 Search PubMed.
- L. Böhmert, M. Girod, U. Hansen, R. Maul, P. Knappe, B. Niemann, S. M. Weidner, A. F. Thünemann and A. Lampen, Nanotoxicology, 2014, 8, 631–642 CrossRef PubMed.
- M. Longmire, P. L. Choyke and H. Kobayashi, Nanomedicine, 2008, 3, 703–717 CrossRef CAS PubMed.
- V. Hornung, F. Bauernfeind, A. Halle, E. O. Samstad, H. Kono, K. L. Rock, K. A. Fitzgerald and E. Latz, Nat. Immunol., 2008, 9, 847–856 Search PubMed.
- W. Wei, Y. Li, M. Lee, N. Andrikopoulos, S. Lin, C. Chen, D. T. Leong, F. Ding, Y. Song and P. C. Ke, Nat. Commun., 2022, 13, 4757 CrossRef CAS PubMed.
- F. Peng, M. I. Setyawati, J. K. Tee, X. Ding, J. Wang, M. E. Nga, H. K. Ho and D. T. Leong, Nat. Nanotechnol., 2019, 14, 279–286 Search PubMed.
- L. Van Cauwenberghe and C. R. Janssen, Environ. Pollut., 2014, 193, 65–70 CrossRef CAS PubMed.
- F. M. Kluxen, Arch. Toxicol., 2019, 93, 2409–2420 CrossRef CAS PubMed.
- I. M. Adjei, B. Sharma and V. Labhasetwar, in Nanomaterial: Impacts on Cell Biology and Medicine, ed. D. G. Capco and Y. Chen, Springer, Netherlands, Dordrecht, 2014, pp. 73–91 DOI:10.1007/978-94-017-8739-0_5.
- B. R. Kiran, H. Kopperi and S. Venkata Mohan, Rev. Environ. Sci. Bio/Technol., 2022, 21, 169–203 CrossRef PubMed.
- K. C. Dibbon, G. V. Mercer, A. S. Maekawa, J. Hanrahan, K. L. Steeves, L. C. M. Ringer, A. J. Simpson, M. J. Simpson, A. A. Baschat, J. C. Kingdom, C. K. Macgowan, J. G. Sled, K. J. Jobst and L. S. Cahill, Biol. Reprod., 2024, 110, 211–218 CrossRef PubMed.
- X. Hua and D. Wang, Rev. Environ. Contam. Toxicol., 2022, 260, 12 CrossRef.
- K. Wang, Y. Du, P. Li, C. Guan, M. Zhou, L. Wu, Z. Liu and Z. Huang, J. Nanobiotechnol., 2024, 22, 96 CrossRef CAS PubMed.
- M. Prüst, J. Meijer and R. H. S. Westerink, Part. Fibre Toxicol., 2020, 17, 24 CrossRef PubMed.
- Q. Wang, J. Wang, H. Chen and Y. Zhang, Environ. Sci. Pollut. Res., 2023, 30, 17360–17373 CrossRef CAS PubMed.
- T. Hirano, Int. Immunol., 2021, 33, 127–148 CrossRef CAS PubMed.
- A. Tylutka, Ł. Walas and A. Zembron-Lacny, Front. Immunol., 2024, 15, 1330386 CrossRef CAS PubMed.
- S. Kany, J. T. Vollrath and B. Relja, Int. J. Mol. Sci., 2019, 20, 6008 CrossRef CAS PubMed.
- K. N. Couper, D. G. Blount and E. M. Riley, J. Immunol., 2008, 180, 5771–5777 CrossRef CAS PubMed.
- D. Lobo-Silva, G. M. Carriche, A. G. Castro, S. Roque and M. Saraiva, J. Neuroinflammation, 2016, 13, 297 CrossRef PubMed.
- V. Carlini, D. M. Noonan, E. Abdalalem, D. Goletti, C. Sansone, L. Calabrone and A. Albini, Front. Immunol., 2023, 14, 1161067 CrossRef CAS PubMed.
- V. Camacho, V. Kuznetsova and R. S. Welner, Front. Immunol., 2021, 12, 772408 CrossRef CAS PubMed.
- G. Arango Duque and A. Descoteaux, Front. Immunol., 2014, 5, 491 Search PubMed.
- A. Markovics, K. S. Rosenthal, K. Mikecz, R. E. Carambula, J. C. Ciemielewski and D. H. Zimmerman, Biomedicines, 2022, 10, 44 CrossRef CAS PubMed.
- C. Q. Yong, S. Valiyaveettil and B. L. Tang, Int. J. Environ. Res. Public Health, 2020, 17, 1509 CrossRef CAS PubMed.
- J. Liu, Z. Liu, Y. Pang and H. Zhou, J. Nanobiotechnol., 2022, 20, 127 CrossRef CAS PubMed.
- J.-H. Woo, H. J. Seo, J.-Y. Lee, I. Lee, K. Jeon, B. Kim and K. Lee, Part. Fibre Toxicol., 2023, 20, 2 CrossRef CAS PubMed.
- M. Chen, Y. Yue, X. Bao, X. Feng, Z. Ou, Y. Qiu, K. Yang, Y. Yang, Y. Yu and H. Yu, Aquacult. Rep., 2022, 27, 101423 Search PubMed.
- C. Milillo, E. Aruffo, P. Di Carlo, A. Patruno, M. Gatta, A. Bruno, M. Dovizio, L. Marinelli, M. P. Dimmito, V. Di Giacomo, C. Paolini, M. Pesce and P. Ballerini, Front. Public Health, 2024, 12, 1385387 CrossRef PubMed.
- F. Clérigo, S. Ferreira, C. Ladeira, A. Marques-Ramos, M. Almeida-Silva and L. A. Mendes, Toxics, 2022, 10, 402 CrossRef PubMed.
- L. Baron, A. Gombault, M. Fanny, B. Villeret, F. Savigny, N. Guillou, C. Panek, M. Le Bert, V. Lagente, F. Rassendren, N. Riteau and I. Couillin, Cell Death Dis., 2015, 6, e1629 CrossRef CAS PubMed.
- M. S. Yee, L.-W. Hii, C. K. Looi, W.-M. Lim, S.-F. Wong, Y.-Y. Kok, B.-K. Tan, C.-Y. Wong and C.-O. Leong, Nanomaterials, 2021, 11, 496 CrossRef CAS PubMed.
- S.-L. Hsieh, S. Hsieh, R.-Q. Xu, Y.-T. Chen, C.-W. Chen, R. R. Singhania, Y.-C. Chen, T.-H. Tsai and C.-D. Dong, Environ. Technol. Innovation, 2023, 30, 103073 CrossRef CAS.
- I. Brandts, R. Solà, M. Garcia-Ordoñez, A. Gella, A. Quintana, B. Martin, A. Esteve-Codina, M. Teles and N. Roher, Environ. Sci.: Nano, 2023, 10, 2245–2258 RSC.
- Z. Fan, Y. Zhang, Y. Fang, H. Zhong, T. Wei, H. Akhtar, J. Zhang, M. Yang, Y. Li, X. Zhou, Z. Sun and J. Wang, J. Hazard. Mater., 2024, 476, 134878 CrossRef CAS PubMed.
- S. Lin, H. Zhang, C. Wang, X. L. Su, Y. Song, P. Wu, Z. Yang, M. H. Wong, Z. Cai and C. Zheng, Environ. Sci. Technol., 2022, 56, 12483–12493 CrossRef CAS PubMed.
- Y. Zhou, L. Zhao, H. Xu, E. G. Xu, M. Li and Y. Wang, Sustainability, 2022, 14, 13405 CrossRef.
- H. Yuan, S. Wen, Y. Zhao, L. Hu and H. Xu, Toxicol. Res., 2023, 12, 446–456 CrossRef CAS PubMed.
- J. Liu, P. Yang, G. Zuo, S. He, W. Tan, X. Zhang, C. Su, L. Zhao, L. Wei, Y. Chen, X. Ruan and Y. Chen, Lipids Health Dis., 2018, 17, 153 CrossRef PubMed.
- X. Shen, X. Liang, X. Ji, J. You, X. Zhuang, Y. Song, H. Yin, M. Zhao and L. Zhao, Food Funct., 2021, 12, 8681–8693 RSC.
- X. Zhang, M. Xia, J. Zhao, Z. Cao, W. Zou and Q. Zhou, Environ. Int., 2022, 158, 106922 CrossRef CAS PubMed.
- H. Mao, Z. Yin, M. Wang, W. Zhang, S. H. A. Raza, F. Althobaiti, L. Qi and J. Wang, Front. Veterinary Sci., 2022, 9, 847363 CrossRef PubMed.
- R. Sussarellu, M. Suquet, Y. Thomas, C. Lambert, C. Fabioux, M. E. J. Pernet, N. Le Goïc, V. Quillien, C. Mingant, Y. Epelboin, C. Corporeau, J. Guyomarch, J. Robbens, I. Paul-Pont, P. Soudant and A. Huvet, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 2430–2435 CrossRef CAS PubMed.
- K. Jomova, R. Raptova, S. Y. Alomar, S. H. Alwasel, E. Nepovimova, K. Kuca and M. Valko, Arch. Toxicol., 2023, 97, 2499–2574 CrossRef CAS PubMed.
- S. Bisht, M. Faiq, M. Tolahunase and R. Dada, Nat. Rev. Urol., 2017, 14, 470–485 CrossRef CAS PubMed.
- K. Sharma, A. Sharma and P. Bhatnagar, Environ. Sci. Pollut. Res., 2024, 31, 23680–23696 CrossRef CAS PubMed.
- P. Chen, B. R. Zirkin and H. Chen, Endocr. Rev., 2020, 41, 22–32 CrossRef PubMed.
- E. Diamanti-Kandarakis, J.-P. Bourguignon, L. C. Giudice, R. Hauser, G. S. Prins, A. M. Soto, R. T. Zoeller and A. C. Gore, Endocr. Rev., 2009, 30, 293–342 CrossRef CAS PubMed.
- N. Engelhard, K. Prchal and M. Nenner, Angew. Chem., Int. Ed. Engl., 1967, 6, 615–626 CrossRef CAS PubMed.
- W. Koh, H. Kwak, E. Cheong and C. J. Lee, Nat. Rev. Neurosci., 2023, 24, 523–539 CrossRef CAS PubMed.
- R. B. Lydiard, J. Clin. Psychiatry, 2003, 64(Suppl 3), 21–27 CAS.
- A.-K. Kraeuter, P. C. Guest and Z. Sarnyai, in Pre-Clinical Models: Techniques and Protocols, ed. P. C. Guest, Springer New York, New York, NY, 2019, pp. 99–103 DOI:10.1007/978-1-4939-8994-2_9.
- E. L. Newman, K. Gupta, J. R. Climer, C. K. Monaghan and M. E. Hasselmo, Front. Behav. Neurosci., 2012, 6, 24 Search PubMed.
- A. Fisahn, F. G. Pike, E. H. Buhl and O. Paulsen, Nature, 1998, 394, 186–189 CrossRef CAS PubMed.
- L. Teles-Grilo Ruivo and J. Mellor, Front. Synaptic Neurosci., 2013, 5, 2 Search PubMed.
- O. A. C. Petroff, Neuroscientist, 2002, 8, 562–573 CrossRef CAS PubMed.
- C. Gottesmann, Neuroscience, 2002, 111, 231–239 CrossRef CAS PubMed.
- H. M. Dusza, E. A. Katrukha, S. M. Nijmeijer, A. Akhmanova, A. D. Vethaak, D. I. Walker and J. Legler, Environ. Health Perspect., 2022, 130, 97006 CrossRef CAS PubMed.
- A. Nandi, L. J. Yan, C. K. Jana and N. Das, Oxid. Med. Cell. Longevity, 2019, 2019, 9613090 Search PubMed.
- P. Diaz-Vivancos, A. de Simone, G. Kiddle and C. H. Foyer, Free Radical Biol. Med., 2015, 89, 1154–1164 CrossRef CAS PubMed.
- K. S. Payne, D. J. Mazur, J. M. Hotaling and A. W. Pastuszak, J. Urol., 2019, 202, 674–681 CrossRef PubMed.
- D. M. Kelly and T. H. Jones, J. Endocrinol., 2013, 217, R25–R45 CAS.
- Y. Okada, in Progress in Brain Research, ed. R. R. Mize, R. E. Marc and A. M. Sillito, Elsevier, 1992, vol. 90, pp. 249–262 Search PubMed.
- S. J. Hutton, L. Kashiwabara, E. Anderson, S. Siddiqui, B. Harper, S. Harper and S. M. Brander, Front. Toxicol., 2024, 6, 1490223 CrossRef PubMed.
- B. Du, T. Li, H. He, X. Xu, C. Zhang, X. Lu, Y. Wang, J. Cao, Y. Lu, Y. Liu, S. Hu, J. Li, L. Li and M. Shi, Int. J. Nanomed., 2024, 19, 7617–7630 CrossRef PubMed.
- K. Płuciennik, P. Sicińska, W. Misztal and B. Bukowska, Cells, 2024, 13, 768 CrossRef PubMed.
- Y. Li, M. Xu, Z. Zhang, G. Halimu, Y. Li, Y. Li, W. Gu, B. Zhang and X. Wang, J. Hazard. Mater., 2022, 424, 127508 CrossRef CAS PubMed.
- Y. Xu, Q. Ou, J. P. van der Hoek, G. Liu and K. M. Lompe, Environ. Sci. Technol., 2024, 58, 991–1009 CrossRef CAS PubMed.
- V. I. Slaveykova and M. Marelja, Microplastics, 2023, 2, 389–410 CrossRef.
- J. Rong, C. Yuan, X. Yin, X. Wu, F. He, Y. Wang, K. S.-Y. Leung and S. Lin, Sci. Total Environ., 2024, 930, 172681 CrossRef CAS PubMed.
- T. Zhang, S. Yang, Y. Ge, X. Wan, Y. Zhu, F. Yang, J. Li, S. Gong, Y. Cheng, C. Hu, Z. Chen, L. Yin, Y. Pu and G. Liang, Part. Fibre Toxicol., 2023, 20, 46 CrossRef CAS PubMed.
- Y. Wu, X. Tan, X. Shi, P. Han and H. Liu, Toxics, 2023, 11, 653 CrossRef CAS PubMed.
- M.-J. Kim, Y. Herchenova, J. Chung, S.-H. Na and E.-J. Kim, Water Res., 2022, 226, 119286 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: The literature screening process for meta-analysis, effect size calculation method, bias calculation, sensitivity analysis, mean effect sizes and 95% confidence intervals (CIs) for different subcategories of toxicity indicators in the 45 selected studies, likelihood ratio tests for different variables in the mixed-effects multivariate model as well as the main parameters used for machine learning and ultimately, the selected hyperparameters. The raw data used for meta-analysis and machine learning, as well as Rstudio and Python code, are provided in the ESI. See DOI: https://doi.org/10.1039/d5nh00024f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |